Abstract
Tolerance of anoxia in maize root tips is greatly improved when seedlings are pretreated with 2 to 4 h of hypoxia. We describe the patterns of protein synthesis during hypoxic acclimation and anoxia. We quantified the incorporation of [35S]methionine into total protein and 262 individual proteins under different oxygen tensions. Proteins synthesized most rapidly under normoxic conditions continued to account for most of the proteins synthesized during hypoxic acclimation, while the production of a very few proteins was selectively enhanced. When acclimated root tips were placed under anoxia, protein synthesis was depressed and no “new” proteins were detected. We present evidence that protein synthesis during acclimation, but not during subsequent anoxia, is crucial for acclimation. The complex and quantitative changes in protein synthesis during acclimation necessitate identification of large numbers of individual proteins. We show that mass spectrometry can be effectively used to identify plant proteins arrayed by two-dimensional gel electrophoresis. Of the 48 protein spots analyzed, 46 were identified by matching to the protein database. We describe the expression of proteins involved in a wide range of cellular functions, including previously reported anaerobic proteins, and discuss their possible roles in adaptation of plants to low-oxygen stress.
Plants cannot survive the prolonged oxygen deficit brought about by flooding. However, the ability of plant tissues such as maize root tips to survive anoxic stress can be increased by hypoxic pretreatment (2–4 kPa partial pressure) (for review, see Drew, 1997). Sachs et al. (1980) reported that after 1 h of anaerobic treatment, the synthesis of most aerobic, soluble proteins in maize seedling primary roots was curtailed, whereas a set of 20 anaerobic proteins was selectively synthesized after 2 h, and after 5 h comprised more than 70% of all soluble proteins synthesized. Most of the anaerobic proteins identified are enzymes involved in sugar metabolism and fermentation, and their synthesis is regulated at both the transcriptional and post-transcriptional levels (Sachs et al., 1996; Drew, 1997; Fennoy et al., 1998).
Gene expression is also altered in hypoxically acclimated maize tissues (Kelley and Freeling, 1982; Saglio et al., 1999). Hypoxic treatment increases transcript levels of alcohol dehydrogenase 1 (adh1), alcohol dehydrogenase 2 (adh2), pyruvate decarboxylase (pdc1), aldolase (ald1), Suc synthase (sus1), and enolase (eno1) (Andrews et al., 1993, 1994a; Zeng et al., 1998). However, no common regulatory pattern for the coordinated transcription of multiple messages was observed in the expression of these genes (for review, see Drew, 1997). Ellis et al. (1999) have shown that the inhibitor cycloheximide prevents hypoxic acclimation in roots and shoots of Arabidopsis, indicating that protein synthesis is important for the acclimation of plants to low-oxygen stress. Furthermore, Xia and Saglio (1992) reported that cycloheximide blocks the induction of a lactate efflux mechanism under hypoxia, suggesting that protein synthesis contributes to improved intracellular pH regulation in hypoxically acclimated roots (Xia and Roberts, 1994, 1996).
The goal of this study was to clarify the role of protein synthesis in the adaptation of maize root tips to low-oxygen stress. We first describe the patterns of protein synthesis in maize root tips prior to, during, and after acclimation to low-oxygen stress. Second, we define when protein synthesis is most critical for improved cytoplasmic pH regulation and survival during anoxia. Third, we report the results of mass spectrometry (MS), two-dimensional isoelectric focusing (IEF) SDS-PAGE, and database searches to identify 46 root tip proteins whose rates of synthesis are altered during hypoxic acclimation.
MATERIALS AND METHODS
Plant Material
Maize (Zea mays L. inbred line B73) kernels were kindly supplied by Pioneer Hi-Bred International (Johnston, IA). Seeds were germinated in plastic trays lined with wet paper towels for 36 h in the dark at 23°C. Seedlings were placed into sterile glass tubes (length, 160 mm; i.d., 2 mm) lined with wicks (width, approximately 1 mm; length, 170 mm) made from chromatography paper (3MM, Whatman, Clifton, NJ) saturated with 0.1 mm CaSO4. Transplanted seedlings were placed upright in a water-saturated chamber and allowed to grow under constant room light for approximately 72 h at 23°C, after which the seedling roots were typically 100 to 120 mm long.
Gas Treatment, Cycloheximide Treatment, and Growth Experiments
Fifteen to 40 germinated seedlings (average root length, 110 mm) were placed into a 75-mm (i.d.) glass funnel with a 10-mL disposable chromatography column (Bio-Rad, Hercules, CA) attached. The roots were submerged in 0.1 mm CaSO4 sparged with either 3% (v/v) O2 balanced with N2 (hypoxia), 99.999% (v/v) N2 (anoxia), or 100% (v/v) O2 (normoxia), depending on the treatment. The gases used in the experiments were first saturated with moisture in a gas washer bottle filled with water. During hypoxic or anoxic treatments, funnels were sealed with rubber stoppers to prevent the entry of O2 from air. To assess the survival of root tips after anoxia, intact seedlings were transferred to a funnel attached to a 110-mL chromatography column (Econo-Column, Bio-Rad) filled with sterile 0.1 mm CaSO4 and bubbled with 100% (v/v) O2. The seedling roots were allowed to grow under normoxic conditions for 26 h. The length of the primary root was measured using a ruler at the beginning and end of the recovery phase. The viability of the root tips was assessed by scoring the number of non-flaccid root tips. It has been demonstrated that the O2 concentration in air-saturated water falls below the critical O2 pressure (the lowest value of the partial O2 pressure that saturates respiration) of submerged maize root tips (Saglio et al., 1984). Consequently, for all normoxic treatments used, including the recovery phase, the CaSO4 medium was sparged with 100% (v/v) O2 to prevent O2 deficit.
In experiments involving cycloheximide (Sigma-Aldrich, St. Louis), the protein translation inhibitor was added to the CaSO4 1 h before a given gas treatment to allow drug uptake by the root tip tissue and to block protein synthesis. Cycloheximide was washed off with distilled water at the end of the treatments, then seedlings were subjected to 13 h of anoxia, followed by 26 h of normoxic recovery. Viability and root elongation rate were assessed at the end of the recovery period. While cycloheximide inhibited protein synthesis effectively, the range of dosages applied was non-lethal for normoxic seedling root tips. In a control experiment, seedling roots were treated with up to 50 μm cycloheximide and normoxia for 18 h; at the end of this period, cycloheximide was washed off and the seedlings were incubated under normoxic conditions for an additional 26-h period. All root tips remained viable at the end of this experiment (data not shown).
In Vivo Labeling, Protein Extraction, and Scintillation Spectroscopy
Fifteen intact seedlings were labeled in a funnel attached to a small disposable column (see above) with roots immersed in 2 mL of 138 μCi/mL (0.117 μm) [35S]Met (Du- Pont/NEN, Wilmington, DE) in 0.1 mm CaSO4 bubbled with appropriate gas. At the end of the labeling period, roots were dipped in ice-cold, sterile water three times, and 5-mm pieces of root apices were cut on an aluminum block over dry ice. The excised root tips were homogenized as described previously (Damerval et al., 1986; Webster et al., 1991b). Undissolved material was removed by a brief centrifugation (5–10 s) at 14,000g. The protein concentration was determined using the protein assay (Bio-Rad), and incorporation of [35S]Met into protein was quantified (Webster et al., 1991b).
Two-Dimensional PAGE and Densitometry
Two-dimensional IEF-SDS-PAGE was essentially as described by O'Farrell (1975) with some modifications (Webster et al., 1991b). Root tip proteins (100 μg per sample) were fractionated by two-dimensional IEF-SDS-PAGE. Gels were either stained with Rapid Coomassie (Research Products International, Mount Prospect, IL) or were silver-stained (Blum et al., 1987) and incubated in Fluoro-Hance (Research Products International) for 30 min. Dried gels were then exposed to X-Omat film (Kodak, Rochester, NY) at −80°C for 95 h.
Fluorographs were scanned (ScanJet 4c/T, Hewlett-Packard, Palo Alto, CA). The scanner output response was linearized by calibration using a reflection density guide (Kodak; Kendrick et al., 1994). Scanned images were saved as tagged image format files, and individual spot intensities were determined using an image analysis program (ImageQuant, Molecular Dynamics, Sunnyvale, CA). Background was subtracted from each spot by the following approach. An arbitrary rectangular region 4 mm2 in size was chosen from a part of the gel where there was no visible protein spots; densitometric volume (intensity × spot area) of this region was divided by its area to give the average background volume/area, and this value was multiplied by the area of a spot that was then subtracted from the reported densitometric volume given by ImageQuant to obtain the normalized volume. The normalized volume was used in all subsequent quantitative gel analyses of individual proteins.
Western-Blot Analyses
Western transfer and immunodetection were carried out as previously described (Webster et al., 1991a) using rabbit polyclonal antisera raised against the following proteins: recombinant eIF-4A, wheat eEF-2 (a gift from Karen Browning, University of Texas, Austin), and maize ADH (a gift from Julia Bailey-Serres, University of California, Riverside; Fennoy and Bailey-Serres, 1995). Binding of primary antibody was visualized using horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) and metal-enhanced diaminobenzidine tetrahydrochloride substrate (Immunopure kit from Pierce Chemical, Rockford, IL).
Estimation of Cytoplasmic pH and Metabolite Analysis by 31P-NMR
NMR spectroscopy of root tips of intact maize seedlings was done essentially as described in Xia and Roberts (1996). Intact seedlings were first treated in glass funnels, as described above. Prior to anoxia, seedlings were transferred into a sealed NMR sample tube, and spectra were obtained at 202.5 MHz on a spectrometer (model GN 500, General Electric, Fairfield, CT). Gases equilibrated with 0.1 mm CaSO4 were used for perfusion with a constant gas stream through the sample tube during the experiment. Cytoplasmic pH was estimated from the chemical shifts of cytoplasmic Pi (Roberts, 1986).
Protein Identification by MS
Individual or pooled gel spots (2–3 spots) from separate Coomassie Blue-stained or silver-stained two-dimensional gels (100 μg protein/gel) were subjected to tryptic digestion using a modified procedure of Rosenfeld et al. (1992). Gel spots containing proteins were excised from gels using a scalpel in a laminar flow hood. The excised gel spots were stored in 100 μL of HPLC-grade water at 4°C until subsequent analyses. The spots were then minced and washed with 25 mm NH4HCO3 in 50% (v/v) acetonitrile. The gel pieces were allowed to dry and then rehydrated in 25 mm NH4HCO3 with 0.5 to 1.0 μg of trypsin at 37°C overnight. After digestion, the digestion solution was separated from the gel slices, and the gel slices were washed with HPLC-grade water once and with 50% (v/v) acetonitrile, 5% (v/v) trifluoroacetic acid three times at room temperature to extract the peptides further. Pooled extracts (including the digestion solution and both the aqueous and organic washes) were concentrated using a Speed-Vac (Savant Instruments, Holbrook, NY). In some cases, samples were further fractionated by reversed phase HPLC on a microbore C18 column (1.0 mm × 15 cm; Vydac, The Separations Group, Hesperia, CA). HPLC fractions were collected and concentrated.
Tryptic peptide masses were measured by analyzing one-twentieth of each concentrated sample after digestion (or one-tenth of each HPLC fraction) using a matrix-assisted laser desorption-ionization delayed extraction reflectron time-of-flight (MALDI-DE-TOF) mass spectrometer equipped with a nitrogen laser (λ = 337 nm) (Voyager-DE STR, PE Biosystems, Framingham, MA). Peptides were co-crystallized 1:1 (v/v) with matrices consisting of saturated α-cyano-4-hydroxycinnamic acid prepared in 50% (v/v) acetonitrile/1% (v/v) trifluoroacetic acid. All MALDI spectra were either externally calibrated using a standard peptide mixture or internally calibrated using trypsin auto-proteolysis products. Mono-isotopic masses from all spectra recorded for a given peptide are reported. For several peptides that exhibited the highest pseudo-molecular ion abundance on MALDI mass spectra, partial amino acid sequence was determined using post-source decay analysis.
Matching of experimental results (measured peptide mass values) with theoretical digests and sequence information obtained from various databases was performed using two sequence database search programs, MS-Fit and MS-Tag (Jimenez et al., 1998; Clauser et al., 1999). These programs were developed by Karl Clauser and Peter Baker of the National Institutes of Health (NIH)/National Science Foundation Mass Spectrometry Facility, University of California, San Francisco, and are available at http://prospector.ucsf.edu/. MS-Fit allows the user to match the observed tryptic peptide masses of an unknown protein to the expected peptide masses of any protein for which amino acid or nucleotide sequence information is available. Database queries were carried out for mono-isotopic peptide masses using the following parameters: peptide mass tolerance of ±50 ppm (ppm = [experimental mass (in daltons) − theoretical mass]/theoretical mass, expressed in parts per million), equivalent to 0.1 D for a 2-kD peptide; the maximum number of missed tryptic cleavages of 2 or 3; and modifications including conversion of peptide N-terminal Gln to pyro-Gln, oxidation of Met, acetylation of the N terminus, and modification of Cys by acrylamide.
Database searches using MS-Tag to match post-source decay (PSD) fragment ions (along with the mass of a precursor ion) used the following parameters: precursor ion mass tolerance of ±100 ppm (measured by MALDI-MS) and PSD fragment ion mass tolerance of ±1,500 ppm. Databases searched included protein databases such as the non-redundant NCBInr compiled by the National Center for Biotechnology Information, and the NIH, and cDNA databases such as dbEST, which is a division of GenBank (NIH genetic sequence database), containing single-pass cDNA sequences or expressed sequence tags.
RESULTS
Acclimation to Anoxic Stress Occurs within 2 to 4 h of Hypoxic Pretreatment
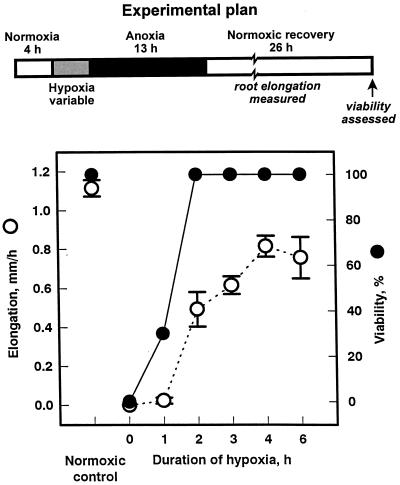
Most previous studies on acclimation have used hypoxic pretreatments lasting 16 h or more (e.g. Saglio et al., 1988; Johnson et al., 1989; Germain et al., 1997; Ellis et al., 1999), although Andrews et al. (1994b) reported that 6 h of hypoxic acclimation significantly improved anoxia tolerance. To study protein synthesis during times most critical for enhanced tolerance of anoxia, we determined the minimal time required for acclimation in hypoxic root tips. Maize seedlings were subjected to 13 h of anoxia, followed by 26 h of recovery under oxygen. Enhanced tolerance of anoxia (acclimation) was assessed primarily by recording survival after the stress and recovery regime. Control (non-acclimated) seedlings could not survive this regimen (Fig. 1, 0 h of hypoxia), whereas as little as 2 h of hypoxic pretreatment led to 100% viability. Acclimation was further assessed by measuring root elongation during the recovery phase. Root elongation improved with increasing duration of hypoxic pretreatment to approximately 70% of normoxic controls with a 4-h pretreatment. Longer hypoxic pretreatments gave no additional improvement. Consequently, a 4-h hypoxic pretreatment was used for the experiments described below.
Figure 1.
Effect of duration of hypoxic pretreatment on maize root tip tolerance to 13 h of anoxia. Intact seedlings were pretreated under hypoxia for various lengths of time, followed by 13 h of anoxia and 26 h of normoxia (see schematic, “Experimental plan”). Tolerance was assessed using root growth and root tip viability assays. Growth data are mean values ± se (n = 10). Viability data are aggregates of three independent experiments, from observations of a total of 30 seedlings for each point. Normoxic control seedlings of the same developmental age were exposed to 100% (v/v) O2 only.
Many Normoxic Proteins Are Synthesized during Hypoxic Acclimation
We looked at changes in protein synthesis that occurred during acclimation to low-oxygen stress. Root tips of intact seedlings subjected to hypoxia were labeled with [35S]Met, and proteins were extracted and separated by two-dimensional IEF-SDS-PAGE (Fig. 2). At the individual protein level, we analyzed 262 proteins with Mrs from 36,000 to 99,000 and pIs from 6.88 to 5.70, where resolution was best and most reproducible. This region of the gel contained approximately 50% of the proteins having pIs between 3 and 10 and Mrs between 20,000 and 200,000, based on the intensity of silver-stained proteins.
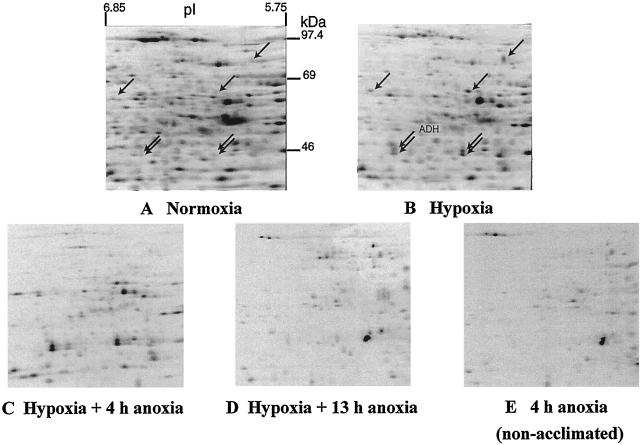
Figure 2.
Effects of low-O2 treatments on patterns of protein synthesis in intact maize root tips. Data are fluorographs of root tip proteins, labeled in vivo with [35S]Met and separated by two-dimensional IEF-SDS-PAGE. Fifteen 6-d-old (post imbibition) seedlings were labeled with [35S]Met during the last 4 h of each treatment. A, Normoxia, 8 h under 100% (v/v) O2. B, Hypoxia, 4 h of O2, 4 h of 3% (v/v) O2. C, Hypoxia plus 4 h of anoxia, 4 h of O2, 4 h of 3% (v/v) O2, 4 h of N2. D, Hypoxia plus 13 h of anoxia, 4 h of O2, 4 h of 3% (v/v) O2, 13 h of N2. E, 4 h of anoxia, 8 h of O2, 4 h of N2 (non-acclimated). Root tip proteins (100 μg per sample) were fractionated by two-dimensional IEF-SDS-PAGE, and labeled proteins were visualized by fluorography using an exposure time of 95 h. Arrows in A and B point to proteins that were induced greater than 2-fold by hypoxic treatment. ADH was identified by western blot and confirmed by MS.
During 4 h of hypoxic acclimation, incorporation of [35S]Met into total, acid-precipitable protein was reduced to 48% to 56% of that in normoxic root tips. Incorporation of label into the 262 proteins resolved in Figure 2 was likewise depressed during hypoxic acclimation, to 53% of that in normoxic controls. Hypoxia depressed the synthesis of most normoxic proteins, while the synthesis of seven proteins, including ADH, was enhanced more than 2-fold (Fig. 2, A and B, arrows). The patterns of protein synthesis in normoxic and hypoxic root tips show clear differences, but also many more similarities than the anaerobic response of whole maize roots described by Sachs et al. (1980), in which aerobic protein synthesis was halted. The labeling of normoxic proteins during acclimation was not due simply to run-off of normoxic protein synthesis during the transition into hypoxia; a virtually identical pattern was obtained when labeling was restricted to the last 30 min of the 4-h hypoxic acclimation (data not shown). The complexity of the acclimation response required quantitative analysis by densitometry.
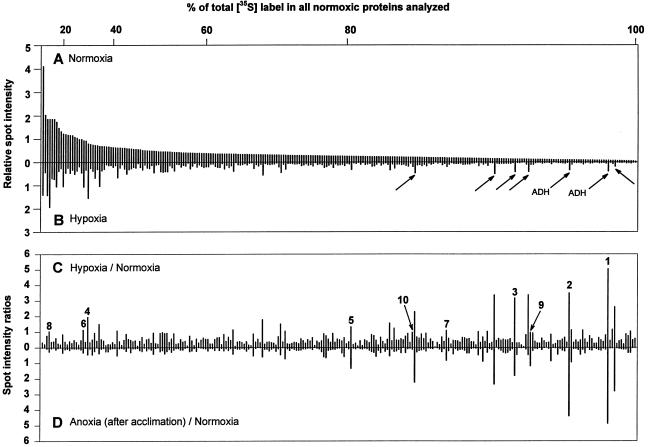
The relative amounts of [35S]Met incorporated into individual proteins during normoxia and hypoxia is shown in Figure 3, A and B. The proteins labeled during hypoxia were also made in normoxia, with less than 10% of these individual proteins being synthesized at a higher rate than in the non-stressed condition (Fig. 3C). Remarkably, the proteins most heavily labeled under normoxic conditions remained the most heavily labeled under hypoxia. For example, these proteins accounting for 20% or 40% of all labeling during normoxia (see axis above Fig. 3A) still accounted for 18% or 38%, respectively, of labeling during hypoxia. The seven most induced proteins (Figs. 2A, 2B, and 3B, arrows) accounted for only about 5% of label in the 262 proteins analyzed.
Figure 3.
Relative incorporation of [35S]Met into individual proteins in maize root tips before, during, and after acclimation. A and B, Relative densitometric intensities of 262 spots from normoxic or hypoxic root tips; spots are ranked from the most to the least intense in the fluorograph of normoxic protein synthesis. The horizontal axis above A shows the percent of radiolabel incorporated into spots to the left of each tick mark. Arrows in B indicate proteins that were induced >2-fold by hypoxic treatment, and correspond to arrows in Figure 2. C, Ratio of hypoxic to normoxic protein synthesis. D, Ratio of anoxic to normoxic protein synthesis in acclimated seedlings. Data for individual labeled proteins in C and D are arranged in the same order as A. Numbered spots in C were identified by MS analysis and are keyed to Table I: 1 and 2, ADH; 3, PDC (inconclusive); 4, actin; 5, GAPC3/4; 6 and 7, GAPC2; 8, GLU1; 9, ADH; 10, malate dehydrogenase precursor. Densities shown are from the gels in Figure 2. Densitometric analysis of three independent replicate experiments with proteins from normoxic and hypoxic root tips gave sd values of ±0.2 for spots of relative intensities between 1 and 4, and sd values of ±0.08 for spots of relative intensities between 0.2 and 0.4.
After Hypoxic Acclimation, Synthesis of Most Proteins Is Further Reduced in Anoxia
When hypoxically acclimated seedlings were subjected to 4 h of anoxia, incorporation of [35S]Met into total root tip protein was reduced to 10% to 15% of that observed in normoxia. At the level of individual proteins, the few labeled most relative to normoxia corresponded to proteins whose synthesis was induced during hypoxia, and the extent of labeling was comparable under hypoxia and anoxia (compare Fig. 3, C and D).
Prolonged anoxic treatment of acclimated root tips gave a very different pattern of protein synthesis (Fig. 2D), which was remarkably similar to the pattern of protein synthesis observed in non-acclimated root tips early in anoxia (Fig. 2E). Given the intolerance of anoxia in non-acclimated root tips, this similarity in protein synthesis patterns suggests that proteins made later in anoxia in acclimated root tips do not contribute to improved tolerance.
Anoxia Tolerance Is Blocked by Cycloheximide When Added during Hypoxic Pretreatment But Not When Added during Anoxia
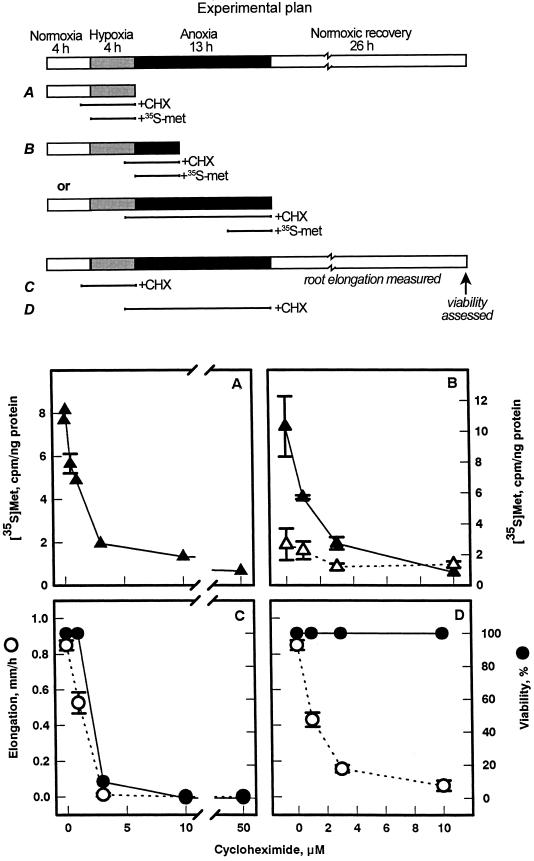
The observation that no “novel” proteins were synthesized under either hypoxia or anoxia within the scope of this study led us to examine when protein synthesis is required for acclimation. Protein synthesis in root tips of intact seedlings was inhibited with cycloheximide (Kerridge, 1958; Lin and Key 1967) added during either the hypoxic pretreatment or the subsequent anoxia.
The efficacy of cycloheximide was assessed from incorporation of [35S]Met into total protein, and tolerance of anoxia was assessed by scoring viability. Cycloheximide substantially inhibited protein synthesis in both hypoxic and anoxic root tips (Fig. 4, A and B). In the absence of protein synthesis during hypoxia, seedlings did not survive subsequent anoxia (Fig. 4C). This result is consistent with earlier studies of acclimation in roots and shoots of Arabidopsis using cycloheximide (Ellis et al., 1999). However, we also found that when cycloheximide was added during anoxia, survival was not affected (Fig. 4D), indicating that the residual protein synthesis in anoxia does not play a critical role in acclimation. The inhibition of root elongation by cycloheximide (Fig. 4D) reflects the dependence of plant growth on protein synthesis (e.g. Black et al., 1967; Coartney et al., 1967), and is not an indicator of viability.
Figure 4.
Effect of cycloheximide (CHX), during hypoxic pretreatment or subsequent anoxia, on protein synthesis and tolerance. Root tips of intact maize seedlings were treated with increasing concentrations of cycloheximide for 1 h prior to and during either 4 h of hypoxia (A and C) or 13 h of anoxia (B and D) (see schematic, “Experimental plan”). Protein synthesis was measured by adding [35S]Met throughout hypoxia (A) and during either the first (▴) or last (▵) 4 h of anoxia (B). Data shown are means ± se. In measurements of root survival (C and D), seedlings were treated sequentially with 4 h of normoxia, 4 h of hypoxia, and 13 h of anoxia, followed by a 26-h normoxic recovery period; cycloheximide was added 1 h prior to and during either 4 h of hypoxia (C) or 13 h of anoxia (D). Cycloheximide was removed at the end of hypoxia (C) and anoxia (D). Growth data are means ± se (n = 10–30); viability data are aggregates of four independent experiments from observations of a total of up to 80 seedlings for each point.
Inhibition of Protein Synthesis during Hypoxic Acclimation Compromises Cytoplasmic pH Regulation under Anoxia
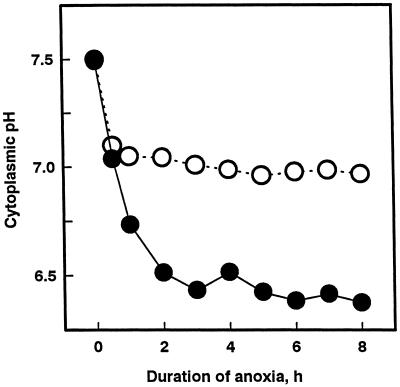
Cytoplasmic acidosis during anoxia is an important determinant of anoxia tolerance (Roberts et al., 1984; Drew, 1997), and we have shown that acclimation of maize root tips to low-oxygen stress is accompanied by a dramatic improvement in cytoplasmic pH regulation (Xia and Roberts, 1994, 1996). In light of the results presented above, we postulated that protein synthesis during acclimation contributes to improved cytoplasmic pH regulation. We tested this hypothesis by determining the effect of cycloheximide added during hypoxic acclimation on cytoplasmic pH regulation during subsequent anoxia using 31P-NMR. Roots so treated exhibited poor cytoplasmic pH regulation under anoxia; cytoplasmic pH fell from 7.5 to 6.5 within 2 h of the onset of anoxic stress (Fig. 5), a pattern of cytoplasmic acidosis characteristic of non-acclimated root tips (Xia and Roberts, 1994, 1996). In contrast, when cycloheximide was added to acclimated roots during subsequent anoxia, root tips exhibited good cytoplasmic pH regulation, maintaining a nearly neutral pH (Fig. 5), similar to regulation in acclimated root tips not exposed to cycloheximide (Xia and Roberts, 1994, 1996).
Figure 5.
Effect of cycloheximide on cytoplasmic pH regulation during anoxia in acclimated root tips. Seedlings were treated with 4 h of normoxia followed by 4 h of hypoxia, then transferred to NMR sample tubes and subjected to anoxia. Cycloheximide (10 μm) was added either 1 h prior to and during hypoxia (●) or 1 h before and during anoxia (○). Cytoplasmic pH was estimated from the chemical shift of the cytoplasmic 31Pi-NMR resonance (Roberts, 1986).
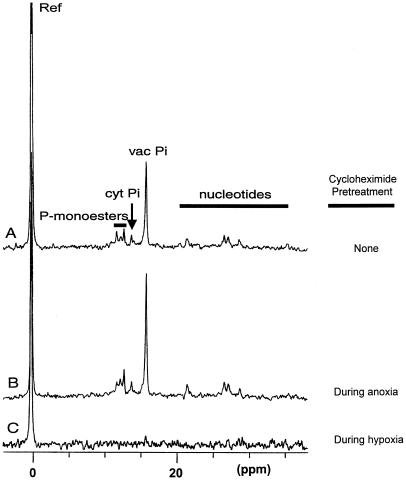
31P-NMR spectra of root tips recorded after these different cycloheximide treatments and a normoxic recovery period confirmed that prevention of cytoplasmic acidosis correlates with tolerance of anoxia. Root tips of acclimated seedlings treated with 10 μm cycloheximide during anoxia retained metabolites such as sugar phosphates and nucleotides and gave distinct cytoplasmic and vacuolar Pi signals, indicating maintenance of the pH gradient between cytoplasm and vacuole (compare Fig. 6, A and B). These spectroscopic signatures are characteristic of living root tips (Roberts and Testa, 1988), and confirm the viability measurements in Figure 4. In contrast, roots that had been exposed to 10 μm cycloheximide during hypoxic pretreatment lost essentially all of these spectroscopic signatures (Fig. 6C). These results indicate that hypoxic protein synthesis during acclimation is required for improved cytoplasmic pH regulation during anoxia, which is crucial for anoxia tolerance.
Figure 6.
Effect of cycloheximide on 31P metabolites in root tips of intact seedlings following anoxia. Maize seedlings were treated for 4 h under normoxia and 4 h of hypoxia in funnels, and then transferred to the NMR sample tubes. Spectra were recorded following 13 h of anoxia and approximately 24 h of normoxic recovery. A, No cycloheximide (control). B, Cycloheximide (10 μm) added during the final hour of hypoxia and throughout anoxia, and then removed after anoxia. C, Cycloheximide (10 μm) added 1 h prior to and during hypoxia, and then removed after hypoxia.
Identification of Maize Root Tip Proteins Synthesized during Hypoxic Acclimation
Having defined the time period when protein synthesis was critical for acclimation to low-oxygen stress, we focussed on identifying which proteins contribute to the adaptive response. The complexity of the pattern of protein synthesis during acclimation (Figs. 2B and 3) required an approach capable of identifying large numbers of proteins with a high rate of success. Previous studies of plant stress responses at the protein level have either described patterns of synthesis of large arrays of proteins on two-dimensional gels, where few if any were identified, or have focused on one or a few known proteins. Neither approach is capable of unraveling complex physiological responses, in which the expression of many genes combines to give improved plant performance. In the present study, we tested a new and promising strategy using MS to analyze tryptic digests of proteins following the methods of Clauser et al. (1995) and Qiu et al. (1998).
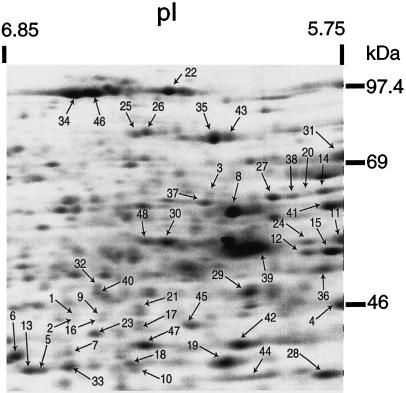
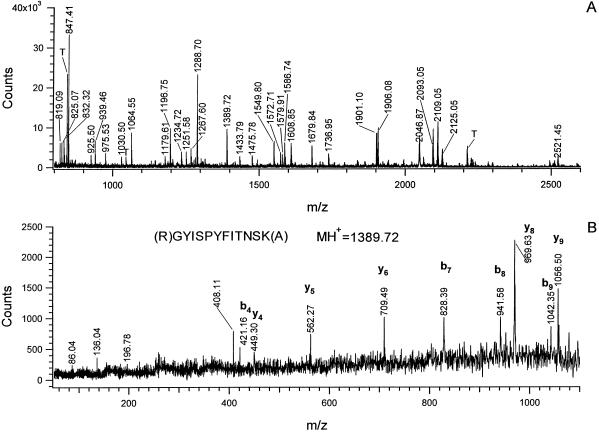
Forty-eight of the 262 protein spots resolved by two-dimensional IEF-SDS-PAGE (shown in Fig. 7) were excised from gels, digested with trypsin, and analyzed by MALDI-MS. These spots were chosen because they were well resolved when visualized with Coomassie or silver staining, and included proteins with a range of Mrs, pIs, and rates of synthesis under hypoxia. Mass spectra such as those shown in Figure 8A were obtained from each spot with sufficient signal to search databases using ProteinProspector (see “Materials and Methods”). The identities of 46 protein spots and the matching sequences for each peptide mass are listed in Table I, ranked in order of their relative rates of synthesis under hypoxia versus normoxia. In two cases, tryptic fragments derived from a single protein spot were matched to two different proteins, indicating comigration (spots 11 and 48). Here, spectral peaks attributed to one protein were subtracted prior to a second database search (Jensen et al., 1997). Additional sequence information for selected peptides (Table I, bold, underlined) was obtained by post-source decay for 20 proteins (see Fig. 8B for a typical PSD spectrum) (Qiu et al., 1998).
Figure 7.
Maize root tip proteins analyzed by MS. Figure is a fluorograph of proteins labeled in vivo during normoxia, and separated by two-dimensional IEF-SDS-PAGE (see Fig. 2A). Proteins are ranked and numbered according to the ratio of [35S]Met incorporation under hypoxia relative to normoxia, with 1 being the highest. Results of the MS analysis are presented in Table I using the same numbering scheme.
Figure 8.
A, MALDI-DE-TOF peptide mass fingerprint spectrum of a peptide mixture from in-gel tryptic digestion of protein spot 41. Masses labeled on the spectrum are the largest in each isotope cluster. Only the mono-isotopic masses were used for database searches. B, MALDI-TOF-PSD spectrum of a peptide with mass at m/z 1,389.72 from the tryptic digestion of spot 41. PSD spectrum was acquired by selecting the specific peptide from the tryptic mixture by precursor ion gating. Fragment ion masses from this spectrum were used as the fragment ion tag for spot 41 in an MS-Tag database search. The partial amino acid sequence deduced from the fragment ion masses and the mono-isotopic mass of the precursor ion are shown above the spectrum. Peptide backbone cleavage ions associated with charge retention at the N terminus are labeled b, while those with C-terminal charge retention are labeled y (for nomenclature of fragment ions, see Biemann, 1990). T, Trypsin autolytic products. I = 86.04, Y = 136.04, IT-H2O = 196.78, PYF = 408.11.
Table I.
Summary of data for 48 maize seedling root tip proteins from 48 two-dimensional PAGE gel spots
| Spot No. | MALDI Massa | Difference from Calculated Mass | Peptide Sequences Matchedb | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| D | D | %d | |||||
| 1 | 901.49 | 0.44 | (K)GQTPVFPR(I) | ADH 1 | 40.9 /42 | 6.43 /6.64 | 505.6 |
| 995.54 | −0.01 | (K)INPQAPLDK(V) | Z. mays | ||||
| 1,083.62 | 0.00 | (R)IIGVDLNPSR(F) | (X04049) | ||||
| 1,186.60 | 0.00 | (K)GTFFGNYKPR(T) | 42% | ||||
| 1,258.60 | 0.00 | (K)THPMNFLNER(T) | Consistent with | ||||
| 1,274.59 | −0.01 | (K)THPMet-oxNFLNER(T) | Western-blot result | ||||
| 1,325.57 | −0.01 | (K)SAESNMet-oxCDLLR(I) | |||||
| 1,340.65 | −0.02 | (R)KFGCTEFVNPK(D) | |||||
| 1,421.73 | −0.01 | (R)TDLPNVVELYMK(K) | |||||
| 1,437.72 | −0.01 | (R)TDLPNVVELYMet-oxK(K) | |||||
| 1,502.80 | 0.00 | (K)FITHSVPFAEINK(A) | |||||
| 1,874.01 | 0.00 | (K)GSTVAVFGLGAVGLAAAEGAR(I) | |||||
| 2,209.01 | −0.06 | (K)DHNKPVQEVLAEMTNGGVDR(S) | |||||
| 2,877.41 | −0.07 | (K)AAVAWEAGKPLSIEEVEVAPPQAMEVR(V) | |||||
| 2,893.41 | −0.07 | (K)AAVAWEAGKPLSIEEVEVAPPQAMet-oxEVR(V) | |||||
| 1,004.54, 1,212.66, 1,290.60, 2,010.00c | |||||||
| 2 | 901.49 | 0.00 | (K)GQTPVFPR(I) | ADH 1 | 40.9 /41 | 6.43 /6.64 | 349.5 |
| 995.53 | −0.02 | (K)INPQAPLDK(V) | Z. mays | ||||
| 1,083.61 | −0.01 | (R)IIGVDLNPSR(F) | (X04049) | ||||
| 1,186.60 | 0.00 | (K)GTFFGNYKPR(T) | 53% | ||||
| 1,212.61 | 0.04 | (K)FGCTEFVNPK(D) | Consistent with | ||||
| 1,258.60 | 0.00 | (K)THPMNFLNER(T) | Western-blot result | ||||
| 1,274.59 | −0.01 | (K)THPMet-oxNFLNER(T) | |||||
| 1,309.58 | −0.01 | (K)SAESNMCDLLR(I) | |||||
| 1,325.57 | −0.01 | (K)SAESNMet-oxCDLLR(I) | |||||
| 1,340.66 | −0.01 | (R)KFGCTEFVNPK(D) | |||||
| 1,421.74 | 0.01 | (R)TDLPNVVELYMK(K) | |||||
| 1,437.73 | 0.00 | (R)TDLPNVVELYMet-oxK(K) | |||||
| 1,502.79 | −0.01 | (K)FITHSVPFAEINK(A) | |||||
| 1,874.01 | 0.00 | (K)GSTVAVFGLGAVGLAAAEGAR(I) | |||||
| 2,144.01 | −0.04 | (K)ILFTSLCHTDVYFWEAK(G) | |||||
| 2,209.00 | −0.07 | (K)DHNKPVQEVLAEMTNGGVDR(S) | |||||
| 2,356.26 | −0.01 | (K)VCVLSCGISTGLGASINVAKPPK(G) | |||||
| 2,877.44 | −0.04 | (K)AAVAWEAGKPLSIEEVEVAPPQAMEVR(V) | |||||
| 2,893.47 | −0.01 | (K)AAVAWEAGKPLSIEEVEVAPPQAMet-oxEVR(V) | |||||
| 1,004.53, 1290.60, 1,783.87, 2,010.02 | |||||||
| 3 | 989.52 | 0.01 | (K)ELLEWGSR(V) | Homologous to PDC2 | 64.3 /64 | 5.90 /6.18 | 316.9 |
| 1,237.67 | 0.04 | (R)VSAANSRPPNPQ(−) | O. sativa | ||||
| 1,876.03 | 0.02 | (R)ILHHTIGLPDFSQELR(C) | (U38199) | ||||
| 2,613.42 | 0.07 | (R)ESKPVYLSISCNLPGLPHPTFSR(D) | 9% | ||||
| 881.28, 1,188.65, 1,589.85, 1,742.96, 2,051.09, 2,308.82, 2,820.40 | Inconclusive search result | ||||||
| 4 | 976.46 | 0.01 | (−)AGFAGDDAPR(A) | Actin | 37.2 /43.5 | 5.28 /5.7 | 195.5 |
| 1,198.72 | 0.01 | (R)AVFPSIVGRPR(H) | Z. mays | ||||
| 1,515.77 | 0.02 | (K)IWHHTFYNELR(V) | (U60511) | ||||
| 1,747.93 | 0.04 | (K)SYELPDGQVITIGAER(F) | 28% | ||||
| 1,954.10 | 0.03 | (R)VAPEEHPVLLTEAPLNPK(A) | |||||
| 3,151.72 | 0.08 | (R)TTGIVLDSGDGVSHTVPIYEGYALPHAILR(L) | |||||
| 873.03, 891.00, 945.57, 1,066.10, 1,132.55, 1,443.71, 1,459.71, 1,531.77 | |||||||
| 5 | 951.48 | 0.01 | (K)EVAVFGCR(N) | GAPC3/4 | 36.4 /35.5 | 7.02 /6.75 | 132.4 |
| 1,133.54 | 0.00 | (K)YDTVHGQWK(H) | Z. mays | ||||
| 1,305.67 | 0.02 | (K)DAPMFVVGVNEK(E) | (U45856, U45857) | ||||
| 1,321.66 | 0.01 | (K)DAPMet-oxFVVGVNEK(E) | 32% | ||||
| 1,434.78 | 0.02 | (R)AASFNIIPSSTGAAK(A) | |||||
| 1,498.87 | 0.02 | (R)VPTVDVSVVDLTVR(L) | |||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,775.84 | 0.03 | (K)LVSWYDNEWGYSTR(V) | |||||
| 2,033.11 | 0.03 | (K)FGIVEGLMet-oxTTVHAITATQK(T) | |||||
| 2,200.05 | 0.00 | (K)GILGYVEEDLVSTDFQGDSR(S) | |||||
| 1,104.63, 1,306.67, 1,319.68 | |||||||
| 6 | 1,133.55 | 0.01 | (K)YDTVHGQWK(H) | GAPC2 | 36.5 /37 | 6.40 /6.85 | 113.6 |
| 1,198.67 | 0.01 | (K)AGIALNDHFIK(L) | Z. mays | ||||
| 1,420.70 | 0.02 | (K)DAPMFVVGVNEDK(Y) | (U45858) | ||||
| 1,434.77 | 0.01 | (R)AASFNIIPSSTGAAK(A) | 35% | ||||
| 1,436.74 | 0.07 | (K)DAPMet-oxFVVGVNEDK(Y) | |||||
| 1,677.03 | 0.06 | (K)TLLFGEKPVTVFGIR(N) | |||||
| 1,788.86 | 0.06 | (K)LVSWYDNEWGYSNR(V) | |||||
| 2,191.03 | 0.04 | (K)GIMGYVEEDLVSTDFTGDSR(S) | |||||
| 2,207.01 | 0.03 | (K)GIMet-oxGYVEEDLVSTDFTGDSR(S) | |||||
| 2,609.49 | 0.11 | (K)VIHDNFGIIEGLMTTVHAITATQK(T) | |||||
| 834.46, 888.52, 1,350.77, 1,422.71, 1,663.02, 1,798.89, 1,804.86, 1,912.02, 1,968.95, 2,625.51 | |||||||
| 7 | 714.44 | −0.01 | (R)VVDLIR(H) | GAPC2 | 36.5 /37 | 6.40 /6.65 | 109.7 |
| 1,133.56 | 0.02 | (K)YDTVHGQWK(H) | Z. mays | ||||
| 1,198.71 | 0.05 | (K)AGIALNDHFIK(L) | (U45858) | ||||
| 1,498.93 | 0.08 | (R)VPTVDVSVVDLTVR(I) | 26% | ||||
| 1,677.09 | 0.12 | (K)TLLFGEKPVTVFGIR(N) | |||||
| 1,788.88 | 0.08 | (K)LVSWYDNEWGYSNR(V) | |||||
| 2,191.07 | 0.08 | (K)GIMGYVEEDLVSTDFTGDSR(S) | |||||
| 2,207.05 | 0.07 | (K)GIMet-oxGYVEEDLVSTDFTGDSR(S) | |||||
| 1,663.05, 1,800.91 | |||||||
| 8 | 807.41 | −0.03 | (R)LDYIQR(H) | GLU1 | 64.2 /60 | 6.23 /6.12 | 104.4 |
| 892.45 | −0.02 | (R)FSISWPR(I) | Z. mays | ||||
| 972.41 | 0.02 | (K)EMGMDAYR(F) | (U44773) | ||||
| 972.41 | −0.02 | (R)GDYPFSMR(S) | 12% | ||||
| 984.50 | −0.02 | (R)YGIVYVDR(N) | |||||
| 988.39 | 0.00 | (K)Emet-oxGMDAYR(F) | |||||
| 988.39 | −0.03 | (R)GDYPFSMet-oxR(S) | |||||
| 1,078.57 | 0.00 | (R)IGLAFDVMGR(V) | |||||
| 1,094.53 | −0.04 | (R)IGLAFDVMet-oxGR(V) | |||||
| 1,126.54 | −0.04 | (R)VPYGTSFLDK(Q) | |||||
| 1,830.94 | −0.01 | (R)SWDINLGWFLEPVVR(G) | |||||
| 750.03, 819.06, 1,014.12, 1,030.09, 1,066.06, 1,111.55, 1,148.53, 1,446.69, 1,927.98 | |||||||
| 9 | 901.49 | 0.00 | (K)GQTPVFPR(I) | ADH1 | 40.9 /43 | 6.43 /6.54 | 97.8 |
| 1,083.63 | 0.01 | (R)IIGVDLNPSR(F) | Z. mays | ||||
| 1,186.62 | 0.02 | (K)GTFFGNYKPR(T) | (X04049) | ||||
| 1,212.59 | 0.01 | (K)FGCTEFVNPK(D) | 27% | ||||
| 1,258.62 | 0.02 | (K)THPMNFLNER(T) | |||||
| 1,274.62 | 0.02 | (K)THPMet-oxNFLNER(T) | |||||
| 1,309.61 | 0.02 | (K)SAESNMCDLLR(I) | |||||
| 1,325.60 | 0.02 | (K)SAESNMet-oxCDLLR(I) | |||||
| 1,340.69 | 0.02 | (R)KFGCTEFVNPK(D) | |||||
| 1,421.76 | 0.03 | (R)TDLPNVVELYMK(K) | |||||
| 1,437.75 | 0.02 | (R)TDLPNVVELYMet-oxK(K) | |||||
| 1,502.83 | 0.03 | (K)FITHSVPFAEINK(A) | |||||
| 1,874.05 | 0.03 | (K)GSTVAVFGLGAVGLAAAEGAR(I) | |||||
| 1,378.67 | |||||||
| 10 | 1,219.72 | 0.01 | (R)LFGVTTLDVVR(A) | Homologous to MDH | 35.9 /35.5 | 8.80 /6.43 | 97.4 |
| 1,318.70 | 0.00 | (R)DDLFNINAGIVK(S) | precursor | ||||
| 1,809.10 | 0.03 | (K)VAILGAAGGIGQPLSLLMK(L) | M. sativa | ||||
| 1,825.10 | 0.04 | (K)VAILGAAGGIGQPLSLLMet-oxK(L) | (AF020271) | ||||
| 1,347.82, 1,861.96, 2,316.21, 2,656.37 | 12% Inconclusive search result | ||||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 11 | 866.39 | −0.01 | (R)EGNDLYR(E) | F1-ATPase, β-subunit | 59 /55 | 6.01 /5.63 | 94.5 |
| 1,134.63 | 0.01 | (K)TNHFLPIHR(E) | Z. mays | ||||
| 1,173.66 | 0.00 | (K)VVDLLAPYQR(G) | (M36087) | ||||
| 1,390.69 | 0.00 | (K)AHGGFSVFAGVGER(T) | 32% | ||||
| 1,399.77 | 0.00 | (R)VGLTGLTVAEHFR(D) | |||||
| 1,492.79 | 0.03 | (R)EMet-oxIESGVIKLDDK(Q) | |||||
| 1,723.93 | 0.01 | (R)LVLEVAQHLGENMet-oxVR(T) | |||||
| 1,864.95 | 0.01 | (R)DAEGQDVLLFIDNIFR(F) | |||||
| 2,044.06 | 0.04 | (R)pyroGluISELGIYPAVDPLDSTSR(M) | |||||
| 2,061.07 | 0.02 | (R)QISELGIYPAVDPLDSTSR(M) | |||||
| 2,186.11 | −0.04 | (R)IPSAVGYQPTLATDLGGLQER(I) | |||||
| 2,589.36 | 0.03 | (K)ITDEFTGAGAIGQVCQVIGAVVDVR(F) | |||||
| 11* | 702.41 | −0.01 | (K)SVIEVR(N) | Homologous to UDP-Glu | 51.6 /55 | 5.20 /5.63 | |
| 790.46 | 0.00 | (K)VANFLAR(F) | pyrophosphorylase | ||||
| 839.51 | 0.00 | (K)AIGINVPR(S) | H. vulgare | ||||
| 1,052.54 | 0.00 | (K)GGTLISYEGR(V) | (X91347) | ||||
| 1,312.76 | 0.00 | (K)VLQLETAAGAAIR(F) | 13% | ||||
| 2,197.98 | −0.05 | (K)YSNSNIEIHTFNQSQYPR(I) | |||||
| 965.543, 1,274.63, 1,520.79, 1,661.82, 2,438.33 | |||||||
| 12 | 675.32 | 0.00 | (K)TGAPCR(S) | ENO1 | 48.1 /52 | 5.20 /5.83 | 94.4 |
| 675.32 | −0.02 | (R)APVEPY(−) | Z. mays | ||||
| 719.38 | −0.01 | (K)ISGDSLK(D) | (X55981) | ||||
| 748.38 | 0.02 | (R)pyro-GluIFDSR(G) | 63% | ||||
| 765.42 | 0.03 | (R)QIFDSR(G) | |||||
| 806.48 | 0.03 | (K)YNQLLR(I) | |||||
| 900.45 | 0.00 | (K)TYDLNFK(E) | |||||
| 946.52 | −0.02 | (K)TCNALLLK(V) | |||||
| 978.52 | 0.02 | (K)FRAPVEPY(−) | |||||
| 992.53 | 0.00 | (K)ARQIFDSR(G) | |||||
| 1,071.52 | 0.00 | (R)AGWGVMASHR(S) | |||||
| 1,087.53 | 0.02 | (R)AGWGVMet-oxASHR(S) | |||||
| 1,143.57 | 0.00 | (K)DKTYDLNFK(E) | |||||
| 1,189.62 | 0.02 | (K)MGVEVYHNLK(S) | |||||
| 1,205.60 | 0.00 | (K)Met-oxGVEVYHNLK(S) | |||||
| 1,513.89 | 0.01 | (K)LGANAILAVSLAVCK(A) | |||||
| 1,535.80 | 0.04 | (R)IEEELGDAAVYAGAK(F) | |||||
| 1,551.89 | 0.03 | (K)IPLYQHIANLAGNK(T) | |||||
| 1,565.94 | 0.04 | (K)AVSNVNNIIGPAIVGK(D) | |||||
| 1,601.94 | 0.09 | (K)VNQIGSVTESIEAVR(M) | |||||
| 1,639.99 | 0.09 | (K)VQIVGDDLLVTNPTR(V) | |||||
| 1,679.99 | 0.03 | (K)KIPLYQHIANLAGNK(T) | |||||
| 1,714.88 | 0.04 | (K)VVIGMet-oxDVAASEFFGEK(D) | |||||
| 1,791.03 | 0.10 | (R)GAVPSGASTGIYEALELR(D) | |||||
| 1,835.95 | 0.07 | (R)GNPTVEVDVGLSDGSYAR(G) | |||||
| 1,903.00 | 0.07 | (K)LAMet-oxQEFMet-oxILPTGASSFK(E) | |||||
| 2,106.21 | 0.10 | (K)EAMet-oxKMGVEVYHNLKSIIK(K) | |||||
| 2,268.13 | 0.01 | (R)SGETEDTFIADLSVGLSTGQIK(T) | |||||
| 2,324.10 | 0.06 | (K)YGQDATNVGDEGGFAPNIQENK(E) | |||||
| 794.41, 1,103.5, 1,604.89, 1,773.86 | |||||||
| 13 | 951.48 | 0.00 | (K)EVAVFGCR(N) | GAPC3/4 | 36.4 /35.5 | 7.02 /6.8 | 90.8 |
| 1,133.55 | 0.01 | (K)YDTVHGQWK(H) | Z. mays | ||||
| 1,305.67 | 0.02 | (K)DAPMFVVGVNEK(E) | (U45856, U45857) | ||||
| 1,321.66 | 0.01 | (K)DAPMet-oxFVVGVNEK(E) | 32% | ||||
| 1,434.77 | 0.01 | (R)AASFNIIPSSTGAAK(A) | |||||
| 1,498.87 | 0.02 | (R)VPTVDVSVVDLTVR(L) | |||||
| 1,775.83 | 0.03 | (K)LVSWYDNEWGYSTR(V) | |||||
| 2,033.09 | 0.02 | (K)FGIVEGLMet-oxTTVHAITATQK(T) | |||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 2,200.02 | −0.02 | (K)GILGYVEEDLVSTDFQGDSR(S) | |||||
| 1,045.57, 1,104.61, 1,149.54, 1,173.82, 1,319.68, 1,987.10 | |||||||
| 14 | 910.50 | −0.01 | (R)VHILTDGR(D) | 2,3-Bisphosphoglycerate- | 60.6 /65 | 5.29 /5.76 | 87.0 |
| 1,087.56 | 0.01 | (K)GVDAQIASGGGR(M) | independent phosphoglycerate mutase | ||||
| 1,188.65 | 0.02 | (R)YLVSPPEIDR(T) | Z. mays | ||||
| 1,304.63 | 0.01 | (K)IYDGDGFNYIK(E) | (M80912) | ||||
| 1,475.67 | 0.03 | (K)ALEYADFDNFDR(V) | 23% | ||||
| 1,557.78 | 0.05 | (−)AcetN-GSSGFSWTLPDHPK(L) | |||||
| 1,589.80 | −0.01 | (K)RGWDAQVLGEAPYK(F) | |||||
| 2,434.32 | 0.08 | (R)DVLDGSSIGFVETLENDLLELR(A) | |||||
| 2,629.60 | 0.08 | (R)IQILTSHTLQPVPVAIGGPGLHPGVK(F) | |||||
| 841.06, 1,482.72, 2,312.20, 2,807.41 | |||||||
| 15 | 675.33 | 0.01 | (K)TGAPCR(S) | ENO1 | 48.1 /52 | 5.20 /5.68 | 85.6 |
| 675.33 | 0.00 | (R)APVEPY(−) | Z. mays | ||||
| 748.36 | 0.00 | (R)pyro-GluIFDSR(G) | (X55981) | ||||
| 765.39 | 0.00 | (R)QIFDSR(G) | 60% | ||||
| 806.45 | 0.00 | (K)YNQLLR(I) | |||||
| 959.55 | 0.00 | (−)Ac-AVTITWVK(A) | |||||
| 978.51 | 0.01 | (K)FRAPVEPY(−) | |||||
| 1,071.52 | 0.00 | (R)AGWGVMASHR(S) | |||||
| 1,087.52 | 0.01 | (R)AGWGVMet-oxASHR(S) | |||||
| 1,551.85 | −0.01 | (K)IPLYQHIANLAGNK(T) | |||||
| 1,601.85 | 0.00 | (K)VNQIGSVTESIEAVR(M) | |||||
| 1,639.90 | 0.00 | (K)VQIVGDDLLVTNPTR(V) | |||||
| 1,679.98 | 0.02 | (K)KIPLYQHIANLAGNK(T) | |||||
| 1,698.88 | 0.04 | (K)VVIGMDVAASEFFGEK(D) | |||||
| 1,714.84 | 0.01 | (K)VVIGMet-oxDVAASEFFGEK(D) | |||||
| 1,790.93 | 0.00 | (R)GAVPSGASTGIYEALELR(D) | |||||
| 1,835.89 | 0.01 | (R)GNPTVEVDVGLSDGSYAR(G) | |||||
| 1,886.97 | 0.03 | (K)LAMQEFMet-oxILPTGASSFK(E) | |||||
| 1,902.94 | 0.01 | (K)LAMet-oxQEFMet-oxILPTGASSFK(E) | |||||
| 2,106.16 | 0.05 | (K)EAMet-oxKMGVEVYHNLKSIIK(K) | |||||
| 2,268.08 | −0.05 | (R)SGETEDTFIADLSVGLSTGQIK(T) | |||||
| 2,324.05 | 0.01 | (K)YGQDATNVGDEGGFAPNIQENK(E) | |||||
| 2,968.39 | 0.09 | (K)SFVSEYPIESIEDPFDQDDWSTYAK(L) | |||||
| 16 | 901.49 | 0.00 | (K)GQTPVFPR(I) | ADH1 | 40.9 /42 | 6.43 /6.54 | 67.3 |
| 1,083.62 | 0.00 | (R)IIGVDLNPSR(F) | Z. mays | ||||
| 1,186.60 | 0.00 | (K)GTFFGNYKPR(T) | (X04049) | ||||
| 1,258.60 | 0.00 | (K)THPMNFLNER(T) | 37% | ||||
| 1,274.59 | −0.01 | (K)THPMet-oxNFLNER(T) | |||||
| 1,309.59 | 0.00 | (K)SAESNMCDLLR(I) | |||||
| 1,325.57 | −0.02 | (K)SAESNMet-oxCDLLR(I) | |||||
| 1,340.67 | 0.01 | (R)KFGCTEFVNPK(D) | |||||
| 1,421.73 | −0.01 | (R)TDLPNVVELYMK(K) | |||||
| 1,437.73 | 0.00 | (R)TDLPNVVELYMet-oxK(K) | |||||
| 1,502.79 | −0.01 | (K)FITHSVPFAEINK(A) | |||||
| 1,874.00 | −0.02 | (K)GSTVAVFGLGAVGLAAAEGAR(I) | |||||
| 2,144.02 | −0.04 | (K)ILFTSLCHTDVYFWEAK(G) | |||||
| 2,208.96 | −0.10 | (K)DHNKPVQEVLAEMTNGGVDR(S) | |||||
| 1,987.08, 1,993.98, 2,010.02 | |||||||
| 17 | 845.53 | 0.01 | (K)SLLIPFR(E) | Glu dehydrogenase | 44.0 /41 | 6.09 /6.42 | 65.6 |
| 888.41 | 0.01 | (R)SHSCDLR(M) | Z. mays | ||||
| 1,065.56 | 0.01 | (R)MGAFTLGVNR(V) | (D49475) | ||||
| 1,081.54 | 0.00 | (R)Met-oxGAFTLGVNR(V) | 40% | ||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,161.62 | 0.00 | (K)TAVANIPYGGAK(G) | |||||
| 1,300.62 | 0.00 | (K)DDGTLASYVGFR(V) | |||||
| 1,555.85 | 0.00 | (R)GVLFATEALLAEHGK(G) | |||||
| 1,760.86 | 0.01 | (K)GGIGCSPGDLSISELER(L) | |||||
| 2,214.15 | −0.01 | (K)FHGYSPAVVTGKPVDLGGSLGR(D) | |||||
| 2,226.10 | 0.01 | (K)YIIEAANHPTDPEADEILSK(K) | |||||
| 2,336.17 | −0.03 | (R)FVIQGFGNVGSWAAQLISEAGGK(V) | |||||
| 2,412.13 | 0.00 | (R)YHHEVDPDEVNALAQLMet-oxTWK(T) | |||||
| 970.54, 1,129.79, 1,262.66, 1,312.74, 1,655.83, 2,284.19 | |||||||
| 18 | 1,219.72 | 0.01 | (R)LFGVTTLDVVR(A) | Homologous to MDH precursor | 35.9 /36.5 | 8.80 /6.44 | 65.3 |
| 1,318.70 | 0.00 | (R)DDLFNINAGIVK(S) | M. sativa | ||||
| 1,809.10 | 0.03 | (K)VAILGAAGGIGQPLSLLMK(L) | (AF020271) | ||||
| 1,825.10 | 0.04 | (K)VAILGAAGGIGQPLSLLMet-oxK(L) | 12% | ||||
| 1,347.82, 1,861.96, 2,316.21, 2,656.37 | Inconclusive search result | ||||||
| 19 | 873.48 | 0.00 | (R)ALGQISER(L) | Cytoplasmic MDH | 35.6 /36.5 | 5.93 /6.14 | 60.8 |
| 877.45 | −0.01 | (K)NVTCLTR(L) | Z. mays | ||||
| 877.45 | −0.01 | (R)KEGMERK(D) | (AF007581) | ||||
| 974.54 | 0.01 | (K)TSTGEKPVR(E) | 39% | ||||
| 1,001.56 | 0.00 | (R)LNVQVSDVK(N) | |||||
| 1,017.52 | 0.00 | (K)EFAPSIPEK(N) | |||||
| 1,346.75 | 0.01 | (K)MELVDAAFPLLK(G) | |||||
| 1,362.74 | 0.01 | (K)Met-oxELVDAAFPLLK(G) | |||||
| 1,373.81 | 0.07 | (K)IVQGLPIDEFSR(K) | |||||
| 1,568.77 | 0.02 | (K)SQASALEAHAAPNCK(V) | |||||
| 1,650.02 | 0.03 | (K)VLVVANPANTNALILK(E) | |||||
| 1,693.83 | 0.03 | (K)FSSALSAASSACDHIR(D) | |||||
| 1,821.93 | 0.03 | (R)KFSSALSAASSACDHIR(D) | |||||
| 2,000.17 | 0.03 | (R)VLVTGAAGQIGYALVPMIAR(G) | |||||
| 2,016.16 | 0.03 | (R)VLVTGAAGQIGYALVPMet-oxIAR(G) | |||||
| 2,379.20 | 0.05 | (R)ELVSDDEWLNGEFITTVQQR(G) | |||||
| 2,466.27 | 0.06 | (K)NVIIWGNHSSSQYPDVNHATVK(T) | |||||
| 2,619.38 | 0.05 | (K)GVVATTDVVEACTGVNVAVMVGGFPR(K) | |||||
| 2,635.39 | 0.06 | (K)GVVATTDVVEACTGVNVAVMet-oxVGGFPR(K) | |||||
| 886.51, 1,277.71, 2,032.14 | |||||||
| 20 | 910.50 | −0.01 | (R)VHILTDGR(D) | 2,3-Bisphosphoglycerate- | 60.6 /64 | 5.29 /5.83 | 57.2 |
| 915.39 | −0.02 | (R)MYVTMDR(Y) | independent phosphoglycerate mutase | ||||
| 1,083.67 | −0.01 | (R)LDQLQLLLK(G) | Z. mays | ||||
| 1,087.55 | 0.00 | (K)GVDAQIASGGGR(M) | (M80912) | ||||
| 1,167.52 | −0.01 | (R)YENDWDVVK(R) | 42% | ||||
| 1,188.64 | 0.01 | (R)YLVSPPEIDR(T) | |||||
| 1,304.62 | 0.01 | (K)IYDGDGFNYIK(E) | |||||
| 1,323.68 | 0.05 | (R)YENDWDVVKR(G) | |||||
| 1,387.66 | 0.00 | (R)YAGMLQYDGELK(L) | |||||
| 1,403.65 | −0.01 | (R)YAGMet-oxLQYDGELK(L) | |||||
| 1,433.71 | 0.00 | (R)GWDAQVLGEAPYK(F) | |||||
| 1,481.71 | 0.01 | (K)FGHVTFFWNGNR(S) | |||||
| 1,557.74 | 0.01 | (−)AcetN-GSSGFSWTLPDHPK(L) | |||||
| 1,589.83 | 0.02 | (K)RGWDAQVLGEAPYK(F) | |||||
| 2,311.17 | 0.00 | (K)ESFESGTLHLIGLLSDGGVHSR(L) | |||||
| 2,420.24 | 0.07 | (R)MVMLAKALEYADNFDRVR(V) | |||||
| 2,434.25 | 0.02 | (R)DVLDGSSIGFVETLENDLLELR(A) | |||||
| 2,629.54 | 0.02 | (R)IQILTSHTLQPVPVAIGGPGLHPGVK(F) | |||||
| 2,721.27 | 0.04 | (K)AHGTAVGLPSDDDMGNSEVGHNALGAGR(I) | |||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 794.36, 1,101.55, 1,202.65, 1,277.71, 1,280.62, 1,458.68, 1,474.67, 1,742.96, 1,794.83, 1,837.08, 1,940.94, 2,230.15, 2,297.16, 2,339.20 | |||||||
| 21 | 993.48 | 0.03 | (R)HAFGDQYR(A) | Homologous to isocitrate dehydrogenase | 46.7 /44 | 6.06 /6.42 | 57.2 |
| 1,510.78 | −0.07 | (R)LVPGWTKPICIGR(H) | (NADP+) | ||||
| 1,797.86 | −0.02 | (K)GGETSTNSIASIFAWTR(G) | N. tabacum | ||||
| 1,149.58, 1,378.57, 2,108.01 | (X77944) | ||||||
| 9% Inconclusive search result | |||||||
| 22 | 622.28 | −0.01 | (R)NFEGR(V) | Homologous to aconitase | 98.2 /90 | 5.79 /6.34 | 54.6 |
| 778.42 | 0.00 | (R)GTFANIR(I) | Arabidopsis | ||||
| 914.58 | 0.01 | (R)ILLESAIR(N) | (AC007170) | ||||
| 945.42 | 0.01 | (K)DFNSYGSR(R) | 21% | ||||
| 1,009.53 | 0.02 | (K)LSVFDAAMR(Y) | |||||
| 1,025.52 | 0.01 | (K)LSVFDAAMet-oxR(Y) | |||||
| 1,073.52 | 0.01 | (R)KDFNSYGSR(R) | |||||
| 1,090.64 | 0.01 | (R)VDKLPYSIR(I) | |||||
| 1,279.57 | −0.02 | (R)DAMNKLGSDSNK(I) | |||||
| 1,292.68 | 0.02 | (K)FYSLPALNDPR(V) | |||||
| 1,497.69 | −0.03 | (R)SDETVAMIEAYLR(A) | |||||
| 1,513.71 | −0.01 | (R)SDETVAMet-oxIEAYLR(A) | |||||
| 1,578.86 | 0.02 | (R)SNLVGMet-oxGIIPLCFK(S) | |||||
| 1,739.82 | −0.02 | (R)ATYESITKGNPMWNK(L) | |||||
| 1,821.00 | 0.09 | (R)RGNDEIMARGTFANIR(I) | |||||
| 2,298.28 | 0.00 | (K)INPLVPVDLVIDHSVQVDVAR(S) | |||||
| 2,453.28 | 0.03 | (R)FDTEVELAYFNHGGILPYVIR(N) | |||||
| 713.36, 727.35, 734.41, 896.47, 1,115.59, 1,141.69, 1,202.61, 1,263.60, 1,306.59, 1,322.61, 1,381.70, 1,527.74, 1,723.84, 1,758.95, 1,804.97, 1,962.98, 2,015.10, 2,031.11, 2,254.07, 2,834.44 | |||||||
| 23 | 868.46 | 0.00 | (R)LFADFQK(R) | Homologous to Asp aminotransferase | 47.5 /40 | 7.64 /6.56 | 53.6 |
| 888.46 | 0.01 | (R)EISHQFK(V) | O. sativa | ||||
| 934.51 | 0.00 | (K)VNVGVGAYR(D) | (D67043) | ||||
| 954.44 | 0.00 | (K)Nmet-oxGLYGQR(A) | 21% | ||||
| 1,300.60 | 0.01 | (K)TFTYYHPESR(G) | |||||
| 1,388.75 | 0.01 | (R)IAAVQALSGTGACR(L) | |||||
| 1,950.93 | −0.01 | (R)IFLEDGHQIGCAQSYAK(N) | |||||
| 2,234.91 | −0.05 | (K)HFPFFDMet-oxAYQGFASGDPER(D) | |||||
| 943.54, 1,257.61, 1,339.69, 1,440.69, 1,456.70, 1,472.73, 1,874.94 | |||||||
| 24 | 790.45 | −0.01 | (K)VANFLAR(F) | Homologous to UDP-Glu pyrophosphorylase | 51.6 /54 | 5.20 /5.83 | 52.7 |
| 949.55 | 0.01 | (K)AGFISLVSR(Y) | H. vulgare | ||||
| 1,052.54 | 0.01 | (K)GGTLISYEGR(V) | (X91347) | ||||
| 1,300.72 | −0.02 | (K)SIPSIVELDSLK(V) | 17% | ||||
| 1,312.78 | 0.02 | (K)VLQLETAAGAAIR(F) | |||||
| 1,358.78 | 0.02 | (R)IVTEDFLPLPSK(G) | |||||
| 2,198.06 | 0.04 | (K)YSNSNIEIHTFNQSQYPR(I) | |||||
| 1,139.72, 1,146.61, 1,271.66, 1,398.73, 1,655.96, 1,790.98, 1,965.06 | |||||||
| 25 | 734.46 | 0.00 | (K)LLSVFR(E) | Homologous to Met synthase | 84.9 /78 | 6.10 /6.44 | 50.3 |
| 1,041.56 | 0.01 | (−)AcetN-ASHIVGYPR(M) | C. roseus | ||||
| 1,096.59 | 0.01 | (K)YLFAGVVDGR(N) | (X83499) | ||||
| 1,096.59 | 0.04 | (K)KISEDDYVK(A) | 12% | ||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,130.58 | 0.00 | (−)MASHIVGYPR(M) | |||||
| 1,130.58 | 0.01 | (R)IPSTEEIADR(I) | |||||
| 1,658.83 | 0.00 | (K)YGAGIGPGVYDIHSPR(I) | |||||
| 1,991.02 | 0.02 | (K)LQEELDIDVLVHGEPER(N) | |||||
| 2,296.33 | 0.10 | (K)LVVSTSCSLLHTAVDLVNEPK(L) | |||||
| 1,199.61, 1,555.73, 1,813.93, 1,864.87, 2,282.20, 2,424.41 | |||||||
| 26 | 684.40 | −0.01 | (R)EGLPLR(K) | Homologous to Met synthase | 84.9 /79 | 6.10 /6.4 | 48.5 |
| 734.45 | −0.01 | (K)LLSVFR(E) | C. roseus | ||||
| 958.53 | −0.02 | (R)GAKTLDLIK(G) | (X83499) | ||||
| 1,041.57 | 0.02 | (−)AcetN-ASHIVGYPR(M) | 21% | ||||
| 1,096.60 | 0.02 | (K)YLFAGVVDGR(N) | |||||
| 1,096.60 | 0.04 | (K)KISEDDYVK(A) | |||||
| 1,470.80 | 0.05 | (−)AcetN-ASHIVGYPRMet-oxGPK(R) | |||||
| 1,470.80 | 0.03 | (R)FETCYQIALAIK(D) | |||||
| 1,658.86 | 0.04 | (K)YGAGIGPGVYDIHSPR(I) | |||||
| 1,699.95 | 0.06 | (−)MASHIVGYPRMGPKR(E) | |||||
| 1,807.02 | −0.03 | (K)ILTALKGVTGFGFDLVR(G) | |||||
| 1,807.02 | 0.06 | (K)GMet-oxLTGPVTILNWSFVR(N) | |||||
| 1,864.93 | −0.06 | (R)KYAEVKPALENMet-oxVSAAK(L) | |||||
| 1,991.06 | 0.05 | (K)LQEELDIDVLVHGEPER(N) | |||||
| 2,296.31 | 0.09 | (K)LVVSTSCSLLHTAVDLVNEPK(L) | |||||
| 1,583.79, 1,814.02, 1,994.03, 2,282.18 | |||||||
| 27 | 664.33 | −0.01 | (K)FDQVR(V) | 2,3-Bisphosphoglycerate- | 60.6 /63 | 5.29 /6 | 48.2 |
| 734.40 | −0.02 | (R)IFAQGAK(L) | independent phosphoglycerate mutase | ||||
| 910.51 | 0.00 | (R)VHILTDGR(D) | Z. mays | ||||
| 931.41 | 0.01 | (R)MYVTMet-oxDR(Y) | M80912 | ||||
| 947.39 | −0.01 | (R)Met-oxYVTMet-oxDR(Y) | 16% | ||||
| 1,083.68 | 0.00 | (R)LDQLQLLLK(G) | |||||
| 1,188.65 | 0.02 | (R)YLVSPPEIDR(T) | |||||
| 1,304.65 | 0.03 | (K)IYDGDGFNYIK(E) | |||||
| 1,403.69 | 0.03 | (R)YAGMet-oxLQYDGELK(L) | |||||
| 1,433.74 | 0.03 | (R)GWDAQVLGEAPYK(F) | |||||
| 1,481.74 | 0.03 | (K)FGHVTFFWNGNR(S) | |||||
| 855.04, 861.06, 864.48, 973.52, 1,030.11, 1,046.60, 1,066.08, 1,101.57, 1,202.64, 1,280.65, 1,326.65, 1,474.70, 1,743.00, 2,338.26, 2,420.33, 2,858.71 | |||||||
| 28 | Not identified | N.A. /34 | N.A. /5.74 | 47.7 | |||
| 29 | 723.37 | 0.00 | (R)SGAYVAR(Q) | Homologous to S-adenosyl Met | 43.2 /46 | 5.74 /6.05 | 46.9 |
| 873.51 | 0.00 | (K)SIVASGLAR(R) | synthetase | ||||
| 979.49 | 0.01 | (K)TAAYGHFGR(D) | O. sativa | ||||
| 995.53 | 0.01 | (K)TQVTVEYR(N) | (Z26867) | ||||
| 1,453.80 | 0.04 | (R)FVIGGPHGDAGLTGR(K) | 42% | ||||
| 1,609.76 | −0.03 | (K)TQVTVEYRNESGAR(V) | |||||
| 1,688.90 | 0.05 | (R)DDPDFTWEVVKPLK(W) | |||||
| 1,937.05 | 0.01 | (K)EHVIKPVIPEQYLDEK(T) | |||||
| 1,963.99 | 0.03 | (K)IIIDTYGGWGAHGGGAFSGK(D) | |||||
| 2,035.10 | 0.03 | (K)ENFDFRPGMIIINLDLK(K) | |||||
| 2,417.35 | 0.09 | (K)VLVNIEQQSPDIAQGVHGHFTK(R) | |||||
| 2,649.44 | 0.11 | (R)VHTVLISTQHDETVTNDEIAADLK(E) | |||||
| 2,649.44 | 0.13 | (K)TAAYGHFGRDDPDFTWEVVKPLK(W) | |||||
| 832.31, 1,141.63, 1,414.72, 1,874.93, 2,206.08 | |||||||
| 30 | 1,074.63 | 0.02 | (K)LAANAFLAQR(I) | Homologous to UDP-Gl dehydrogenase | 52.9 /53 | 5.74 /6.3 | 46.6 |
| 1,353.66 | 0.02 | (K)AADLTYWESAAR(M) | G. max | ||||
| 1,632.78 | 0.00 | (K)FLNASVGFGGSCFQK(D) | (U53418) | ||||
| 1,784.93 | 0.05 | (K)IFDNMQKPAFVFDGR(N) | 10% | ||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,800.90 | 0.03 | (K)IFDNMet-oxQKPAFVFDGR(N) | Inconclusive search result | ||||
| 908.49, 1,365.68, 1,386.69, 1,398.67, 1,739.95, 2,004.05, 3,050.56 | |||||||
| 31 | 1,228.63 | 0.00 | (R)VEIIANDQGNR(T) | Heat shock protein 70 | 70.6 /72 | 5.22 /5.66 | 44.3 |
| 1,278.62 | −0.01 | (R)MVNHFVQEFK(R) | Z. mays | ||||
| 1,294.66 | −0.03 | (K)EIAEAYLGSTIK(N) | (P11143) | ||||
| 1,294.66 | 0.04 | (R)Met-oxVNHFVQEFK(R) | 24% | ||||
| 1,313.63 | 0.01 | (R)FEELNMDLFR(K) | |||||
| 1,329.62 | 0.00 | (R)FEELNMet-oxDLFR(K) | |||||
| 1,412.76 | 0.01 | (K)SSVHDVVLVGGSTR(I) | |||||
| 1,473.70 | 0.02 | (R)TTPSYVGFTDTER(L) | |||||
| 1,659.91 | 0.02 | (R)QATKDAGVIAGLNVMet-oxR(I) | |||||
| 1,659.91 | 0.01 | (R)IINEPTAAAIAYGLDK(K) | |||||
| 1,675.76 | 0.03 | (K)ATAGDTHLGGEDFDNR(M) | |||||
| 1,680.86 | 0.03 | (K)NAVVTVPAYFNDSQR(Q) | |||||
| 2,658.32 | 0.05 | (K)EQVFSTYSDNQPGVLIQVYEGER(A) | |||||
| 1,197.69, 1,390.63, 1,426.78, 1,437.75, 1,487.72 | |||||||
| 32 | 993.46 | 0.01 | (R)HAFGDQYR(A) | Homologous to isocitrate dehydrogenase | 46.7 /47 | 6.06 /6.56 | 43.4 |
| 1,033.59 | 0.01 | (R)NILNGTVFR(E) | (NADP) | ||||
| 1,117.59 | 0.01 | (K)YFDLGLPHR(D) | N. tabacum | ||||
| 1,170.54 | 0.02 | (K)SEGGYVWACK(N) | (X77944) | ||||
| 1,355.70 | 0.01 | (K)TIEAEAAHGTVTR(H) | 23% | ||||
| 1,510.88 | 0.02 | (R)LVPGWTKPICIGR(H) | |||||
| 1,797.90 | 0.02 | (K)GGETSTNSIASIFAWTR(G) | |||||
| 2,122.02 | 0.02 | (R)DHYLNTEEFIDAVADELK(A) | |||||
| 855.06, 1,034.58, 1,319.78, 1,378.68, 1,484.72, 1,495.84, 1,607.82 | |||||||
| 33 | 1,219.73 | 0.02 | (K)LFGVTTLDVVR(V) | Homologous to MDH | 35.7 /35.5 | 9.18 /6.64 | 39.9 |
| 1,347.81 | 0.01 | (K)KLFGVTTLDVVR(V) | B. napus (X89451) | ||||
| 1,825.80 | 0.70 | (K)VAILGAAGGIGQPLSLLMet-oxK(L) | Homologous to MDH precursor | 35.8 /35.5 | 8.00 /6.64 | ||
| M. sativa | |||||||
| (AF020271) | |||||||
| 1389.99 | 0.30 | (K)RTQDGGTEVVQAK(A) | Homologous to MDH | 38.5 /35.5 | 8.77 /6.64 | ||
| C. reinhardtii | |||||||
| (U40212) | |||||||
| 1,318.71 | 0.01 | (R)DDLFNINAGIVK(T) | Homologous to MDH glyoxysomal precursor | 37.3 /35.5 | 6.99 /6.64 | ||
| G. max | |||||||
| (P37228) | |||||||
| 34 | 744.39 | 0.00 | (R)FFAFGR(V) | Homologous to elongation factor 2 | 93.8 /90 | 5.93 /6.63 | 39.4 |
| 744.39 | 0.02 | (R)DDPKNR(S) | B. vulgaris | ||||
| 890.52 | 0.01 | (K)FSVSPVVR(V) | (Z97178) | ||||
| 1,039.67 | 0.01 | (R)IRPVLTVNK(M) | 15% | ||||
| 1,119.50 | −0.01 | (K)EGALAEENMR(G) | Consistent with Western-blot result | ||||
| 1,401.71 | 0.02 | (R)GFVQFCYEPIK(Q) | |||||
| 1,424.63 | −0.05 | (R)LWGENFFDPATK(K) | |||||
| 2,116.05 | −0.01 | (R)GHVFEEMQRPGTPLYNIK(A) | |||||
| 2,132.01 | −0.05 | (R)GHVFEEMet-oxQRPGTPLYNIK(A) | |||||
| 2,257.08 | −0.12 | (K)STLTDSLVAAAGIIAQEVAGDVR(M) | |||||
| 2,674.26 | −0.12 | (R)ITDGALVVVDCIEGVCVQTETVLR(Q) | |||||
| 2,931.34 | −0.15 | (R)KGNDYLINLIDSPGHVDFSSEVTAALR(I) | |||||
| 865.45, 955.53, 1,484.73, 1,797.91, 2,357.15 | |||||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 35 | 629.29 | −0.01 | (R)NDQPR(F) | Homologous to Met synthase | 84.9 /77 | 6.10 /6.17 | 36.8 |
| 684.40 | 0.00 | (R)EGLPLR(K) | C. roseus | ||||
| 734.46 | 0.00 | (K)LLSVFR(E) | (X83499) | ||||
| 812.50 | 0.00 | (R)EGLPLRK(A) | 23% | ||||
| 1,021.56 | 0.01 | (K)SWLAFAAQK(V) | |||||
| 1,041.58 | 0.04 | (−)AcetN-ASHIVGYPR(M) | |||||
| 1,096.62 | 0.04 | (K)YLFAGVVDGR(N) | |||||
| 1,470.79 | 0.04 | (−)AcetN-ASHIVGYPRMet-oxGPK(R) | |||||
| 1,470.79 | 0.02 | (R)FETCYQIALAIK(D) | |||||
| 1,658.90 | 0.07 | (K)YGAGIGPGVYDIHSPR(I) | |||||
| 1,791.02 | 0.05 | (K)GMLTGPVTILNWSFVR(N) | |||||
| 1,807.02 | −0.03 | (K)ILTALKGVTGFGFDLVR(G) | |||||
| 1,807.02 | 0.06 | (K)GMet-oxLTGPVTILNWSFVR(N) | |||||
| 1,848.94 | 0.06 | (K)AEHAFYLDWAVHSFR(I) | |||||
| 1,848.94 | −0.05 | (R)KYAEVKPALENMVSAAK(L) | |||||
| 1,977.05 | 0.07 | (R)KAEHAFYLDWAVHSFR(I) | |||||
| 1,991.07 | 0.06 | (K)LQEELDIDVLVHGEPER(N) | |||||
| 2,310.37 | 0.07 | (K)LNLPVLPTTTIGSFPQTLELR(R) | |||||
| 2,438.49 | 0.09 | (K)KLNLPVLPTTTIGSFPQTLELR(R) | |||||
| 1,100.5817, 1,293.66, 1,326.64, 1,517.86, 1,555.76, 1,745.03, 1,830.03, 1,957.08, 2,087.98, 2,103.98, 2,124.11, 2,178.13, 2,268.19 | |||||||
| 36 | 796.40 | −0.01 | (R)VFDMet-oxLR(R) | Translational initiation factor 4A | 47.0 /49 | 5.38 /5.75 | 35.7 |
| 976.58 | 0.02 | (K)GVAINFVTR(D) | Z. mays | ||||
| 1,053.59 | 0.02 | (R)pyroGluSLRPDNIK(M) | (U73459) | ||||
| 1,104.67 | 0.01 | (R)KGVAINFVTR(D) | 56% | ||||
| 1,114.70 | 0.01 | (R)VLITTDLLAR(G) | Consistent with Western-blot result | ||||
| 1,156.62 | 0.03 | (K)VHACVGGTSVR(E) | |||||
| 1,366.61 | 0.01 | (−)AcetN-AGMet-oxAPEGSQFDAK(H) | |||||
| 1,461.89 | 0.04 | (R)ILASGVHVVVGTPGR(V) | |||||
| 1,553.79 | 0.06 | (K)pyroGluFYVNVDKEDWK(L) | |||||
| 1,570.79 | 0.03 | (K)QFYVNVDKEDWK(L) | |||||
| 1,579.87 | 0.03 | (K)DQIYDIFQLLPSK(I) | |||||
| 1,587.76 | 0.05 | (K)Met-oxFVLDEADEMet-oxLSR(G) | |||||
| 1,800.82 | 0.07 | (R)DHTVSATHGDMet-oxDQNTR(D) | |||||
| 1,827.99 | 0.05 | (R)GIYAYGFEKPSAIQQR(G) | |||||
| 2,059.15 | 0.06 | (K)IQVGVFSATMPPEALEITR(K) | |||||
| 2,075.12 | 0.03 | (K)IQVGVFSATMet-oxPPEALEITR(K) | |||||
| 2,911.53 | 0.04 | (R)GIDVQQVSLVINYDLPTQPENYLHR(I) | |||||
| 1,563.87, 2,239.10, 2,807.32 | |||||||
| 37 | Not identified | N.A. /62 | N.A. /6.2 | 35.4 | |||
| 38 | 910.49 | −0.02 | (R)VHILTDGR(D) | 2,3-Bisphosphoglycerate- | 60.6 /63 | 5.29 /5.91 | 34.6 |
| 1,083.67 | 0.00 | (R)LDQLQLLLK(G) | independent phosphoglycerate mutase | ||||
| 1,188.64 | 0.01 | (R)YLVSPPEIDR(T) | Z. mays | ||||
| 1,304.62 | 0.00 | (K)IYDGDGFNYIK(E) | (M80912) | ||||
| 1,403.65 | 0.00 | (R)YAGMet-oxLQYDGELK(L) | 15% | ||||
| 1,433.69 | −0.01 | (R)GWDAQVLGEAPYK(F) | |||||
| 1,589.84 | 0.03 | (K)RGWDAQVLGEAPYK(F) | |||||
| 2,420.27 | 0.10 | (R)MVMLAKALEYADFDNFDRVR(V) | |||||
| 861.07, 1,030.11, 1,066.07, 1,101.58, 1,165.57, 1,280.64, 1,320.60, 1,365.65, 1,475.67, 1,482.71 | |||||||
| 39 | 765.38 | −0.01 | (R)QIFDSR(G) | ENO2 | 48.1 /53 | 5.71 /6.03 | 34.5 |
| 806.45 | 0.00 | (K)YNQLLR(I) | Z. mays | ||||
| 1,533.87 | 0.05 | (R)IEEELGAIAVYAGAK(F) | (U17973) | ||||
| 1,551.87 | 0.01 | (R)IPLYQHIANLAGNK(Q) | 36% | ||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,573.90 | 0.06 | (K)VNQIGSVTESIEAVK(M) | |||||
| 1,790.99 | 0.06 | (R)AAVPSGASTGVYEALELR(D) | |||||
| 1,984.97 | 0.06 | (R)GNPTVEVDVFCSDGTFAR(A) | |||||
| 2,252.16 | 0.03 | (R)SGETEDTFIADLAVGLSTGQIK(T) | |||||
| 2,557.41 | 0.13 | (K)MTEEIGEQVQIVGDDLLVTNPTR(V) | |||||
| 2,573.38 | 0.10 | (K)Met-oxTEEIGEQVQIVGDDLLVTNPTR(V) | |||||
| 2,986.53 | 0.15 | (K)SFVSEYPIVSIEDPFDQDDWVHYAK(M) | |||||
| 742.18, 877.05, 893.02, 1,658.89, 1,716.91, 1,830.03, 2,268.2, 2,384.06, 2,807.44 | |||||||
| 40 | 993.46 | 0.00 | (R)HAFGDQYR(A) | Homologous to isocitrate | 46 | 6.56 | 31.8 |
| 1,007.59 | 0.03 | (K)WPLYLSTK(N) | dehydrogenase | ||||
| 1,033.60 | 0.02 | (R)NILNGTVFR(E) | (NADP+) | ||||
| 1,117.59 | 0.01 | (K)YFDLGLPHR(D) | N. tabacum | ||||
| 1,147.52 | −0.02 | (K)CATITPDEAR(V) | (X77944) | ||||
| 1,170.51 | −0.01 | (K)SEGGYVWACK(N) | 27% | ||||
| 1,355.71 | 0.02 | (K)TIEAEAAHGTVTR(H) | |||||
| 1,510.89 | 0.03 | (R)LVPGWTKPICIGR(H) | |||||
| 1,797.93 | 0.05 | (K)GGETSTNSIASIFAWTR(G) | |||||
| 2,122.05 | 0.06 | (R)DHYLNTEEFIDAVADELK(A) | |||||
| 729.43, 1,151.61, 1,319.77, 1,378.70, 1,484.72, 1,495.86, 1,607.84, 1,710.74 | |||||||
| 41 | 633.32 | 0.00 | (K)SIEER(A) | Mitochondrial chaperonin 60 | 60.9 /61 | 5.67 /5.73 | 29.4 |
| 678.36 | 0.01 | (K)FGVEAR(A) | Z. mays | ||||
| 847.41 | 0.00 | (K)APGFGENR(K) | (L21006) | ||||
| 939.46 | 0.00 | (K)AIFTEGCK(S) | 35% | ||||
| 975.53 | 0.03 | (K)APGFGENRK(A) | |||||
| 1,064.55 | 0.01 | (K)LQTANFDQK(I) | |||||
| 1,196.75 | 0.01 | (K)IGVQIIQNALK(T) | |||||
| 1,251.58 | −0.01 | (K)SVAAGMet-oxNAMDLR(R) | |||||
| 1,267.60 | 0.02 | (K)SVAAGMet-oxNAMet-oxDLR(R) | |||||
| 1,288.70 | 0.01 | (R)NVVIEQSFGAPK(V) | |||||
| 1,389.72 | 0.01 | (R)GYISPYFITNSK(A) | |||||
| 1,433.79 | 0.02 | (R)GISMet-oxAVDAVVTNLK(S) | |||||
| 1,549.81 | 0.02 | (K)ELDKLQTANFDQK(I) | |||||
| 1,608.85 | 0.01 | (K)CELEDPLILIHDKK(V) | |||||
| 1,736.95 | 0.03 | (K)CELDDPLILIHEKK(I) | |||||
| 1,906.08 | 0.04 | (K)TPVHTIASNAGVEGAVVVGK(L) | |||||
| 2,093.05 | 0.03 | (R)MISTSEEIAQVGTISANGER(E) | |||||
| 2,109.05 | 0.04 | (R)Met-oxISTSEEIAQVGTISANGER(E) | |||||
| 2,521.45 | 0.03 | (K)QRPLLIVAEDVESEALGTLIINK(L) | |||||
| 925.50, 1,586.74, 1,679.84, 2,046.87, 2,125.05 | |||||||
| 42 | 839.44 | 0.00 | (R)GYPFSLR(E) | Golgi associated protein se-wap41 | 41.2 /38.5 | 5.75 /6.06 | 26.5 |
| 989.55 | 0.01 | (K)ASNPFVNLK(K) | Z. mays | ||||
| 1,152.65 | 0.05 | (K)LGTIDPYFVK(L) | (U89897) | ||||
| 1,152.65 | 0.02 | (K)LGTIDPYFVK(L) | 44% | ||||
| 1,244.66 | 0.02 | (K)CYIYLSGQVK(E) | |||||
| 1,283.74 | 0.02 | (K)DELDIVIPTIR(N) | |||||
| 1,501.73 | 0.03 | (K)VPEGFDYELYNR(N) | |||||
| 1,636.83 | 0.07 | (K)YIYTIDDDCFVAK(D) | |||||
| 2,124.11 | 0.08 | (R)DLIGPAMet-oxYFGLMGDGQPIGR(Y) | |||||
| 2,140.08 | 0.06 | (R)DLIGPAMet-oxYFGLMetoxGDGQPIGR(Y) | |||||
| 2,292.15 | 0.02 | (K)NLLSPSTPFFFNTLYDPYR(E) | |||||
| 2,292.15 | −0.05 | (K)GIFWQEDIIPFFQNVTIPK(D) | |||||
| 3,019.74 | 0.09 | (−)Acet-AGTVTVPGSSTPSTPLLKDELDIVIPTIR(N) | |||||
| 2,077.79, 3,057.74 | |||||||
| 43 | 734.45 | −0.01 | (K)LLSVFR(E) | Homologous to | 84.9 /77 | 6.10 /6.14 | 24.5 |
| 1,021.56 | 0.01 | (K)SWLAFAAQK(V) | Met synthase | ||||
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 1,041.56 | 0.02 | (−)AcetN-ASHIVGYPR(M) | C. roseus | ||||
| 1,096.59 | 0.01 | (K)YLFAGVVDGR(N) | (X83499) | ||||
| 1,096.59 | 0.04 | (K)KISEDDYVK(A) | 21% | ||||
| 1,658.86 | 0.03 | (K)YGAGIGPGVYDIHSPR(I) | |||||
| 1,791.03 | 0.07 | (K)GMLTGPVTILNWSFVR(N) | |||||
| 1,806.98 | −0.06 | (K)ILTALKGVTGFGFDLVR(G) | |||||
| 1,806.98 | 0.03 | (K)GMet-oxLTGPVTILNWSFVR(N) | |||||
| 1,848.91 | 0.03 | (K)AEHAFYLDWAVHSFR(I) | |||||
| 1,848.91 | −0.08 | (R)KYAEVKPALENMVSAAK(L) | |||||
| 1,977.00 | 0.02 | (R)KAEHAFYLDWAVHSFR(I) | |||||
| 1,991.04 | 0.03 | (K)LQEELDIDVLVHGEPER(N) | |||||
| 2,310.32 | 0.02 | (K)LNLPVLPTTTIGSFPQTLELR(R) | |||||
| 2,438.42 | 0.02 | (K)KLNLPVLPTTTIGSFPQTLELR(R) | |||||
| 1,100.57, 1,199.58, 1,293.66, 1,829.99, 2,087.92, 2,103.92, 2,124.06, 2,178.08, 2,230.20, 2,268.14 | |||||||
| 44 | 1,219.73 | 0.03 | (K)LFGVTTLDVVR(V) | Homologous to MDH | 35.7 /34.5 | 9.18 /6.04 | 21.4 |
| 1,318.72 | 0.01 | (R)DDLFNINAGIVK(N) | B. napus | ||||
| 918.43, 1,807.91, 1,834.94, 2,656.38, 2,672.38 | (X89451) | ||||||
| 45 | 1,340.77 | 0.02 | (K)KFEAEIYVLTK(D) | Homologous to | 49.4 /41 | 6.25 /6.24 | 21.0 |
| 1,474.84 | 0.03 | (R)QVGVPSLVCFLNK(V) | mitochondrial elon- | ||||
| 1,603.87 | 0.04 | (R)GITIATAHVEYETAK(R) | gation factor Tu | ||||
| 1,632.84 | 0.04 | (K)LMDAVDEYIPDPVR(V) | Arabidopsis | ||||
| 1,648.84 | 0.05 | (K)Lmet-oxDAVDEYIPDPVR(V) | (AC004044) | ||||
| 1,759.98 | 0.05 | (K)RGITIATAHVEYETAK(R) | 15% | ||||
| 1,759.98 | 0.05 | (R)GITIATAHVEYETAKR(H) | |||||
| 1,795.99 | −0.01 | (K)KILDNGQAGDNVGLLLR(G) | |||||
| 851.51, 1,561.82, 1,589.82, 1,691.95, 1,740.96, 1,877.94, 1,891.97, 2,101.09 | |||||||
| 46 | 744.39 | 0.00 | (R)FFAFGR(V) | Homologous to elonga- | 93.8 /90 | 5.93 /6.57 | 20.2 |
| 744.39 | 0.02 | (R)DDPKNR(S) | tion factor 2 | ||||
| 890.52 | 0.01 | (K)FSVSPVVR(V) | B. vulgaris | ||||
| 1,039.69 | 0.03 | (R)IRPVLTVNK(M) | (Z97178) | ||||
| 1,304.03 | −0.57 | (K)EGALAEENMR(G) | 13% | ||||
| 2,132.01 | −0.05 | (R)GHVFEEMet-oxQRPGTPLYNIK(A) | Consistent with Western- | ||||
| 2,257.13 | −0.08 | (K)STLTDSLVAAAGIIAQEVAGDVR(M) | blot result | ||||
| 2,931.53 | 0.04 | (R)KGNDYLINLIDSPGHVDFSSEVTAALR(I) | |||||
| 861.08, 890.52, 955.54, 1,039.69, 1,484.75, 1,797.99, 2,038.03 | |||||||
| 47 | 703.35 | 0.00 | (K)ERNER(Y) | Golgi associated protein | 41 /38.5 | 5.75 /6.39 | 19.4 |
| 793.39 | 0.01 | (R)EGADFVR(G) | se-wap41 | ||||
| 826.41 | 0.00 | (K)ASCISFK(D) | Z. mays | ||||
| 839.45 | 0.01 | (R)GYPFSLR(E) | (U89897) | ||||
| 989.54 | 0.00 | (K)ASNPFVNLK(K) | 55% | ||||
| 1,021.44 | −0.01 | (R)CFGYMet-oxVSK(K) | |||||
| 1,152.60 | 0.00 | (R)YVDAVMet-oxTIPK(G) | |||||
| 1,180.64 | 0.01 | (K)DINALEQHIK(N) | |||||
| 1,201.64 | 0.00 | (K)TGLPYIWHSK(A) | |||||
| 1,239.61 | 0.03 | (R)NLDFLEMet-oxWR(A) | |||||
| 1,244.67 | 0.04 | (K)CYIYLSGQVK(E) | |||||
| 1,283.75 | 0.03 | (K)DELDIVIPTIR(N) | |||||
| 1,501.75 | −0.02 | (K)CYIYLSGQVKEK(L) | |||||
| 1,636.82 | 0.06 | (K)YIYTIDDDCFVAK(D) | |||||
| 1,775.87 | 0.08 | (K)GTLFPMet-oxCGMet-oxNLAFDR(D) | |||||
| 2,124.11 | 0.08 | (R)DLIGPAMet-oxYFGLMGDGQPIGR(Y) | |||||
| 2,140.02 | 0.00 | (R)DLIGPAMet-oxYFGLMet-oxGDGQPIGR(Y) | |||||
| 2,292.17 | −0.04 | (K)GIFWQEDIIPFFQNVTIPK(D) | |||||
| 853.44, 1,335.70, 1,677.00, 2,020.19, 3,046.82 | |||||||
| 48 | 892.49 | 0.00 | (K)LELAQYR(E) | F1-ATPase, α-subunit | 55.2 /54.5 | 5.85 /6.4 | 15.3 |
| Spot No. | MALDI Mass | Difference from Calculated Mass | Peptide Sequences Matched | Protein Identified Species (GenBank Accession No.) % of Sequence Covered | Theoretical/Observed
|
Spot
Intensity Ratio
|
|
|---|---|---|---|---|---|---|---|
| Mr/kD | pI | Hypoxic Normoxic
|
|||||
| 972.56 | 0.01 | (R)VVSVGDGIAR(V) | Z. mays | ||||
| 1,026.60 | 0.01 | (K)AVDSLVPIGR(G) | (P05494) | ||||
| 1,203.69 | 0.03 | (R)AAELTTLLESR(M) | 20% | ||||
| 1,300.74 | −0.01 | (K)TAIAIDTILNQK(Q) | |||||
| 1,537.76 | 0.02 | (R)EAFPGDVFYLHSR(L) | |||||
| 1,721.86 | −0.01 | (K)pyro-GluIVVIYAAVNGFCDR(M) | |||||
| 1,738.86 | −0.04 | (K)QIVVIYAAVNGFCDR(M) | |||||
| 2,308.14 | −0.02 | (R)EVAAFAQFGSDLDAATQALLNR(G) | |||||
| 48e | 789.42 | −0.01 | (R)VVGDPFR(K) | RF2 | 59.4 /54.5 | 6.69 /6.4 | |
| 870.55 | −0.02 | (K)IILELAAK(S) | Putative aldehyde dehydrogenase | ||||
| 917.52 | 0.00 | (R)VVGDPFRK(G) | Z. mays | ||||
| 946.50 | 0.00 | (K)TFPTLDPR(T) | (U43082) | ||||
| 1,105.62 | 0.00 | (K)FKDLNEVIK(R) | 31% | ||||
| 1,386.69 | −0.07 | (R)APAGAPPAAPSAPRR(T) | |||||
| 1,437.73 | 0.04 | (R)YGVDGGATLVTGGDR(L) | |||||
| 1,671.98 | 0.04 | (K)IAQEEIFGPVQSILK(F) | |||||
| 1,703.86 | 0.07 | (K)GVEQGPQIDDEQFNK(I) | |||||
| 1,895.96 | 0.05 | (R)TGEVIAHVAEGDAEDINR(A) | |||||
| 1,961.99 | 0.06 | (K)GFYIQPTIFSDVQDGMet-oxK(I) | |||||
| 2,556.40 | 0.13 | (R)ANASQYGLAAGVFTNSLDTANTLTR(A) | |||||
| 1,020.60, 1,062.61, 1,112.65, 1,214.71, 1,353.66, 1,365.70, 1,376.78, 1,398.70, 1,790.95, 2,004.05 | |||||||
Monoisotopic mass.
Sequences determined by PSD are in bold and underlined. Amino acids before and after a peptide sequence are in parentheses. AcetN-, acet pyro-Glu, pyroglutamate.
Unassigned peaks. Major peaks in italics.
Data obtained from densitometric analysis of individual spots (see Fig. 3).
Co-migrating protein.
Most of the root tip proteins identified are soluble metabolic enzymes. These included three anaerobic proteins: ADH1 (Sachs et al., 1980) (spots 1, 2, 9, and 16), ENO1 (enolase 1; Lal et al., 1998) (spots 12 and 15), and GAPC (Russell and Sachs, 1991) (spots 5–7 and 13). All three proteins showed comparable or increased synthesis during hypoxic acclimation relative to normoxia (Table I). A fourth protein whose synthesis was significantly induced during hypoxic acclimation (Table I, spot 3) was tentatively identified as pyruvate decarboxylase (PDC), which is also an anaerobic protein (Kelley, 1989; Kelley et al., 1991; Peschke and Sachs, 1993). This assignment was based on matches of four mass peaks to rice PDC sequences, three of which also matched maize PDC1, and on the pI and Mr of spot 3, which are comparable to the predicted values for PDC1 (Table I). However, as complete sequences for other maize PDC genes are not available, and two major peptide masses could not be assigned, this identification is inconclusive. In addition to these anaerobic proteins, two abundant proteins, actin (spot 4) and β-d-glucosidase (GLU1) (spot 8), were also synthesized at high rates during both normoxia and hypoxia (Fig. 3 and Table I).
Proteins with crucial roles in both cytoplasmic and organellar translation (eIF-4A, spot 36; eEF-2, spots 34 and 46; and mitochondrial elongation factor Tu, spot 45) were also identified. The synthesis of these factors was substantially repressed by hypoxia (Table I), which may contribute to the overall reduction in protein synthesis during low-oxygen stress. In addition, we identified proteins involved in oxidative phosphorylation (subunits of the F1-ATPase, spots 11 and 48), protein folding (mitochondrial chaperonin 60, spot 41), intracellular trafficking (Golgi-associated protein se-wap41, spots 42 and 47), and heat stress (HSP 70, spot 31).
For 20 proteins, identities were assigned by matching to homologous sequences from other species. In cases in which homologies from more than one species were matched, only the match that gave the highest MOWSE score (Pappin et al., 1995) is listed (Table I). With the exception of malate dehydrogenase, maize sequences for these proteins were either absent from the databases or incomplete. For example, spots 25, 26, 35, and 43 were identified as homologous to Met synthase from plants other than maize. Three of these spots also had one or two masses that matched a partial maize Met synthase sequence (GenBank accession no. AF093539), but these limited matches gave much lower MOWSE scores.
Multiple isoforms of many proteins were identified. These were not due to allelic variation, because we used the inbred maize line B73. Rather, they may have resulted from post-translational modifications and/or expression of genetically distinct isoforms. For example, phosphorylated proteins are readily separated on two-dimensional gels, due to an acidic pI shift. This phenomenon may account for several of the isoform pairs we identified (Fig. 7); e.g. Met synthase (spots 35, 43, and 25, 26), EF-2 (34, 46), ENO1 (12, 15), and GAPC3/4 (5, 13). More definitive was our identification of genetically distinct isoforms. Even small variations in primary amino acid sequence can give substantial differences in the number of unique peptide masses generated from each isoform. GAPC2 differs from GAPC1 by only 2.7% in primary sequence (Manjunath and Sachs, 1997), but 50% of the matched peptides were unique to GAPC2 (spots 6 and 7).
Conversely, we were unable to distinguish GAPC3 from GAPC4 because these isozymes differ by only two amino acids (0.6%) (Manjunath and Sachs, 1997), and none of the nine matches were unique to either isozyme (spots 5 and 13). These nine matches covered 32% of the protein sequence of GAPC3/4. We were also able to identify and distinguish ENO1 (spots 12, 15) and ENO2 (spot 39), which differ by 10.5% in sequence (Lal et al., 1998). ENO1 was preferentially synthesized during hypoxia (Table I). Similarly, observed peptides in spots 1, 2, 9, and 16 were identified as ADH1, since most peptide masses matched were unique to ADH1. Maize ADH1 and ADH2 share 87% sequence homology at the amino acid level (Dennis et al., 1985). Finally, for spot 8, two of the peptide masses measured could only be matched to GLU1 but not GLU2, indicating that GLU1 was the isozyme observed. A primary sequence homology of 88% is shared between GLU1 and GLU2 (Esen and Shahid, 1992; Bandaranayake and Esen, 1996).
These results demonstrate that MS can be used successfully to identify plant proteins arrayed by two-dimensional IEF-SDS-PAGE and to study complex patterns of gene expression at the protein level.
DISCUSSION
Low-oxygen stress has been shown to trigger many basic cellular responses in plants. These include early events, within 1 min to tens of minutes, of changes in free cytosolic calcium (Subbaiah et al., 1994), pH, metabolism (for review, see Xia and Roberts, 1996), and translation (for review, see Vayda and Webster, 1998). Changes in gene expression at the levels of transcription and translation have generally been studied in plants stressed for several hours (for review, see Sachs et al., 1996; Drew, 1997; Vayda and Webster, 1998). Tolerance of low-oxygen stress varies with plant species, age, cell type, and acclimation conditions, and the root tip is particularly sensitive (Drew, 1997).
In this study we first defined the minimum time period required for acclimation in the root tip as being within the first 4 h of hypoxia (Fig. 1). Experiments with the inhibitor cycloheximide support a model for acclimation in which protein synthesis during hypoxia is required for improved cytoplasmic pH regulation (Xia and Saglio, 1992) (Fig. 5) and survival (Ellis et al., 1999) (Fig. 4) during subsequent anoxia. Our results also suggest that after hypoxic acclimation, protein synthesis during anoxia is not required for effective cytoplasmic pH regulation (Fig. 5) or anoxia tolerance (Fig. 4). We consequently conclude that, although many different and complex patterns of protein synthesis occur at different oxygen tensions and times (Fig. 2), those changes in protein synthesis that occur during the first 4 h of hypoxia are most critical for acclimation.
Fermentation of sugars plays a crucial role in plant survival under anoxia (Schwartz, 1969; Bouny and Saglio, 1996; Drew, 1997), and the expression of genes involved in fermentation has been extensively studied at the mRNA and protein levels (Sachs et al., 1996). Our results are consistent with a model in which these enzymes play a role in the root tip acclimation response, because the anaerobic proteins ADH (Sachs et al., 1980), glyceraldehyde-3-P dehydrogenase (GAPC) (Russell and Sachs, 1991), and enolase (Lal et al., 1998) are among the proteins preferentially synthesized during hypoxic acclimation (Table I). However, there are several reasons to think that acclimation requires more than just enhanced fermentation via increased levels of enzymes.
First, acclimated maize root tips do not have a higher initial rate of fermentation than non-acclimated root tips; it is their ability to sustain fermentation under prolonged anoxia that is enhanced (Bouny and Saglio, 1996). Second, anaerobic proteins do not appear to be key regulatory enzymes in glycolysis (Miernyk, 1990), so increasing the amounts of these enzymes would not significantly enhance flux. Third, some of the enzymes involved in fermentation may also have other quite different biological functions (for review, see Jeffery, 1999), which may also contribute to plant survival under anoxia. For example, GAPC, in addition to catalyzing a reaction in glycolysis and gluconeogenesis, has been shown to exhibit protein kinase activity (Duclos-Vallee et al., 1998), to bind RNA (Nagy and Rigby, 1995), and to enhance ribozyme (Sioud and Jespersen, 1996) and phosphotransferase (Engel et al., 1998) activities. Elucidation of the multifaceted properties of the proteins synthesized during hypoxic acclimation would help our understanding of the mechanism of acclimation. Fourth, during hypoxic acclimation many more proteins are synthesized than contribute to fermentation, in contrast to the anaerobic response (Sachs et al., 1996). Identification of the anaerobic proteins among the large number of normoxic proteins made during acclimation was only possible following detailed quantitative analysis of patterns of protein synthesis. The induced proteins synthesized during acclimation comprise only a very small percentage of total protein synthesis, and there is no basis to infer that this small component contributes exclusively to anoxia tolerance. Finally, other cellular activities have been implicated in tolerance of low-oxygen stress, including pH regulation (Fig. 7) (Xia and Roberts, 1996), signal transduction (Subbaiah et al., 1994), and post-anoxic oxidative stress resistance (Crawford and Braendle, 1996). We observed that the enzyme GLU1 is synthesized at high rates during acclimation (Fig. 3 and Table I). This abundant protein has been implicated in hormone metabolism (Brzobohaty et al., 1993) and protection against pathogens (Cicek and Esen, 1998).
The complexity of the acclimation response described here argues against a simple relationship between the level of expression of any single gene and anoxia tolerance. Rather, multiple suites of gene products may combine to provide tolerance, and the understanding of this process requires analysis of global patterns of gene expression. At present, it is not possible to correlate increased or decreased rates of synthesis of particular proteins with their importance in the adaptive response. Many processes, including transcription, translation, post-translational modification, subcellular localization of gene products, and protein degradation, contribute to the control of gene function by regulating levels and activities of the protein products of genes. The complexity of these processes is indicated by the lack of correlation between levels of specific mRNAs and their corresponding protein (Andrews et al., 1994a; Gygi et al., 1999). Our assignments of spots on two-dimensional gels to specific proteins will be valuable in future studies of these gene products. In addition to determination of the rates of synthesis or turnover of individual proteins from radiolabeling studies, such as described here, information on protein accumulation and post-translational modifications (e.g. phosphorylation) can be readily obtained using two-dimensional gels.
The combination of two-dimensional IEF-SDS-PAGE and MS is a powerful approach to studying complex patterns of gene expression at the level of proteins (Cantor and Little, 1998), and provides both higher sensitivity and higher throughput than is possible with Edman degradation (Guerreiro et al., 1997; Damerval and Le Guilloux, 1998; Santoni et al., 1998; Kamatsu et al., 1999). Our results here show that this approach can be successfully applied to gene expression in plants: most proteins resolved on two-dimensional gels gave high-quality mass spectra, and most of these spectra allowed identification (Table I). Our continued work using this approach will be greatly aided by ongoing plant genome sequencing efforts (Walbot, 1999).
ACKNOWLEDGMENT
We thank Julia Bailey-Serres for helpful discussions.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative-Competitive Grants Program (grant no. 98351006146 to J.K.M.R.) and by the National Institutes of Health NCRR (grant no. RR 01614 to A.L.B.).
LITERATURE CITED
- Andrews DL, Cobb BG, Johnson JR, Drew MC. Hypoxic and anoxic induction of alcohol dehydrogenase in roots and shoots of seedlings of Zea mays. Plant Physiol. 1993;101:407–414. doi: 10.1104/pp.101.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, Drew MC, Johnson JR, Cobb BG. The response of maize seedlings of different ages to hypoxic and anoxic stress. Plant Physiol. 1994a;105:53–60. doi: 10.1104/pp.105.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, MacAlpine DM, Johnson JR, Kelley PM, Cobb BG, Drew MC. Differential induction of mRNAs for the glycolytic and ethanolic fermentative pathways by hypoxia and anoxia in maize seedlings. Plant Physiol. 1994b;106:1575–1582. doi: 10.1104/pp.106.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake H, Esen A. Nucleotide sequence of a beta-glucosidase (glu2) cDNA from maize (accession no. U44087) (PGR 96-009) Plant Physiol. 1996;110:1048. [Google Scholar]
- Biemann K. Nomenclature for peptide fragment ions (positive ions) Methods Enzymol. 1990;193:886–887. doi: 10.1016/0076-6879(90)93460-3. [DOI] [PubMed] [Google Scholar]
- Black M, Bullock C, Chantler EN, Clarke RA, Hanson AD, Jolley GM. Effect of inhibitors of protein synthesis on the plastic deformation and growth of plant tissues. Nature. 1967;215:1289–1290. [Google Scholar]
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Bouny JM, Saglio PH. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzobohaty B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science. 1993;262:1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- Cantor RC, Little DP. Massive attack on high-throughput biology. Nat Genet. 1998;20:5–6. doi: 10.1038/1651. [DOI] [PubMed] [Google Scholar]
- Cicek M, Esen A. Structure and expression of a dhurrinase (beta-glucosidase) from sorghum. Plant Physiol. 1998;116:1469–1478. doi: 10.1104/pp.116.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauser KR, Baker P, Burlingame AL. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem. 1999;71:2871–2882. doi: 10.1021/ac9810516. [DOI] [PubMed] [Google Scholar]
- Clauser KR, Hall SC, Smith DM, Webb JW, Andrews LE, Tran HM, Epstein LB, Burlingame AL. Rapid mass spectrometric peptide sequencing and mass matching for characterization of human melanoma proteins isolated by two-dimensional PAGE. Proc Natl Acad Sci USA. 1995;92:5072–5076. doi: 10.1073/pnas.92.11.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coartney JS, Morre DJ, Key JL. Inhibition of RNA synthesis and auxin-induced cell wall extensibility and growth by actinomycin D. Plant Physiol. 1967;42:434–439. doi: 10.1104/pp.42.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RMM, Braendle R. Oxygen deprivation stress in a changing environment. J Exp Bot. 1996;47:145–159. [Google Scholar]
- Damerval C, de Vienne D, Zivy M, Thiellement H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis. 1986;7:52–54. [Google Scholar]
- Damerval C, Le Guilloux M. Characterization of novel proteins affected by the o2 mutation and expressed during maize endosperm development. Mol Gen Genet. 1998;257:354–361. doi: 10.1007/s004380050657. [DOI] [PubMed] [Google Scholar]
- Dennis ES, Sachs MM, Gerlach WL, Finnegan EJ, Peacock WJ. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985;13:727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Duclos-Vallee JC, Capel F, Mabit H, Petit MA. Phosphorylation of the hepatitis B virus core protein by glyceraldehyde-3-phosphate dehydrogenase protein kinase activity. J Gen Virol. 1998;79:1665–1670. doi: 10.1099/0022-1317-79-7-1665. [DOI] [PubMed] [Google Scholar]
- Ellis MH, Dennis ES, Peacock WJ. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999;119:57–64. doi: 10.1104/pp.119.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M, Seifert M, Theisinger B, Seyfert U, Welter C. Glyceraldehyde-3-phosphate dehydrogenase and Nm23–H1/nucleoside diphosphate kinase A: two old enzymes combine for the novel Nm23 protein phosphotransferase function. J Biol Chem. 1998;273:20058–20065. doi: 10.1074/jbc.273.32.20058. [DOI] [PubMed] [Google Scholar]
- Esen A, Shahid M. Purification and partial characterization of maize (Zea maysL.) beta-glucosidase. Plant Physiol. 1992;98:174–182. doi: 10.1104/pp.98.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J. Post-translational regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Nong T, Bailey-Serres J. Transcriptional and post-translational processes regulate gene expression in oxygen-deprived roots of maize. Plant J. 1998;15:727–735. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Germain V, Ricard B, Raymond P, Saglio PH. The role of sugars, hexokinase, and sucrose synthase in the determination of hypoxically induced tolerance to anoxia in tomato roots. Plant Physiol. 1997;114:167–175. doi: 10.1104/pp.114.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro N, Redmond JW, Rolfe BG, Djordjevic MA. New Rhizobium leguminosarumflavonoid-induced proteins revealed by proteome analysis of differentially displayed proteins. Mol Plant-Microbe Interact. 1997;10:506–516. doi: 10.1094/MPMI.1997.10.4.506. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferey CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Podtelejnikov AV, Mann M. Identification of the components of simple protein mixtures by high-accuracy peptide mass mapping and database searching. Anal Chem. 1997;69:4741–4750. doi: 10.1021/ac970896z. [DOI] [PubMed] [Google Scholar]
- Jimenez CR, Huang L, Qiu Y-C, Burlingame AL. Application of mass spectrometry in protein identification. In: Speicher DW, editor. Current Protocols in Protein Science, Unit 16. New York: Wiley; 1998. pp. 16.1.1–16.6.7. [Google Scholar]
- Johnson J, Cobb GB, Drew MC. Hypoxic induction of anoxia tolerance in root tips of Zea mays. Plant Physiol. 1989;91:837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatsu S, Muhammad A, Rakwal R. Separation and characterization of proteins from green and etiolated shoots of rice (Oryza sativaL.): towards a rice proteome. Electrophoresis. 1999;20:630–636. doi: 10.1002/(SICI)1522-2683(19990301)20:3<630::AID-ELPS630>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kelley PM. Maize pyruvate decarboxylase mRNA is induced anaerobically. Plant Mol Biol. 1989;13:213–222. doi: 10.1007/BF00016139. [DOI] [PubMed] [Google Scholar]
- Kelley PM, Freeling M. A preliminary comparison of maize anaerobic and heat shock proteins. In: Schlesinger MJ, Ashburner M, Tissiéres A, editors. Heat Shock from Human to Bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. pp. 315–319. [Google Scholar]
- Kelley PM, Godfrey K, Lal SK, Alleman M. Characterization of the maize pyruvate decarboxylase gene. Plant Mol Biol. 1991;17:1259–1261. doi: 10.1007/BF00028743. [DOI] [PubMed] [Google Scholar]
- Kendrick NC, Johansen JJ, Lee PR, Santek DA. Optimization of an HP Scanjet for quantification of protein electrophoresis gels. Anal Biochem. 1994;219:297–304. doi: 10.1006/abio.1994.1269. [DOI] [PubMed] [Google Scholar]
- Kerridge D. The effect of actidione and other antifungal agents on nucleic acid and protein synthesis in Saccharomyces carlsbergensis. J Gen Microbiol. 1958;19:497–506. doi: 10.1099/00221287-19-3-497. [DOI] [PubMed] [Google Scholar]
- Lal SK, Lee C, Sachs MM. Differential regulation of enolase during anaerobiosis in maize. Plant Physiol. 1998;118:1285–1293. doi: 10.1104/pp.118.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Key JL. Dissociation and assembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967;26:237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Manjunath S, Sachs MM. Molecular characterization and promoter analysis of the maize cytosolic glyceraldehyde 3-phosphate dehydrogenase gene family and its expression during anoxia. Plant Mol Biol. 1997;33:97–112. doi: 10.1023/a:1005729112038. [DOI] [PubMed] [Google Scholar]
- Miernyk JA. Glycolysis, the oxidative pentose phosphate pathway and anaerobic respiration. In: Dennis DT, Turpin DH, editors. Plant Physiology, Biochemistry and Molecular Biology. Essex, UK: Longman; 1990. pp. 89–90. [Google Scholar]
- Nagy E, Rigby WFC. Glyceraldehyde-3-phosphate dehydrogenase selectively binds Au-rich RNA in the NAD+-binding region (Rossmann fold) J Biol Chem. 1995;270:2755–2769. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pappin DJC, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1995;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Peschke VM, Sachs MM. Multiple pyruvate decarboxylase genes in maize are induced by hypoxia. Mol Gen Genet. 1993;240:206–212. doi: 10.1007/BF00277058. [DOI] [PubMed] [Google Scholar]
- Qiu Y-C, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivoin mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- Roberts JKM. NMR methods for determination of intracellular pH. In: Linskens HF, Jackson J, editors. Modern Methods of Plant Analysis, New Series. 2: Nuclear Magnetic Resonance. Berlin: Springer-Verlag; 1986. pp. 106–126. [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci USA. 1984;81:6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Testa MP. 31P-NMR spectroscopy of roots of intact corn seedlings. Plant Physiol. 1988;86:1127–1130. doi: 10.1104/pp.86.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J, Capdevielle J, Cuillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Russell DA, Sachs MM. The maize glyceraldehyde-3-phosphate dehydrogenase gene family: organ-specific expression and genetic analysis. Mol Gen Genet. 1991;229:219–228. doi: 10.1007/BF00272159. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Saglio P, Germain V, Ricard B. The role of enzyme induction in the improvement of tolerance to anoxia. In: Lerner HR, editor. Plant Responses to Environmental Stresses. New York: Marcel Dekker; 1999. pp. 373–393. [Google Scholar]
- Saglio PH, Drew MC, Pradet A. Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiol. 1988;86:61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Rancillac M, Bruzan F, Pradet A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984;76:151–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni V, Rouquie D, Doumas P, Mansion M, Boutry M, Degand H, Dupree P, Packman L, Sherrier J, Prime T, Bauw G, Posada E, Rouze P, Dehais P, Sanoun I, Barlier I, Rossignol M. Use of a proteome strategy for tagging proteins present at the plasma membrane. Plant J. 1998;16:633–641. doi: 10.1046/j.1365-313x.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Schwartz D. An example of gene fixation resulting from selective advantage in suboptimal conditions. Am Nat. 1969;103:479–481. [Google Scholar]
- Sioud M, Jespersen L. Enhancement of hammerhead ri-bozyme catalysis by glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1996;257:775–789. doi: 10.1006/jmbi.1996.0201. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994;105:369–376. doi: 10.1104/pp.105.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayda ME, Webster C. Translational regulation during periods of environmental stress. In: Bailey-Serres J, Gallie DR, editors. A Look Beyond Transcription: Mechanisms Determining mRNA Stability and Translation in Plants. Rockville, MD: American Society of Plant Physiologists; 1998. pp. 102–114. [Google Scholar]
- Walbot V. Genes, genomes, genomics: what can plant biologists expect from the 1998 National Science Foundation Plant Genome Research Program? Plant Physiol. 1999;119:1151–1156. doi: 10.1104/pp.119.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Gaut RL, Browning KS, Ravel JM, Roberts JKM. Hypoxia enhances phosphorylation of eukaryotic initiation factor 4A in maize root tips. J Biol Chem. 1991a;266:23341–23346. [PubMed] [Google Scholar]
- Webster C, Kim C-Y, Roberts JKM. Elongation and termination reactions of proteins synthesis on maize root tip polyribosomes studied in a homologous cell-free system. Plant Physiol. 1991b;96:418–425. doi: 10.1104/pp.96.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J-H, Roberts JKM. Improved cytoplasmic pH regulation, increased lactate efflux, and reduced cytoplasmic lactate levels are biochemical traits expressed in root tips of whole maize seedlings acclimated to a low-oxygen environment. Plant Physiol. 1994;105:651–657. doi: 10.1104/pp.105.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J-H, Roberts JKM. Regulation of H+extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiol. 1996;111:227–233. doi: 10.1104/pp.111.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J-H, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Differential regulation of sugar-sensitive sucrose synthase by hypoxia and anoxia indicate complementary transcriptional and posttranslational response. Plant Physiol. 1998;116:1573–1583. doi: 10.1104/pp.116.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]