Synopsis
Parasites can represent formidable selection pressures for hosts, but the cost of infection is sometimes difficult to demonstrate in natural populations. While parasite exploitation strategies may, in some instances, actually inflict low costs on their hosts, the response of hosts to infection is also likely to determine whether or not these costs can be detected. Indeed, costs of infection may be obscured if infected individuals in the wild are those that are the most tolerant, rather than the most susceptible, to infection. Here we test this hypothesis in two natural populations of Anolis sagrei, one of the most common anole lizard of the Bahamas. Plasmodium parasites were detected in > 7% of individuals and belonged to two distinct clades: P. mexicanum and P. floriensis. Infected individuals displayed greater body condition than non-infected ones and we found no association between infection status, stamina, and survival to the end of the breeding season. Furthermore, we found no significant difference in the immuno-competence (measured as a response to phytohemagglutinin challenge) of infected versus non-infected individuals. Taken together, our results suggest that the infected individuals that are caught in the wild are those most able to withstand the cost of the infection and that susceptible, infected individuals have been removed from the population (i.e., through disease-induced mortality). This study highlights the need for caution when interpreting estimates of infection costs in natural populations, as costs may appear low either when parasites exploitation strategies truly inflict low costs on their hosts or when those costs are so high that susceptible hosts are removed from the population.
Introduction
Harboring parasites is energetically costly to hosts, not only because they exploit host resources, but also because they cause damage to host tissues and activate costly immune responses (Sheldon and Verhulst 1996; Bonneaud et al. 2012). Access to limited resources means that any reallocation of energy to parasite proliferation, tissue repair or immune activation will divert it away from other fitness-associated traits, such as physical activity, thereby giving rise to the physiological constraints underlying life-history trade-offs (e.g., between survival and reproduction) (Bonneaud et al. 2003; van der Most et al. 2011). While evidence for energetic costs of infection is accumulating (Eraud et al. 2005; Bonneaud et al. 2016), the impact of infection on other fitness-associated traits remains difficult to demonstrate in natural populations (Knowles et al. 2009). One key reason is that it is unclear whether infection in wild-caught individuals reflects increased susceptibility or heightened tolerance to parasites. In both of these cases, wild-caught individuals that are not infected will comprise of resistant, as well as unexposed hosts. However, whether infection reflects susceptibility or tolerance will have consequences for the pool of infected individuals, since susceptible individuals that are infected will be removed from the population (i.e., through disease-induced mortality) in the latter, but not in the former case. Because energy should become limiting primarily in infections of resistant and susceptible hosts (due to protective immune activity and pathogenesis, respectively; Bonneaud et al. 2012), and less so of tolerant individuals (Råberg et al. 2007), trade-offs resulting from infection may therefore not always be apparent in the wild.
Plasmodium parasites, which are transmitted to vertebrate hosts by haematophagous dipteran vectors during blood meals, have the potential to cause high levels of morbidity and mortality in natural populations (Van Riper et al. 1986). Pathogenesis is caused primarily by the high metabolic demands of Plasmodium proliferation, hemoglobin catabolism for the biosynthesis of parasite amino acids, and massive lysis of infected erythrocytes, all of which give rise to shortages of oxygen and glucose necessary for cellular metabolism in host tissues (Roth 1990; Mackintosh et al. 2004; Olszewski et al. 2009). Consequently, Plasmodium infections have been shown to be associated with substantial metabolic complications in a range of organisms, in part, due to a mismatch between oxygen supplies and requirements of host tissues (Li et al. 2008; Olszewski and Llinas 2011). For instance, in humans, severe malaria is marked by low blood glucose levels (hypoglycaemia) and build-up of lactate in the body (lactic acidosis) due to increased anaerobic glycolysis (Planche et al. 2005). Western Fence Lizards (Sceloporus occidentalis) infected with Plasmodium mexicanum, displayed a 25% reduction in hemoglobin concentration and 30% increase in oxygen consumption following physical exertion relative to uninfected individuals, evidencing similar increased reliance on anaerobic metabolism and greater costs of recovery (Scholnick et al. 2010). Plasmodium infection also increased the cost of recovery following physical activity in S. occidentalis, with infected lizards displaying heightened blood glucose and lactate levels relative to non-infected ones (Scholnick et al. 2012). Such metabolic complications are expected to impair the physical activity of Plasmodium-infected hosts and, accordingly, classical symptoms of severe malaria in humans include muscle aches, contractures, fatigue, and weakness (Miller et al. 1989).
Plasmodium infections have been associated with cardiac dysfunction and shown to have detrimental effects on skeletal muscles in both humans (Miller et al. 1989; Nguah et al. 2012; Yeo et al. 2013; Marrelli and Brotto 2016) and animals (Carmona et al. 1996; Vuong et al. 1999; Brotto et al. 2005; Scholnick et al. 2012). While such pathogenic effects are thought to be primarily driven by tissue hypoxia (Yeo et al. 2013), investigation of the contractile function and biochemical properties of the skeletal muscles of mice infected with P. berghei revealed direct effects on the contractile machinery itself (Brotto et al. 2005). Indeed, the leg muscles of infected mice displayed a significant loss of essential contractile proteins that was likely responsible for a 50% decrease in contractile force, heightened fatigue, and lower recovery from fatigue. Atlantic canary (Serinus canaria) infected with P. cathemerium exhibited similar skeletal muscle compromise, with marked alterations in their contractile and sarcotubular systems (Carmona et al. 1996). Such muscle cell damage is thought to result from the inflammatory and oxidative stress triggered during malaria (Callahan et al. 2001; Clark and Cowden 2003; Pabon et al. 2003). Despite measurable effects on muscle function in humans and animals in the laboratory, there remains considerable variation in estimates of the impact of Plasmodium on physical activity in natural populations (Merino et al. 2000; Schall and Pearson 2000; Knowles et al. 2010).
Impacts of Plasmodium infection on activity in the wild have been investigated as direct measures of locomotor capacity, as well as indirectly by evaluating effects on higher-level phenotypes mediated by physical performance (e.g., reproductive effort). For instance, natural Plasmodium infections were found associated with reduced stamina in both western fence and rainbow (Agama agama) lizards (Schall 1990). However, there was no association between Plasmodium infection status and sprint speed in western fence lizards (Schall 1990), or locomotor activity in Spiny lizards (Sceloporus jarrovii) (Halliday et al. 2014). Plasmodium infection nevertheless impacted social interactions in western fence lizards, with infected males being more often socially submissive, less socially active, and less able to maintain territories and defend access to females (Schall and Dearing 1987; Schall and Sarni 1987). Plasmodium infections have also been shown to have mix effects on reproductive success in the wild. Female blue tits (Cyanistes caeruleus) that were infected and treated with an anti-malarial drug displayed increased hatching success, provisioning rates and fledging success relative to infected females that were untreated (Knowles et al. 2010). In contrast, the same population of blue tits also exhibited a positive association between reproductive effort (measured as clutch size) and parasitaemia (Knowles et al. 2011), and no association was reported between infection status and reproductive performance in red-billed gulls (Larus scopulinus) (Cloutier et al. 2011). The association between Plasmodium infection status and physical activity is likely to be, in large part, dependent on the actual cost of the parasite’s exploitation strategy. But greater virulence may not necessarily be associated with greater measurable costs if virulence is so high that infected individuals that are susceptible are removed from the population, thus biasing the pool of infected individuals towards those that are able to withstand the cost of infection.
We investigated whether infection with Plasmodium signals increased susceptibility or heightened tolerance in natural populations of Anolis sagrei lizards. To do so, we screened wild-caught lizards for Plasmodium parasites and examined links between infection status, body condition, locomotor performance (stamina), and survival to the end of the breeding season. We predicted that, if infection signals increased susceptibility to Plasmodium (hereafter: the susceptibility hypothesis), infected lizards should exhibit reduced body condition, locomotor performance, and survival relative to non-infected ones. Conversely, a lack of association or positive associations between infection status and those traits would support the hypothesis that, under natural conditions, wild-caught infected individuals are those that are able to tolerate the costs of infection (hereafter: the tolerance hypothesis). In addition, we predicted that the immuno-competence of infected individuals would be lower than that of non-infected individuals if infection reflects greater susceptibility (Navarro et al. 2003). To test this additional prediction, we challenged all individuals with phytohemagglutinin (PHA), which stimulates the infiltration and/or proliferation of various immune cells, including T lymphocytes (Licastro et al. 1993; Martin et al. 2006), and is hence commonly used in eco-immunology to estimate cell-mediated immunity (for e.g., Gonzalez et al. 1999; Martin et al. 2003; Svensson et al. 2001; Mugabo et al. 2015; Bowers et al. 2014).
Methods
Study system and field methods
The brown anole, A. sagrei, is a small (40–70 mm snout-vent-length; SVL) semi-arboreal lizard, and is one of the most common anoles in the Bahamas (Losos 2009). We studied wild populations of A. sagrei at two sites of the Bahamas: Regatta Point on the large island of Great Exuma (23°30′25.1″N 75°45′58.3″W) and Stocking Island (23°32′N 75°46′W), a ∼1 km2 island <2 km offshore. We captured a total of 343 individuals, 130 from Regatta Point (66 females and 64 males) and 207 from Stocking Island (52 females and 155 males) during spring (May–June) 2005. Upon capture, we measured body mass (nearest g) and assigned each individual with a unique four-color combination of elastomer markings, which were injected into the underside of the hind- and forelimbs. Blood was drawn from the postorbital sinus and stored in PBS/EDTA buffer at −20 °C, and we measured immune-competence using a PHA assay (see below). All lizards were then released back to their site of capture and a subset of them (from Regatta Point only) was recaptured 2 weeks later to measure running endurance.
Most lizards (∼90%; Cox and Calsbeek 2010) in our study population mature and die in a single year. We therefore estimated fitness as survival from initial capture (sub-adulthood) in late May–early June to our population censuses conducted during late September–early October. This 4-month period accounts for survival to maturity and to the end of the first breeding season. Lizards that we did not recapture were considered to have died; this is a reasonable assumption since emigration from islands is extremely rare, except perhaps during hurricanes (Calsbeek and Smith 2003), of which none occurred during this study. Moreover, although the majority of surviving lizards were recaptured within the first 2 days of our census, we searched an additional 3 weeks to ensure the recapture of every marked lizard. Censuses continued until two consecutive days with no new recaptured individuals. In total, we recaptured 108 individuals, including 47 on Regatta Point (19 females and 26 males) and 60 on Stocking Island (12 females and 48 males).
Screening for Plasmodium infection
DNA was extracted for all samples from whole blood following a DNeasy kit protocol (Qiagen, Valencia, CA, USA). We used primers and methods described in Perkins and Schall (2002) to detect Haemoproteus and Plasmodium parasites, which are euprotista belonging to the phylum apicomplexa. The PCR products were run on 2% agarose gels and stained with ethidium bromide for UV detection. Negative results were confirmed by repeated PCR. The PCR products were purified using a MinElute Qiagen® kit following manufacturer’s instructions. We identified lineages by sequencing the fragments (BigDye (R) version 1.1 sequencing kit, Applied Biosystems) on an ABI PRISM 3100 (TM) sequencing robot (Applied Biosystems). Distinct sequences found several times in independent PCRs, either within a same individual or in several different individuals, were considered to be verified (V). Unique sequences, which only differed from verified sequences by one nucleotide, were also found. However, a single nucleotide divergence may be attributed to a Taq polymerase incorporation error during amplification or to another type of PCR error (jumping PCR, heteroduplex artifact) and these haplotypes are therefore considered non-verified (NV). Sequences are deposited in GenBankTM with the following accession numbers DQ846851-DQ846861 and DQ986492-DQ986495.
Immune response
In vivo cell-mediated immune response was assessed using a PHA assay (Goto et al. 1978). Because males are larger than females, we challenged males with 0.20 mg PHA in 0.02 ml PBS and females with 0.10 mg PHA in 0.01 ml PBS, injected in the left hind-foot pad. We injected the same volume of PBS in the right hind-foot pad as a control. We recorded the thickness of each footpad with dial-calipers (±0.01 mm) at the site of PHA injection, before and again 24 h following injection. We assessed the intensity of the response to PHA as the difference in swelling between the PHA-injected and the control footpad. Swelling was measured in a total of 194 individuals, including 77 from Regatta Point (39 females and 38 males) and 118 from Stocking Island (9 females and 109 males). All individuals were released back at their site of capture following immune measure.
Stamina
Individuals on Regatta Point were re-captured after 2 weeks to ensure full recovery from immune measurements. Stamina was then measured by running lizards to exhaustion on an electrical treadmill (0.4km/h) (Perry et al. 2004). Because anoles do not run well on level surfaces (Perry et al. 2004), we set the treadmill at a 20° incline. We motivated lizards to run by manually tapping the hind limb. Lizards were considered to have run to exhaustion after three failed attempts to induce running, and/or the loss of the lizard’s natural righting response. Stamina was measured as the time to exhaustion (in seconds) in a total of 127 individuals from Regatta Point only (64 females and 63 males).
Phylogenetic and statistical analyses
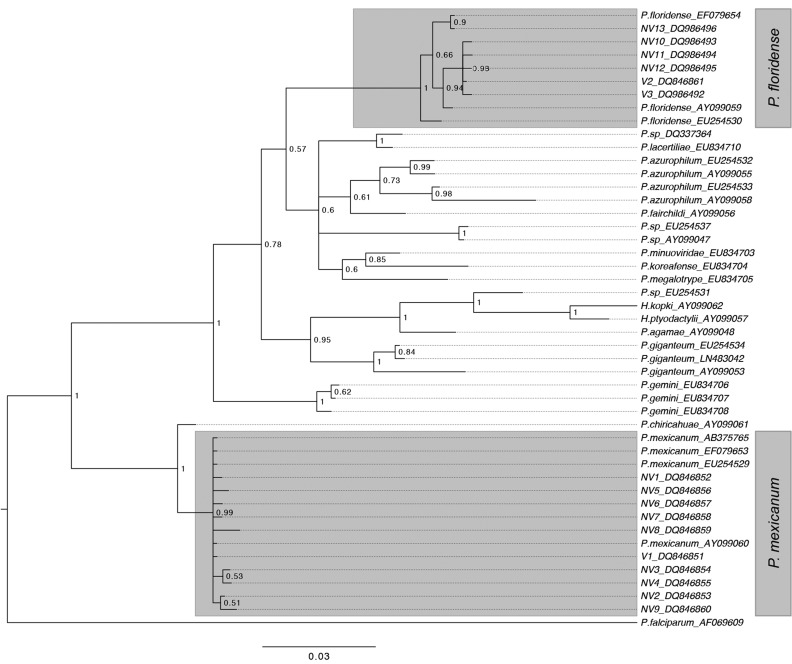
The phylogeny of the isolates was reconstructed using a Bayesian approach in MrBayes v.3.2.6 (Huelsenbeck and Ronquist 2001) and includes reptilian malaria isolates available on Genbank, as well as Plasmodium falciparum, which is used as an outgroup. The phylogeny is based on 598 bp of the cytB gene. Genbank accession numbers are included in the tree annotation (Fig. 1). The tree was reconstructed using a gamma-distributed, site-specific, general time-reversible model, with parameters estimated from the data during the analysis. We ran two runs of two chains for 20,000,000 MCMC generations, sampling trees every 20,000 generations. The tree was then plotted using Figtree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Fig. 1.
Phylogenetic tree 15 Plasmodium isolates found in A. sagrei based on cytochrome b sequences. The phylogeny of the cytochrome b gene was reconstructed using a Bayesian approach. Sequences from known lizard malaria parasites were included for comparison, and human P. falciparum was used as an out-group. V1 belongs to the monophyletic group of P. mexicanum, while V2 and V3 verified lineages belonged to the monophyletic group of P. floridense. GenBank accession numbers of all sequences are indicated. Numbers on interior branches indicate Bayesian support.
All statistical analyses were conducted in R 3.3.2 (R Core Team 2016). Out of the 25 individuals that tested positive for Plasmodium infection, only one was female. As a result, all analyses were done on males only. First, we tested whether body condition was affected by infection status using linear regressions with body condition as the response variable and with infection status as the explanatory term. Body condition was calculated as (body mass/SVL2) with body mass in milligrams and SVL in millimeters; by doing so, our analysis controls for any differences in SVL that are generated by differences in age and/or growth rate. To test for differences in stamina as a function of infection status, we then used a linear regression with stamina as the response variable and with infection status and body mass as the explanatory terms. We investigated whether individuals experience different survival probability depending on their infection status using a logistic regression with survival to the end of the breeding season as the response variable and with infection status and body mass as the explanatory terms. Finally, we modelled differences in immune response using a linear regression that included immune swelling as the response variable and with infection status and body mass as the explanatory variables. Figures 2–4 were made using the package ggplot2 (Wickham 2009).
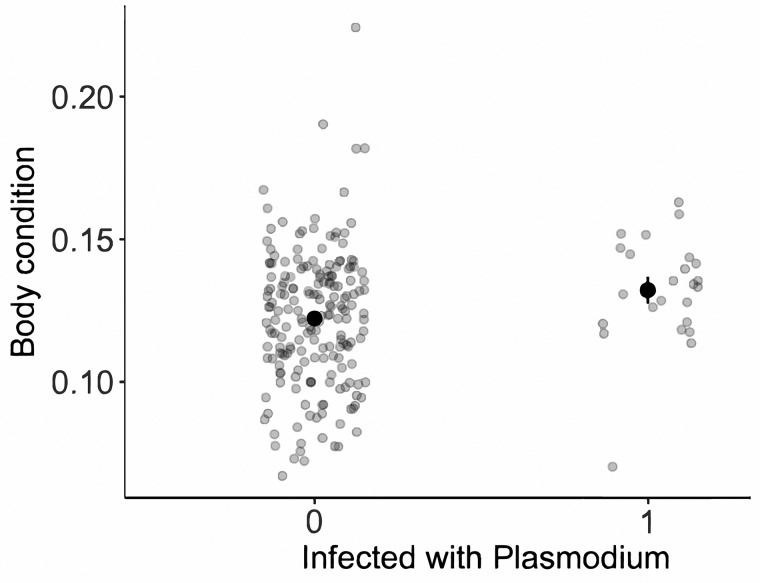
Fig. 2.
Association between Plasmodium infection status and body condition in male A. sagrei. The darker symbols show the predicted means and se, and the lighter symbols show the raw values.
Results
Out of 337 individuals, 25 (7.4%) were infected with Plasmodium lineages, with prevalence differing significantly between sites and reaching 12% on Regatta Point and 5% on Stocking Island (χ2 = 4.3, df = 1, P = 0.04). Of the 25 infected lizards, only one was a female from Stocking Island. Out of the 24 males infected, 15 (63%) were from Regatta Point and nine (38%) from Stocking Island. Sequencing Plasmodium infections in all 25 infected individuals yielded 15 unique sequences (597 bp), only three of which were verified mitochondrial malaria lineages (Fig. 1). All sequences belonged to two well-supported monophyletic clusters of Plasmodium lineages, with V1 and NV1-9 belonging to the clade containing P. mexicanum and V2, V3, and NV10-13 belonging to the clade containing P. floridense group. No individual was found to be co-infected with P. mexicanum and P. floridense.
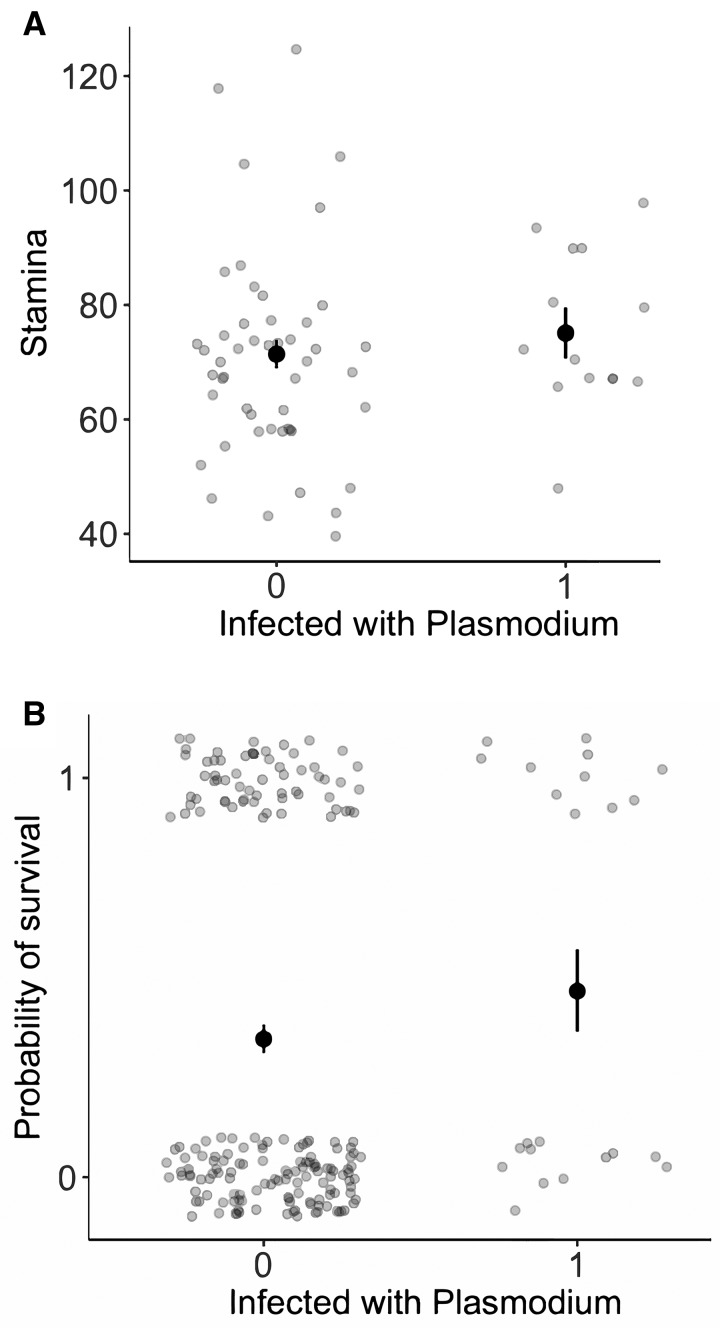
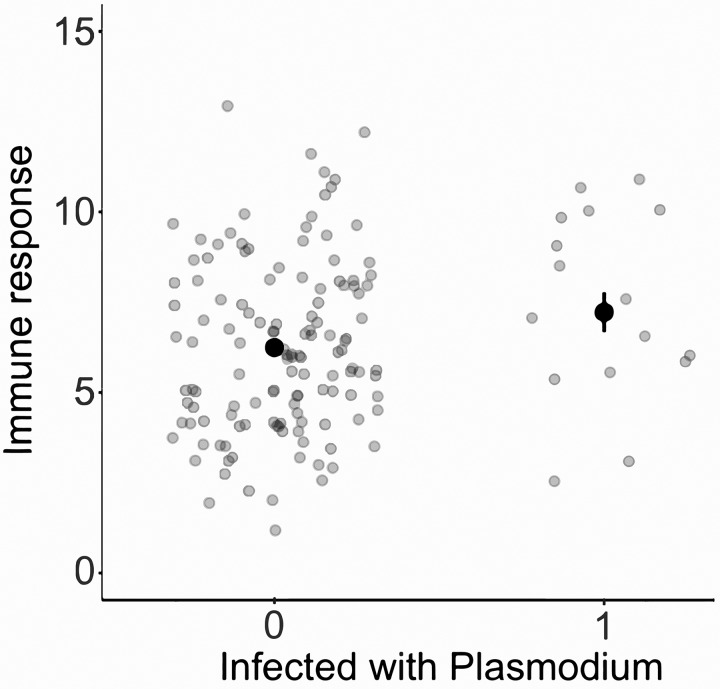
We found no compelling support for the susceptibility hypothesis: in no case was there a negative association between infection status and host traits, and any non-significant associations were all in a positive direction (Table 1). Males that were infected were found to be in significantly better body condition than non-infected males (linear regression; infection status: t1,215 = 2.0, P = 0.04; R2 = 0.02; Fig. 2). There was no effect of infection status on male stamina (linear regression; infection status: t1,60 = 0.8, P = 0.46; body mass: t1,60 = 2.2, P < 0.04; R2 = 0.09; Fig. 3A). Similarly, there was no association between survival to the next breeding season and infection status (logistic regression; infection status: z1,202 = 1.1, P = 0.26, relative odds ratio = 1.7 (CI = 0.68–4.0); body mass: z1,202 = 1.2, P = 0.24, relative odds ratio = 1.0 (CI = 0.99–1.0); Fig 3B). Finally, immune swelling in response to PHA tended to be higher in infected males, but this effect was not significant (linear regression; infection status: t1,143 = 1.73, P = 0.09; body mass: t1,143 = 5.3, P < 0.001; site: t1,143 = 0.6, P = 0.54; R2 = 0.19; Fig. 4).
Table 1.
Model estimates and standard errors for each of four models testing the association between infection status with Plasmodium parasites and host traits
| Response variable | Explanatory variables | Estimate | SE |
|---|---|---|---|
| Body condition | Infection status | 0.01 | 0.005 |
| Stamina | Infection status | 3.81 | 5.09 |
| Body mass | 0.04 | 0.02 | |
| Survival | Infection status | 0.50 | 0.45 |
| Body mass | 0.001 | 0.001 | |
| Immune response | Infection status | 1.02 | 0.60 |
| Body mass | 0.007 | 0.001 |
Fig. 3.
Association between Plasmodium infection status and (A) stamina (in s) and (B) survival to the next breeding season in male A. sagrei. The darker symbols show the predicted means and se, and the lighter symbols show the raw values. In (B), note the dispersion of observations around 0 (no survival) and 1 (survived) to improve the visualization of results.
Fig. 4.
Association between Plasmodium infection status and immune swelling (in mm) to PHA in male A. sagrei. The darker symbols show the predicted means and se, and the lighter symbols show the raw values.
Discussion
Plasmodium infections were detected in >7% of wild-caught A. sagrei, with prevalence ranging from 12% on the main island of Great Exuma (Regatta Point) to 5% on the more remote Stocking Island. Lizards were infected either with P. mexicanum or with P. floridense, and both Plasmodium clades were found at both sites. Despite demonstrated costs of Plasmodium infection in other taxa in both laboratory and natural settings, we found that infected male had higher body condition than non-infected ones. Furthermore, infection with Plasmodium was not associated with reduced stamina, survival, or immune swelling to PHA and any trend was in a positive direction in contrast to the predictions of the susceptibility hypothesis (Table 1). Although these trends were not significant in the opposite direction to those expected under the susceptibility hypothesis, power analyses revealed that considerably more individuals would be required to obtain significance for each parameter tested (e.g., 463 for stamina and 322 for immune response). Our results are therefore consistent with the prediction that wild-caught lizards infected with Plasmodium are tolerant, rather than susceptible, to the parasite.
While studies on humans and laboratory animals demonstrate measurable costs of Plasmodium infections with detrimental consequences on host traits (e.g., body condition, physical activity), evidence of such effects in natural populations remains mixed (Merino et al. 2000; Schall and Pearson 2000; Knowles et al. 2010). For several years now, this has fueled debate as to whether or not Plasmodium infections are actually truly costly in the wild (Asghar et al. 2011). Comparisons across host populations and Plasmodium lineages reveal that costs of infection can, in fact, vary markedly. For example, the widespread population declines and extinctions suffered by the Hawaiian avifauna as a result of the introduction of P. relictum attests to the fact that infections may be more costly in recently exposed hosts (Van Riper et al. 1986). Furthermore, the fitness consequences of infection may also vary depending on the Plasmodium lineage involved. Lesser Kestrels (Falco naumanni) displayed reduced fledging numbers only when infected with one of two Plasmodium lineages detected in this species (Ortego et al. 2008). Interestingly, while on the whole correlative studies estimating the cost of Plasmodium infection remain inconclusive, experimental manipulations of Plasmodium infection through the administration of anti-malarial medication demonstrate that chronic infections with Plasmodium can indeed have significant effects on host fitness (Marzal et al. 2005; Knowles et al. 2010). As a result, the absence of measurable cost to Plasmodium infection in natural populations does not necessarily imply that there is no cost per se. Rather our ability to estimate this cost will depend on whether we are able to sample all the individuals of the population that have been infected, or whether our sample includes only the subset of individuals that can sustain the costs of infection.
Tolerance is the ability to limit the damages caused by infection for a given parasite load (Råberg et al. 2009). In order words, while tolerant individuals are not able to control their parasite burden, they are able to diminish the associated pathogenic effects. Accordingly, an experimental infection of five strains of mice with P. chabaudi revealed measurable differences in tolerance to infection, with the most tolerant mice strains exhibiting reduced loss of both body mass and red blood cells relative to the least tolerant ones (Råberg et al. 2007). Tolerance therefore has the potential to lessen, if not erase, the cost of infection in wild populations. The lack of associations between stamina, survival and Plasmodium infection status in our populations of A. sagrei evidence an absence of measurable costs of infection. Furthermore, we found that, in fact, infected individuals were in better body condition than non-infected ones. Taken together, these results suggest that wild-caught infected A. sagrei encompass the individuals that are able to bear the cost of infection by Plasmodium parasites, rather than those that are the most susceptible to infection. While we cannot fully exclude the possibility that infected A. sagrei are those that are quantitatively resistant to infection (i.e., able to limit parasite growth; Gandon and Michalakis 2000; Sepil et al. 2013) rather than tolerant, the absence of measurable costs of infection expected as a result of immune activity suggests that this is unlikely to be the case.
That Plasmodium-infected lizards are the most tolerant rather than the most susceptible is further supported by the fact that infected individuals did not display reduced immuno-competence relative to non-infected ones. The link between infection status and measures of immune capability (i.e., immuno-competence) is still debated and questions remain as to whether measures of immunity mirror an individual’s health (i.e., whether or not it is currently infected), or whether these measures are indicative of the individuals’ ability to control and clear parasites (reviewed in Biard et al. 2015). The PHA-induced swelling test stimulates the infiltration and/or proliferation of various immune cells, including T lymphocytes (Licastro et al. 1993; Martin et al. 2006), and is hence commonly used in eco-immunology to estimate cell-mediated immunity (e.g., Bowers et al. 2014; Mugabo et al. 2015). Links between the response to PHA and infection status with various parasites is mixed, with some studies showing positive associations and others reporting negative ones (Biard et al. 2015). However, the one study that has tested links with haemosporidian parasites (genus Haemoproteus) found that infected house sparrows (Passer domesticus) had lower PHA responses and that individuals in better body condition had stronger immune responses to PHA than individuals in lower condition (Navarro et al. 2003). That our study shows a trend for infected A. sagrei to display increased immune responses to PHA relative to non-infected ones is therefore more consistent with the hypothesis that infected lizards are tolerant rather than susceptible to infection. Experimental work is, however, now required to fully understand the link between infection status with hemosporidians (including Plasmodium) and response to PHA.
Our study highlights the need to take into account the complexity of host–parasite co-evolutionary interactions when evaluating the costs of infection. Virulence, which is strictly defined as parasite-induced host mortality but which can be more broadly thought of as the fitness cost of infection to the host, is a product of both parasite and host behavior and hence an outcome of their interaction (Poulin and Combes 1999; Alizon et al. 2009; Bull and Lauring 2014). As a result, we will only gain a complete understanding of disease virulence and the intensity of parasite-driven selection, if we measure infection costs in an unbiased sample of the host population. However, we are at risk of under-estimating those costs when virulence is such that all susceptible hosts are removed from the population (i.e., through mortality) and that the only infected individuals remaining are the tolerant ones.
Acknowledgments
This project benefited from the excellent assistance of Yuri Springer and Delphin Ruché in the field. Special thanks go to Nancy Bottomley, Regatta Point in Georgetown, Exuma for logistical support and permission to work on her land. We thank the department of agriculture and the people of the Bahamas for permission to conduct this research. Research was performed under the University of California, Los Angeles’s Institutional Animal Care and Use Committee (protocol 2004-47-04).
Funding
This work was supported by a National Geographic Society [grant #8002-06 to R.C.]; a Natural Environment Research Council [research grant NE/M00256X to C.B.]; The symposium was supported by National Science Foundation [grant # IOS-1637160]; Company of Biologists [grant EA1233] both Simon Lailvaux and Jerry Husak; and bySociety for Integrative and Comparative Biology divisions DAB, DCB, DEC, DEDE, DEE, DNB, and DVM.
References
- Alizon S, Hurford A, Mideo N, Van Baalen M.. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evolution Biol 22:245–59. [DOI] [PubMed] [Google Scholar]
- Asghar M, Hasselquist D, Bensch S.. 2011. Are chronic avian haemosporidian infections costly in wild birds? J Avian Biol 42:530–7. [Google Scholar]
- Biard C, Monceau K, Motreuil S, Moreau J.. 2015. Interpreting immunological indices: the importance of taking parasite community into account. An example in blackbirds Turdus merula. Methods Ecol Evol 6:960–72. [Google Scholar]
- Bonneaud C, Balenger SL, Hill GE, Russell AF.. 2012. Experimental evidence for distinct costs of pathogenesis and immunity against a natural pathogen in a wild bird. Mol Ecol 21:4787–96. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G.. 2003. Assessing the cost of mounting an immune response. Am Nat 161:367–79. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Wilson RS, Seebacher F.. 2016. Immune-Challenged Fish Up-Regulate Their Metabolic Scope to Support Locomotion. PLoS One 11:e0166028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK.. 2014. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95:3027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto MAP, Marrelli MT, Brotto LS, Jacobs-Lorena M, Nosek TM.. 2005. Functional and biochemical modifications in skeletal muscles from malarial mice. Exp Physiol 90:417–25. [DOI] [PubMed] [Google Scholar]
- Bull JJ, Lauring AS.. 2014. Theory and empiricism in virulence evolution. PLoS Pathog 10: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G.. 2001. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24:210–7. [DOI] [PubMed] [Google Scholar]
- Calsbeek R, Smith TB.. 2003. Ocean currents mediate evolution in island lizards. Nature 426:552–5. [DOI] [PubMed] [Google Scholar]
- Carmona M, Finol HJ, Marquez A, Noya O.. 1996. Skeletal muscle ultrastructural pathology in Serinus canarius infected with Plasmodium cathemerium. J Submicrosc Cytol Pathol 28:87–91. [PubMed] [Google Scholar]
- Clark IA, Cowden WB.. 2003. The pathophysiology of falciparum malaria. Pharmacol Ther 99:221–60. [DOI] [PubMed] [Google Scholar]
- Cloutier A, Mills JA, Yarrall JW, Baker AJ.. 2011. Plasmodium infections of red-billed gulls (Larus scopulinus) show associations with host condition but not reproductive performance. J R Soc NZ 41:261–77. [Google Scholar]
- Cox RM, Calsbeek R.. 2010. Severe costs of reproduction persist in anolis lizards despite the evolution of a single-egg clutch. Evolution 64:1321–30. [DOI] [PubMed] [Google Scholar]
- Eraud C, Duriez O, Chastel O, Faivre B.. 2005. The energetic cost of humoral immunity in the Collared Dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade-offs? Funct Ecol 19:110–8. [Google Scholar]
- Gandon S, Michalakis Y.. 2000. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc R Soc Lond B Biol Sci 267:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Sanz I, Rojas N, Silva V, Kirsten L, Bustamante M.. 1999. In vitro inhibition of lymphocyte proliferation by low density lipoproteins. Revista Medica De Chile 127:1305–11. [PubMed] [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y.. 1978. Suppression of phytohemagglutinin skin-response in thymectomized chickens. Poultry Sci 57:246–50. [DOI] [PubMed] [Google Scholar]
- Halliday WD, Paterson JE, Patterson LD, Cooke SJ, Blouin-Demers G.. 2014. Testosterone, body size, and sexual signals predict parasite load in Yarrow's Spiny Lizards (Sceloporus jarrovii). Canad J Zool 92:1075–82. [Google Scholar]
- Huelsenbeck JP, Ronquist F.. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–5. [DOI] [PubMed] [Google Scholar]
- Knowles SCL, Nakagawa S, Sheldon BC.. 2009. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct Ecol 23:405–15. [Google Scholar]
- Knowles SCL, Palinauskas V, Sheldon BC.. 2010. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evolution Biol 23:557–69. [DOI] [PubMed] [Google Scholar]
- Knowles SCL, Wood MJ, Alves R, Wilkin TA, Bensch S, Sheldon BC.. 2011. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol Ecol 20:1062–76. [DOI] [PubMed] [Google Scholar]
- Li JV, Wang Y, Saric J, Nicholson JK, Dirnhofer S, Singer BH, Tanner M, Wittlin S, Holmes E, Utzinger J.. 2008. Global metabolic responses of NMRI mice to an experimental Plasmodium berghei infection. J Proteom Res 7:3948–56. [DOI] [PubMed] [Google Scholar]
- Licastro F, Davis LJ, Morini MC.. 1993. Lectins and superantigens - membrane interactions of these compounds with t-lymphocytes affect immune-responses. Intl J Biochem 25:845–52. [DOI] [PubMed] [Google Scholar]
- Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, Los Angeles and London: University of California Press; p. 528. [Google Scholar]
- Mackintosh CL, Beeson JG, Marsh K.. 2004. Clinical features and pathogenesis of severe malaria. Trend Parasitol 20:597–603. [DOI] [PubMed] [Google Scholar]
- Marrelli MT, Brotto M.. 2016. The effect of malaria and anti-malarial drugs on skeletal and cardiac muscles. Malaria J 15:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M.. 2006. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20:290–9. [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M.. 2003. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond B Biol Sci 270:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzal A, de Lope F, Navarro C, Moller AP.. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–5. [DOI] [PubMed] [Google Scholar]
- Merino S, Moreno J, Sanz JJ, Arriero E.. 2000. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc R Soc Lond B Biol Sci 267:2507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, White NJ, Lott JA, Roberts JM, Greenwood BM.. 1989. Biochemical-evidence of muscle injury in african children with severe malaria. J Infect Dis 159:139–42. [DOI] [PubMed] [Google Scholar]
- Mugabo M, Perret S, Decenciere B, Meylan S, Le Galliard JF.. 2015. Density-dependent immunity and parasitism risk in experimental populations of lizards naturally infested by ixodid ticks. Ecology 96:450–60. [DOI] [PubMed] [Google Scholar]
- Navarro C, Marzal A, de Lope F, Moller AP.. 2003. Dynamics of an immune response in house sparrows Passer domesticus in relation to time of day, body condition and blood parasite infection. Oikos 101:291–8. [Google Scholar]
- Nguah SB, Feldt T, Hoffmann S, Pelletier D, Ansong D, Sylverken J, Mehrfar P, Herr J, Thiel C, Ehrhardt S, et al. 2012. Cardiac function in Ghanaian children with severe malaria. Intensive Care Med 38:2032–41. [DOI] [PubMed] [Google Scholar]
- Olszewski KL, Llinas M.. 2011. Central carbon metabolism of Plasmodium parasites. Mol Biochem Parasitol 175:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski KL, Morrisey JM, Wilinski D, Burns JM, Vaidya AB, Rabinowitz JD, Linas M.. 2009. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe 5:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Cordero PJ, Aparicio JM, Calabuig G.. 2008. Consequences of chronic infections with three different avian malaria lineages on reproductive performance of Lesser Kestrels (Falco naumanni). J Ornithol 149:337–43. [Google Scholar]
- Pabon A, Carmona J, Burgos LC, Blair S.. 2003. Oxidative stress in patients with non-complicated malaria. Clin Biochem 36:71–8. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Schall JJ.. 2002. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol 88:972–8. [DOI] [PubMed] [Google Scholar]
- Perry G, Levering K, Girard I, Garland T.. 2004. Locomotor performance and social dominance in male Anolis cristatellus. Anim Behav 67:37–47. [Google Scholar]
- Planche T, Dzeing A, Ngou-Milama E, Kombila M, Stacpoole PW.. 2005. Metabolic complications of severe malaria Curr Top Microbiol Immunol 295:105–36. [DOI] [PubMed] [Google Scholar]
- Poulin R, Combes C.. 1999. The concept of virulence: Interpretations and implications - Comment. Parasitol Today 15:474–5. [DOI] [PubMed] [Google Scholar]
- Råberg L, Graham AL, Read AF.. 2009. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc B Biol Sci 364:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Sim D, Read AF.. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318:812–4. [DOI] [PubMed] [Google Scholar]
- Roth E, 1990. Plasmodium-falciparum carbohydrate-metabolism - a connection between host-cell and parasite. Blood Cells 16:453–60. [PubMed] [Google Scholar]
- Schall JJ. 1990. Virulence of lizard malaria - the evolutionary ecology of an ancient parasite host association. Parasitology 100:S35–52. [DOI] [PubMed] [Google Scholar]
- Schall JJ, Dearing MD.. 1987. Malarial parasitism and male competition for mates in the western fence lizard, Sceloporus-occidentalis. Oecologia 73:389–92. [DOI] [PubMed] [Google Scholar]
- Schall JJ, Pearson AR.. 2000. Body condition of a Puerto Rican anole, Anolis gundlachi: Effect of a malaria parasite and weather variation. J Herpetol 34:489–91. [Google Scholar]
- Schall JJ, Sarni GA.. 1987. Malarial parasitism and the behavior of the lizard, Sceloporus-occidentalis. Copeia 84–93. [Google Scholar]
- Scholnick DA, Gilpin NT, Manivanh RV.. 2012. Disruption to Recovery Metabolism in the Fence Lizard Sceloporus occidentalis Infected with the Malarial Parasite Plasmodium mexicanum. J Herpetol 46:643–7. [Google Scholar]
- Scholnick DA, Manivanh RV, Savenkova OD, Bates TG, McAlexander SL.. 2010. Impact of malarial infection on metabolism and thermoregulation in the fence lizard Sceloporus occidentalis from Oregon. J Herpetol 44:634–40. [Google Scholar]
- Sepil I, Lachish S, Hinks AE, Sheldon BC.. 2013. Mhc supertypes confer both qualitative and quantitative resistance to avian malaria infections in a wild bird population. Proc R Soc Lond B Biol Sci 280:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S.. 1996. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–21. [DOI] [PubMed] [Google Scholar]
- Svensson E, Sinervo B, Comendant T.. 2001. Density-dependent competition and selection on immune function in genetic lizard morphs. Proc Natl Acad Sci U S A 98:12561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2016. R version 3.3.2 (2016-10-31) – “Sincere Pumpkin Patch”. Platform: x86_64-apple-darwin13.4.0 (64-bit). The R Foundation for Statistical Computing.
- van der Most PJ, de Jong B, Parmentier HK, Verhulst S.. 2011. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct Ecol 25:74–80. [Google Scholar]
- Van Riper C, Van Riper SG, Goff ML, Laird M.. 1986. The epizootiology and ecological significance of malaria in hawaiian land birds. Ecol Monogr 56:327–44. [Google Scholar]
- Vuong PN, Richard F, Snounou G, Coquelin F, Renia L, Gonnet F, Chabaub AG, Landau I.. 1999. Development of irreversible lesions in the brain, heart and kidney following acute and chronic murine malaria infection. Parasitology 119:543–53. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York (NY: ): Springer. [Google Scholar]
- Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM.. 2013. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 207:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]