Abstract
Plants synthesize numerous classes of natural products that accumulate during development and are thought to function as constitutive defenses against herbivores and pathogens. However, little information is available about how the levels of such defenses are regulated. We measured the accumulation of monoterpenes, a model group of constitutive defenses, in peppermint (Mentha × piperita L.) leaves and investigated several physiological processes that could regulate their accumulation: the rate of biosynthesis, the rate of metabolic loss, and the rate of volatilization. Monoterpene accumulation was found to be restricted to leaves of 12 to 20 d of age, the period of maximal leaf expansion. The rate of monoterpene biosynthesis determined by 14CO2 incorporation was closely correlated with monoterpene accumulation, as determined by gas chromatographic analysis, and appeared to be the principal factor controlling the monoterpene level of peppermint leaves. No significant catabolic losses of monoterpenes were detected throughout leaf development, and monoterpene volatilization was found to occur at a very low rate, which, on a monthly basis, represented less than 1% of the total pool of stored monoterpenes. The composition of volatilized monoterpenes differed significantly from that of the total plant monoterpene pool, suggesting that these volatilized products may arise from a separate secretory system. With the demonstration that the rate of biosynthesis is the chief process that determines monoterpene accumulation in peppermint, efforts to improve production in this species can now focus on the genes, enzymes, and cell differentiation processes that regulate monoterpene biosynthesis.
Plants produce an enormous variety of natural products that are thought to play a critical role in defense against herbivores and pathogens (Wink, 1999). These metabolites may be synthesized constitutively in specific organs or at specific stages of development, or their production may be induced by herbivore or pathogen attack. Considerable information is available about the mechanisms by which plant damage induces the synthesis of defensive metabolites (Karban and Baldwin, 1997). By comparison, much less is known about what controls the formation of constitutive defenses. Compounds such as monoterpenes (Gambliel and Cates, 1995), napthoquinones (Brigham et al., 1999), pyrrolizidine alkaloids (Hartmann and Dierich, 1998), and glucosinolates (Blake-Kalff et al., 1998) accumulate during normal root or shoot development in thousands of plant taxa, but the physiological and molecular mechanisms that regulate the production of these natural products have seldom been examined.
One of the best-studied examples of constitutive plant defenses are the monoterpenes, the C10 members of the terpenoid (isoprenoid) family of natural products. Monoterpenes are colorless, lipophilic, volatile substances that have been implicated as defenses against a variety of herbivores and pathogens (Langenheim, 1994). Known from species of Pinaceae, Lamiaceae, Rutaceae, Myrtaceae, Asteraceae, and many other plant families (Charlwood and Charlwood, 1991), they are responsible for many of the characteristic odors of plants (Hay and Waterman, 1993). Monoterpenes are also frequent constituents of oils and resins, and their accumulation is often associated with complex secretory structures such as glandular trichomes, secretory cavities, or resin ducts (Fahn, 1979).
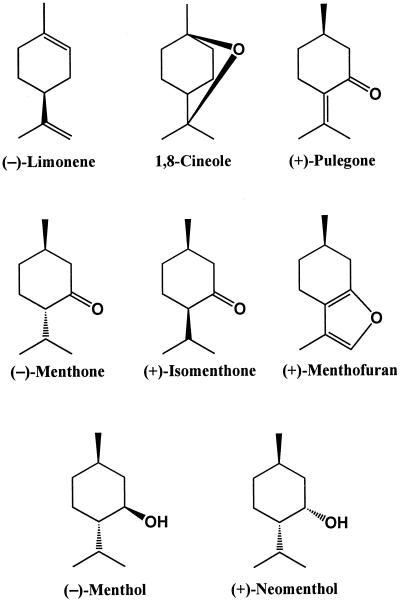
From an economic standpoint, the most important monoterpene-producing species is peppermint (Mentha × piperita L.), a perennial herb of the Lamiaceae that produces high levels of p-menthane monoterpenes (Fig. 1) in glandular trichomes found on the surfaces of leaves, young stems, and parts of the inflorescence (Amelunxen, 1965). During leaf development, the total content of monoterpenes increases with age (Burbott and Loomis, 1969; Croteau and Martinkus, 1979), and the composition of monoterpenes is significantly altered. Limonene and menthone are the major monoterpenes present in the youngest leaves. The proportion of limonene declines rapidly with development, while menthone increases in prominence and declines only at later stages as menthol becomes the dominant monoterpene constituent (Burbott and Loomis, 1969; Croteau and Martinkus, 1979; Brun and Voirin, 1991).
Figure 1.
Major monoterpene constituents of peppermint leaves.
The pathway of monoterpene biosynthesis in peppermint has been well established by in vivo and cell-free studies (Kjonaas and Croteau, 1983; Kjonaas et al., 1985; Croteau and Venkatachalam, 1986), and all of the enzymes involved have been described (Kjonaas et al., 1982, 1985; Croteau and Venkatachalam, 1986; Karp et al., 1990; Croteau et al., 1991; Alonso et al., 1992; Rajaonarivony et al., 1992; Colby et al., 1993). In addition, the site of monoterpene biosynthesis has been specifically localized to the secretory cells of the glandular trichomes (Gershenzon et al., 1989; McCaskill et al., 1992). However, the physiological factors regulating monoterpene accumulation are poorly known. The accumulation of any metabolite is controlled by the balance between the rate of formation and the rate of loss, a consequence of direct release into the environment and/or catabolism. For volatile compounds such as monoterpenes, emission into the atmosphere may be a major route of loss from plants (Lerdau et al., 1997). However, catabolism must also be considered, because, while short-term monoterpene turnover in peppermint has been ruled out (Mihaliak et al., 1991), a pathway for the long-term degradation of monoterpenes in mature leaves of this species has been previously described (Croteau, 1988).
In this study, the pattern of monoterpene accumulation in the developing leaves of peppermint was measured, and the rates of monoterpene synthesis, loss, and volatile emission were determined at various stages during development to evaluate the influence of these processes on monoterpene yield. The results define the physiological factors that control the accumulation of a major class of defensive compounds in plants, and provide the necessary foundation for further regulatory studies at the biochemical and molecular levels. Given the economic importance of monoterpenes for the fragrance, flavor, and pharmaceutical industries, knowledge of the processes that control monoterpene accumulation in plants can be of value in increasing the yields of these commercially valuable natural products.
MATERIALS AND METHODS
Plant Material
Peppermint (Mentha × piperita L.) was propagated from rhizomes and raised in a plant growth chamber equipped with a mixture of fluorescent and incandescent lights (16-h photoperiod, 350 μmol m−2 s−1 of photosynthetically active radiation [PAR] at plant height) and a temperature cycle of 22°C/10°C (day/night). Plants were grown in peat moss:pumice:sand (55:35:10, v/v), watered daily, and fertilized on alternate days with a complete fertilizer (N:P:K, 20:20:20, v/v) plus iron chelate and micronutrients. To study developmental changes in monoterpene accumulation and the rate of monoterpene biosynthesis, a cohort of leaves was utilized that was initiated on 3-week-old stems. Samples of this cohort were removed at ages ranging from 5 to 55 d, at which time the majority of leaves of this group had senesced or abscised.
Monoterpene Extraction and Analysis
Fresh leaves of different ages were soaked in 5 mL of diethyl ether for 1 h, and then again in a second portion of diethyl ether for 1 h at room temperature. A mixture of 1.25 μmol of isobutyl benzene and 1.25 μmol of camphor was added to the combined extract for each age group as an internal standard for the quantification of monoterpene olefins and oxygenated monoterpenes, respectively, followed by concentration under nitrogen to approximately 3 mL and treatment with 25 mg of activated charcoal. After filtration and washing with 1 mL of water, the organic extract was passed through a short column of anhydrous sodium sulfate and silica gel in a Pasteur pipette and concentrated further to 0.5 mL.
Gas chromatography was performed on a model HP5890 gas chromatograph (Hewlett-Packard, Palo Alto, CA) with an AT-1000 column (polyethylene glycol ester, 30-m × 0.25-mm i.d., 0.2-μm film thickness, Alltech, Deerfield, IL) operated with hydrogen (1.5 mL min−1) as a carrier, a split injector (injector temperature 220°C, injection volume 2 μL, split ratio 75:1) flame ionization detector (300°C), and a temperature program from 45°C (5-min hold) to 150°C at 10°C min−1, and to 220°C at 50°C min−1 (with a 10-min hold). Components were identified by comparison of retention times and mass spectra with authentic standards from our own collection (Kjonaas et al., 1985), and were quantified by comparison of detector response with that of the appropriate internal standard. Gas chromatography-mass spectrometry analysis was performed on a Hewlett-Packard 5840A–5985B system at 70 eV, with the same column and separation conditions described above.
Rate of Monoterpene Synthesis
Pulse-labeling experiments were conducted using 14CO2 with rooted plants in a 40-L plexiglass chamber. A pulse of 37 MBq of 14CO2 was administered by acidification (with 1 mL of perchloric acid) of Na214CO3 (20 GBq mmol−1; DuPont/NEN, Wilmington, DE) dissolved in 0.5 mL of water. After the plants were placed in the chamber and the door was sealed, the acid was added to a beaker containing the Na214CO3 solution by injection through a septum inlet in the chamber wall. Plants were exposed to 14CO2 for 5 min under incandescent lights providing 250 μmol m−2 s−1 PAR. Temperature was maintained at 22°C by the use of water-filled trays placed on top of the chamber through which the light was filtered. 14CO2 concentration in the chamber was measured by sampling air through the septum with a gastight syringe. Air samples were transferred to septum-capped, glass scintillation vials containing 0.2 mL of 1 n KOH and allowed to stand for 30 min to permit the trapping of 14CO2 as carbonate. After the addition of scintillation cocktail (10 mL of 0.4% [w/v] DuPont/NEN Omnifluor in toluene/ethanol, 7:3, v/v), samples were analyzed in a liquid scintillation counter (Tricarb 460 CD, Packard Instruments, Meriden, CT) with a 14C counting efficiency of 91%. Plants absorbed 20% to 25% of the administered 14CO2 pulse. After the pulse, unincorporated 14CO2 was exhausted from the chamber into a 10 n KOH trap, and the plants were left under the incandescent lights in a fume cabinet for 6 h. Replicate samples of leaves of the various ages were then harvested for analysis. Each sample, consisting of four to 20 leaves depending on leaf size, was immediately weighed and frozen at −20°C. At least three samples were analyzed for each age group.
Radiolabeled monoterpenes were extracted from peppermint leaves by simultaneous steam distillation-pentane extraction using a Likens-Nickerson apparatus (J&W Scientific, Folsom, CA) equipped with a standard condenser that was cooled with ice water. The leaves were heated to reflux in a flask with 30 mL of distilled water and 3 μmol of camphor as an internal standard. The organic phase consisted of 10 mL of pentane. Both solvents were heated for 30 min after refluxing had begun, and the pentane layer was then collected, dried over anhydrous sodium sulfate, and concentrated to 4 mL under a stream of nitrogen. A portion was removed for liquid scintillation counting, and the remainder analyzed by gas chromatography as described above to evaluate losses during extraction and concentration.
Rate of Monoterpene Loss
A 5-min pulse of 14CO2 similar to that described above was administered to 5-week-old peppermint stems. Plants were then kept in a fume cabinet under lights for 3 d and then transferred to a confined greenhouse, where they were allowed to grow for an additional 6 weeks. The greenhouse had supplemental light (16-h photoperiod, 350–550 μmol m−2 s−1 of PAR at plant height) and a 30°C/15°C (day/night) temperature cycle. Samples were taken periodically from a group of leaves that were approximately 2 weeks old at the time of pulsing, and thus (according to measurements of the rate of monoterpene biosynthesis; see Fig. 2) at the stage of maximal monoterpene production. This was the same cohort of leaves used for measurements of monoterpene accumulation and the rate of biosynthesis. At least three samples were harvested at each time point, with each sample consisting of a pair of leaves from a single stem. To determine whether there were significant differences between time points, statistical analyses were performed using SAS software (SAS Institute, Cary, NC).
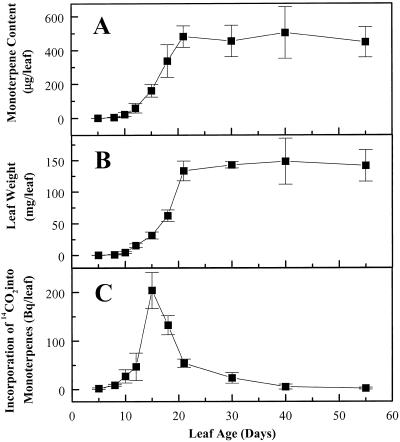
Figure 2.
Changes in monoterpene content (A), leaf weight (B), and rate of monoterpene biosynthesis (C) during peppermint leaf development. Monoterpenes were extracted with diethyl ether and analyzed by gas chromatography. To determine the rate of monoterpene biosynthesis, leaves of various ages were exposed to a 5-min pulse of 14CO2, and the incorporation of 14C into monoterpenes was measured after a 6-h chase period. Each data point is the mean of three to six independent measurements. Bars indicate sd.
Rate of Monoterpene Volatilization
The monoterpenes volatilized from peppermint were collected by headspace sorption from intact plants enclosed in a 40-L plexiglass chamber lined with a 0.005-cm layer of transparent polyvinylfluoride film (Tedlar, DuPont/NEN). A constant stream of air flowing at 1.1 L min−1 was drawn through the chamber with two small diaphragm vacuum pumps (no. 8803, Welch Vacuum Technology, Skokie, IL) connected in parallel. Flow was carefully regulated by controlling the pump speed and adjusting a valve on the inlet side of the chamber. The air exiting the chamber was passed through an adsorbent trap consisting of a 200- × 7-mm glass tube containing 150 mg of Tenax GC (a polymer of 2,6-diphenyl-p-phenylene oxide, 60/80 mesh, Alltech) and 150 mg of Super Q (a polymer of divinylbenzene, 80/100 mesh, Alltech) held in place with plugs of silanized glass wool. Preliminary trials showed that the combination of these two adsorbents trapped the full spectrum of peppermint monoterpenes with higher efficiency than either one alone.
An additional adsorbent trap of identical construction was placed at the inlet of the chamber to purify incoming air. Trials with two such traps connected in series at the chamber exit indicated that there was no detectable “breakthrough” (i.e. no loss of volatile monoterpenes from the first trap due to overloading, even when collections were carried out for periods of up to 8 h). Prior to initial use, the adsorbents were extracted exhaustively with diethyl ether and pentane, and before every subsequent use they were washed free of residual material with 50 mL of diethyl ether and dried with a stream of compressed air. In addition to the adsorbent traps, all fittings and connecting tubes were of glass. The entire volatile collection apparatus was contained in a controlled environment room that was adjusted such that light (16-h photoperiod) inside the plant chamber was maintained at an intensity of 350 μmol m−2 s−1 PAR with a constant temperature of 24°C.
Plants were placed in the chamber prior to volatile collection and left undisturbed with the chamber cover open for at least 2 h to ensure that any volatiles released by handling had dissipated. After the chamber was sealed and the airflow initiated, actual collection was not begun for an additional 2 h to ensure that an equilibrium concentration of volatiles had been reached in the chamber. Collection was then initiated for 3 to 6 h to accumulate sufficient material for accurate analysis. Trapped volatiles were desorbed from the trap with 25 mL of diethyl ether, and the sample was concentrated to 1 mL under a stream of nitrogen, and analyzed by gas chromatography as described above. Internal standards (75 μg each of isobutyl benzene and fenchone) were added prior to desorption to adjust for losses during sample processing. Tared vials of 2-carene and camphor (monoterpenes not found in peppermint) were also placed directly in the plant chamber to assess recovery of standard monoterpenes during the collection process. Collections made without plants (with pots and soil, with empty pots, or with an empty chamber) established that the background of monoterpenes present was negligible, and that the “carryover” from one collection to the next was insignificant.
RESULTS AND DISCUSSION
Monoterpenes in Peppermint Leaves Accumulate with Leaf Development
The pattern of monoterpene accumulation in peppermint leaves was measured by following a single cohort of leaves from initiation to senescence. Leaves were harvested at nine different stages during development, and the monoterpenes were extracted and analyzed by gas chromatography. The cohort of leaves chosen was initiated on 3-week-old (10- to 15-cm) stems and reached full expansion 21 d later when stems were 20 to 25 cm in length. Flower buds first appeared at the stem apex when leaves were 30 to 35 d old (stems 30–35 cm tall), and flowering commenced at approximately 45 to 50 d. By the time leaves were 55 d old, they had begun to senesce and abscise.
The monoterpene content of young leaves increased rapidly for the first 21 d of leaf development, then leveled off and was stable for the remainder of leaf life (Fig. 2A). Leaf weight showed a nearly identical trend (Fig. 2B). Similar profiles of monoterpene accumulation have been described for leaves and fruits of other species, including dill (Anethum graveolens) (Porter et al., 1983), garden sage (Salvia officinalis) (Croteau et al., 1981), lemongrass (Cymbopogon flexuosus) (Singh et al., 1989), and caraway (Carum carvi) (Bouwmeester et al., 1998). In all of these taxa, the monoterpene content increases during the early stages of organ development and then remains relatively constant over the rest of organ life. In contrast, several studies of peppermint and other Lamiaceae have reported that monoterpene content declines as leaves age. These results are probably attributable to unusual growth conditions, such as extensive overhead irrigation (Croteau, 1977a), or to sampling schemes in which leaves of different ages were all harvested from the same stem at the same time (Srivastava et al., 1990; Srivastava and Luthra, 1991). The latter sampling method does not represent a true developmental gradient, since it is known that peppermint leaves initiated at early growth stages never attain monoterpene levels as high as leaves initiated at later stages (Burbott and Loomis, 1969).
The developmental changes in monoterpene accumulation in peppermint were accompanied by alterations in the monoterpene composition. The proportions of limonene, menthofuran, and pulegone declined as leaves aged, while those of 1,8-cineole, menthol, and neomenthol increased substantially (Table I). The major constituent, menthone, which was present in 5-d-old leaves at 36% of total monoterpenes, increased to approximately 75% at 15 d and then declined to 10% by the end of the study. Among the minor constituents, β-pinene, myrcene, and linalool showed increased percentages during development. Some of these compositional shifts have been documented in previous studies (Grahle and Holtzel, 1963; Brun and Voirin, 1991; Court et al., 1993; Voirin and Bayet, 1996; Rohloff, 1999).
Table I.
Changes in monoterpene composition during leaf development in peppermint
| Compound | Leaf Age (d)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 8 | 12 | 15 | 18 | 21 | 30 | 40 | 55 | |
| % of total monoterpenes | |||||||||
| α-Pinene | 1.5 | 1.2 | 1.1 | 0.8 | 1.5 | 1.4 | 1.8 | 1.6 | 2.0 |
| β-Pinene | 1.1 | 1.3 | 1.1 | 1.0 | 1.7 | 1.9 | 2.4 | 2.3 | 2.9 |
| Sabinene | 1.0 | 0.3 | 0.6 | 0.5 | 0.8 | 1.0 | 1.3 | 1.3 | 1.6 |
| Myrcene | tra | tr | 0.4 | 0.3 | 0.4 | 0.5 | 0.6 | 0.6 | 0.7 |
| Limonene | 26.8 | 19.9 | 13.0 | 5.0 | 2.4 | 1.4 | 2.4 | 1.3 | 1.8 |
| 1,8-Cineole | tr | 0.5 | 0.8 | 2.4 | 6.0 | 8.5 | 12.0 | 11.7 | 15.6 |
| Menthone | 36.4 | 53.6 | 66.0 | 75.5 | 58.5 | 63.5 | 40.2 | 33.3 | 10.0 |
| Menthofuran | 3.9 | 1.9 | 2.8 | 1.4 | 4.6 | 1.5 | tr | tr | tr |
| Isomenthone | 3.5 | 3.5 | 4.2 | 4.5 | 3.7 | 4.2 | 3.4 | 3.7 | 3.6 |
| Linalool | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.8 | 0.6 | 1.0 |
| Neomenthol | tr | tr | tr | tr | tr | tr | 0.8 | 2.6 | 4.6 |
| Menthol | tr | tr | tr | tr | 1.6 | 2.6 | 12.0 | 35.2 | 54.0 |
| Pulegone | 24.9 | 16.9 | 8.5 | 7.1 | 17.5 | 11.8 | 21.1 | 4.6 | 0.8 |
| α-Terpineol | tr | tr | 0.1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.3 | 0.2 |
| Piperitone | 0.4 | 0.8 | 1.2 | 1.1 | 0.8 | 1.0 | 0.7 | 0.9 | 1.2 |
Diethyl ether extracts prepared from leaves of different ages were analyzed by gas chromatography. Each value is the mean of at least three separate analyses. “tr” indicates that <0.1% was detected.
tr, Trace (<0.1% detected).
Monoterpene Biosynthesis Is Restricted to a Brief Period Early in Leaf Development
The ontogenetic profile of monoterpene accumulation in peppermint may be influenced by both monoterpene synthesis and loss. To examine the rate of monoterpene biosynthesis, rooted plants were exposed to a 5-min pulse of 14CO2. Leaves of different ages from the same cohort as that used to study changes in monoterpene content were harvested 6 h after 14CO2 exposure, and the monoterpenes were isolated by simultaneous steam distillation-pentane extraction. The 14C content of each extract was determined by liquid scintillation counting, and radio-gas chromatography was used to measure the percentage of radioactivity attributable to monoterpenes. There was a sharp peak of biosynthetic activity centered at 15 d, when leaves were still expanding, but only very low rates of biosynthesis were observed in leaves younger than 12 or older than 20 d (Fig. 2C). The rapid decline in biosynthetic rate between 15 and 20 d coincides with the leveling off of leaf monoterpene content (Fig. 2A) and the cessation of leaf expansion (Fig. 2B).
These results are consistent with those of several previous investigations on plant terpene formation. The biosynthesis of monoterpenes in S. officinalis leaves (Croteau et al., 1981), Majorana hortensis leaves (Croteau, 1977b), C. carvi fruits (Bouwmeester et al., 1998), C. flexuosus blades (Singh et al., 1989), and maritime pine (Pinus pinaster) foliage (Bernard-Dagan et al., 1982) is also restricted to a short interval during organ ontogeny. Among other terpenes, the biosynthesis of sesquiterpenes in Heterotheca subaxillaris (Mihaliak and Lincoln, 1989), diterpenes in Newcastelia viscida (Dell and McComb, 1978), and triterpenes in Euphorbia lathyris (Koops and Groeneveld, 1991) is also confined to early development. A 14CO2 pulse-labeling experiment previously conducted with peppermint showed similar trends (Srivastava and Luthra, 1991). However, these latter results were deemed unreliable because cuttings rather than rooted plants were used (peppermint cuttings pulsed with 14CO2 exhibit an artifactual turnover of monoterpenes [Mihaliak et al., 1991]), and because the monoterpene extracts were not examined for radiochemical purity.
The peak period of monoterpene biosynthesis in peppermint coincides with the time when the secretory cells of the glandular trichomes are metabolically active. Monoterpene synthesis in this species is localized to the secretory cells of glandular trichomes (Gershenzon et al., 1989; McCaskill et al., 1992), and the monoterpenes are discharged into a surmounting subcuticular storage compartment formed by expansion of the cuticle (Amelunxen, 1965). Anatomical studies indicated that the formation of Lamiaceae glands and filling of the subcuticular space occur only in actively growing, protodermal regions of the leaf surface (Werker et al., 1993); tracking the distribution of various gland developmental stages during peppermint leaf development has shown that 2-week-old leaves have especially high proportions of filling glands (G. Turner, J. Gershenzon, and R. Croteau, unpublished data). The occurrence of monoterpene biosynthesis in other species is also associated with the metabolic activity of glandular trichomes or other specialized secretory structures, such as secretory cavities and resin ducts, in which monoterpenes are synthesized and sequestered. Such structures commonly differentiate in young, expanding tissue (Werker and Fahn, 1981; Charon et al., 1986; Russin et al., 1988), which may account for the fact that monoterpene biosynthesis is often highest in immature tissue.
Rate of Monoterpene Loss Is Negligible throughout Leaf Development
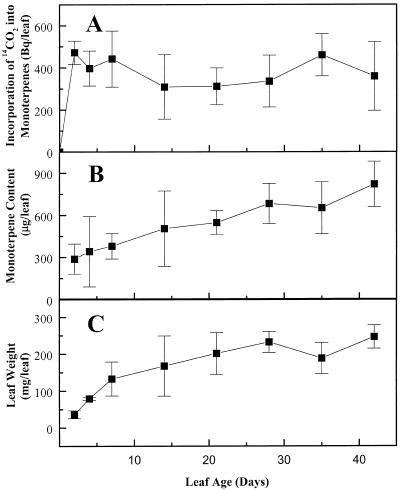
To determine the rate of monoterpene loss, similar pulse-labeling experiments were employed, except that plants were allowed to grow for 6 more weeks following the initial 5-min pulse of 14CO2. Samples of a single, marked group of leaves (2 weeks old at the time of pulsing) were harvested periodically for monoterpene extraction and determination of 14C content. There was a rapid incorporation of 14C into monoterpenes during the first 2 d following the pulse, but no further significant changes in the radioactivity of extracted monoterpenes were observed over the remainder of the time course (Tukey's studentized range test, P > 0.05) (Fig. 3A). Thus, the metabolic pools of monoterpenes in peppermint leaves appear to be stable, and do not exhibit any detectable turnover. In contrast, the weight and total monoterpene content of these leaves increased steadily over the period of measurement (Fig. 3, B and C).
Figure 3.
Lack of monoterpene turnover in peppermint leaves. Plants were exposed to a 5-min pulse of 14CO2, and samples were harvested over the next 6 weeks for determination of monoterpene content. Incorporation of 14C into monoterpenes (A) did not change significantly (Tukey's studentized range test, P > 0.05) over the time course of the experiment, indicating the lack of detectable monoterpene loss. In contrast, total monoterpene content (B) and leaf weight (C) increased steadily over the time course of the experiment. Each data point represents the mean of at least three independently measured samples, each consisting of a pair of leaves from a single stem. Bars indicate sd.
These results extend those of an earlier study in which peppermint plants exhibited no significant losses of radiolabeled monoterpenes over 29 h following a 5-min pulse of 14CO2 (Mihaliak et al., 1991). They are also in accord with recent experiments conducted to examine terpenoid turnover conducted with lodgepole pine (Pinus contorta), Australian tea tree (Melaleuca alternifolia), garden sage (S. officinalis), and common tansy (Tanacetum vulgare) (Gershenzon et al., 1993). None of these species, which span a range of taxonomically distant plant families, contain different types of secretory structures, and include plants that contain sesquiterpenes and diterpenes as well as monoterpenes, exhibited significant losses of terpenoids over a period of 10 to 14 d following an initial 14CO2 pulse. In contrast, detached stems of peppermint studied under similar conditions displayed pronounced monoterpene turnover, an artifact also observed in numerous other investigations with detached tissues of other monoterpene-containing species (Francis and O'Connell, 1969; Croteau et al., 1972; Njar et al., 1989).
In the current study, peppermint leaves showed no significant loss of monoterpenes during an interval of 6 weeks after pulse labeling. At the end of this period, the group of marked leaves under study was 8 weeks old, and the experiment was terminated because most of the remaining marked leaves had begun to senesce. Previous investigations have indicated that peppermint monoterpenes can be metabolically degraded at later stages of leaf development (Croteau, 1988). A catabolic pathway was described involving the sequential reduction and glucosylation of menthone to neomenthol glucoside (Croteau and Martinkus, 1979), which is transported to the rhizome and degraded (Croteau et al., 1984; Croteau and Sood, 1985). Although the present study provided no evidence for monoterpene catabolism, the large variances in monoterpene incorporation after pulse labeling (Fig. 3C) may have prevented its detection. Alternatively, the monoterpene degradation enzymes previously described may function not to degrade stored monoterpenes, but to detoxify monoterpenes that have come into contact with living cells after damage to the secretory structures. Monoterpenes such as limonene, linalool, and isomenthone have been demonstrated to be toxic to plant tissues (Brown et al., 1987), and disruption of monoterpene-containing secretory structures has been reported to cause damage to surrounding cells (Shomer and Erner, 1989; Loveys et al., 1992).
Rate of Monoterpene Volatilization Is Low and Does Not Significantly Influence Accumulation
Many species of plants release volatile monoterpenes into the atmosphere (Lerdau et al., 1997). Therefore, any consideration of the factors that regulate monoterpene accumulation in peppermint would be incomplete without an assessment of the rate of volatilization. The volatilization of peppermint monoterpenes was quantified by dynamic headspace sampling (Dobson, 1991) under a controlled-environment regime. Potted plants were placed in a chamber through which a stream of filtered air was passed. The air exiting the chamber passed through a cartridge packed with adsorbents to trap volatile organic compounds. Monoterpenes and other adsorbed substances were desorbed from the trap with organic solvent and analyzed by gas chromatography. Intact plants rather than cut leaves or stems were used, since detached tissues may have altered volatilization rates (Mookherjee et al., 1989; Nielsen et al., 1995).
Preliminary observations indicated that the rate of monoterpene volatilization from peppermint plants varied with light, temperature, time of day, and stage of development. For the purposes of this study, nonflowering, 6-week-old plants were measured under light and temperature conditions that were virtually identical to those used in the other experiments described here. The volatilization rate during the light period was 1.22 μg h−1 plant−1, which can be expressed as 4.95 μg h−1 m−2 leaf area or 0.254 μg h−1 per g−1 dry weight. During the dark period, the volatilization rate was slightly higher at 1.73 μg h−1 plant−1, or 7.02 μg h−1 m−2 leaf area and 0.36 μg h−1 per g−1 dry weight. According to the calculations in Table II, the monoterpenes emitted as volatiles represent only a small fraction of the total pool of monoterpenes present in the plant. Extrapolation over a typical 6-month growing period leads to the conclusion that <5% of total monoterpenes would be released to the atmosphere. This proportion could be somewhat higher under conditions of elevated temperature (Dement et al., 1975; Loreto et al., 1996) or higher humidity (Croteau, 1977a).
Table II.
Comparison of monoterpene content and monoterpene volatilization rate of peppermint shoots
| Parameter | Measurement |
|---|---|
| Shoot monoterpene content | |
| Monoterpene content of average leaf (n = 50) | 658 ± 115 μg |
| Average number of leaves per plant (n = 6) | 185 ± 66.9 leaves |
| Monoterpene content of average stem (n = 50) | 2.78 ± 0.47 mg m−1 |
| Average length of total stems per plant (n = 6) | 3.59 ± 0.77 m |
| Total shoot monoterpene content per plant = (Ia × Ib) + (Ic × Id) | 132 mg |
| Rate of monoterpene loss | |
| Average daytime volatilization rate per plant (n = 18) | 1.22 ± 0.64 μg h−1 |
| Average nighttime volatilization rate per plant (n = 6) | 1.73 ± 0.60 μg h−1 |
| Average monoterpene loss over 24-h period per plant = (IIa × 16) + (IIb × 8) | 33.4 μg |
| Percentage | |
| Total shoot monoterpenes volatilized per day = (IIc)/(Ie) | 0.025% |
| Total shoot monoterpenes volatilized per month = (IIc × 30)/(Ie) | 0.759% |
Measurements were performed on a set of six plants that were 6 weeks old, 25 cm tall, and had not yet begun to flower. Stored monoterpenes were extracted by soaking in diethyl ether and analyzed by gas chromatography. Volatiles were collected by headspace sorption from intact plants at 24°C (see “Materials and Methods” for details). Each plant was measured three times during the light period and once during the dark period for a span of 3 to 6 h at a time. All values are given as mean ± sd.
The low rate of monoterpene volatilization measured is consistent with the results of the 14CO2 pulse experiments (Fig. 3), which showed no significant loss of labeled monoterpenes over 6 weeks of leaf development. Peppermint monoterpenes are stored in glandular trichomes within a subcuticular compartment that remains intact unless the leaf is damaged (Amelunxen, 1965; G. Turner, J. Gershenzon, and R. Croteau, unpublished results), and therefore their persistence is not surprising. The rate of monoterpene volatilization from peppermint foliage is also lower than rates reported for most other monoterpene-emitting species, including Salvia mellifera (Dement et al., 1975), Citrus sp. (Winer et al., 1992), Eucalyptus globulus (Evans et al., 1982), Quercus ilex (Street et al., 1997), and various conifers (Janson, 1993; Staudt et al., 1997).
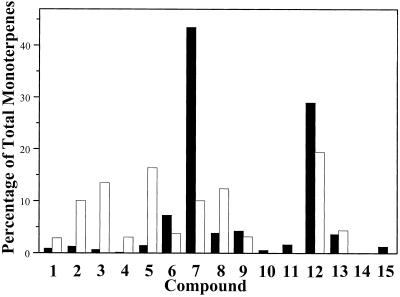
The composition of the monoterpenes emitted from peppermint is quite different from the composition of the total monoterpenes present in the plant (Fig. 4). Compared with the total pool, the mix of emitted monoterpenes contains higher proportions of menthofuran (12.4% versus 3.9%) and three olefins, β-pinene (10.1% versus 1.3%), sabinene (13.5% versus 0.7%), and limonene (16.4% versus 1.5%). In contrast, the two major monoterpenes found in mature leaves, menthone and menthol, are found in much lower proportions in the emitted mix than in the total pool (menthone at 10.1% versus 43.5%, and menthol at 19.5% versus 29.0%). If peppermint monoterpenes are ranked in order of volatility based on vapor pressure at 25°C (a ranking roughly approximating elution order on gas chromatography as indicated on the “compound” axis of Fig. 4), it can be seen that the more volatile substances are better represented in the mixture of emitted monoterpenes than in the mixture of total monoterpenes. Therefore, emission could occur directly from the total stored pool with greater representation of those compounds with greater volatility. However, two compounds of relatively low volatility, menthofuran and the α,β-unsaturated ketone, pulegone, are actually more abundant in the emitted mixture than in the total pool, suggesting that the membranes of the storage compartment might be selectively more permeable to some monoterpenes or that the emitted substances may be associated with an entirely different secretory compartment than the stored monoterpenes.
Figure 4.
Comparison of the composition of monoterpenes stored (black bars) and emitted (white bars) by peppermint shoots. Aerial parts of 6-week-old plants were examined. Stored monoterpenes were extracted with diethyl ether and analyzed by gas chromatography. Emitted monoterpenes were collected by headspace sorption from intact plants (see “Materials and Methods” for details). Each value is the mean of five determinations. Key to compounds (in order of elution on gas chromatography, which approximates the range from most to least volatile): 1, α-Pinene; 2, β-pinene; 3, sabinene; 4, myrcene; 5, limonene; 6, 1,8-cineole; 7, menthone; 8, menthofuran; 9, isomenthone; 10, linalool; 11, neomenthol; 12, menthol; 13, pulegone; 14, α-terpineol; and 15, piperitone.
The existence of a separate compartment for the synthesis of emitted (as distinct from stored) monoterpenes has been inferred from previous studies with other plant species. For example, herbivore damage to cotton (Gossypium hirsutum) foliage results in the immediate release of α-pinene, β-pinene, myrcene, limonene, and some sesquiterpenes from stored pools located in subepidermal glands (Loughrin et al., 1994). Herbivory to cotton also induces the release of a second set of monoterpenes and sesquiterpenes, including β-ocimene and linalool, that are largely absent from the stored pools and whose emission is not observed until 2 to 4 d after initial damage (Loughrin et al., 1994; Röse et al., 1996). An elegant series of [13C]CO2 tracer studies conducted following herbivore damage to cotton (Pare and Tumlinson, 1997) revealed that the immediately emitted terpenes arise from stored pools, whereas most of the later-emitted compounds are synthesized de novo just prior to their release. These late-emitted terpenes, like the monoterpenes released from peppermint, may be part of a secretory system that is distinct from that producing the stored terpenes, and under the control of different physiological factors. Thus, their emission would have no effect on the monoterpenes of stored pools. In the case of peppermint, it is possible that the emitted monoterpenes derive from the smaller, sparsely distributed capitate glands, whereas the bulk of the monoterpene pool is produced and permanently stored in the peltate glandular trichomes (Fahn, 1979).
CONCLUSIONS
The accumulation of monoterpenes in developing peppermint leaves could, in theory, be influenced by both the rate of monoterpene synthesis and the rate of monoterpene loss. However, no evidence for significant loss of monoterpenes during leaf development as a result of volatilization, metabolic degradation, or other routes was observed. The pattern of monoterpene accumulation can be explained solely by changes in the rate of monoterpene biosynthesis. The large increase in monoterpene content of 12- to 20-d-old leaves coincides with the peak period of monoterpene biosynthesis. Prior to this stage, monoterpene biosynthesis and accumulation are negligible, while after this stage, the rate of synthesis declines precipitously and monoterpene accumulation ceases. The preeminent role of biosynthesis in controlling monoterpene accumulation in peppermint has stimulated further interest in glandular trichome development (G. Turner, J. Gershenzon, and R. Croteau, in preparation) and in those factors that regulate the rate of monoterpene biosynthesis, including the activities of individual pathway enzymes and the expression of the corresponding structural genes, as described in the following paper (McConkey et al., 2000).
ACKNOWLEDGMENTS
We thank Greg Wichelns for growing the plants, Colette Gibbons and Diego Rivera for technical assistance, and G. John Murtagh for advice on the collection of volatiles.
Footnotes
This work was supported in part by the U.S. Department of Energy Division of Energy Biosciences, by the Mint Industry Research Council, and by the Agricultural Research Center (project no. 0268), Washington State University.
LITERATURE CITED
- Alonso WR, Rajaonarivony JIM, Gershenzon J, Croteau R. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) and spearmint (Mentha spicata) J Biol Chem. 1992;267:7582–7587. [PubMed] [Google Scholar]
- Amelunxen F. Elektronenmikroskopische Untersuchungen an den Drüsenschuppen von Mentha piperita L. Planta Med. 1965;13:457–473. [Google Scholar]
- Bernard-Dagan C, Pauly G, Marpeau A, Gleizes M, Carde J-P, Baradat P. Control and compartmentation of terpene biosynthesis in leaves of Pinus pinaster. Physiol Veg. 1982;20:775–795. [Google Scholar]
- Blake-Kalff MMA, Harrison KR, Hawkesford MJ, Zhao FJ, McGrath SP. Distribution of sulfur within oilseed rape leaves in response to sulfur deficiency during vegetative growth. Plant Physiol. 1998;118:1337–1344. doi: 10.1104/pp.118.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Gershenzon J, Konings MCJM, Croteau R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway: I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998;117:901–912. doi: 10.1104/pp.117.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham LA, Michaels PJ, Flores HE. Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiol. 1999;119:417–428. doi: 10.1104/pp.119.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JT, Hegarty PK, Charlwood BV. The toxicity of monoterpenes to plant cell cultures. Plant Sci. 1987;48:195–201. [Google Scholar]
- Brun N, Voirin B. Chemical and morphological studies of the effects of aging on monoterpene composition in Mentha × piperita leaves. Can J Bot. 1991;69:2271–2278. [Google Scholar]
- Burbott AJ, Loomis WD. Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol. 1969;44:173–179. doi: 10.1104/pp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood BV, Charlwood KA. Monoterpenoids. In: Charlwood BV, Banthorpe DV, editors. Terpenoids. London: Academic Press; 1991. pp. 43–98. [Google Scholar]
- Charon J, Launay J, Vindt-Balguerie E. Ontogenese des canaux secreteurs d'origine primaire dans le bourgeon de Pin maritime. Can J Bot. 1986;64:2955–2964. [Google Scholar]
- Colby SM, Alonso WR, Katahira EJ, McGarvey DJ, Croteau R. 4S-Limonene synthase from the oil glands of spearmint (Mentha spicata): cDNA isolation, characterization, and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem. 1993;268:23016–23024. [PubMed] [Google Scholar]
- Court WA, Roy RC, Pocs R. Effect of harvest date on the yield and quality of the essential oil of peppermint. Can J Plant Sci. 1993;73:815–824. [Google Scholar]
- Croteau R. Effects of irrigation method on essential oil yield and rate of oil evaporation in mint grown under controlled conditions. Hortscience. 1977a;12:563–565. [Google Scholar]
- Croteau R. Site of monoterpene biosynthesis in Majorana hortensis leaves. Plant Physiol. 1977b;59:519–520. doi: 10.1104/pp.59.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R. Catabolism of monoterpenes in essential oil plants. In: Lawrence BM, Mookherjee BD, Willis BJ, editors. Flavors and Fragrances: A World Perspective. Proceedings of the 10th International Congress of Essential Oils, Fragrances and Flavors, Washington, DC, November, 1986. Amsterdam: Elsevier Science Publishers; 1988. pp. 65–84. [Google Scholar]
- Croteau R, Burbott AJ, Loomis WD. Biosynthesis of mono- and sesqui-terpenes in peppermint from glucose-14C and 14CO2. Phytochemistry. 1972;11:2459–2467. [Google Scholar]
- Croteau R, Felton M, Karp F, Kjonaas R. Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis) Plant Physiol. 1981;67:820–824. doi: 10.1104/pp.67.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Karp F, Wagschal KC, Satterwhite DM, Hyatt DC, Skotland CB. Biochemical characterization of a spearmint mutant that resembles peppermint in monoterpene content. Plant Physiol. 1991;96:744–752. doi: 10.1104/pp.96.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Martinkus C. Metabolism of monoterpenes: demonstration of (+)-neomenthyl-β-d-glucoside as a major metabolite of (−)-menthone in peppermint (Mentha piperita) Plant Physiol. 1979;64:169–175. doi: 10.1104/pp.64.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Sood VK. Metabolism of monoterpenes: evidence for the function of monoterpene catabolism in peppermint (Mentha piperita) rhizomes. Plant Physiol. 1985;77:801–806. doi: 10.1104/pp.77.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Sood VK, Renstrom G, Bhushan R. Metabolism of monoterpenes: early steps in the metabolism of d-neomenthyl-β-d-glucoside in peppermint (Mentha piperita) rhizomes. Plant Physiol. 1984;76:647–653. doi: 10.1104/pp.76.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Venkatachalam KV. Metabolism of monoterpenes: demonstration that (+)-cis-isopulegone, not piperitenone, is the key intermediate in the conversion of (−)-isopiperitenone to (+)-pulegone in peppermint (Mentha piperita) Arch Biochem Biophys. 1986;249:306–315. doi: 10.1016/0003-9861(86)90007-x. [DOI] [PubMed] [Google Scholar]
- Dell B, McComb AJ. Biosynthesis of resin terpenes in leaves and glandular hairs of Newcastelia viscida. J Exp Bot. 1978;29:89–95. [Google Scholar]
- Dement WA, Tyson BJ, Mooney HA. Mechanism of monoterpene volatilization in Salvia mellifera. Phytochemistry. 1975;14:2555–2557. [Google Scholar]
- Dobson HEM. Analysis of flower and pollen volatiles. In: Linskens HF, Jackson JF, editors. Essential Oils and Waxes. Berlin: Springer-Verlag; 1991. pp. 231–251. [Google Scholar]
- Evans RC, Tingey DT, Gumpertz ML, Burns WF. Estimates of isoprene and monoterpene emission rates in plants. Bot Gaz. 1982;143:304–310. [Google Scholar]
- Fahn A. Secretory Tissues in Plants. London: Academic Press; 1979. [Google Scholar]
- Francis MJO, O'Connell M. The incorporation of mevalonic acid into rose petal monoterpenes. Phytochemistry. 1969;8:1705–1708. [Google Scholar]
- Gambliel HA, Cates RG. Terpene changes due to maturation and canopy level in Douglas-fir (Pseudotsuga menziesii) flush needle oil. Biochem Syst Ecol. 1995;23:469–476. [Google Scholar]
- Gershenzon J, Maffei M, Croteau R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata) Plant Physiol. 1989;89:1351–1357. doi: 10.1104/pp.89.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Murtagh GJ, Croteau R. Absence of rapid terpene turnover in several diverse species of terpene-accumulating plants. Oecologia. 1993;96:583–592. doi: 10.1007/BF00320517. [DOI] [PubMed] [Google Scholar]
- Grahle A, Holtzel C. Photoperiodische Abhaengigkeit der Bildung des aetherischen Oels bei Mentha piperita L. Naturwissenschaften. 1963;50:552. [Google Scholar]
- Hartmann T, Dierich B. Chemical diversity and variation of pyrrolizidine alkaloids of the senecionine type: biological need or coincidence. Planta. 1998;206:443–451. [Google Scholar]
- Hay RKM, Waterman PG, editors. Volatile Oil Crops: Their Biology, Biochemistry and Production. Essex, UK: Longman Scientific & Technical; 1993. [Google Scholar]
- Janson RW. Monoterpene emission from Scots pine and Norwegian spruce. J Geophys Res. 1993;98:2839–2850. [Google Scholar]
- Karban R, Baldwin IT. Induced Responses to Herbivory. University of Chicago Press; 1997. [Google Scholar]
- Karp F, Mihaliak CA, Harris JL, Croteau R. Monoterpene biosynthesis: specificity of the hydroxylation of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata) and perilla (Perilla frutescens) leaves. Arch Biochem Biophys. 1990;276:219–226. doi: 10.1016/0003-9861(90)90029-x. [DOI] [PubMed] [Google Scholar]
- Kjonaas R, Croteau R. Demonstration that limonene is the first cyclic intermediate in the biosynthesis of oxygenated p-menthane monoterpenes in Mentha piperita and other Mentha species. Arch Biochem Biophys. 1983;220:79–89. doi: 10.1016/0003-9861(83)90389-2. [DOI] [PubMed] [Google Scholar]
- Kjonaas R, Martinkus-Taylor C, Croteau R. Metabolism of monoterpenes: conversion of l-menthone to l-menthol and d-neomenthol by stereospecific dehydrogenases from peppermint (Mentha piperita) leaves. Plant Physiol. 1982;69:1013–1017. doi: 10.1104/pp.69.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjonaas RB, Venkatachalam KV, Croteau R. Metabolism of monoterpenes: oxidation of isopiperitenol to isopiperitenone, and subsequent isomerization to piperitenone by soluble enzyme preparations from peppermint (Mentha piperita) leaves. Arch Biochem Biophys. 1985;238:49–60. doi: 10.1016/0003-9861(85)90139-0. [DOI] [PubMed] [Google Scholar]
- Koops AJ, Groeneveld HW. Triterpenoid biosynthesis in the etiolated seedling of Euphorbia lathyris: developmental changes and the regulation of local triterpenoid production. J Plant Physiol. 1991;138:142–149. [Google Scholar]
- Langenheim JH. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- Lerdau M, Guenther A, Monson R. Plant production and emission of volatile organic compounds. Bioscience. 1997;47:373–383. [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D. Influence of environmental factors and air composition on the emission of α-pinene from Quercus ilex leaves. Plant Physiol. 1996;110:267–275. doi: 10.1104/pp.110.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids from herbivore-injured cotton plants. Proc Natl Acad Sci USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveys BR, Robinson SP, Brophy JJ, Chacko EK. Mango sapburn: components of fruit sap and their role in causing skin damage. Aust J Plant Physiol. 1992;19:449–457. [Google Scholar]
- McCaskill D, Gershenzon J, Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- McConkey M, Gershenzon J, Croteau R. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint (Mentha × piperita L.) Plant Physiol. 2000;122:215–223. doi: 10.1104/pp.122.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaliak CA, Gershenzon J, Croteau R. Lack of rapid monoterpene turnover in rooted plants: implications for theories of plant chemical defense. Oecologia. 1991;87:373–376. doi: 10.1007/BF00634594. [DOI] [PubMed] [Google Scholar]
- Mihaliak CA, Lincoln DE. Changes in leaf mono- and sesquiterpene metabolism with nitrate availability and leaf age in Heterotheca subaxillaris. J Chem Ecol. 1989;15:1579–1588. doi: 10.1007/BF01012385. [DOI] [PubMed] [Google Scholar]
- Mookherjee BD, Trenkle RW, Wilson RA. Live vs. dead: Part II. A comparative analysis of the headspace volatiles of some important fragrance and flavor raw materials. J Ess Oil Res. 1989;2:85–90. [Google Scholar]
- Nielsen JK, Jakobsen HB, Friis P, Hansen K, Moller J, Olsen CE. Asynchronous rhythms in the emission of volatiles from Hesperis matronalis flowers. Phytochemistry. 1995;38:847–851. [Google Scholar]
- Njar VCO, Arnold LM, Banthorpe DV, Branch SA, Christie AC, Marsh DC. Metabolism of exogenous monoterpenes and their epoxides in seedlings of Pinus pinaster Ait. J Plant Physiol. 1989;135:628–630. [Google Scholar]
- Pare PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter NG, Shaw ML, Shaw GJ, Ellingham PJ. Content and composition of dill herb oil in the whole plant and the different plant parts during crop development. N Z J Agric Res. 1983;26:119–127. [Google Scholar]
- Rajaonarivony JIM, Gershenzon J, Croteau R. Characterization and mechanism of (4S)-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) Arch Biochem Biophys. 1992;296:49–57. doi: 10.1016/0003-9861(92)90543-6. [DOI] [PubMed] [Google Scholar]
- Rohloff J. Monoterpene composition of essential oil from peppermint (Mentha × piperita L.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J Agric Food Chem. 1999;47:3782–3786. doi: 10.1021/jf981310s. [DOI] [PubMed] [Google Scholar]
- Röse USR, Manukian A, Heath RR, Tumlinson JH. Plant Physiol 111: 487–495. 1996. Volatile semiochemicals released from undamaged cotton leaves: a systemic response of living plants to caterpillar damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin WA, Uchytil TF, Feistner G, Durbin RD. Developmental changes in content of foliar secretory cavities of Tagetes erecta (Asteraceae) Am J Bot. 1988;75:1787–1793. [Google Scholar]
- Shomer I, Erner Y. The nature of oleocellosis in citrus fruits. Bot Gaz. 1989;150:281–288. [Google Scholar]
- Singh N, Luthra R, Sangwan RS. Effect of leaf position and age on the essential oil quantity and quality in lemongrass (Cymbopogon flexuosus) Planta Med. 1989;55:254–256. doi: 10.1055/s-2006-961997. [DOI] [PubMed] [Google Scholar]
- Srivastava NK, Luthra R. Distribution of photosynthetically fixed 14CO2 into essential oil in relation to primary metabolites in developing peppermint (Mentha piperita) Plant Sci. 1991;76:153–157. [Google Scholar]
- Srivastava NK, Luthra R, Naqvi A. Relationship of photosynthetic carbon assimilation to essential oil accumulation in developing leaves of Japanese mint. Photosynthetica. 1990;24:406–411. [Google Scholar]
- Staudt M, Bertin N, Hansen U, Seufert G, Ciccioli P, Foster P, Frenzel B, Fugit J-L. Seasonal and diurnal patterns of monoterpene emission from Pinus pinea (L.) under field conditions. Atmos Environ. 1997;31:145–156. [Google Scholar]
- Street RA, Owen S, Duckham SC, Boissard C, Hewitt CN. Effect of habitat and age on variations in volatile organic compound (voc) emissions from Quercus ilex and Pinus pinea. Atmos Environ. 1997;31:89–100. [Google Scholar]
- Voirin B, Bayet C. Developmental changes in the monoterpene composition of Mentha × piperita leaves from individual peltate trichomes. Phytochemistry. 1996;43:573–580. [Google Scholar]
- Werker E, Fahn A. Secretory hairs of Inula viscosa (L.) Ait.: development, ultrastructure, and secretion. Bot Gaz. 1981;142:461–476. [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. Ann Bot. 1993;71:43–50. [Google Scholar]
- Winer AM, Arey J, Atkinson R, Aschmann SM. Emission rates of organics from vegetation in California's Central Valley. Atmos Environ. 1992;26A:2647–2659. [Google Scholar]
- Wink M, editor. Biochemistry of Plant Secondary Metabolism. Sheffield, UK: Sheffield Academic Press; 1999. [Google Scholar]