Abstract
Background
Oxidative stress occurs in white adipose tissue and dysregulates the expression of adipokines secreted from adipocytes. Since adipokines influence inflammation, supplementation with antioxidants might be beneficial for preventing oxidative stress-mediated inflammation in adipocytes and inflammation-associated complications. β-Carotene is the most prominent antioxidant carotenoid and scavenges reactive oxygen species in various tissues. The purpose of this study was to determine whether β-carotene regulates the expression of adipokines, such as adiponectin, monocyte chemoattractant protein-1 (MCP-1), and regulated on activation, normal T cell expressed and secreted (RANTES) in 3T3-L1 adipocytes treated with glucose/glucose oxidase (G/GO).
Methods
3T3-L1 adipocytes were cultured with or without β-carotene and treated with G/GO, which produces H2O2. mRNA and protein levels in the medium were determined by a real-time PCR and an ELISA. DNA binding activities of transcription factors were assessed using an electrophoretic mobility shift assay.
Results
G/GO treatment increased DNA binding affinities of redox-sensitive transcription factors, such as NF-κB, activator protein-1 (AP-1), and STAT3. G/GO treatment reduced the expression of adiponectin and increased the expression of MCP-1 and RANTES. G/GO-induced activations of NF-κB, AP-1, and STAT3 were inhibited by β-carotene. G/GO-induced dysregulation of adiponectin, MCP-1, and RANTES were significantly recovered by treatment with β-carotene.
Conclusions
β-Carotene inhibits oxidative stress-induced inflammation by suppressing pro-inflammatory adipokines MCP-1 and RANTES, and by enhancing adiponectin in adipocytes. β-Carotene may be beneficial for preventing oxidative stress-mediated inflammation, which is related to adipokine dysfunction.
Keywords: Adipocytes, Adipokines, Beta carotene, Oxidative stress
INTRODUCTION
Reactive oxygen species (ROS) act as signaling mediators invloved in various diseases.1 Elevated production of ROS activates inflammatory pathways via modulating the expression of cytokines.2 ROS-mediated cytokine production is regulated by the activation of redox-sensitive transcription factors, such as NF-κB, activator protein-1 (AP-1), and STAT3,3–6 which orchestrates and amplifies inflammatory signaling. Antioxidants can inhibit this inflammatory process by reducing excess ROS, and thereby alleviate disease complications.7 Therefore, a significant effort has been made to find potent and feasible antioxidants.
In addition to its traditional role as an energy storage site, white adipose tissue actively participates in inflammation by producing and secreting a variety of signaling molecules, collectively called adipokines.8 Dysregulated production and/or release of the adipokines due to dysfunctional adipocytes may lead to inflammation-associated conditions.9 Pro-inflammatory adipokines include monocyte chemoattractant protein-1 (MCP-1), which recruits monocytes into adipocytes,10 and regulated on activation, normal T cell expressed and secreted (RANTES), which has chemotactic activity for various subsets of leukocytes.11 Adiponectin, an adipocyte-derived anti-inflammatory factor, promotes macrophage polarization toward an anti-inflammatory phenotype,12 and reduces cytokine expression from macrophages.13 In studies using adipose tissue from obese mice and 3T3-L1 adipocytes, a high level of ROS resulted in increased expression of MCP-1, and decreased expression of adiponectin,14,15 suggesting a differential role of ROS in the expression of adipokines. Inhibition of ROS production in the adipose tissues of obese mice attenuated the dysregulation of adipokines and improved the expression of markers for diabetes and hyperlipidemia.15 Therefore, the use of adipose tissue-targeting antioxidants may be a reasonable therapeutic approach for improving complications derived from dysfunctional adipocytes. AP-1, NF-κB, and STAT3 are the main transcription factors that modulate the expression of a number of adipokines; increasing MCP-1 and RANTES, but decreasing adiponectin.16,17 Therefore, it is necessary to determine the role of these redox-sensitive transcription factors on the expression of adiponectin, MCP-1, and RANTES in adipocytes.
β-Carotene is the major carotenoid present in various tissues including adipose tissue.18 Studies have indicated that β-carotene is involved in adipose tissue processes, such as adipogenesis, oxidative stress, and the regulation of adipose tissue-derived signaling pathways.19 Moreover, the anti-inflammatory properties of β-carotene have been demonstrated in different contexts and cell types.20 Previously, we reported that β-carotene inhibits the activation of mitogen-activated protein kinases, NF-κB, and AP-1 in gastric epithelial AGS cells infected with Helicobacter pylori.21 Therefore, β-carotene may inhibit ROS-induced pro-inflammatory signaling in 3T3-L1 adipocytes. In the present study, we examined whether β-carotene attenuates ROS-induced activation of transcription factors, such as NF-κB, AP-1, and STAT3 in 3T3-L1 adipocytes. Furthermore, we determined the effect of β-carotene on the dysregulated expression of adipokines including adiponectin, MCP-1, and RANTES in adipocytes.
MATERIALS AND METHODS
1. Cell culture
The 3T3-L1 preadipocytes were kindly provided by Dr. Jae Woo Kim at Yonsei University College of Medicine (Seoul, Korea). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Sigma, St. Louis, MO, USA). The cells were incubated in a CO2 incubator at 37°C. To induce adipocyte differentiation, 3T3-L1 preadipocytes were further cultured in DMEM supplemented with 10% (v/v) FBS (Gibco, Grand Island, NY, USA), 520 μM isobutylmethylxanthine, 1 μM dexamethasone, and 167 nM insulin (Sigma). On day 2, the medium was changed to medium that contained 10% FBS and 167 nM insulin. On day 4, the medium was again replaced with DMEM that contained only 10% FBS, which was subsequently changed every 2 days until the cell were used for experiments.
2. Experimental protocol
To induce oxidative stress, differentiated 3T3-L1 cells were treated with β-D-glucose (10 mM) and glucose oxidase (10 mU/mL) (G/GO; Sigma) which produces H2O2.22 To examine the anti-inflammatory effect of β-carotene (prepared in tetrahydrofuran), the 3T3-L1 cells were pretreated with β-carotene at a final concentration of 5 μM or 10 μM for 2 hours before the G/GO treatment.
3. Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed as previously described.23 Briefly, nuclear proteins were isolated, and incubated with [32P]dATP-labeled gel shift oligonucleotides: NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Promega, Madison, WI, USA), AP-1 (5′-CGCTTGATAGTCAGCCGGAA-3′; Promega), and STAT3 (5′-GATCCTTCTGGGAATTCCTAGATC-3′; Santa Cruz Biotechnology, Dallas, TX, USA). Samples were subjected to electrophoretic separation on a nondenaturing 5% acrylamide gel. Then gels were dried at 80°C for 1 hour and exposed to radiography film for 6 to 18 hours at −70°C with intensifying screens.
4. Quantitative real-time PCR
Total RNA was extracted from cells using the TRI reagent (Sigma). RNA were converted into cDNA using a Primescript RT Master Mix Kit (Takara Bio, Shiga, Japan). The cDNA was used as a template for real-time PCR using a SYBR Green Real-time PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan). β-Actin was used as a housekeeping gene for normalization. The primers used in PCR were as follows: adiponectin, forward 5′-CCCAAGGGAACTTGTGCAGGT TGGATG -3′ and reverse 5′-GTTGGTATCATGGTAGAGAAGAA AGCC -3′ (634-bp product); MCP-1, forward 5′-TGATCCCAATGAG TAGGCTGGAG -3′ and reverse 5′-ATGTCTGGACCCATTCCTTCT TG -3′ (132-bp product); RANTES, forward 5′-GCCCACGTCAAGG AGTATTTC -3′ and reverse 5′-AACCCACTTCTTCTCTGGGTTG -3′ (112-bp product); β-actin, forward 5′-GTGCTGTCCCTGTATGC CTCTG-3′ and reverse 5′-AACCGCTCGTTGCCAATAGTG-3′ (350-bp product).
5. ELISA
The levels of MCP-1, RANTES, and adiponectin in the cell culture media were determined using an ELISA kit (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instruction.
6. Statistical analysis
All values are expressed as mean ± SEM of three independent experiments. Statistical differences were determined by one-way ANOVA, followed by Newman-Keul’s post hoc test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
1. Effect of glucose/glucose oxidase treatment on the activations of redox-sensitive transcription factors and the expressions of adipokines in 3T3-L1 cells
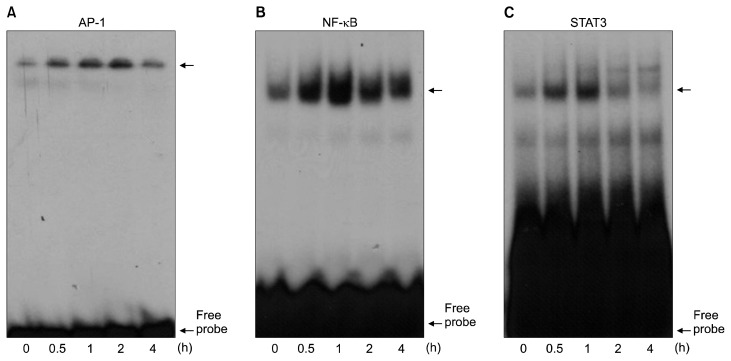
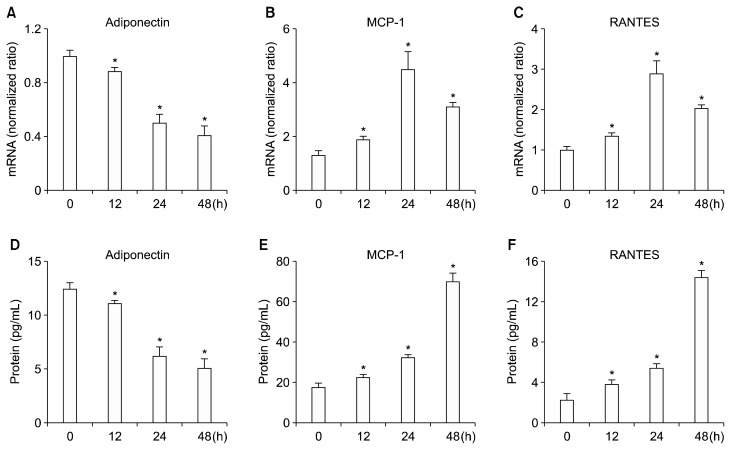
To examine the effect of G/GO treatment on the activations of redox-sensitive and pro-inflammatory transcription factors, AP-1, NF-κB, and STAT3, we treated 3T3-L1 cells with G/GO for 0.5, 1, 2, and 4 hours, and conducted an EMSA to determine their DNA binding affinities. The binding affinity of AP-1, NF-κB, and STAT3 to DNA increased with time and peaked at 1 hour after G/GO treatment (Fig. 1). These results suggest that oxidative stress, induced by G/GO treatment, activated transcription factors AP-1, NF-κB, and STAT3. Additionally, the mRNA expression of adiponectin was found to be reduced following G/GO treatment (~50% for the 24 hours culture) (Fig. 2A). On the other hand, mRNA expression of MCP-1 and RANTES increased following G/GO treatment (Fig. 2B and 2C). Specifically, for the 24-hour culture, mRNA expression levels of MCP-1 and RANTES were about 4.5-fold and 3-fold higher than the levels at 0 hour, respectively. The secreted protein levels, determined by ELISA, were consistent with their mRNA levels. Following G/GO treatment, adiponectin levels in the media were less (~40% for the 48-hour culture) than the levels at 0 hour (Fig. 2D). Conversely, MCP-1 and RANTES levels in the media were markedly increased (~3.5-fold increase for MCP-1; ~5-fold increase for RANTES for the 48-hour culture) (Fig. 2E and 2F). Collectively, these data indicate that G/GO-mediated H2O2 production activates pro- inflammatory transcription factors, AP-1, NF-κB, and STAT3, which further induces the dysregulated expression of adipokines, such as adiponectin, MCP-1, and RANTES in 3T3-L1 adipocytes.
Figure 1.
The effect of glucose/glucose oxidase (G/GO) treatment on the DNA binding activities of activator protein-1 (AP-1), NF-κB, and STAT3 in 3T3-L1 adipocytes. Cells were treated with G/GO for the indicated time periods, and then nuclear proteins were isolated. DNA binding activities of (A) AP-1, (B) NF-κB, and (C) STAT3 were determined using electrophoretic mobility shift assay.
Figure 2.
The effect of glucose/glucose oxidase (G/GO) treatment on the expressions of adiponectin, monocyte chemoattractant protein-1 (MCP-1), and regulated on activation, normal T cell expressed and secreted (RANTES) in 3T3-L1 adipocytes. Cells were treated with G/GO for the indicated time periods. mRNA expression of (A) adiponectin, (B) MCP-1, and (C) RANTES were determined by real-time PCR. β-Actin was used as a housekeeping gene for normalization. Protein levels in cell culture media for (D) adiponectin, (E) MCP-1, and (F) RANTES were measured using ELISA. *P < 0.05 vs. 0 hour.
2. Effect of β-carotene on the activations of redox-sensitive transcription factors and the expressions of adipokines in glucose/glucose oxidase-treated 3T3-L1 cells
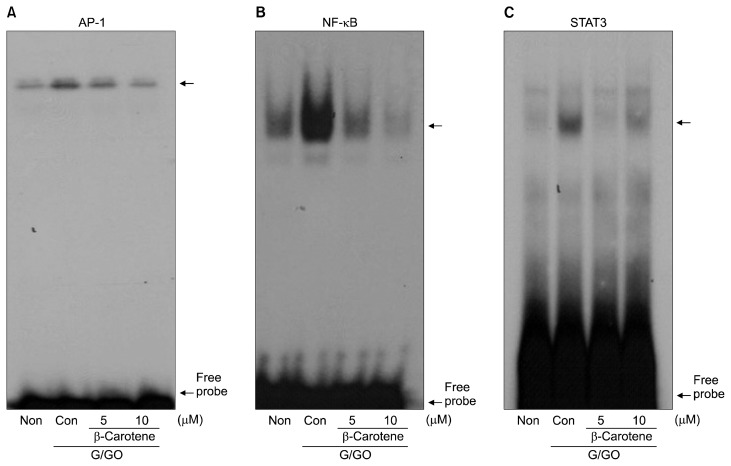
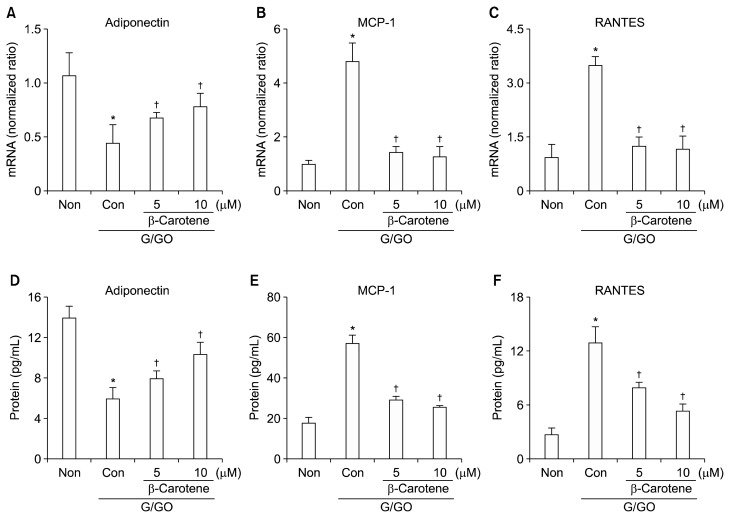
To determine the effect of β-carotene on inflammatory markers, adipocytes were pretreated with β-carotene for 2 hours before G/GO treatment. The G/GO-induced increase in DNA binding activities of AP-1, NF-κB, and STAT3 were markedly attenuated by β-carotene treatment (Fig. 3). Reduced adiponectin expression by G/GO treatment was significantly recovered by β-carotene both at the mRNA (Fig. 4A) and protein levels (Fig. 4D) in a dose-dependent manner. β-Carotene inhibited G/GO- induced induction of MCP-1 and RANTES dose-dependently (Fig. 4B, 4C, 4E, and 4F). Collectively, these results demonstrate that the anti-inflammatory effect of β-carotene against ROS-mediated inflammation is facilitated by suppressing the activation of AP-1, NF-κB, and STAT3 in 3T3-L1 adipocytes.
Figure 3.
The effect of β-carotene on the DNA binding activities of activator protein-1 (AP-1), NF-κB, and STAT3 in glucose/glucose oxidase (G/GO)-treated 3T3-L1 adipocytes. Cells were pretreated with β-carotene at a final concentration of 5 and 10 μM. After 2 hours, cells were treated with G/GO for 1 hour, and then nuclear proteins were isolated. DNA binding activities of (A) AP-1, (B) NF-κB, and (C) STAT3 were determined using electrophoretic mobility shift assay.
Figure 4.
The effect of β-carotene on the expressions of adiponectin, monocyte chemoattractant protein-1 (MCP-1), and regulated on activation, normal T cell expressed and secreted (RANTES) in glucose/glucose oxidase (G/GO)-treated 3T3-L1 adipocytes. Cells were pretreated with β-carotene at a final concentration of 5 and 10 μM for 2 hours, followed by G/GO treatment for 24 hours (mRNA level) and 48 hours (protein level). mRNA expression of (A) adiponectin, (B) MCP-1, and (C) RANTES were determined by real-time PCR. β-Actin was used as a housekeeping gene for normalization. Protein levels in cell culture media for (D) adiponectin, (E) MCP-1, and (F) RANTES were measured using ELISA. Non, none (untreated cells); Con, control (G/GO-treated cells without β-carotene treatment). *P < 0.05 vs. Non, †P < 0.05 vs. Con.
DISCUSSION
Increased oxidative stress in the white adipose tissue is linked to the pathogenesis of obesity-related disorders.15 In part, this event is mediated by adipokines, which are produced and released by adipocytes. High level of oxidative stress generated by adipose tissue lead to the expression of pro-inflammatory adipokines, such as MCP-1 and RANTES via the activations of transcription factors, such as NF-κB, AP-1, and STAT3, which recruit immune cells and worsen local inflammation.6 Anti- inflammatory adipokine adiponection is inversely related to the activation of these redox-sensitive transcription factors.24,25 Therefore, suppression of adipocyte-derived oxidative stress and/or inflammation has been considered a therapeutic target for preventing inflammatory complications including diabetes and obesity-related disorders.15
In the present study, we demonstrated that the anti-inflammatory action of β-carotene in 3T3-L1 adipocytes. Similar to our results, lycopene, a carotenoid with antioxidant properties, suppressed TNF-α-induced inflammation in 3T3-L1 cells via modulating the NF-κB pathway.26 The anti-inflammatory effect of the carotenoids may be attributed to its antioxidant effect, as proven in numerous in vitro and in vivo models.27 As some carotenoids containing electrophilic groups have been suggested to directly interact with the cysteine residues of NF-κB subunits (p65) to inhibit their activation,28 there may be a direct effect on inflammatory mediators besides scavenging ROS.
Clinical studies have shown that a higher dietary consumption of carotenoids is associated with lower levels of inflammation in relation to obesity.29 Moreover, a recent study reported that the concentration of β-carotene was significantly lower in the adipocytes of obese groups than in the adipocytes of non-obese groups.30 Thus, it may be important that there is a sufficient intake of β-carotene through food or supplementation for the obese population to prevent adverse health consequences.
In the present study, the differentiated 3T3-L1 cells were exposed to β-D-glucose (G; 10 mM) and glucose oxidase (GO; 10 mU/mL). In our previous study,22 G (10 mM) produces H2O2 with the increase in amounts of GO added. G (10 mM) reacted with 1.25 mg of silica-immobilized GO to produce about 100 μM of H2O2 in gastric epithelial AGS cells. Other study31 showed that pretreatment of β-carotene (20 μM) decreased ROS levels up to 25% in AGS cells treated with H2O2 (100 μM). Furthermore, pretreatment of β-carotene (10 μM) decreased ROS levels in H. pylori-infected gastric epithelial AGS cells.21 These studies support the present results showing that β-carotene inhibits ROS-mediated activation of AP-1, NF-κB, and STAT3 in the differentiated 3T3-L1 cells. Earlier, we demonstrated that G (10 mM)/GO (5 mU/mL) induced cell death in pancreatic acinar AR42J cells at 12 hour-culture.32 Therefore, long-term treatment of G (10 mM)/GO (10 mU/mL) may induce cell death in 3T3-L1 cells. Further study should be performed to determine cell viability and ROS levels in the systems of 3T3-L1 cells treated with G/GO and/or β-carotene.
In conclusion, β-carotene inhibits oxidative stress-induced dysregulation in the expressions of adipokines (adiponectin, MCP-1, and RANTES) and the activations of redox-sensitive transcription factors (AP-1, NF-κB, and STAT3) in 3T3-L1 adipocytes. Supplementation of β-carotene may prevent and inhibit adipose tissue-derived inflammation and inflammatory complications.
ACKNOWLEDGMENTS
The authors appreciate Dr. Jae Woo Kim at Yonsei University College of Medicine (Seoul, Korea) for providing 3T3-L1 preadipocytes and technical support for adipocyte culture.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–47. 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 2.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 2011;208:417–20. 10.1084/jem.20110367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991;10: 2247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem 1995;270:11727–30. 10.1074/jbc.270.20.11727 [DOI] [PubMed] [Google Scholar]
- 5.He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 2010;17:286–97. 10.1016/j.ccr.2009.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014;24:R453–62. 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84. 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911–9;quiz 920. 10.1016/j.jaci.2005.02.023 [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Yong W, Wu X, Yu Y, Lv J, Liu C, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun 2008;369:471–7. 10.1016/j.bbrc.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 11.Madani R, Karastergiou K, Ogston NC, Miheisi N, Bhome R, Haloob N, et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am J Physiol Endocrinol Metab 2009;296:E1262–8. 10.1152/ajpendo.90511.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010;285:6153–60. 10.1074/jbc.M109.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000; 96:1723–32. [PubMed] [Google Scholar]
- 14.Kamigaki M, Sakaue S, Tsujino I, Ohira H, Ikeda D, Itoh N, et al. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2006;339:624–32. 10.1016/j.bbrc.2005.11.059 [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–61. 10.1172/JCI21625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347–55. 10.1079/BJN20041213 [DOI] [PubMed] [Google Scholar]
- 17.Ehsan M, Singh KK, Lovren F, Pan Y, Quan A, Mantella LE, et al. Adiponectin limits monocytic microparticle-induced endothelial activation by modulation of the AMPK, Akt and NFκB signaling pathways. Atherosclerosis 2016;245:1–11. 10.1016/j.atherosclerosis.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem 1990;8:1–10. [PubMed] [Google Scholar]
- 19.Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids in adipose tissue biology and obesity. Subcell Biochem 2016;79:377–414. 10.1007/978-3-319-39126-7_15 [DOI] [PubMed] [Google Scholar]
- 20.Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34:907–29. 10.1016/j.nutres.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 21.Jang SH, Lim JW, Kim H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cy-clooxygenase-2 in human gastric epithelial AGS cells. J Physiol Pharmacol 2009;60 Suppl 7:131–7. [PubMed] [Google Scholar]
- 22.Yu JH, Kang SG, Jung UY, Jun CH, Kim H. Effects of omega-3 fatty acids on apoptosis of human gastric epithelial cells exposed to silica-immobilized glucose oxidase. Ann N Y Acad Sci 2009; 1171:359–64. 10.1111/j.1749-6632.2009.04703.x [DOI] [PubMed] [Google Scholar]
- 23.Kim MH, Kim A, Yu JH, Lim JW, Kim H. Glutamine deprivation induces interleukin-8 expression in ataxia telangiectasia fibroblasts. Inflamm Res 2014;63:347–56. 10.1007/s00011-013-0706-0 [DOI] [PubMed] [Google Scholar]
- 24.Fang F, Bae EH, Hu A, Liu GC, Zhou X, Williams V, et al. Deletion of the gene for adiponectin accelerates diabetic nephropathy in the Ins2 (+/C96Y) mouse. Diabetologia 2015;58:1668–78. 10.1007/s00125-015-3605-9 [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Chen Q, Pu H, Wei Q, Duan M, Zhang C, et al. Adiponectin improves NF-κB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis 2016;15:33. 10.1186/s12944-016-0202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, et al. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem 2011;22:642–8. 10.1016/j.jnutbio.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 27.Pryor WA, Stahl W, Rock CL. Beta carotene: from biochemistry to clinical trials. Nutr Rev 2000;58:39–53. 10.1111/j.1753-4887.2000.tb07810.x [DOI] [PubMed] [Google Scholar]
- 28.Linnewiel K, Ernst H, Caris-Veyrat C, Ben-Dor A, Kampf A, Salman H, et al. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med 2009;47: 659–67. 10.1016/j.freeradbiomed.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 29.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106 Suppl 3:S5–78. 10.1017/S0007114511005460 [DOI] [PubMed] [Google Scholar]
- 30.Östh M, Öst A, Kjolhede P, Strålfors P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS One 2014;9:e85610. 10.1371/journal.pone.0085610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, Seo JH, Kim H. β-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J Nutr Sci Vitaminol (Tokyo) 2011;57: 216–23. 10.3177/jnsv.57.216 [DOI] [PubMed] [Google Scholar]
- 32.Song JY, Lim JW, Kim H, Morio T, Kim KH. Oxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cells. J Biol Chem 2003; 278:36676–87. 10.1074/jbc.M303692200 [DOI] [PubMed] [Google Scholar]