Abstract
Background
Epidemiological studies recognize cannabis intake as a risk factor for schizophrenia, yet the majority of adolescents who use marijuana do not develop psychosis. Similarly, the abuse of synthetic cannabinoids poses a risk for psychosis. For these reasons, it is imperative to understand the effects of adolescent cannabinoid exposure in susceptible individuals.
Methods
We recently developed a novel rodent model of schizophrenia susceptibility, the F2 methylazoxymethanol acetate rat, where only a proportion (~40%) of rats display a schizophrenia-like phenotype. Using this model, we examined the effects of adolescent synthetic cannabinoid exposure (0.2 mg/kg WIN55, 212-2, i.p.) or adolescent endocannabinoid upregulation (0.3 mg/kg URB597, i.p.) on dopamine neuron activity and amphetamine sensitivity in adulthood.
Results
Adolescent synthetic cannabinoid exposure significantly increased the proportion of susceptible rats displaying a schizophrenia-like hyperdopaminergic phenotype after puberty without producing any observable alterations in control rats. Furthermore, this acquired phenotype appears to correspond with alterations in parvalbumin interneuron function within the hippocampus. Endocannabinoid upregulation during adolescence also increased the proportion of susceptible rats developing an increase in dopamine neuron activity; however, it did not alter the behavioral response to amphetamine, further emphasizing differences between exogenous and endogenous cannabinoids.
Conclusions
Taken together, these studies provide experimental evidence that adolescent synthetic cannabinoid exposure may contribute to psychosis in susceptible individuals.
Keywords: URB597; WIN55,212-2; methylazoxymethanol acetate; electrophysiology; parvalbumin
Significance Statement
Schizophrenia is likely the result of interactions between genetic predispositions and environmental insults. In a rat model predisposed to aberrant dopamine system function, exposure during adolescence to a synthetic cannabinoid significantly enhances the proportion of these animals that develop a schizophrenia-like phenotype in adulthood. Notably, the same treatment has minimal effects on control animals. Therefore, adolescent exposure to synthetic cannabinoids may contribute to psychosis in susceptible individuals. Understanding these interactions may allow us to guide policy and health recommendations about synthetic cannabinoid use.
Introduction
Schizophrenia is a debilitating disease emerging during early adulthood and characterized by symptoms including hallucinations, social withdrawal, and cognitive impairment (American Psychiatric Association, 2000). Clinical observations indicate that acute or chronic cannabis administration can induce behavioral and cognitive deficits that resemble those observed in major psychiatric disorders like schizophrenia (Emrich et al., 1997). In addition, epidemiological studies suggest that frequent cannabis use is associated with a greater risk to develop schizophrenia or psychosis (Arseneault et al., 2002; Henquet et al., 2005). Indeed, cannabis users tend to develop schizophrenia at a younger age and suffer from more psychotic relapses (Linszen et al., 1994; Veen et al., 2004). Similarly, synthetic cannabinoids have been associated with psychosis and psychosis-like conditions (van Amsterdam et al., 2015), and exogenous cannabinoid agonists, administered during adolescence to rodents or nonhuman primates, produce a schizophrenia-like phenotype in adulthood (Rubino et al., 2009; Cass et al., 2014; Verrico et al., 2014).
It should be mentioned that a causal link between adolescent cannabis use and schizophrenia has not been conclusively demonstrated, and there is controversy over the nature of this association (Hall and Degenhardt, 2008; Hill, 2014; Haney and Evins, 2016). Indeed, a shared genetic vulnerability between cannabis use and psychosis might explain this relationship (Power et al., 2014; Carey et al., 2016). Adolescent cannabis exposure is neither necessary nor sufficient to induce schizophrenia (Arseneault et al., 2004). Indeed, the majority of cannabis users never develop schizophrenia nor do they present mild psychotic symptoms, suggesting that gene/environment interactions are responsible for the susceptibility to cannabis-induced psychosis (Degenhardt et al., 2003; Henquet et al., 2005; French et al., 2015). The use of synthetic cannabinoids has increased among adolescents, likely due to their high potency, availability, perceived safety, and ability to circumvent common drug tests (Every-Palmer, 2011; Castellanos and Gralnik, 2016). The potential for greater cannabinoid receptor activation than delta-9-tetrahydracannabinol (THC) may explain synthetic cannabinoids’ strong association with acute psychosis in vulnerable individuals and the general population (Castellanos and Gralnik, 2016; Fattore, 2016). The long-term consequences of adolescent exposure to synthetic cannabinoids, however, are relatively unknown.
There is also a disconnect between clinical studies, which point to cannabis consumption as a risk factor for schizophrenia, and preclinical studies showing that adolescent cannabinoid exposure induces a schizophrenia-like phenotype in essentially all animals (Rubino et al., 2009; Leweke and Schneider, 2011; Zamberletti et al., 2014). Thus, to better examine the mechanisms by which adolescent cannabinoid exposure contributes to schizophrenia, it is essential to use a model of susceptibility that recapitulates the clinical observations. The methylazoxymethanol acetate (MAM)-treated rat is a developmental disruption model of schizophrenia whereby a mitotoxin (MAM) is administered to pregnant females on gestational day 17 (Moore et al., 2006; Lodge and Grace, 2009). This model displays histological, neurophysiological, and behavioral deficits in the F1 generation, which are analogous to schizophrenia symptoms observed in patients (Penschuck et al., 2006). This phenotype, however, is observed in most (if not all) of the experimental animals. Given that MAM is a potent methylating agent that induces epigenetic alterations in the F1 generation (Perez et al., 2016), we examined whether schizophrenia-like neurophysiological deficits could be inherited through generations and found that a subpopulation of F2 (and F3) MAM rats displayed a robust schizophrenia-like hyperdopaminergic phenotype characterized by increased dopamine neuron activity (Perez et al., 2016). As this phenotype was observed in only a subset of rats, we hypothesized that these animals provide a valid model of susceptibility to schizophrenia to examine potential gene/environment interactions. In this work, we used this novel model to show that synthetic cannabinoid exposure during adolescence increases the proportion of F2 MAM rats displaying a schizophrenia-like hyperdopaminergic phenotype in adulthood without affecting control littermates. Our data indicate that this phenotype is associated with a decrease in inhibitory interneuron function in the hippocampus, a key pathological deficit in schizophrenia (Lodge et al., 2009; Lodge and Grace, 2011; Perez and Lodge, 2013; Boley et al., 2014). Given the purported beneficial effects of endogenous cannabinoid upregulation in schizophrenia (Seillier et al., 2010; Seillier et al., 2013; Aguilar et al., 2014), we also examined the effect of administration of the endocannabinoid-enhancing drug URB597 during adolescence, a critical period for endocannabinoid system development. Interestingly, endocannabinoid upregulation during adolescence also increased the proportion of susceptible rats developing a hyperdopaminergic phenotype. Taken together, these data demonstrate that adolescent cannabinoid exposure (both exogenous and endogenous) can contribute to the development of schizophrenia-like hyperdopaminergic symptoms in susceptible individuals.
Methods
All experiments were conducted in accordance with the guidelines outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center San Antonio.
Animals
Albino Sprague-Dawley rats were used for all experiments. MAM treatments were performed as described previously (Moore et al., 2006). Timed pregnant rats were injected with MAM (25 mg/kg i.p.) or saline (1 mL/kg i.p.) on gestational day 17. All rats were weaned at postnatal day (PND) 21. Once F1 MAM and saline rats had reached sexual maturity, they were mated with controls to generate the second filial generation (F2). The phenotype of F1 animals was not verified in this experiment, as gestational disruption with MAM reliably induces a schizophrenia-like phenotype in all subjects (Moore et al., 2006; Perez and Lodge, 2013). Nine F1 MAM animals were bred with control mates to produce 49 F2 MAM males. Ten F1 saline (SAL) animals were bred with control mates to produce 51 F2 SAL males. All adult F2 animals in the WIN cohort were subjected to the experimental recordings described in detail below. One F2 SALxWIN rat was removed from the dataset as a significant outlier (2.55 dopamine cells/track, Grubbs’ outlier test, P<.05). No other outliers were detected in the dataset.
Adolescent Drug Administration
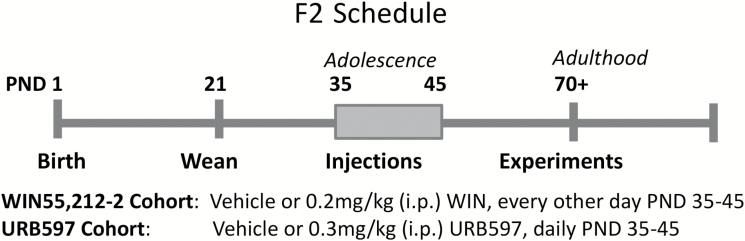
Adolescent F2 MAM and F2 SAL rats received daily injections of either vehicle (1 mL/kg i.p.), the cannabinoid (CB1 and CB2 receptor) agonist WIN55,212-2 (0.2 mg/kg i.p. every other day), or the fatty acid amide hydrolase (FAAH) inhibitor URB597 (0.3 mg/kg i.p. daily) during PND 35 to 45 (Figure 1). URB597 increases endogenous cannabinoid signaling and produces antipsychotic effects in rodents (Seillier et al., 2010; Aguilar et al., 2014). URB597 was dissolved in 10% Tween-80, 10% polyethylene glycerol, 80% saline; WIN55,212-2 was dissolved in 1% dimethyl sulfoxide (in saline). Many preclinical studies report schizophrenia-like phenotypes after administering cannabinoids at higher doses (1.2 mg/kg WIN55,212-2) for 20+ days during adolescence (Leweke and Schneider, 2011; Gomes et al., 2015). Similarly, WIN55,212-2 (2 mg/kg, i.p., 3–5 injections) administered during the adolescent period of PND 35 to 45 in rats or PND 30 to 35 in mice is sufficient to evoke schizophrenia-like changes in adult rodents (Gleason et al., 2012; Cass et al., 2014). In our preliminary studies, 1.2 mg/kg WIN55,212-2 (i.p.) administration for 5 days during PND35-45 was sufficient to augment dopamine population activity in a majority of control rats. We used a lower dose of WIN55,212-2 to more accurately recapitulate cannabinoid receptor activation by marijuana and to avoid induction of a schizophrenia-like phenotype in control animals. Indeed, 0.5 mg/kg WIN55,212-2 (i.p.) has been used in rats as a correlate of a moderately low exposure to cannabis in humans (Mereu et al., 2003), and chronic administration of 0.2 mg/kg WIN55,212-2 (i.p.) was reported to have minimal behavioral effects in rats (Bambico et al., 2010). The dose of URB597 (0.3 mg/kg i.p.) was based on our previous studies showing antipsychotic-like activity in an adult rat model of schizophrenia (Seillier et al., 2010, 2013; Aguilar et al., 2014). In rodents, this dose of URB597 inhibits FAAH in vivo and upregulates brain endocannabinoid anandamide for hours without producing changes in locomotion, body temperature, or food intake (Kathuria et al., 2003).
Figure 1.
Experimental timeline. Adolescent F2 methylazoxymethanol acetate (MAM) or saline rats were injected with vehicle, WIN55,212-2 (0.2 mg/kg, i.p. every other day) or URB597 (0.3 mg/kg, i.p. daily) from postnatal day (PND) 35 to 45 and tested in adulthood (PND 70+). Behavioral experiments were run at least 7 days before dopamine recordings.
Extracellular Dopamine Cell Recordings
Adult male F2 MAM and F2 SAL rats were anesthetized with 8% chloral hydrate (400 mg/kg i.p.) and placed in a stereotaxic apparatus with body temperature held at 37oC. Supplemental chloral hydrate was administered as needed to suppress the limb compression withdrawal reflex during recordings (tested every 20 minutes). Chloral hydrate induces general anesthesia without significantly affecting spontaneous dopamine neuron activity (Hyland et al., 2002). Glass micro-electrodes (impedance 6–14 MΩ) were lowered into the ventral tegmental area (VTA) in the right hemisphere (coordinates from bregma: A/P -5.3 mm, M/L +0.6 mm, D/V -6.5 to -9.0 mm). Three-minute-long extracellular recordings were taken from spontaneously active dopamine neurons. These neurons were identified using electrophysiological parameters recorded with open-filter settings (low pass: 30 Hz; high pass: 30 kHz) as previously reported (Grace and Bunney, 1983). Dopamine neurons can be identified using electrophysiological criteria, if applied carefully (Ungless and Grace, 2012). Population activity (number of spontaneously active dopamine cells per vertical electrode track), basal firing rate, and the proportion of action potentials occurring in bursts were examined as previously reported (Grace and Bunney, 1983). Five to 9 electrode tracks (separated by 0.2 mm) were recorded in each animal.
Behavior
Adult male F2 MAM or F2 saline (SAL) rats (300–425 g) were acclimated to the testing room for at least 1 hour. Rats were then placed in an open field arena (17.5 x 17.5 x 12 inches; MED Associates) under bright light, and their locomotion was measured in the x-y plane by recording beam breaks with the Open Field Activity software (Med Associates). Following a 45-minute baseline recording, rats received an injection of amphetamine (0.5 mg/kg i.p.) and their locomotor activity was recorded for 45 minutes. Rats then received an additional injection of 2 mg/kg amphetamine and locomotor activity was again recorded for 45 minutes. Dopamine cell recordings in these animals were carried out at least 7 days later to avoid any confounding effects of amphetamine.
Histology
After the electrophysiological recordings, all rats were deeply anesthetized, decapitated, and the brain was collected. The ventral hippocampus was rapidly dissected from the left hemisphere and immediately frozen at -80oC for protein analysis. To verify electrode track placement within the VTA, the right hemisphere was collected, fixed for at least 48 hours in 8% (wt/vol) paraformaldehyde, and cryoprotected in 25% (wt/vol) sucrose until saturated. Right hemispheres were sectioned (25-µm coronal sections), mounted on gelatin-chrom alum-coated slides, and stained with Cresyl violet. All histological analyses were performed with reference to a stereotaxic atlas (Paxinos and Watson, 2009).
Western Blot
The ventral hippocampus was homogenized in 500 μL of ice-cold homogenization buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, pH 7.4) containing 1% protease inhibitor cocktail (Sigma-Aldrich). Homogenates were centrifuged at 12000 G for 10 minutes at 4oC, and the supernatant was collected and analyzed for parvalbumin (PV) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. After being heated at 90oC for 10 minutes in Laemmli sample buffer + 5% dithiothreitol, proteins (25 μg per well) were separated (40–50 minutes at 150 V) on Any kD Mini-PROTEAN TGX gels (Bio-Rad) in Tris/glycine/SDS buffer (Bio-Rad) and subsequently transferred onto nitrocellulose/filter paper sandwiches, 0.2 um (Bio-Rad), with ice-cold transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% [wt/vol] ethanol, pH 8.3) for 1 hour at 100 V. Membranes were blocked for 30 minutes (PV: 3% nonfat milk in TBST; GAPDH: 5% bovine serum albumin in TBST; TBST: 15 mM Tris-HCl, 137 mM NaCl, 0.1% Tween 20, pH 7.4) and incubated for 1 hour in primary anti-PV (1:5000, rabbit, 23oC) or anti-GAPDH (1:1000, mouse, 4oC) antibody. Membranes were washed in TBST (3x 10 minutes) before incubation for 1 hour at 23oC in secondary antibody (PV: 1:10000, goat anti-rabbit conjugated to HRP; GAPDH: 1:5000, goat anti-mouse conjugated to HRP). Membranes were rinsed again in TBST (3x 10 minutes) and then in Pierce ECL Western Blotting substrate (Thermo Scientific; no. 32106) for 1 minute, exposed to high-performance chemiluminescent film (GE Healthcare; Amersham Hyperfilm ECL; 28906839), and the band density quantified with ImageJ. After the quantification of PV expression, membranes were stripped for 15 minutes in Restore Western Blot Stripping Buffer (Thermo Scientific) and reprobed for GAPDH.
Analysis
Electrophysiological analysis of putative dopamine neuron activity was performed with LabChart software v7.1 (ADInstruments) and plotted using GraphPad Prism (GraphPad Software). All data are presented as the mean±SE, with n values representing the number of animals per experimental group. For firing rate and burst firing analyses, n values indicate the number of cells recorded. Statistical analyses were carried out using SigmaPlot (Systat Software, Inc) and Graphpad Prism. Dopamine neuron activity and western blot data were analyzed by 2-way ANOVA. Variables were strain (F2 Saline / F2 MAM) and treatment (vehicle / URB597 or vehicle / WIN55,212-2). Posthoc comparisons were performed using the Holm-Sidak method. The percentage of rats that developed a schizophrenia-like hyperdopaminergic phenotype was analyzed by the chi square test of independence followed by Marasculio’s posthoc. The average dopamine population activity in each subset (<1.5 or ≥1.5 cells per track) was analyzed by 1-way ANOVA and the Holm-Sidak posthoc test. Locomotor activity was analyzed by 3-way ANOVA (variables: treatment, amphetamine dose, time).
Chemicals
MAM was purchased from Midwest Research Institute. URB597 was from Cayman Chemical Company. WIN55,212-2 mesylate was from Tocris Bioscience. Goat anti-mouse IgG HRP (A4416) antibody, chloral hydrate, D-Amphetamine sulfate, and Dulbecco’s phosphate-buffered saline were from Sigma-Aldrich. Anti-parvalbumin (aab11427), anti-GAPDH (ab9484), and goat anti-rabbit IgG HRP (ab6721) antibodies were from Abcam.
Results
Adolescent Exposure to WIN55,212-2 Induces a Schizophrenia-Like Hyperdopaminergic Phenotype in F2 MAM Rats
WIN55,212-2 administration during adolescence induced a persistent increase in dopamine neuron population activity in susceptible F2 MAM, but not F2 SAL, rats (Figure 2A). Two-way ANOVA revealed a main effect of strain [F(1,51)=10.96; P=.0017; 2-way ANOVA; Figure 2A] with no significant interaction (P=.10). The Holm-Sidak multiple comparison test revealed the F2 MAM x WIN group had significantly higher dopamine population activity (1.642±0.12 cells/track, n=14) than the F2 SAL x VEH (1.134±0.08 cells/track, n=14; P<.05) and F2 SAL x WIN groups (1.084±0.09 cells/track, n=13; P<.01), but not F2 MAM x VEH littermates (1.319±0.14 cells/track, n=14; Figure 2A). The firing rate of dopamine neurons was unchanged by all treatments (Figure 2B, 2-way ANOVA), but a main effect of strain was observed, such that F2 MAM rats had significantly less bursting (32.34±1.79%) compared with F2 SAL rats [39.08±2.09%; F(1,408)=6.376, P<.05; 2-way ANOVA; Figure 2C]. The sample sizes were as follows: F2 SAL VEH n=14 rats, 96 cells; F2 SAL WIN n=13 rats, 84 cells; F2 MAM VEH n=14 rats, 113 cells; F2 MAM WIN n=14 rats, 125 cells.
Figure 2.
(A) Adolescent exposure to WIN55,212-2 increases dopamine neuron population activity in “susceptible” F2 methylazoxymethanol acetate (MAM) but not F2 saline rats. The firing rate and burst firing of putative dopamine neurons are presented in B and C, respectively. * Represents a significant difference from F2 saline (SAL) x vehicle (VEH) and F2 SAL x WIN groups (P<.05, 2-way ANOVA, Holm-Sidak). # Represents a main effect of strain (P<.05, 2-way ANOVA). Error bars indicate SEM. The sample sizes per group were as follows: F2 SAL VEH, n=14 rats, 96 cells; F2 SAL WIN, n=13 rats, 84 cells; F2 MAM VEH, n = 14 rats, 113 cells; F2 MAM WIN, n=14 rats, 125 cells.
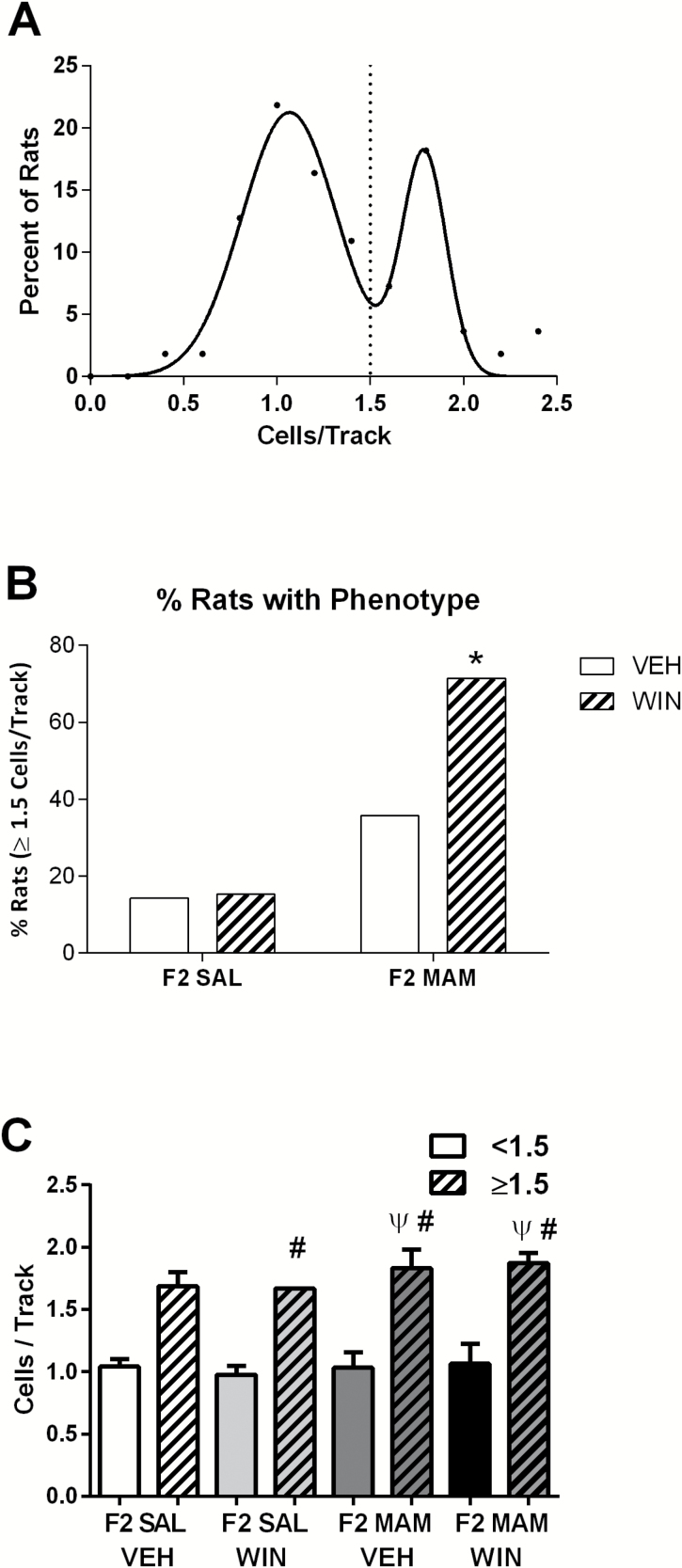
As only a proportion of F2 MAM animals inherits a schizophrenia-like phenotype (Perez et al., 2016), we examined the distribution of dopamine neuron activity across all rats tested. Consistent with our published data, we found a bimodal distribution (Figure 3A: R2=0.96) of VTA dopamine neuron population activity that was best fit by the sum of 2 Gaussians (extra sum-of-squares F test: P=.0001) that separated at ~1.5 cells/track (Perez et al., 2016).
Figure 3.
Adolescent exposure to WIN55,212-2 increases the proportion of F2 methylazoxymethanol acetate (MAM) rats displaying a schizophrenia-like phenotype. (A) Dopamine population activity recorded from all animals (F2 saline [SAL] and F2 MAM) follows a bimodal distribution (R2=0.96) and a split around 1.5 spontaneously active dopamine cells per track. Some F2 MAM animals inherit a schizophrenia-like hyperdopaminergic phenotype (augmented dopamine population activity) from their F1 MAM parent (right distribution), while others show a phenotype closer to 1.0 cells/track, like their control parent (left distribution). (B) Percentage of rats with ≥1.5 dopamine cells per track for each treatment group. (C) Average dopamine cells per track (<1.5 solid colors, ≥1.5 striped) in each treatment group. * Represents a significant difference from F2 SAL x Vehicle and F2 SAL x WIN groups (chi square, Marasculio’s posthoc). Ψ Represents a significant difference from the F2 SAL VEH <1.5 cells/track subgroup (solid white), while # represents a significant difference from a treatment’s respective <1.5 cells/track subgroup (corresponding solid color) (1-way ANOVA, P<.0001, Holm-Sidak). The proportion of animals with ≥1.5 dopamine cells per track was: 2/14 (F2 SAL VEH), 2/13 (F2 SAL WIN), 5/14 (F2 MAM VEH), 10/14 (F2 MAM WIN).
The percentage of F2 MAM rats displaying a schizophrenia-like hyperdopaminergic phenotype (defined by augmented dopamine population activity) doubled from 36% to 71% after adolescent treatment with 0.2 mg/kg WIN55,212-2 (Figure 3B). A chi square test of independence was used to compare the percentage values of rats developing schizophrenia-like hyperdopaminergic phenotypes in each experimental group [χ2 (3, n=55)=13.0833, α=0.0045]. Specifically, the F2 MAM x WIN group had a significantly higher proportion of animals with ≥1.5 dopamine cells per track (71%) than either the F2 SAL x VEH (14%; χ2=14, α=0.0029; Marasculio’s posthoc) or the F2 SAL x WIN (15%; χ2=12.7727,α=0.0052; Marasculio’s posthoc) groups (Figure 3B). The average number of dopamine cells/track recorded in each subset is shown in Figure 3C.
Adolescent Exposure to WIN55,212-2 Increases Amphetamine Sensitivity in F2 MAM Rats
The locomotor response to amphetamine was tested following 2 increasing doses (0.5 and 2 mg/kg, i.p.) administered 45 minutes apart (Figure 4). Here we demonstrate that WIN-treated F2 MAM rats display a hyper-responsivity to amphetamine administration, indicative of a sensitized dopaminergic system. In F2 SAL rats, we found a significant interaction between adolescent WIN and amphetamine dose [F(2,729)=8.687, P<.001; 3-way ANOVA]. Specifically, after 2 mg/kg amphetamine, the F2 SAL x WIN group (3929±112.3 cm) traveled significantly less than their F2 SAL x VEH littermates (4682±108.4 cm; Holm-Sidak:=4.823, P<.001; Figure 4A). There was also a significant interaction between adolescent WIN and amphetamine dose in F2 MAM rats [F(2,729)= 3.452, P<.05; 3-way ANOVA]. At baseline, F2 MAM rats treated with WIN55,212-2 during adolescence traveled significantly more distance (2072±126.3 cm) than their F2 MAM littermates treated with vehicle (1630±122.0 cm; Holm-Sidak: t=2.515, P<.05; Figure 4B). After 2 mg/kg amphetamine, F2 MAM rats treated with WIN55,212-2 in adolescence traveled significantly more distance (5270±126.3 cm) than their littermates treated with vehicle (4318±122.0 cm; Holm-Sidak:=5.416, P<.001; Figure 4B). There was no difference between groups (either F2 SAL or F2 MAM rats) after 0.5 mg/kg amphetamine. To further examine the relationship between dopamine neuron activity and amphetamine responsivity, we divided the groups into susceptible (>1.5 cells/track) and nonsusceptible (<1.5 cells/track) and examined locomotor activity in these populations. Unfortunately, the variability in behavior between individual rats coupled with the decrease in sample size made these data difficult to interpret (not shown).
Figure 4.
Adolescent exposure to WIN55,212-2 increases the locomotor response to amphetamine in F2 methylazoxymethanol acetate (MAM) rats. The average locomotor activity of rats across baseline, 0.5 mg/kg, and 2 mg/kg (i.p.) amphetamine challenges was recorded. While F2 saline rats were less sensitive to 2 mg/kg amphetamine after adolescent WIN55,212-2 treatment (A), the “susceptible” F2 MAM rats displayed increased locomotor response to amphetamine (B). † Represents a significant difference from vehicle group for entire period following 2 mg/kg amphetamine (P<.05, 3-way ANOVA). * Represents a significant difference from vehicle group for entire baseline period (P<.05, 3-way ANOVA). Error bars indicate SEM. Sample sizes were SAL VEH, n=15; SAL WIN, n=14; MAM VEH, n=15; MAM WIN, n=14.
Adolescent Cannabinoid Treatment Decreases Parvalbumin Expression in the Hippocampus
PV expression in the ventral hippocampus was measured by western blot, using GAPDH as a loading control (Figure 5A). F2 rats, regardless of strain, show decreased PV protein expression in response to adolescent exposure to WIN55,212-2 [main effect of WIN; F(1,40)=5.645, P=.0224; 2-way ANOVA; Figure 5B]. The graph in Figure 5C shows a linear regression between the data collected from adolescent vehicle and WIN treatments within each strain. In F2 MAM rats, WIN treatment during adolescence decreased PV expression (Figure 5B), which was associated with increased dopamine cells per track (Figure 5C), consistent with previously published correlations (Perez et al., 2016). Sample sizes were: SAL VEH, n=10; SAL WIN, n=13; MAM VEH, n=8; MAM WIN, n=13.
Figure 5.
Adolescent exposure to WIN55,212-2 decreases parvalbumin (PV) expression in the ventral hippocampus. A representative blot is depicted in A, whereas the quantification of these data are presented in B. Two-way ANOVA showed a main effect of adolescent WIN (P<.05). (C) Consistent with previous reports (Perez et al., 2016), a negative relationship between PV expression and dopamine cells per track is observed only in F2 methylazoxymethanol acetate (MAM) rats. † Represents a significant difference from vehicle group (P<.05, 2-way ANOVA). Error bars indicate SEM. Sample sizes were saline (SAL) vehicle (VEH), n=10; SAL WIN, n=13; MAM VEH, n=8; MAM WIN, n=13. The uncropped blot for A is included as supplementary material.
Adolescent URB597 Increases the Proportion of F2 MAM Rats with a Schizophrenia-like Hyperdopaminergic Phenotype
As previous studies have shown antipsychotic-like activity of URB597 (Seillier et al., 2013; Aguilar et al., 2014), we examined the effect of this drug when administered during adolescence. Although URB597 in adolescence did not significantly increase adult dopamine neuron population activity in either F2 SAL (Veh, 1.360±0.09 cells; URB, 1.368±0.13 cells; 2-way ANOVA) or F2 MAM rats (Veh, 1.345±0.15 cells; URB, 1.587±0.13 cells; 2-way ANOVA) (Figure 6A), after this treatment the proportion of F2 MAM rats displaying a schizophrenia-like hyperdopaminergic phenotype (≥1.5 dopamine cells/track) doubled from 38% to 78% [Figure 7B: χ2 (3, n=37)=4.9001, α= 0.1793]. This trend was comparable to that observed in rats treated with WIN55,212-2 during adolescence (Figure 3B). Firing rate and burst firing of dopaminergic cells were unchanged in all treatment groups (Figure 6B-C, 2-way ANOVA). We again examined the distribution of dopamine neuron activity across all rats tested in this cohort. Consistent with our published data (Perez et al., 2016), we found a bimodal distribution (Figure 7A: R2 = 0.96) of VTA dopamine neuron population activity that was best fit by the sum of 2 Gaussians (Extra sum-of-squares F test: P=.0134) that separated at ~1.5 cells/track. Sample size per experimental group was: F2 SAL x Veh, n=8 rats (68 cells recorded); F2 SAL x URB, n=12 rats (107 cells recorded); F2 MAM x Veh, n=8 rats (67 cells recorded); F2 MAM x URB, n=9 rats (84 cells recorded).
Figure 6.
Adolescent exposure to the fatty acid amide hydrolase inhibitor URB597 does not significantly alter the average dopamine cells per track (A), firing rate (B), or burst firing (C) characteristics of the dopamine cells. Error bars indicate SEM. Sample sizes were: saline (SAL) vehicle (VEH), n=8; SAL URB, n=12; methylazoxymethanol acetate (MAM) VEH, n=8; MAM URB, n=9.
Figure 7.
Adolescent exposure to URB597 increases the proportion of “susceptible” F2 methylazoxymethanol acetate (MAM) rats with increased dopamine population activity in adulthood, while having no effect in F2 saline rats (B). (A) Dopamine population activity recorded from all animals in this cohort follows a bimodal distribution (R2=0.96) and a split around 1.5 spontaneously active dopamine cells per track. (B) Percentage of rats with ≥1.5 dopamine cells per track for each treatment group. (C) Average dopamine cells per track (<1.5 solid colors, ≥1.5 striped) in each treatment group. Ψ Represents a significant difference from the F2 saline (SAL) vehicle (VEH) <1.5 cells/track subgroup (solid white), while # represents a significant difference from a treatment’s respective <1.5 cells/track subgroup (corresponding solid color) (1-way ANOVA, P<.0001, Holm-Sidak). The proportion of animals with ≥1.5 dopamine cells per track was: 3/8 (F2 SAL VEH), 4/12 (F2 SAL URB), 3/8 (F2 MAM VEH), 7/9 (F2 MAM URB).
The locomotor response to amphetamine was tested as described above. Interestingly, and in contrast to the effect of WIN55,212-2, there were no observable increases in the locomotor response to amphetamine following adolescent exposure to URB-597 (not shown). One potential reason for this discrepancy is based on recent data from the Grace laboratory suggesting opposing effects of dopamine neuron activity in the VTA and SNc that are mediated by distinct regions of the ventral pallidum (Bortz and Grace, 2017). Thus, the lack of effect on amphetamine-induced behavior may be due to URB-597’s robust targeting of the VP following systemic administration as we have reported previously (Aguilar et al., 2014). Indeed, this observation is consistent with recent physiological reports demonstrating qualitative and quantitative differences between exogenous and endogenous cannabinoids (Aguilar et al., 2016).
Discussion
The adolescent use of cannabinoids has been consistently associated with increased risk for developing schizophrenia or psychosis, although it is neither necessary nor sufficient (Arseneault et al., 2004; Bechtold et al., 2015). This apparent lack of causality has generated many hypotheses (for review, see Haney and Evins, 2016). While schizophrenia-like traits may increase the likelihood of cannabis use, we posit that adolescent cannabinoid exposure contributes to the etiology of schizophrenia only in individuals with an underlying genetic predisposition (Caspi et al., 2005; Henquet et al., 2005; French et al., 2015). This hypothesis is consistent with longitudinal studies showing that adolescent cannabis use increased the incidence of schizophrenia only in patients with specific catechol-methyl-transferase polymorphisms (Caspi et al., 2005).
Several preclinical studies have examined the effects of early-life cannabinoid administration in adult animals. Adolescent administration of THC or the synthetic cannabinoid agonist WIN55,212-2 lead to a variety of schizophrenia-like deficits in adult rodents that may be reversed by atypical antipsychotic treatment (Rubino et al., 2009; Leweke and Schneider, 2011). However, these deficits occur in all experimental animals, whereas clinical observations indicate that cannabinoid exposure during adolescence does not induce schizophrenia in the majority of people (Caspi et al., 2005; Bechtold et al., 2015). In this study, we used a novel rodent model of schizophrenia susceptibility to examine the consequences of adolescent cannabinoid exposure. Specifically, we demonstrate that administration of a synthetic cannabinoid to adolescent rats, at a dose and duration that do not produce overt physiological changes in controls, dramatically increased the proportion of F2 MAM rats displaying schizophrenia-like hyperdopaminergic phenotypes. Indeed, WIN55,212-2 doubled the percentage of F2 MAM rats showing aberrant dopamine neuron population activity in adulthood without dramatically altering their firing rate or burst firing characteristics. It should be noted that previous studies have demonstrated that WIN55,212-2 acutely activates dopamine neurons in adult rats (French et al., 1997). This acute and transient increase in firing rate is likely responsible for the acute psychotomimetic effects of synthetic cannabinoids whereby the data presented here are in line with findings from other rodent models and selective and persistent increases in the number of spontaneously active neurons are observed (Lodge and Grace, 2007; Aguilar et al., 2014; Gomes et al., 2015). Dopamine neuron population activity (i.e., the number of dopamine neurons firing spontaneously) is thought to provide a gain of function to the dopamine system; thus, an increase in this measure would ascribe the same (enhanced) salience to all environmental stimuli, a mechanism that may underlie the positive symptoms of schizophrenia (for review, see Lodge and Grace, 2011).
The increase in dopamine neuron population activity is likely responsible for the aberrant response to psychomotor stimulants, such as amphetamine, seen in patients with schizophrenia (Laruelle et al., 1996) and rodent models (Lodge and Grace, 2011). Our studies showed that adolescent WIN55,212-2 administration increased the locomotor response to a low dose of amphetamine in F2 MAM rats but not in F2 controls. Although F2 MAM x WIN rats exhibited an increased locomotor activity at baseline, this effect was greatly exaggerated following exposure to 2 mg/kg amphetamine. These data suggest that F2 MAM rats have a sensitized dopamine system, which is consistent with the increased dopamine population activity reported above and with previously reported data in F1 generation MAM rats.
Using a variety of rodent models, we previously demonstrated that increased dopamine neuron population activity is directly attributable to a pathologically enhanced drive from the ventral hippocampus (Lodge and Grace, 2007; Aguilar et al., 2014). Aberrant activity in the anterior hippocampus (the analogous region in primates) has been repeatedly reported in patients with schizophrenia by imaging studies (Kawasaki et al., 1992) and correlated with symptom severity (Schobel et al., 2009). The cause of ventral hippocampus hyperactivity in rodent models has not been conclusively elucidated; however, it likely involves a decrease in inhibitory interneuron function in this area. PV is a calcium binding protein found in fast-firing interneurons that has been consistently implicated in the pathophysiology of schizophrenia based on postmortem studies (Lewis et al., 2005). In addition, decreased PV expression or PV interneuron activity in the hippocampus has been associated with impaired downstream regulation of dopamine activity in the VTA (Shah and Lodge, 2013; Boley et al., 2014; Perez et al., 2016) and schizophrenia-like behavioral deficits (Nguyen et al., 2014). We previously showed that the F1 generation of MAM-treated rats have decreased expression of PV protein in the ventral hippocampus (Lodge et al., 2009), a region where PV levels significantly increase during adolescence (Caballero et al., 2013). In F2 MAM rats, we also observed a negative correlation between PV expression in this area and dopamine neuron activity (Perez et al., 2016). In this study, we showed that adolescent WIN55,212-2 administration to F2 MAM rats produced a significant decrease of PV expression in the ventral hippocampus as well as a concomitant increase in dopamine neuron population activity. These findings are consistent with studies carried out in other labs showing that adolescent THC administration decreases the cell size of PV-containing interneurons in the prefrontal cortex of catechol-methyl-transferase knockout mice (Behan et al., 2012). Adolescent exposure to cannabinoids likely disturbs endocannabinoid signaling with potential consequences on synaptic pruning, dendritic plasticity, and maturation of limbic and cortical regions (Malone et al., 2010). This may affect the excitability of circuits, potentially leading to oxidative damage that affects the vulnerable fast-spiking PV interneurons in a wide variety of preclinical models (Steullet et al., 2017). It is important to note that the decrease in hippocampal PV expression occurred in both F2 MAM and control rats, albeit this was more pronounced in the F2 MAM animals (~22% decrease vs ~10% decrease from vehicle-treated littermates). Thus, it is possible that adolescent cannabinoid exposure represents one of the multiple “hits” (both genetic and environmental) that are required to reduce PV function to a level sufficient to alter neuronal activity and behavior. Indeed, a minor decrease in PV expression (such as that seen in control animals following WIN55,212-2) appears insufficient to produce enduring changes in dopamine activity (Figure 2A). Moreover, PV is a calcium binding protein and, as such, its expression can be regulated to compensate for increased fast-spiking interneuron activity (Hu et al., 2014). Thus, it is possible that the behavioral paradigms and anesthesia may have influenced PV expression per se. Given that all treatment groups underwent the same procedures, the reported effects are likely driven by treatment.
It is important to note that these rats were bred from F1 MAM mothers and F1 saline fathers. We previously demonstrated (Perez et al., 2016), and consistent with the results presented here, that F2 MAM rats from F1 MAM mothers do not display a significant decrease in PV expression nor a significant increase in dopamine cells per track. As such, they represent a useful model of schizophrenia “susceptibility” to examine the consequence of environmental manipulations.
While a large body of evidence indicates that exogenous cannabinoids in adulthood may exacerbate schizophrenia symptoms (Mason et al., 2009), enhanced endogenous cannabinoid transmission (through FAAH inhibition) has been shown to alleviate some schizophrenia-like behaviors (Leweke et al., 2012; Iseger and Bossong, 2015). We previously demonstrated that the FAAH inhibitor URB597 (0.3 mg/kg i.p.) can alleviate both social withdrawal (Seillier et al., 2013) and aberrant dopamine neuron population activity (Aguilar et al., 2014) in the phencyclidine (PCP) rodent model of schizophrenia. Similarly, initial clinical trials with cannabidiol (a marijuana plant ingredient with FAAH inhibitory activity) have produced promising results in adult patients with schizophrenia (Leweke et al., 2012). It has been suggested that targeting the prepsychotic or prodromal phases of the illness may be an effective way to prevent the transition to schizophrenia (Phillips et al., 2002). For this reason, we also examined what effects URB597 administration might have during adolescence in the F2 MAM model of susceptibility.
Interestingly, while URB597 decreased aberrant dopamine neuron population activity in adult PCP-treated rats (Aguilar et al., 2014), we found that F2 MAM rats exposed to URB597 during adolescence are twice as likely to present augmented dopamine population activity than their vehicle-treated littermates. The reason for this discrepancy likely lies in the physiological changes that the endogenous cannabinoid system undergoes throughout adolescence. During this time, CB1 receptor expression increases dramatically (Rodriguez de Fonseca et al., 1993; Mato et al., 2003; Verdurand et al., 2011) and the endocannabinoid anandamide reaches its highest concentration levels in limbic brain regions (Ellgren et al., 2008). Therefore, altering endogenous cannabinoid signaling during this critical period may produce different effects than those seen in adulthood. For this reason, FAAH inhibitors may be contraindicated for the pharmacological treatment of prodromal patients.
In summary, our study indicates that adolescent synthetic cannabinoid exposure (both exogenous and endogenous) can induce a schizophrenia-like hyperdopaminergic phenotype in a novel rodent model of schizophrenia susceptibility at doses and treatment regimens that have no observable effects in control animals. These data more closely recapitulate the human condition, where cannabis use in adolescence is a risk factor without being necessary or sufficient to induce psychosis (Arseneault et al., 2004; Hall and Degenhardt, 2008). Such a model could be used to examine mechanistic alterations underlying gene/environment interactions that contribute to the pathophysiology of schizophrenia. Indeed, we showed that adolescent synthetic cannabinoid exposure in this model decreases PV expression in the ventral hippocampus, a key deficit linked to dysregulation of the dopamine system in schizophrenia (Lewis et al., 2005; Shah and Lodge, 2013; Boley et al., 2014).
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
This work was supported by the US Department of Veterans Affairs and by the National Institute of Mental Health at the National Institutes of Health (grant nos. R01MH090067 to D.J.L., R01MH091130 to A.G., F31MH105166 to D.D.A., and MH016259-37 to M.E. Shenton).
Current address (D.D.A.): Laboratory of Neuroscience, VA Boston Healthcare System and Department of Psychiatry, Harvard Medical School, West Roxbury, MA
Statement of Interest
None.
References
- Aguilar DD, Chen L, Lodge DJ(2014)Increasing endocannabinoid levels in the ventral pallidum restore aberrant dopamine neuron activity in the subchronic PCP rodent model of schizophrenia. Int J Neuropsychopharmacol 18. doi:10.1093/ijnp/pyu035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar DD, Giuffrida A, Lodge DJ(2016)THC and endocannabinoids differentially regulate neuronal activity in the prefrontal cortex and hippocampus in the subchronic PCP model of schizophrenia. J Psychopharmacol 30:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000)Diagnostic and statistical manual of mental disorders, 4th ed Text Revision (DSM-IV-TR).Washington, DC: American Psychiatric Association. [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE(2002)Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM(2004)Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184:110–117. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G(2010)Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis 37:641–655. [DOI] [PubMed] [Google Scholar]
- Bechtold J, Simpson T, White HR, Pardini D(2015)Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol Addict Behav 29:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan AT, Hryniewiecka M, O’Tuathaigh CM, Kinsella A, Cannon M, Karayiorgou M, Gogos JA, Waddington JL, Cotter DR(2012)Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on indices of dopaminergic, endocannabinoid and gabaergic pathways. Neuropsychopharmacology 37:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boley AM, Perez SM, Lodge DJ(2014)A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr Res 157:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz DM, Grace AA(2017)Medial septum differentially regulates spontaneous dopamine neuron activity in the ventral tegmental area and substantia nigra pars compacta via distinct neurochemical pathways. Poster presented at Society for Neuroscience; Program No. 435.02. Washington, DC: Neuroscience Meeting Planner. [Google Scholar]
- Caballero A, Diah KC, Tseng KY(2013)Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus 23:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CE, Agrawal A, Bucholz KK, Hartz SM, Lynskey MT, Nelson EC, Bierut LJ, Bogdan R(2016)Associations between polygenic risk for psychiatric disorders and substance involvement. Front Genet 7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW(2005)Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 57:1117–1127. [DOI] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY(2014)CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry 19:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos D, Gralnik LM(2016)Synthetic cannabinoids 2015: an update for pediatricians in clinical practice. World J Clin Pediatr 5:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M(2003)Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend 71:37–48. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL(2008)Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol 18:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich HM, Leweke FM, Schneider U(1997)Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol Biochem Behav 56:803–807. [DOI] [PubMed] [Google Scholar]
- Every-Palmer S.(2011)Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend 117:152–157. [DOI] [PubMed] [Google Scholar]
- Fattore L.(2016)Synthetic cannabinoids-further evidence supporting the relationship between cannabinoids and psychosis. Biol Psychiatry 79:539–548. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X(1997)Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8:649–652. [DOI] [PubMed] [Google Scholar]
- French L, et al. 2015) Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry 72:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason KA, Birnbaum SG, Shukla A, Ghose S(2012)Susceptibility of the adolescent brain to cannabinoids: long-term hippocampal effects and relevance to schizophrenia. Transl Psychiatry 2:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Guimaraes FS, Grace AA(2015)Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS(1983)Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L(2008)Cannabis use and the risk of developing a psychotic disorder. World Psychiatry 7:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Evins AE(2016)Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology 41:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van Os J(2005)Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 330:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN.(2014)Clearing the smoke: what do we know about adolescent cannabis use and schizophrenia?J Psychiatry Neurosci 39:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P(2014)Interneurons. Fast-spiking, parvalbumin⁺ gabaergic interneurons: from cellular design to microcircuit function. Science 345:1255263. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R(2002)Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114:475–492. [DOI] [PubMed] [Google Scholar]
- Iseger TA, Bossong MG(2015)A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res 162:153–161. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D(2003)Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Suzuki M, Takashima T(1992)Regional cerebral blood flow in patients with schizophrenia. a preliminary report. Eur Arch Psychiatry Clin Neurosci 241:195–200. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB(1996)Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkötter J, Hellmich M, Koethe D(2012)Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Schneider M(2011)Chronic pubertal cannabinoid treatment as a behavioural model for aspects of schizophrenia: effects of the atypical antipsychotic quetiapine. Int J Neuropsychopharmacol 14:43–51. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW(2005)Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME(1994)Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 51:273–279. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA(2009)A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 29:2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA(2007)Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27:11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA(2009)Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res 204:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA(2011)Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T(2010)Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol 160:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason O, Morgan CJ, Dhiman SK, Patel A, Parti N, Patel A, Curran HV(2009)Acute cannabis use causes increased psychotomimetic experiences in individuals prone to psychosis. Psychol Med 39:951–956. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A(2003)Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci 17:1747–1754. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, Ghiglieri V, Tanganelli S, Gessa GL, Cuomo V(2003)Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. PNAS 100:4915–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA(2006)A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry 60:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Morrissey MD, Mahadevan V, Cajanding JD, Woodin MA, Yeomans JS, Takehara-Nishiuchi K, Kim JC(2014)Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. J Neurosci 34:14948–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C(2009)The rat brain in stereotaxic coordinates, compact 6th ed Sydney: Academic Press. [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT(2006)Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci 23:279–284. [DOI] [PubMed] [Google Scholar]
- Perez SM, Aguilar DD, Neary JL, Carless MA, Giuffrida A, Lodge DJ(2016)Schizophrenia-like phenotype inherited by the F2 generation of a gestational disruption model of schizophrenia. Neuropsychopharmacology 41:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SM, Lodge DJ(2013)Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry 18:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, Yung AR, Yuen HP, Pantelis C, McGorry PD(2002)Prediction and prevention of transition to psychosis in young people at incipient risk for schizophrenia. Am J Med Genet 114:929–937. [DOI] [PubMed] [Google Scholar]
- Power RA, Verweij KJ, Zuhair M, Montgomery GW, Henders AK, Heath AC, Madden PA, Medland SE, Wray NR, Martin NG(2014)Genetic predisposition to schizophrenia associated with increased use of cannabis. Mol Psychiatry 19:1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ(1993)Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4:135–138. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D(2009)Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19:763–772. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA(2009)Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A(2010)Inhibition of fatty-acid amide hydrolase and cb1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol 13:373–386. [DOI] [PubMed] [Google Scholar]
- Seillier A, Martinez AA, Giuffrida A(2013)Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB₁ receptors: implications for schizophrenia. Neuropsychopharmacology 38:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Lodge DJ(2013)A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 3:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L, Maynard TM, Meyer U, Morishita H, O’Donnell P, Puhl M, Cuenod M, Do KQ(2017)Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry 22:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA(2012)Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amsterdam J, Brunt T, van den Brink W(2015)The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol 29:254–263. [DOI] [PubMed] [Google Scholar]
- Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS(2004)Cannabis use and age at onset of schizophrenia. Am J Psychiatry 161:501–506. [DOI] [PubMed] [Google Scholar]
- Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire MC, Greguric I, Zavitsanou K(2011)Comparison of cannabinoid CB(1) receptor binding in adolescent and adult rats: a positron emission tomography study using [F]MK-9470. Int J Mol Imaging 2011:548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA(2014)Repeated δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. American Journal of Psychiatry 171:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo L Jr, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D(2014)Alterations of prefrontal cortex gabaergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis 63:35–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.