Abstract
Cancer is the second leading cause of death worldwide, and it is estimated that Human papillomavirus (HPV) related cancers account for 5% of all human cancers. Current HPV vaccines are extremely effective at preventing infection and neoplastic disease; however, they are prophylactic and do not clear established infections. Therapeutic vaccines which trigger cell-mediated immune responses for the treatment of established infections and malignancies are therefore required. The E6 and E7 early genes are ideal targets for vaccine therapy due to their role in disruption of the cell cycle and their constitutive expression in premalignant and malignant tissues. Several strategies have been investigated for the development of therapeutic vaccines, including live-vector, nucleic acid, peptide, protein-based and cell-based vaccines as well as combinatorial approaches, with several vaccine candidates progressing to clinical trials. With the current understanding of the HPV life cycle, molecular mechanisms of infection, carcinogenesis, tumour biology, the tumour microenvironment and immune response mechanisms, an approved HPV therapeutic vaccine seems to be a goal not far from being achieved. In this article, the status of therapeutic HPV vaccines in clinical trials are reviewed, and the potential for plant-based vaccine production platforms described.
Keywords: Therapeutic vaccine, HPV, E6 and E7, Cervical cancer, Plant-based production
1. Introduction
Cancer is a global leading cause of death [1], and it is estimated that Human papillomavirus (HPV) related cancers account for 5% of all human cancers [2], [3]. Cervical cancer is an important disease, more so than other cancers (breast, colorectal) as it affects women below the age of 45, resulting in more life years lost [4], [5]. HPV is the most common cause of cervical cancer, the 4th most common cancer in women, which results in an estimated 528,000 cases and 266,000 deaths every year [6]. There are at least 170 HPV genotypes described, which are categorised into two groups: these are the low-risk types, including HPV-6/11/40/42/43/44/54/61 and -72 which cause genital warts, and high-risk (hr) types including HPV-16/18/31/35/39/45/51/52/56/58/66 and -68, which are responsible for 99.7% of cervical cancer cases [7], [8], [9]. HPV-16 and -18 are the most prevalent types associated with cervical cancer worldwide, causing more than 70% of cases. HPVs are also responsible for many penile, vulvar and anal carcinomas and contribute to over 40% of oropharyngeal cancers [10], [11]. Persistent infection with hrHPVs results in the development of squamous intraepithelial lesions (SILs): in the cervix, these are also called cervical intraepithelial neoplasia, CIN; and in the vulva, vulval intraepithelial neoplasia (VIN). SILs can progress to malignant cancers [9].
1.1. HPV structure and pathogenesis
HPVs are small non-enveloped double-stranded DNA viruses with a genome size of approximately 8 kb [12]. The genome encodes for six early regulatory proteins - E1, E2, E4, E5, E6 and E7 - and the two late structural proteins L1 and L2. The early genes encode proteins responsible for viral DNA replication, transcription and oncogenic transformation, and the late genes encode the virus capsid proteins [13], [14]. The capsid is 50–60 nm in diameter and is arranged in a T = 7 quasi-icosahedral formation consisting of 360 copies of L1 that assemble into 72 pentamers, with up to 72 copies of L2 integrated into each capsid [15], [16].
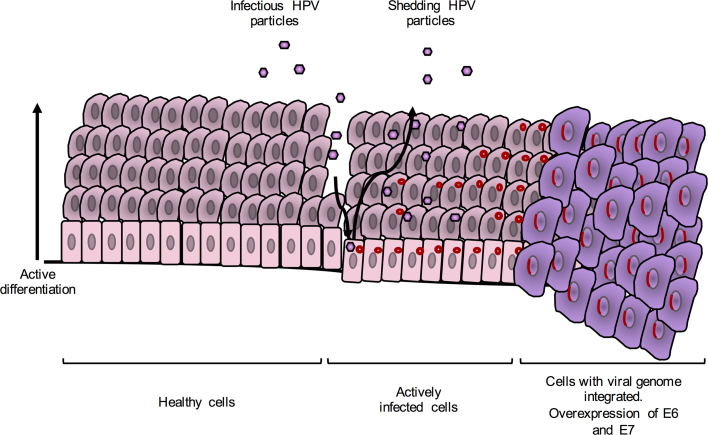
HPV infects basal epithelial cells through anatomically accessible points such as microlesions in the skin, genital organs and oropharyngeal areas. Capsid proteins L1 and L2 attach to epithelial cell receptors and a long process of entry commences, resulting in cytoplasmic uncoating of the virus and entry of its genome into the nucleus of the infected cell, where it is transcribed and then replicated. Early proteins are expressed first and regulate the host cell life cycle and genome replication. They also regulate the expression of late proteins in a cell differentiation-dependent manner: L1 and L2 are only expressed in mature squamous cells. Maturation of virions occurs after terminal differentiation of epithelial cells, and their release coincides with natural shedding of senescent cells at the end of the epithelial cell life cycle [9]. Most infections are cleared by the immune system [17], [18]; however, some benign cervical lesions progress to cancer. Continuous infection results in low-grade CIN 1 lesions. Progression to high-grade CIN 2/3 lesions caused by hrHPVs leads to invasive cervical cancer (ICC), where the viral genome may integrate into the host genome [9] (Fig. 1). In a typical hrHPV carcinogenesis, the genome of the virus is integrated into the host's chromosomal DNA and the E2 sequence is disrupted during the linearisation of the genome. The E2 protein is the transcriptional repressor of E6 and E7; therefore, expression from these genes becomes constitutive once E2 is disrupted. The E6 protein subsequently promotes the degradation of the host apoptosis regulator protein p53, and activates telomerase which results in extended cell life. The E7 protein targets the tumour suppressor retinoblastoma protein (pRb) for degradation and leads to the transition of the cell life cycle to the S-phase and subsequent host cell genome replication. E6 and E7 disrupt the cell cycle regulation and promote prolonged host cell life, leading to genomic instability and eventually cancer [9].
Fig. 1.
The life-cycle of a typical hr-HPV. Infection occurs at basal epithelial cells through anatomically accessible points such as microlesions. The genomes of HPVs stay as episomes in the host's cell nuclei. Cells proliferate and differentiate. The expression of structural proteins, L1 and L2, viral assembly and release only occur at late stages of the cell life cycle. Integration of the viral genome into the host's genome leads to overexpression of E6 and E7 which disrupt the cell life cycle regulation which promotes prolonged cell life leading to genomic instability and cancer. At this stage no viral structural proteins are expressed. Adapted from Moody and Laimins [10].
1.2. Prophylactic vaccines and limitations
Currently, there are three commercially available HPV vaccines: these are Cervarix®, a bivalent HPV-16/18 vaccine; Gardasil®, a quadrivalent HPV-6/11/16/18 vaccine; and Gardasil®9, a nonavalent HPV-6/11/16/18/31/33/45/52/58 vaccine. All exploit the fact that HPV L1 protein can form virus-like particles (VLPs) when expressed alone in a variety of cell types, that are morphologically and antigenically highly similar to native virions [19]. These three vaccines effectively prevent HPV infections caused by the targeted types by eliciting the production of neutralising antibodies that bind to the viral particles and block their entrance into host cells [20], [21], [22]. However, these vaccines are not effective at eliminating pre-existing infections, since the target antigens, L1 capsid proteins, are not expressed in infected basal epithelial cells [23], [24], [25]. Therefore, the large number of individuals already infected with HPV do not benefit from the current vaccines. Developing countries carry the greatest burden of HPV infections and malignancies due to their lack of resources to implement efficient vaccination and screening programmes [8]. Thus, many women only detect infections when they have already progressed past CIN 1 or when cancer has already developed. Additionally, the high cost of these vaccines puts them out of the reach of low-income populations. Although HPV immunization programmes have been implemented in 76 countries and territories worldwide, only 1% of women in low and low-middle income countries are covered by these programmes [26]. Furthermore, the incidence of human immunodeficiency virus (HIV) has been shown to influence HPV acquisition, the prevalence of multiple HPV types, persistence of infection and alters the carcinogenicity of hrHPV types [27], [28], [29], [30], [31], [32]. A meta-analysis looking at the carcinogenicity of HPV in HIV positive women worldwide, showed HPV-16, -18 and particularly HPV-45 in African women accounted for a greater proportion of HPV infection in ICC compared to normal cytology, and other high-risk types accounted for important proportions of other low and high-grade lesions [33]. Therefore, therapeutic vaccines that broadly target oncogenic HPV types, and that are inexpensive, are urgently required.
1.3. Therapeutic vaccines
Cell-mediated rather than humoral immune responses are important for the clearance of established infections. It has been observed that spontaneous clearance and slow progression of HPV infections are associated with a strong cell-mediated immune response involving mainly T-helper type 1 cells and cytotoxic T-cells derived from CD4+ and CD8+ T-cells, respectively [34]. The HPV E6 and E7 oncoproteins are essential for the onset and maintenance of malignancy; thus, they are unlikely to escape immune responses by mutation. They are also expressed constitutively and at high levels, and therefore represent near-ideal targets for the development of therapeutic vaccines against established HPV infections and lesions [9], [35], [36]. Other proteins useful for targeting of early viral infections are E1 (viral helicase) and E2 which are expressed at higher levels than E6 and E7 at very early stages before viral genome integration [37], [38]. An ideal therapeutic vaccine would target these proteins to induce strong tumour-specific T-cell type 1 and cytotoxic lymphocyte (CTL) responses able to kill infected and malignant cells [34]. However, there are currently no HPV therapeutic vaccines approved for use in humans. Nevertheless, there have been numerous and extensive studies that have generated promising vaccine candidates tested in clinical trials [39], [40], [41]. Despite the current success of these vaccine candidates, there remains the concern that conventional expression methods might result in expensive products [42], [43] making them inaccessible to the poorer countries who have the highest incidences.
1.4. Reducing vaccine production cost
Plants represent an attractive alternative production platform due to their scalability, rapid production and low risk of contamination [44], [45], [46], compared to traditional microbial fermentation or mammalian and insect cell expression systems. Additionally, they contain the necessary eukaryotic machinery for protein modification such as protein folding and glycosylation [44]. It has been estimated that there could be a greater than 50% reduction in cost of goods, particularly associated with upstream processes, by using plants for production of vaccines [47].
Plants can be adapted to express heterologous proteins via transgenic or transient expression systems. Transgenic expression requires the integration of a transgene into the nuclear or plastid genomes of plants. This is achieved via transformation with the soil pathogen Agrobacterium tumefaciens, or by use of biolistics or microparticle bombardment [48], [49]. Transient expression of recombinant proteins is facilitated mainly through Agrobacterium-mediated somatic gene transfer, where a T-DNA derived from the tumour-inducing (Ti) plasmid harbouring the gene of interest is transferred into the plant host genome with the help of virulence genes [50]. This system is advantageous as it allows for rapid protein production several days after cloning compared to the months needed for transgenic expression, expression levels are not affected by transgene insertions, and higher protein expression levels can generally be achieved compared to those achieved in transgenic plants [44], [51]. The use of plant virus-based vectors is another method used for transient expression: this involves the use of infectious modified plant viruses (e.g. geminiviruses, tobamoviruses and bromoviruses [52], [53], [54], [55]) to deliver a transgene to plant host cells, without the need for stable integration [56]. These vectors can potentially allow systemic spread of the virus, which is advantageous as it means very little inoculum is required. Several plant-made vaccines against cancer have been developed using viral vectors and peptide/protein-based strategies, and it is thought that these have potential for reaching the market [57].
In this article we review the various therapeutic vaccine strategies that have been explored, including live vector, nucleic acid, protein, whole cell and combinatorial vaccines. Their advantages and disadvantages are highlighted, some important examples of each strategy are discussed, and ongoing clinical trials are summarised. In addition, the current status of plant-made therapeutic vaccine candidates will be highlighted.
2. Therapeutic vaccine strategies
2.1. Live vector vaccines
Live vector vaccines are recombinant bacterial or viral vectors that can replicate inside the host cells, facilitating the spread of antigens. They can drive antigen presentation through both major histocompatibility complex (MHC) class I and class II pathways, stimulating CD8+ cytotoxic T-cells and CD4+ helper T-cells respectively, thus providing high levels of immunogenicity [35].
2.1.1. Bacterial vectors
Bacterial vector species include Listeria monocytogenes, Lactobacillus casei, Lactobacillus lactis and Salmonella. Listeria is a promising vector due to properties such as its ability to infect macrophages without being captured by phagocytosis, and its ability to direct antigen processing via MHC I and MHC II pathways [58], [59]. It also evades phagosomal lysis through secretion of listeriolysin O (LLO) [60]. L. monocytogenes (Lm) is of particular interest for vaccine development since it is able to act as a natural adjuvant. A phase I clinical trial of an E7-based vaccine called Lm-LLO-E7 in 15 patients with metastatic or advanced cervical cancer, showed an increase in E7-specific IFNγ+ T cells in 3 patients and reduction in tumour size in 4 patients [61]. Based on this study, Advaxis Inc. have designed and planned additional phase I and/or II clinical trials with ADXS11-001, in patients with metastatic anal cancer, squamous cell carcinoma (SCC) of the rectum, metastatic cervical cancer, head and neck cancer, or SCC or non-SCC of the cervix (NCT01671488, NCT02164461, NCT02291055, NCT01266460, NCT02399813 and NCT02002182) [40], [62]. A phase III trial for high-risk and advanced cervical cancer (AIM2CERV) is currently recruiting participants (NCT02853604). Oral administration of a L. casei bacterial vector vaccine GLBL101c, expressing a modified HPV-16 E7, was recently tested in a phase I/IIa clinical trial in 17 patients with HPV16+ CIN3. There was a significant increase in E7 cell mediated immunity, with 9 patients showing regression to CIN2, and 5 progressing to LSIL. No adverse side effects were experienced by any patients and this study was the first report of a therapeutic HPV vaccine to induce anti-neoplasm mucosal immunity [63].
2.1.2. Viral vectors
The efficacy of viral vectors such as adenoviruses, alphaviruses, fowlpox and vaccinia viruses has been investigated in preclinical models [25], [62], [64]. Vaccinia virus vectors have shown the most promise for antigen-specific immunotherapy [25], [39]. In clinical trials, recombinant modified vaccinia virus Ankara (MVA) viral vectors expressing HPV-16 or -18 E6 or E7 (TA-HPV) showed HPV-specific CTL responses in 28% of patients with advanced cervical cancer in a phase I/II study [65], [66], and at least a 40% reduction in lesions in 83% of patients aged 42–54 with high-grade vulval or vaginal intraepithelial neoplasia in a phase II study [67]. Most recently, a vaccine based on HPV-16 E2 (MVA E2) was shown to have 90% efficacy in the treatment of HPV-induced anogenital intraepithelial lesions in a phase III study in 1356 male and female patients [68]. Additionally, all males showed complete eradication of lesions, and HPV-specific CTL T cell responses were observed. E2 is a protein inhibitor for the expression of E6 and E7 [69], [70], and has been shown to arrest cell growth and induced apoptosis of cancer cells [71]. Therefore, vaccination with E2 may suppress E6 and E7 activity in the infected host, thereby reducing the transformation ability of infected cells and survival of HPV tumour cells. Another MVA vector - TG4001, based on HPV-16 E6/E7 and IL-2 - showed 10 out of 21 patients as clinical responders after 6 months, regression of CIN 2/3 in 7 out of 10 patients and 7 out of 8 patients showed no relapse of CIN 2/3 or HPV-16 infection after 12 months [72].
RNA replicon vaccines can be derived from RNA alphaviruses such as Sindbis virus (SIN), Venezuelan equine encephalitis virus (VEE) and Semliki forest virus (SFV) [73]. RNA replicons are capable of self-replication, resulting in sustained antigen expression and thus an increase in immunogenicity [35]. Mice vaccinated with recombinant SIN replicon VP22-E7 particles expressing the herpes simplex virus type 1 tegument protein linked to HPV-16 E7 showed a significant increase in E7-specific CD8+ T cells, and there was a strong antitumour effect [74]. A VEE E7 replicon particle (E7-VRP) vaccine prevented tumour development in all mice and showed complete protection from tumour challenge after 3 months. In a different therapeutic experiment, E7-VRP also eliminated 7-day established tumours in 67% of tumour-bearing mice [75]. In another study, a VEE E6/E7 vaccine protected 100% of mice from C3 or TC-1 tumour challenge, and eradicated C3 tumours in 90% of mice [76]. Immunization of mice with SFV replicons encoding E6 and E7 resulted in strong HPV-specific CTL responses, and the regression and elimination of established tumours [77], [78], [79], [80]. Vvax001, a replication incompetent SFV HPV-16 E6/E7 vector, is being tested in humans for the first time. A phase I trial to investigate the immune modulating effects and safety of Vvax001 is currently recruiting participants (NCT03141463).
However, there remain challenges in the use of live vector vaccines due to potential dominance of the immune response to the viral vector instead of the HPV antigen, pre-existing immunity, the generation of neutralising antibodies which restrict repeated therapy, and the potential pathogenicity of the vector particularly in immunocompromised individuals [35], [81], [82].
2.2. Subunit vaccines
Subunit vaccines are antigens delivered in the form of peptides or whole proteins. They are regarded as safer than live vector vaccines for they are present in the host cells transiently, decreasing the chances of toxicity.
2.2.1. Peptide vaccines
Peptide vaccines are stable, safe and easy to produce; however, they are often MHC specific and need to match the patient's human leukocyte antigen (HLA) type for effective presentation. Therefore, immunogenic epitopes need to be identified for each individual which makes this strategy impractical to implement in mass vaccinations [83], [84]. In addition, they have poor immunogenicity and require administration with molecules or adjuvants such as cytokines and Toll-like receptor (TLR) ligands, to improve vaccine potency for strong CD8+ T cell responses [35], [40], [85]. To overcome the HLA limitation, synthetic long overlapping peptides (SLPs) that contain E6/E7 peptides have been used and been shown to improve T cell responses. In a phase II clinical trial, vaccination of women positive for HPV-16 CIN 3 with a mixture of HPV-16 E6 and E7 SLPs and adjuvant induced the activation of CD4+ T-cells and CTL responses. At the end of the trial, 79% of women vaccinated showed positive clinical responses and 45% had complete regression of the lesions 12 months after vaccination [86]. In another phase II clinical trial, patients that had advanced or recurrent HPV-16-associated cervical cancer were treated with a mixture of 13 overlapping SLPs covering the entire sequences of HPV-16 E6 and E7. Of 16 patients tested, 56% showed vaccine-induced HPV-specific T-cell proliferation, and 85% of the 13 patients tested showed vaccine-induced immune responses. Furthermore, those with a longer survival time showed a stronger response than those that lived relatively shorter after vaccination, as seen by increased lymphocyte stimulation and anti-tumour agents such as INF-γ and α, and IL production [87]. A phase II trial with ISA101 (9 HPV-16 E6 and 4 E7 SLPs) and the drug Nivolumab is ongoing in the treatment of patients with solid tumours (NCT02426892). A phase I clinical study using PepCan, a vaccine that consists of 4 HPV-16 E6 synthetic peptides and a novel adjuvant Candin, in patients with HSIL showed that 45% of patients had regression of disease, and significant decreases in viral load [88]. A 2-arm therapy phase II trial to evaluate the efficacy and safety of PepCan (arm 1 - PepCan; arm 2 - Candin) is currently recruiting participants (NCT02481414). Another phase I study to determine the biological activity of Hespecta (HPV-16 E6 71–95 and E6 127–158 SLPs coupled to Amplivant, a synthetic TLR 2 ligand), in the treatment of HPV+ tumours or premalignant lesions is recruiting patients (NCT02821494). A phase Ib/II trial in HLA-A*02+ patients with incurable HPV+ head and neck, cervical, or anal cancer is currently recruiting participants (NCT02865135). This study aims to test the safety and efficacy of DPX-E7, a synthetic peptide consisting of HPV16-E7 amino acids 11–19, in combination with cyclophosphamide. The future of peptide-based vaccines is dependent on their immunogenicity and antigen presentation.
2.2.2. Protein-based vaccines
Unlike peptide-based vaccines, protein-based vaccines contain all antigenic HLA epitopes therefore are not MHC restricted; however, they show low immunogenicity and promote antibody responses over T cell responses due to presentation to the MHC II complex [84]. Efforts to increase immunogenicity and presentation to the MHC I pathway and activation of CD8+ T cells include the creation of fusion proteins to target antigen to dendritic cells (DCs) and the use of adjuvants [35], [89]. TA-CIN is a subunit vaccine (fusion protein composed of HPV-16 L2, E6, and E7) and has been proven safe in a number of clinical trials [90], [91], [92]. In a phase II trial treating VIN2/3, it was shown that after vaccination with TA-CIN there was an increase in CD4+ and CD8+ T cells and complete regression of VIN in 63% of patients 1 year after vaccination [93]. A phase I study to evaluate the safety of TA-CIN as an adjuvant therapy in patients with HPV-16 associated cervical cancer is expected to commence in November 2017 (NCT02405221). Fusion proteins targeting proteins to the endoplasmic reticulum (ER) have also shown improved CTL responses. A HPV-16 E7 fusion peptide, TVGV-1 (TheVax Genetics Vaccine Co.), with GP100 adjuvant, demonstrated strong HPV-specific E7 CTL responses and protection from tumour challenge and a phase IIa clinical trial is currently underway in patients with HSIL (NCT02576561). Several other clinical trials are ongoing testing the potential of therapeutic protein vaccines [39], [40]. Overall, the enhancement of immunogenicity and CD8+ T cell responses is key to the future of protein-based vaccines.
Granadillo et al. [94] developed a novel fusion protein vaccine consisting of the HPV-16 E7 protein and a peptide derived from the Limulus polyphemus anti-lipopolysaccharide factor (LALF31–52) in an E. coli expression system. LALF31–52 is a small and hydrophobic peptide that can penetrate cell membranes, and which has immunomodulatory properties [95]. Fusion of LALF31–52 to E7 (LALF-E7) increased the immunogenicity and aided in antigen presentation of E7. In a prophylactic experiment, vaccinated mice were protected against E7-expressing TC-1 cell tumour challenges. In a therapeutic experiment, tumour-bearing mice showed tumour-specific immune responses and tumour regression. The tumour protection and tumour regression were significantly higher when compared to control mice vaccinated with E7 or LALF alone. Furthermore, the anti-tumour effect of LALF-E7 was comparable in the presence or absence of an adjuvant [94]. These results confirmed the potentiating effect of the cell-penetrating peptide, and showed that LALF-E7 is promising as a HPV therapeutic vaccine.
2.2.3. Plant peptide/protein-based vaccines
The use of plant-based viral vector vaccines for protein production could both increase vaccine yield and shorten production time [55]. Plant-made early protein-derived vaccines tested in mice have elicited humoral and cell-mediated immune responses, and protection against tumour challenge. HPV-16 E7 expressed using a potato virus X (PVX) replicating vector in Nicotiana benthamiana showed E7-specific CTL responses in mice. The animals showed both Th-1 and Th-2 responses, and after challenge with C3 cells, tumour growth was inhibited in 40% of mice [96]. Tumour growth was further inhibited in 80% of mice in another study by the same group, where E7 expression was enhanced 5-fold by targeting to the plant secretory pathway [97]. The authors also suggested that plant extracts may have adjuvanting properties as mice showed a stronger immune response when vaccinated with the leaf extracts without adjuvant, and at a 20-fold lower dose than is known to prevent tumour growth, compared to the positive control group vaccinated with Escherichia coli produced E7 and Quil A adjuvant [97]. In another study, the HPV-16 E7 mutant E7GGG (E7 without a Rb binding site) fused to Clostridium thermocellum β-1,3- 1,4-glucanase or LicKM was expressed via recombinant tobacco mosaic virus (TMV). Vaccinated mice had strong humoral and cell mediated responses and tumour growth was inhibited in all animals [98], [99].
DNA encoding E7GGG was introduced into the chloroplast genome of the unicellular alga Chlamydomonas reinhardtii by homologous recombination. Sixty percent of mice injected with purified protein were protected from tumour growth, and growth was slowed in mice that developed tumours compared to the control group [100], showing that this vaccine candidate expressed in an algal system retained its therapeutic relevance. Additionally, the E7 protein in this study was soluble compared to most other E7 proteins that are insoluble, giving it the advantage of more cost-effective downstream processing. C. reinhardtii is easy to grow and transform and fully amenable to Good Manufacturing Practice (GMP) guidelines, making this a potential ideal system for vaccine production [100].
More recently, HPV-16 E7 protein bodies made in N. benthamiana by Agrobacterium-mediated transient expression were shown to elicit tumour regression in mice [101]. A shuffled E7 sequence that has no transformation ability, but which contains natural CTL epitopes [102], was fused to Zera® (Zip® Solutions, Spain), a peptide that has been shown to result in the high-level accumulation of recombinant proteins in the ER. These are localised in large, stable protein bodies that are protected from degradation and are easy to purify [103], [104]. Humoral and potent cellular immune responses were stimulated in a murine model and results were comparable to a gold standard DNA vaccine previously tested. The use of Incomplete Freund's adjuvant (IFA) in the presence of Zera® did not enhance immune responses significantly, suggesting Zera® has adjuvanting properties. This vaccine has the potential to further enhance immunogenicity using a DNA/fusion protein prime-boost regimen [101].
Yanez et al. [105] have successfully expressed the LALF-E7 vaccine described above in N. benthamiana. The highest expression levels were obtained with a self-replicating plant expression vector and chloroplast targeting, which increased accumulation 27-fold over cytoplasmic localisation. LALF32–51 -E7 was indeed targeted to the chloroplasts by the chloroplast transit peptide used, and moreover formed aggregated PB-like structures [106]. Production and extraction of the antigen was scaled up and purification was optimised by affinity chromatography; however, further optimisation of purification protocols is required before determining its therapeutic potential in animal studies.
2.3. Nucleic acid vaccines
2.3.1. DNA vaccines
DNA vaccines are safe, easy to manufacture and purify, promote MHC I antigen presentation, and unlike live vector and protein vaccines, do not produce neutralising antibodies to the vector, allowing for repeated vaccination [107]. DNA vaccines have been extensively studied and proven to be safe in clinical studies [39], [40]. The potential risk of DNA plasmids integrating into the host genome for HPV vaccines in particular has been addressed with the use of modified E6 and E7 genes that do not encode proteins with oncogenic transformation properties. However, DNA vaccines alone have been found to be poorly immunogenic. To increase their potency, strategies such as increasing the number of antigen expressing/antigen-loaded DCs, improving antigen presentation and processing, and enhancing DC and T cell interaction have been developed [108], [109]. To enhance antigen processing and presentation by DCs, Kim et al. [110] designed the DNA vaccine GX-188E (Genexine, Inc.) that co-expressed the HPV-16 and -18 antigens E6 and E7, and the Fms-like tyrosine kinase-3 ligand (Flt3L). The latter is a known DC activator and is commonly used in anti-cancer vaccine designs [111], [112], [113]. A phase I study in 9 patients with HPV-16/18+ CIN 3 elicited HPV-16 and -18 E6 and E7 specific cell-mediated responses including IFN-γ-secreting CD8+ and CD4+ T-cells, and showed HPV-specific polyfunctional CD8+ T cell responses, with 7 patients showing complete lesion regression at the end of the study [110]. A phase II trial in women with HPV-16/18+ CIN 3 lesions (NCT02139267) has been completed and another phase II trial in women with HPV-16/18+ CIN 2, CIN2/3 or CIN 3 (NCT02596243) is ongoing, with expected completion in 2018. A phase Ib/II trial to investigate the safety and efficacy of GX-188E administered intramuscularly with local administration of immunomodulators GX-I7 or Imiquimod in women with CIN 3, is currently recruiting participants (NCT03206138).
Vaccine delivery by electroporation, encapsulation, gene gun or laser therapy have also been described as methods to enhance immunogenicity [41] as they can increase the number of antigen-expressing/antigen-loaded DCs [114]. VGX-3100 (Inovio Pharmaceuticals, Inc.) is a DNA vaccine based on HPV-16/18 E6/E7, and is delivered via intramuscular injection followed by electroporation in the form of a small electrical charge. A phase I clinical trial in 18 patients who had been previously treated for CIN 2/3 showed that 14 patients (78%) developed HPV-specific CD8+ T cell responses, 17 patients (94%) had increased HPV-16 E7 antibody titres and all patients had increased HPV-18 E7 antibody titres. Furthermore, 12 patients (67%) and 7 patients (39%) had increased HPV-16 E6 or HPV-18 E6 antibody titres, respectively [115]. A follow-up phase IIb trial was conducted based on the robust antigen-specific immune responses observed and the potential to contribute to the eradication of HPV-infected cells and lesion regression. In a randomized, double blind, placebo controlled study, patients with CIN 2/3 lesions showed greater HPV-specific humoral and T cell immune responses and lesion regression accompanied by viral clearance [116]. VGX-3100 is the most successful DNA vaccine to date; it is being tested in a phase I/IIa trial in patients with HPV- associated head and neck cancer (NCT02163057) [117], as well as in a phase I/IIa trial in women with new, recurrent or persistent cervical cancer (NCT02172911). A phase III randomized, double blind, placebo controlled study (REVEAL 1) in women with confirmed CIN 2 or 3 is currently recruiting participants (NCT03185013). Another DNA vaccine, VB10.16, is in phase I/IIa to test its safety and immunogenicity in patients with CIN 2/3 (NCT02529930).
Most recently, genome editing tools such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats/Cas9 protein (CRISPR/Cas9) in the treatment of HPV-induced cancers have been shown to induce apoptosis, inhibit growth and reduce tumorigenicity in HPV+ cell lines, and to reduce the viral load in transgenic mouse models [118], [119], [120], [121], [122], [123], [124]. A phase I study to determine the efficacy of ZFN-603 and ZFN-758, which can cleave HPV-16/18 E7, in the treatment of cervical precancerous lesions has recently been completed (NCT02800369). A phase I study to test the efficacy and safety of TALEN-HPV-16 or -18 E6/E7 plasmid or CRISPR/cas9-HPV-16 or -18 E6/E7 plasmid in the treatment of HPV-related CIN is expected to begin in January 2018 (NCT03057912).
2.3.2. RNA replicon-based DNA vaccines
Single-stranded RNA vaccines have similar properties to DNA vaccines in that they are safe, do not generate neutralising antibodies and therefore can be administered multiple times. Additionally, replicating versions based on RNA viruses can replicate in various cell types [125] and pose no risk of chromosomal integration or cellular transformation. However, they are difficult to make and also cannot spread intercellularly. Suicidal DNA vaccines, where an RNA replicon is encoded in a DNA vaccine, have been designed to overcome this problem. Suicidal DNA is translated into RNA in transfected cells and triggers apoptosis, ensuring genomic integration cannot occur. However, due to apoptosis of transfected DCs, this strategy has led to poor immunogenicity [40]. To overcome this issue, the inclusion of an anti-apoptotic gene in suicidal DNA to enhance survival of APCs has been described in a preclinical model [126]. The use of a flavivirus Kunjin (KUN) vector [125], [127], also allows and prolongs direct antigen presentation by transfected DCs. The latter approach elicited E7-specific T cell responses and was protective in mice challenged with an E7-expressing tumour [127]. RNA vaccines for other cancers have progressed to clinical trials [73], [128]; however, there remains a lot of work to be done in the development of HPV RNA vaccines.
2.4. Cell-based vaccines
Cell based vaccines involve the isolation of target cells such as DCs or T cells from the patient, manipulation ex vivo, and transfer back to the patient for treatment.
2.4.1. Dendritic cell-based vaccines
The use of DCs is attractive because these cells are the major antigen presenting cells that can activate CD4+ and CD8+ T-cells [89], bypassing the need to enhance antigen visibility that is faced by protein or DNA vaccines. DCs are advantageous as they can act as natural adjuvants and increase the potency of the specific antigen [129]. DCs can either be loaded with HPV-specific peptide/protein antigens or transduced so as to express antigens, which are then delivered back to the patient [130]. A phase I study with full length HPV-16 and -18 E7 and keyhole limpet hemocyanin (KLH) showed an increase in E7-specific CD4+ T cells in patients with stage Ib or IIa cervical cancer, and 8 out of 10 patients showed production of E7-specific CD8+ T cells [131]. Additionally, the vaccine was well tolerated in all patients. In a similar study of DCs loaded with HPV-16 or -18 E7 co-administered with IL-2, specific CD4+ T cell responses were detected in 2 out of 4 patients and E7-specific CD8+ responses observed in all patients [132]. Pre-immune DCs (PIDCs) (cells lacking full T cell co-stimulatory activity, [89]) have also been shown to be effective in the treatment of patients with advanced cervical cancer. PIDCs pulsed with HPV-16 E6 or E7 induced specific immune responses in 63% (E6) or 58% (E7) of patients [133]. Other antigen presenting cells have been used to evaluate the immune response in patients with advanced or recurrent cervical cancer. Participants are currently being recruited for a phase I trial for BVAC-C, a recombinant HPV16/18 E6/E7 expressing adenovirus-infected B cells and monocytes (NCT02866006).
DC-based vaccines have several limitations, however, as they are restricted in their capacity for large-scale production due to the requirement of harvesting sufficient DCs from each patient, do not have a defined route for vaccination (critical for priming of T cells), show inconsistent vaccine quality due to variations in cell culture technique, and they have a limited lifespan due to T-cell mediated apoptosis [40], [41], [62]. To address their short life span, short interfering RNA (siRNAs) targeting pro-apoptotic molecules have been explored, which have shown enhanced E7-specific CD8+ activation and anti-tumour effects in mice [134], [135], [136]. Additionally, handling of human cells ex-vivo requires high technical competence, is labour-intensive, time consuming and costly, making this strategy impractical to implement for large scale vaccinations [35], [36]. The use of PIDCs over mature DCs has the potential to reduce vaccine production cost [133]; however, further clinical study into whether DC maturation is necessary to generate an immune response is required.
2.4.2. Adoptive cell transfer
Adoptive cell transfer (ACT) involves the generation of antigen-specific CTLs ex vivo, which are then used in vivo to enhance immunogenicity. This technique is advantageous as it generates antigen-specific CTLs that can be produced in large quantities in vitro; CTLs can be engineered or activated ex vivo to obtain antigenic functions; and it allows for manipulation of the host before cell transfer to eliminate suppressor cells such as regulatory T cells, or Tregs [62]. A pilot study in 9 patients with metastatic cervical cancer showed complete regression in 2 patients after treatment with HPV-16 E6 and E7 reactive CTLs [137]. In another study, T cell receptors against E6 were introduced into CTLs, which killed HPV+ cells from cervical and head and neck cancer cell lines [138]. Most recently, a phase I/II clinical trial (NCT02280811) targeting several HPV-associated cancers (cervical, anal, vaginal and oropharyngeal) showed regression of metastatic HPV+ carcinoma in 2 out of 12 patients, suggesting that T cell receptor therapy can facilitate epithelial cancer regression [139]. A phase I trial using T cells genetically engineered with T cell receptors targeting HPV-16 E7 with or without programmed cell death protein 1 (PD-1) blocking drugs (PD-1 is an inhibitory receptor on T cells), in patients with cervical, vaginal, anal, penile or oropharyngeal cancers, is currently recruiting participants (NCT02858310). A phase I trial using HPV-16 E6 T cell receptors for the treatment of high-grade VIN is also recruiting participants (NCT03197025).
A new experimental treatment is using HPV-specific T-cells (HPVST) derived from the blood of patients with HPV cancers, and determining if these cells can survive in the blood and affect the HPV-associated tumour [140]. Additionally, to make the T cells more active, they have been engineered to make them resistant to transforming growth factor beta (TGF-β) which is produced by HPV cancers [141]. A phase I trial in patients with relapsed HPV-associated cancers (HESTIA) is currently recruiting participants to investigate the safest dose, side effects and how long HPV-16/18 E6/E7 HPVSTs last in the body (NCT02379520).
Most recently, chimaeric antigen receptor (CAR) T cell therapy is being investigated in the treatment of cervical cancer. CAR T cells are designed to express receptors that redirect polyclonal T cells to surface-exposed tumour-associated antigens (TAAs) for subsequent tumour elimination [142]. A phase I/II study is currently recruiting participants to assess the feasibility, safety and efficacy of CAR T cells in patients with TAAs GD2, PSMA, Muc1, Mesothelin or other markers positive for cervical cancer (NCT03356795).
However, it is thought that CTLs alone may not be enough to eliminate cancer cells in patients with advanced disease. HPV cancers evade the immune system and treatment of solid tumours remains a challenge. Effective T cell therapy will need to improve and address concerns such as tumour heterogeneity, antigen escape, an immunosuppressive microenvironment, inhibition of T cell localisation [139], [140], [141] and methods to improve the state of leaky and fragile blood vessels that supply tumour cells [143], [144].
2.5. Combining different therapeutic strategies
The diversity of current therapeutic vaccine candidates for HPV-related malignancies offers an opportunity to combine some of the strategies and to better exploit their potential. Combination treatments could be the key to successful treatment of HPV lesions [145].
2.5.1. Prime-boost regimens
Heterologous prime-boost regimens can be used to enhance vaccine potency: for example, one may prime the immune system with a DNA-based vaccine, followed by a boost with a virus-based vaccine. Evaluation in clinical trials of prime-boost regimens has also been explored. In a phase II clinical trial, TA-CIN fusion protein vaccine was boosted with recombinant vaccinia virus TA-HPV in 29 patients with anogenital intraepithelial neoplasia, with 5 patients showing increased HPV-16 antigen-specific T cell-mediated immune responses [146], [147]. However, this result was not significant over TA-HPV vaccination alone. In another study, 10 patients with HPV-16+ high grade VIN were this time primed with TA-HPV and boosted with TA-CIN. Nine patients showed HPV-16 specific T-cell responses and 3 patients had a significant reduction in lesions. However, these results did not show a direct correlation between clinical and immunological responses [90]. More recently, in a phase I trial (NCT00788164) in patients with CIN 3, a pNGVL4a-sig/E7(detox)/HSP70 DNA prime followed by TA-HPV boost in combination with imiquimod is being used to evaluate therapy. pNGVL4a-sig/E7(detox)/HSP70 DNA vaccine has previously been shown to elicit systemic HPV-specific CD8 T-cell responses in women with CIN 2/3 that could traffic to the lesion, and regression was observed in some patients [148].
2.5.2. Immunomodulators
Vaccines with immunomodulatory agents that influence the tumour microenvironment can increase the success of therapeutic vaccines. There are several targets for immune modulation, including Tregs, tumour-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) [149]. Tregs release immunosuppressive cytokines (e.g. IL-10) and transforming growth factors which can affect T cell function. Depletion of CD4+ and CD25+ Tregs have been shown to enhance E7-HSP70 vaccine potency [150]. Therefore, controlling the effects of these factors could deprive tumour cells of important growth factors and enhance the antitumour response of therapeutic vaccine candidates [149], [151], [152]. IRX-2, a biological product containing multiple cytokines (e.g. IL-2, IL-β, IFN-γ and TNF-α) and produced by stimulation of mononuclear cells, has been shown to activate different cells of the immune system [153], [154] and the ability to potentiate antitumor effects of immune cells, such as upregulation of key signalling molecules' expression on DCs [155], [156]. IRX-2 in combination with cyclophosphamide, indomethacin, zinc-containing multivitamins and omeprazole (IRX-2 Regimen) has been studied in patients with squamous cell carcinoma of the head and neck [157], [158], [159], [160], and shown promise as a novel immunotherapeutic. A phase II trial in patients with CIN or VIN 3 is currently recruiting participants (NCT03267680).
In plants, the immunomodulatory properties of N. benthamiana leaf extracts has been demonstrated by Di Bonito et al. [161], who tested the effect of leaf extracts containing E7 on human monocyte derived DCs. The vaccine NbPVX-E7 (HPV-16 E7 protein expressed by a PVX-derived vector) was able to prime human blood-derived lymphocytes from healthy individuals and induce E7-specific cytotoxic activity, thereby showing the potential for their use in the immunotherapy of HPV-related lesions.
2.5.3. Checkpoint inhibitors
Immune checkpoint inhibitors disrupt cell signalling which prevents cancer cells from avoiding T-cell mediated death [162], [163]. PD-1 and cytotoxic T lymphocyte-associated transmembrane receptor 4 (CTLA-4) are immune checkpoint receptors expressed on the surface of CTLs, and interact with PD-L1 (programmed death-ligand 1) and CD80/CD86, respectively, on APCs resulting in attenuation of CTL activation [164]. Checkpoint inhibitors are advantageous over ACT therapies as they can easily be used across a number of tumour types, and do not rely on cancer-specific antigens [62]. A phase I study testing Ipilimumab, an anti-CTLA-4 monoclonal antibody, together with chemoradiation therapy is being tested in patients with advanced cervical cancer (NCT01711515). Ipilimumab is also being tested in a phase II trial in patients with HPV+ metastatic or recurrent cervical cancer (NCT01693783). A phase Ib/II trial testing TG4001 in combination with the Avelumab (human anti-PD-L1 IgG1 monoclonal antibody), in patients with oropharyngeal squamous cell carcinoma of the head and neck or metastatic malignancies is recruiting participants (NCT03260023). Dual checkpoint inhibition is being tested in 2 phase I studies with MEDI4736 (anti-PD-L1 antibody) and tremelimumab (anti-CTLA-4 antibody), in patients with advanced solid tumours (NCT02261220) and cervical cancer (NCT01975831).
2.5.4. Non-specific immunostimulants
TLR agonists are immunopotentiators which have been shown to increase activation of cell-mediated responses and viral clearance [165]. Wick and Webb [166] designed a broad-spectrum HPV therapeutic vaccine, Pentarix, consisting of the E7 sequences from the major hrHPV types 16, 18, 31, 45 and 52 that account for more than 80% of cervical cancers. They previously showed that proteins can elicit a strong cell-mediated response extracellularly when accompanied by a TLR3 agonist, poly(I:C) [167]. Here they also tested the TLR9 agonist CpG DNA. Mice vaccinated with Pentarix and either one of the adjuvants showed regression of TC-1-induced tumours after one week of vaccination. They had a complete tumour regression by week three post treatment, and remained tumour-free for at least three months. Furthermore, they showed that cluster immunization of mice with Pentarix + poly(I:C) increased the cell-mediated responses in mice as well as the rate of tumour regression compared to mice vaccinated only once. Their findings suggested that the vaccine was able to elicit a cell-mediated response strong enough to overcome the immunosuppressive tumour microenvironment. Pentarix could be used as a broad spectrum treatment for a variety of HPV-related pre- and cancerous conditions [166]. A patent for Pentarix was filed in 2012; however, no information on future clinical trials was available.
2.5.5. Therapeutic vaccines with other therapies
Therapeutic treatments such as chemotherapy and radiotherapy have been used in conjunction with candidate therapeutic vaccines. In a phase II clinical trial, women with VIN 2 and 3 were treated with the topical immunomodulator imiquimod, followed by 3 doses of TA-CIN. One year after the last immunization, 63% of patients showed lesion clearance, and had increased CD4+ and CD8+ T cells compared to non-responders. In addition, 36% of patients showed HPV-16 clearance and 79% were symptom-free [93]. In ongoing trials, ISA101/ISA101b in combination with chemotherapy drugs carboplatin and paclitaxel, and with or without bevacizumab, is currently being tested in patients with cervical cancer (NCT02128126) in a phase I/II study. Another phase Ib/IIa study is recruiting patients with recurrent/metastatic HPV-associated head and neck squamous cancer, to test the anti-tumour activity and immunogenicity of MEDI0457 (VGX-3100 DNA vaccine) in combination with durvalumab (anti-PD-L1 monoclonal antibody) (NCT03162224).
2.5.6. Combination prophylactic and therapeutic vaccines
Combination vaccines would be beneficial in providing immediate impact and long-term protection through mass immunization of pre-adolescents and older women who have previously been exposed to HPV infection [168]. Chimaeric proteins and pseudovirions (PsVs) delivering a DNA vaccine are current strategies to produce combination vaccines.
2.5.6.1. Chimaeric vaccines
Several groups have developed L1/E7 chimaeras where full length or N-terminal regions of E7 have been fused to the L1 C-terminus [169], [170], [171], [172], [173], [174], [175], [176] or fused to the C-terminal of L2 to create L1/L2/E7 chimaeras [177], [178], [179], [180], [181]. A clinical study in 39 women with CIN 2/3 showed that vaccination with L1:E7 chimaeras induced high anti-L1 antibody titres and low anti-E7 antibody titres, in addition to cellular immune responses to both L1 and E7. Improvement to CIN 1 or normal tissue was seen in 39% of patients compared to 25% in the placebo group, with 56% of patients being HPV-16 DNA negative at the end of the trial. However, clinical efficacy was not significant [173].
In plants, L1:E6:E7 chimaeras have been produced in transgenic tomato [182]. The chimaeras elicited neutralising antibodies and elicited cytotoxic T cell responses to L1 and E6/E7 in mice. In a follow up study looking at the persistence of specific IgG antibodies and the therapeutic potential of L1:E6:E7, IgG antibodies were shown to be persistent for over 1 year and there was a 57% reduction in tumour growth in immunized mice [183].
2.5.6.2. Pseudovirions (PsVs) in gene delivery
HPV PsVs have emerged as a viable means of DNA and RNA delivery into several cell types and tissues. PsVs have several advantages as they have been shown to act as adjuvants and can facilitate the activation and maturation of APCs such as DCs [180], [184], [185], [186], and do not have safety concerns, unlike live vector vaccines [185]. Packaging of plasmid DNA allows for stimulation of both humoral and cellular immunity [187]. In vivo, PsVs have been used in gene therapy experiments for ovarian cancer. HPV-16 PsVs were used to deliver a herpes simplex thymidine kinase (HSV-tk) gene to ovarian tumour cells in mice and were shown to have antitumour effects [188]. Delivery of the model ovalbumin (OVA) antigen in HPV-16 PsVs was also shown to generate OVA-specific CD8+ T cells immune responses in mice, the highest number of OVA-specific CD8+ T cells compared to DNA delivery by other methods [185], [189], and potent neutralising responses when DNA was co-administered with capsid proteins [190]. An alternative approach using short hairpin RNA (shRNA) has also been reported. HPV-31 PsVs encoding shRNA against E6 or E7 were used to silence E6 or E7 expression in cervical carcinoma cells. shRNA was delivered to CaSki and TC-1 HPV+ cells and resulted in the degradation of E6 and E7 mRNAs, with more significant cell death observed when E7 expression was suppressed, and in vivo testing in mice resulting in the dramatic inhibition of tumour growth [191].
HPV PsVs have recently been produced in plants for the first time [192]. Secreted alkaline phosphatase and luciferase encoding reporter plasmids were designed based on the genome of the bean yellow dwarf geminivirus and successfully packaged in plant-made PsVs. These PsVs were shown to be capable of infecting mammalian cells, could deliver a functional reporter plasmid for use in pseudovirion-based neutralisation assays and could be neutralised by anti-L1 monoclonal antibodies. This shows the potential of these PsVs to package and deliver HPV nucleic acid therapeutics. Additionally, plant made PsVs could offer a cheaper alternative to current PsV production methods that require cultured mammalian cells and expensive media and transfection reagents.
A summary of the mechanism of action of an ideal HPV therapeutic vaccine is shown in Fig. 2. Furthermore, a summary of HPV therapeutic vaccines in ongoing clinical trials is given in Table 1.
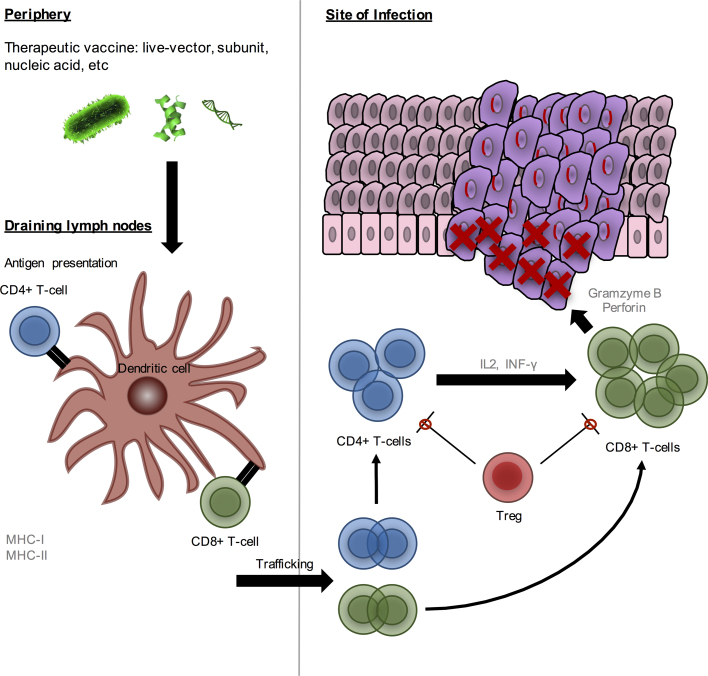
Fig. 2.
Ideal mechanism of a therapeutic vaccine against HPV-related malignancies. An ideal HPV therapeutic vaccine would elicit a strong cell-mediated immune response where CD4+ T-cells would provide support to CD8+ T-cells by secreting cytokines such as IFN-γ and IL2 labelling infected and malignant cells. Cytotoxic CD8+ T-cells would eliminate infected cells by secreting high amounts of granzyme B and perforin which lead to cell death. The response would be effective even in the presence of immunosuppressive cells [36].
Table 1.
Most recently completed and ongoing clinical trials.
| Vaccine type | Vaccine name | Vaccine description | HPV-related disease | Clinical phase | Status | Expected completion | Clinical trial number |
|---|---|---|---|---|---|---|---|
| Bacterial vector | ADXS11-001 | Attenuated live Listeria Encoding HPV 16 E7 vector | Anal cancer | I/II | Active, not recruiting | February 2018 | NCT01671488 |
| Cervical carcinoma | I and II | Active, not recruiting | December 2018 | NCT02164461 | |||
| Cervical cancer | I and II | Active, recruiting | December 2019 | NCT02291055 | |||
| Head and neck cancer | |||||||
| Cervical cancer | II | Active, not recruiting | October 2018 | NCT01266460 | |||
| Cervical cancer | III | Active, recruiting | June 2021 | NCT02853604 | |||
| Anal and rectal cancer | II | Active, not recruiting | March 2022 | NCT02399813 | |||
| Head and neck cancer, oropharyngeal squamous cell carcinoma | II | Active, not recruiting | August 2019 | NCT02002182 | |||
| Viral vector | Vvax001 | Replication incompetent SFV HPV-16 E6/E7 vector | Malignant cervical lesions | I | Active, recruiting | December 2017 | NCT03141463 |
| TG4001 | MVA HPV-16 E6/E7/IL-2 vector | Oropharyngeal squamous cell carcinoma of the head and neck | Ib/II | Active, recruiting | May 2021 | NCT03260023 | |
| Peptide-based | DPX-E7 | Synthetic HPV16-E7 peptide 11–19 nanomer | Head and neck cancer, cervical cancer, anal cancer | Ib/II | Active, recruiting | May 2023 | NCT02865135 |
| ISA 101 | 9 HPV-16 E6 and 4 E7 SLPs | Solid tumours | II | Active, not recruiting | December 2018 | NCT02426892 | |
| PepCan | HPV16 E6 peptides combined with Candida skin testing reagent Candin | Cervical intraepithelial neoplasia | II | Active, recruiting | August 2020 | NCT02481414 | |
| Hespecta | 2 HPV-16 E6 SLPs conjugated to Amplivant | HPV+ tumours or malignant lesions | I | Active, recruiting | December 2017 | NCT02821494 | |
| Protein | TA-CIN | HPV-16 L2, E6, and E7 fusion protein | Cervical cancer | I | Not open | November 2022 | NCT02405221 |
| TVGV-1 | HPV-16 E7 fusion protein | High-grade squamous intraepithelial lesions | II | Active, not recruiting | September 2018 | NCT02576561 | |
| DNA | GX-188E | Plasmid encoding HPV-16 and −18 E6 and E7 proteins fused to Fms-like tyrosine kinase-3 ligand | Cervical intraepithelial neoplasia | II | Completed | March 2016 | NCT02139267 |
| Cervical intraepithelial neoplasia | II | Active, not recruiting | August 2018 | NCT02596243 | |||
| Cervical intraepithelial neoplasia 3 | Ib/II | Active, recruiting | October 2018 | NCT03206138 | |||
| VGX-3100 | Mixture of HPV-16/18 E6/E7 plasmids | Head and neck squamous cell cancer | I/IIa | Active, not recruiting | October 2017 | NCT02163057 | |
| Cervical cancer | I/IIa | Active, not recruiting | March 2018 | NCT02172911 | |||
| High-grade squamous intraepithelial lesion of the cervix | III | Active, recruiting | August 2020 | NCT03185013 | |||
| VB10.16 | DNA vaccine | High-grade cervical intraepithelial neoplasia | I/IIa | Active, recruiting | December 2018 | NCT02529930 | |
| ZFN-603 and ZFN-758 | HPV-16/18 E7 ZFNs | HPV malignant neoplasm | I | Active, not recruiting | July 2017 | NCT02800369 | |
| N/A | HPV 16/18 E6/E7 TALEN or CRISPR/Cas9 plasmids | HPV malignant neoplasm | I | Not open | January 2019 | NCT03057912 | |
| DC-based | BVAC-C | Recombinant HPV16/18 E6/E7 expressing adenovirus-infected B cells and monocytes | Uterine cervical neoplasms | I | Active, recruiting | August 2017 | NCT02866006 |
| ACT | N/A | T cell receptor immunotherapy targeting HPV-16 E6 | Cervical, anal, vaginal and oropharyngeal | I/II | Completed | June 2016 | NCT02280811[139] |
| T cell receptor immunotherapy targeting HPV-16 E6 | Vulvar high-grade squamous intraepithelial lesions | I | Active, recruiting | February 2020 | NCT03197025 | ||
| HPV-16/18 E6/E7-Specific T Lymphocytes | Carcinoma (oropharyngeal, cervical, anal, vulval, penile | I | Active, recruiting | October 2033 | NCT02379520 | ||
| E7 T cell receptor cells | Cervical, vaginal, anal, penile and oropharyngeal cancer | I | Active, recruiting | January 2026 | NCT02858310 | ||
| Cervical cancer-specific CAR-T cells | Cervical cancer | I/II | Active, recruiting | December 2020 | NCT03356795 | ||
| Combination therapy | N/A | pNGVL4a-sig/E7(detox)/HSP70 DNA prime, TA-HPV boost | High-grade cervical dysplasia | I | Active, recruiting | July 2017 | NCT00788164 |
| N/A | IRX-2 Regimen | Cervical or vulvar squamous intraepithelial neoplasia 3 | II | Active, recruiting | November 2022 | NCT03267680 | |
| Ipilimumab | anti-CTLA-4 monoclonal antibody + chemoradiotherapy | Advanced cervical cancer | I | Active, not recruiting | March 2017 | NCT01711515 | |
| Ipilimumab | anti-CTLA-4 monoclonal antibody | Metastatic or recurrent cervical cancer | II | Active, not recruiting | December 2017 | NCT01693783 | |
| MEDI4736 + Tremelimu- mab | anti-PD-L1 antibody + anti-CTLA-4 antibody | Advanced solid tumours | I | Active, not recruiting | January 2018 | NCT02261220 | |
| October 2017 | NCT01975831 | ||||||
| ISA101/ ISA101b | HPV-16 E6/E7 SLP vaccine + Carboplatin and Paclitaxel with or without Bevacizumab | Advanced or recurrent cervical cancer | I/II | Active, not recruiting | April 2021 | NCT02128126 | |
| MEDI0457 + Durvalumab | VGX-3100 DNA vaccine + anti-PD-L1 monoclonal antibody | Head and neck cancer | Ia/IIb | Active, recruiting | July 2019 | NCT03162224 |
N/A, not applicable.
3. Future perspectives for HPV therapeutics
With several candidate vaccines in clinical trials, it is likely that we will see a therapeutic HPV vaccine on the market soon. However, current therapeutic strategies remain expensive due to systems of manufacture, meaning even if a vaccine were to be commercialised, its cost would render it less accessible to populations in developing countries where the burden of cervical cancer is highest. To expand the impact of therapeutic vaccines in developing countries, plant-made products are a promising technology to offer inexpensive and efficacious pharmaceuticals [55]. Additionally, cold-chain vaccine delivery is necessary to maintain vaccine shelf life, and contributes to the inaccessibility of vaccine products in developing countries due to poor infrastructure. Plant-produced vaccines can potentially remove the need for cold-chain transportation and storage as vaccine antigens bioencapsulated in plant tissue could be used for oral administration or expressed in seeds that remain stable at ambient temperature, and be effectively delivered to the gut without degradation [193], [194], [195]. Although no plant-made therapeutic for HPV-related disease has reached clinical trial, there are several plant-based therapeutics that show potential as vaccine candidates as they have been shown to elicit HPV-specific CTL responses and result in tumour regression in animal studies. It could be that plant-produced therapeutic vaccines are the ideal niche for plant-made pharmaceuticals (PMPs), given that market sizes and therefore production volumes will be far smaller than for prophylactic vaccines [43], [46].
Recently, the use of plant viruses has been explored in cancer immunotherapy. A wide range of viral nanoparticles (VNPs) has been described and tailored for specific applications. VNPs can target and elicit highly localised immune responses to solid tumours [196] and have also been used in drug delivery and tissue imaging [197]. Plant-derived VNPs are advantageous for use in cancer therapy due to their inexpensive and highly scalable manufacture, stability and adaptability to engineering, their non-toxicity and a higher degree of biocompatibility because they are naturally occurring unlike other nanomaterials [198]. PVX nanofilaments carrying trastuzumab (a monoclonal antibody used as a targeted therapy in breast cancer patients) have been shown to induce apoptosis in breast cancer cell lines [199]. The use of tobacco mosaic virus (TMV)-derived particles for the delivery of drugs for chemotherapeutic treatments has been investigated, and the TMV-mediated in vivo delivery of phenanthriplatin has been confirmed in a triple negative breast cancer mouse model [200]. Cowpea mosaic virus (CMPV) nanoparticles have also been shown to stimulate the immune system to attack a number of cancers. Empty (eCPMV) particles have been produced in N. benthamiana [201] and have recently been used as targets for tumour immunotherapy via in situ vaccination to alter the tumour microenvironment. eCPMV particles were shown to induce potent anti-tumour immune responses in melanoma, ovarian, colon and breast cancer models [202]. Additionally, the delivery of nucleic acid therapies using plant viruses has recently been reviewed by Lam and Steinmetz [203], and this system is said to have significant advantages over conventional mammalian viral vectors due to factors such as high production yields, increased safety due to no replication in mammalian cells, and no risk of insertional mutagenesis. This is a relatively new field which shows significant promise; however, more research is required [203].
4. Conclusions
There is an urgent need for therapeutic HPV vaccines to reduce the burden of cervical cancer, as current vaccines have no therapeutic effects. To date, no therapeutic vaccine has been approved for commercial use in the treatment of HPV infections and related malignancies. Nevertheless, numerous clinical trials show the progress that has been made using several vaccine strategies and two vaccine candidates, VGX-3100 DNA vaccine and ADXS11-001 bacterial vector vaccine, are both in phase III clinical trials, showing that there is promise of a vaccine in the near future. Plant expression systems have successfully been used to produce biologically relevant products and offer a platform to manufacture cheaper vaccines: these would be accessible to a greater population, specifically in low-middle income countries where the disease burden is greatest.
Funding
This work was supported by the Poliomyelitis Research Foundation (PRF) (15/12), the National Research Foundation of South Africa (NRF) and the Cancer Association of South Africa (CANSA) (2015). AC was supported by grants from the Carnegie Corporation of New York, the PRF and the University of Cape Town. RL and RJRY were supported by grants from the PRF and NRF.
References
- 1.Abubakar I., Tillmann T., Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017 doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbyn M. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22(12):2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 5.Yang B.H. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. Int. J. Cancer. 2004;109(3):418–424. doi: 10.1002/ijc.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers E.-M. Cross-roads in the classification of papillomaviruses. Virology. 2013;445(1):2–10. doi: 10.1016/j.virol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Parkin D.M., Bray F. The burden of HPV-related cancers. Vaccine. 2006;24:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 9.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 10.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer. 2010;10(8):550. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 11.Walboomers J.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.de Villiers E.M. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Brentjens M.H. Human papillomavirus: a review. Dermatol. Clin. 2002;20(2):315–331. doi: 10.1016/s0733-8635(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 14.Münger K., Howley P.M. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89(2):213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 15.Buck C.B. Arrangement of L2 within the papillomavirus capsid. J. Virol. 2008;82(11):5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck C.B. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 17.Goodman M.T. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68(21):8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa M.I. Persistence and clearance of human papillomavirus infection: a prospective cohort study. Am. J. Obstet. Gynecol. 2008;199(6):617.e1–617.e7. doi: 10.1016/j.ajog.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Kirnbauer R. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA. 1992;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper D.M. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 21.Joura E.A. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 22.Villa L.L. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 23.Hildesheim A. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am. J. Obstet. Gynecol. 2016;215(2):212.e1–212.e15. doi: 10.1016/j.ajog.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildesheim A. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 25.Hung C.-F. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin. Biol. Ther. 2008;8(4):421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni L. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob. Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 27.Allan B. Cervical human papillomavirus (HPV) infection in South African women: implications for HPV screening and vaccine strategies. J. Clin. Microbiol. 2008;46(2):740–742. doi: 10.1128/JCM.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifford G.M. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: a nested case‐control study in the Swiss HIV cohort study. Int. J. Cancer. 2016;138(7):1732–1740. doi: 10.1002/ijc.29913. [DOI] [PubMed] [Google Scholar]
- 29.Massad L.S. Association of cervical precancer with human papillomavirus types other than 16 among HIV co-infected women. Am. J. Obstet. Gynecol. 2016;214(3):354.e1–354.e6. doi: 10.1016/j.ajog.2015.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massad L.S. Incidence of cervical precancers among HIV-seropositive women. Am. J. Obstet. Gynecol. 2015;212(5):606.e1–606.e8. doi: 10.1016/j.ajog.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poljak M., Šterbenc A., Lunar M.M. Prevention of human papillomavirus (HPV)-related tumors in people living with human immunodeficiency virus (HIV) Expert Rev. Anti-Infect. Ther. 2017 doi: 10.1080/14787210.2017.1392854. just-accepted. [DOI] [PubMed] [Google Scholar]
- 32.Rowhani-Rahbar A. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J. Infect. Dis. 2007;196(6):887–894. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 33.Clifford G.M., Tully S., Franceschi S. Carcinogenicity of human Papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin. Infect. Dis. 2017;64(9):1228–1235. doi: 10.1093/cid/cix135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Burg S.H., Melief C.J. Therapeutic vaccination against human papilloma virus induced malignancies. Curr. Opin. Immunol. 2011;23(2):252–257. doi: 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Lin K. Perspectives for preventive and therapeutic HPV vaccines. J. Formos. Med. Assoc. 2010;109(1):4–24. doi: 10.1016/s0929-6646(10)60017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow M.P., Yan J., Sardesai N.Y. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev. Vaccin. 2013;12(3):271–283. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]
- 37.Yang L. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA. 1993;90(11):5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M. Šmídková et al. Plant production of vaccine against HPV: a new perspectives. In: Human Papillomavirus and Related Diseases-From Bench to Bedside-A Clinical Perspective, InTech, 2012.
- 39.Vici P. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev. Vaccin. 2016;15(10):1327–1336. doi: 10.1080/14760584.2016.1176533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang A. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 2016;23(1):75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H.J., Kim H.-J. Current status and future prospects for human papillomavirus vaccines. Arch. Pharm. Res. 2017:1–14. doi: 10.1007/s12272-017-0952-8. [DOI] [PubMed] [Google Scholar]
- 42.Giorgi C., Franconi R., Rybicki E.P. Human papillomavirus vaccines in plants. Expert. Rev. Vaccin. 2010;9(8):913–924. doi: 10.1586/erv.10.84. [DOI] [PubMed] [Google Scholar]
- 43.Rybicki E.P. Plant-based vaccines against viruses. Virol. J. 2014;11(1):205. doi: 10.1186/s12985-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer R. Plant-based production of biopharmaceuticals. Curr. Opin. Plant Biol. 2004;7(2):152–158. doi: 10.1016/j.pbi.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Merlin M. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. BioMed. Res. Int. 2014;2014:136419. doi: 10.1155/2014/136419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rybicki E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010;8(5):620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nandi S. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. MAbs. 2016 doi: 10.1080/19420862.2016.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen G., Wright M.S. Recent advances in the transformation of plants. Trends Plant Sci. 1999;4(6):226–231. doi: 10.1016/s1360-1385(99)01412-0. [DOI] [PubMed] [Google Scholar]
- 49.Lessard P.A. Manipulating gene expression for the metabolic engineering of plants. Metab. Eng. 2002;4(1):67–79. doi: 10.1006/mben.2001.0210. [DOI] [PubMed] [Google Scholar]
- 50.Tzfira T. Agrobacterium T-DNA integration: molecules and models. Trends Genet. 2004;20(8):375–383. doi: 10.1016/j.tig.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Kapila J. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997;122(1):101–108. [Google Scholar]
- 52.Gleba Y., Klimyuk V., Marillonnet S. Magnifection—a new platform for expressing recombinant vaccines in plants. Vaccine. 2005;23(17):2042–2048. doi: 10.1016/j.vaccine.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Hefferon K.L., Fan Y. Expression of a vaccine protein in a plant cell line using a geminivirus-based replicon system. Vaccine. 2004;23(3):404–410. doi: 10.1016/j.vaccine.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 54.Komarova T.V. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccin. 2010;9(8):859–876. doi: 10.1586/erv.10.85. [DOI] [PubMed] [Google Scholar]
- 55.Hefferon K. Plant virus expression vectors: a powerhouse for global health. Biomedicines. 2017;5(3):44. doi: 10.3390/biomedicines5030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porta C., Lomonossoff G.P. Viruses as vectors for the expression of foreign sequences in plants. Biotechnol. Genet. Eng. Rev. 2002;19(1):245–292. doi: 10.1080/02648725.2002.10648031. [DOI] [PubMed] [Google Scholar]
- 57.Wong-Arce A., González-Ortega O., Rosales-Mendoza S. Plant-made vaccines in the fight against cancer. Trends Biotechnol. 2017 doi: 10.1016/j.tibtech.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Goossens P.L. Listeria monocytogenes: a live vector able to deliver heterologous protein within the cytosol and to drive a CD8 dependent T cell response. Biologicals. 1995;23(2):135–143. doi: 10.1006/biol.1995.0024. [DOI] [PubMed] [Google Scholar]
- 59.Gunn G.R. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001;167(11):6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 60.Schnupf P., Portnoy D.A. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9(10):1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Maciag P.C., Radulovic S., Rothman J. The first clinical use of a live-attenuated listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27(30):3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 62.Skeate J.G. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum. Vaccin. Immunother. 2016;12(6):1418–1429. doi: 10.1080/21645515.2015.1136039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawana K. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32(47):6233–6239. doi: 10.1016/j.vaccine.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Monahan S., Salgaller M. Viral vectors for gene transfer into antigen presenting cells. Curr. Opin. Mol. Ther. 1999;1(5):558–564. [PubMed] [Google Scholar]
- 65.Borysiewicz L. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 66.Kaufmann A.M. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)−16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin. Cancer Res. 2002;8(12):3676–3685. [PubMed] [Google Scholar]
- 67.Baldwin P.J. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin. Cancer Res. 2003;9(14):5205–5213. [PubMed] [Google Scholar]
- 68.Rosales R. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum. Gene Ther. 2014;25(12):1035–1049. doi: 10.1089/hum.2014.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doorbar J. Model systems of human papillomavirus‐associated disease. J. Pathol. 2016;238(2):166–179. doi: 10.1002/path.4656. [DOI] [PubMed] [Google Scholar]
- 70.Doorbar J. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 71.Desaintes C. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 1997;16(3):504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brun J.-L. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am. J. Obstet. Gynecol. 2011;204(2):169.e1–169.e8. doi: 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Lundstrom K. Replicon RNA Viral Vectors as Vaccines. Vaccines. 2016;4(4):39. doi: 10.3390/vaccines4040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng W.-F. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22–antigen fusion. Hum. Gene Ther. 2002;13(4):553–568. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 75.Velders M.P. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001;61(21):7861–7867. [PubMed] [Google Scholar]
- 76.Cassetti M.C. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine. 2004;22(3):520–527. doi: 10.1016/j.vaccine.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Daemen T. Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Ther. 2002;9(2):85. doi: 10.1038/sj.gt.3301627. [DOI] [PubMed] [Google Scholar]
- 78.Daemen T. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine. 2003;21(11):1082–1088. doi: 10.1016/s0264-410x(02)00558-3. [DOI] [PubMed] [Google Scholar]
- 79.Daemen T. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization. Antivir. Ther. 2004;9(5):733–742. [PubMed] [Google Scholar]
- 80.Riezebos-Brilman A. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12(18):1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 81.Kumar S., Biswas M., Jose T. HPV vaccine: current status and future directions. Med. J. Armed Forces India. 2015;71(2):171. doi: 10.1016/j.mjafi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medina E., Guzmán C.A. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine. 2001;19(13):1573–1580. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 83.Kast W.M. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J. Immunol. 1994;152(8):3904–3912. [PubMed] [Google Scholar]
- 84.Su J.-H. Immunotherapy for cervical cancer. BioDrugs. 2010;24(2):109–129. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khong H., Overwijk W.W. Adjuvants for peptide-based cancer vaccines. J. Immunother. Cancer. 2016;4(1):56. doi: 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenter G.G. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 87.van Poelgeest M.I. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013;11(1):88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coleman H.N. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol. Immunother. 2016;65(5):563–573. doi: 10.1007/s00262-016-1821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 90.Davidson E.J. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22(21):2722–2729. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 91.de Jong A. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20(29–30):3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 92.van der Burg S. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001;19(27):3652–3660. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 93.Daayana S. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer. 2010;102(7):1129. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Granadillo M. A novel fusion protein-based vaccine comprising a cell penetrating and immunostimulatory peptide linked to human papillomavirus (HPV) type 16 E7 antigen generates potent immunologic and anti-tumor responses in mice. Vaccine. 2011;29(5):920–930. doi: 10.1016/j.vaccine.2010.11.083. [DOI] [PubMed] [Google Scholar]
- 95.Vallespi M.G. A Limulus antilipopolysaccharide factor-derived peptide exhibits a new immunological activity with potential applicability in infectious diseases. Clin. Diagn. Lab. Immunol. 2000;7(4):669–675. doi: 10.1128/cdli.7.4.669-675.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franconi R. Plant-derived human papillomavirus 16 E7 oncoprotein induces immune response and specific tumor protection. Cancer Res. 2002;62(13):3654–3658. [PubMed] [Google Scholar]
- 97.Franconi R. Exploiting the plant secretory pathway to improve the anticancer activity of a plant-derived HPV16 E7 vaccine. Int. J. Immunopathol. Pharmacol. 2006;19(1):187–197. [PubMed] [Google Scholar]
- 98.Massa S. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine. 2007;25(16):3018–3021. doi: 10.1016/j.vaccine.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 99.Venuti A. An E7-based therapeutic vaccine protects mice against HPV16 associated cancer. Vaccine. 2009;27(25–26):3395–3397. doi: 10.1016/j.vaccine.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 100.Demurtas O.C. A Chlamydomonas-derived human papillomavirus 16 E7 vaccine induces specific tumor protection. PLoS One. 2013;8(4):e61473. doi: 10.1371/journal.pone.0061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whitehead M. Human papillomavirus (HPV) type 16 E7 protein bodies cause tumour regression in mice. BMC Cancer. 2014;14:367–381. doi: 10.1186/1471-2407-14-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohlschlager P. An improved rearranged human papillomavirus type 16 E7 DNA vaccine candidate (HPV-16 E7SH) induces an E7 wildtype-specific T cell response. Vaccine. 2006;24(15):2880–2893. doi: 10.1016/j.vaccine.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 103.Geli M.I., Torrent M., Ludevid D. Two structural domains mediate two sequential events in [gamma]-Zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell. 1994;6(12):1911–1922. doi: 10.1105/tpc.6.12.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torrent M. Eukaryotic protein production in designed storage organelles. BMC Biol. 2009;7:5–18. doi: 10.1186/1741-7007-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yanez R.J. Expression optimization of a cell membrane-penetrating human papillomavirus type 16 therapeutic vaccine candidate in Nicotiana benthamiana. PLoS One. 2017;12(8):e0183177. doi: 10.1371/journal.pone.0183177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yanez R.J. LALF32‐51‐E7, a HPV‐16 therapeutic vaccine candidate, forms protein body‐like structures when expressed in Nicotiana benthamiana leaves. Plant Biotechnol. J. 2017 doi: 10.1111/pbi.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S.-J. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J. Gynecol. Oncol. 2016;27(5):e51. doi: 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hung C., Wu T. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr. Opin. Mol. Ther. 2003;5(1):20–24. [PubMed] [Google Scholar]
- 109.Tsen S.W. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev. Vaccin. 2007;6(2):227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim T.J. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014:5. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnard Z. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery. 2012;71(3):E749–E756. doi: 10.1227/NEU.0b013e318260fd73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lynch D.H. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat. Med. 1997;3(6):625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 113.Riediger C. Fms-like tyrosine kinase 3 receptor ligand (Flt3L)-based vaccination administered with an adenoviral vector prevents tumor growth of colorectal cancer in a BALB/c mouse model. J. Cancer Res. Clin. Oncol. 2013;139(12):2097–2110. doi: 10.1007/s00432-013-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang C.-F. DNA vaccines for cervical cancer. Am. J. Transl. Res. 2010;2(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- 115.Bagarazzi M.L. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4(155) doi: 10.1126/scitranslmed.3004414. (p. 155ra138-155ra138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trimble C.L. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aggarwal C. Immunotherapy with VGX-3100 (HPV16 and HPV18 plasmids) + INO-9012 (DNA encoding IL-12) in human papillomavirus (HPV) associated head and neck squamous cell carcinoma (HNSCCa): interim safety and immunogenicity results. J. Immunother. Cancer. 2015;3(Suppl 2) (p. P426-P426) [Google Scholar]
- 118.Ding W. Zinc finger nucleases targeting the human papillomavirus E7 oncogene induce E7 disruption and a transformed phenotype in HPV16/18-positive cervical cancer cells. Clin. Cancer Res. 2014;20(24):6495–6503. doi: 10.1158/1078-0432.CCR-14-0250. [DOI] [PubMed] [Google Scholar]
- 119.Hu Z. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J. Clin. Investig. 2015;125(1):425–436. doi: 10.1172/JCI78206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Z. Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/612823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kennedy E.M. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-Guided endonuclease. J. Virol. 2014;88(20):11965–11972. doi: 10.1128/JVI.01879-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shankar S. TALEN based HPV-E7 editing triggers necrotic cell death in cervical cancer cells. Sci. Rep. 2017;7:5500. doi: 10.1038/s41598-017-05696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu L. deletion of HPV18 E6 and E7 genes using dual sgRNA-directed CRISPR/Cas9 inhibits growth of cervical cancer cells. Int. J. Clin. Exp. Med. 2017;10(6):9206–9213. [Google Scholar]
- 124.Zhen S. In vitro and in vivo synergistic therapeutic effect of cisplatin with human papillomavirus16 E6/E7 CRISPR/Cas9 on cervical cancer cell line. Transl. Oncol. 2016;9(6):498–504. doi: 10.1016/j.tranon.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Varnavski A.N., Young P.R., Khromykh A.A. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J. Virol. 2000;74(9):4394–4403. doi: 10.1128/jvi.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim T. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene Ther. 2004;11(3):336–342. doi: 10.1038/sj.gt.3302164. [DOI] [PubMed] [Google Scholar]
- 127.Herd K.A. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology. 2004;319(2):237–248. doi: 10.1016/j.virol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 128.Sebastian M. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer. 2014;14(1):748. doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santin A.D. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr. Pharm. Des. 2005;11(27):3485–3500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 130.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Interact. Immune Cancer Cells. 2014:75–89. [Google Scholar]
- 131.Santin A.D. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J. Virol. 2008;82(4):1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santin A.D. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecol. Oncol. 2006;100(3):469–478. doi: 10.1016/j.ygyno.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 133.Rahma O.E. Pre-immature dendritic cells (PIDC) pulsed with HPV16 E6 or E7 peptide are capable of eliciting specific immune response in patients with advanced cervical cancer. J. Transl. Med. 2014;12(1):353. doi: 10.1186/s12967-014-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ahn Y.H. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor‐β receptor on dendritic cells potentiates tumour antigen‐specific CD8+ T cell immunity. Clin. Exp. Immunol. 2015;181(1):164–178. doi: 10.1111/cei.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim J.H. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8+ T cell-mediated cell death. Immunol. Lett. 2009;122(1):58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Peng S. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum. Gene Ther. 2005;16(5):584–593. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stevanović S. Complete regression of metastatic cervical cancer after treatment with human papillomavirus–targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015;33(14):1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Draper L.M. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin. Cancer Res. 2015;21(19):4431–4439. doi: 10.1158/1078-0432.CCR-14-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hinrichs C.S. A Phase I/II Clinical Trial of E6 T-cell Receptor Gene Therapy for Human Papillomavirus (HPV)-Associated Epithelial Cancers. J. Clin. Oncol. 2017;35(15):3009. [Google Scholar]
- 140.Ramos C.A. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes for adoptive immunotherapy of HPV-associated malignancies. J. Immunother. 2013;36(1):66. doi: 10.1097/CJI.0b013e318279652e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.C.A. Ramos, C.M. Rooney, N. Narala, Generation of HPV-Specific T-Cells. Google Patents, 2017.
- 142.Newick K. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 143.Cantelmo A.R. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30(6):968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim S.J. Tumor vessel normalization by the PI3K inhibitor HS-173 enhances drug delivery. Cancer Lett. 2017;403:339–353. doi: 10.1016/j.canlet.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 145.Ma W., Melief C.J., van der Burg S.H. Control of immune escaped human papilloma virus is regained after therapeutic vaccination. Curr. Opin. Virol. 2017;23:16–22. doi: 10.1016/j.coviro.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 146.Fiander A.N. Prime‐boost vaccination strategy in women with high‐grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int. J. Gynecol. Cancer. 2006;16(3):1075–1081. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 147.Smyth L.J. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res. 2004;10(9):2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 148.Alvarez R.D. A pilot study of pNGVL4a-CRT/E7 (detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecol. Oncol. 2016;140(2):245–252. doi: 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chuang C.-M. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+ CD25+ T regulatory cells. Vaccine. 2009;27(5):684–689. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim R. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 152.Pittet M.J. Behavior of immune players in the tumor microenvironment. Curr. Opin. Oncol. 2009;21(1):53–59. doi: 10.1097/CCO.0b013e32831bc38a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Egan J.E. IRX-2, a novel in vivo immunotherapeutic, induces maturation and activation of human dendritic cells in vitro. J. Immunother. 2007;30(6):624–633. doi: 10.1097/CJI.0b013e3180691593. [DOI] [PubMed] [Google Scholar]
- 154.Czystowska M. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16(5):708–718. doi: 10.1038/cdd.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rapidis A.D., Wolf G.T. Immunotherapy of head and neck cancer: current and future considerations. J. Oncol. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schilling B. IRX-2, a novel immunotherapeutic, enhances functions of human dendritic cells. PLoS One. 2013;8(2):e47234. doi: 10.1371/journal.pone.0047234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Freeman S.M. A phase 1 safety study of an IRX-2 regimen in patients with squamous cell carcinoma of the head and neck. Am. J. Clin. Oncol. 2011;34(2):173–178. doi: 10.1097/COC.0b013e3181dbb9d8. [DOI] [PubMed] [Google Scholar]
- 158.Wolf G.T. Novel neoadjuvant immunotherapy regimen safety and survival in head and neck squamous cell cancer. Head Neck. 2011;33(12):1666–1674. doi: 10.1002/hed.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Berinstein N.L. Increased lymphocyte infiltration in patients with head and neck cancer treated with the IRX-2 immunotherapy regimen. Cancer Immunol. Immunother. 2012;61(6):771–782. doi: 10.1007/s00262-011-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whiteside T.L. A short course of neoadjuvant IRX-2 induces changes in peripheral blood lymphocyte subsets of patients with head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2012;61(6):783–788. doi: 10.1007/s00262-011-1136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Bonito P. Immunomodulatory activity of a plant extract containing human papillomavirus 16-E7 protein in human monocyte-derived dendritic cells. Int. J. Immunopathol. Pharmacol. 2009;22(4):967–978. doi: 10.1177/039463200902200412. [DOI] [PubMed] [Google Scholar]
- 162.Dine J. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac. J. Oncol. Nurs. 2017;4(2):127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.La‐Beck N.M. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacother.: J. Hum. Pharmacol. Drug Ther. 2015;35(10):963–976. doi: 10.1002/phar.1643. [DOI] [PubMed] [Google Scholar]
- 164.Callahan M.K., Postow M.A., Wolchok J.D. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front. Oncol. 2015;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shen K.-Y. Self-adjuvanting lipoimmunogens for therapeutic HPV vaccine development: potential clinical impact. Expert Rev. Vaccin. 2015;14(3):383–394. doi: 10.1586/14760584.2015.966696. [DOI] [PubMed] [Google Scholar]
- 166.Wick D.A., Webb J.R. A novel, broad spectrum therapeutic HPV vaccine targeting the E7 proteins of HPV16, 18, 31, 45 and 52 that elicits potent E7-specific CD8T cell immunity and regression of large, established, E7-expressing TC-1 tumors. Vaccine. 2011;29(44):7857–7866. doi: 10.1016/j.vaccine.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 167.Wick D.A. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly (I: C) Vaccine. 2011;29(5):984–993. doi: 10.1016/j.vaccine.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 168.Schiller J.T., Davies P. Delivering on the promise: hpv vaccines and cervical cancer. Nat. Rev. Microbiol. 2004;2(4):343–347. doi: 10.1038/nrmicro867. [DOI] [PubMed] [Google Scholar]
- 169.Bian T. Human papillomavirus type 16 L1E7 chimeric capsomeres have prophylactic and therapeutic efficacy against papillomavirus in mice. Mol. Cancer Ther. 2008;7(5):1329–1335. doi: 10.1158/1535-7163.MCT-07-2015. [DOI] [PubMed] [Google Scholar]
- 170.Freyschmidt E.-J. Activation of dendritic cells and induction of T cell responses by HPV 16 L1/E7 chimeric virus-like particles are enhanced by CpG ODN or sorbitol. Antivir. Ther. 2004;9:479–490. [PubMed] [Google Scholar]
- 171.Jochmus I. Chimeric virus-like particles of the human papillomavirus type 16 (HPV 16) as a prophylactic and therapeutic vaccine. Arch. Med. Res. 1999;30(4):269–274. doi: 10.1016/s0188-0128(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 172.Kaufmann A. HPV16 L1E7 chimeric virus‐like particles induce specific HLA‐restricted T cells in humans after in vitro vaccination. Int. J. Cancer. 2001;92(2):285–293. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1181>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 173.Kaufmann A.M. Vaccination trial with HPV16 L1E7 chimeric virus‐like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int. J. Cancer. 2007;121(12):2794–2800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 174.Kuck D. Efficiency of HPV 16 L1/E7 DNA immunization: influence of cellular localization and capsid assembly. Vaccine. 2006;24(15):2952–2965. doi: 10.1016/j.vaccine.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 175.Müller M. Chimeric papillomavirus-like particles. Virology. 1997;234(1):93–111. doi: 10.1006/viro.1997.8591. [DOI] [PubMed] [Google Scholar]
- 176.Schäfer K. Immune response to human papillomavirus 16 L1E7 chimeric virus‐like particles: induction of cytotoxic T cells and specific tumor protection. Int. J. Cancer. 1999;81(6):881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 177.Da Silva D.M., Schiller J.T., Kast W.M. Heterologous boosting increases immunogenicity of chimeric papillomavirus virus-like particle vaccines. Vaccine. 2003;21(23):3219–3227. doi: 10.1016/s0264-410x(03)00237-8. [DOI] [PubMed] [Google Scholar]
- 178.Greenstone H.L. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA. 1998;95(4):1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qian J. Combined prophylactic and therapeutic cancer vaccine: enhancing CTL responses to HPV16 E2 using a chimeric VLP in HLA‐A2 mice. Int. J. Cancer. 2006;118(12):3022–3029. doi: 10.1002/ijc.21781. [DOI] [PubMed] [Google Scholar]
- 180.Rudolf M.P. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J. Immunol. 2001;166(10):5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 181.Wakabayashi M.T. Comparison of human papillomavirus type 16 L1chimeric virus-like particles versus L1/L2 chimeric virus-like particles in tumor prevention. Intervirology. 2002;45(4–6):300–307. doi: 10.1159/000067921. [DOI] [PubMed] [Google Scholar]
- 182.Paz De la R.G. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virol. J. 2009;6:2–12. doi: 10.1186/1743-422X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Monroy-Garcia A. Immunization with an HPV-16 L1-based chimeric virus-like particle containing HPV-16 E6 and E7 epitopes elicits long-lasting prophylactic and therapeutic efficacy in an HPV-16 tumor mice model. Arch. Virol. 2014;159(2):291–305. doi: 10.1007/s00705-013-1819-z. [DOI] [PubMed] [Google Scholar]
- 184.Lenz P. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 2001;166(9):5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 185.Peng S. Efficient delivery of DNA vaccines using human papillomavirus pseudovirions. Gene Ther. 2010;17(12):1453–1464. doi: 10.1038/gt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang R. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 2004;78(20):11152–11160. doi: 10.1128/JVI.78.20.11152-11160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gurunathan S., Klinman D.M., Seder R.A. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 188.Hung C.-F. Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene. PLoS One. 2012;7(7):e40983. doi: 10.1371/journal.pone.0040983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peng S. DNA vaccines delivered by human papillomavirus pseudovirions as a promising approach for generating antigen-specific CD8+ T cell immunity. Cell Biosci. 2011;1(1):26–36. doi: 10.1186/2045-3701-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang B. Co-administration with DNA encoding papillomavirus capsid proteins enhances the antitumor effects generated by therapeutic HPV DNA vaccination. Cell Biosci. 2015;5(1):35–44. doi: 10.1186/s13578-015-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bousarghin L. Inhibition of cervical cancer cell growth by human papillomavirus virus–like particles packaged with human papillomavirus oncoprotein short hairpin RNAs. Mol. Cancer Ther. 2009;8(2):357–365. doi: 10.1158/1535-7163.MCT-08-0626. [DOI] [PubMed] [Google Scholar]
- 192.Lamprecht R.L. Production of human papillomavirus pseudovirions in plants and their use in pseudovirion-based neutralisation assays in mammalian cells. Sci. Rep. 2016;6:e20431. doi: 10.1038/srep20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waheed M.T. Need of cost-effective vaccines in developing countries: what plant biotechnology can offer? SpringerPlus. 2016;5:65. doi: 10.1186/s40064-016-1713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sack M. The increasing value of plant-made proteins. Curr. Opin. Biotechnol. 2015;32:163–170. doi: 10.1016/j.copbio.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chan H.T., Daniell H. Plant‐made oral vaccines against human infectious diseases—are we there yet? Plant Biotechnol. J. 2015;13(8):1056–1070. doi: 10.1111/pbi.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peruzzi P.P., Chiocca E.A. Cancer immunotherapy: a vaccine from plant virus proteins. Nat. Nanotechnol. 2016;11(3):214–215. doi: 10.1038/nnano.2015.306. [DOI] [PubMed] [Google Scholar]
- 197.Steinmetz N.F. Viral Nanoparticles in Drug Delivery and Imaging. Mol. Pharm. 2013;10:1–2. doi: 10.1021/mp300658j. [DOI] [PubMed] [Google Scholar]
- 198.Steinmetz N.F. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed.: Nanotechnol. Biol. Med. 2010;6(5):634–641. doi: 10.1016/j.nano.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Esfandiari N. A new application of plant virus nanoparticles as drug delivery in breast cancer. Tumor Biol. 2016;37(1):1229–1236. doi: 10.1007/s13277-015-3867-3. [DOI] [PubMed] [Google Scholar]
- 200.Czapar A.E. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano. 2016;10(4):4119–4126. doi: 10.1021/acsnano.5b07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Saunders K., Sainsbury F., Lomonossoff G.P. Efficient generation of cowpea mosaicvirus empty virus-like particles by the proteolytic processing of precursors in insect cells and plants. Virology. 2009;393(2):329–337. doi: 10.1016/j.virol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 202.Lizotte P. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016;11(3):295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lam P., Steinmetz N.F. Plant Viral and Bacteriophage Delivery of Nucleic Acid Therapeutics. Nanomed. Nanobiotechnol. 2018;10(1):e1487. doi: 10.1002/wnan.1487. [DOI] [PubMed] [Google Scholar]