Abstract
While previous studies have shown intergenerational transmission of birth weight from mother to child, only one study has assessed whether this continuity persists across three generations. We used the Aberdeen Maternity and Neonatal Databank to examine the intergenerational correlations of birth weight, birth weight adjusted for gestational age and sex, and small- and large for gestational age births among 1457 grandmother-mother-grandchild triads across three generations. All participants were born between 1950 and 2015. The intergenerational transmission was examined with linear regression analyses. Our findings showed that grandmaternal birth weight was associated with grandchild birth weight, independently of prenatal and sociodemographic covariates and maternal birth weight (B=0.12 Standard deviation units, 95% Confidence Interval=0.07, 0.18). Similar intergenerational continuity was found for birth weight adjusted for sex and gestational age, and for small for gestational age births. To conclude, birth weight and fetal growth show intergenerational continuity across three generations. The developmental origins of birth weight and hence later health and disease are already present in earlier generations.
Keywords: Fetal Growth, Birth Weight, Cross-generational, Longitudinal, Grandmaternal, Grandchild, Small for Gestational Age
Individual differences in birth weight and fetal growth predict premature mortality and increased risk of cardiovascular disease across the lifespan(1–3). Thus to increase our understanding of the developmental pathways to chronic illnesses, it is important to understand the developmental pathways to fetal growth.
Several studies have supported small to moderate-sized intergenerational associations of birth weight and suboptimal fetal growth [indexed most commonly as small-for gestational age (SGA; defined as birth weight predicted by sex and gestational age at ≤-2 standard deviation(SD)s or below the 10th percentile] or large for gestational age (LGA; defined as birth weight predicted by sex and gestational age at ≥2 SD or above the 90th percentile) births] of parents and their children (4–19), with correlations between maternal and child birth weight typically ranging between .20 and .25. The intergenerational associations are noticeably stronger for maternal than paternal birth weight(12,20), suggesting that maternal genetic and/or fetal environmental factors may explain this continuity. Indeed, while recent genetic studies have identified a small genetic component in birth weight and fetal growth(21), prenatal and/or postnatal environmental factors can strengthen or attenuate the familial transmission of birth weight(22).
Although transmission of birthweight across two generations has been repeatedly reported(4,6,8–11,14–18,23), to our knowledge only one study has examined continuity across three generations(20). This study(20) examined the intergenerational associations of birth size among 28152 grandparent-grandchild pairs and reported modest correlations between grandparental and grandchild birth size, with stronger associations found for birth weight along the maternal line. This study, however, only had data from grandparents and grandchildren with no data about the second generation, and hence could not examine whether this intergenerational association was mediated by birth weight in the intermediate generation(20).
Furthermore, the underlying mechanisms of this intergenerational continuity of birthweight are unclear. It is not known whether these associations are mediated by prenatal environmental adversities and/or whether this is due to genetic or epigenetic inheritance. Only few studies (6,11,16,18,24)have examined whether this intergenerational continuity is explained by socioeconomic or prenatal environmental adversities (e.g. cardiometabolic pregnancy disorders, maternal smoking during pregnancy) that are known predictors of birth weight and fetal growth(18,25,26). These studies suggest that these factors do not explain the intergenerational continuity(6,11,16,18,24).
Here we used Aberdeen Maternity and Neonatal Databank (AMND)-data to examine the intergenerational transmission of birth weight and fetal growth across three generations. We hypothesized that birth weight is transmitted across three generations. Based on previous evidence, we also hypothesized that the associations are at most partially mediated by prenatal and sociodemographic factors and thus emerge also independently of assessed covariates. We also examined whether grandmaternal birth weight predicts grandchild birthweight independently of birth weight in the intermediate generation.
Methods
The study sample
The AMND comprises maternal and neonatal data from medical records of all pregnancies in Aberdeen Maternity Hospital, Aberdeen Scotland since 1950(27). The database includes pregnancy, delivery and baby records on multiple aspects of obstetric and perinatal health and development. The Aberdeen Maternity Hospital is the only maternity hospital in Aberdeen and around 99% of the births in the city take place at this hospital(27). The city of Aberdeen also has a rather stable population, with less than 4% of those born in the Aberdeen Maternity Hospital migrating out of this region(27). Therefore, the AMND is a representative dataset of the whole population of Aberdeen. Perinatal data are coded by trained professionals using a stringent, standardized procedure. Complete computerized data on reproductive histories allow identification of families and thus intergenerational studies(27).
Web Figure 1 shows the flowchart of the participant selection for the current study. We excluded multiple births, stillbirths and neonatal deaths from our analyses. To avoid the possibility of having dependent observations of siblings in the same analytic pool, we included only the first pregnancies of the mothers in the dataset in each generation. If a mother’s first pregnancy in the dataset had been a stillbirth, she was included in the study with her first live-born offspring. With these criteria, birth weight data from three generations was available for 1457 triads [grandmother, mother, and the child of the third generation] born 1950-2015. The study was approved by the AMND Steering Committee.
Neonatal characteristics
Birth weight was measured in grams and gestation length in weeks. Fetal growth was defined in sex and birth order -stratified models by the residuals of birth weight predicted by gestational age according to Scottish norms(28), and used both as a continuous variable [referred to as birth weight SD score] and as classified into three categories; SGA ≤-2 SD: appropriate for gestational age (-2 to 2 SD) and LGA (≥ 2 SD).
Covariates
Data on other prenatal and sociodemographic factors were extracted from the AMND database. These included maternal age at delivery (years), height, body mass index: weight(kilograms)/height(meters)2] in pregnancy and its gestational week of measurement (<20 weeks, 20-24 weeks, 25-39 weeks and ≥30 weeks), hypertensive pregnancy disorders [preeclampsia, gestational hypertension, pre-existing(essential or renal) hypertension and normotensive], gestational or pre-existing diabetes, socioeconomic deprivation status categories [low (category 1), intermediate (categories 2-3) and high (categories ≥4)] parity (primiparous vs. other), maternal smoking during pregnancy (never smoked/ex-smoker/smoked throughout pregnancy), labour type (elective cesarean section/induced labour/spontaneous birth) child sex (boy/girl) and year of birth. Due to low levels of socioeconomic deprivation in the first generation of the three-generation dataset, the categories of intermediate and high socioeconomic deprivation were combined in this generation. Child sex only varied in the final generation of the datasets, since in earlier generations all participants were also participating mothers. For all categorical covariates, we dummy-coded participants with missing data into a separate category.
Statistical Analyses
Pearson correlation analyses- and t- and Analysis of variance-tests were conducted to assess the associations of the covariates with birth weight and birth weight SD score across generations. The intergenerational continuity of birth weight and fetal growth was examined also in linear regression models where birth weight and birth weight SD score in the third generation were the outcomes. First regression models included maternal age at delivery, socioeconomic deprivation level, year of delivery and parity in grandmaternal, maternal, and child’s generation and child’s sex in the final generation as covariates. Second models included model one covariates and maternal smoking, hypertensive disorders during pregnancy, gestational or pre-existing diabetes and labour type in each generation. Third regression models included also maternal birth weight as a covariate. Fourth models adjusted further for maternal height and body mass index during pregnancy and gestation of weight measurement in each generation. To account for skewness, maternal body mass index during pregnancy in each generation was rank-normalized according to Blom’s formula.
To assess whether any associations between grandmaternal birth weight on child birth weight were mediated through maternal birth weight, mediation analyses were performed with the bootstrapping method with 5000 re-samples and bias-corrected confidence intervals, using the PROCESS macro for mediation analyses developed by Andrew Hayes and colleagues (29,30). The mediation analyses were ran with only the birth weight variables included, with no covariates.
All continuous independent and dependent variables were standardized into SD units (mean=0, SD=1) for linear regression and mediation analyses to facilitate comparison of the strength of the associations. We also ran the analyses by using the categorical indices of fetal growth (SGA/appropriate for gestational age/LGA birth) as predictors of birth weight in the forthcoming generations.
We also examined the intergenerational continuity of birth weight and birth weight SD score across 2-generations in the grandmother-mother and mother-child dyads of the dataset, using the same factors of the examined generations as described above as covariates.
Results
Table 1 shows the characteristics of the study sample, and Web Table 1 shows the associations of the covariates with birth weight and fetal growth in each generation.
Table 1.
Characteristics of the Study Sample. The Aberdeen Maternity and Neonatal Databank-Study 1950-2015.
| Study Generation | Grandmaternal | Maternal | Child | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Data Available (No.) | No. | % | Mean (SD) | Data Available (No.) | No. | % | Mean (SD) | Data Available (No.) | No. | % | Mean (SD) |
| Maternal Prenatal Body Mass Indexa | 1,449 | 23.3 (3.7) | 1,454 | 23.9 (3.7) | 1,454 | 25.2 (5.2) | ||||||
| Maternal Height (cm) | 1,457 | 157.8 (5.9) | 1,457 | 159.4 (6.0) | 1,457 | 163.4 (6.4) | ||||||
| Maternal Prenatal Weight (kg) | 1,449 | 58.1 (9.6) | 1,454 | 60.8 (10.4) | 1,454 | 67.4 (14.8) | ||||||
| Gestation Weight Measured | 1,449 | 1,454 | 1,454 | |||||||||
| <20 Weeks | 960 | 66.3 | 999 | 68.7 | 1365 | 93.9 | ||||||
| 20-24 Weeks | 273 | 18.8 | 352 | 24.2 | 40 | 2.8 | ||||||
| 25-29 Weeks | 137 | 9.5 | 62 | 4.3 | 20 | 1.4 | ||||||
| 30≥ Weeks | 79 | 5.5 | 41 | 2.8 | 29 | 2.0 | ||||||
| Maternal Smoking During Pregnancy | 162 | 1010 | 1,447 | |||||||||
| Never Smoked | 105 | 64.8 | 316 | 31.3 | 870 | 60.1 | ||||||
| Ex-Smoker | 0 | 0 | 21 | 2.1 | 158 | 10.9 | ||||||
| Smoked Throughout Pregnancy | 57 | 35.2 | 673 | 66.6 | 419 | 29.0 | ||||||
| Year of Delivery | 1,457 | 1,957(5) | 1,457 | 1,981(6) | 1,457 | 2,005(6) | ||||||
| Maternal Age at Delivery (years) | 1,455 | 25.3 (5.2) | 1,457 | 23.2 (4.2) | 1,457 | 23.9 (5.1) | ||||||
| Parity | 1,457 | 1,457 | ||||||||||
| Primiparous | 628 | 43.1 | 899 | 61.7 | 1,382 | 94.9 | ||||||
| Multiparous | 829 | 56.9 | 558 | 38.3 | 75 | 5.1 | ||||||
| Labour Type | 1,457 | 1,457 | 1,457 | |||||||||
| Elective Caesarean Section | 18 | 1.2 | 55 | 3.8 | 62 | 4.3 | ||||||
| Induced Delivery | 282 | 19.4 | 442 | 30.3 | 467 | 32.1 | ||||||
| Spontaneous | 1,157 | 79.4 | 960 | 65.9 | 928 | 63.7 | ||||||
| Maternal Hypertensive Disorders in Pregnancy | 1,457 | 1,457 | 1,457 | |||||||||
| Gestational Hypertension | 198 | 13.6 | 340 | 23.3 | 166 | 11.4 | ||||||
| Preeclampsia | 48 | 3.3 | 76 | 5.2 | 90 | 6.2 | ||||||
| Essential Hypertension | 0 | 0.0 | 4 | 0.3 | 5 | 0.3 | ||||||
| Normotension | 1,211 | 83.1 | 1,037 | 71.2 | 1,196 | 82.1 | ||||||
| Maternal Pre-Existing or Gestational Diabetes | 1,457 | 1,457 | 1,457 | |||||||||
| Yes | 0 | 0.0 | 6 | 0.4 | 17 | 1,2 | ||||||
| No | 1,457 | 100.0 | 1,451 | 99.6 | 1,440 | 98.8 | ||||||
| Socioeconomic Deprivation Level | 1,055 | 1392 | 1,423 | |||||||||
| Low (=1) | 1,013 | 96.0 | 380 | 27.3 | 204 | 14.3 | ||||||
| Intermediate (2-3) | 42 | 4.0 | 337 | 24.2 | 531 | 37.3 | ||||||
| High (≥4) | 675 | 48.5 | 688 | 48.3 | ||||||||
| Child Sex | 1,457 | 1,457 | 1,457 | |||||||||
| Girls | 1,457 | 100.0 | 1,457 | 100.0 | 721 | 49.5 | ||||||
| Boys | 0 | 0.0 | 0 | 0.0 | 736 | 50.5 | ||||||
| Child Gestational Age (weeks) | 1,382 | 40.4 (1.7) | 1,457 | 39.6 (1.8) | 1,457 | 39.4 (1.9) | ||||||
| Child Birth Weight (grams) | 1,457 | 3,240.7 (460.2) | 1,457 | 3,198.4 (494.8) | 1,457 | 3,315.8 (562.5) | ||||||
| Child Birth Weight SD score | 1,280 | -0.04 (0.96) | 1,429 | -0.10 (1.00) | 1,426 | -0.07 (1.01) | ||||||
| Small For Gestational Age Birth | 25 | 2.0 | 39 | 2.7 | 39 | 2.7 | ||||||
| Appropriate For Gestational Age Birth | 1226 | 95.8 | 1,361 | 95.2 | 1,353 | 94.9 | ||||||
| Large For Gestational Age Birth | 29 | 2.3 | 29 | 2.0 | 34 | 2.4 | ||||||
Body mass index=weight (kg)/height (metres)2. Abbreviations: kg=kilograms; cm=centimeters; m=metres; SD=standard deviation
Birth weight and fetal growth across three generations
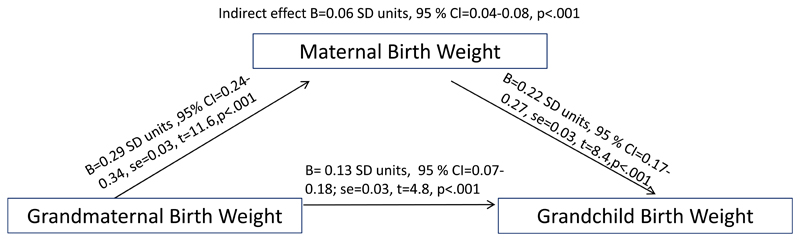
Higher grandmaternal birth weight and increased grandmaternal SD birth weight score were correlated with higher child birth weight and higher child SD birth weight score (Table 2). In linear regression analyses, these associations were independent of all the sociodemographic and prenatal covariates. Correlation coefficients and regression coefficients in models 1-2 varied between .17 and .19 SD units. Furthermore, although maternal birth weight partly explained the intergenerational continuity, evident associations remained also in the third regression models. The observed associations were also independent of maternal body size in adulthood in each generation, since the addition of maternal height and body mass index in pregnancy in models 4 did not influence the strength of the associations. In analytic models 3-4, 1 SD increases in grandmaternal birth weight and grandmaternal birth weight SD score were independently associated with .12 SD unit increases in child birth weight and birth weight SD score (Table 2). Mediation analyses shown in Figure 1 illustrates that the association between grandmaternal and child birth weight was partially mediated by maternal birth weight, and that there was also an independent association between grandmaternal and child birth weights.
Table 2.
The Associations of Grandmaternal and Child Birth Weight and Fetal Growth in the Aberdeen Maternity and Neonatal Databank-Study 1950-2015. Pearson Correlation Coefficients, Mean Group Values And Regression Coefficients And Their 95 % Confidence Intervals From Linear Regression Analyses Where All Continuous Variables Are Expressed in Standard Deviation Units.
| Univariate analyses | Model 1a | Model 2b | Model 3c | Model 4d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grandmaternal Birth Weight | No. | r= | P | B | 95 % CI | B | 95 % CI | B | 95 % CI | B | 95 % CI |
| Child Birth Weight as Measured | |||||||||||
| As Measured | 1457 | 0.19 | <0.001 | 0.18 | 0.13, 0.23 | 0.18 | 0.13, 0.23 | 0.12 | 0.07, 0.18 | 0.12 | 0.07, 0.18 |
| Adjusted for sex and gestational age | 1280 | 0.18 | <0.001 | 0.17 | 0.11, 0.22 | 0.17 | 0.11, 0.22 | 0.12 | 0.06, 0.17 | 0.12 | 0.06, 0.17 |
| Child Birth Weight Adjusted for sex and gestational age | |||||||||||
| As Measured | 1426 | 0.19 | <0.001 | 0.19 | 0.13, 0.24 | 0.18 | 0.13, 0.23 | 0.12 | 0.07, 0.18 | 0.12 | 0.07, 0.18 |
| Adjusted for sex and gestational age | 1252 | 0.18 | <0.001 | 0.18 | 0.13, 0.23 | 0.17 | 0.12, 0.23 | 0.12 | 0.07, 0.17 | 0.12 | 0.06, 0.18 |
B=unstandardized regression coefficient; CI=Confidence Interval; r=Pearson correlation coefficient; SD=Standard deviation
Regression model 1 is adjusted for child sex in the grandchild’s generation, year of delivery, maternal age at delivery, socioeconomic deprivation level of the family and parity in the grandmother’s, mother’s and child’s generation.
Model 2 includes model 1 covariates and maternal hypertensive disorders during pregnancy, pre-existing or gestational diabetes, labour type and maternal smoking during pregnancy in grandmaternal, maternal and child’s generations.
Model 3 is adjusted for model 2 covariates and also for birth weight in the intermediate generation.
Model 4 is adjusted for model 3 covariates and maternal height, body mass index and gestation of weight measurement during pregnancy.
Figure 1.
The direct and indirect effects of grandmaternal birth weight on grandchild birth weight. The figure shows the results of the mediation analyses, performed with the bootstrapping method with 5000 resamples, where maternal birth weight was examined as a mediator of the associations between grandmaternal birth weight and the birth weight of the grandchild. As estimates of the strength of the associations, we provide unstandardized regression coefficients (B) in standard deviation (SD) units, their 95 % confidence intervals (CI) and standard errors (se) and p-values of direct and indirect effects from the mediation analyses. All continuous variables are expressed in SD units. The predictive power of the whole regression analysis model where both grandmaternal and maternal birth weight are used as predictors of child birth weight is specified with the following estimates: F (2, 1454) =64.0, R2=8.1%, p<0.001.
Grandmaternal SGA birth predicted smaller child birth weight and lower child birth weight SDS score independently of sociodemographic and prenatal covariates, of birth weight in the intermediate generation and of maternal body size in adulthood within each generation (Table 3). Grandmaternal SGA birth was associated with between -.44 and -.66 SDs lower child birth weight and birth weight SD scores in the regression models 1-4. In contrast, grandmaternal LGA birth was not associated with child birth weight or birth weight SD score (Table 3).
Table 3.
The Associations of Grandmaternal Appropriateness of Birth Weight for Gestation and Child Birth Weight and Fetal Growth in the Aberdeen Maternity and Neonatal Databank-Study 1950-2015. Pearson Correlation Coefficients, Mean Group Values And Regression Coefficients And Their 95 % Confidence Intervals from Linear Regression Analyses Where Continuous Variables Are Expressed in Standard Deviation Units.
| Univariate analyses | Model 1a | Model 2b | Model 3c | Model 4d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grandmother’s appropriateness of birth weight for gestation | N | Mean (g) | P | B | 95 % CI | B | 95 % CI | B | 95 % CI | B | 95 % CI |
| Child Birth weight as measured | |||||||||||
| Small For Gestational Age | 25 | 2968.4 | 0.002 | -0.60 | -0.99, -0.21 | -0.64 | -1.03, -0.26 | -0.49 | -0.87, -0.11 | -0.49 | -0.88, -0.10 |
| Appropriate For Gestational Age | 1226 | 3320.1 | Referent | ||||||||
| Large for Gestational age | 29 | 3454.3 | 0.20 | 0.30 | -0.07, 0.66 | 0.30 | -0.06, 0.66 | 0.13 | -0.23, 0.48 | 0.06 | -0.29, 0.41 |
| Child Birth Weight Adjusted for Sex and Gestational Age | |||||||||||
| N | Mean (SD:s) | P | B | 95 % CI | B | 95 % CI | B | 95 % CI | B | 95 % CI | |
| Small For Gestational Age | 25 | -0.72 | 0.001 | -0.66 | -1.06, -0.27 | -0.66 | -1.05, -0.27 | -0.49 | -0.88, -0.11 | -0.44 | -0.83, -0.04 |
| Appropriate For Gestational Age | 1198 | -0.06 | Referent | ||||||||
| Large for Gestational Age | 29 | 0.14 | 0.27 | 0.27 | -0.10, 0.64 | 0.29 | -0.07, 0.65 | 0.11 | -0.24, 0.46 | 0.06 | -0.29, 0.41 |
B=unstandardized regression coefficient; CI=Confidence Interval; g=grams SD=Standard deviation
Regression model 1 is adjusted for child sex in the grandchild’s generation, year of delivery, maternal age at delivery, socioeconomic deprivation level of the family and parity in the grandmother’s, mother’s and child’s generation.
Model 2 includes model 1 covariates and maternal hypertensive disorders during pregnancy, pre-existing or gestational diabetes, labour type and maternal smoking during pregnancy in grandmaternal, maternal and child’s generations.
Model 3 is adjusted for model 2 covariates and also for birth weight in the intermediate generation.
Model 4 is adjusted for model 3 covariates and maternal height, body mass index during pregnancy and gestation of weight measurement during pregnancy.
Birth weight and fetal growth across two generations
Web Table 2 and Web Table 3 show that both across the first and second and second and third generations of the sample, higher maternal birth weight and birth weight SD score predicted higher child birth weight and larger child birth weight SD score, independently of the assessed covariates. Maternal SGA birth also independently predicted higher child birth weight and birth weight SD score, and maternal LGA birth predicted higher child birth weight and/or higher child SD birth weight score.
Discussion
In this longitudinal study including births among three generations over 60 years, birth weight and birth weight adjusted for sex and gestational age, indexing fetal growth, showed intergenerational transmission from grandmother to grandchild. This intergenerational continuity of birth weight and fetal growth was independent of sociodemographic and perinatal factors in each generation as well as maternal body size in adulthood and cardiometabolic health during pregnancy. Although partial mediation was evident, higher grandmaternal birth weight predicted higher grandchild birth weight also independently of birth weight in the intermediate generation. Corresponding intergenerational continuity was found for birth weight and fetal growth assessed linearly and for suboptimal fetal growth as indexed by SGA birth.
A novel key finding of our study was that grandmaternal birth weight predicted the birth weight and fetal growth of the grandchildren, independently of perinatal and sociodemographic covariates and of birth weight in the intermediate generation. The previous study(20) in a relatively larger sample where the grandparents were born 1915-1929 showed more modest (correlation coefficients .13 compared to .18-.19 in our sample) intergenerational continuity from grandmaternal to grandchild birth weight. They suggested that this continuity was mostly due to fetal or maternal genetic factors, but sociodemographic and maternal behavioral factors during pregnancy partially mediated the association(20). In contrast, in our study with a rich dataset including multiple sociodemographic and perinatal confounders affecting fetal growth, we could show that the strength of the associations remained unchanged after adjusting for sociodemographic and perinatal factors and maternal cardiometabolic health during pregnancy. While differences in the birth years of the studied cohorts and in their sociodemographic characteristics may explain the partial differences in findings between the studies, both studies showed that grandmaternal birth weight independently predicts grandchild birth weight. Furthermore, while the earlier study lacked birth weight data in the intermediate generation(20), we found that the association between grandmaternal and grandchild birth weight was only partially mediated by birth weight in the intermediate generation, and that an independent association across three generations was also present.
As another novel finding, we demonstrated that suboptimal fetal growth is also transmitted across three generations. Namely, grandmaternal SGA birth predicted smaller birth weight and slower fetal growth within the grandchildren. In contrast, LGA birth showed no significant associations across three generations. It may be that with the increasing obesity rates(31,32), the factors underlying LGA births have changed across time more than those underlying SGA birth, contributing to the higher intergenerational transmission of SGA births. Notably, the observed associations of grandmaternal SGA birth with grandchild birth size were independent of all the assessed covariates. Further, the strength of these intergenerational associations of suboptimal fetal growth were marked; grandmaternal SGA births predicted 0.4-0.7 SD units lower birth weights and slower fetal growth in the grandchildren. This suggests that grandmaternal SGA birth is among the key risk factors for suboptimal fetal growth within the grandchild’s generation.
On the other hand, our findings of .2-.3-sized correlations of birth weight and fetal growth in mothers and children correspond with previous findings from multiple studies, including two studies using the AMND dataset(4,6–10,12,14–17). Also corresponding with previous findings(7,9,18,19), both SGA and LGA births predicted individual differences in fetal growth and birth weight within the next generation and these associations were of similar or larger magnitude to those reported in previous studies (7,9,18,19).
The underlying mechanisms for the intergenerational transmission of birth weight and fetal growth may include genetic and epigenetic inheritance. According to twin studies, genome-wide and epigenome-wide association studies, hereditary vulnerabilities, certain single nucleotide polymorphisms and epigenetic DNA methylation and gene expression changes each predict individual differences in birth weight(21,33–37). Yet, other things being equal, genetic transmission should lead to estimated transmission from grandparent to grandchild of approximately 25% of the transmission from parent to child, while the associations we found across three generations were of higher magnitude, approximately 65-75% of the two-generation correlations. Although maternal cardiometabolic health, smoking during pregnancy and other sociodemographic and perinatal covariates showed expected associations with offspring birth weight and fetal growth, they did not explain the intergenerational continuity of birth weight and fetal growth. Yet, considering that grandmaternal birth weight predicted grandchild birth weight independently of birth weight in the intermediate generation, one may speculate whether epigenetic inheritance plays a role in explaining the relatively high intergenerational transmission across three generations. Do some environmental factors occurring during the pregnancy in the first generation lead to changes in gene expression that become stable and are then reflected in birth size across generations? Further studies are needed to explore these potential epigenetic and genetic mechanisms.
The strengths of our study include the large and geographically representative study sample, the extensive data on perinatal and sociodemographic covariates and the reliably coded data on perinatal characteristics. Our longitudinal study has one of the longest follow-ups in the field and thus enabled, to our knowledge, for the first time thorough examination of transmission of birth weight across three generations. The limitations of the study include having no paternal or grandpaternal birth weight data or data on maternal nutrition and psychological well-being during pregnancy, which are known to influence birth outcome(38,39). Although previous evidence does suggest that birth weight is more evidently transmitted across generations through the maternal than paternal line of heritage(11,20), information on paternal birth size would have enabled us to more precisely assess the contributory factors to the intergenerational transmission of birth weight. Information on maternal nutrition and psychological well-being would have added further information on the potential mechanistic pathways. Furthermore, data on maternal smoking during pregnancy was only available from year 1965 onwards. This important confounder affecting birth weight was thus incomprehensively assessed in our cohort. We focused our analyses on first pregnancies of the mothers in the dataset, which led to overrepresentation of primiparous pregnancies in our sample. This also led to the mean maternal age at delivery being slightly younger than for the whole Scottish population(40), which was exacerbated by the length of follow up, spanning births over 66 years rather than throughout the reproductive cycle of mothers within each generation. Maternal age at delivery is also associated with family’s socioeconomic position(41), and parity, maternal age and socioeconomic position each predict individual differences in birth weight(41). Hence, the overrepresentation of younger mothers may limit the generalizability of the findings, and further studies should assess whether corresponding findings emerge in more multiparous samples, among older mothers and among individuals from different socioeconomic backgrounds. Future studies should also study the intergenerational transmission of other birth size measures such as length, ponderal index, and head circumference at birth and gestation length.
To conclude, our findings highlighted the marked intergenerational continuity of birth weight and fetal growth across three generations. This transmission occurred independently of maternal, sociodemographic and perinatal characteristics and suggests that the developmental origins of fetal growth, and hence later life health and disease, are evident already generations earlier.
Supplementary Material
Acknowledgements
Funding and acknowledgements: The study was funded by grants from Tommy’s, the British Heart Foundation and the Medical Research Council (MR/N022556/1), the Academy of Finland and University of Helsinki. We especially want to acknowledge our appreciation for all the support of Tommy’s.
Abbreviations
- AMND
Aberdeen Maternity and Neonatal Databank
- LGA
large for gestational age
- SD
standard deviation
- SGA
small for gestational age
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Contributor Information
Marius Lahti-Pulkkinen, University/British Heart Foundation Centre for Cardiovascular Science, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, United Kingdom; Tommy’s Centre for Maternal and Fetal Health, Medical Research Unit Centre for Reproductive Health, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, Scotland, UK; Department of Psychology and Logopedics, University of Helsinki, Helsinki, Finland.
Sohinee Bhattacharya, Obstetric Epidemiology, Division of Applied Health Sciences, Dugald Baird Centre for Research on Women's Health, University of Aberdeen, Aberdeen, UK.
Katri Räikkönen, Department of Psychology and Logopedics, University of Helsinki, Helsinki, Finland.
Clive Osmond, Medical Research Council Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom.
Jane E Norman, Tommy’s Centre for Maternal and Fetal Health, Medical Research Unit Centre for Reproductive Health, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, Scotland, UK.
Rebecca M Reynolds, University/British Heart Foundation Centre for Cardiovascular Science, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, United Kingdom; Tommy’s Centre for Maternal and Fetal Health, Medical Research Unit Centre for Reproductive Health, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, Scotland, UK.
References
- 1.Barker DJP, Osmond C, Forsén TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 2.Class QA, Rickert ME, Lichtenstein P, et al. Birth weight, physical morbidity, and mortality: A population-based sibling-comparison study. Am J Epidemiol. 2014;179(5):550–558. doi: 10.1093/aje/kwt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S-F, Shu L, Sheng J, et al. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J Dev Orig Health Dis. 2014;5(6):408–19. doi: 10.1017/S2040174414000440. [DOI] [PubMed] [Google Scholar]
- 4.Agnihotri B, Antonisamy B, Priya G, et al. Europe PMC Funders Group Intergenerational Study of Trends in Human Birth Weight across Two Successive Generations. Indian J Pediatr. 2009;75(39):111–117. doi: 10.1007/s12098-008-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bladh M, Josefsson A, Carstensen J, et al. Intergenerational cohort study of preterm and small-for-gestational-age birth in twins and singletons. Twin Res Hum Genet. 2015;18(5):581–590. doi: 10.1017/thg.2015.60. [DOI] [PubMed] [Google Scholar]
- 6.Carr-Hill R, Campbell DM, Hall MH, et al. Is birth weight determined genetically? Br Med J. 1987;295(6600):687–689. doi: 10.1136/bmj.295.6600.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrillio SM, Rankin KM, David RJ, et al. Small-for-Gestational Age and Preterm Birth Across Generations: A Population-Based Study of Illinois Births. Matern Child Health J. 2014;18(10):2456–2464. doi: 10.1007/s10995-014-1484-1. [DOI] [PubMed] [Google Scholar]
- 8.Collins JWJ, David RJ, Prachand NG, et al. Low birth weight across generations. Matern Child Health J. 2003;7(4):229–237. doi: 10.1023/a:1027371501476. [DOI] [PubMed] [Google Scholar]
- 9.Cnattingius S, Villamor E, Lagerros YT, et al. High birth weight and obesity—a vicious circle across generations. Int J Obes. 2012;36(10):1320–1324. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 10.Horta BL, Gigante DP, Osmond C, et al. Intergenerational effect of weight gain in childhood on offspring birthweight. Int J Epidemiol. 2009;38(3):724–732. doi: 10.1093/ije/dyp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyppönen E, Power C. An intergenerational study of birthweight: Investigating the birth order effect. BJOG An Int J Obstet Gynaecol. 2004;111(4):377–379. doi: 10.1111/j.1471-0528.2004.00089.x. [DOI] [PubMed] [Google Scholar]
- 12.Hyppönen E, Power C, Smith GD. Parental growth at different life stages and offspring birthweight: An intergenerational cohort study. Paediatr Perinat Epidemiol. 2004;18(3):168–177. doi: 10.1111/j.1365-3016.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff MA, Schulsinger C, Mednick BR, et al. Preterm and small-for-gestational-age birth across generations. Am J Obstet Gynecol. 1997;176(3):521–526. doi: 10.1016/s0002-9378(97)70540-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuzawa CW, Eisenberg DTA. Intergenerational predictors of birth weight in the philippines: Correlations with mother’s and father’s birth weight and test of maternal constraint. PLoS One. 2012;7(7):1–9. doi: 10.1371/journal.pone.0040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarron P, Smith GD, Hattersley aT, et al. Type 2 diabetes in grandparents and birth weight in offspring and grandchildren in the ALSPAC study. J Epidemiol Community Health. 2004;58(6):517–522. doi: 10.1136/jech.2003.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton SMB, De Stavola BL, Leon DA. Intergenerational determinants of offspring size at birth: A life course and graphical analysis using the aberdeen children of the 1950s study (ACONF) Int J Epidemiol. 2014;43(3):749–759. doi: 10.1093/ije/dyu028. [DOI] [PubMed] [Google Scholar]
- 17.Ogonowski J, Miazgowski T. Intergenerational transmission of macrosomia in women with gestational diabetes and normal glucose tolerance. Eur J Obstet Gynecol Reprod Biol. 2015;195:113–116. doi: 10.1016/j.ejogrb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Selling KE, Carstensen J, Finnstrom O, et al. Intergenerational effects of preterm birth and reduced intrauterine growth: A population-based study of Swedish mother-offspring pairs. BJOG An Int J Obstet Gynaecol. 2006;113(4):430–440. doi: 10.1111/j.1471-0528.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Winkvist A, Mogren I, Högberg U. Familial patterns in birth characteristics: Impact on individual and population risks. Int J Epidemiol. 1998;27(2):248–254. doi: 10.1093/ije/27.2.248. [DOI] [PubMed] [Google Scholar]
- 20.De Stavola BL, Leon DA, Koupil I. Intergenerational correlations in size at birth and the contribution of environmental factors. Am J Epidemiol. 2011;174(1):52–62. doi: 10.1093/aje/kwr032. [DOI] [PubMed] [Google Scholar]
- 21.Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538(7624):248–252. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake AJ, Walker BR. The intergenerational effects of fetal programming: Non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180(1):1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 23.Conley D, Bennett NG. Birth weight and income: interactions across generations. J Health Soc Behav. 2001;42(4):450–465. [PubMed] [Google Scholar]
- 24.Ogonowski J, Miazgowski T, Engel K, et al. Birth weight predicts the risk of gestational diabetes mellitus and pregravid obesity. Nutrition. 2014;30(1):39–43. doi: 10.1016/j.nut.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 25.He X-J, Qin F-Y, Hu C-L, et al. Is gestational diabetes mellitus an independent risk factor for macrosomia: a meta-analysis? Arch Gynecol Obstet. 2015;291(4):729–735. doi: 10.1007/s00404-014-3545-5. [DOI] [PubMed] [Google Scholar]
- 26.Knopik VS, Marceau K, Palmer RHC, et al. Maternal Smoking During Pregnancy and Offspring Birth Weight: A Genetically-Informed Approach Comparing Multiple Raters. Behav Genet. 2016;46(3):353–364. doi: 10.1007/s10519-015-9750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayorinde AA, Wilde K, Lemon J, et al. Data Resource Profile: The Aberdeen Maternity and Neonatal Databank (AMND) Int J Epidemiol. 2016;45(2):389–394. doi: 10.1093/ije/dyv356. [DOI] [PubMed] [Google Scholar]
- 28.Carr-Hilll RA, Pritchard CW. Reviewing birthweight standards. Br J Obstet Gynaecol. 1983;90:718–725. doi: 10.1111/j.1471-0528.1983.tb09301.x. [DOI] [PubMed] [Google Scholar]
- 29.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 30.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67:451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 31.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Baumbach J, Vandin F, et al. Differentially Methylated Genomic Regions in Birth-Weight Discordant Twin Pairs. Ann Hum Genet. 2016;80(2):81–87. doi: 10.1111/ahg.12146. [DOI] [PubMed] [Google Scholar]
- 34.Damcott CM. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Diabetes. 2013;62(8):2994. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubois L, Ohm Kyvik K, Girard M, et al. Genetic and environmental contributions to weight, height, and bmi from birth to 19 years of age: An international study of over 12,000 twin pairs. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel SM, Joubert BR, Wu MC, et al. Neonatal genome-wide methylation patterns in relation to birth weight in the norwegian mother and child cohort. Am J Epidemiol. 2014;179(7):834–842. doi: 10.1093/aje/kwt433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai PC, Van Dongen J, Tan Q, et al. DNA Methylation Changes in the IGF1R Gene in Birth Weight Discordant Adult Monozygotic Twins. Twin Res Hum Genet. 2015;18(6):1–12. doi: 10.1017/thg.2015.76. [DOI] [PubMed] [Google Scholar]
- 38.Jarde A, Morais M, Kingston D, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2016;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- 39.Stevens B, Buettner P, Watt K, et al. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: A systematic review and meta-analysis. Matern Child Nutr. 2015;11(4):415–432. doi: 10.1111/mcn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NHS National Services Scotland: Information Services Division. Births in Scottish Hospitals. Year ending 31 March 2016. [Accessed 3th October 2017];2016 Online Publication. http://www.isdscotland.org/Health-Topics/Maternity-and-Births/Publications/2016-11-29/2016-11-29-Births-Report.pdf.
- 41.Fall CHD, Sachdev HS, Osmond C, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: A prospective study in five low-income and middle-income countries (COHORTS collaboration) Lancet Glob Heal. 2015;3(7):e366–e377. doi: 10.1016/S2214-109X(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.