Abstract
Frailty, the state of increased vulnerability to physical stressors as a result of progressive and sustained degeneration in multiple physiological systems, is common in those with chronic kidney disease (CKD). In fact, the prevalence of frailty in the older adult population is reported to be 11%, whereas the prevalence of frailty has been reported to be greater than 60% in dialysis-dependent CKD patients. Frailty is independently linked with adverse clinical outcomes in all stages of CKD and has been repeatedly shown to be associated with an increased risk of mortality and hospitalization. In recent years there have been efforts to create an operationalized definition of frailty to aid its diagnosis and to categorize its severity. Two principal concepts are described, namely the Fried Phenotype Model of Physical Frailty and the Cumulative Deficit Model of Frailty. There is no agreement on which frailty assessment approach is superior, therefore, for the time being, emphasis should be placed on any efforts to identify frailty. Recognizing frailty should prompt a holistic assessment of the patient to address risk factors that may exacerbate its progression and to ensure that the patient has appropriate psychological and social support. Adequate nutritional intake is essential and individualized exercise programmes should be offered. The acknowledgement of frailty should prompt discussions that explore the future care wishes of these vulnerable patients. With further study, nephrologists may be able to use frailty assessments to inform discussions with patients about the initiation of renal replacement therapy.
Keywords: CKD, dialysis, elderly, exercise, frailty, nutrition
Introduction
Frailty is a state of increased vulnerability to physical stressors, such as illness or trauma, with an associated increased risk of poor clinical outcomes [1]. This occurs as the result of a progressive and sustained degeneration in multiple physiological systems and, some would argue, also the result of a decline in psychological health and inadequate social support [1–3]. In isolation these deficits may not be considered severe enough to be classified as a disease state or to require the individual to need additional care [2]. It is the accumulation of multiple deficits across various systems that is thought to be fundamental to the development of frailty [2]. In recent years there have been efforts to create an operationalized definition of frailty to aid in its diagnosis and to categorize its severity. Two principal concepts are described: the Fried Phenotype Model of Frailty, which focuses on physical frailty, and the more holistic Cumulative Deficit Model of Frailty, also known as the Frailty Index, which takes into account a broad range of medical and psychological conditions and considers functional impairments [2, 4–8].
Frailty is common in those with chronic kidney disease (CKD). The prevalence of frailty in the community-dwelling older adult population is reported to be 11%, whereas studies have reported a frailty prevalence of > 60% in dialysis-dependent CKD patients [9–11]. The Atherosclerosis Risk in Communities (ARIC) Study demonstrated that frailty is strongly associated with progressive renal impairment [12]. Furthermore, frailty is independently linked with adverse clinical outcomes in all stages of CKD and has been repeatedly shown to be associated with an increased risk of mortality and hospitalization [9, 10, 13–16].
Given the convincing relationship between frailty and adverse outcomes in those with CKD, nephrologists should be more aware of the concept of frailty. This is particularly true during interactions with other health care providers, such as general practitioners and geriatric medicine physicians, who will assess frailty in the renal population and use the diagnosis as part of decision making. The European Renal Best Practice (ERBP) Working Group recently released a clinical practice guideline on the management of older patients with CKD Stage 3b or higher [17]. They emphasized the importance of assessing functional decline in older frail patients with advanced CKD, although they conceded that there was insufficient evidence to recommend a specific frailty scoring tool [17]. So where does this leave the practising nephrologist? How should we screen for frailty in those with CKD? Are scores and tools better than clinical acumen, and if so, which should be used? What’s more, if we identify frailty, what can we do for our patients? Here we provide a focused review of frailty in the renal population with an emphasis on learning points for the general nephrologist. We also provide a brief review of the recent literature and highlight areas of uncertainty and controversy with a need for further research.
Why does physical frailty occur in those with CKD?
Patients with advanced CKD often have a reduced energy intake that contributes to sarcopenia and, subsequently, physical frailty [18, 19]. Studies have shown that there is in fact a progressive decline in food intake with deteriorating kidney function [18, 20, 21]. The reduced energy intake is frequently due to anorexia, which is present in up to one-third of patients with end-stage renal disease (ESRD) [18, 22]. The loss of appetite that occurs in CKD is likely multifactorial; potential contributors include the uraemic milieu, inflammation, superimposed illnesses, medications and coexistent low mood [18, 19]. It has been postulated that the accumulation of uraemic toxins may cause defects in the hypothalamic regulation of appetite [18]. Furthermore, cognitive impairment is more common in the CKD population and may lead to reduced food intake [23]. Another dietary challenge for those with CKD is maintaining adequate protein and energy intake while restricting dietary phosphate intake to prevent the development of secondary hyperparathyroidism and CKD–mineral bone disease [24]. This is particularly difficult in those that are dialysis-dependent as there are unavoidable losses of amino acids during the dialysis procedure [25].
Physical activity tends to decrease with ageing and this decline is more marked for individuals with CKD [26–29]. Notably, patients with dialysis-dependent CKD who maintain physical activity have superior gait speed, leg strength and lean body mass [19, 30, 31]. Furthermore, physical inactivity is associated with increased mortality in those with CKD, as in the general population [29, 32]. Hence physical inactivity may be partly responsible for the reduced lean body mass and, in turn, the development of sarcopenia and frailty in patients with CKD.
Studies have demonstrated a correlation between pro-inflammatory cytokines and white blood cell count with frailty in older adults [33–36]. There are increased levels of pro-inflammatory cytokines in CKD, including IL-6 and tumour necrosis factor alpha (TNF-α) [18, 37–39]. This is likely secondary to a combination of impaired clearance of cytokines with progressive renal impairment and exposure to inflammatory stimulants, such as uraemic toxins, dialysis and concomitant infections [19, 37]. The signalling of the anabolic hormones insulin and insulin-like growth factor 1 (IGF)-1 is impaired by these pro-inflammatory cytokines by increasing the activity of glucocorticoids and by directly causing skeletal muscle resistance to insulin and IGF-1 [18, 19, 37, 38, 40]. This incites muscle protein breakdown via the caspase-3 and ubiquitin proteasome system [38]. The inflammatory state is also associated with an increase in resting energy expenditure that may contribute to the imbalance of muscle protein homeostasis and, in turn, the frailty syndrome [18, 19].
Metabolic acidosis develops with progressive renal impairment as the ability of nephrons to excrete the daily acid load is impaired [41]. Metabolic acidosis activates caspase-3 and the ubiquitin proteasome system, inhibits intracellular signalling of insulin and IGF-1 and increases adrenal glucocorticoid production [18, 19, 38]. All of the above result in a state of protein catabolism that, if it persists, can lead to sarcopenia [41].
Prolactin retention occurs with progressive renal impairment [18]. This impairs the production of gonadotropic hormones such as testosterone [18]. Testosterone is an anabolic hormone that promotes muscle protein synthesis [18]. Testosterone deficiency is frequently present in male individuals with ESRD and is independently associated with adverse outcomes [42]. In earlier stages of CKD, testosterone level was an independent predictor of muscle mass and strength [43]. Thus low levels of testosterone in men are likely a factor in the pathophysiology of sarcopenia and, subsequently, frailty.
Low 25-hydroxyvitamin D [25(OH)D] levels are associated with frailty in the older population [35, 44]. 25(OH)D is hydroxylated to the more active 1,25-dihydroxyvitamin D [1,25(OH)2 D] in the proximal tubule of the kidney [45]. Levels of 1,25(OH)2 D decrease with progressive renal impairment, thus deficiency of 1,25(OH)2 D is common in those with CKD [46]. Evidence suggests that vitamin D may act directly on skeletal muscle through genomic and non-genomic pathways, ultimately affecting contractile muscle function and muscle metabolism [45]. Gordon et al. [47] demonstrated that 1,25(OH)2 D is a determinant of physical function and muscle size in those with CKD. It is therefore conceivable that vitamin D deficiency may be a factor in the development of frailty in CKD, although further study is needed.
Finally, cellular senescence, loss of telomeric structures, mitochondrial dysfunction, increased free radical production and poor DNA repair capability are important in the ageing process and in the development of frailty [48]. These processes occur prematurely in those with CKD and are thought to be the result of the uraemic milieu [49, 50]. They ultimately lead to sarcopenia, vascular dysfunction and progressive organ damage [49, 50]. Although not exhaustive, Figure 1 summarizes fundamental mechanisms involved in the pathophysiology of physical frailty in those with CKD.
Fig. 1.
Putative mechanisms involved in the pathophysiology of physical frailty in CKD.
Frailty assessment for the nephrologist
In their seminal paper, Fried et al. [4] described the Frailty Phenotype (FP) as ‘a clinical syndrome involving at least three of the following: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed and low physical activity’ (Table 1). They demonstrated that their definition of physical frailty, although having some overlap with disability and comorbidity, was a distinct syndrome and independently predictive of adverse outcomes, including falls, hospitalization and death [4]. Furthermore, the presence of one or two of their frailty criterion, termed intermediate frailty (or pre-frailty), was predictive of becoming frail over the subsequent 3–4 years [4].
Table 1.
| Phenotype model of physical frailty | Cumulative Deficit Model of Frailty |
|---|---|
Clinical syndrome involving at least three of the following:
|
A Frailty Index score is calculated by totaling the number of deficits from a predetermined list of ≥ 30 clinical variables including a broad range of medical and psychological conditions and functional impairments. |
The FP has been used in several studies involving patients with CKD. Roshanravan et al. [51] reviewed the outcomes for those categorized as frail by the FP in patients with CKD Stages 1–4. They established that the FP is associated with a 2.5-fold [95% confidence interval (CI) 1.4–4.4] increased risk of death or requiring dialysis in those with CKD [51]. Bao et al. [9] evaluated the outcomes of those diagnosed as frail at dialysis initiation. They demonstrated that frailty at dialysis initiation was associated with an increased risk of mortality [hazard ratio [HR] 1.57 (95% CI 1.25–1.97)] [9]. They also determined that frailty at dialysis initiation was an independent risk factor for first hospitalization [HR 1.26 (95% CI 1.09–1.45)] [9]. McAdams-DeMarco et al. [13] assessed the association between frailty and mortality and hospitalization risk in those established on dialysis. The authors categorized participants as either non-frail, intermediately frail or frail [13]. They found that the proportion of participants admitted to hospital on two or more occasions over the subsequent year after enrolment was 43% for frail dialysis-dependent CKD patients compared with 28% for non-frail dialysis-dependent CKD patients [13]. They also showed that the 3-year mortality was 40% for frail dialysis-dependent CKD patients [13]. Notably, 34% of those categorized as intermediately frail died within the 3-year follow-up period, compared with only 16% of those that were categorized as non-frail [13]. This study thus suggests that differentiating degrees of frailty may offer even greater clinical utility. In an additional study, McAdams-DeMarco et al. [52] reviewed the number of falls occurring over a 6.7-month follow-up period of 95 dialysis-dependent CKD patients. They used the phenotype definition of frailty and demonstrated that frailty predicted a 3.09-fold (95% CI 1.38–6.90) greater number of falls in a dialysis-dependent CKD population [52]. There was no difference in the association between frailty and falls for younger and older participants [52].
Notwithstanding the value of the FP, the measures of weakness and walking speed present practical issues, specifically the time and equipment needed to complete the assessments. Therefore, several studies of CKD populations have used modified versions of the FP, often substituting questionnaire-based assessments for the objective measures of weakness and slowness [9, 10, 53–55]. It has been suggested that questionnaire-based methods of assessing frailty are more likely to overestimate the prevalence of frailty, although they still appear to be predictive of outcomes [56–58]. Johansen et al. [10] reviewed the outcomes of those fulfilling the criteria of a modified version of the FP with dialysis-dependent CKD. They demonstrated that a modified FP was independently associated with an increased mortality risk in dialysis-dependent CKD [HR 2.24 (95% CI 1.60–3.15)] [10]. They also demonstrated that frailty was associated with an increased risk of the combined endpoint of hospitalization or death [HR 1.56 (95% CI 1.36–1.79)] [10]. Interestingly, these outcomes were unrelated to participant age [10]. Mansur et al. [53] reviewed the association of frailty with quality of life in CKD patients. They used a modified version of the phenotype definition of frailty that relied on self-reporting of all domains. They demonstrated that frailty correlated with poorer health-related quality of life as measured by the 36-item Short Form Health Survey (SF-36) [53]. Lee etal. [59] performed a similar cross-sectional study in 2015. They also demonstrated that a modified version of the FP was associated with lower health-related quality of life scores as assessed using the SF-36 [59].
Mitnitski et al. [7] described a contrasting and more holistic approach to assessing frailty in the general older patient, namely the Cumulative Deficit Model of Frailty. Rockwood et al. [5] further developed this model, including a total of 70 variables consisting of a variety of medical and psychological conditions and functional impairments. The total number of deficits for an individual patient was divided by all the predetermined clinical variables to calculate a Frailty Index (FI) score [5]. Rockwood et al. [6] subsequently compared the FI with the FP. They performed both measures on 2305 individuals ≥ 70 years of age from the Canadian Study of Health and Aging [6]. They demonstrated that these operational definitions of frailty correlated moderately well with each other [6]. They categorized participants as robust, pre-frail (intermediate frailty) and frail as per the FP [6]. They demonstrated that increasing FI scores correlated with worse outcomes, specifically with respect to survival and institutionalization [6]. The FI accurately predicted outcomes within categories of the FP, suggesting that the FI may be a more precise measure [6]. Hubbard et al. [60] demonstrated that frailty can be measured in CKD using an FI. Within their study there was agreement between an FI and a modified version of the FP [60]. The FI is challenging to implement into routine clinical care, as at least 30 variables are required to calculate the score [61–64]. However, with the advent of electronic patient records it may be possible to overcome this challenge. Clegg et al. [65] demonstrated that data from primary health care electronic records can be used to create a FI that is predictive of mortality, hospitalization and nursing home admission.
Clinicians’ perception of frailty does not correlate well with measured frailty, therefore there definitely is merit in formally assessing frailty [66]. Unfortunately, it has not been agreed what precise operational definition of frailty should be adopted. There is certainly overlap between both these concepts of frailty. However, in the general older population and in the CKD population, the prevalence of frailty differs depending on the approach employed [11, 67]. A study performed by Drost et al. [68] effectively demonstrated the inconsistencies in frailty identification when using different operational definitions of frailty. Their study population included 95 patients that were either dialysis-dependent or who had advanced CKD not yet necessitating dialysis [68]. They demonstrated a frailty prevalence of 37% when using the FI and 27% when using the FP criteria, perhaps because the FI is a more holistic approach to the concept of frailty [68]. There have been more CKD studies to date that assess frailty using the FP rather than the Cumulative Deficit Model of Frailty [67]. There is some progress towards a consensus in gerontology, namely that it is useful to identify physical frailty for which a targeted management plan can be developed [69, 70]. Given that currently there is no overall agreement as to which concept of frailty is superior and as both approaches are associated with clinical outcomes, arguably it is more important that efforts are made to identify frailty, regardless of the adopted methodology.
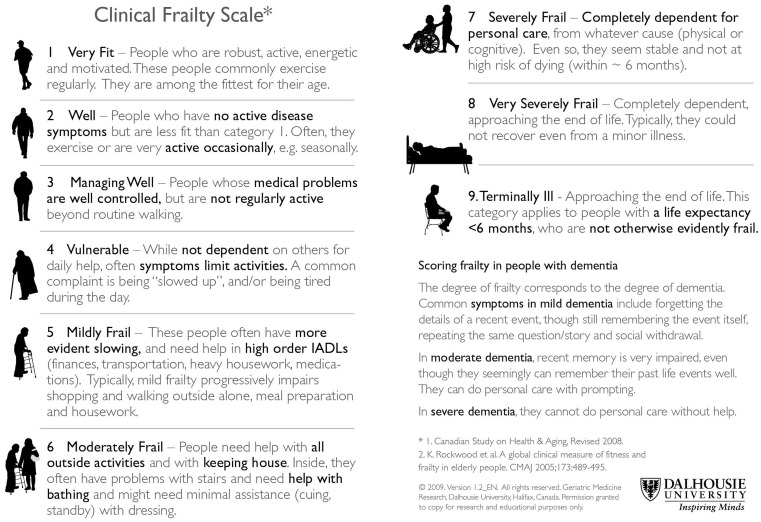
Several frailty screening tools have been developed, although not all have been used in CKD cohorts [61, 71–77]. Following on from their work with the FI, Rockwood et al. [5] developed the Clinical Frailty Scale (CFS), which is a frailty screening tool that relies on clinical judgement alone. In its original form, the CFS was a 7-point scale with descriptors for levels of frailty [5]. It has since been updated to include nine descriptors (Figure 2) [61]. In their 2005 study, Rockwood et al. [5] demonstrated that the CFS correlated well with the FI in the general population. Higher scores on the CFS were also associated with an increased risk of death [HR 1.30 (95% CI 1.27–1.33)] and institutionalization [HR 1.46 (95% CI 1.39–1.53)] [5]. Alfaadhel et al. [76] demonstrated that CFS scores at dialysis initiation are associated with mortality. A subsequent study showed that the CFS performed in patients pre-dialysis is an independent predictor of mortality [78]. Iyasere et al. [75] performed the CFS within their study that compared the quality of life and physical function in older patients on assisted peritoneal dialysis and haemodialysis. The authors demonstrated that higher CFS scores are associated with worse health-related quality of life scores [75]. We believe that the CFS is a promising frailty screening tool that could be incorporated into routine clinical nephrology care. Further research is required to establish the construct validity and interrater reliability of the CFS within CKD populations given the inherent subjective nature of the tool.
Fig. 2.
The 9-point Clinical Frailty Scale was adapted from the 7-point scale used in the Canadian Study of Health and Aging [5] and has been reprinted with permission of Geriatric Medicine Research, Dalhousie University, Halifax, Nova Scotia, Canada.
How should we care for the frail patient with CKD?
The management of frailty is multifaceted and multidisciplinary given that frailty is the result of multiple deficits. A cornerstone of the management of frailty is a holistic medical review. Within gerontology, the Comprehensive Geriatric Assessment (CGA) is advocated [1, 79–82]. The CGA is a multidisciplinary, systematic approach to identify the medical, psychosocial and functional needs of older adults [79–81]. This allows the formulation of a targeted management plan that should address current medical conditions and include a medication review, fall prevention measures and anticipatory care planning (Table 2) [79–83]. The use of the CGA in the management of older adults has been associated with improved outcomes, both in terms of physical function and survival [79–81]. Recent studies have demonstrated that it is feasible to use a CGA within nephrology care, although further research is required to assess outcomes [84, 85]. We encourage nephrology services to consider the development of management pathways with local gerontology departments so that patients identified as frail receive specialist geriatric assessment.
Table 2.
Approach to the management of frail patients with CKD
| Practice points |
1. Holistic assessment and targeted management strategy, including:
|
2. Nutrition
|
3. Timely care of complications of CKD
|
| 4. Individualized exercise training programme |
| 5. Shared decision with the patient regarding the appropriateness of renal replacement therapy |
Undernutrition is a key contributor to the development of sarcopenia and frailty in those with CKD [18, 19, 70]. First and foremost, it is important to address possible causes for reduced appetite, including medications, uraemia, metabolic acidosis, intercurrent illness and comorbid conditions such as depression [24]. Nutritional supplementation may enhance protein anabolism in the general older patient [86, 87]. A Cochrane review illustrated that calorie supplementation in older adults does lead to a modest but consistent gain in weight [88]. Cheu et al. [89] demonstrated that oral nutritional supplementation is associated with positive outcomes, specifically, fewer hospital admissions, in those with ESRD and hypoalbuminaemia, although they did not show any significant effect on survival. The risks of undernutrition and protein-energy wasting may outweigh the benefits of strict phosphate control in the frail CKD cohort, therefore dietary phosphate restriction should be individualized to allow adequate nutritional intake [17, 90–93]. In fact, recent guidelines state that ‘preserving nutritional status should prevail over any other dietary restriction’ [17]. There is a need for further research that investigates the benefits of phosphate and potassium restriction compared with dietary advice more focused at maintaining adequate nutrition. Intradialytic parenteral nutrition has been used in dialysis-dependent CKD, although the evidence to date is limited [19, 24, 94, 95]. It may improve nutritional status, but it has yet to be shown to have any beneficial effects on survival [19, 24, 94, 95].
Good and timely care of the complications of CKD is essential to limit the propagation of protein-energy wasting, sarcopenia and frailty [19]. As mentioned earlier, metabolic acidosis develops as renal function declines and is thought to contribute to the development of sarcopenia [41]. Oral sodium bicarbonate treatment in those with mild acidosis is associated with an improvement in nutritional parameters and in muscle strength [96, 97]. Most guidelines currently recommend administering oral sodium bicarbonate when the serum bicarbonate concentration falls below 22 mmol/L, though the target serum bicarbonate level is not well-defined [41, 98]. It is also important to avoid periods of significant fluid overload that can stimulate the inflammatory cascade and subsequent protein catabolism [24]. This requires judicious fluid management that may include fluid restriction, diuretic therapy and renal replacement therapy [24]. Lastly, uraemia leads to protein catabolism and subsequent sarcopenia, therefore the timing of dialysis is likely important—this is discussed in more detail below [18, 19].
Exercise has well-established, multifaceted benefits for patients with all stages of CKD, including improvements in muscle strength, cardiovascular function, physical function and health-related quality of life [99–101]. Aerobic, resistance and combined exercise programmes have been investigated and all have demonstrated benefits for those with CKD [102–112]. Several studies have examined the effects of intradialytic exercise programmes [104, 106, 108, 109, 112]. For example, Konstantinidou et al. [104] examined the effect of different programmes and concluded that exercising during non-dialysis days was most effective, but exercising during dialysis was both effective and preferable. So it seems that regardless of the form or mode of exercise, exercise is beneficial for those with CKD. Exercise training is also associated with improved functional performance in frail older adults [113–117]. Although studies to date have not directly targeted frailty status as a primary outcome in frail adults with CKD, it is conceivable that exercise may improve physical frailty in this patient group, provided there is appropriate consideration of the individual patient’s medical condition and functional needs. Further work is required to explore the feasibility of a targeted exercise program for frail patients with CKD.
There are potential pharmacological options for frailty under investigation in those with CKD, although further evidence is required before they can be recommended in routine clinical practice [109, 118–123]. Vitamin D replacement is currently recommended for those that are frail and vitamin D deficient, although a randomized controlled trial has not yet been performed [70, 123]. The evidence so far has demonstrated that vitamin D supplementation for those with native vitamin D deficiency is associated with improved outcomes in the elderly, including a reduced risk of falls and risk of hip fractures, improved muscle strength and balance and reduced mortality [70, 124–128]. Vitamin D supplementation in a CKD cohort was also linked with an improvement in physical performance [129].
Finally, how does dialysis affect the trajectory of frailty and should this influence the timing of dialysis or indeed the decision to commence dialysis at all? Kurella Tamura et al. [130] assessed the functional status of elderly nursing home residents before and after commencing dialysis. They demonstrated that dialysis was associated with a sustained decline in functional ability rather than an improvement that would be expected if uraemia alone was thought to be the cause of their poor performance status [130]. van Loon et al. [131] also demonstrated that physical performance declines while on haemodialysis, especially for older patients. These reports appear at odds with a study by John et al. [132] that demonstrated that muscle loss is more pronounced pre-dialysis and that this actually may be ameliorated once dialysis has been established. One of the main difficulties in establishing how dialysis affects the trajectory of frailty is that few studies to date have measured frailty directly and over a sustained period before and after dialysis initiation. A recent study by Johansen et al. [133] directly assessed frailty, although participants were already established on dialysis at the time of assessment. They demonstrated that there is variability in frailty scores over time, with a roughly equal difference in those whose frailty scores improved and those that worsened [133]. It thus remains unclear precisely how dialysis affects the trajectory of frailty in patients with advanced CKD. Moreover, in the absence of randomized controlled trials, it is unclear if dialysis, regardless of the modality, offers a significant survival benefit over conservative management for frail older patients with advanced CKD [17, 134, 135]. We recommend that future studies involving patients with advanced CKD assess the FP so that more direct comparisons and more definite conclusions can be made. For the time being, the decision to commence renal replacement therapy should be made in collaboration with the individual patient, outlining the perceived risks and benefits within the context of the limited evidence currently available. If a patient opts for renal replacement therapy, with the lack of a clear consensus, the choice of modality should be governed by patient preference and individual circumstances. We believe that the acknowledgement of frailty in these discussions provides a meaningful opportunity to discern future care wishes of these vulnerable patients.
Conclusion
Frailty is not just a problem faced by geriatricians. Frail patients with CKD are more likely to require hospitalization and more likely to die than their non-frail counterparts. Therefore, nephrologists should actively attempt to identify these vulnerable patients. With no agreement on which frailty assessment approach is superior, for the time being emphasis should be placed on any efforts to identify frailty. Recognizing frailty should prompt a holistic assessment of the patient to address risk factors that may exacerbate its progression and to ensure they have appropriate psychological and social support. Adequate nutritional intake is essential and individualized exercise programmes should be offered. In the same way that the assessment of frailty may be used to guide chemotherapy decisions, with further study nephrologists may be able to use frailty assessments to inform discussions with patients about dialysis initiation [136, 137]. Finally, acknowledging frailty should prompt discussions with patients that establish future care wishes.
Conflict of interest statement
None declared. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
References
- 1. Clegg A, Young J, Iliffe S. et al. Frailty in elderly people. Lancet 2013; 381: 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rockwood K, Mitnitski A.. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–727 [DOI] [PubMed] [Google Scholar]
- 3. Levers MJ, Estabrooks CA, Ross Kerr JC.. Factors contributing to frailty: literature review. J Adv Nurs 2006; 56: 282–291 [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 5. Rockwood K, Song X, MacKnight C. et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rockwood K, Andrew M, Mitnitski A.. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007; 62: 738–743 [DOI] [PubMed] [Google Scholar]
- 7. Mitnitski AB, Mogilner AJ, Rockwood K.. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walston JD, Bandeen-Roche K.. Frailty: a tale of two concepts. BMC Med 2015; 13: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bao Y, Dalrymple L, Chertow GM. et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 2012; 172: 1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johansen KL, Chertow GM, Jin C. et al. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18: 2960–2967 [DOI] [PubMed] [Google Scholar]
- 11. Collard RM, Boter H, Schoevers RA. et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60: 1487–1492 [DOI] [PubMed] [Google Scholar]
- 12. Ballew SH, Chen Y, Daya NR. et al. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2016; 67: 218–22626250781 [Google Scholar]
- 13. McAdams-DeMarco MA, Law A, Salter ML. et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61: 896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kallenberg MH, Kleinveld HA, Dekker FW. et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—a systematic review. Clin J Am Soc Nephrol 2016; 11: 1624–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker SR, Gill K, Macdonald K. et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol 2013; 14: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen Z, Ruan Q, Yu Z. et al. Chronic kidney disease-related physical frailty and cognitive impairment: a systemic review. Geriatr Gerontol Int 2017; 17: 529–544. [DOI] [PubMed] [Google Scholar]
- 17. Farrington K, Covic A, Aucella F. et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2). Nephrol Dial Transplant 2016; 31(Suppl 2): ii1–ii66 [DOI] [PubMed] [Google Scholar]
- 18. Carrero JJ, Stenvinkel P, Cuppari L. et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013; 23: 77–90 [DOI] [PubMed] [Google Scholar]
- 19. Kim JC, Kalantar-Zadeh K, Kopple JD.. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013; 24: 337–351 [DOI] [PubMed] [Google Scholar]
- 20. Ikizler TA, Greene JH, Wingard RL. et al. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol 1995; 6: 1386–1391 [DOI] [PubMed] [Google Scholar]
- 21. Duenhas MR, Draibe SA, Avesani CM. et al. Influence of renal function on spontaneous dietary intake and on nutritional status of chronic renal insufficiency patients. Eur J Clin Nutr 2003; 57: 1473–1478 [DOI] [PubMed] [Google Scholar]
- 22. Bossola M, Tazza L, Giungi S. et al. Anorexia in hemodialysis patients: an update. Kidney Int 2006; 70: 417–422 [DOI] [PubMed] [Google Scholar]
- 23. Berger I, Wu S, Masson P. et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016; 14: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ritter CS, Slatopolsky E.. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikizler TA, Cano NJ, Franch H. et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013; 84: 1096–1107 [DOI] [PubMed] [Google Scholar]
- 26. Johansen KL, Chertow GM, Ng AV. et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 2000; 57: 2564–2570 [DOI] [PubMed] [Google Scholar]
- 27. Johansen KL, Chertow GM, Kutner NG. et al. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int 2010; 78: 1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowlby W, Zelnick LR, Henry C. et al. Physical activity and metabolic health in chronic kidney disease: a cross-sectional study. BMC Nephrol 2016; 17: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beddhu S, Baird BC, Zitterkoph J. et al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009; 4: 1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Majchrzak KM, Pupim LB, Sundell M. et al. Body composition and physical activity in end-stage renal disease. J Ren Nutr 2007; 17: 196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kutsuna T, Matsunaga A, Matsumoto T. et al. Physical activity is necessary to prevent deterioration of the walking ability of patients undergoing maintenance hemodialysis. Ther Apher Dial 2010; 14: 193–200 [DOI] [PubMed] [Google Scholar]
- 32. Roshanravan B, Robinson-Cohen C, Patel KV. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ershler WB, Keller ET.. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000; 51: 245–270 [DOI] [PubMed] [Google Scholar]
- 34. Leng SX, Hung W, Cappola AR. et al. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 2009; 64: 499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puts MT, Visser M, Twisk JW. et al. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol 2005; 63: 403–411 [DOI] [PubMed] [Google Scholar]
- 36. Schaap LA, Pluijm SM, Deeg DJ. et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009; 64: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrero JJ, Stenvinkel P.. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial 2010; 23: 498–509 [DOI] [PubMed] [Google Scholar]
- 38. Wang XH, Mitch WE.. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Domański M, Ciechanowski K.. Sarcopenia: a major challenge in elderly patients with end-stage renal disease. J Aging Res 2012; 2012: 754739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu Z, Wang H, Lee IH. et al. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 2009; 119: 3059–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kraut JA, Madias NE.. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67: 307–317 [DOI] [PubMed] [Google Scholar]
- 42. Carrero JJ, Qureshi AR, Nakashima A. et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26: 184–190 [DOI] [PubMed] [Google Scholar]
- 43. Cigarran S, Pousa M, Castro MJ. et al . Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr 2013; 23: e89–95 [DOI] [PubMed] [Google Scholar]
- 44. Bruyere O, Cavalier E, Buckinx F. et al. Relevance of vitamin D in the pathogenesis and therapy of frailty. Curr Opin Clin Nutr Metab Care 2017; 20: 26–29 [DOI] [PubMed] [Google Scholar]
- 45. Molina P, Carrero JJ, Bover J. et al. European Renal Nutrition (ERN) and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Working Groups of the European Renal Association-European Dialysis Transplant Association (ERA-EDTA). Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J Cachexia Sarcopenia Muscle 2017; 8: 686–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Górriz JL, Molina P, Bover J. et al. Characteristics of bone mineral metabolism in patients with stage 3–5 chronic kidney disease not on dialysis: results of the OSERCE study. Nefrologia 2013; 33: 46–60 [DOI] [PubMed] [Google Scholar]
- 47. Gordon PL, Doyle JW, Johansen KL.. Association of 1, 25-dihydroxyvitamin D levels with physical performance and thigh muscle cross-sectional area in chronic kidney disease stage 3 and 4. J Ren Nutr 2012; 22: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walston J, Hadley EC, Ferrucci L. et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54: 991–1001 [DOI] [PubMed] [Google Scholar]
- 49. Kooman JP, Kotanko P, Schols AM. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 50. Kooman JP, Broers NJH, Usvyat L. et al. Out of control: accelerated aging in uremia. Nephrol Dial Transplant 2013; 28: 48–54 [DOI] [PubMed] [Google Scholar]
- 51. Roshanravan B, Khatri M, Robinson-Cohen C. et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012; 60: 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McAdams-DeMarco MA, Suresh S, Law A. et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013; 14: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mansur HN, Colugnati FA, Grincenkov FR. et al. Frailty and quality of life: a cross-sectional study of Brazilian patients with pre-dialysis chronic kidney disease. Health Qual Life Outcomes 2014; 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delgado C, Grimes BA, Glidden DV. et al. Association of frailty based on self-reported physical function with directly measured kidney function and mortality. BMC Nephrol 2015; 16: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delgado C, Shieh S, Grimes B. et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol 2015; 42: 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Painter P, Kuskowski M.. A closer look at frailty in ESRD: getting the measure right. Hemodial Int 2013; 17: 41–49 [DOI] [PubMed] [Google Scholar]
- 57. Johansen KL, Dalrymple LS, Glidden D. et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 2016; 11: 626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johansen KL, Dalrymple LS, Delgado C. et al. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis 2014; 64: 600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee SJ, Son H, Shin SK.. Influence of frailty on health-related quality of life in pre-dialysis patients with chronic kidney disease in Korea: a cross-sectional study. Health Qual Life Outcomes 2015; 13: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hubbard RE, Peel NM, Smith M. et al. Feasibility and construct validity of a frailty index for patients with chronic kidney disease. Australas J Ageing 2015; 34: E9–12 [DOI] [PubMed] [Google Scholar]
- 61. Moorhouse P, Rockwood K.. Frailty and its quantitative clinical evaluation. J R Coll Physicians Edinb 2012; 42: 333–340 [DOI] [PubMed] [Google Scholar]
- 62. Rockwood K, Mitnitski A.. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27: 17–26 [DOI] [PubMed] [Google Scholar]
- 63. Searle SD, Mitnitski A, Gahbauer EA. et al. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Song X, Mitnitski A, Rockwood K.. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010; 58: 681–687 [DOI] [PubMed] [Google Scholar]
- 65. Clegg A, Bates C, Young J. et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salter ML, Gupta N, Massie AB. et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr 2015; 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chowdhury R, Peel NM, Krosch M. et al. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr 2016; 68: 135–142 [DOI] [PubMed] [Google Scholar]
- 68. Drost D, Kalf A, Vogtlander N. et al. High prevalence of frailty in end-stage renal disease. Int Urol Nephrol 2016; 48: 1357–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodríguez-Mañas L, Féart C, Mann G. et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013; 68: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morley JE, Vellas B, Abellan van Kan G. et al. Frailty consensus: a call to action. J Am Med Direct Assoc 2013; 14: 392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Loon IN, Goto NA, Boereboom FTJ. et al. Frailty screening tools for elderly patients incident to dialysis. Clin J Am Soc Nephrol 2017; 12: 1480–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bohm C, Storsley L, Tangri N.. The assessment of frailty in older people with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24: 498–504 [DOI] [PubMed] [Google Scholar]
- 73. Clegg A, Rogers L, Young J.. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age Ageing 2015; 44: 148–152 [DOI] [PubMed] [Google Scholar]
- 74. van Munster BC, Drost D, Kalf A. et al. Discriminative value of frailty screening instruments in end-stage renal disease. Clin Kidney J 2016; 9: 606–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iyasere OU, Brown EA, Johansson L. et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 2016; 11: 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alfaadhel TA, Soroka SD, Kiberd BA. et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015; 10: 832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. de Vries NM, Staal JB, van Ravensberg CD. et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011; 10: 104–114 [DOI] [PubMed] [Google Scholar]
- 78. Pugh J, Aggett J, Goodland A. et al. Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J 2016; 9: 324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stuck AE, Siu AL, Wieland GD. et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993; 342: 1032–1036 [DOI] [PubMed] [Google Scholar]
- 80. Ellis G, Whitehead MA, Robinson D. et al. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ 2011; 343: d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Welsh TJ, Gordon AL, Gladman JR.. Comprehensive geriatric assessment—a guide for the non-specialist. Int J Clin Pract 2014; 68: 290–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Turner G, Clegg A.. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014; 43: 744–747 [DOI] [PubMed] [Google Scholar]
- 83.Fit for Frailty, Part I: Consensus best practice guidance for the care of older people living in community and outpatient settings—a report from the British Geriatrics Society 2014. http://www.bgs.org.uk/campaigns/fff/fff_full.pdf (12 October 2017, date last accessed)
- 84. Parlevliet JL, Buurman BM, Pannekeet MMH. et al. Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: a cross-sectional comparative and feasibility study. BMC Nephrol 2012; 13: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hall RK, Haines C, Gorbatkin SM. et al. Incorporating geriatric assessment into a nephrology clinic: preliminary data from two models of care. J Am Geriatr Soc 2016; 64: 2154–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Paddon-Jones D, Rasmussen BB.. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009; 12: 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Paddon-Jones D, Short KR, Campbell WW. et al. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 2008; 87: 1562s–1566s [DOI] [PubMed] [Google Scholar]
- 88. Milne AC, Potter J, Vivanti A. et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2009; 2: CD003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cheu C, Pearson J, Dahlerus C. et al. Association between oral nutritional supplementation and clinical outcomes among patients with ESRD. Clin J Am Soc Nephrol 2013; 8: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kalantar-Zadeh K, Tortorici AR, Chen JLT. et al. Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial 2015; 28: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lertdumrongluk P, Rhee CM, Park J. et al. Association of serum phosphorus concentration with mortality in elderly and nonelderly hemodialysis patients. J Ren Nutr 2013; 23: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shinaberger CS, Greenland S, Kopple JD. et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008; 88: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lynch KE, Lynch R, Curhan GC. et al. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol 2011; 6: 620–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cano NJM, Fouque D, Roth H. et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 2007; 18: 2583–2591 [DOI] [PubMed] [Google Scholar]
- 95. Dukkipati R, Kalantar-Zadeh K, Kopple JD.. Is there a role for intradialytic parenteral nutrition? A review of the evidence. Am J Kidney Dis 2010; 55: 352–364 [DOI] [PubMed] [Google Scholar]
- 96. Abramowitz MK, Melamed ML, Bauer C. et al. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 2013; 8: 714–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Brito-Ashurst I, Varagunam M, Raftery MJ. et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(Suppl 3): S1–201 [PubMed] [Google Scholar]
- 99. Heiwe S, Jacobson SH.. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 100. Heiwe S, Jacobson SH.. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 10: CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Segura-Orti E. [Exercise in haemodyalisis patients: a literature systematic review]. Nefrologia 2010; 30: 236–246 [DOI] [PubMed] [Google Scholar]
- 102. Leehey DJ, Moinuddin I, Bast JP. et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol 2009; 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Koufaki P, Mercer TH, Naish PF.. Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin Physiol Funct Imaging 2002; 22: 115–124 [DOI] [PubMed] [Google Scholar]
- 104. Konstantinidou E, Koukouvou G, Kouidi E. et al. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 2002; 34: 40–5 [DOI] [PubMed] [Google Scholar]
- 105. DePaul V, Moreland J, Eager T. et al. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis 2002; 40: 1219–1229 [DOI] [PubMed] [Google Scholar]
- 106. van Vilsteren MC, de Greef MH, Huisman RM.. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 107. Rossi AP, Burris DD, Lucas FL. et al. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol 2014; 9: 2052–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cheema B, Abas H, Smith B. et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007; 18: 1594–1601 [DOI] [PubMed] [Google Scholar]
- 109. Johansen KL, Painter PL, Sakkas GK. et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 2006; 17: 2307–2314 [DOI] [PubMed] [Google Scholar]
- 110. Castaneda C, Gordon PL, Parker RC. et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004; 43: 607–616 [DOI] [PubMed] [Google Scholar]
- 111. Watson EL, Greening NJ, Viana JL. et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis 2015; 66: 249–257 [DOI] [PubMed] [Google Scholar]
- 112. Segura-Ortí E, Kouidi E, Lisón JF.. Effect of resistance exercise during hemodialysis on physical function and quality of life: randomized controlled trial. Clin Nephrol 2009; 71: 527–537 [DOI] [PubMed] [Google Scholar]
- 113. Singh NA, Quine S, Clemson LM. et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc 2012; 13: 24–30 [DOI] [PubMed] [Google Scholar]
- 114. Theou O, Stathokostas L, Roland KP. et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011; 2011: 569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. de Labra C, Guimaraes-Pinheiro C, Maseda A. et al. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr 2015; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Binder EF, Schechtman KB, Ehsani AA. et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 2002; 50: 1921–1928 [DOI] [PubMed] [Google Scholar]
- 117. Marijke JM, Paw MCA, de Jong N, Stevens M. et al. Development of an exercise program for the frail elderly. J Aging Phys Activity 2001; 9: 452–465 [Google Scholar]
- 118. Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ. et al. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc 2006; 54: 1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Iglesias P, Diez JJ, Fernandez-Reyes MJ. et al. Recombinant human growth hormone therapy in malnourished dialysis patients: a randomized controlled study. Am J Kidney Dis 1998; 32: 454–463 [DOI] [PubMed] [Google Scholar]
- 120. Fouque D, Peng SC, Shamir E. et al. Recombinant human insulin-like growth factor-1 induces an anabolic response in malnourished CAPD patients. Kidney Int 2000; 57: 646–654 [DOI] [PubMed] [Google Scholar]
- 121. Niemczyk S, Sikorska H, Wiecek A. et al. A super-agonist of growth hormone-releasing hormone causes rapid improvement of nutritional status in patients with chronic kidney disease. Kidney Int 2010; 77: 450–458 [DOI] [PubMed] [Google Scholar]
- 122. Basaria S, Coviello AD, Travison TG. et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363: 109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stenvinkel P, Carrero JJ, von Walden F. et al. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant 2016; 31: 1070–1077 [DOI] [PubMed] [Google Scholar]
- 124. Murad MH, Elamin KB, Abu Elnour NO. et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96: 2997–3006 [DOI] [PubMed] [Google Scholar]
- 125. Bischoff-Ferrari HA, Willett WC, Orav EJ. et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012; 367: 40–49 [DOI] [PubMed] [Google Scholar]
- 126. Rejnmark L, Avenell A, Masud T. et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab 2012; 97: 2670–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Muir SW, Montero-Odasso M.. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2011; 59: 2291–2300 [DOI] [PubMed] [Google Scholar]
- 128. Gillespie LD, Robertson MC, Gillespie WJ. et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012; 15:CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Taskapan H, Baysal O, Karahan D. et al. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol 2011; 76: 110–116 [DOI] [PubMed] [Google Scholar]
- 130. Kurella Tamura M, Covinsky KE, Chertow GM. et al. Functional status of elderly adults before and after initiation of dialysis. New Engl J Med 2009; 361: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. van Loon I, Hamaker ME, Boereboom FTJ. et al. A closer look at the trajectory of physical functioning in chronic hemodialysis. Age Ageing 2017; 6: 1–6 [DOI] [PubMed] [Google Scholar]
- 132. John SG, Sigrist MK, Taal MW. et al. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an observational study. PLoS One 2013; 8: e65372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Johansen KL, Dalrymple LS, Delgado C. et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. O’Connor NR, Kumar P.. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med 2012; 15: 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Foote C, Kotwal S, Gallagher M. et al. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: a systematic review and meta-analysis. Nephrology 2016; 21: 241–253 [DOI] [PubMed] [Google Scholar]
- 136. Handforth C, Clegg A, Young C. et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2014; 26: 1091–1101 [DOI] [PubMed] [Google Scholar]
- 137. Rodriguez Villarreal I, Ortega O, Hinostroza J. et al. Geriatric assessment for therapeutic decision-making regarding renal replacement in elderly patients with advanced chronic kidney disease. Nephron Clin Pract 2014; 128: 73–78 [DOI] [PubMed] [Google Scholar]