Abstract
Background:
Reconstruction of nervous system is a great challenge in the therapeutic medical field. Nerve tissue engineering is a novel method to regenerate nervous system in human health care. Tissue engineering has introduced novel approaches to promote and guide peripheral nerve regeneration using submicron and nanoscale fibrous scaffolds.
Materials and Methods:
In this study, 9 wt% poly(3-hydroxybutyrate) (PHB) solutions with two different ratios of chitosan (CTS) (15%, and 20%) were mixed in trifluoroacetic acid as a cosolvent. Thereafter, random and aligned PHB/CTS scaffolds were fabricated by electrospinning method in an appropriate condition.
Results:
Average diameters for aligned PHB, PHB/CTS 85:15 and PHB/CTS 80:20 were obtained as 675 nm, 740.3 nm, and 870.74 nm, which was lesser than random fibers. The solution components entity authenticity was approved by Fourier transform infrared. The addition of CTS decreased both water droplet contact angle from 124.79° to 43.14° in random and 110.87° to 33.49° in aligned PHB/CTS fibrous scaffold. Moreover, alignment of fibers causes tremendous increase in hydrophilicity of fibrous PHB/CTS substrate. Tensile strength increased from 6.41 MPa for random to 8.73 MPa for aligned PHB/CTS 85:15.
Conclusions:
Our results indicated that aligned PHB/CTS 85:15 nanofibers are the desired scaffold than the random PHB/CTS nanofibers for application in nerve tissue regeneration.
Keywords: Chitosan, nerve tissue engineering, poly(3-hydroxybutyrate), scaffold
Introduction
The rehabilitation of partial or complete loss of nerve action is an important phenomenon because of the complexity of the human adult nervous system. Neural reconstruction of the human adult is often limited. Versatile therapeutic methods such as nerve autografts, allografts, and xenografts have been used to treatment damage or permanent functional loss in the peripheral nervous system; but the donor grafts shortage, loss of function at donor sites with multiple surgeries requirement for nerve autografts and immunological rejections with disease transfer chances of allografts and xenografts limited these treatments.[1,2,3]
Tissue engineering is a novel medical therapy field which would reconstruct injured or missing tissues in the body using polymeric scaffold. Bioengineered scaffolds can help to repair long peripheral nerve gaps by employing suitable polymer compositions within the body. Polymeric scaffolds develop tissue regeneration by Mimic cellular environment that would be suitable for cell attachment, proliferation, and differentiation. A scaffold which fabricated from synthetic polymers alone has poor cell functionality because of hydrophobic structure and lack of surface cell recognition sites.[1,2,3,4,5,6,7,8]
Poly(3-hydroxybutyrate) (PHB) is a synthetic particular polymer and member of the polyhydroxyalkanoate family. It is bacterially derived copolymer produced by fermentation of sugars, lipids, and other functional groups.[9] PHB have valid mechanical properties for fabrication of neural scaffolds. It is also known to have good biocompatibility, and its degradation behavior (resistant to hydrolytic degradation) matches better for long duration treatment strategies of tissues like the nerve.[10]
Synthetic polymers with their hydrophobic properties were not employed alone in producing neural scaffold. So by incorporation of natural polymers could improve the biocompatibility and biodegradability of the electrospun scaffolds. One of the most effective methods to develop novel scaffold is alloying of synthetic and natural polymers that can promote cell adhesion and the degradation rate.
Chitosan (CTS) as natural polymer, usually derived from shells of crustaceans own features such as biocompatibility and nonmalignant degradation products but have poor mechanical properties and fast degradation rate. CTS is a natural polymer provides a microenvironment with desirable physicochemical properties that promote cell growth and attachment for the reason of owing structure similar to glycosaminoglycan (a serious component of the natural extracellular matrix [ECM]).[7,11]
Recently, a random electrospun PHB/CTS alloy was fabricated which is produced a hydrophilic fibrous scaffolds with higher mass loss rates for cartilage tissue engineering application.[12]
One of the advanced methods for fabrication fibrous scaffold with desired fiber diameter and alignment is electrospinning. In this process, polymer solutions convert to the uniform fibers with nanometer-scale diameters using electric force. Solution properties, distance between needle and collector, apparatus parameter, ambient condition effect on morphology and architecture of scaffold produced by electrospinning.[8,13]
In addition to general requirements for scaffold used in tissue engineering such as biocompatibility and biodegradability, neural scaffold should have other necessary properties. The effective feature of fibrous scaffold fabricated by electrospinning cause cell attachment and outgrowth is the orientation of fibers. According to contact guidance theory the ability of cell for migrating depend on chemical, morphology and/or mechanical properties of the surface. Therefore, the suitable substrate for nutrition outgrowth is aligned fibers.[1,2,3,4,5]
For neural tissue engineering studies, aligned fibrous scaffold-like polycaprolactone (PCL)-gelatin, PCL-collagen, polyhydroxybutyrate-co-valerate (PHBV)/collagen, poly (L-lactic acid), and poly lactic-co-glycolic acid/gelatin fabricated and they promoted nerve cells adhesion and outgrowth compared to random fibers.[2,5,6,14,15]
In this study, we fabricated a scaffold with PHB and CTS in two different ratios of 85:15 and 80:20 by electrospinning to provide aligned and random fibrous scaffold. The morphology, mechanical, chemical, and physical properties of random and aligned electrospun PHB and PHB/CTS fibrous scaffold were assessed and their result compared with each other.
Materials and Methods
Sample preparation
PHB powder (molecular weight [MW] =300,000; CAS number = 3-00-26063) was prepared from Sigma-Aldrich, USA. Medium MW CTS with deacetylation degree of 75%–85% and trifluoroacetic acid (TFA) with 99% purity were also purchased from Sigma-Aldrich, USA.
Nine percent (w/v) of PHB was dissolved in TFA (Sigma-Aldrich, USA) and stirred for 1 h. Then, CTS with a ratio of 15% and 20% (w/v) was added to PHB. For electrospinning of aligned and random PHB and PHB/CTS solution, 1 ml of the solution was placed in a 1 ml syringe connected to a blunt-end 21-gauge needle (21 kV) voltage was applied to the tip of the needle of syringe. Random nanofibers were electrospun on a collector covered with aluminum foil; but to produce aligned nanofibers, a rotating disc with 1500 rpm rotating speed and the needle tip distance from a collector with 20 cm, was used. Solutions were fed at a constant feed rate of 1 ml/h using a syringe pump (KD Scientific, Inc., Holliston, MA, USA). Subsequently, the fibers were vacuum dried to remove any residual solvent and used for further experiments.
Scanning electron microscopy
The morphology of random and aligned fibrous scaffolds was observed by scanning electron microscopy (SEM) (Seron Technologies AIS2100, South Korea) with an accelerating voltage of 10 kV, after sputter coating with gold (Quorum Technologies-EMITECH SC7620, England). The diameter of the fibers was measured from SEM images using the ImageJ software.
Water contact angle measurements
The water contact angle (WCA) of random and aligned PHB and PHB/CTS fibrous scaffolds was measured according to sessile drop WCA measurement using video contact angle instrument. Distilled water drop mounted onto the sample surface of the scaffold at 0, 5, and 10 s was recorded, and the surface contact angles were measured. Five samples were employed for each test. The average value was expressed with 6 standard deviation.
Attenuated total reflection Fourier transform infrared
Chemical analysis of PHB/CTS nanofibrous scaffolds was performed by attenuated total reflection Fourier transform infrared (FTIR) spectroscopy to obtain chemical interactions between the PHB and CTS using Nicolet Spectrometer system (Thermo, USA) over a spectra range of 4000–400/cm-1.
Mechanical evaluation
Tensile tests of the nanofibrous scaffolds was performed using a table top tensile tester (Instron 5943, USA) uniaxial testing machine and a load cell of 50 N capacity on two samples for each ratio of both random and aligned PHB and PHB/CTS (85:15), (80:20) fibrous scaffold prepared in the form of rectangular shape 20 mm × 30 mm dimensions, under a crosshead speed of 10 mm/min.
Statistical analysis
Statistically significant differences were determined with a two-tailed Student's t-test between four groups and a one-ways ANOVA was used to compare the means of different data sets, and statistical significance was accepted at a 0.05 confidence level. The results are reported as mean ± mean transit time and trypan blue. P < 0.05 was considered statistically significant.
Results
Scanning electron microscopy
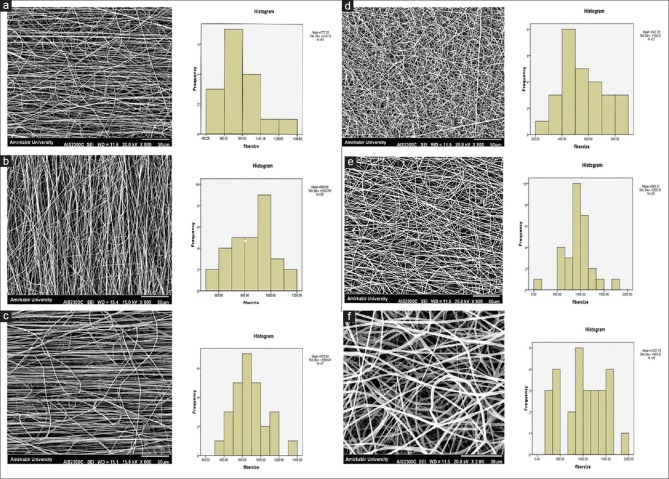
As shown in Figure 1, the average diameter of random fibers estimated to be 559.04 nm for PHB, 770.86 nm for PHB/15% CTS, and 862.66 nm for PHB/20% CTS. The average diameters for aligned PHB, PHB/CTS 85:15 and PHB/CTS 80:20 were obtained as 675 nm, 740.3 nm, and 870.74 nm, respectively. This results is showing that there is no significant differences in the diameter were observed in random and aligned PHB and PHB/CTS nanofibers (P > 0.05).
Figure 1.
Scanning electron microscopy images of aligned and random pure poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate)/chitosan fibrous scaffolds: (a) Aligned poly(3-hydroxybutyrate), (b) aligned poly(3-hydroxybutyrate)/15% chitosan, (c) aligned poly(3-hydroxybutyrate)/20% chitosan, (d) random poly(3-hydroxybutyrate), (e) random poly(3-hydroxybutyrate)/15% chitosan, (f) random poly(3-hydroxybutyrate)/20% chitosan
Water contact angle measurements
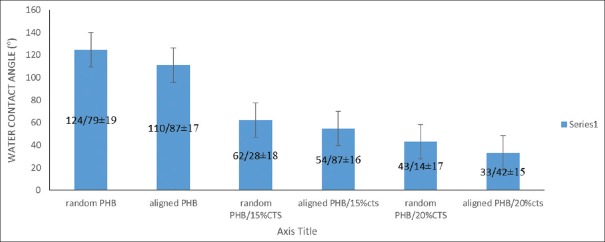
Contact angle measurements carried out on aligned and random electrospun PHB/CTS. Results of contact angle measurement presented in Figure 2 revealed that, the fibrous scaffolds containing CTS more hydrophilic than the pure fibers (P < 0.05). This figure is also showed that aligned fibers have more hydrophilicity than random one (P < 0.05).
Figure 2.
Contact angles of water droplets on the surfaces of random poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate)/chitosan alloy films
Attenuated total reflection Fourier transform infrared
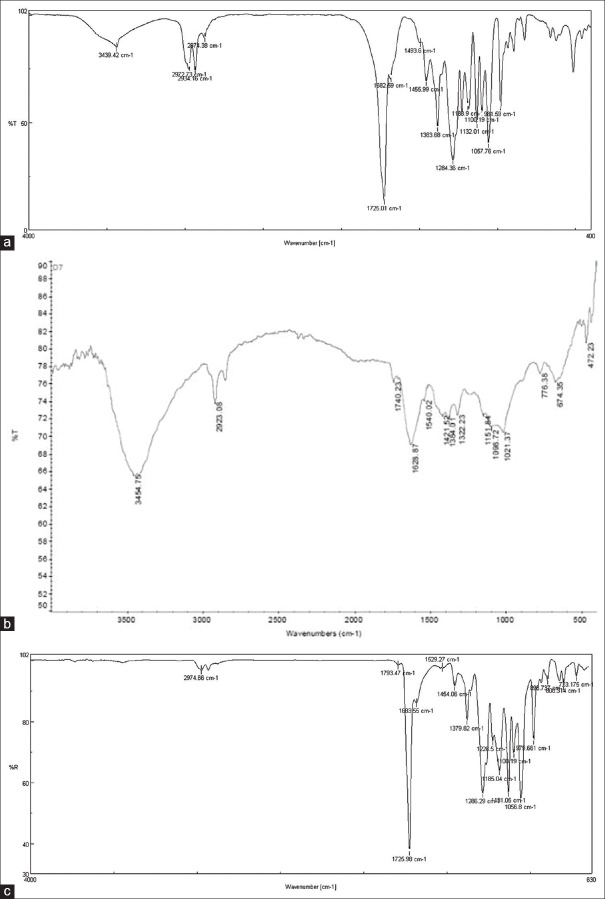
Figure 3 illustrates the FTIR spectra for electrospun PHB, CTS, PHB/CTS alloy and used to investigate crystallinity and interactions between the polymers. The FTIR spectrum of PHB [Figure 3a] has a major characteristic absorption peak at about 1724/cm-1, which is belonged to stretching vibration of carbonyl (C = O) group. Meanwhile, the crystalline phase of PHB indicated by peaks at about 973, 1293 and 1731/cm-1 and peak at 1186/ cm-1 arises from the amorphous phase. In addition, the peak observed at about 1378/ cm-1 is related to the symmetric stretching vibration of the CH3 group.
Figure 3.
Fourier transform infrared spectra of (a) poly(3-hydroxybutyrate), (b) chitosan and (c) poly(3-hydroxybutyrate)/chitosan alloy
CTS has characteristic absorptions peak at about 3400 cm-1, 1650 cm-1, and 1550 cm-1. The 3400 cm-1 peak is assigned to the O–H and the symmetric stretching vibration of N–H groups and the other two bands are corresponding to the tension– C = O (amide I), amide II, and the N–H stretching [Figure 3b]. The C = O absorption peak at about 1731 cm-1 for pure PHB shifted to higher wavenumbers in PHB/CTS blend scaffolds proportional to percentages of CTS [Figure 3b]. Increasing the wavenumber of the C = O group is a result of disturbances in the crystalline phase of PHB after addition of CTS. Furthermore, peaks at about 973 cm-1 and 1293 cm-1 also shifted to higher wavenumbers. Presence and weakening of these peaks as a result of addition of CTS are attributed to a decrease in crystallinity of PHB.
Mechanical evaluation
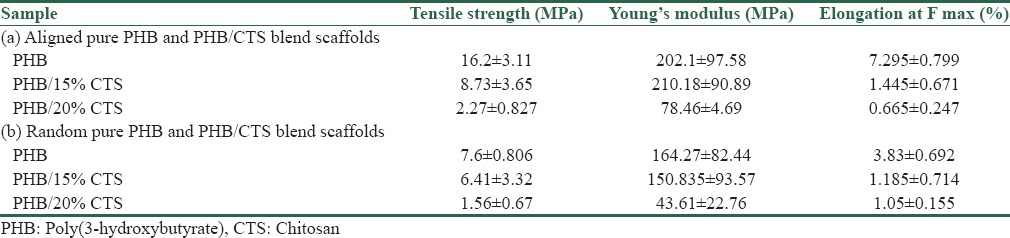
The tensile strength and Young's modulus of the random and align electrospun PHB, PHB/CTS 85:15, and PHB/CTS 80:20 fibers presented in Table 1. As shown in this table, the mechanical properties of the randomly oriented and aligned PHB/CTS electrospun fibers differed considerably. The Young's modulus and tensile strength for the aligned electrospun fibers were all greater than those for the random fibers. The presence of CTS with weak mechanical properties in polymer alloy caused a reduction in mechanical strength. CTS concentrations of >15% in the polymer alloy had a undesired effect on the production of oriented fibers (P < 0.05).
Table 1.
Mechanical properties of (a) aligned and (b) random pure poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate)/chitosan blend scaffolds
Discussion
An ideal scaffold for nerve tissue regeneration that improves the functional nerve repair should have biological and physiochemical properties, permeable for oxygen and nutrition, unique mechanical properties together. These features can maintain the ECM with tremendous potential for regenerative and cell supporting.[16]
Moreover, an efficient scaffold should provide axonal growth and guidance by appropriate surface characteristics, this is mainly due to axonal guidance is an important phenomenon that controls and maintains neural circuits during development and it is guided by various ECM proteins present within the nervous system.[17,18]
According to the results, if the electrospinning jet is not interrupted, a long continuous fiber strand is produced as the solvent evaporates and higher alignment can be obtained. The probability of produce a short continuous fiber strand and fiber breakage increase by decreasing CTS content due to the decrease in solution viscosity and therefore, the fiber alignment also decreased. The SEM images show that by using of suitable electrospinning condition, the fibers will have a uniform and same diameter in both random and aligned mat.
The contact angle for random PHB scaffolds was obtained at 124.79° which confirms that this scaffold was highly hydrophobic and nonadsorbent to water. The aligned scaffolds PHB/20% CTS were hydrophilic compared to random PHB/20% CTS scaffolds, with a contact angle value of 33.42°. The hydrophilicity of scaffolds contained CTS was higher than the other scaffolds in both random and aligned, which again confirmed the hydrophilicity of PHB/CTS scaffolds due to the increasing concentration of CTS within these scaffolds.
FTIR results showed that increasing in CTS amount; raise the contribution of the amorphous fraction. The results show that the crystallinity of PHB was suppressed by CTS in the alloy fibers via the hydrogen bonds. In addition, the crystalline-related peaks of PHB weakened in the PHB/CTS alloy fibers. Presence and weakening of these peaks as a result of the addition of CTS are attributed to a decrease in crystallinity of PHB.[12]
Ikejima et al. reported the same conclusion for PHB/CTS alloys with 10%–50% wt. of PHB. The infrared spectrum of a semi-crystalline polymer should be composed of at least two major “crystalline” and “amorphous” phases. The lowest band related to the crystalline phase because this band is not noted in the molten state. The other ones are involved in the amorphous phase. The carbonyl band of PHB divides into three components, i.e., crystalline, free amorphous and interacting amorphous bands. As shown in Figure 3, no peaks could be realized which related to “interacting” amorphous phase in the PHB/CTS alloys. Thus, there is no intermolecular interaction between PHB and CTS in the amorphous phase. Other researchers in this field reported the same conclusion.[19]
Identification of PHB crystallization using FTIR spectrum has been initially established by Bloembergen et al. Furthermore, C = O absorption peak of pure PHB in the PHB/CTS alloys shifted to higher wave number to the percentages of CTS in PHB/CTS alloy scaffolds. This chemical interaction acts as a bridge between two polymers and accordingly, decreases the crystallinity, aiding the miscibility of the two polymers together.
About results of mechanical properties, sufficient tensile strength is necessary for a peripheral nerve scaffold, since it is critical that these scaffolds tolerate the surgical procedures, subsequent tissue movements associated with patient movement, especially when tissue begins to infiltrate through the scaffolds and axonal extension occurs.[20]
The increase in tensile strength of aligned PHB/15% CTS nanofibers is likely due to the orientation of fibers. The orientation of the electrospun fibers directly affects the mechanical strength of the scaffolds.[4]
The tensile strength of aligned PHB/15% CTS was even higher than the tensile strength of random PCL/gelatin and PHBV/collagen scaffolds fabricated by other researchers.[2,5]
The mechanical evaluation result showed that with higher concentrations of CTS, the tensile strength of the fibrous scaffold decreased due to low mechanical properties of CTS. Moreover, the tensile strength and Young's modulus of aligned nanofibers was higher compared to the similar formulation in random nanofibers, so aligned fibers are more suitable for the regeneration of stronger and stiffer tissues such as the nerve. It is generally accepted that Young's modulus of peripheral nerves (e.g., a rabbit tibial nerve) in the longitudinal direction is in the range of 0.50 MPa.[21]
Conclusions
Aligned nanofibrous scaffolds in nanoscale dimensions are desirable for directionally aligned tissues such as the nerve. The contact angle illustrates that hydrophilicity of scaffolds were increased by increasing in CTS concentration and alignment of fibers. The tensile strength and Young's modulus of aligned nanofibers were higher compared to the similar formulation in random nanofibers. The results demonstrated that the aligned forms of the PHB/CTS (15%) nanocomposites indicated more suitable morphology, hydrophilicity, mechanical properties, with regard to nerve tissue engineering applications. PHB/CTS 85:15 nanofibers own better morphology, hydrophilicity, mechanical properties, and properties than PHB/CTS 80:20. It is concluded that aligned PHB/15% CTS fibers are more suitable for the regeneration of stronger and stiffer tissues such as the nerve. The results reported that the concentration of CTS and alignment in the biocomposite were strongly effect nanofibrous scaffolds.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cao H, Liu T, Chew SY. The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev. 2009;61:1055–64. doi: 10.1016/j.addr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(3-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–9. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Cunha C, Panseri S, Antonini S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed Nanotechnol Biol Med. 2011;7:50–9. doi: 10.1016/j.nano.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ranjbar-Mohammadi M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Gum tragacanth/poly(l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr Polym. 2016;140:104–12. doi: 10.1016/j.carbpol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran MP, Vatankhah E, Ramakrishna S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol Bioeng. 2013;110:2775–84. doi: 10.1002/bit.24937. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A, Bhattarai1 N, Zhang M. Fabrication and cellular compatibility of aligned chitosan – PCL fibers for nerve tissue regeneration. Carbohydr Polym. 2011;85:149–56. [Google Scholar]
- 8.Zhu W, Masood F, Brien J, Zhang LG. Highly aligned nanocomposite scaffolds by electrospinning and electrospraying for neural tissue regeneration. Nanomed Nanotechnol Biol Med. 2015;11:693–704. doi: 10.1016/j.nano.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Amass W, Amass A, Tighe B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int. 1998;47:89–144. [Google Scholar]
- 10.Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7:2249–58. doi: 10.1021/bm060317c. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Wang A, Jing D, Gong Y, Zhao N, Zhang X. Novel biodegradable films and scaffolds of chitosan blended with poly (3-hydroxybutyrate) J Biomater Sci Polym Ed. 2005;16:1379–94. doi: 10.1163/156856205774472308. [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi D, Karbasi S, Razavi SH, Mohammadi S, Shokrgozar MA, Bonakdar SH. Electrospun poly (hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J Appl Polym Sci. 2016;133:44171. [Google Scholar]
- 13.Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015;46:1–24. [Google Scholar]
- 14.Mehrasaa M, Asadollahia MA, Ghaedib K, Salehic H, Arpanaeid A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int J Biol Macromol. 2015;79:687–95. doi: 10.1016/j.ijbiomac.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 15.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–25. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Zhou-Ping T, Xing-Yong C, Rong-Hua T. Current application of scaffold materials for nerve tissue engineering. J Clin Rehabil Tissue Eng Res. 2008;12:189–92. [Google Scholar]
- 17.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 18.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 19.Ikejima T, Yagi K, Inoue Y. Thermal properties and crystallization behavior of poly(3-hydroxybutyric acid) in blends with chitin and chitosan. Macromol Chem Phys. 1999;200:413–21. [Google Scholar]
- 20.Ma M, Wei P, Wei T, Ransohoff RM, Jakeman LB. Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol. 2004;474:469–86. doi: 10.1002/cne.20149. [DOI] [PubMed] [Google Scholar]
- 21.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–30. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]