SUMMARY
Calorie restriction (CR) is a dietary intervention with potential benefits for healthspan improvement and lifespan extension. In 53 (34 CR and 19 Control) non-obese adults, we tested the hypothesis that energy expenditure (EE) and its endocrine mediators are reduced with a CR diet over two-years. Approximately 15% CR was achieved over two-years resulting in an average 8.7 kg weight loss whereas controls gained 1.8 kg. In the CR group, EE measured over 24-hours or during sleep were approximately 80–120 kcal/d lower than expected on the basis of weight loss indicating sustained metabolic adaptation over two years. This metabolic adaptation was accompanied by significantly reduced thyroid axis activity and reactive oxygen species (F2 isoprostane) production. Findings from this two-year CR trial in healthy, non-obese humans provides new evidence of persistent metabolic slowing accompanied by reduced oxidative stress which support the rate of living and oxidative damage theories of mammalian aging.
eTOC Blurb

Calorie restriction (CR) has been shown to have health benefits and to extend lifespan in diverse species. XXX et al conducted a two-year CR trial in healthy, non-obese humans and found evidence that prolonged CR enhances resting energy efficiency, resulting in decreased oxidative damage to tissues and organs.
INTRODUCTION
For the past 40 years, aging research has focused on the mechanisms underlying the beneficial health impact of a sustained reduction in caloric intake below usual levels, while maintaining adequate intake of essential nutrients. Observations in a variety of laboratory animals indicate that calorie restriction (CR), beginning early or in mid-life and sustained for a substantial portion of the lifespan, increases longevity in a wide variety, but not all, species (Speakman and Mitchell, 2011). While the field of CR research eagerly awaits final lifespan data from the two remaining colonies of CR primates (Colman et al., 2009; Mattison et al., 2012), despite differences in study designs, current data support the observation that sustained CR extends life without chronic disease and promotes a more youthful physical and mental functionality (Mattison et al., 2017). In terms of CR in humans, few controlled clinical trials exist and much of what is known has been derived from observational and cross-sectional studies of individuals who are long-lived such as the centenarians who reside in Okinawa, Japan (Willcox et al., 2006) or individuals self-practicing CR who are members of the calorie restriction optimal nutrition (CRON) society (Fontana et al., 2004).
A variety of mechanisms have been proposed as mediators of the effects of CR on lifespan. Old, but arguably one of the most prevailing theories supporting lifespan extension with CR is a hybrid between two longstanding hypotheses of aging; the “rate of living” and the “oxidative damage” theories of aging. Pearl (Pearl, 1928) in 1928, proposed the idea that mammalian longevity is inversely related to their metabolic rate per unit of tissue mass, hence “rate of living”. Several decades later, Harman proposed the oxidative damage theory of aging and suggested that reactive oxygen species (ROS) – byproducts of oxidative phosphorylation in mitochondria – damage DNA, lipids and proteins, all leading to accelerated biological aging (Harman, 1956). There are data from studies in rodents (Hambly and Speakman, 2005), non-human primates (Blanc et al., 2003; Ramsey et al., 1997a) and humans (Heilbronn et al., 2006) indicating that CR results in a decrease in metabolic rate that is greater than that expected on the basis of loss of tissue mass (Heilbronn et al., 2006; McCarter and Palmer, 1992a). This phenomenon referred to as metabolic adaptation, was associated with less oxidative damage to DNA in our 6-month pilot study of CR in humans (Heilbronn et al., 2006). The CR field has also focused on the ability for CR to attenuate age-related changes in physiological and endocrine factors that are known to change with age such as core body temperature, plasma insulin, DHEAS and thyroid hormones (Roth et al., 2002b) as well as endocrine mediators of metabolic slowing such as plasma leptin (Rosenbaum et al., 2005).
Phase 1 CALERIE or the Comprehensive Assessment of the Long term Effects of Reducing Intake of Energy studies were the first randomized controlled trials to test the metabolic effects of CR in non-obese humans (Das et al., 2009; Heilbronn et al., 2006; Weiss et al., 2006). Then, the phase 2 CALERIE study, a 2-year 25% caloric restriction prescription in non-obese volunteers, was shown to be safe and without any untoward effects on quality of life (Martin et al., 2016; Rickman et al., 2011; Rochon et al., 2011; Romashkan et al., 2016). Importantly, the study confirmed the presence of a CR-induced decrease in total daily energy expenditure measured by doubly labeled water after 12 and 24 months (measured CR was 12% on average) indicating a decrease in physical activity and/or a metabolic adaptation. However, in the CR group compared to the control group, resting metabolic rate adjusted for loss of fat-free and fat masses was only lower during the weight loss phase, i.e. at 12 months of intervention (Ravussin et al., 2015) but not a year later. Furthermore, reductions in core body temperature were noted in the CR group but were not different from the controls and changes in oxidative damage were not assessed.
As an ancillary study of the multi-center CALERIE phase 2 trial, individuals studied at Pennington Biomedical Research Center underwent additional procedures to assess changes in the different components of sedentary energy expenditure measured in a metabolic chamber (more precise measure of daily energy expenditure including sleeping metabolic rate) after 1 and 2 years of CR. Importantly, we also measured changes in potential metabolic mediators and biomarkers of aging mediators such as core temperature, thyroid hormones, leptin and insulin as well as downstream effectors including lipid peroxidation and DNA damage, markers of oxidative stress. We hypothesized that after one year, energy expenditure would be lower than that expected on the basis of the loss in energetically active tissues (fat-free mass and fat mass) and that this metabolic adaptation will still be present after another year of sustained CR and weight stability as previously shown in obese individuals (Rosenbaum et al., 2008). Furthermore, following from our 6-month pilot CR study, we hypothesized a reduction in oxidative damage after 1 and 2 years of CR. Taken together, such results would speak in favor of the long-standing hypotheses of biological aging stating that prolonged CR enhances energy efficiency at rest and therefore results in less ROS production and reduced oxidative damage to tissues and organs, thus a combination of the “rate of living” and the “oxidative damage” theories of aging. To test this hypothesis, we delivered a highly controlled and intensive behavioral intervention targeting a 25% CR diet over 2 years and obtained reliable measurements of the most robust component of daily sedentary energy expenditure, i.e. energy metabolism during sleep, measured in a room calorimeter. Hormonal mediators of metabolism were measured along with urinary F2 isoprostane excretion as an index of oxidative damage.
RESULTS AND DISCUSSION
This paper summarizes the findings of the first randomized clinical trial to test whether CR in young, healthy individuals sustained over two years provides support for the rate of living and oxidative damage theories of aging.
Study subjects and throughput
Out of 80 eligible subjects, 73 consented to the ancillary study and 2 subjects did not complete the baseline metabolic chamber measurement (Figure 1). During the course of the 2-year study, 3 subjects failed to complete all 3 chamber stays (2 in the CR group and 1 in the control), 7 subjects dropped (6 in the CR group and 1 in the control), leaving a group of 60 subjects with complete data available for analysis. Based on adherence to the prescription (weight change), the final data analyses were performed on 53 subjects (36 females, mean age=40±6y), of which 34 subjects were randomized to the CR intervention and 19 subjects to the ad libitum control group. The 53 participants were objectively selected on the basis of adherence to their assigned treatment groups whereby the criteria for adherence was >5% weight loss at either Y1 or Y2 for CR participants (3 excluded) and ≤5% weight loss at either Y1 or Y2 for control participants (4 excluded). Per design, all subjects were healthy with 22 (41.5%) being normal weight and 31 (58.5%) slightly overweight at screening. The cohort was 39.8±6.3 years of age at randomization and majority were female (67.9%) and White (73.6%).
Figure 1.
Subject throughput from enrolment (N=71) to data analysis (N=53). Analyses were performed on 53 men and women who on the basis of an objective pre-analytical criterion (weight change) were determined to be adherent to their assigned treatment groups.
Prescribed Two Year CR Resulted in Sustained Weight Loss
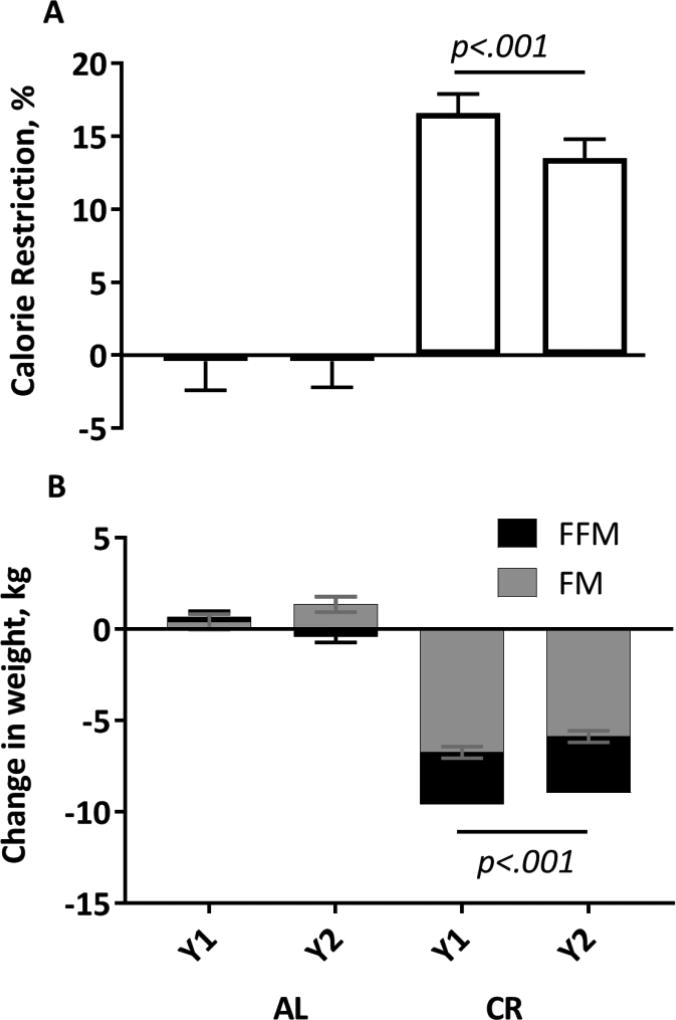
As anticipated by the study design and use of a mathematical model to guide weight loss during the CR intervention (STAR Methods), after Y1, the CR group achieved a 16.5±1.5% reduction in energy intake (or CR) from baseline with an overall 14.8±1.5% CR over the entire 2-year intervention (Figure 2A). Despite a slight tendency to gain weight, there was no change in energy intake in the Control group from baseline at either time point (Y1: −1.8±2.0, Y2: −1.7±2.0 %CR). Subjects in the CR group experienced a significant weight loss at Y1 (−9.4 ± 0.4 kg) which was maintained at Y2 (−8.7 ± 0.4 kg, Figure 2B). Subjects in the Control group essentially maintained weight during the 2-year period (Figure 2B). In the CR group, the majority of weight loss (70.7%) was fat mass (Y1: −6.7±0.3; Y2: −5.9±0.3, kg, p<.0001 from baseline, within group effect), however a significant loss in fat-free mass (Y1: −2.9±0.2; Y2: −3.1±0.2, kg, p<.0001 from baseline, within group effect) was also observed from baseline at both time points (Figure 2B).
Figure 2.
Percent of calorie restriction (A) achieved after 1 and 2 years of calorie restriction and the resulting change in fat mass (FM) and fat free mass (FFM), panel B. N=53; 34 CR, 19 Control. P-value for statistically significant treatment group effects, adjusted for multiple comparisons is shown. The change in weight, FM and FFM were all significantly different between the CR and control group (p<.0001 for all, treatment main effect).
CR for Two Years Resulted in Metabolic Adaptation Measured in a Metabolic Chamber
Sedentary 24-hour energy expenditure (24hEE) was significantly reduced from baseline in both the CR and Control groups at Y1 and Y2 (Table 1), whereas SleepEE was reduced from baseline only in the CR group at both time points (Table 1). In response to the reduced body weight, we observed an approximate 10% drop in absolute energy expenditure during sleep. After taking the loss of metabolic tissues (fat-free and fat masses) into account, SleepEE was still reduced by ~7% in the CR group indicating a metabolic adaptation in comparison to the control group (Figure 3A, p<.02). Similarly, 24hEE adjusted for changes in body composition (FFM and FM) was significantly decreased from baseline at Y1 and Y2 but not differently from the control group (Figure 3B, p>.55).
Table 1.
Physical Characteristics of 53 Men and Women during Weight Maintenance at Baseline and Following 1 and 2 Years of CR
| Ad Libitum Group (Control, n = 19) | Calorie Restriction Group (n = 34) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | ΔY1 | ΔY2 | Baseline | ΔY1 | ΔY2 | |
| Age (years) | 39.0 ± 5.4 | – | – | 40.2 ± 6.7 | – | – |
|
| ||||||
| Weight (kg) | 71.0 ± 8.3 | 0.6 ± 0.6 | 1.8 ± 0.6 | 71.9 ± 8.7 | −9.4 ± 0.4a | −8.7 ± 0.4a |
|
| ||||||
| BMI (kg/m2) | 25.5 ± 1.6 | 0.2 ± 0.2 | 0.5 ± 0.2 | 25.7 ± 1.5 | −3.4 ± 0.2a | −3.2 ± 0.2a |
|
| ||||||
| Body fat (%) | 32.9 ± 5.5 | 0.2 ± 0.4 | 1.4 ± 0.4a | 34.2 ± 6.6 | −5.6 ± 0.3a | −4.5 ± 0.3a |
|
| ||||||
| Fat mass (kg) | 23.4 ± 4.0 | 0.4 ± 0.4 | 1.4 ± 0.4a | 24.7 ± 5.0 | −6.7 ± 0.3a | −5.9 ± 0.3a |
|
| ||||||
| Fat-free mass (kg) | 48.3 ± 8.1 | 0.3 ± 0.3 | −0.1 ± 0.3 | 47.8 ± 8.7 | −2.9 ± 0.2a | −3.1 ± 0.2a |
|
| ||||||
| Energy requirement (kcal/day) | 1782 ± 242 | – | – | 1747 ± 248 | – | – |
|
| ||||||
| 24hEE (kcal/day) | 1893 ± 250 | −90 ± 28a | −81 ± 28a | 1834 ± 244 | −209 ± 21a | −186 ± 21a |
|
| ||||||
| SleepEE (kcal/day) | 1523 ± 219 | 12 ± 25 | −6 ± 25 | 1530 ± 197 | −170 ± 18a | −160 ± 18a |
|
| ||||||
| T3 (ng/dL) | 121.4 ± 20.1 | −9.1 ± 3.6a | −13.3 ± 3.6a | 115.9 ± 24.4 | −23.5 ± 2.7a | −29.9 ± 2.7a |
|
| ||||||
| T4 (µg/dL) | 7.6 ± 0.9 | 0.01 ± 0.22 | 0.14 ± 0.22 | 7.1 ± 1.5 | −0.29 ± 0.17 | −0.73 ± 0.17a |
|
| ||||||
| TSH (µIU/mL) | 1.63 ± 1.25 | −0.04 ± 0.11 | −0.26 ± 0.11a | 1.31 ± 0.63 | −0.15 ± 0.09 | −0.16 ± 0.08 |
|
| ||||||
| Leptin (ng/dL) | 183.6 ± 146.0 | −0.52 ± 1.6 | −0.63 ± 1.6 | 193.9 ± 171.5 | −11.4 ± 1.2a | −9.3 ± 1.2a |
|
| ||||||
| Insulin (µIU/mL) | 6.5 | 0.7 ± 0.5 | 0.5 ± 0.5 | 5.1 ± 2.4 | −1.5 ± 0.4a | 0.2 ± 0.4 |
|
| ||||||
| 2,3-dinor-iPF(2α)-III (ng/mg Cr) | 2.17 ± 1.03 | −0.09 ± 0.17 | −0.07 ± 0.17 | 2.16 ± 0.87 | −0.42 ± 0.12a | −0.49 ± 0.12a |
Baseline data are presented as means ± SD. Change from baseline data is the adjusted LS mean ± SE from the mixed linear models, which includes the baseline value as a covariate. BMI, body mass index; 24hEE, 24-hr energy expenditure; SleepEE, energy expenditure during sleep (02:00–05:00 hr); T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Denotes significant within group change from baseline (p < 0.05).
Figure 3.
A comparison of metabolic adaptation in sleep energy expenditure (panel A) and 24-hour energy expenditure (panel B) between the AL (control, n=19 ■) and CR (n=34, □) groups, after 1 and 2 years of calorie restriction. Metabolic adaptation was considered to represent the change in energy expenditure after adjusting for the changes in fat-free mass, fat mass, age and sex and the metabolic adaptation at baseline (see methods, Sedentary 24-hour Energy Expenditure, for calculation). P-values for statistically significant treatment group effects, adjusted for multiple comparisons are shown.
An ongoing debate among metabolism, obesity and aging investigators is whether a chronic deficit in energy intake leads to metabolic slowing or a decreased “rate of living”. This phenomenon which has been termed “metabolic adaptation,” defines a reduction in energy expenditures that is larger than expected for the metabolic mass of the organism following a reduction in the respiring mass due to a caloric deficit (Heilbronn and Ravussin, 2003). It is thought that the rate of biological aging may be delayed by prolonged CR through a reduction in the “rate of living,” (Sacher and Duffy, 1979) leading ultimately to reduced oxidative damage. Together these theories imply that increased metabolism (above what is required to support the respiring mass) and the resultant increased production of reactive oxygen species (ROS) leads to a shorter life span unless these ROS are removed by antioxidant mechanisms.
Support for the rate of living hypothesis as a mechanism which may extend lifespan in mammals is debated (Speakman and Mitchell, 2011). Indeed some of this conflict is due to discrepancies in the different degrees and duration of the imposed CR, the timing of the energy expenditure evaluation following the initiation of CR, and whether energy expenditure was measured under basal or free-living conditions, which includes physical activity. However, probably the most likely source of conflicting evidence is owed to conclusions being drawn from energy expenditure measurements without appropriate statistical approaches used to account for the losses of metabolic tissues, which also are reduced with CR (Ravussin and Bogardus, 1989; Speakman et al., 2002). For instance, regarding the observations in rodents, the early seminal work of McCarter showed that the basal metabolic rate in rats following a 40% CR for six months (McCarter et al., 1985) and across the entire life span (McCarter and Palmer, 1992b) was comparable when compared to rats fed ad libitum. These data were however, soon after criticized for the assumption and statistical consideration that oxygen consumption should be adjusted for functional metabolic mass (Lynn and Wallwork, 1992). In contrast to McCarter’s study, the evaluation of 24h energy metabolism in rats after 11 weeks of moderate (25%) and severe (50%) CR showed a decrease in oxygen uptake (Ballor, 1991), that after adjustment for the change in body mass, was only observed in severe CR. In agreement with the necessity for more severe CR in rats, a 14% lowering of the metabolic rate (adjusted for body size) was observed following a 60% CR for six weeks, in comparison to counterparts fed ad libitum (Gonzales-Pacheco et al., 1993). Of the three non-human primate colonies, a 30% CR diet had no impact on oxygen consumption (measured over 36 hours) after 1 year in comparison to controls (Kemnitz et al., 1993). However, energy expenditure (night time and 24h) examined in the same cohort after 30 months was significantly lower in CR monkeys (Ramsey et al., 1997b). This agrees with a reduction in total daily energy expenditure (adjusted for fat-free mass) observed in a different colony undergoing a 26–31% CR for more 10 years. Yet, despite a consistently lower 24h energy expenditure in a third colony exposed to a 30% CR, the reduced EE was not different from the control animals (Lane et al., 1995).
In our earlier pilot study, which achieved 19% CR but across only 6 months, we observed a significant metabolic adaptation of energy expenditure (24h and Sleep) measured in a metabolic chamber which was paralleled by a reduced oxidative damage to DNA (Heilbronn et al., 2006). The multi-center CALERIE trial in 218 individuals, using a measure of resting metabolic rate with a bed-side indirect calorimeter (ventilated hood), observed a metabolic adaptation after 1 year in comparison to the controls (ad libitum diet) but this adaptation was no longer significant after another year of weight stability (Ravussin et al., 2015).
Believed to be a mechanism for energy conservation, metabolic adaptation has been the focus of weight loss (not specifically CR) in overweight or obese individuals undergoing intensive dietary interventions (Johannsen et al., 2012; Knuth et al., 2014; Rosenbaum et al., 2008). Weight-loss induced metabolic slowing has been reported in overweight/obese individuals with weight loss maintenance persistent for up to 7 years (Rosenbaum et al., 2008). However, the weight loss literature has not focused on quantifying the downstream effects on oxidative damage, which is the other key assumption in a reduced energy flux for delaying biological aging and potentially extending lifespan.
Physical activity
Recent investigations in non-human primates (Yamada et al., 2013) allude to an increased physical activity at a lower metabolic cost with sustained CR. We observed no significant treatment effect for activity-related EE (AREE; p=.20). AREE in the CR group was not changed at Y1 (−101±55 kcal/d, p=.07, within group effect) but was significantly decreased from baseline at Y2 (−119±55 kcal/d, p=.03, within group effect). In the control group, there was no change in AREE from baseline at either time point. The mean change in spontaneous physical activity (SPA, kcal/d) measured in the metabolic chamber was not different between the CR and Control groups (p=.46, treatment main effect). SPA was significantly decreased from baseline in the CR group at both Y1 and Y2 (Y1: −29±9; Y2: −30±9, kcal/d, p<.01 for both, within group effect), suggesting a reduced energy cost of activity in the chamber.
The lack of effect of CR on physical activity related energy expenditure was echoed in our preliminary 6 month study (Redman et al., 2009). Although the measurement of spontaneous physical activity in the metabolic chamber alludes to a possible reduction in activity with CR, physical activity was unfortunately not objectively measured in the present study while participants were free-living.
Mediators of Energy Metabolism, Biomarkers of Aging and Relationship with Metabolic Adaptation
As expected from the differential changes in fat mass between the treatment groups, there was a significant treatment effect for the change in leptin (p<.0001, treatment main effect) with significant reductions from baseline in the CR group at both Y1 and at Y2 and no observed changes in the Control group (Figure 4A). T3 concentrations were significantly decreased from baseline at Y1 and Y2 in the CR group (Y1: −23.5±2.7; Y2: −29.9±2.7, ng/dL, p<.0001 for both, within group effect) and that was significantly different from the change in the Control group (p<.01, treatment main effect). Similarly, there was a significant treatment effect observed for the change in T4 concentrations (Figure 4B, p=.02) and the post-hoc comparison revealed that the change in T4 concentration from baseline in the CR group was only significant at Y2 (p<.0001). This reduced activity of the thyroid axis was not supported by a change in TSH (Y1: −0.15±0.09; Y2: −0.16±0.08, uIU/mL, p<.10 for both, within group effect) or reverse T3 (data not shown). Sympathetic nervous system activity assessed through excretion of urinary catechomines over 24-hours during the chamber was not changed from baseline for epinephrine or norepinephrine in either group at Y1 or Y2. Core temperature recorded over 24-hours was not changed during the weight loss phase (Y1: −0.01±0.03 °C, p=.88, within group effect), however with weight maintenance and sustained CR (Y2), there was a trend for core temperature to be decreased from baseline (Y2: −0.06±0.03 °C, p=.07, within group effect). When day-time (0800–2200h) and night-time (0200–0500h) temperature were considered separately, no change in day-time temperature was observed in the CR or Control at either time point and therefore the reduced 24h core temperature in the CR group at Y2 was attributed to a reduction in core body temperature recorded at night (Y1: −0.05±0.05, p=.30; Y2: −0.10±0.05, °C, p=.05, within group effect).
Figure 4.
A comparison of changes in the potential mediators of metabolic adaptation; leptin (panel A) and thyroxine (T4, panel B), and the association between metabolic adaptation in Sleep EE with percent change from baseline in leptin concentrations at year 1 (Y1) (panel C) and percent change from baseline in thyroxine concentrations (T4) at year 2 (Y2), panel D. AL (control, ■) and CR groups (□). P-values for statistically significant treatment group effects, adjusted for multiple comparisons are shown. Scatterplots show the linear regression model with 95% confidence interval. N=53; 34 CR, 19 Control.
While there was no observed treatment group effect on the changes in fasting concentrations of DHEAS, there was a significant interaction (diet intervention group by time) for fasting insulin (p<.05), with a significant reduction in insulin concentration in the CR group at Y1 which was no longer evident at Y2 (Y1: −1.5±0.4, p<.001; Y2: 0.15±0.4, µIU/mL, p=.70, within group effect). Furthermore, there was a significant increase in adiponectin concentrations (HMW) from baseline in the CR group (Y1: 1188±275, Y2: 1185±271, ng/mL, p<.001 for both, within group effect) which was different from Control group (Y1: 23±363; Y2: −722±363, ng/mL, within group effect) and at both time points (treatment main effect, p<.001).
During the weight loss phase (Y1), the metabolic adaption in SleepEE (SleepEE residual) was associated with greater reductions in leptin (Figure 4C, r=0.35; p=.01), but not in the thyroid axis activity (T3, T4, TSH or rT3). At Y2, however when weight loss was maintained, the relation between metabolic adaptation in SleepEE and leptin was no longer significant (p=.22), but the metabolic adaptation was correlated with the reduction in T4 concentrations (Figure 4D r=0.33; p=.02).
In comparison to the Control group, significant reductions in hormonal mediators of energy metabolism including leptin, and thyroid hormones (T3 and T4) and an increase in adiponectin were observed in the CR group. However, the relationship of the changes in these hormones to the metabolic adaption differed in relation to CR during weight loss (Year 1) compared to CR during weight loss maintenance (Year 2). The CR group also demonstrated attenuation in two well-described biomarkers of aging; night-time core body temperature and fasting insulin.
There appear to be two distinct hormonal mechanisms during CR that potentially influence the development and sustenance of the metabolic effects. During the weight loss phase, we observed a 28% reduction in fat mass and a parallel reduction in leptin. We have previously shown that leptin is a determinant of metabolic adaptation during CR (Lecoultre et al., 2011), independent of the changes in fat-free mass and fat mass. Indeed, the change in leptin after 1 year of CR was significantly associated with the metabolic adaptation during sleep, however this relationship was no longer evident after year 2, when weight and fat mass loss were maintained but the metabolic adaptation still present. The mechanisms linking leptin to metabolic adaptation are unclear even if leptin replacement in obese individuals who had undergone a 10% weight loss rescued in part the metabolic adaptation and reduction in thyroid hormones (Rosenbaum et al., 2002). With prolonged CR and weight loss maintenance the hormone milieu changes with less contribution from obesity-related hormones (insulin, leptin) and greater contribution from metabolic hormones (T3 and T4). A reduction in thyroid axis activity is a hallmark feature of the hypometabolic state with weight loss and has been described as a biomarker aging (Roth et al., 2002b). In the non-human primate colonies (DeLany et al., 1999; Lane et al., 1996; Ramsey et al., 2000; Roth et al., 2002a) exposed to CR diets and in individuals either naturally-exposed to CR or self-practicing CR diets (Fontana et al., 2006; Soare et al., 2011), reduced thyroid hormones and lower core body temperatures are also reported. Whether these biomarkers are necessary drivers for maintaining metabolic adaptation or a consequence is unknown and cannot be discerned from our study.
Markers of oxidative stress
Urinary F2-isoprostane excretion (2,3-dinor-iPF(2α)-III) was significantly reduced from baseline at Y1 and Y2 in the CR group (Y1: −0.42±0.12; Y2: −0.49±0.12 ng/mg Cr, p<.01 for both, within group effect) and not changed in the Control group (p>.5 for both time points, data not shown). The pairwise comparison showed that the difference in 2,3-dinor-iPF(2α)-III concentrations was significant between CR and Control at Y2 (p<.05). Similarly, the three additional isomers of F2-isoprostanes (iPF(2α)-III, iPF(2 α)-VI, and 8,12-iso-iPF(2α)-VI) were significantly reduced from baseline in the CR group at Y2 (p<.05, within group effect) and not changed in the control group, but no treatment effects were observed. The change in 2,3-dinor-iPF(2α)-III concentrations from baseline to Y2 (expressed as percent change) within the CR group was associated with 24hEE metabolic adaptation (r=0.33, p=.05) and percent CR achieved (r=−36, p=.05). Serum protein carbonyl concentrations were not changed from baseline at Y1 or Y2 in either the CR or Control group.
Urinary F2-isoprostane excretion including four isomers of (F2-isoprostanes) was significantly reduced following the weight loss phase and remained significantly lower than baseline after year 2. Importantly, the drop in 2,3-dinor-iPF(2 α)-III, our primary measure of oxidative damage was associated with metabolic adaptation in 24-hour energy expenditure and the degree of calorie restriction. In contrast, no changes in plasma protein carbonyl levels were found in either group. This discordance has also been observed in previous studies. However urinary F2-isoprostanes are thought to be more sensitive biomarkers of oxidative stress in both animals (Kadiiska et al., 2005) and humans (Il'yasova et al., 2010) and are sensitive to changes in age and caloric restriction (Ward et al., 2005), whereas protein carbonyls are less sensitive to oxidative assault (Il'yasova et al., 2009; Kadiiska et al., 2005).
The “free radical theory of aging” or “oxidative stress” hypothesis is a well-supported theory of aging. It is widely accepted that the metabolic rate of an organism is a major factor in the rate of aging and is inversely related to its lifespan (Sohal and Allen, 1985). Additionally, since 1% to 3% of consumed oxygen is associated with the production of reactive oxygen species (ROS), namely superoxide (O2•−), hydrogen peroxide (H2O2•−), and the hydroxyl ion (OH•−) (Alexeyev et al., 2004), the production of these highly reactive molecules is thought to be proportional to the metabolic rate of an organism. Numerous studies have shown that modulation of the oxidative stress of an organism through prolonged CR retards the aging process in various species, including some mammals (Sohal and Weindruch, 1996; Weindruch et al., 1986) and possibly humans (Heilbronn et al., 2006). In support for a CR-induced metabolic slowing and reduction in oxidative stress, the CR group in this investigation had a significant reduction in urinary 2,3-dinor-iPF(2 α)-III isoprostane excretion after both 1 and 2 years and three other F2 isomers after 2 years. The reductions in isoprostane excretion were significantly associated with metabolic slowing (metabolic adaptation) in 24-hour energy expenditure at Year 2. This finding may emphasize the necessity for long-term studies of sustained CR in humans. Interestingly, reduced levels of oxidative damage have not been reported in centenarians (Klapcinska et al., 2000; Paolisso et al., 1998). Furthermore, in a large study of nonagenarians residing in Louisiana, we did not find an association between a reduced age-related decline in energy metabolism and oxidative damage to DNA (Frisard et al., 2007). This could bring into question the validity of the oxidative damage theory of aging particularly as it relates to the rate of living, or alternatively it could point to technical limitations in the measurement of ROS with only indirect biomarkers including urinary protein carbonylation and/or F2-isoprostane excretion rates and/or measurement of energy expenditure (bedside calorimeter versus metabolic chamber). These methods may be either insensitive to the small changes to energy efficiency (metabolic adaptation) with CR, or alternatively ROS accumulation may not be attenuated with CR but buffered by the antioxidant defenses of the organism (Schachter et al., 1993). Indeed, observational studies in individuals who live to over 100 years have higher levels of several antioxidant molecules (Mecocci et al., 2000) including those individuals residing on Okinawa (Suzuki et al., 2010) who have been exposed to natural CR across most of their lifespan.
In the exploration of a potential mechanism linking the rate of living and oxidative stress theories to explain the benefit of prolonged CR on aging, it is not surprising that the work has focused on mitochondria. Mitochondrial metabolism is the major endogenous source of ROS production (Fulle et al., 2004). However, the findings from the CR studies do not explain how changes in mitochondrial metabolism lead to lower ROS production. For example, CR in rats has been shown to reduce mitochondrial proton leak and production of hydrogen peroxide (Hagopian et al., 2005), although ROS generation is proportional to transmembrane potential and is down-regulated by proton leak (Brookes, 2005; Stowe and Camara, 2009). In monkeys and humans undergoing CR of a shorter duration, CR has been shown to induce robust increases in PGC-1 and mitochondrial biogenesis (Civitarese et al., 2007; McKiernan et al., 2012; Stein et al., 2012). As a result of mitochondrial biogenesis, CR results in improved mitochondrial function or mitochondrial efficiency, decreased total body oxygen consumption, and therefore decreased production of ROS (Civitarese et al., 2007). Indeed an increase in skeletal muscle work efficiency has been observed in obese individuals with a metabolic adaptation following weight loss (Rosenbaum et al., 2003). Furthermore, in our previous 6 month study (Heilbronn et al., 2006), we observed a reduced energy cost of physical activity after CR.
Overall Conclusions and Limitations of Study
In summary, according to the rate of living theory, those individuals who are the most efficient at utilizing energy should experience the greatest longevity. Observational studies of human aging have shown higher mass-adjusted metabolic rate (24hEE or Resting EE) is associated with disease burden (Fabbri et al., 2015; Schrack et al., 2014) and is a predictor of early mortality (Jumpertz et al., 2011; Ruggiero et al., 2008). Interventions with the capacity to induce a sustained slowing of energy metabolism such as calorie restriction should remain a focus of longevity research because randomized clinical trials and cohort studies are lacking. With careful phenotyping of energy metabolism, biomarkers of aging and oxidative stress, this modest, two-year study of human calorie restriction identified a reduction in the rate of living along with a reduction in systemic oxidative stress. The duration of imposed CR being for only two years clearly limits any extrapolation or speculation of the impact of CR on longevity in humans. Notably, many biomarkers of aging (that could be a consequence of the overall improved metabolic profile commensurate with adipose tissue loss) were also improved in these young, healthy individuals. There is a clear need for continued investigations of calorie restriction in humans since the non-human primate data is not entirely conclusive on the extension in the average and maximal lifespan but provides strong evidence for extensive health benefits including improved quality of life. The CALERIE study did not prescribe a particular diet composition and thereby this research cannot be extrapolated to infer diet recommendations to promote healthier aging apart from a reduction in calories. Future research on CR for healthy aging would benefit from considering the diet quality of individuals who have successfully defied the aging process (e.g. antioxidant content). Finally, future studies of CR may benefit from a combined approach with use of a CR mimetic such as resveratrol or greater attention to the dietary prescription which would include foods that will increase antioxidant defense systems.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
The dataset pertaining to the current study is available upon written request. Resources will be shared in accordance with appropriate data use agreements and IRB approvals for secondary analyses. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Leanne Redman (leanne.redman@pbrc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Study Design
CALERIE 2 (Rochon et al., 2011) was a two year multi-center, parallel-group, randomized controlled trial that recruited healthy individuals to receive an intervention aimed at reducing energy intake by 25% (CR group) or to maintain habitual energy intake on an ad libitum basis (control group). Two hundred and twenty individuals from Pennington Biomedical Research Center (Baton Rouge, LA), Washington University (St. Louis, MO) and Tufts University (Boston, MA) were randomized in this multi-center study (NCT00427193) for which Duke University, (Durham, NC) was the coordinating center (Rochon et al., 2011). The present ancillary study (NCT02695511) was approved by the IRB of the Pennington Biomedical Research Center and offered only to the 80 individuals enrolled in the parent study at this site. Interested individuals provided written informed consent for the additional visits and procedures. Following baseline assessments, participants were randomized to adhere for two years to a diet that targeted 25% calorie restriction (CR group) or calorie intake ad libitum (AL; Control group) according to a 2:1 allocation in favor of the CR group. Randomization was stratified by study site, sex and BMI dichotomized into normal weight (22.0≤ BMI<25.0 kg/m2) and overweight (25.0≤ BMI<28.0 kg/m2). Ancillary testing included an additional outpatient visit and a 24-hour stay in a metabolic chamber at baseline, and after 1 year (Y1; 12 months) and 2 years (Y2; 24 months) of intervention. The Clinic staff involved in the collection of study outcomes was blinded to the treatment group assignments.
Participants
Men and women were aged 20 to 50 years and 20 and 47 years, respectively, and had body mass index (BMI) between 22.0 to 27.9 kg/m2 at the initial screening visit. Potential participants in the ancillary study were excluded for claustrophobia, contraindications to MRI and history of blood clotting disorders. The CONSORT diagram summarizing throughput of participants in the study is provided in Figure 1 and the characteristics of the participants at baseline is summarized in Table 1.
METHOD DETAILS
Study Interventions
From day 1, the CR intervention targeted a sustained 25% restriction of energy intake prescribed on the basis of the energy requirements determined from two, 14-day doubly labeled water measures at baseline (Redman et al., 2014; Rickman et al., 2011). The goal for the intervention was adherence to a mathematically predicted weight loss trajectory that reached 15.5% below baseline weight after one year of intervention followed by maintenance of this weight over the second year (Pieper et al., 2011). Participants received a weekly weight loss graph that showed a targeted weight range which was used as the primary tool to maintain adherence during the intervention. Because of the variability in projected weight loss needed to achieve 25% CR, participants were also provided with guidance indicating a “zone of acceptable weight loss” which ranged from 12 to 22%. Nutritional and behavioral guidance was customized and modified to decrease the degree to which weight change differed from the target. Adherence to 25% CR was further fostered by provision of meals for the first 27 days of the study. Participants were fed their assigned caloric prescription in the form of three, 9-day diets. The food provision was used to educate on portion size, energy content and anticipated diet changes necessary to maintain 25% CR with different types of dietary patterns. The behavioral intervention included delivery of a structured curriculum in regular group and individual meetings with interventionists (clinical psychologists and nutritionists) from a standardized treatment manual developed specifically for the study (Rickman et al., 2011). Participants randomized to the control group were advised to continue their current diets on a completely ad libitum basis. No specific level of physical activity was required or recommended for either group. All participants received a multivitamin (Nature Made Multi Complete, Pharmavite LLC, Mission Hills, CA) and calcium supplement (1000mg/d, Douglas laboratories, Pittsburgh, PA) to foster nutritional adequacy of the self-selected diets.
Energy Intake and Calorie Restriction
Energy intake was calculated at baseline by total daily energy expenditure (doubly labeled water) and during the trial between baseline and Y1 as well as baseline and Y2 by the intake/balance method derived from total daily energy expenditure (doubly labeled water) and the changes in energy content of fat mass (9,300 kcal/kg) and fat-free mass (1,100 kcal/kg) from DXA (Racette et al., 2012). The percent reduction in energy intake (%CR) achieved during each interval was defined as; %CR = 100 × (Energy intake at baseline − EI during Interval) / Energy intake at baseline.
Total Daily Energy Expenditure
For each doubly labeled water (DLW) assessment, two baseline urine samples were collected before subjects consumed an oral cocktail containing 0.1 g of 2H2O at 99.98 atom % 2H and 0.16 g of 100% 18O per kg body weight. After dosing, participants were asked to void their bladder at approximately 1–3 h after ingestion (this sample was discarded) and to collect six additional, timed urine samples: two approximately 4.5h and 6h after dosing, two on day 7, and two on day 14. Measurement of hydrogen and oxygen isotope enrichments were measured by gas-isotope-ratio mass spectrometry at the USDA/ARS Children’s Nutrition Research Center Stable Isotope Laboratory (Houston, TX) (Racette et al., 1994; Wong et al., 1992). Carbon dioxide production rate (VCO2) was calculated from the fractional turnover rates of 2H (kH) and 18O (kO) (Racette et al., 1994) and converted to TDEE based on an energy equivalent of a liter of CO2 to be 3.815/RQ + 1.2321 where the RQ was determined for each individual using food diaries and changes in body composition.
Anthropometrics and Body Composition
Metabolic body weight was measured (Scale Tronix 5200, White Plains, NY) in the morning after an overnight fast and voiding while wearing a surgical gown which was subtracted from the total weight. Body composition (fat, lean, and bone) was measured by dual X-ray absorptiometry (DXA; Hologic QDR 4500A; Hologic, Bedford, MA) according to a standardized protocol and all scans were analyzed at a centralized reading center (University of CA, San Francisco) using Hologic software version Apex 3.3.
Sedentary 24-hour Energy Expenditure
Participants entered a metabolic chamber at approximately 0800h after an overnight fast for measurement of 24 hour sedentary energy expenditure (24hEE) and sleeping energy expenditure (SleepEE). Meals were prepared by the metabolic kitchen and served according to a fixed schedule. At baseline, the energy intake provided was estimated according to an equation and adjusted during the day on the basis of actual measured energy expenditure of the first 7 hours of measurement (Nguyen et al., 2003). For the subsequent chambers, the energy content of the food was held constant for control participants and was 75% of measured baseline 24-hour energy expenditure for CR participants. SleepEE was assessed between 0200–0500h for those minutes that activity is less than 1%. During their stay in the chamber, no exercise will be allowed. The change in 24hEE and SleepEE is expressed as the residual EE which is the difference between the measured value and the value predicted for the EE measurement (on the basis of weight and body composition) at each time point. The predicted values were derived from a linear regression at baseline for the 71 participants using fat-free mass, fat mass, age and sex as covariates;
24hEE (kcal/d) = 1100 + 17.2 (fat-free mass, kg) + 4.6 (fat mass, kg) − 1.9 (age, y) − 167 (sex; 1=female, 0=male); R2=0.70, p<.0001.
SleepEE (kcal/d) = 749 + 17.6 (fat-free mass, kg) + 3.2 (fat mass, kg) − 2.6 (age, y) − 58 (sex; 1=female, 0=male); R2=0.70, p<.0001.
The difference in the residual EE (follow-up minus baseline) was then used as a marker of the extent to which energy expenditure adapted to calorie restriction independently from the changes in body mass and body composition with negative values indicating metabolic adaptation (Galgani and Santos, 2016).
Core body temperature
Core body temperature (VitalSense, Mini-Mitter, Bend, OR) was measured and recorded every minute during the energy expenditure measurement in the metabolic chamber. Mean temperature over 24-hours as well as mean day time (0800–2230h) and night time (0200–0500h) temperatures were calculated.
Physical activity
The energy cost of physical activity, termed activity related energy expenditure (AREE) was calculated as the cost of daily activities beyond sleep using linear regression model of total daily energy expenditure by doubly labeled water measures (TDEE) and SleepEE at baseline: TDEE (kcal/d) = 859 + 1.1 (SleepEE, kcal/d) + 4.1 (age, y) − 340 (sex; 1=female, 0=male); R2=0.66, p<.0001. AREE is positive for subjects with higher physical activity than average and negative for subjects with lower physical activity than average (Redman et al., 2009). Second, the calories expended in spontaneous physical activity (SPA) were determined by microwave motion detectors and indirect calorimetry in the metabolic chamber.
Oxidative Stress
Our primary measure of oxidative damage, urinary 2,3-dinor-iPF(2α)-III was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a Shimadzu 20A series LC and Applied Biosystems API 4000 QTrap MS/MS instruments as previously described (Il'yasova et al., 2010). We also measured three additional isomers of F2-isoprostanes; iPF(2α)-III, iPF(2 α)-VI, and 8,12-iso-iPF(2α)-VI as exploratory variables. Urine specimens were diluted to 0.65 mg/mL creatinine, and samples with creatinine levels equal to or below this value were analyzed without dilution. Sample preparation included addition of internal standards [iPF(2α)-III-d4, 8,12-iso-iPF(2α)-VI-d11, iPF(2α)-VI-d4] and 10 µL 1M HCl; washing of samples (500 µL) with 1 mL hexane; extraction of the analytes by ethyl acetate/hexane mixture (3/1, v/v); evaporation of the liquid and resuspension of the residue in 150 µL of a mixture containing 70% mobile phase A (0.1% formic acid in water) and 30% methanol. Using LC-MS/ MS, 100 µL of sample were injected into two solid core C18 columns (Phenomenex Kinetex C18, 150 × 4.6 mm) in series to achieve chromatographic separation of the F2-isoprostane isomers. The mass spectrometer was operated in negative mode with the following MRM transitions (m/z): 353/193 [iPF(2α)-III], 357/197 [iPF(2α)-III-d4], 325/237 [2,3-dinor-iPF(2α)-III], 353/115 [iPF(2α)-VI and 8,12-iso-iPF(2α)-VI], 364/115 [iPF(2α)-VI-d11], and 357/115 [8,12-iso-iPF(2α)-VI-d4]. Calibration samples covering the expected range of concentrations were prepared by adding pure material into pooled human urine, injected before and after the patient samples. Lower limits of quantification (LLOQ, >80 % accuracy) were 0.007, 0.34, 0.25, and 0.12 mg/mL for iPF(2α)-III, 2,3-dinor-iPF(2α)-III, iPF(2α)-VI, and 8,12-iso-iPF(2α)-VI, respectively. As a complimentary measure of oxidative damage, serum protein carbonyls were determined using a modified 2,4-dinitrophenylhydrazine assay (Mates et al., 2000).
Clinical Chemistry
Fasting blood samples were collected and the following assays were performed at the CALERIE central biochemistry laboratory at Vermont University or at the Clinical Chemistry Core at Pennington Biomedical Research Center: thyroid stimulating hormone (TSH) and triiodothyronine (T3) by chemiluminescent immunoassay (ADVIA Centaur, Bayer Health Care, Deerfield, IL); thyroxine (T4) by particle-enhanced immunonephelometric assay, (BN II, Siemens, Deerfield, IL); reverse T3 by multiplex immunoassay (Bio-Plex, Bio-Rad Laboratories, Hercules, CA); leptin by multiplex immunoassay (Bio-Plex, Bio-Rad Laboratories, Hercules, CA); and insulin by chemiluminescent immunoassay (Elecsys 2010, Roche Diagnostics, Indianapolis, IN). Nitrogen, creatinine, norepinephrine and epinephrine were measured in a 24 hour pooled urine sample collected during the chamber stay.
QUANTIFICATION AND STATISTICAL ANALYSIS
Sample size estimation
This study was powered on the ability to detect a significant adaptation in energy metabolism (24hEE, SleepEE) from baseline and to detect differences in this adaptation between the two diet groups (AL vs CR). Sample size estimates were derived from the data obtained in our 6 month pilot study where the standard deviation in EE was assumed to be 140 kcal/d. Anticipating that a maximum of 75 subjects (50 in CR and 25 in control) would enroll in the ancillary study, the minimal detectable metabolic adaptation within groups is 60 kcal/d and between groups is 100 kcal/d to achieve a power ≥ 80%.
Statistical Analysis
All analyses were carried out using SAS/STAT software, Version 9.4 of the SAS System for Windows© (SAS Institute Inc. Cary, NC, USA) and tests were evaluated using significance level of α=.05. The per-protocol analysis (see “study subjects and throughput in the Results Section) comprised of computing the change from baseline to Y1 and Y2 in all outcomes which were investigated for fixed effects (treatment group, time) and a treatment-by-time interaction using linear mixed models for repeated measures. The models included the baseline outcome value as a covariate. A random subject effect was also included to account for intra-individual correlations over time. Two-sample t-tests derived from least squares means (LSM) were used to compare adjusted mean changes between treatment groups (AL vs CR) and to test for group differences in adjusted mean change at Y1 and Y2. This same method was used to model and assess differences in percent change from baseline. Finally, Pearson’s correlation analysis was used to assess relationships between %CR and metabolic adaptation, change from baseline in clinical chemistries and for Spearman’s correlation analysis was used to examine relationships between %CR, metabolic adaptation and isoprostane concentrations which were non-normally distributed.
Highlights.
Calorie restriction (CR) extends maximum lifespan in most species
Young, healthy individuals achieved 15% CR and 8 kg weight loss over 2-years
Energy expenditure (24-hour and sleep) was reduced beyond weight loss
Oxidative stress was also reduced supporting two longstanding theories of aging
Acknowledgments
This work was funded by National Institutes of Health: R01 AG029914 (Ravussin, E); U01 AG020478 (Ravussin, E) and supported in part by P30DK072476 (Pennington/Louisiana NORC); U54 GM104940 (Louisiana Clinical and Translational Science Center). We are indebted to the commitment of the study participants who invested over two years of their life to participate in this clinical trial. The efforts of the CALERIE data coordinating center (James Rochon, William Krauss and Manjushri Bhapkah) is also acknowledged and greatly appreciated. We thank Dr. Donald Ingram for his critical review and thoughtful insights which helped to shape this paper. The CALERIE Phase 2 trial (NCT00427193) and this ancillary study (NCT02695511) are registered as clinical trials at clinicaltrials.gov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
E.R. and L.M.R.: designed the study, obtained funding, conducted the research, interpreted the results and wrote the manuscript; S.R.S., C.K.M. designed the study, obtained funding, conducted the research; J.H.B. performed the statistical analysis. All authors reviewed and approved the final draft of the manuscript.
DECLARATION OF INTERESTS
The authors declare no financial conflicts of interest in association with the research described in this paper.
References
- Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond) 2004;107:355–364. doi: 10.1042/CS20040148. [DOI] [PubMed] [Google Scholar]
- Ballor DL. Effect of dietary restriction and/or exercise on 23-h metabolic rate and body composition in female rats. J Appl Physiol. 1991:801–806. doi: 10.1152/jappl.1991.71.3.801. [DOI] [PubMed] [Google Scholar]
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Saltzman E, Gilhooly CH, DeLany JP, Golden JK, Pittas AG, Dallal GE, Bhapkar MV, Fuss PJ, Dutta C, et al. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity (Silver Spring) 2009;17:2019–2024. doi: 10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA. Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci. 1999:B5–11. doi: 10.1093/gerona/54.1.b5. discussion B12–13. [DOI] [PubMed] [Google Scholar]
- Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Energy Metabolism and the Burden of Multimorbidity in Older Adults: Results From the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2015;70:1297–1303. doi: 10.1093/gerona/glu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisard MI, Broussard A, Davies SS, Roberts LJ, 2nd, Rood J, de Jonge L, Fang X, Jazwinski SM, Deutsch WA, Ravussin E. Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. J Gerontol A Biol Sci Med Sci. 2007;62:752–759. doi: 10.1093/gerona/62.7.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Galgani JE, Santos JL. Insights about weight loss-induced metabolic adaptation. Obesity (Silver Spring) 2016;24:277–278. doi: 10.1002/oby.21408. [DOI] [PubMed] [Google Scholar]
- Gonzales-Pacheco DM, Buss WC, Koehler KM, Woodside WF, Alpert SS. Energy restriction reduces metabolic rate in adult male Fisher-344 rats. J Nutr. 1993:90–97. doi: 10.1093/jn/123.1.90. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Harper ME, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- Hambly C, Speakman JR. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes Res. 2005;13:1548–1557. doi: 10.1038/oby.2005.190. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Il'yasova D, Mixon G, Wang F, Marcom PK, Marks J, Spasojevich I, Craft N, Arredondo F, DiGiulio R. Markers of oxidative status in a clinical model of oxidative assault: a pilot study in human blood following doxorubicin administration. Biomarkers. 2009;14:321–325. doi: 10.1080/13547500902946757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'yasova D, Spasojevic I, Wang F, Tolun AA, Base K, Young SP, Marcom PK, Marks J, Mixon G, DiGiulio R, et al. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer Epidemiol Biomarkers Prev. 2010;19:1506–1510. doi: 10.1158/1055-9965.EPI-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumpertz R, Hanson RL, Sievers ML, Bennett PH, Nelson RG, Krakoff J. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab. 2011;96:E972–976. doi: 10.1210/jc.2010-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993:B17–26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Klapcinska B, Derejczyk J, Wieczorowska-Tobis K, Sobczak A, Sadowska-Krepa E, Danch A. Antioxidant defense in centenarians (a preliminary study) Acta Biochim Pol. 2000;47:281–292. [PubMed] [Google Scholar]
- Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, Abumrad NN, Ravussin E, Hall KD. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring) 2014;22:2563–2569. doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Tilmont EM, Rumpler WV, Ingram DK, Roth GS, Cutler RG. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995:B295–302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011;96:E1512–1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn WS, Wallwork JC. Does food restriction retard aging by reducing metabolic rate? J Nutr. 1992:1917–1918. doi: 10.1093/jn/122.9.1917. [DOI] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, Perez-Gomez C, Olalla L, Segura JM, Blanca M. Allergy to drugs: antioxidant enzymic activities, lipid peroxidation and protein oxidative damage in human blood. Cell Biochem Funct. 2000;18:77–84. doi: 10.1002/(SICI)1099-0844(200006)18:2<77::AID-CBF851>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985:E488–490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. The American journal of physiology. 1992a;263:E448–452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol. 1992b:E448–452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, Aiken JM, Weindruch R, Anderson RM. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp Gerontol. 2012;47:229–236. doi: 10.1016/j.exger.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, Straatman M, Monti D, Stahl W, Sies H, et al. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Tagliamonte MR, Rizzo MR, Manzella D, Gambardella A, Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. J Am Geriatr Soc. 1998;46:833–838. doi: 10.1111/j.1532-5415.1998.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Pearl R. The rate of living. University Press; 1928. [Google Scholar]
- Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, Martin C, Kraus W, Das S, Williamson D, et al. Development of adherence metrics for caloric restriction interventions. Clinical trials. 2011;8:155–164. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302:E441–448. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. The American journal of physiology. 1994;267:E585–590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. Energy expenditure of adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol. 1997a;272:E901–907. doi: 10.1152/ajpendo.1997.272.5.E901. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. Energy expenditure of adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol. 1997b:E901–907. doi: 10.1152/ajpendo.1997.272.5.E901. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Bogardus C. Relationship of Genetics, Age, and Physical-Fitness to Daily Energy-Expenditure and Fuel Utilization. Am J Clin Nutr. 1989:968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–78. doi: 10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemporary Clinical Trials. 2011;32:874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, Bhapkar M, Kraus WE. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget. 2016 doi: 10.18632/oncotarget.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- Roth GS, Handy AM, Mattison JA, Tilmont EM, Ingram DK, Lane MA. Effects of dietary caloric restriction and aging on thyroid hormones of rhesus monkeys. Horm Metab Res. 2002a;34:378–382. doi: 10.1055/s-2002-33469. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002b;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Metter EJ, Melenovsky V, Cherubini A, Najjar SS, Ble A, Senin U, Longo DL, Ferrucci L. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher GA, Duffy PH. Genetic relation of life span to metabolic rate for inbred mouse strains and their hybrids. Fed Proc. 1979:184–188. [PubMed] [Google Scholar]
- Schachter F, Cohen D, Kirkwood T. Prospects for the genetics of human longevity. Hum Genet. 1993;91:519–526. doi: 10.1007/BF00205074. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. "IDEAL" aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:667–672. doi: 10.1111/jgs.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY) 2011;3:374–379. doi: 10.18632/aging.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Molecular aspects of medicine. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Selman C, McLaren JS, Harper EJ. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132:1583S–1597S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging cell. 2012;11:644–650. doi: 10.1111/j.1474-9726.2012.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Willcox DC, Rosenbaum MW, Willcox BJ. Oxidative stress and longevity in okinawa: an investigation of blood lipid peroxidation and tocopherol in okinawan centenarians. Curr Gerontol Geriatr Res. 2010;2010:380460. doi: 10.1155/2010/380460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts LJ, 2nd, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2005;60:847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- Wong WW, Clarke LL, Llaurador M, Klein PD. A new zinc product for the reduction of water in physiological fluids to hydrogen gas for 2H/1H isotope ratio measurements. Eur J Clin Nutr. 1992;46:69–71. [PubMed] [Google Scholar]
- Yamada Y, Colman RJ, Kemnitz JW, Baum ST, Anderson RM, Weindruch R, Schoeller DA. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp Gerontol. 2013;48:1226–1235. doi: 10.1016/j.exger.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]