Key Points
Question
Can facial atrophic scars be treated effectively, from both a functional and an aesthetic perspective, with the use of condensed nanofat combined with conventional fat grafts?
Findings
Twenty-five atrophic scars on a total of 20 patients were included in the study. The patients’ and clinician observers’ scores significantly improved after treatment with fat grafting using condensed nanofat and conventional fat grafts, and the overall Patient and Observer Scar Assessment Scale score also significantly improved.
Meaning
In this study, we achieved good results when treating atrophic scars with condensed nanofat combined with fat grafts, suggesting that this technique may be a useful addition to the treatment options for this type of scar.
Abstract
Importance
In addition to the physical deformity, there is often great psychological burden of facial scars for patients. In this study, we use condensed nanofat combined with fat grafts in a novel technique to improve atrophic facial scars by raising both the surface and the bottom of the affected area.
Objective
To assess whether the use of condensed nanofat combined with fat grafting can be effective in treating atrophic facial scars from both an aesthetic and a functional perspective.
Design, Setting, and Participants
In this prospective case series of 20 patients with 25 atrophic facial scars, each scar was treated with condensed nanofat combined with fat grafts at the Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, Zhejiang, China. Postoperative results were evaluated by the patients themselves and by 3 senior plastic surgeon observers.
Main Outcomes and Measures
Multiple preoperative and postoperative examinations included the use of the Patient and Observer Scar Assessment Scale (POSAS) to evaluate both the functional and aesthetic aspects of the atrophic facial scars. Punch biopsy specimens were stained for the presence of melanin, elastic fibers, and cytokeratin (CK) 14 and CK19. Images were analyzed using ImageJ software, and the data were analyzed by paired sample t test.
Results
Twenty patients (6 men and 14 women; mean age, 38.25 years; age range, 21-62 years) with a total of 25 atrophic facial scars were treated between March 2014 and December 2016. The patients’ mean (SD) scar assessment scores were significantly decreased postoperatively in the final examination for color, 6.40 (0.51) vs 2.40 (0.24) (P < .001); stiffness, 7.20 (0.37) vs 3.20 (0.20) (P < .001); thickness, 5.80 (0.73) vs 1.80 (0.37) (P = .001); and irregularity, 5.20 (0.49) vs 2.20 (0.37) (P = .003); and the observers’ scores were also significantly decreased for pigmentation, 4.40 (0.51) vs 2.00 (0.32) (P = .004); thickness, 3.00 (0.32) vs 1.80 (0.20) (P = .03); relief, 4.40 (0.51) vs 2.40 (0.24) (P = .003); and pliability, 4.20 (0.37) vs 1.40 (0.24) (P < .001). In the final follow-up examinations, a significantly improved overall POSAS score was found among both patients, 28.80 (1.02) vs 12.20 (0.80) (P < .001), and observers, 18.00 (0.71) vs 9.20 (0.37) (P = .001). Enhancement of Fontana-Masson staining of melanin in the basal cell layer was observed postoperatively, and a significant postoperative change was detected for the mean (SD) values of average optical density from the preoperative measurement, 0.671 (0.083) vs 0.844 (0.110) (P = .01). The sebaceous glands and sweat glands that were not found in the preoperative images were seen postoperatively by immunohistochemical staining with CK14 and CK19.
Conclusions and Relevance
Our preliminary clinical and pathological results indicate that the use of condensed nanofat combined with fat grafts may be an effective approach to treating atrophic facial scars from both an aesthetic and a functional perspective.
Level of Evidence
4.
This case series evaluates the use of condensed nanofat combined with fat grafts to treat atrophic facial scars.
Introduction
Scarring is the pathological outcome of the repair and reconstruction after tissue damage and may affect the patient’s personal appearance and physical function, causing deformity and possibly a great psychological burden, especially when the scar is on the face.1,2 Three of the most common types of scars are hypertrophic, atrophic, and keloid scars. For hypertrophic scars and keloids, studies have found that the abnormal accumulation of extracellular matrix (ECM), composed mainly of overproduced collagen, is one of the main causes.3,4 Scar tissue is not as flexible as normal skin, lacks elasticity, and does not have a normal blood supply, sweat glands, or hair.5,6,7 An atrophic scar is sunken, visibly pigmented or hypopigmented, and often associated with acne.8,9 Deficiency of collagen and other fibrous tissues may result in an atrophic scar.10 Although none of the currently available treatments can achieve complete resolution any scars, there are some ways to minimize their appearance, such as steroid injections, laser treatment, radiotherapy, and injectable fillers.11,12,13
For long-term scar treatment, for which medical and surgical therapies seem to be ineffective, autologous fat grafts have been used successfully to repair tissue damage. In recent years, treatment of different scar types with autologous fat graft injection has been widely used.14,15,16,17 Klinger et al18 used autologous fat grafts to treat retractile and painful scars that compromised normal daily activity or mobility of the involved joint, and they found a qualitative improvement in all treated scars. After treating 35 facial scars by autologous lipofilling, Pallua et al19 demonstrated that the scar quality improved in all cases. Sarangal et al20 reconstructed a depressed, depigmented, irregular vertical scar in the center of the forehead with a suspension of noncultured epidermal melanocytes and dermal fat grafting.
It should be noted that autologous fat grafts are made up of fat cells, ECM, and adipose-derived stem cells (ADSCs).21,22 Since ADSCs have been shown to play a role in antiaging and skin regeneration, the scar-remodeling effect of fat grafts may be related to ADSCs. Through intralesional injection of ADSCs to hypertrophic scars in rabbit ears, Zhang et al23 have added support to this hypothesis. Tonnard et al24 have produced nanofat for skin rejuvenation, which could be injected with a thinner needle and still contain therapeutic quantities of ADSCs.24 However, nanofat contains much oil and is too bulky to be injected into scars. Based on these factors, we devised a new approach to remove oil and obtain condensed nanofat to treat atrophic facial scars. We used pathological examination, photographic documentation, and the Patient and Observer Scar Assessment Scale (POSAS) to evaluate the effect of this treatment.
Methods
Patients
A total of 20 nonsmoking patients (6 men and 14 women) with atrophic facial scars (N = 25) were included in this prospective clinical study between March 2014 and December 2016 in Sir Run Run Shaw Hospital. The 25 scars included were hypopigmented (n = 23) and mixed (n = 2). Patient ages ranged from 21 to 62 years (mean age, 38.25 years). Every patient was treated 1 or 2 times (14 patients once, 6 patients twice), multiple treatments separated by an interval of 3 to 12 months (mean interval, 6.15 months).
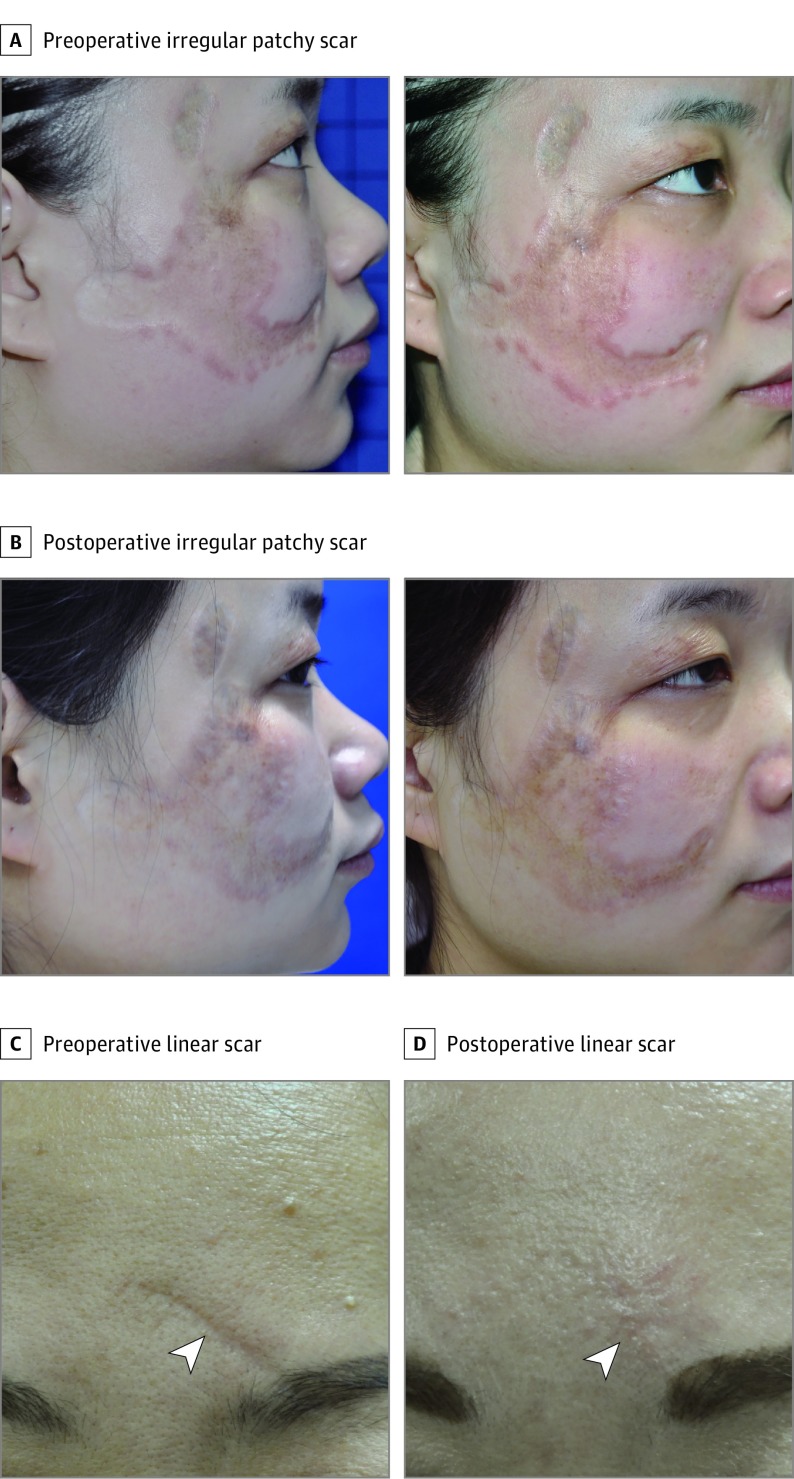
Among these patients, the most common cause of the facial scars was surgical sutures (n = 12); other causes were burns (n = 8), trauma (n = 3), and acne (n = 2). In addition to 1 irregular and patchy scar (area of approximately 6 × 6 cm) (Figure 1A and B), there were 24 linear scars ranging in length from 1 to 5 cm (mean length, 2.45 cm) (Figure 1C and D; eFigures 1 and 2 in the Supplement). All scars had formed over 3 to 26 years (mean formation time, 7.45 years) and were stable.
Figure 1. Clinical View of 2 Types of Atrophic Scars.
A and B, Case 1 shows an irregular and patchy scar on the right cheek preoperatively (A) and 6 months postoperatively (B). Case 2 shows a linear atrophic scar (arrowhead) on the forehead preoperatively (C) and 14 months postoperatively (D).
All included patients had no local ulcers, infectious diseases, autoimmune diseases, or organic diseases, and received neither immunosuppressant therapy nor local radiotherapy preoperatively. All participants provided written informed consent for participation in this study, which was approved by the ethics committee of Sir Run Run Shaw Hospital.
Liposuction Procedure
According to the Coleman protocol,25,26,27 liposuction was performed through a small incision in the sterilized paraumbilical region after infiltration of a solution of 20 mL of lidocaine, 0.5%, and 1 mL of 1:1000 epinephrine per 1000 mL of saline with a 3.0-mm multihole aspiration cannula manually connected to a 20-mL Luer-Lok syringe (Fisher Scientific). The infiltration step minimized bleeding and reduced pain. The piston of the syringe was then pulled back gently to create a light negative pressure to create suction. After saline rinsing and filtering, we performed preliminary centrifugation during which the 20-mL Luer-Lok syringes were centrifuged at 3000 rpm for 3 minutes (Era Beili Centrifuge Co Ltd). The oil layer was decanted, and the aqueous component drained. For mechanical emulsification, 2 20-mL Luer-Lok syringes with appropriate fat grafts were connected to the Tulip transfer connector with three 1.4-mm holes (Black Tie Medical). Mechanical emulsification was performed by pushing the fat through the connector forward and backward between the 2 syringes 30 times to produce nanofat.24 To remove additional oil and obtain more highly purified nanofat, the nanofat was centrifuged again at 3000 rpm for 3 minutes after emulsification. After removal of the oil layer and the aqueous component, the condensed nanofat was collected.
Surgical Procedure
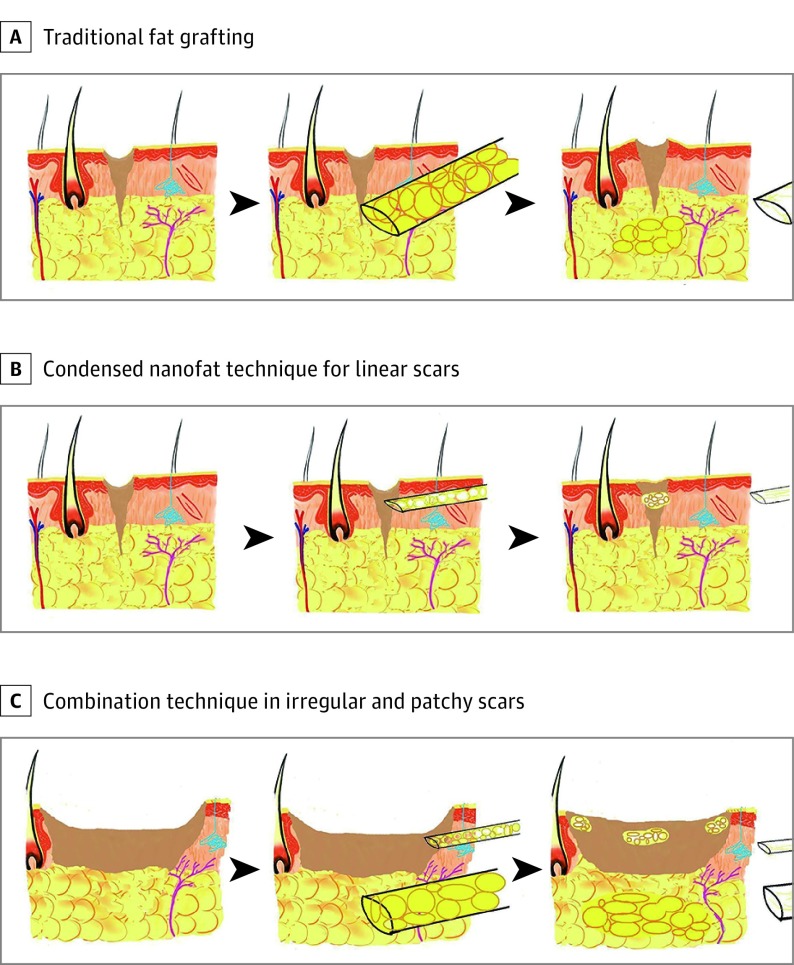
Initially, the whole face was sterilized with benzalkonium chloride. Under local anesthesia, subcisions and microincisions were made with an 18-gauge needle to break underlying adhesions of the scar and make space for the fat graft combined with condensed nanofat injection for the irregular and patchy scar. A syringe with a blunt 1.2-mm cannula was used to inject fat grafts into the tissue underlying the scar to achieve visible elevation as the syringe was withdrawn, and the piston was pushed forward. Otherwise, we injected condensed nanofat grafts intradermally into scars using 29-gauge insulin syringes (BD [Becton, Dickinson, and Company]) to increase the thickness of tissue under the epidermis and dressed condensed nanofat on the surface of incisions. However, for linear scars, only the condensed nanofat grafts were needed (Figure 2). Finally, the scars were dressed with sterile gauze without compression. Postoperative oral antibiotic was taken for 3 days.
Figure 2. Illustrated Differences Between the Traditional Fat Graft and the Technique Using Condensed Nanofat Combined With Fat Graft.
A, The commonly used autologous fat grafting procedure is achieved through subcutaneous injection, but the cannula is not thin enough to inject fat grafts into a scar to elevate the surface of the scar. B, For the nanofat technique in linear scars, the condensed nanofat is injected into the scar with 29-gauge insulin syringes to elevate the surface. C, For the nanofat combination technique in irregular and patchy scars, the fat graft is injected into the tissue under the scar, and the condensed nanofat is injected into the scar to elevate both the surface and the bottom of scar.
Pathological Examination
Punch biopsies (2 × 2 mm) were taken in the same scar area before and 6 months after the procedure and used for Verhoeff–Van Gieson staining, Fontana-Masson staining, and immunohistochemical analysis. All biopsy samples were fixed in 10% formalin at 4°C overnight, embedded in paraffin, sectioned, and then examined histologically.
For special staining, slides were deparaffinized and hydrated to distilled water. According to the Verhoeff–Van Gieson staining kit protocol (Polysciences Inc), slides were incubated in Verhoeff solution for 1 hour and then washed in tap water. Tissue sections were differentiated in ferric chloride, 2%, solution until gray background was analyzed under the microscope. After thorough rinsing in distilled water, tissue sections were treated with sodium thiosulfate, 5%, for 1 minute to remove iodine. The slides were thoroughly washed with water and then counterstained in Counterstain I for 5 minutes. Finally, slides were washed, dehydrated, cleared in xylene, and mounted for analysis.
According to the Fontana-Masson stain protocol (Abcam), slides were incubated in Fontana ammoniacal silver solution for 15 minutes. Following thorough rinsing in distilled water, slides were incubated in gold chloride solution for 2 minutes. Slides were rinsed again in distilled water and then incubated in sodium thiosulfate, 2%, solution for 2 minutes. Slides were washed well in distilled water and counterstained with neutral red stain for 1 minute. Finally, slides were washed, dehydrated in absolute alcohol, cleared, and mounted for analysis.
For immunohistochemical analysis, slides were fixed in paraformaldehyde, 4%, deparaffinized, rehydrated, and then immersed in the distilled water. Following citrate incubation and blocking with 5% rabbit serum for 30 minutes, slides were incubated at 4°C overnight with the following antibodies against cytokeratin (CK) 14 and CK19 (both at 1:200 concentration; ProteinTech). The paraffin sections were incubated with secondary antibody (1:200, ProteinTech) for 30 minutes and then developed with DAB for 10 minutes. After staining with hematoxylin-eosin for 1 minute, slides were mounted for analysis.
Follow-up Examination
For clinical examinations, photographs were taken preoperatively and 6 months postoperatively for comparison under the same environmental conditions and standard camera settings. At the same time, the scars were evaluated by both patients and clinician observers using the POSAS. This scale grades 6 properties of the scar in the patient scale—color, pliability, thickness, relief, itching, and pain—and 5 scar properties in the observer scale—vascularization, pigmentation, pliability, thickness, and relief.28,29 The clinical examinations and observer scar assessments were performed independently by 3 senior plastic surgeons.
Statistical Analysis
Images of immunohistochemical and specific staining of tissue were analyzed using ImageJ for Mac OS X, version 1.49 (National Institutes of Health). Tissue area was measured using 3 random fields per slide viewed at high magnification (×100, ×200, and ×400). Paired sample t testing with the SPSS statistical package, version 18.0 (SPSS Inc), were used to compare the preoperative average optical density (AOD) of melanin (integrated density per unit area) and fractional area fraction of elastic fibers with the 6-month postoperative measures. All the figures were generated by Prism 6 (GraphPad) and Photoshop CC (Adobe Systems). Data are expressed as mean (SD) values; P < .05 was considered statistically significant.
Results
Follow-up Examination With POSAS
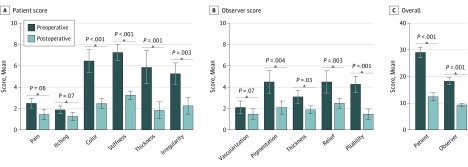
We observed a marked improvement in the quality of the scars in all facial scars (N = 25). The improvements could also be found in the scar assessment scores assigned by both the patients and the observers (Figure 3). The patients’ scores were also significantly improved at the final examination for color, 6.40 (0.51) vs 2.40 (0.24) (P < .001); stiffness, 7.20 (0.37) vs 3.20 (0.20) (P < .001); thickness, 5.80 (0.73) vs 1.80 (0.37) (P = .001); and irregularity, 5.20 (0.49) vs 2.20 (0.37) (P = .003) (Figure 3A). The observers’ scar assessment scores were also significantly improved for pigmentation, 4.40 (0.51) vs 2.00 (0.32) (P = .004); thickness, 3.00 (0.32) vs 1.80 (0.20) (P = .03); relief, 4.40 (0.51) vs 2.40 (0.24) (P = .003); and pliability, 4.20 (0.37) vs 1.40 (0.24) (P < .001) (Figure 3B). In the final follow-up examinations, a significantly improved overall POSAS score was found among both patients, 28.80 (1.02) vs 12.20 (0.80) (P < .001), and observers, 18.00 (0.71) vs 9.20 (0.37) (P = .001) (Figure 3C).
Figure 3. Patient and Observer Scar Assessment Scale Scores.
Pathological Examination
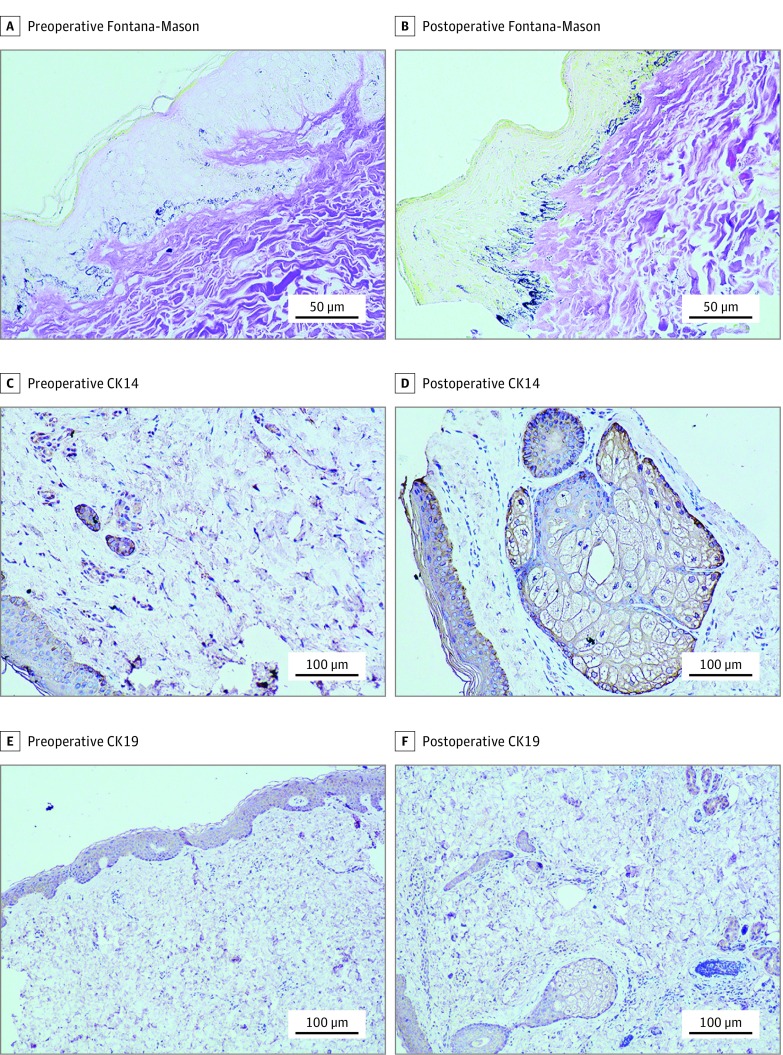
We observed an enhancement of Fontana-Masson staining of melanin in the basal cell layer postoperatively (Figure 4A and B), and a statistically significant increase was detected in the mean (SD) AOD of melanin between preoperative and postoperative evaluation, 0.671 (0.083) vs 0.844 (0.110) (P = .01) (Table). We observed no difference in Verhoeff-Van Gieson staining of elastic fibers between preoperative and postoperative evaluation and found no significant change in the fractional area occupied by elastic fibers (Table).
Figure 4. Histopathologic Findings for Melanin and Sebaceous and Sweat Glands.
A and B, Under Fontana-Masson staining, an increase in melanin is seen in the basal cell layer between the preoperative (A) and 6-month postoperative (B) specimens (original magnification ×400). C and D, Under cytokeratin (CK) 14 staining, almost no sebaceous or sweat glands were observed preoperatively (C), but they were clearly visualized 6 months postoperatively (D) (original magnification ×200). E and F, Under CK19 staining, almost no sebaceous or sweat glands were observed preoperatively (E), but they were clearly visualized 6 months postoperatively (F) (original magnification ×100).
Table. Mean (SD) Differences in Melanin AOD and Area Fraction of Elastic Fibers Between Preoperative and Postoperative Measurementsa.
| Measurement Characteristic | Melanin | Elastic Fibers |
|---|---|---|
| Preoperative | 0.671 (0.083) | 0.096 (0.014) |
| Six-month postoperative | 0.844 (0.110) | 0.081 (0.042) |
| t value | 4.253 | −0.699 |
| P value | .01 | .52 |
| Melanin AOD/elastic fibers (95% CI) | 0.173 (0.068-0.277) | −0.016 (−0.073 to 0.042) |
Abbreviation: AOD, average optical density (integrated density per unit area).
Data are calculated from analyses of AOD for melanin and density for elastic fibers per unit area.
Moreover, we detected changes in sebaceous glands and sweat glands postoperatively by immunohistochemical staining using CK14 and CK19 (Figure 4C-F). We observed no dermal papillae, sebaceous glands, or sweat glands preoperatively, but sebaceous and sweat glands appeared postoperatively.
Discussion
An atrophic scar is one in which the healed surface of the skin is below the level of normal dermal tissue, so the healed tissue of an atrophic scar must be built up above the level of the normal surrounding skin. The commonly used autologous fat grafting procedure uses subcutaneous injection, but the cannula is not thin enough to inject fat grafts into a scar to elevate the surface of the scar. Tenna et al30 report effectively treating atrophic facial scars with subcutaneous infiltration of nanofat and platelet-rich plasma combined with fractional carbon dioxide laser resurfacing. In the present study, we treated atrophic facial scars with condensed nanofat combined with fat grafts, a novel method that raises both the surface and the bottom of scars, and we observed both aesthetic and functional improvement postoperatively in the treated scars.
Microfat is processed into nanofat by means of emulsification, which destroys large adipocytes while ADSCs are preserved. This mechanical process can be understood as the process of purifying ADSCs.24 However, facial atrophic scars are almost always small and linear. Conventional nanofat is not small enough to pass through 29-gauge insulin syringes, and the conventional preparation contains many damaged oil droplets, which may stimulate the inflammatory response of the recipient’s body.31 In the present study, to remove the oil, obtain condensed nanofat made up of smaller components, and enrich the ADSCs, we performed a second centrifugation. In addition, ADSCs secrete various growth factors, such as vascular endothelial growth factor, hepatocyte growth factor, transforming growth factor (TGF)-β, and platelet-derived growth factor.31,32 Studies have demonstrated that growth factors may have antifibrotic effects.33,34 Furthermore, Spiekman et al35 have also observed that an ADSC-conditioned medium inhibits TGF-β1–induced adverse differentiation and function of adult human dermal fibroblasts and TGF-β1–induced contraction in keloid scar–derived fibroblasts to remodel the scar.35 Therefore, we hypothesized that condensed nanofat may be effective in treating atrophic scars.
Melanocyte death and disruption of melanogenesis have been shown to affect skin pigmentation.36 A hypopigmented scar is often the result of deep skin damage, which may lead to reduction or loss of local melanin secretion. Alternatively, the scar tissue itself may become a barrier to melanin transfer and melanocyte migration.37 The 25 scars included in the present study were hypopigmented or mixed (mixed scars contained both hypopigmented and hyperpigmented tissue). All participating patients were satisfied with the postoperative scar color, which was reflected in the color parameter of the patient POSAS score (Figure 3A). Observers also reported improvement in scar pigmentation, which was reflected in the pigmentation parameter of the observer scar score (Figure 3B). In addition, we analyzed the AOD of melanin via Fontana-Masson staining using ImageJ software and detected a significant increase from preoperative measurement in the postoperative AOD of melanin (0.17; 95% CI, 0.068 to 0.277; P = .01) (Table). Lim et al38 have observed that ADSCs improve the efficacy of melanocyte transplantation in nude mouse skin. Therefore, we speculated that the ADSCs injected into a hypopigmented scar might positively influence peripheral melanocytes to migrate to the basal cell layer or to differentiate into melanocytes themselves. Further studies are needed to investigate and better understand the mechanisms.
Scars are often stiff because scar tissue is composed of collagen overexpressed relative to the tissue it has replaced and lacks elastic fibers.39 Preoperatively, all the patients in the present study considered their scars to be hard and sunken, but after the treatment, they were all satisfied with the improvement in scar stiffness; this was reflected in the stiffness parameter of the patient POSAS score (Figure 3A). The observers perceived improvement in postoperative scar relief and pliability, as reflected in the relief and pliability parameter of the observer POSAS score (Figure 3B). However, we did not observe a difference in elastic fibers based on Verhoeff-Van Gieson staining between preoperative and postoperative measurements and did not find a significant change in the fractional area occupied by elastic fibers (Table). Valerie et al40 have observed that elastin levels, and the organization of elastic fibers tend to return to normal after the injection of ADSCs in vocal fold scars in rabbits. Jeong et al41 have demonstrated that scars in nude mice treated with human ADSCs showed higher collagen density and better outcomes with regard to tropoelastin and fibrillin-1. However, these results are contradictory to our results. We think that this discrepancy might relate to the small sample size of scars (N = 25) in our study and that future studies should include larger samples. It could also be noted that the change in scar stiffness might be due to the subcisions and microincisions made in the scars during surgery.
Epidermal appendages in the dermis, such as sebaceous glands, sweat glands, and hair follicles, are almost nonexistent in scar tissues.42 Before the treatment in the present study, we did not detect dermal papillae, sebaceous glands, or sweat glands by immunohistochemical staining with CK14 and CK19 (Figure 4C-F), but sebaceous and sweat glands appeared postoperatively. In patients with atrophic scars, the injury is often deep, reaching the muscle tissue, and can damage epidermal appendages. Previous studies have demonstrated that mesenchymal stem cells may regenerate sweat glands and sebaceous glands.43,44,45 Our results indicate that the ADSCs in condensed nanofat can regenerate sebaceous glands and sweat glands to convert atrophic scar tissue to normal skin.
Limitations
Although we have found an effective therapy for scars, the study has some limitations. First, we focused only on procedures and clinical efficiency rather than the mechanism of our treatment. Second, only 25 atrophic scars were included in this study, and only 1 of them required the combination treatment. Therefore, larger studies that evaluate the mechanism are needed.
Conclusions
In summary, our preliminary clinical and pathological results indicate that use of condensed nanofat may be an effective approach to treating atrophic scars, from both an aesthetic and a functional perspective. It is unfortunate that we have not investigated the mechanism underlying the results of our treatment and that the sample is limited. Therefore, further studies and larger samples are needed to investigate and better understand the mechanisms associated with condensed nanofat and the remodeling of atrophic scars to render the results even more reliable and predictable for patients.
eFigure 1. A linear atrophic scar on the left temporal region preoperatively (A), immediately postoperatively (B), and 1 year postoperatively (C)
eFigure 2. A linear atrophic scar on the forehead preoperatively (A), immediately postoperatively (B), and 1 year postoperatively (C)
References
- 1.Ladak A, Tredget EE. Pathophysiology and management of the burn scar. Clin Plast Surg. 2009;36(4):661-674. [DOI] [PubMed] [Google Scholar]
- 2.Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63(6):1015-1021. [DOI] [PubMed] [Google Scholar]
- 4.Huang D, Shen KH, Wang HG. Pressure therapy upregulates matrix metalloproteinase expression and downregulates collagen expression in hypertrophic scar tissue. Chin Med J (Engl). 2013;126(17):3321-3324. [PubMed] [Google Scholar]
- 5.Roten SV, Bhat S, Bhawan J. Elastic fibers in scar tissue. J Cutan Pathol. 1996;23(1):37-42. [DOI] [PubMed] [Google Scholar]
- 6.Fu XB, Sun TZ, Li XK, Sheng ZY. Morphological and distribution characteristics of sweat glands in hypertrophic scar and their possible effects on sweat gland regeneration. Chin Med J (Engl). 2005;118(3):186-191. [PubMed] [Google Scholar]
- 7.Zhu X, Zhuo S, Zheng L, et al. . Quantified characterization of human cutaneous normal scar using multiphoton microscopy. J Biophotonics. 2010;3(1-2):108-116. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick S, Heath R, Shah M. Abnormal pigmentation within cutaneous scars: A complication of wound healing. Indian J Plast Surg. 2012;45(2):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozali MV, Zhou B. Effective treatments of atrophic acne scars. J Clin Aesthet Dermatol. 2015;8(5):33-40. [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbrocini G, Annunziata MC, D’Arco V, et al. . Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010(5):893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MA, Davidson TM. Scar management: prevention and treatment strategies. Curr Opin Otolaryngol Head Neck Surg. 2005;13(4):242-247. [DOI] [PubMed] [Google Scholar]
- 12.Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci. 2014;29(6):751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnani LR, Schweiger ES. Fractional CO2 lasers for the treatment of atrophic acne scars: a review of the literature. J Cosmet Laser Ther. 2014;16(2):48-56. [DOI] [PubMed] [Google Scholar]
- 14.Negenborn VL, Groen JW, Smit JM, Niessen FB, Mullender MG. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions: a systematic review. Plast Reconstr Surg. 2016;137(1):31e-43e. [DOI] [PubMed] [Google Scholar]
- 15.Condé-Green A, Marano AA, Lee ES, et al. . Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg. 2016;137(1):302-312. [DOI] [PubMed] [Google Scholar]
- 16.Gentile P, De Angelis B, Pasin M, et al. . Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg. 2014;25(1):267-272. [DOI] [PubMed] [Google Scholar]
- 17.Piccolo NS, Piccolo MS, Piccolo MT. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin Plast Surg. 2015;42(2):263-283. [DOI] [PubMed] [Google Scholar]
- 18.Klinger M, Caviggioli F, Klinger FM, et al. . Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24(5):1610-1615. [DOI] [PubMed] [Google Scholar]
- 19.Pallua N, Baroncini A, Alharbi Z, Stromps JP. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg. 2014;67(8):1033-1037. [DOI] [PubMed] [Google Scholar]
- 20.Sarangal R, Yadav S, Sakral A, Dogra S. Noncultured epidermal-melanocyte cell suspension and dermal-fat grafting for the reconstruction of an irregular, atrophic, and depigmented forehead scar: an innovative approach. J Cosmet Dermatol. 2015;14(4):332-335. [DOI] [PubMed] [Google Scholar]
- 21.Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. . Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382(9898):1113-1120. [DOI] [PubMed] [Google Scholar]
- 22.Tan SS, Ng ZY, Zhan W, Rozen W. Role of adipose-derived stem cells in fat grafting and reconstructive surgery. J Cutan Aesthet Surg. 2016;9(3):152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132(4):1017-1026. [DOI] [PubMed] [Google Scholar]
- 25.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19(5):421-425. [DOI] [PubMed] [Google Scholar]
- 26.Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33(4):567-577. [DOI] [PubMed] [Google Scholar]
- 27.Coleman SR, Katzel EB. Fat grafting for facial filling and regeneration. Clin Plast Surg. 2015;42(3):289-300, vii. vii. [DOI] [PubMed] [Google Scholar]
- 28.Draaijers LJ, Tempelman FR, Botman YA, et al. . The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113(7):1960-1965. [DOI] [PubMed] [Google Scholar]
- 29.Idriss N, Maibach HI. Scar assessment scales: a dermatologic overview. Skin Res Technol. 2009;15(1):1-5. [DOI] [PubMed] [Google Scholar]
- 30.Tenna S, Cogliandro A, Barone M, et al. . Comparative study using autologous fat grafts plus platelet-rich plasma with or without fractional CO2 laser resurfacing in treatment of acne scars: analysis of outcomes and satisfaction with FACE-Q. Aesthetic Plast Surg. 2017;41(3):661-666. [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Mineda K, Eto H, et al. . Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133(3):303e-313e. [DOI] [PubMed] [Google Scholar]
- 32.Pallua N, Pulsfort AK, Suschek C, Wolter TP. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg. 2009;123(3):826-833. [DOI] [PubMed] [Google Scholar]
- 33.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VL, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120(2):330-337. [DOI] [PubMed] [Google Scholar]
- 34.Dong LH, Jiang YY, Liu YJ, et al. . The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep. 2015;5:8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134(4):699-712. [DOI] [PubMed] [Google Scholar]
- 36.Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17(2):96-110. [DOI] [PubMed] [Google Scholar]
- 37.Açikel C, Ulkür E, Güler MM. Treatment of burn scar depigmentation by carbon dioxide laser-assisted dermabrasion and thin skin grafting. Plast Reconstr Surg. 2000;105(6):1973-1978. [DOI] [PubMed] [Google Scholar]
- 38.Lim WS, Kim CH, Kim JY, Do BR, Kim EJ, Lee AY. Adipose-derived stem cells improve efficacy of melanocyte transplantation in animal skin. Biomol Ther (Seoul). 2014;22(4):328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen BE, Geronemus RG, McDaniel DH, Brauer JA. The role of elastic fibers in scar formation and treatment. Dermatol Surg. 2017;43(suppl 1):S19-S24. [DOI] [PubMed] [Google Scholar]
- 40.Valerie A, Vassiliki K, Irini M, Nikolaos P, Karampela E, Apostolos P. Adipose-derived mesenchymal stem cells in the regeneration of vocal folds: a study on a chronic vocal fold scar. Stem Cells Int. 2016;2016(5):9010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong JH, Fan Y, You GY, Choi TH, Kim S. Improvement of photoaged skin wrinkles with cultured human fibroblasts and adipose-derived stem cells: a comparative study. J Plast Reconstr Aesthet Surg. 2015;68(3):372-381. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Chen Y, Fu X. Sweat gland regeneration after burn injury: is stem cell therapy a new hope? Cytotherapy. 2015;17(5):526-535. [DOI] [PubMed] [Google Scholar]
- 43.Al-Zaid T, Vanderweil S, Zembowicz A, Lyle S. Sebaceous gland loss and inflammation in scarring alopecia: a potential role in pathogenesis. J Am Acad Dermatol. 2011;65(3):597-603. [DOI] [PubMed] [Google Scholar]
- 44.Ma K, Tan Z, Zhang C, Fu X. Mesenchymal stem cells for sweat gland regeneration after burns: from possibility to reality. Burns. 2016;42(3):492-499. [DOI] [PubMed] [Google Scholar]
- 45.Al-Refu K. Stem cells and alopecia: a review of pathogenesis. Br J Dermatol. 2012;167(3):479-484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. A linear atrophic scar on the left temporal region preoperatively (A), immediately postoperatively (B), and 1 year postoperatively (C)
eFigure 2. A linear atrophic scar on the forehead preoperatively (A), immediately postoperatively (B), and 1 year postoperatively (C)