Key Points
Question
Are time-updated heart rate and change in heart rate over time associated with outcomes in participants from a community-based cohort?
Findings
In 15 680 participants enrolled in the Atherosclerosis Risk in Communities cohort study, time-updated heart rate and change in heart rate over a median time interval of 3.0 years were associated with outcomes such as all-cause death, incident heart failure, and noncardiovascular death.
Meaning
Higher resting heart rate and increases in heart rate over time are associated with risk, supporting the importance of measuring and noting a change in resting heart rate in everyday clinical practice.
Abstract
Importance
Time-updated heart rate (HR) and temporal change in HR (ΔHR) are associated with outcome in individuals with established heart failure (HF). Whether these factors are associated with outcomes in a community-based cohort is unclear.
Objective
To determine whether the time-updated analysis of resting HR, defined as the most recent HR value measured before occurrence of an event or the end of study, and ΔHR over time are associated with outcomes in a community-based cohort.
Design, Setting, and Participants
A total of 15 680 participants were enrolled in the Atherosclerosis Risk in Communities cohort study, with HR recorded at baseline and during 3 follow-up visits from 1987 to 1998, with a median interval between visits of 3.0 (interquartile range, 2.9-4.0) years. The ΔHR was calculated by assessing a change in HR from the preceding visit. Participants were followed up until December 31, 2014, equating to 28 years of follow-up. The present study was conducted from March 2014 to June 2016 with updated analysis.
Main Outcomes and Measures
Baseline HR, time-updated HR, and ΔHR associated with outcomes, adjusted for established baseline and time-updated risk factors and medications. The main outcomes measures included all-cause mortality, incident HF, incident myocardial infarction, stroke, and cardiovascular and noncardiovascular death.
Results
Of the 15 680 participants, 8656 (55.2%) were women, mean (SD) age was 54 (6) years, and 4218 (26.9%) were African American. Time-updated HR and ΔHR were associated with death, incident HF, incident myocardial infarction, stroke, and cardiovascular and noncardiovascular death compared with baseline HR. For example, a ΔHR from the preceding visit was significantly associated with increased risk of all-cause mortality (adjusted hazard ratio, 1.12; 95% CI, 1.10-1.15; P < .001 for every 5-bpm increase in HR from the preceding visit) and time-updated HR was also significantly associated with increased risk of all-cause mortality (adjusted hazard ratio, 1.14; 95% CI, 1.12-1.17; P < .001 for every 5-bpm higher time-updated HR).
Conclusions and Relevance
In a community-based cohort, time-updated HR and ΔHR are associated with mortality and nonfatal outcomes of incident HF, myocardial infarction, and stroke.
This cohort study evaluates the use of resting heart rate over time for determination of cardiovascular outcomes in analysis of a community-based population.
Introduction
Resting heart rate (HR) is a known risk factor for adverse outcomes in individuals with cardiovascular (CV) disease and overall within the general population. In addition to being a marker for increased occurrence of adverse outcomes, resting HR may be viewed as a modifiable risk factor in some populations, such that lowering resting HR in individuals with heart failure (HF) is associated with better outcomes, but in those with stable coronary artery disease, lowering resting HR was not associated with better outcomes.
The association between changes in HR and mortality has been assessed in individuals without known CV disease and in those with hypertension. However, the approach used in these studies included assessing changes in HR at 2 points only or by averaging follow-up HR. Our group has shown that using a time-updated analysis and calculating changes in HR from a preceding clinic visit over a median of 3 months was associated with outcomes in individuals with HF, such that a rise in HR was associated with adverse outcomes and a drop in HR was associated with better outcomes. The availability of strategies to monitor and track HR provides a logical rationale for determining the utility of temporal changes in HR as a biomarker for severity of CV or non-CV disease.
The objective of this study was to use a time-updated approach to determine whether actual temporal changes in HR from the preceding visit are of prognostic importance in participants of the Atherosclerosis Risk in Communities (ARIC) study, a large, multicenter, biracial, community-based cohort, with clinical assessments conducted over 12 years and outcomes surveillance spanning more than 2 decades.
Methods
Study Sample
The design of the ARIC study has been described previously. In brief, participants were recruited from 4 communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland) to take part in a prospective study of CV disease. Participants underwent a standardized evaluation of CV risk factors at each of the following examinations: visit 1 in 1987-1989, visit 2 in 1990-1992, visit 3 in 1993-1995, and visit 4 in 1996-1998. Thus, the median time interval between visits for participants was a median of 3.0 years, with an interquartile range (IQR) of 2.9 to 4.0 years. The CV examination included a 12-lead electrocardiogram, from which the HR data were derived. The present study was conducted from March 2014 to June 2016 with updated analysis. The study was approved by each site’s institutional review board (The Johns Hopkins University, Wake Forest University, University of Mississippi Medical Center, and University of Minnesota), and written informed consent was signed by all participants and, when required, proxies.
HR and Calculation of Temporal Changes in HR
To assess temporal changes in HR, we created a time-updated covariate representing the most recent available HR value for each participant over the course of the study. We termed this covariate time-updated HR. We used the data from visits 1 to 4, such that resting HR was updated up to 3 times after baseline for each participant. For participants with missing HR data at visits 2 to 4, the last HR recorded was carried forward as the most recent HR available.
We calculated temporal changes in resting HR from the preceding visit (change in HR [ΔHR]) by subtracting the time-updated visit HR value from the value from the preceding visit. The median time interval between visits was 3 (IQR, 2.9-4.0) years. Thus, temporal changes in HR occurred over a median of 3.0 (2.9-4.0) years.
Ascertainment of Outcomes
Participants were followed up longitudinally from the first examination and through to December 31, 2014, equating to 28 years of follow-up, for the incidence of new CV events, ascertained according to previously defined criteria and death outlined in a previous publication from our group. Incident CV events included coronary heart disease, HF, and stroke. The cause of death was defined using International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes.
Statistical Analysis
We modeled change in HR as a continuous covariate. Time-updated HR was modeled continuously similar to our group’s previous analysis of resting baseline HR. Because participants’ changing postbaseline HR values may cause them to appear in more than 1 HR category, only the total time spent in each category and the associated numbers of events are presented for the time-updated analysis. We also simultaneously looked at the characteristics of the participants who had a change in their HR from the preceding visit (eTables 1-3 in the Supplement).
We related resting baseline HR, time-updated HR, and change in HR to several outcomes outlined above. Incidence rates were calculated per 1000 patient-years. The association between outcomes and resting baseline HR, time-updated HR, and change in HR was assessed using multivariable Cox proportional hazards models. As continuous covariates, the estimated hazard ratio for each of these covariates corresponds to a 5-bpm difference in HR. The multivariable analysis adjusted for the established predictors of outcome. These included sex and race and time-updated variables such as age, body mass index, diabetes status, estimated glomerular filtration rate estimated by the Modified Diet in Renal Disease equation, systolic blood pressure, and pulse pressure. Levels of activity at work and leisure and sports activity at baseline together with alcohol use status and smoking status at the baseline visit were also included. We also considered time-updated use of HR-limiting drugs, such as β-blockers, calcium channel blockers, digoxin, antianginals, antiarrhythmics, and antihypertensives, which would control for starting or stopping these drugs. Our model also adjusted for the presence of atrial fibrillation at baseline and follow-up visits. Interval nonfatal myocardial infarction was also included in the model when we considered end points that did not include nonfatal myocardial infarction. We controlled for baseline HR when modeling for the time-updated HR; however, we replaced baseline HR with the HR from the preceding visit when modeling change in HR.
An adjusted model using a restricted cubic spline with 5 knots was constructed to flexibly display the association between the hazards of developing the outcome and continuous covariate of time-updated HR, using a reference value of 60 bpm. For change in HR, 0 was used as the reference. Interaction testing was used to determine whether the association between change in HR and outcomes varied in different subgroups (participants with or without diabetes at any time, β-blocker use at any time, and African American vs non–African American). Continuous variables are described using mean (SD) and categorical variables are expressed as counts and percentages. When the median value is reported, the IQR is also given.
All P values were 2-sided, and P < .05 was used to determine statistical significance. Analysis was performed using Stata, version 13.1 (StataCorp LP).
Results
The baseline characteristics of the study group at visits 1 to 4 are presented in Table 1. At visit 1, the mean (SD) age of the cohort at baseline was 54 (6) years, 8656 (55.2%) were women, and 4218 (26.9%) were African American. A total of 5451 (34.9%) of the population at baseline had hypertension. With respect to HR-limiting medications, 1646 (10.5%) were receiving β-blocker therapy; 538 (3.4%), calcium-channel blockers; and 248 (1.6%), digoxin. At the follow-up visits, there was an increase in body mass index, prevalence of hypertension and diabetes, and use of β-blockers and calcium-channel blockers. The frequency of atrial fibrillation was low at less than 1.0% for all visits; however, it increased from 0.2% at visit 1 to 0.7% at visit 4.
Table 1. Characteristics of Participants at Visits 1 to 4.
| Characteristic | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| No. of participants | 15 680 | 14 288 | 12 750 | 11 553 |
| Age, mean (SD), y | 54 (6) | 57 (6) | 60 (6) | 63 (6) |
| Sex, No. (%) | ||||
| Women | 8656 (55.2) | 7911 (55.4) | 7086 (55.6) | 6457 (56.0) |
| Men | 7026 (44.8) | 6377 (44.6) | 5664 (44.4) | 5096 (44.1) |
| African American, No. (%) | 4218 (26.9) | 3535 (24.7) | 2914 (22.9) | 2612 (22.6) |
| BMI, mean (SD) | 27 (5) | 28 (5) | 29 (6) | 29 (6) |
| Diabetes, No. (%) | 1858 (11.9) | 2149 (15.1) | 1960 (15.5) | 1920 (14.9) |
| Hypertension, No. (%) | 5451 (34.9) | 5131 (36.0) | 5197 (41.0) | 5492 (47.8) |
| Smoking, No. (%) | 4099 (26.2) | 3199 (22.4) | 2258 (17.7) | 1704 (14.9) |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 121 (19) | 122 (19) | 125 (19) | 128 (19) |
| Diastolic | 74 (11) | 72 (10) | 72 (11) | 71 (10) |
| Heart rate, mean (SD), bpm | 67 (10) | 66 (10) | 66 (10) | 63 (10) |
| eGFR<60 mL/min, No. (%) | 461 (3.0) | 690 (4.8) | NA | 954 (8.3) |
| β-Blocker, No. (%) | 1646 (10.5) | 1421 (9.9) | 1361 (10.7) | 1481 (12.8) |
| Calcium antagonist, No. (%) | 538 (3.4) | 1028 (7.2) | 1486 (11.7) | 1520 (13.2) |
| Digoxin, No. (%) | 248 (1.6) | 280 (2.0) | 287 (2.3) | 287 (2.5) |
| Atrial fibrillation, No. (%) | 28 (0.2) | 57 (0.4) | 72 (0.6) | 86 (0.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; eGFR, estimated glomerular filtration rate; NA, not available.
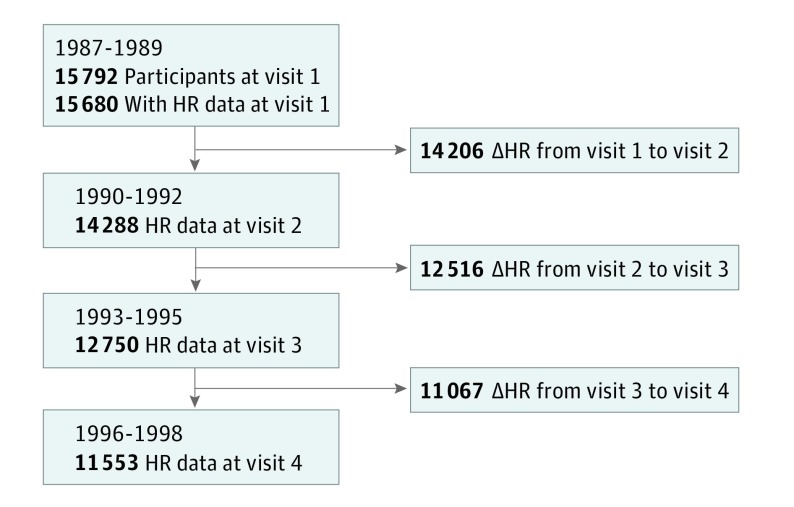
Figure 1 demonstrates the number of participants with HR data available at each visit and the total number of participants with changes in HR available from a preceding visit. The change in HR reflects changes in HR occurring over a median of 3.0 years (IQR, 2.9-4.0 years).
Figure 1. Flow Diagram.
Number of participants with heart rate (HR) data available at each visit and the total number of changes in HR (ΔHR) from a preceding visit.
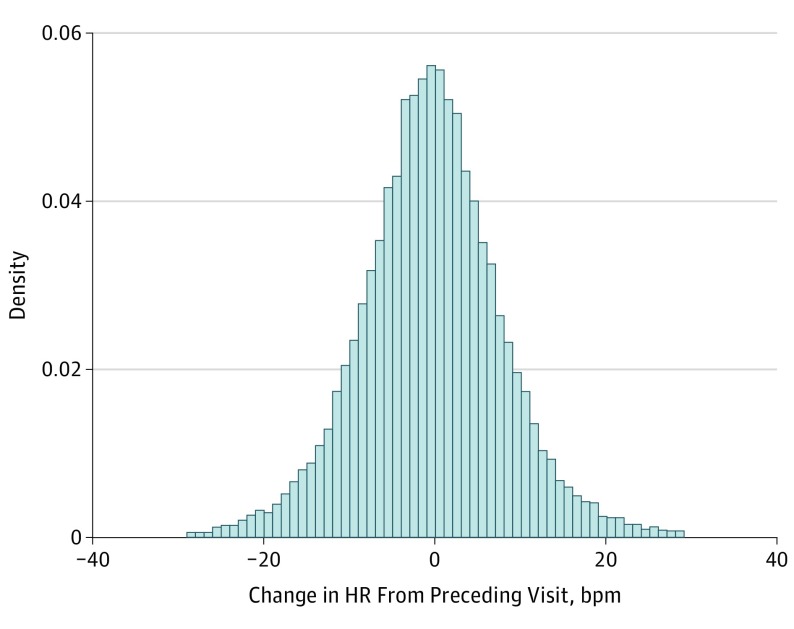
The median values of the time-updated HR measured at any visit for the cohort was 1 bpm lower compared with the baseline HR (65; IQR, 58 to 72 bpm vs 66; IQR, 60 to 73 bpm). The distribution of change in HR is summarized in Figure 2. Most participants had drop in HR by 1 bpm (IQR, −6 to 4) over the median time interval of 3.0 (IQR, 2.9 to 4.0) years.
Figure 2. Distribution of Changes in Heart Rate (HR) From Preceding Visit.
Most participants had a 1-bpm drop in HR from the preceding visit. Median, −1 (interquartile range, −6 to 4) bpm.
With follow-up of 28 years, a total of 6467 participants died and 2939 experienced incident HF. The number of events for other outcomes is summarized in Table 2. As a continuous covariate, increases in HR from the preceding visit were associated with all outcome measures (Table 2). Each 5-bpm increase in HR from the preceding visit was associated with 12% (95% CI, 10%-15%) higher risk of all-cause mortality and a 13% (95% CI, 9%-16%) higher risk of incident HF. Furthermore, each 5-bpm increase HR from the preceding visit was also associated with a 9% (95% CI, 4%-13%) higher risk of myocardial infarction, 6% (95% CI, 1%-11%) higher risk of stroke, 13% (95% CI, 8%-17%) higher risk of CV death, 12% (95% CI, 10%-15%) higher risk of non-CV death, and 8% (95% CI, 3%-13%) higher risk of cancer death (Table 2).
Table 2. Association Between Heart Rate Covariates and Outcomes.
| Outcomea | Events, No. | Event Rate (95% CI)b | Adjusted Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline HR | P Value | Time-Updated HR | P Value | ΔHR | P Value | |||
| All-cause mortality | 6467 | 18 (18-19) | 1.07 (1.05-1.09) | <.001 | 1.14 (1.12-1.17) | <.001 | 1.12 (1.10-1.15) | <.001 |
| Incident heart failure | 2939 | 11 (10-11) | 1.07 (1.04-1.10) | <.001 | 1.17 (1.14-1.20) | <.001 | 1.13 (1.09-1.16) | <.001 |
| Myocardial infarction | 1921 | 6 (5-6) | 1.03 (1.00-1.06) | .03 | 1.08 (1.04-1.12) | <.001 | 1.09 (1.04-1.13) | <.001 |
| Stroke | 1359 | 4 (4-4) | 1.04 (1.01-1.08) | .02 | 1.06 (1.01-1.10) | .01 | 1.06 (1.01-1.11) | .02 |
| CV death | 1685 | 5 (5-5) | 1.05 (1.02-1.09) | .003 | 1.15 (1.11-1.20) | <.001 | 1.13 (1.08-1.17) | <.001 |
| Non-CV death | 4782 | 14 (13-14) | 1.07 (1.05-1.09) | <.001 | 1.14 (1.11-1.17) | <.001 | 1.12 (1.10-1.15) | <.001 |
| Cancer death | 1463 | 4 (4-4) | 1.03 (0.99-1.07) | .09 | 1.09 (1.05-1.14) | <.001 | 1.08 (1.03-1.13) | .001 |
Abbreviations: CV, cardiovascular; HR, heart rate; ΔHR, change in HR from the preceding visit.
For outcomes without myocardial infarction, interval nonfatal myocardial infarction was also included in the model. Participants with prevalent heart failure were not included in the analysis for incident heart failure.
Event rate per 1000 patient-years. Adjusted for sex; race; time-updated age, diabetes status, body mass index, estimated glomerular filtration rate, systolic blood pressure, pulse pressure, and atrial fibrillation status; time-updated use of β-blockers, digoxin, antianginals, antihypertensives, and antiarrhythmics; activity levels at work, leisure, and sports activity at leisure time at baseline; and alcohol use and smoking status at baseline. For changes in HR from the preceding visit, preceding HR was used in the model instead of baseline HR.
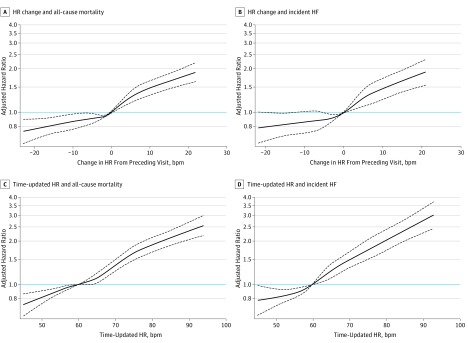
The association between change in HR and all-cause mortality was near linear (Figure 3A). Any rise in HR above a preceding HR value was associated with elevated risk and, conversely, any drop in HR from the preceding HR value was significantly associated with lower risk. A similar finding was observed for the end point of incident HF: lowering of HR of more than 12 bpm was significantly associated with a lower risk of incident HF (Figure 3B).
Figure 3. Association Between Changes in Heart Rate (HR) From Preceding Visit and End Points.
The adjusted cubic spline model demonstrates the flexible association between changes in HR from the preceding visit and the hazard of all-cause mortality (A) and hazard of incident HF (B), when no change in HR is taken as the reference (ie, 0 bpm). Both curves display a near linear relationship between change in HR and the end points of all-cause mortality and incident heart failure (HF), such that any rise in HR (>1 bpm) from the preceding visit appears to increase the risk significantly. However, any drop in HR from the preceding visit significantly reduced risk of all-cause mortality, and a drop in HR from the preceding visit of more than 12 bpm is associated with a significant reduction in risk of incident HF. The adjusted cubic spline model demonstrates the flexible relationship between time-updated HR (ie, most recent HR value before an event or end of the study) and the hazard of all-cause mortality (C) and hazard of incident HF (D) when a resting HR of 60 bpm is taken as the reference. Time-updated HR between 50 and 60 bpm during follow-up was associated with a lower risk of all-cause mortality and risk of incident HF compared with a HR of 60 bpm. A time-updated HR between 60 and 66 bpm was not associated with an increase in risk of all-cause mortality compared with a HR of 60 bpm; however, the risk of incident HF was greater above a HR of 60 bpm compared with a HR of 60 bpm. The most recent resting HR above 66 bpm during follow-up was associated with a higher risk of all-cause mortality. The dashed black curves represent the upper and lower 95% confidence limits. The horizontal blue line represents the hazard ratio of 1. The domain was defined by excluding the smallest 1% and the largest 1% of values of change in HR values.
The use of β-blockers at any time in the study influenced the association between change in HR and all-cause mortality (P for interaction, <.01) and also between time-updated HR and all-cause mortality (P for interaction, <.01) such that for participants receiving a β-blocker, the association between change in HR and all-cause mortality and between time-updated and all-cause mortality was no longer significant (eTable 4 in the Supplement). The use of β-blockers at any time in the study did not affect the association between baseline HR and all-cause mortality (P for interaction, .21). Diabetes status did not influence the association between change in HR from the preceding visit and outcomes (P for interaction, .89 for all-cause mortality and .28 for incident HF), nor did smoking status or race.
Participants who had an increase in heart rate from the preceding visit had a low resting heart rate at the preceding visit and were more frequently receiving β-blocker therapy. These data are presented in eTables 1-3 in the Supplement.
As a continuous covariate, time-updated HR, which represents the most recent HR before an event or the end of the follow-up, and also baseline HR were associated with both all-cause mortality and incident HF (Table 2). For every 5-bpm higher time-updated HR, the risk of all-cause mortality and incident HF was 14% (95% CI, 12%-17%) and 17% (95% CI, 13%-20%) higher, respectively. However, the magnitude of risk was less with baseline HR compared with the time-updated HR (Table 2).
In the adjusted restricted cubic spline models, we found that time-updated HR values between 50 to 60 bpm were associated with a lower risk of all-cause mortality and risk of incident HF relative to a time-updated HR of 60 bpm (Figure 3C and D). A time-updated HR value between 60 and 66 bpm was not associated with an increase in risk of all-cause mortality, but a time-updated HR value above 66 bpm relative to a value of 60 bpm was associated with a higher risk of all-cause mortality. The risk of incident HF was greater above a time-updated HR of 60 bpm relative to time-updated HR of 60 bpm (Figure 3C and D).
Discussion
In a large, community-based study comprising a significant biracial population, resting HR at baseline or time updated and changes in HR over a median of 3.0 (IQR, 2.9-4.0) years were associated with CV and non-CV outcomes. Increases in HR were associated with higher risk, and reduction in HR was associated with lower risk. Both time-updated HR, which represents the most recent available HR value, and temporal changes in HR were associated with a greater magnitude of risk compared with baseline HR. These findings suggest that HR may be a useful and easily measured biomarker of general ill health.
A novel aspect of our study includes the use of the time-updated approach of calculating actual changes in HR from the preceding visit at multiple visits; to our knowledge, this approach has not been used by any previous study assessing the association between resting HR or changes in HR and outcomes in the community population. Another novel aspect of our study is that we associated change in HR to incidence of diseases such as HF, myocardial infarction, stroke, and non-CV death.
Similar to previous epidemiologic studies, our study suggests that high resting HR is associated with an elevated risk of mortality, with increased sympathetic activity as a proposed mechanism. Our study confirms that temporal changes in HR are of prognostic importance in a community population. Temporal increases in HR over time may reflect increased sympathetic activity through the development of subclinical underlying CV or non-CV disease, including cancers or reduced fitness. In contrast, a drop in HR may reflect improving cardiac function, physical fitness, and lower sympathetic tone. Heart rate–limiting drugs, such as β-blockers and digoxin, are possible explanations for reduction in HR from the preceding visit, especially as β-blocker use became more frequent over the course of the study. However, the use of β-blockers and digoxin was time-updated, which would account for these medications being started or stopped and were included within the model used for the outcome analysis. In our analysis, we found that β-blocker use at any time during the study altered the association between change in HR and outcome, such that in participants receiving a β-blocker, which ranged from 10% to 13% of participants across the visits, the association between change in HR and all-cause mortality and incident HF was no longer significant (eTable 4). The latter factor may be related to the relatively lower use of β-blocker therapy compared with our group’s previous study of change in HR in participants with HF in which use of β-blocker therapy was more widespread (>50%) and did not affect the association between change in HR and outcome. Another factor that might play a role in change in HR over time (and its association with outcomes) might be physical activity; however, we adjusted for this variable in our model by taking information about physical activity from visits 1 and 3. Because the increase in HR from the preceding visit was also associated with an increased risk of non-CV death, the change in HR is likely to be a nonspecific signal of deteriorating health.
Our observation is in keeping with 2 longitudinal studies looking at participants without known CV disease, the first of which showed that a change in HR between 2 visits over a 5-year period in 5139 healthy, middle-aged men was associated with mortality over a 20-year follow-up. In another large epidemiologic study, which included 29 325 individuals without known CV disease, an increase in resting HR between 2 visits over a 10-year period was associated with a higher risk of all-cause mortality and death from ischemic heart disease. Our data are also consistent with those in a study performed in 4065 hypertensive participants, where change in HR was calculated by measuring the difference between the HR at the baseline visit and the mean HR at a final follow-up visit occurring at a median of 2.5 years. The investigators found that an increase in HR of greater than 5 bpm between these 2 times was associated with mortality. Thus, compared with the studies reported above, our study has incremental value in that we used a novel time-updated approach to calculate actual changes in HR, not just between 2 points, but between multiple visits, with changes in HR over a median of 3.0 years being associated with outcomes over 28 years of follow-up. In keeping with other published studies, our findings showed that a higher resting HR and a temporal increase in HR were associated with increased risk, but a novel finding in our study was the association of a temporal drop in HR over time being associated with a lower number of events. To our knowledge, this has not been reported in any previous study. Furthermore, we demonstrated that the most recent available HR (ie, time-updated HR) before an event or at the end of the study is associated with greater magnitude of risk compared with baseline HR. This finding is in keeping with our group’s previous article, suggesting that the most recent value of HR before an event or at the end of the follow-up period is more informative of outcome than the baseline resting HR value in participants with HF.
Our observation suggests that monitoring of HR over time as a biomarker of health status in the clinic setting or remotely may be useful in identifying participants at greatest risk of CV, such as incident HF, or non-CV events, such as chronic obstructive pulmonary disease, infections, and cancers. Heart rate is of importance, especially as it is potentially a modifiable risk factor in selected populations and these individuals could be considered for interventions that might improve their disease trajectory. Whether remote monitoring devices that can assess HR more continuously might improve risk prediction in the community will need further study.
It is not clear why β-blocker therapy significantly interacts with the association between change in HR or time-updated HR and all-cause mortality, such that participants receiving β-blockers no longer had a significant association between change in HR and all-cause mortality or time-updated HR and all-cause mortality. This finding is not in keeping with results in our group’s previous analyses of patients with HF with preserved or reduced ejection fraction.
Strengths and Limitations
A limitation that should be considered is the lack of information on doses of HR-limiting drugs, such as β-blockers, that could not be accounted for in the analysis. However, our study has several strengths, including the fact that it is a large population including white and African American participants followed up for up to 28 years with many outcome events. We relied on electrocardiogram-derived HR at each visit. This variable should have been measured in similar ways, at different times of the day, and under similar circumstances. However, an advantage of our analysis, including a time-updated analysis, is that it uses HR data from all visits and also allows the calculation of temporal changes in HR (the actual difference in HR between visits) and relating these to all subsequent events. The low number of visits allowed us to describe the characteristics of participants who experience a temporal change in HR to understand which of them had a temporal change in HR.
Conclusions
In a community-based cohort, the most recent resting HR and temporal change in HR are associated with outcomes; higher resting HR and temporal increases in HR are associated with increased risk of adverse outcomes. Our findings further support the importance of measuring resting HR in everyday clinical practice, and potentially with remote monitoring, as a way to identify individuals who could be at greatest risk of CV and non-CV events.
eTable 1. Characteristics of Participants at Visit 1 Categorized by Change in Heart Rate Between Visit 1 and 2
eTable 2. Characteristics of Participants at Visit 2 Categorized by Change in Heart Rate Between Visit 2 and 3
eTable 3. Characteristics of Participants at Visit 3 Categorized by Change in Heart Rate Between Visit 3 and 4
eTable 4. Beta-Blocker Use and Effect on Relationship Between Heart Rate Covariates and All-Cause Mortality
References
- 1.Castagno D, Skali H, Takeuchi M, et al. ; CHARM Investigators . Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) program. J Am Coll Cardiol. 2012;59(20):1785-1795. [DOI] [PubMed] [Google Scholar]
- 2.Böhm M, Swedberg K, Komajda M, et al. ; SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376(9744):886-894. [DOI] [PubMed] [Google Scholar]
- 3.Fox K, Borer JS, Camm AJ, et al. ; Heart Rate Working Group . Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823-830. [DOI] [PubMed] [Google Scholar]
- 4.Poole-Wilson PA, Uretsky BF, Thygesen K, Cleland JG, Massie BM, Rydén L; Atlas Study Group. Assessment of treatment with lisinopril and survival; mode of death in heart failure: findings from the ATLAS trial. Heart. 2003;89(1):42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26(7-8):637-644. [DOI] [PubMed] [Google Scholar]
- 6.Swedberg K, Komajda M, Böhm M, et al. ; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875-885. [DOI] [PubMed] [Google Scholar]
- 7.Swedberg K, Komajda M, Böhm M, et al. ; SHIFT Investigators . Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose? findings from the SHIFT (Systolic Heart Failure Treatment with the I(f) Inhibitor Ivabradine Trial) study. J Am Coll Cardiol. 2012;59(22):1938-1945. [DOI] [PubMed] [Google Scholar]
- 8.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150(11):784-794. [DOI] [PubMed] [Google Scholar]
- 9.Fox K, Ford I, Ferrari R. Ivabradine in stable coronary artery disease. N Engl J Med. 2014;371(25):2435. [DOI] [PubMed] [Google Scholar]
- 10.Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306(23):2579-2587. [DOI] [PubMed] [Google Scholar]
- 11.Jouven X, Empana JP, Escolano S, et al. . Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol. 2009;103(2):279-283. [DOI] [PubMed] [Google Scholar]
- 12.Paul L, Hastie CE, Li WS, et al. . Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension. 2010;55(2):567-574. [DOI] [PubMed] [Google Scholar]
- 13.Vazir A, Claggett B, Jhund P, et al. . Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. Eur Heart J. 2015;36(11):669-675. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 15.Cheng S, Claggett B, Correia AW, et al. . Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities study. Circulation. 2014;130(10):820-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black A, Murray L, Cardwell C, Smith GD, McCarron P. Secular trends in heart rate in young adults, 1949 to 2004: analyses of cross sectional studies. Heart. 2006;92(4):468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazir A, Claggett B, Pitt B, et al. . Prognostic importance of temporal changes in resting heart rate in heart failure and preserved ejection fraction: from the TOPCAT Study. JACC Heart Fail. 2017;5(11):782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Participants at Visit 1 Categorized by Change in Heart Rate Between Visit 1 and 2
eTable 2. Characteristics of Participants at Visit 2 Categorized by Change in Heart Rate Between Visit 2 and 3
eTable 3. Characteristics of Participants at Visit 3 Categorized by Change in Heart Rate Between Visit 3 and 4
eTable 4. Beta-Blocker Use and Effect on Relationship Between Heart Rate Covariates and All-Cause Mortality