Key Points
Question
What pharmacological treatments are associated with the highest likelihood of hemodynamically significant patent ductus arteriosus (PDA) closure in premature infants?
Findings
In this network meta-analysis that included 68 randomized trials with 4802 infants, a high dose of oral ibuprofen was associated with a statistically significantly higher likelihood of hemodynamically significant PDA closure vs standard doses of intravenous ibuprofen (odds ratio, 3.59) or intravenous indomethacin (odds ratio, 2.35). Placebo or no treatment was not associated with an increased likelihood of mortality, necrotizing enterocolitis, or intraventricular hemorrhage.
Meaning
A high dose of oral ibuprofen may offer the highest likelihood of hemodynamically significant PDA closure in preterm infants. Conservative management of hemodynamically significant PDA is not likely to increase morbidity and mortality.
Abstract
Importance
Despite increasing emphasis on conservative management of patent ductus arteriosus (PDA) in preterm infants, different pharmacotherapeutic interventions are used to treat those developing a hemodynamically significant PDA.
Objectives
To estimate the relative likelihood of hemodynamically significant PDA closure with common pharmacotherapeutic interventions and to compare adverse event rates.
Data Sources and Study Selection
The databases of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until August 15, 2015, and updated on December 31, 2017, along with conference proceedings up to December 2017. Randomized clinical trials that enrolled preterm infants with a gestational age younger than 37 weeks treated with intravenous or oral indomethacin, ibuprofen, or acetaminophen vs each other, placebo, or no treatment for a clinically or echocardiographically diagnosed hemodynamically significant PDA.
Data Extraction and Synthesis
Data were independently extracted in pairs by 6 reviewers and synthesized with Bayesian random-effects network meta-analyses.
Main Outcomes and Measures
Primary outcome: hemodynamically significant PDA closure; secondary: included surgical closure, mortality, necrotizing enterocolitis, and intraventricular hemorrhage.
Results
In 68 randomized clinical trials of 4802 infants, 14 different variations of indomethacin, ibuprofen, or acetaminophen were used as treatment modalities. The overall PDA closure rate was 67.4% (2867 of 4256 infants). A high dose of oral ibuprofen was associated with a significantly higher odds of PDA closure vs a standard dose of intravenous ibuprofen (odds ratio [OR], 3.59; 95% credible interval [CrI], 1.64-8.17; absolute risk difference, 199 [95% CrI, 95-258] more per 1000 infants) and a standard dose of intravenous indomethacin (OR, 2.35 [95% CrI, 1.08-5.31]; absolute risk difference, 124 [95% CrI, 14-188] more per 1000 infants). Based on the ranking statistics, a high dose of oral ibuprofen ranked as the best pharmacotherapeutic option for PDA closure (mean surface under the cumulative ranking [SUCRA] curve, 0.89 [SD, 0.12]) and to prevent surgical PDA ligation (mean SUCRA, 0.98 [SD, 0.08]). There was no significant difference in the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage with use of placebo or no treatment compared with any of the other treatment modalities.
Conclusions and Relevance
A high dose of oral ibuprofen was associated with a higher likelihood of hemodynamically significant PDA closure vs standard doses of intravenous ibuprofen or intravenous indomethacin; placebo or no treatment did not significantly change the likelihood of mortality, necrotizing enterocolitis, or intraventricular hemorrhage.
Trial Registration
PROSPERO Identifier: CRD42015015797
This network meta-analysis of randomized trials compares the efficacy and safety of indomethacin, ibuprofen, and acetaminophen for closure of clinically or echocardiographically diagnosed hemodynamically significant patent ductus arteriosus (PDA) in preterm infants.
Introduction
A common early cardiovascular problem of prematurely born infants is hemodynamically significant patent ductus arteriosus (PDA). The utility of active management and the timing and modality of PDA treatment have been debated. Persistent ductal shunting may lead to pulmonary overcirculation, increasing the risk of bronchopulmonary dysplasia; conversely, shunting may induce systemic hypoperfusion, increasing the risk of necrotizing enterocolitis, intraventricular hemorrhage, renal failure, and death. Nonsteroidal anti-inflammatory drugs along with other pharmacotherapeutic agents have been used to close PDAs to prevent such complications. However, conservative management of PDA without the use of pharmacotherapeutic agents has recently increased. The hypothesis is that a large proportion of the PDAs that occur in preterm infants would spontaneously close within the first few days, thereby having minimal effect on clinical outcomes. As a result, emphasis has been placed on targeted pharmacotherapeutic treatment of PDAs when deemed hemodynamically significant by the clinician based on clinical and echocardiographic parameters. However, lack of pharmacokinetic and pharmacodynamic data on nonsteroidal anti-inflammatory drug use in preterm infants has led to the use of different drugs in varying doses and routes of administration. The 2 most commonly used treatment options are standard doses of intravenous ibuprofen and intravenous indomethacin.
The availability of different management options poses a challenge for neonatologists when making evidence-based management decisions after diagnosing hemodynamically significant PDAs. The dilemma is whether to use pharmacotherapy at all, and if a decision is made to treat the PDA medically, what should be the ideal choice of pharmacotherapy. Therefore, a comprehensive systematic review and Bayesian network meta-analysis was conducted to summarize the evidence from randomized clinical trials comparing placebo, indomethacin, ibuprofen, and acetaminophen for the treatment of hemodynamically significant PDAs in preterm infants.
Methods
The network meta-analysis protocol is available in Supplement 1 and has been published. This study complies with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research guidance on network meta-analysis and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for reporting of systematic reviews incorporating network meta-analysis of health care interventions. The differences between the protocol and the final article are summarized in Supplement 2.
Eligibility Criteria
Studies were included if they were randomized clinical trials that enrolled preterm infants with a gestational age younger than 37 weeks at birth or low-birth-weight infants (<2500 g) who were treated with either intravenous or oral formulations of indomethacin, ibuprofen, or acetaminophen compared with another medication, placebo, or no treatment for hemodynamically significant PDA diagnosed clinically or echocardiographically during the neonatal period (defined as <28 days of life; a full glossary of abbreviations and acronyms, including medication doses and routes, appears in eTable 1 in Supplement 3). Studies were excluded in which a medication was used prophylactically (ie, within the first 24 hours of life without documented clinical or echocardiographic evidence of hemodynamically significant PDA) or surgery was a primary treatment modality.
Primary and Secondary Outcomes
Fourteen outcomes were defined a priori, which included 3 effectiveness outcomes and 11 adverse events (Table). The primary outcome was hemodynamically significant PDA closure within 1 week of administration of the first dose of the intervention and defined echocardiographically (as physical closure of PDA or change from hemodynamically significant to nonsignificant status based on a priori–defined parameters) or clinically (disappearance of cardiac murmur). The other 2 effectiveness outcomes were need for repeat pharmacotherapy and surgical ligation.
Table. A Priori–Defined Outcome Measures.
| Outcome Measure | Definition |
|---|---|
| Primary Outcome | |
| Effectiveness outcome | |
| PDA closurea | Closure within 1 wk of administration of the first dose (PDA diagnosed either clinically or by echocardiographic criteria) |
| Secondary Outcomes | |
| Effectiveness outcomes | |
| Need for repeat pharmacotherapya | No. of neonates who require a repeat course following initial treatment of persistent hemodynamically significant PDA |
| Need for surgical closure of the PDAa | No. of neonates who require closure following failure of pharmacological PDA closure |
| Adverse Events | |
| Neonatal mortalitya | Death at postmenstrual age of 36 wks or before hospital discharge |
| Gastrointestinal events | |
| Necrotizing enterocolitisa | No. of neonates with ≥stage 2 based on the Bell criteria |
| Intestinal perforation | No. of neonates with event |
| Gastrointestinal bleeding | No. of neonates with event |
| Time to full enteral feedings | Postnatal age at stopping parenteral nutrition and achievement of full enteral feedings |
| Bronchopulmonary dysplasiaa | No. of neonates who require oxygen at postmenstrual age of 36 weeks |
| Neurological events | |
| Intraventricular hemorrhagea | No. of neonates with any grade based on the Papile criteria |
| Severe intraventricular hemorrhage | No. of neonates with grades 3-4 based on the Papile criteria |
| Periventricular leukomalacia | No. of neonates with any grade documented on cranial ultrasound |
| Neurodevelopmental disability | No. of children with any reported disability at 1-2 y of age (ie, motor, cognitive, sensory impairments) |
| Oliguriaa | No. of neonates with reduced urine output defined as <1 mL/kg/h |
Abbreviation: PDA, patent ductus arteriosus.
Indicates an outcome included in the network meta-analysis
The adverse events were death at postmenstrual age of 36 weeks or before hospital discharge, necrotizing enterocolitis (≥stage 2 based on the Bell criteria), bronchopulmonary dysplasia (defined as oxygen use at postmenstrual age of 36 weeks), intraventricular hemorrhage (any grade based on the Papile criteria), and oliguria (defined as urine output <1 mL/kg/h). The 6 outcomes that were not included in the quantitative synthesis due to lack of sufficient data were severe intraventricular hemorrhage, periventricular leukomalacia, neurodevelopmental disability, intestinal perforation, gastrointestinal bleeding, and time to full enteral feedings (Table).
Information Sources and Trial Search
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched electronically from inception until August 15, 2015, and updated on December 31, 2017, prior to the final data analysis (eTable 2 in Supplement 3). Registered details of selected trials in the US National Institutes of Health resource (http://www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) search portal were sought.
Additional related trials were sought from personal communication with experts in the field, reviewing the reference lists of relevant articles, abstracts, and conference proceedings (European Society for Pediatric Research and US pediatric academic societies from 1990-2017). There were no language restrictions.
Study Selection and Risk of Bias
The retrieved titles, abstracts, and full text were screened by 2 independent reviewers in duplicate (S.M., I.D.F., M.E.T., A.M.Z., Y.Z., B.S.) to assess their eligibility. The risk of bias for the eligible studies was assessed according to a modified and validated version of the Cochrane Collaboration risk of bias tool by 2 independent reviewers (eFigure 1 and eText 1 in Supplement 3).
Data extraction was performed by 6 reviewers (S.M., I.D.F., M.E.T., A.M.Z., Y.Z., B.S.) using a prespecified standardized data extraction form and working independently in pairs and in duplicate. Discrepancies were resolved through discussion or in consultation with a third reviewer (S.M. or I.D.F.).
Data Synthesis and Analysis
For each outcome, an initial pairwise meta-analysis was conducted using a random-effects model for every direct pairwise comparison, followed by a Bayesian random-effects network meta-analysis to compare all interventions simultaneously using the Markov-chain Monte Carlo method conducted under the assumption of transitivity.
Transitivity was defined as the assumption that the studies were sufficiently similar in their distribution of effect modifiers so that indirect comparisons could be used as a valid method to compare 2 treatment options. Transitivity was assessed by subjectively comparing the distribution of the population, the intervention, and the methodological characteristics of the studies. The consistency assumption among the combined sources of evidence in the network was first evaluated globally for the entire network using the design × treatment interaction model, and then locally for each treatment comparison using the node-splitting model.
The mean surface under the cumulative ranking (SUCRA) curve for each intervention was calculated. Based on the mean SUCRA values, heat maps were generated to efficiently recognize what were most likely the best and worst interventions for each outcome. For both the meta-analysis and the network meta-analysis, Bayesian hierarchical models with noninformative priors assigned to all model parameters were used.
For each meta-analysis, the I2 statistic was used to assess the heterogeneity of the trials. In the network meta-analysis, a common within-network heterogeneity was assumed because the treatments were of similar nature. A series of 100 000 simulations was used to allow convergence, and after thinning of 10 and discarding the first 20 000 simulations, the outputs were produced. The model convergence was assessed on the basis of Gelman and Rubin diagnostic tests.
Odds ratios (ORs) and 95% credible intervals (95% CrIs) were estimated from the medians and the 2.5th and 97.5th percentiles of the posterior distributions in the simulations. A network absolute risk difference was calculated from the network OR estimates using an assumed control risk that was derived by dividing the total event number by the total infant number in the control groups in the network.
Network Sensitivity and Meta-Regression Analyses
The following potential sources of heterogeneity were identified a priori: gestational age, birth weight, different doses of the interventions, age at the time of administration of the first dose of the intervention, echocardiographic findings, and risk of bias. The overall risk of bias for each study was assessed by taking the average of the 3 most important risk of bias items identified by expert consensus (ie, sequence generation, allocation concealment, and blinding).
Sensitivity analyses were conducted for all outcomes including only the high-quality studies (those with low risk and probably low risk of bias). When at least 10 studies were available, network meta-regression was conducted assuming a common fixed coefficient across comparisons to explore the effect of gestational age, birth weight, age of treatment initiation, and year of publication on the most important clinical outcomes (ie, PDA closure, need for repeat pharmacotherapy, mortality, and necrotizing enterocolitis).
All analyses were performed using WinBUGS version 1.4.3 and OpenBUGS version 3.2.3 revision 1012 (MRC Biostatistics Unit), NetMetaXL, GeMTC GUI, and RStudio packages. The design × treatment model was performed in Stata version 15 (StataCorp) using the network command.
Assessment of the Quality of the Evidence
The quality of the evidence for each direct, indirect, and network effects estimate was evaluated for the primary and main secondary outcomes according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method for network meta-analysis. The quality of the evidence for the direct estimates started as high and was decreased to moderate, low, or very low based on risk of bias, imprecision, heterogeneity, indirectness, and publication bias.
Publication bias was assessed by visual inspection of asymmetry in the funnel plots. The quality of the evidence for estimates of the indirect and network effects were computed from the direct estimates by evaluating each indirect comparison from the network geometry, qualitative assessment of intransitivity, and quantitative assessment of incoherence based on the inconsistency test.
Results
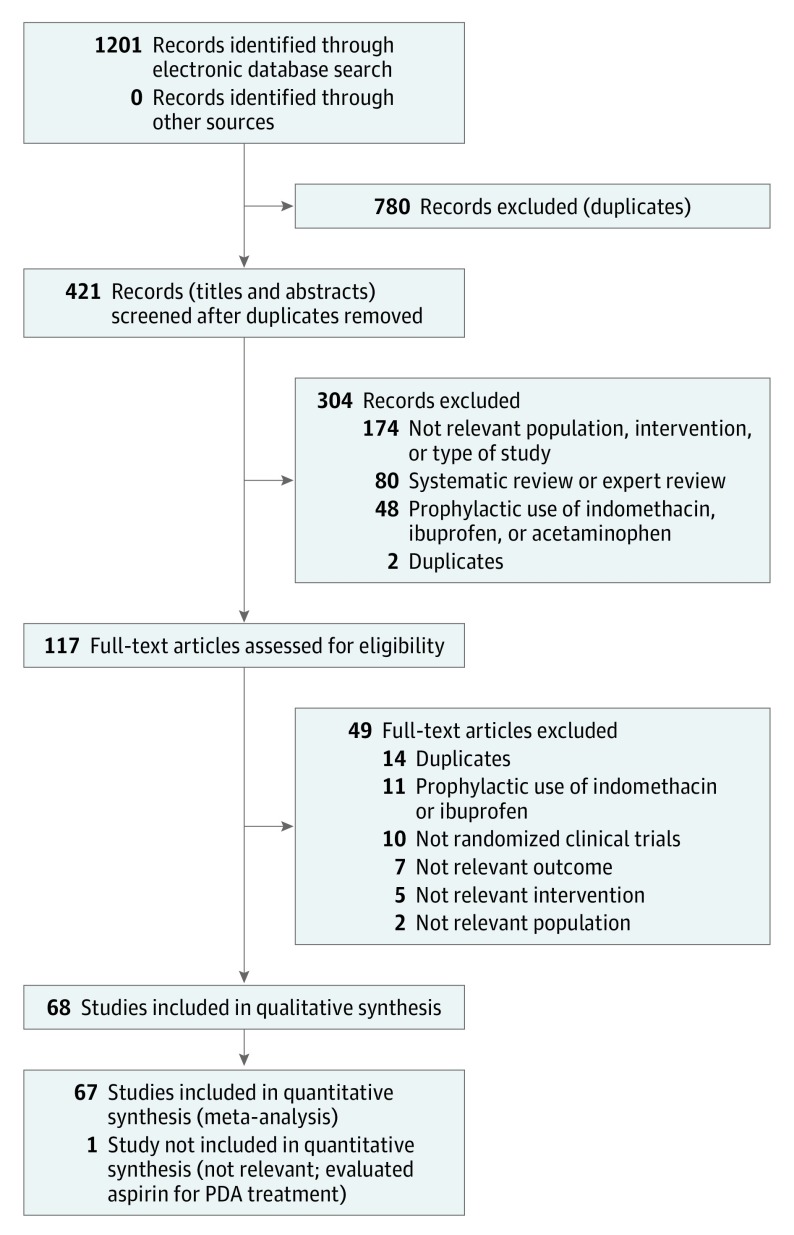
Among 1201 records retrieved, 68 randomized clinical trials met inclusion criteria and included 4802 preterm infants. Details regarding the study selection appear in the flow diagram (Figure 1). Forty-nine studies were excluded after full-text screening (eTable 3 in Supplement 3). The clinical and methodological characteristics of the included studies appear in eTable 4 in Supplement 3. The studies were published between 1980 and 2017. Sixty-one of the 68 studies were published in English. The remaining were published in Polish, Turkish, Persian, Spanish, Korean, Chinese, and French.
Figure 1. Literature Search and Study Selection Flow Diagram.
PDA indicates patent ductus arteriosus.
Fourteen different variations of indomethacin, ibuprofen, or acetaminophen were used as treatment modalities across the studies. The variations included differences in route of administration (intravenous or oral), dose of medication (standard dose, high dose, prolonged course), method of administration (bolus dose, continuous infusion), and cointerventions (concomitant use of furosemide, dopamine, or echocardiographically guided indomethacin infusion). The dosage for intravenous indomethacin was defined as 0.1 to 0.3 mg/kg administered intravenously every 12 to 24 hours for a total of 3 doses. A standard dose of ibuprofen was defined as 10 mg/kg followed by 5 mg/kg administered every 12 to 24 hours for a total of 3 doses (both intravenous and oral administrations). A high dose of ibuprofen was defined as 15 to 20 mg/kg followed by 7.5 to 10 mg/kg administered every 12 to 24 hours for a total of 3 doses (both intravenous and oral administrations). The detailed definitions of the different doses and methods of administration of the medications appear in eTable 1 in Supplement 3.
One study used aspirin for the treatment of PDA. This study was excluded from the analysis due to lack of relevance in the current context. Intravenous indomethacin was the most commonly used intervention (in 38 studies), followed by standard doses of intravenous ibuprofen (used in 23 studies) and oral ibuprofen (used in 21 studies). Oral acetaminophen was used in 5 studies and higher doses of intravenous and oral ibuprofen were used in 1 and 3 studies, respectively (eTable 4 in Supplement 3).
The criteria for hemodynamically significant PDA varied across studies and appear in eTable 4 in Supplement 3. A PDA diameter of more than 1.5 mm and a ratio of 1.4 or greater for left atrium to aortic root were the 2 most commonly used echocardiographic criteria for defining hemodynamically significant PDA (eTable 4 in Supplement 3). Sixteen studies were found to have a low risk of bias (eFigure 2 in Supplement 3). Twenty-eight studies had a risk of bias that was considered probably low, whereas 21 studies had a risk of bias that was considered probably high. Three studies did not report any of sequence generation, allocation concealment, or blinding and were therefore judged to have a high risk of bias (eFigures 2 and 3 in Supplement 3).
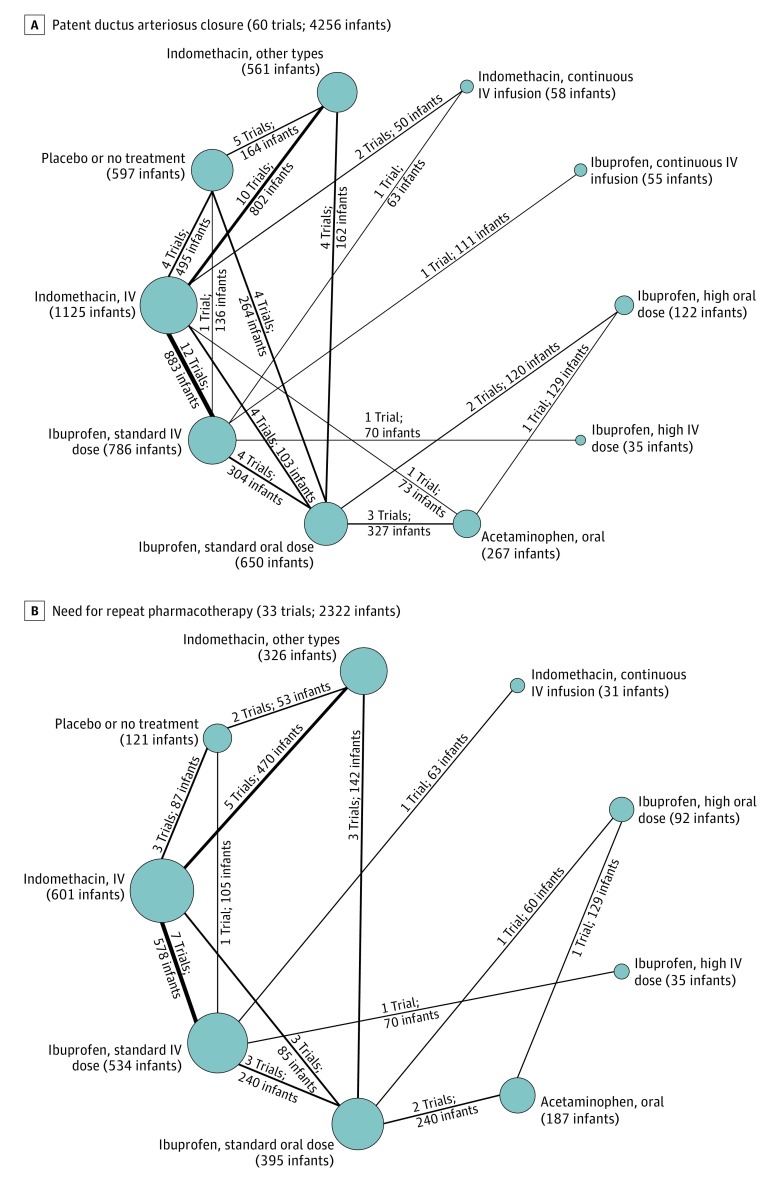
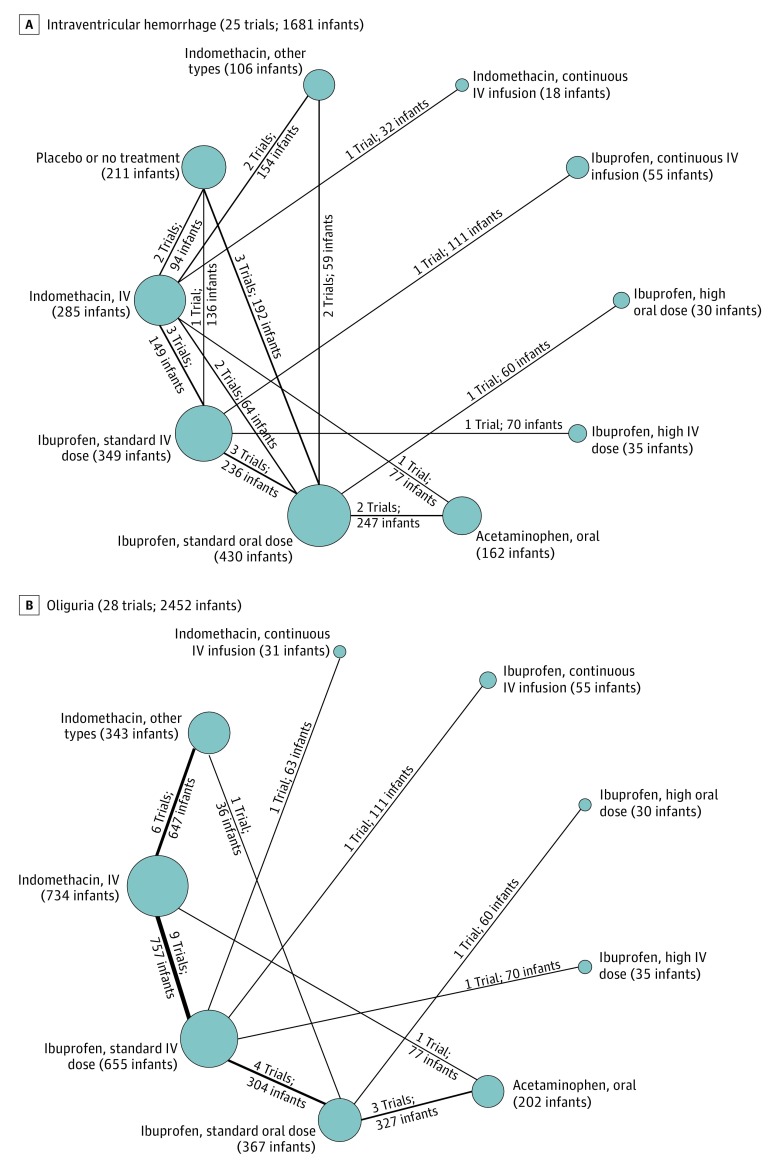
The Network Plots
Head-to-head comparisons between the different therapeutic options were depicted as network plots for each outcome (Figures 2, 3, 4, and 5). In the figures, (1) each node indicates a treatment modality and is sized proportionally to the number of infants who received the treatment modality and (2) each line connecting 2 nodes indicates a direct comparison between 2 modalities. The thickness of each line is proportional to the number of trials directly comparing the 2 modalities.
Figure 2. Network Plots for Patent Ductus Arteriosus Closure and Need for Repeat Pharmacotherapy.
These 2 outcome measures for treatment of hemodynamically significant patent ductus arteriosus were evaluated in the Bayesian network meta-analysis. Each node indicates a treatment modality and is sized proportionally to the number of infants who received the treatment modality. Each line connecting 2 nodes indicates a direct comparison between 2 modalities, and the thickness of each is proportional to the number of trials directly comparing the 2 modalities. Seldom-used variations of indomethacin were condensed into a single node termed indomethacin, other types. A standard dose of ibuprofen is 10 mg/kg followed by 5 mg/kg every 12 to 24 hours for a total of 3 doses. A high dose of ibuprofen is 15 to 20 mg/kg followed by 7.5 to 10 mg/kg every 12 to 24 hours for a total of 3 doses. IV indicates intravenous.
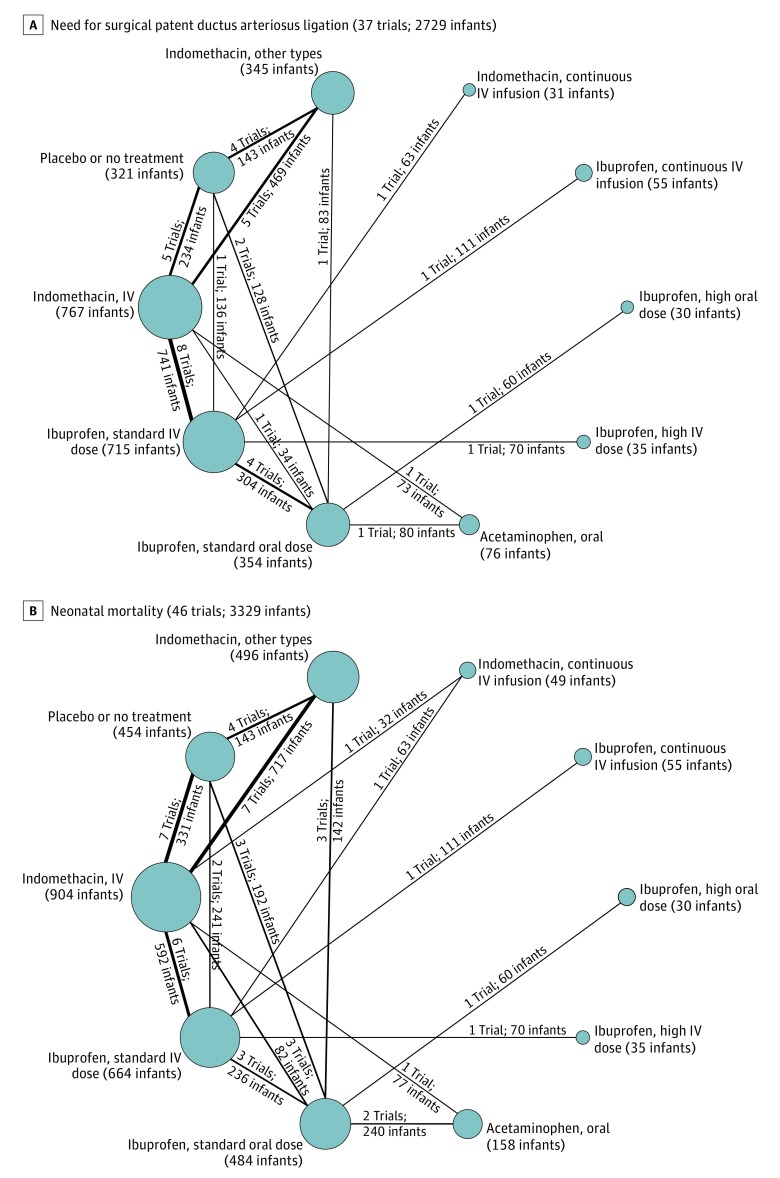
Figure 3. Network Plots for Surgical Patent Ductus Arteriosus Ligation and Neonatal Mortality.
These 2 outcome measures for treatment of hemodynamically significant patent ductus arteriosus were evaluated in the Bayesian network meta-analysis. Each node indicates a treatment modality and is sized proportionally to the number of infants who received the treatment modality. Each line connecting 2 nodes indicates a direct comparison between 2 modalities, and the thickness of each is proportional to the number of trials directly comparing the 2 modalities. Seldom-used variations of indomethacin were condensed into a single node termed indomethacin, other types. A standard dose of ibuprofen is 10 mg/kg followed by 5 mg/kg every 12 to 24 hours for a total of 3 doses. A high dose of ibuprofen is 15 to 20 mg/kg followed by 7.5 to 10 mg/kg every 12 to 24 hours for a total of 3 doses. IV indicates intravenous.
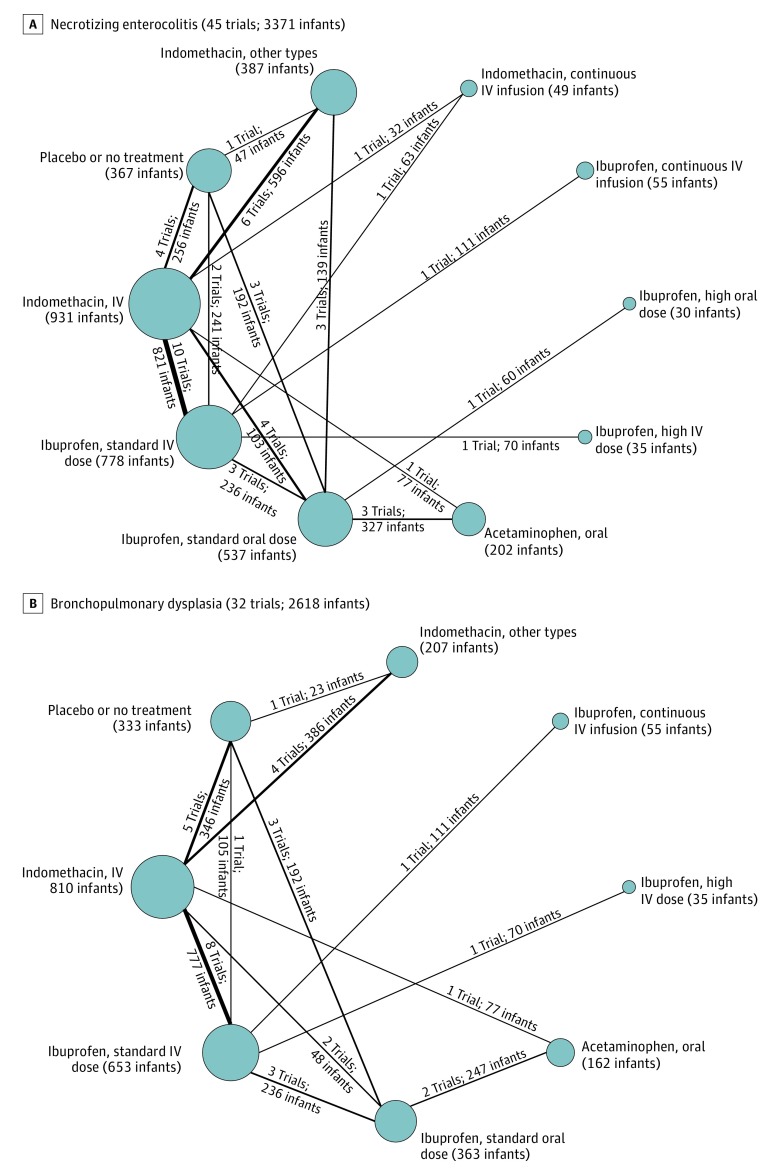
Figure 4. Network Plots for Necrotizing Enterocolitis and Bronchopulmonary Dysplasia.
These 2 outcome measures for treatment of hemodynamically significant patent ductus arteriosus were evaluated in the Bayesian network meta-analysis. Each node indicates a treatment modality and is sized proportionally to the number of infants who received the treatment modality. Each line connecting 2 nodes indicates a direct comparison between 2 modalities, and the thickness of each is proportional to the number of trials directly comparing the 2 modalities. Seldom-used variations of indomethacin were condensed into a single node termed indomethacin, other types. A standard dose of ibuprofen is 10 mg/kg followed by 5 mg/kg every 12 to 24 hours for a total of 3 doses. A high dose of ibuprofen is 15 to 20 mg/kg followed by 7.5 to 10 mg/kg every 12 to 24 hours for a total of 3 doses. IV indicates intravenous.
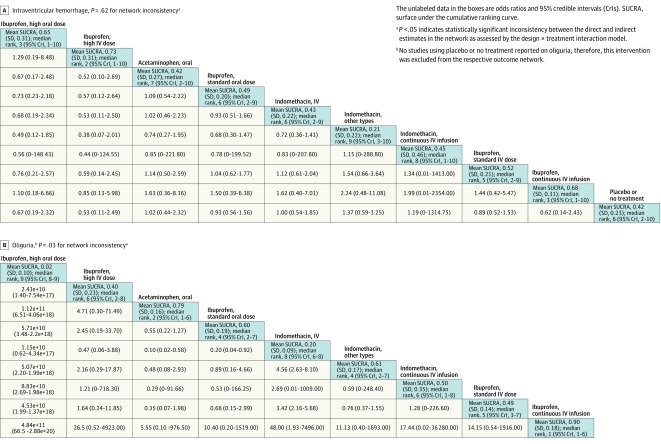
Figure 5. Network Plots for Intraventricular Hemorrhage and Oliguria.
These 2 outcome measures for treatment of hemodynamically significant patent ductus arteriosus were evaluated in the Bayesian network meta-analysis. Each node indicates a treatment modality and is sized proportionally to the number of infants who received the treatment modality. Each line connecting 2 nodes indicates a direct comparison between 2 modalities, and the thickness of each is proportional to the number of trials directly comparing the 2 modalities. Seldom-used variations of indomethacin were condensed into a single node termed indomethacin, other types. A standard dose of ibuprofen is 10 mg/kg followed by 5 mg/kg every 12 to 24 hours for a total of 3 doses. A high dose of ibuprofen is 15 to 20 mg/kg followed by 7.5 to 10 mg/kg every 12 to 24 hours for a total of 3 doses. IV indicates intravenous.
Seldom-used variations of indomethacin were condensed into a single node named other types of indomethacin to make the results more relevant in the current clinical context (eTable 1 in Supplement 3). Similarly, placebo or no treatment were combined into a single node. Therefore, the final network meta-analysis was conducted with 10 nodes, each depicting a treatment modality (Figures 2, 3, 4, and 5).
PDA Closure, Need for Repeat Pharmacotherapy, and Surgical Ligation
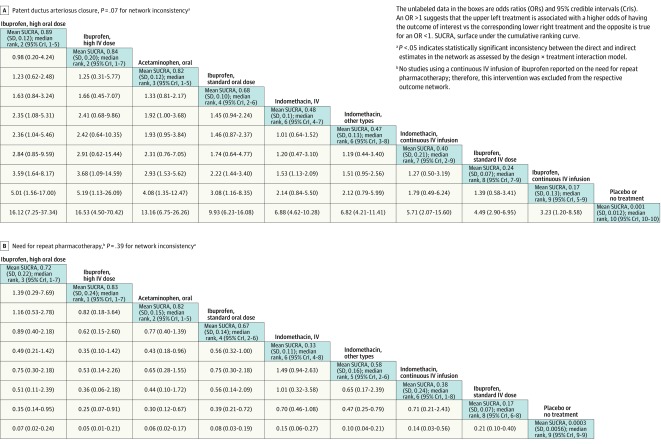
A total of 60 studies including 4256 infants reported the primary outcome. The overall PDA closure rate was 67.4% (2867 of 4256 infants) in all studies combined and 38% in the placebo or no treatment group. A high dose of oral ibuprofen was associated with a significantly higher odds of PDA closure vs a standard dose of intravenous ibuprofen (OR, 3.59; 95% CrI, 1.64-8.17; absolute risk difference, 199 [95% CrI, 95-258] more per 1000 infants) and a standard dose of intravenous indomethacin (OR, 2.35 [95% CrI, 1.08-5.31]; absolute risk difference, 124 [95% CrI, 14-188] more per 1000 infants) (Figure 6A; eTables 5-6, eFigures 4-5, and eText 2 in Supplement 3).
Figure 6. Network Effect Estimates and Ranking Statistics for Patent Ductus Arteriosus Closure and the Need for Repeat Pharmacotherapy.
Compared with a standard dose of intravenous ibuprofen, the following were associated with a significantly higher odds of PDA closure: a high dose of intravenous ibuprofen (OR, 3.68 [95% CrI, 1.09-14.59]; absolute risk difference, 201 [95% CrI, 18-281] more per 1000 infants), oral acetaminophen (OR, 2.93 [95% CrI, 1.53-5.62]; absolute risk difference, 177 [95% CrI, 83-236] more per 1000 infants), and a standard dose of oral ibuprofen (OR, 2.22 [95% CrI, 1.44-3.40]; absolute risk difference, 142 [95% CrI, 72-194] more per 1000 infants) (Figure 6A).
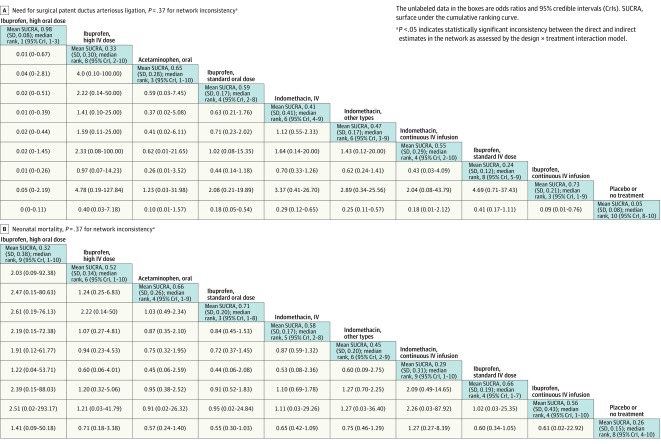
The network OR for each possible comparison for all 8 outcomes along with their mean SUCRA values and median ranks appear in Figures 6, 7, 8, and 9. Based on mean SUCRA values, a high dose of oral ibuprofen was ranked as the best treatment option for PDA closure (mean SUCRA, 0.89 [SD, 0.12]) and for reducing surgical PDA ligation (mean SUCRA, 0.98 [SD, 0.08]). In terms of reducing the need for repeat pharmacotherapy, a high dose of intravenous ibuprofen (mean SUCRA, 0.83 [SD, 0.24]) and oral acetaminophen (mean SUCRA, 0.82 [SD, 0.15]) were ranked as the best (Figure 6B; eTables 5-10 and eFigures 4-9 in Supplement 3).
Figure 7. Network Effect Estimates and Ranking Statistics for Need for Surgical Patent Ductus Arteriosus Ligation and Neonatal Mortality.
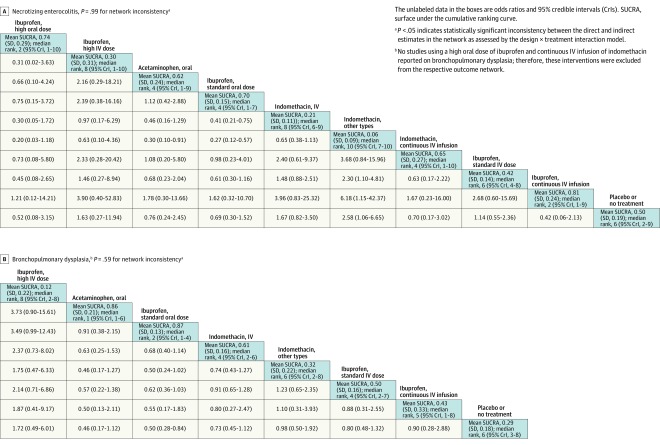
Figure 8. Network Effect Estimates and Ranking Statistics for Necrotizing Enterocolitis and Bronchopulmonary Dysplasia.
Figure 9. Network Effect Estimates and Ranking Statistics for Intraventricular Hemorrhage and Oliguria.
Adverse Events
Neonatal mortality was reported in 46 studies (3329 infants). The incidence of death was 11.9% in all studies and 17.4% in the placebo or no treatment group. Although a standard dose of oral ibuprofen ranked best in terms of preventing mortality (mean SUCRA, 0.71 [SD, 0.20]), there was no statistically significant difference between any of the treatment modalities in the network in relation to neonatal mortality (Figure 7B; eTables 11-12 and eFigures 10-11 in Supplement 3).
Incidence of necrotizing enterocolitis was reported in 45 studies (3371 infants). The overall incidence of necrotizing enterocolitis was 8.7% and it was 6.5% in the placebo or no treatment group. Continuous infusion of intravenous ibuprofen (mean SUCRA, 0.81 [SD, 0.24]) was associated with the lowest incidence of necrotizing enterocolitis (Figure 8A; eTables 13-14 and eFigures 12-13 in Supplement 3). A standard dose of intravenous ibuprofen (mean SUCRA, 0.42 [SD, 0.14]) and a high dose of intravenous ibuprofen (mean SUCRA, 0.30 [SD, 0.31]) and a standard dose of intravenous indomethacin (mean SUCRA, 0.21 [SD, 0.11]) ranked worse than placebo or no treatment (mean SUCRA, 0.50 [SD, 0.19]) in terms of necrotizing enterocolitis incidence; however, the differences in their effect estimates failed to reach statistical significance (Figure 8A).
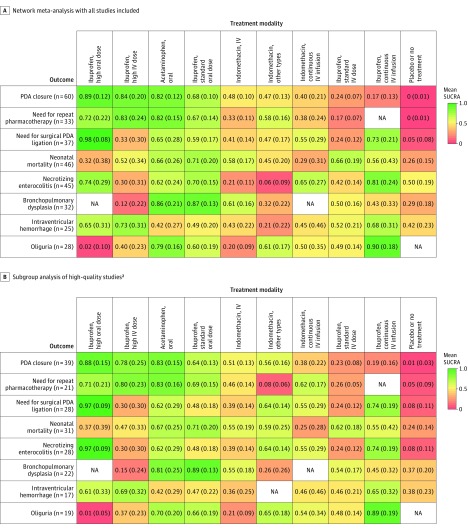
A standard dose of oral ibuprofen was associated with the lowest incidence of bronchopulmonary dysplasia (mean SUCRA, 0.87 [SD, 0.13]; Figure 8B). A high dose of intravenous ibuprofen was associated with the lowest incidence of intraventricular hemorrhage (mean SUCRA, 0.73 [SD, 0.31]; Figure 9A). A continuous infusion of intravenous ibuprofen was associated with the lowest incidence of oliguria (mean SUCRA, 0.90 [SD, 0.18]; Figure 9B) (eTables 15-20 and eFigures 14-19 in Supplement 3). Heat maps depicting the hierarchy of the 10 treatment modalities according to mean SUCRA values across all 8 outcomes appear in Figure 10. Due to the paucity of data, quantitative synthesis was not performed on the remaining a priori–defined outcomes (Table).
Figure 10. Heat Maps of 10 Treatment Modalities Studied in Preterm Infants With Hemodynamically Significant PDA for 8 Outcomes.
Each column represents a treatment modality and each row represents an outcome. For each outcome (column 1), the No. of studies included in the analysis is presented in parentheses. IV indicates intravenous; PDA, patent ductus arteriosus; SUCRA, surface under the cumulative ranking curve. Each box is colored according to the mean SUCRA value of the corresponding treatment and outcome. The color scale consists of values that represent mean SUCRA which range from 0 (red, indicating a treatment is always last) to 1 (green, indicating a treatment is always first). Uncolored boxes labeled NA (data not available) show that the underlying treatment was not included for that particular outcome. The values in each box represent the mean (SD) SUCRA value of the corresponding treatment and outcome. A standard dose of ibuprofen is 10 mg/kg followed by 5 mg/kg every 12 to 24 hours for a total of 3 doses. A high dose of ibuprofen is 15 to 20 mg/kg followed by 7.5 to 10 mg/kg every 12 to 24 hours for a total of 3 doses.
aHigh-quality studies indicates there is a low or probably low risk of bias.
Assessment of the Quality of the Evidence
For the primary outcome of PDA closure, there were 17 direct comparisons and 45 possible comparisons in the network. Using the GRADE assessment methods, the quality of evidence for 6 comparisons was judged to be of high quality, 14 of moderate quality, 20 of low quality, and 5 of very low quality (eTable 5 in Supplement 3). The quality of the evidence for a number of secondary outcome comparisons (especially adverse events) was rated as low or very low due to the imprecise effect estimates as evidenced by the wide 95% CrIs (eTables 7, 9, 11, 13, 15, 17, 19 in Supplement 3). For the global assessment of network inconsistency using the design × treatment interaction model, only the oliguria network effect estimate showed significant inconsistency (P = .03; Figure 9B).
Sensitivity Analyses
The sensitivity analyses for the outcomes were only conducted for the high-quality studies (Figure 10; eTables 21-36 and eFigures 20-27 in Supplement 3). A high dose of oral ibuprofen still ranked as the best treatment option for PDA closure (mean SUCRA, 0.88 [SD, 0.15]) and reducing surgical ligation (mean SUCRA, 0.97 [SD, 0.09]) (eTables 22 and 26 in Supplement 3). It also emerged as the best-ranked treatment for preventing necrotizing enterocolitis (mean SUCRA, 0.97 [SD, 0.09]) vs a continuous intravenous infusion of ibuprofen (mean SUCRA, 0.74 [SD, 0.19]) (eTable 30 in Supplement 3).
Meta-Regression Analysis
In the meta-regression analysis exploring the effects of potential sources of heterogeneity, such as gestational age, birth weight, and year of publication, a high dose of oral ibuprofen remained the best-ranked treatment for PDA closure (eTables 37-44 and eText 3 in Supplement 3). Even after controlling for potential effect modifiers, a high dose of oral ibuprofen still had a significantly higher odds of PDA closure compared with standard doses of intravenous ibuprofen and intravenous indomethacin (eTable 37 in Supplement 3).
Discussion
In this network meta-analysis, a high dose of oral ibuprofen was found to be associated with the best odds of hemodynamically significant PDA closure among all available pharmacotherapeutic options. The quality of evidence was high or moderate for 20 of the 45 comparisons for the primary outcome, whereas it was uniformly lower for most of the secondary outcomes in light of the imprecision resulting from wide 95% CrIs on the network meta-analysis.
Management of PDA has evolved during the last 4 decades from requiring prophylactic closure using pharmacotherapy or surgical intervention to one that is amenable to more conservative management strategies. Conservative management strategies have ranged from targeted pharmacotherapy (based on echocardiographic or clinical criteria for hemodynamic significance) to no PDA treatment combined with cointerventions such as fluid restriction and ventilator adjustments. Despite ranking worst in terms of PDA closure, placebo or no treatment was not associated with a higher odds of death, necrotizing enterocolitis, or intraventricular hemorrhage compared with any other treatment modality. This raises the question whether active pharmacological closure of hemodynamically significant PDA necessarily improves clinical outcomes. With increasing emphasis on conservative management of PDA, these results may encourage researchers to revisit placebo-controlled trials against newer pharmacotherapeutic options.
Targeted PDA treatment has become the preferred approach; therefore, the question of choice of pharmacotherapy has become more important. A number of Cochrane systematic reviews of randomized clinical trials have provided head-to-head comparisons of the various management options. They concluded that ibuprofen was as effective as indomethacin for PDA closure, whereas the former reduced the risk of necrotizing enterocolitis and transient renal insufficiency. There was insufficient evidence to suggest benefit of any of the variations in treatment with a standard dose of indomethacin. Oral acetaminophen was found to be as effective as oral ibuprofen for PDA closure based on only 2 unblinded randomized clinical trials.
However, none of the reviews conducted an in-depth comparison of the different doses and modes of administration for the different medications. In regard to the multiple treatment comparisons, only 1 network meta-analysis compared intravenous indomethacin, intravenous ibuprofen, and placebo for hemodynamically significant PDA but did not include evidence for acetaminophen. Oral acetaminophen has recently emerged as a new treatment option as well as higher doses of oral ibuprofen. Use of a network meta-analysis framework has enabled comparisons among currently used PDA treatment modalities, which has increased the statistical power by taking advantage of direct and indirect treatment comparisons.
In this network meta-analysis, a high dose of oral ibuprofen (15-20 mg/kg followed by 7.5-10.0 mg/kg every 12-24 hours for a total of 3 doses) was found to be associated with a significantly higher likelihood of PDA closure than 2 of the most widely used forms of pharmacotherapy (ie, standard doses of intravenous ibuprofen and intravenous indomethacin). The ibuprofen dose that is traditionally used (10 mg/kg, 5 mg/kg, and 5 mg/kg, each given at 24-hour intervals) is based on old pharmacokinetic data obtained from the experiences of preterm infants. More recent pharmacokinetic studies have shown benefit from using higher doses. In a double-blind dose-finding study, Desfrere et al showed that among infants with a gestational age of younger than 27 weeks, the estimated minimum effective dose regimen of 20 mg/kg, 10 mg/kg, and 10 mg/kg had a higher estimated probability of success (54.8%; 95% CrI, 22%-84%) compared with the conventional dose regimen (30.6%; 95% CrI, 13%-56%). The results of this network meta-analysis are consistent with the above pharmacokinetic data.
Apart from the high dose of oral ibuprofen, oral acetaminophen also consistently ranked high across all effectiveness outcomes, suggesting that it could be an alternative to intravenous ibuprofen and intravenous indomethacin for hemodynamically significant PDA closure. In contrast, the standard dose of intravenous ibuprofen generally ranked just above placebo across all effectiveness outcomes, suggesting that the standard intravenous doses may be ineffective in achieving PDA closure beyond the first few days of life. In the 2015 Cochrane review, intravenous ibuprofen was significantly less efficacious than oral ibuprofen (relative risk, 0.37; 95% confidence interval, 0.23-0.61) in achieving PDA closure. Similar findings were observed in this network meta-analysis in which the intravenous formulation ranked below the oral formulation across most outcomes. Although this finding may appear counterintuitive, available pharmacokinetic data support this observation. Pacifici postulated that a slower absorption rate along with a longer half-life prolong the time of contact with the PDA, leading to higher responsiveness of oral ibuprofen compared with the intravenous formulation.
Despite supporting pharmacokinetic evidence, clinicians have often been reluctant to use oral ibuprofen formulations due to concerns about necrotizing enterocolitis. In this network meta-analysis, a high dose of oral ibuprofen was not associated with an increased incidence of necrotizing enterocolitis (Figure 8A). In the sensitivity analysis of the high-quality studies (Figure 10B), high-dose oral ibuprofen was associated with the best cumulative probability for preventing necrotizing enterocolitis, suggesting that hemodynamically significant PDA in itself is probably a significant risk factor for necrotizing enterocolitis and closing it successfully when hemodynamically significant could in turn reduce the risk of necrotizing enterocolitis.
Despite ranking lower than a high dose of oral ibuprofen across the effectiveness outcomes, a standard dose of oral ibuprofen ranked as the best treatment for preventing death (Figure 10). This apparent paradox in the network meta-analysis results was likely artifactual due to substantial imprecision in the effect estimates for the secondary outcomes as evidenced by the wide 95% CrIs of the ranking statistics (Figure 7B). No statistically significant difference in mortality rates was observed with any of the interventions based on the available evidence, which suggests that active pharmacological closure of a hemodynamically significant PDA may not be associated with lower mortality in preterm infants.
The overall high-ranking probabilities across outcomes suggest that high and standard doses of oral ibuprofen and oral acetaminophen could be effective alternatives to the standard regimens of intravenous ibuprofen and intravenous indomethacin currently used to close a hemodynamically significant PDA (Figure 10). Well-designed randomized clinical trials with optimal sample sizes to detect clinically important differences in effectiveness and safety using such medications are needed to confirm or refute the validity of the network meta-analysis results.
Limitations
This study has several limitations. First, this network meta-analysis was based on the assumption of transitivity, which in turn was based on the assumption that population and intervention characteristics were largely similar across the studies. This transitivity assumption could have been violated due to variation in gestational age, birth weight, timing of treatment, or associated cointerventions, which have changed during the last 4 decades. This was accounted for in the meta-regression analysis conducted for the most important outcomes and controlling for the effect modifiers.
Second, the ranking order of interventions was based on mean SUCRA values, which does not necessarily imply that a higher-ranked intervention was statistically significantly better than a lower-ranked intervention. In addition to the absolute ranks, the dispersion around the ranking statistics and the absolute risk differences between interventions should be taken into account when choosing a pharmacotherapeutic option for hemodynamically significant PDA treatment.
Third, limited sample size resulted in substantial imprecision in the effect estimates for a number of the secondary outcomes in the primary analyses as well as many of the analyses restricted to the higher-quality studies, precluding derivation of meaningful inferences. Clinical outcomes (such as necrotizing enterocolitis, bronchopulmonary dysplasia, intraventricular hemorrhage, and mortality) beyond immediate PDA closure should be explored in future studies.
Conclusions
A high dose of oral ibuprofen was associated with a higher likelihood of hemodynamically significant PDA closure vs standard doses of intravenous ibuprofen or intravenous indomethacin; placebo or no treatment did not significantly change the likelihood of mortality, necrotizing enterocolitis, or intraventricular hemorrhage.
Trial protocol
Differences between trial protocol and article
eTable 1. Glossary of abbreviations, acronyms (including dosage & routes of administration of medications) and terms
eTable 2. Electronic database search strategies
eTable 3. List of excluded studies after full text screening
eTable 4. Clinical & methodological characteristics of included studies
eTable 5. GRADE assessment of the quality of evidence (QoE) for the network for PDA closure
eTable 6. Ranking statistics for each treatment modality for PDA closure
eTable 7. GRADE assessment of the quality of evidence (QoE) for the network for need for repeat pharmacotherapy
eTable 8. Ranking statistics for each treatment modality for outcome need for repeat pharmacotherapy
eTable 9. GRADE assessment of the quality of evidence (QoE) for the network for need for surgical PDA ligation
eTable 10. Ranking statistics for each treatment modality for need for surgical PDA ligation
eTable 11. GRADE assessment of the quality of evidence (QoE) for the network for neonatal mortality
eTable 12. Ranking statistics for each treatment modality for neonatal mortality
eTable 13. GRADE assessment of the quality of evidence (QoE) for the network for risk of NEC
eTable 14. Ranking statistics for each treatment modality for risk of necrotizing enterocolitis (NEC)
eTable 15. GRADE assessment of the quality of evidence (QoE) for the network for risk of BPD
eTable 16. Ranking statistics for each treatment modality for risk of bronchopulmonary dysplasia (BPD)
eTable 17. GRADE assessment of the quality of evidence (QoE) for the network for risk of IVH
eTable 18. Ranking statistics for each treatment modality for risk of intraventricular hemorrhage (IVH)
eTable 19. GRADE assessment of the quality of evidence (QoE) for the network for risk of Oliguria
eTable 20. Ranking statistics for each treatment modality for risk of Oliguria
eTable 21. Network effect estimates for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 22. Ranking statistics for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 23. Network effect estimates for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 24. Ranking statistics for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 25. Network effect estimates for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 26. Ranking statistics for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 27. Network effect estimates for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 28. Ranking statistics for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 29. Network effect estimates for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 30. Ranking statistics for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 31. Network effect estimates for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 32. Ranking statistics for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 33. Network effect estimates for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 34. Ranking statistics for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 35. Network effect estimates for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 36. Ranking statistics for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 37. Meta-regression analysis results for outcome: PDA closure
eTable 38. Meta-regression analysis corresponding SUCRA values: PDA closure
eTable 39. Meta-regression analysis results for outcome: need for repeat pharmacotherapy
eTable 40. Meta-regression analysis corresponding SUCRA values: need for repeat pharmacotherapy
eTable 41. Meta-regression analysis results for outcome: neonatal mortality
eTable 42. Meta-regression analysis corresponding SUCRA values: neonatal mortality
eTable 43. Meta-regression analysis results for outcome: risk of necrotizing enterocolitis
eTable 44. Meta-regression analysis corresponding SUCRA values: risk of necrotizing enterocolitis
eFigure 1. Specific instructions for estimating unclearly reported blinding status
eFigure 2. Detailed risk of bias assessment of individual studies
eFigure 3. Assessment summary across the risk of bias items
eFigure 4. Network meta-analysis forest plots for outcome: PDA closure
eFigure 5. Ranking probability (rankogram) of each treatment modality for PDA closure
eFigure 6. Network meta-analysis forest plots for outcome: need for repeat pharmacotherapy
eFigure 7. Ranking probability (rankogram) of each treatment modality for need for repeat pharmacotherapy
eFigure 8. Network meta-analysis forest plots for outcome: need for surgical PDA ligation
eFigure 9. Ranking probability (rankogram) of each treatment modality for need for surgical PDA ligation
eFigure 10. Network meta-analysis forest plots for outcome: neonatal Mortality
eFigure 11. Ranking probability (rankogram) of each treatment modality for neonatal mortality
eFigure 12. Network meta-analysis forest plots for outcome: risk of necrotizing enterocolitis (NEC)
eFigure 13. Ranking probability (rankogram) of each treatment modality for risk of NEC
eFigure 14. Network meta-analysis forest plots for outcome: risk of bronchopulmonary dysplasia (BPD)
eFigure 15. Ranking probability (rankogram) of each treatment modality for risk of BPD
eFigure 16. Network meta-analysis forest plots for outcome: risk of intraventricular hemorrhage (IVH)
eFigure 17. Ranking probability (rankogram) of each treatment modality for risk of IVH
eFigure 18. Network meta-analysis forest plots for outcome: risk of oliguria
eFigure 19. Ranking probability (rankogram) of each treatment modality for risk of oliguria
eFigure 20. Rankogram for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 21. Rankogram for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 22. Rankogram for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 23. Rankogram for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 24. Rankogram for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 25. Rankogram for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 26. Rankogram for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 27. Rankogram for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eText 1. Risk of bias assessment of eligible studies
eText 2. Guide to interpreting network meta-analysis results (rankograms; SUCRA; network GRADE) (includes rankogram example figure; SUCRA example table; network plot example for first-order loop; network plot example for second-order loop)
eText 3. Guide to interpreting meta-regression results (included example tables A & B)
eReferences
References
- 1.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36(2):123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40(2):184-188. [DOI] [PubMed] [Google Scholar]
- 3.Brown ER. Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. J Pediatr. 1979;95(5 pt 2):865-866. [DOI] [PubMed] [Google Scholar]
- 4.Lipman B, Serwer GA, Brazy JE. Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatrics. 1982;69(6):778-781. [PubMed] [Google Scholar]
- 5.Letshwiti JB, Semberova J, Pichova K, Dempsey EM, Franklin OM, Miletin J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. 2017;104:45-49. [DOI] [PubMed] [Google Scholar]
- 6.Vanhaesebrouck S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe MR, Theyskens C. Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F244-F247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra S, Rønnestad A, Holmstrøm H. Management of patent ductus arteriosus in preterm infants—where do we stand? Congenit Heart Dis. 2013;8(6):500-512. [DOI] [PubMed] [Google Scholar]
- 8.Mercanti I, Ligi I, Boubred F, et al. Ibubrofen in the treatment of patent ductus arteriosus in preterm infants: what we know, what we still do not know. Curr Pharm Des. 2012;18(21):3007-3018. [DOI] [PubMed] [Google Scholar]
- 9.Mercanti I, Boubred F, Simeoni U. Therapeutic closure of the ductus arteriosus: benefits and limitations. J Matern Fetal Neonatal Med. 2009;22(suppl 3):14-20. [DOI] [PubMed] [Google Scholar]
- 10.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956-964. [DOI] [PubMed] [Google Scholar]
- 11.Mitra S, Thabane L, Florez ID, Tamayo ME, Aune D Systematic review and network meta-analysis of IV indomethacin versus IV Ibuprofen versus oral Ibuprofen versus oral acetaminophen versus placebo for treatment of symptomatic patent ductus arteriosus in preterm infants. http://www.crd.york.ac.ukdisplay_record.php?ID=CRD42015015797. Accessed October 10, 2016.
- 12.Mitra S, Florez ID, Tamayo ME, et al. Effectiveness and safety of treatments used for the management of patent ductus arteriosus (PDA) in preterm infants: a protocol for a systematic review and network meta-analysis. BMJ Open. 2016;6(7):e011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014;17(2):157-173. [DOI] [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723-1729. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://training.cochrane.org/handbook. Accessed October 13, 2017.
- 19.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol. 2012;65(3):262-267. [DOI] [PubMed] [Google Scholar]
- 20.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105-3124. [DOI] [PubMed] [Google Scholar]
- 21.Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308(12):1246-1253. [DOI] [PubMed] [Google Scholar]
- 22.Baker SG, Kramer BS. The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C? BMC Med Res Methodol. 2002;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130-137. [DOI] [PubMed] [Google Scholar]
- 24.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42(1):332-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. [DOI] [PubMed] [Google Scholar]
- 27.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. [DOI] [PubMed] [Google Scholar]
- 28.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;(4):457-472. [Google Scholar]
- 29.Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316(6):611-624. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325-337. [Google Scholar]
- 32.Brown S, Hutton B, Clifford T, et al. A Microsoft Excel-based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Syst Rev. 2014;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285-299. [DOI] [PubMed] [Google Scholar]
- 34.Palmer T, Sterne J. Meta-Analysis in Stata: An Updated Collection From the Stata Journal: 2. College Station, TX: Stata Press; 2016. [Google Scholar]
- 35.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 37.Adamska E, Helwich E, Rutkowska M, Zacharska E, Piotrowska A. Comparison of the efficacy of ibuprofen and indomethacin in the treatment of patent ductus arteriosus in prematurely born infants [in Polish]. Med Wieku Rozwoj. 2005;9(3 pt 1):335-354. [PubMed] [Google Scholar]
- 38.Akisü M, Özyürek R, Dorak C, Parlar A, Kültürsay N. Enteral ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm newborn infants [in Turkish]. Çocuk Sagligi Hastalik Derg. 2001;44:56-60. [Google Scholar]
- 39.Aly H, Lotfy W, Badrawi N, Ghawas M, Abdel-Meguid IE, Hammad TA. Oral ibuprofen and ductus arteriosus in premature infants: a randomized pilot study. Am J Perinatol. 2007;24(5):267-270. [DOI] [PubMed] [Google Scholar]
- 40.Aranda JV, Clyman R, Cox B, et al. A randomized, double-blind, placebo-controlled trial on intravenous ibuprofen L-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 hours of birth in extremely low-birth-weight infants. Am J Perinatol. 2009;26(3):235-245. [DOI] [PubMed] [Google Scholar]
- 41.Baenziger O, Waldvogel K, Ghelfi D, Arbenz U, Fanconi S. Can dopamine prevent the renal side effects of indomethacin? a prospective randomized clinical study. Klin Padiatr. 1999;211(6):438-441. [DOI] [PubMed] [Google Scholar]
- 42.Bagheri MM, Niknafs P, Sabsevari F, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iran J Pediatr. 2016;26(4):e3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagnoli F, Rossetti A, Messina G, Mori A, Casucci M, Tomasini B. Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side-effects in VLBW and ELBW newborns. J Matern Fetal Neonatal Med. 2013;26(4):423-429. [DOI] [PubMed] [Google Scholar]
- 44.Betkerur MV, Yeh TF, Miller K, Glasser RJ, Pildes RS. Indomethacin and its effect on renal function and urinary kallikrein excretion in premature infants with patent ductus arteriosus. Pediatrics. 1981;68(1):99-102. [PubMed] [Google Scholar]
- 45.Cherif A, Khrouf N, Jabnoun S, et al. Randomized pilot study comparing oral ibuprofen with intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Pediatrics. 2008;122(6):e1256-e1261. [DOI] [PubMed] [Google Scholar]
- 46.Chotigeat U, Jirapapa K, Layangkool T. A comparison of oral ibuprofen and intravenous indomethacin for closure of patent ductus arteriosus in preterm infants. J Med Assoc Thai. 2003;86(suppl 3):S563-S569. [PubMed] [Google Scholar]
- 47.Christmann V, Liem KD, Semmekrot BA, van de Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatr. 2002;91(4):440-446. [DOI] [PubMed] [Google Scholar]
- 48.Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One. 2013;8(11):e77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dani C, Vangi V, Bertini G, et al. High-dose ibuprofen for patent ductus arteriosus in extremely preterm infants: a randomized controlled study. Clin Pharmacol Ther. 2012;91(4):590-596. [DOI] [PubMed] [Google Scholar]
- 50.Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or intravenous indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatr. 2015;52(7):573-578. [DOI] [PubMed] [Google Scholar]
- 51.Ding YJ, Han B, Yang B, Zhu M. NT-proBNP plays an important role in the effect of ibuprofen on preterm infants with patent ductus arteriosus. Eur Rev Med Pharmacol Sci. 2014;18(18):2596-2598. [PubMed] [Google Scholar]
- 52.Erdeve O, Yurttutan S, Altug N, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F279-F283. [DOI] [PubMed] [Google Scholar]
- 53.Fakhraee SH, Badiee Z, Mojtahedzadeh S, Kazemian M, Kelishadi R. Comparison of oral ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9(5):399-403. [PubMed] [Google Scholar]
- 54.Fesharaki HJ, Nayeri FS, Asbaq PA, Amini E, Sedagat M. Different doses of ibuprofen in the treatment of patent ductus arteriosus: a randomized controlled trial. Tehran Univ Med J. 2012;70(8):488-493. [Google Scholar]
- 55.Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102(6):895-906. [DOI] [PubMed] [Google Scholar]
- 56.Ghanem S, Mostafa M, Shafee M. Effect of oral ibuprofen on patent ductus arteriosus in premature newborns. J Saudi Heart Assoc. 2010;22(1):7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gimeno Navarro A, Cano Sánchez A, Fernández Gilino C, et al. Ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm infants [in Spanish]. An Pediatr (Barc). 2005;63(3):212-218. [DOI] [PubMed] [Google Scholar]
- 58.Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral versus intravenous ibuprofen in very low birth weight preterm infants with patent ductus arteriosus. J Pediatr. 2011;158(4):549-554.e1. [DOI] [PubMed] [Google Scholar]
- 59.Hammerman C, Aramburo MJ. Prolonged indomethacin therapy for the prevention of recurrences of patent ductus arteriosus. J Pediatr. 1990;117(5):771-776. [DOI] [PubMed] [Google Scholar]
- 60.Hammerman C, Glaser J, Schimmel MS, Ferber B, Kaplan M, Eidelman AI. Continuous versus multiple rapid infusions of indomethacin: effects on cerebral blood flow velocity. Pediatrics. 1995;95(2):244-248. [PubMed] [Google Scholar]
- 61.Hammerman C, Shchors I, Jacobson S, et al. Ibuprofen versus continuous indomethacin in premature neonates with patent ductus arteriosus: is the difference in the mode of administration? Pediatr Res. 2008;64(3):291-297. [DOI] [PubMed] [Google Scholar]
- 62.Jegatheesan P, Ianus V, Buchh B, et al. Increased indomethacin dosing for persistent patent ductus arteriosus in preterm infants: a multicenter, randomized, controlled trial. J Pediatr. 2008;153(2):183-189. [DOI] [PubMed] [Google Scholar]
- 63.Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F99-F104. [DOI] [PubMed] [Google Scholar]
- 64.Krauss AN, Fatica N, Lewis BS, et al. Pulmonary function in preterm infants following treatment with intravenous indomethacin. Am J Dis Child. 1989;143(1):78-81. [DOI] [PubMed] [Google Scholar]
- 65.Lago P, Bettiol T, Salvadori S, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. Eur J Pediatr. 2002;161(4):202-207. [DOI] [PubMed] [Google Scholar]
- 66.Lago P, Salvadori S, Opocher F, Ricato S, Chiandetti L, Frigo AC. Continuous infusion of ibuprofen for treatment of patent ductus arteriosus in very low birth weight infants. Neonatology. 2014;105(1):46-54. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Rajadurai VS, Tan KW, Wong KY, Wong EH, Leong JY. Randomized trial of prolonged low-dose versus conventional-dose indomethacin for treating patent ductus arteriosus in very low birth weight infants. Pediatrics. 2003;112(2):345-350. [DOI] [PubMed] [Google Scholar]
- 68.Lee SJ, Kim JY, Park EA, Sohn S. The pharmacological treatment of patent ductus arteriosus in premature infants with respiratory distress syndrome: oral ibuprofen vs indomethacin [in Korean]. Korean J Pediatr. 2008;51(9):956-963. [Google Scholar]
- 69.Lin YJ, Chen CM, Rehan VK, et al. Randomized trial to compare renal function and ductal response between indomethacin and ibuprofen treatment in extremely low birth weight infants. Neonatology. 2017;111(3):195-202. [DOI] [PubMed] [Google Scholar]
- 70.Lin XZ, Chen HQ, Zheng Z, Li YD, Lai JD, Huang LH. Therapeutic effect of early administration of oral ibuprofen in very low birth weight infants with patent ductus arteriosus [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(7):502-505. [PubMed] [Google Scholar]
- 71.Merritt TA, Harris JP, Roghmann K, et al. Early closure of the patent ductus arteriosus in very low-birth-weight infants: a controlled trial. J Pediatr. 1981;99(2):281-286. [DOI] [PubMed] [Google Scholar]
- 72.Monset-Couchard M, Dias-Mançano D, Murat I, Relier JP. Controlled trial of intravenous lyophilized indomethacin in the treatment of persistent ductus arteriosus in premature infants [in French]. Pediatrie. 1983;38(6):365-377. [PubMed] [Google Scholar]
- 73.Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr. 1997;131(4):549-554. [DOI] [PubMed] [Google Scholar]
- 74.Mullett MD, Croghan TW, Myerberg DZ, Krall JM, Neal WA. Indomethacin for closure of patent ductus arteriosus in prematures. Clin Pediatr (Phila). 1982;21(4):217-220. [DOI] [PubMed] [Google Scholar]
- 75.Nestrud RM, Hill DE, Arrington RW, et al. Indomethacin treatment in patent ductus arteriosus: a double-blind study utilizing indomethacin plasma levels. Dev Pharmacol Ther. 1980;1(2-3):125-136. [PubMed] [Google Scholar]
- 76.Neu J, Ariagno RL, Johnson JD, et al. A double blind study of the effects of oral indomethacin in preterm infants with patent ductus arteriosus who failed medical management. Pediatr Pharmacol (New York). 1981;1(3):245-249. [PubMed] [Google Scholar]
- 77.Oncel MY, Yurttutan S, Erdeve O, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164(3):510-4.e1. [DOI] [PubMed] [Google Scholar]
- 78.Osborn DA, Evans N, Kluckow M. Effect of early targeted indomethacin on the ductus arteriosus and blood flow to the upper body and brain in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F477-F482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel J, Roberts I, Azzopardi D, Hamilton P, Edwards AD. Randomized double-blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res. 2000;47(1):36-42. [DOI] [PubMed] [Google Scholar]
- 80.Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr. 1999;135(6):733-738. [DOI] [PubMed] [Google Scholar]
- 81.Pistulli E, Hamiti A, Buba S, Hoxha A, Kelmendi N, Vyshka G. The association between patent ductus arteriosus and perinatal infection in a group of low birth weight preterm infants. Iran J Pediatr. 2014;24(1):42-48. [PMC free article] [PubMed] [Google Scholar]
- 82.Pourarian Sh, Pishva N, Madani A, Rastegari M. Comparison of oral ibuprofen and indomethacin on closure of patent ductus arteriosus in preterm infants. East Mediterr Health J. 2008;14(2):360-365. [PubMed] [Google Scholar]
- 83.Pourarian S, Takmil F, Cheriki S, Amoozgar H. The effect of oral high-dose ibuprofen on patent ductus arteriosus closure in preterm infants. Am J Perinatol. 2015;32(12):1158-1163. [DOI] [PubMed] [Google Scholar]
- 84.Rennie JM, Cooke RW. Prolonged low dose indomethacin for persistent ductus arteriosus of prematurity. Arch Dis Child. 1991;66(1):55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhodes PG, Ferguson MG, Reddy NS, Joransen JA, Gibson J. Effects of prolonged versus acute indomethacin therapy in very low birth-weight infants with patent ductus arteriosus. Eur J Pediatr. 1988;147(5):481-484. [DOI] [PubMed] [Google Scholar]
- 86.Romagnoli C, Zecca E, Papacci P, et al. Furosemide does not prevent indomethacin-induced renal side effects in preterm infants. Clin Pharmacol Ther. 1997;62(2):181-186. [DOI] [PubMed] [Google Scholar]
- 87.Rudd P, Montanez P, Hallidie-Smith K, Silverman M. Indomethacin treatment for patent ductus arteriosus in very low birthweight infants: double blind trial. Arch Dis Child. 1983;58(4):267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JK. Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. J Med Assoc Thai. 2008;91(suppl 3):S28-S34. [PubMed] [Google Scholar]
- 89.Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929-35.e1. [DOI] [PubMed] [Google Scholar]
- 90.Su BH, Peng CT, Tsai CH. Echocardiographic flow pattern of patent ductus arteriosus: a guide to indomethacin treatment in premature infants. Arch Dis Child Fetal Neonatal Ed. 1999;81(3):F197-F200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indometacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F94-F99. [DOI] [PubMed] [Google Scholar]
- 92.Su PH, Chen JY, Su CM, Huang TC, Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003;45(6):665-670. [DOI] [PubMed] [Google Scholar]
- 93.Supapannachart S, Limrungsikul A, Khowsathit P. Oral ibuprofen and indomethacin for treatment of patent ductus arteriosus in premature infants: a randomized trial at Ramathibodi Hospital. J Med Assoc Thai. 2002;85(suppl 4):S1252-S1258. [PubMed] [Google Scholar]
- 94.Tammela O, Ojala R, Iivainen T, et al. Short versus prolonged indomethacin therapy for patent ductus arteriosus in preterm infants. J Pediatr. 1999;134(5):552-557. [DOI] [PubMed] [Google Scholar]
- 95.van Overmeire B, Brus F, van Acker KJ, et al. Aspirin versus indomethacin treatment of patent ductus arteriosus in preterm infants with respiratory distress syndrome. Pediatr Res. 1995;38(6):886-891. [DOI] [PubMed] [Google Scholar]
- 96.Van Overmeire B, Follens I, Hartmann S, Creten WL, Van Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. 1997;76(3):F179-F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343(10):674-681. [DOI] [PubMed] [Google Scholar]
- 98.Van Overmeire B, Van de Broek H, Van Laer P, Weyler J, Vanhaesebrouck P. Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr. 2001;138(2):205-211. [DOI] [PubMed] [Google Scholar]
- 99.Yadav S, Agarwal S, Maria A, et al. Comparison of oral ibuprofen with oral indomethacin for PDA closure in Indian preterm neonates: a randomized controlled trial. Pediatr Cardiol. 2014;35(5):824-830. [DOI] [PubMed] [Google Scholar]
- 100.Yanagi RM, Wilson A, Newfeld EA, Aziz KU, Hunt CE. Indomethacin treatment for symptomatic patent ductus arteriosus: a double-blind control study. Pediatrics. 1981;67(5):647-652. [PubMed] [Google Scholar]
- 101.Yang B, Gao X, Ren Y, Wang Y, Zhang Q. Oral paracetamol vs oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: a randomized controlled trial. Exp Ther Med. 2016;12(4):2531-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeh TF, Luken JA, Thalji A, Raval D, Carr I, Pildes RS. Intravenous indomethacin therapy in premature infants with persistent ductus arteriosus—a double-blind controlled study. J Pediatr. 1981;98(1):137-145. [DOI] [PubMed] [Google Scholar]
- 103.Yeh TF, Wilks A, Singh J, Betkerur M, Lilien L, Pildes RS. Furosemide prevents the renal side effects of indomethacin therapy in premature infants with patent ductus arteriosus. J Pediatr. 1982;101(3):433-437. [DOI] [PubMed] [Google Scholar]
- 104.Zanardo V, Vedovato S, Lago P, Piva D, Faggian D, Chiozza L. Effects of ibuprofen and indomethacin on urinary antidiuretic hormone excretion in preterm infants treated for patent ductus arteriosus. Fetal Diagn Ther. 2005;20(6):534-539. [DOI] [PubMed] [Google Scholar]
- 105.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2015;(2):CD003481. [DOI] [PubMed] [Google Scholar]
- 106.Herrera C, Holberton J, Davis P. Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2007;(2):CD003480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low-birth-weight infants. Cochrane Database Syst Rev. 2015;(3):CD010061. [DOI] [PubMed] [Google Scholar]
- 108.Jones LJ, Craven PD, Attia J, Thakkinstian A, Wright I. Network meta-analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F45-F52. [DOI] [PubMed] [Google Scholar]
- 109.Dornelles LV, Corso AL, Silveira RdeC, Procianoy RS. Comparison of two dose regimens of ibuprofen for the closure of patent ductus arteriosus in preterm newborns. J Pediatr (Rio J). 2016;92(3):314-318. [DOI] [PubMed] [Google Scholar]
- 110.Van Overmeire B, Touw D, Schepens PJ, Kearns GL, van den Anker JN. Ibuprofen pharmacokinetics in preterm infants with patent ductus arteriosus. Clin Pharmacol Ther. 2001;70(4):336-343. [PubMed] [Google Scholar]
- 111.Hirt D, Van Overmeire B, Treluyer J-M, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Desfrere L, Zohar S, Morville P, et al. Dose-finding study of ibuprofen in patent ductus arteriosus using the continual reassessment method. J Clin Pharm Ther. 2005;30(2):121-132. [DOI] [PubMed] [Google Scholar]
- 113.Barzilay B, Youngster I, Batash D, et al. Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2012;97(2):F116-F119. [DOI] [PubMed] [Google Scholar]
- 114.Pacifici GM. Clinical pharmacology of ibuprofen in preterm infants: a meta-analysis of published data. http://www.gnresearch.org/doi/10.5935/MedicalExpress.2014.02.02. Accessed September 30, 2016.
- 115.Tatli MM, Kumral A, Duman N, Demir K, Gurcu O, Ozkan H. Spontaneous intestinal perforation after oral ibuprofen treatment of patent ductus arteriosus in two very-low-birthweight infants. Acta Paediatr. 2004;93(7):999-1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Differences between trial protocol and article
eTable 1. Glossary of abbreviations, acronyms (including dosage & routes of administration of medications) and terms
eTable 2. Electronic database search strategies
eTable 3. List of excluded studies after full text screening
eTable 4. Clinical & methodological characteristics of included studies
eTable 5. GRADE assessment of the quality of evidence (QoE) for the network for PDA closure
eTable 6. Ranking statistics for each treatment modality for PDA closure
eTable 7. GRADE assessment of the quality of evidence (QoE) for the network for need for repeat pharmacotherapy
eTable 8. Ranking statistics for each treatment modality for outcome need for repeat pharmacotherapy
eTable 9. GRADE assessment of the quality of evidence (QoE) for the network for need for surgical PDA ligation
eTable 10. Ranking statistics for each treatment modality for need for surgical PDA ligation
eTable 11. GRADE assessment of the quality of evidence (QoE) for the network for neonatal mortality
eTable 12. Ranking statistics for each treatment modality for neonatal mortality
eTable 13. GRADE assessment of the quality of evidence (QoE) for the network for risk of NEC
eTable 14. Ranking statistics for each treatment modality for risk of necrotizing enterocolitis (NEC)
eTable 15. GRADE assessment of the quality of evidence (QoE) for the network for risk of BPD
eTable 16. Ranking statistics for each treatment modality for risk of bronchopulmonary dysplasia (BPD)
eTable 17. GRADE assessment of the quality of evidence (QoE) for the network for risk of IVH
eTable 18. Ranking statistics for each treatment modality for risk of intraventricular hemorrhage (IVH)
eTable 19. GRADE assessment of the quality of evidence (QoE) for the network for risk of Oliguria
eTable 20. Ranking statistics for each treatment modality for risk of Oliguria
eTable 21. Network effect estimates for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 22. Ranking statistics for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 23. Network effect estimates for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 24. Ranking statistics for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 25. Network effect estimates for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 26. Ranking statistics for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 27. Network effect estimates for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 28. Ranking statistics for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 29. Network effect estimates for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 30. Ranking statistics for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 31. Network effect estimates for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 32. Ranking statistics for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 33. Network effect estimates for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 34. Ranking statistics for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 35. Network effect estimates for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 36. Ranking statistics for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eTable 37. Meta-regression analysis results for outcome: PDA closure
eTable 38. Meta-regression analysis corresponding SUCRA values: PDA closure
eTable 39. Meta-regression analysis results for outcome: need for repeat pharmacotherapy
eTable 40. Meta-regression analysis corresponding SUCRA values: need for repeat pharmacotherapy
eTable 41. Meta-regression analysis results for outcome: neonatal mortality
eTable 42. Meta-regression analysis corresponding SUCRA values: neonatal mortality
eTable 43. Meta-regression analysis results for outcome: risk of necrotizing enterocolitis
eTable 44. Meta-regression analysis corresponding SUCRA values: risk of necrotizing enterocolitis
eFigure 1. Specific instructions for estimating unclearly reported blinding status
eFigure 2. Detailed risk of bias assessment of individual studies
eFigure 3. Assessment summary across the risk of bias items
eFigure 4. Network meta-analysis forest plots for outcome: PDA closure
eFigure 5. Ranking probability (rankogram) of each treatment modality for PDA closure
eFigure 6. Network meta-analysis forest plots for outcome: need for repeat pharmacotherapy
eFigure 7. Ranking probability (rankogram) of each treatment modality for need for repeat pharmacotherapy
eFigure 8. Network meta-analysis forest plots for outcome: need for surgical PDA ligation
eFigure 9. Ranking probability (rankogram) of each treatment modality for need for surgical PDA ligation
eFigure 10. Network meta-analysis forest plots for outcome: neonatal Mortality
eFigure 11. Ranking probability (rankogram) of each treatment modality for neonatal mortality
eFigure 12. Network meta-analysis forest plots for outcome: risk of necrotizing enterocolitis (NEC)
eFigure 13. Ranking probability (rankogram) of each treatment modality for risk of NEC
eFigure 14. Network meta-analysis forest plots for outcome: risk of bronchopulmonary dysplasia (BPD)
eFigure 15. Ranking probability (rankogram) of each treatment modality for risk of BPD
eFigure 16. Network meta-analysis forest plots for outcome: risk of intraventricular hemorrhage (IVH)
eFigure 17. Ranking probability (rankogram) of each treatment modality for risk of IVH
eFigure 18. Network meta-analysis forest plots for outcome: risk of oliguria
eFigure 19. Ranking probability (rankogram) of each treatment modality for risk of oliguria
eFigure 20. Rankogram for PDA closure on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 21. Rankogram for need for repeat pharmacotherapy on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 22. Rankogram for need for surgical PDA ligation on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 23. Rankogram for neonatal mortality on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 24. Rankogram for risk of necrotizing enterocolitis on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 25. Rankogram for risk of bronchopulmonary dysplasia on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 26. Rankogram for risk of intraventricular hemorrhage on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eFigure 27. Rankogram for risk of oliguria on sensitivity analysis (‘low’ & ‘probably low’ risk of bias studies)
eText 1. Risk of bias assessment of eligible studies
eText 2. Guide to interpreting network meta-analysis results (rankograms; SUCRA; network GRADE) (includes rankogram example figure; SUCRA example table; network plot example for first-order loop; network plot example for second-order loop)
eText 3. Guide to interpreting meta-regression results (included example tables A & B)
eReferences