Abstract
The Niemann–Pick type C1 (NPC1) protein regulates the transport of cholesterol and fatty acids from late endosomes/lysosomes and has a central role in maintaining lipid homeostasis. NPC1 loss-of-function mutations in humans cause NPC1 disease, a rare autosomal-recessive lipid-storage disorder characterized by progressive and lethal neurodegeneration, as well as liver and lung failure, due to cholesterol infiltration. In humans, genome-wide association studies and post–genome-wide association studies highlight the implication of common variants in NPC1 in adult-onset obesity, body fat mass, and type 2 diabetes. Heterozygous human carriers of rare loss-of-function coding variants in NPC1 display an increased risk of morbid adult obesity. These associations have been confirmed in mice models, showing an important interaction with high-fat diet. In this review, we describe the current state of knowledge for NPC1 variants in relationship to pleiotropic effects on metabolism. We provide evidence that NPC1 gene variations may predispose to common metabolic diseases by modulating steroid hormone synthesis and/or lipid homeostasis. We also propose several important directions of research to further define the complex roles of NPC1 in metabolism. This review emphasizes the contribution of NPC1 to obesity and its metabolic complications.
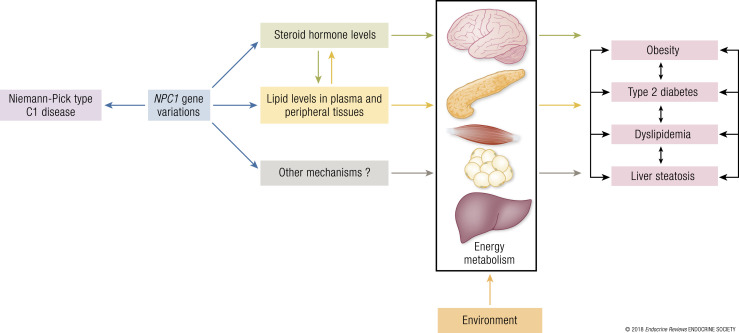
This article reviews the implication of the NPC1 gene in obesity and its metabolic complications and proposes a mechanistic model to explain the link between NPC1 and metabolism.
Essential Points
Niemann–Pick C1 (NPC1), a protein coded by the NPC1 gene, locates in the membrane of late endosomes/lysosomes and regulates the efflux of cholesterol and fatty acids from these vesicles
NPC1 loss-of-function mutations cause NPC1 disease, a rare autosomal recessive lipid storage neurodegenerative disorder characterized by an accumulation of cholesterol and other lipids in late endosomes/lysosomes
Humans and mice affected with NPC1 disease develop metabolic phenotypes, including weight loss, abnormal blood lipid levels, and liver steatosis
NPC1 haploinsufficiency is associated with weight gain, insulin resistance, and obesity in mice and humans.
Single nucleotide polymorphisms in the NPC1 gene are associated with obesity, body fat mass variations, dyslipidemia, insulin resistance, and type 2 diabetes in humans and mice
The effect of NPC1 on metabolism could be explained by its influence on steroid hormones production and/or blood and peripheral lipid levels
Cholesterol is an essential structural component of mammalian cell membranes. It affects membrane fluidity, curvature, and the function of associated peripheral and transmembrane proteins. Cholesterol also serves as a signaling molecule that regulates gene expression in diverse metabolic pathways. In certain tissues (e.g., liver, adrenals, gonads, kidney, skin), cholesterol is a precursor for the synthesis of bioactive molecules (e.g., bile acids, steroid hormones, vitamin D) (1). Hence, to maintain optimal health and prevent human disease, the concentration of cellular and plasma cholesterol is tightly regulated. Deleterious mutations in genes that regulate cholesterol homeostasis result in severe hereditary disorders, including familial hypercholesterolemia and Niemann–Pick type C (NPC) disease (2, 3).
In this review, we describe the current state of knowledge for the NPC intracellular cholesterol transporter 1 (NPC1) gene and protein in relationship to metabolic disease predisposition in mice and humans. We also propose a mechanistic model that may explain how the loss of function/expression of the NPC1 protein (induced by genetic variations) affects steroid hormone and lipid homeostasis, and thereby increases susceptibility to cardiometabolic diseases in an obesogenic environment. Finally, we propose several important directions of research to further define the complex roles of NPC1 in metabolism.
Cholesterol Sources, Transport, and Homeostasis in the Organism
In humans, ~50% (36% to 74%) of ingested dietary cholesterol (300 mg/d recommended in adults) is absorbed by enterocytes (4). In addition to this exogenous source (diet), endogenous cholesterol synthesis represents a major source of cholesterol in mammalian organisms (1000 mg produced per day on average). In humans, nearly all cells are capable of synthesizing cholesterol using the condensation of acetyl–coenzyme A (CoA) and acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) as a precursor of the mevalonate pathway. This synthesis is highly regulated by HMG-CoA reductase, an enzyme that is the target to one of the most commonly used classes of lipid-lowering drugs, the HMG-CoA reductase inhibitors, also known as statins. The liver, small intestine, adrenal cortex, and reproductive organs have the highest rates of de novo cholesterol synthesis in the body. However, unlike the adrenal cortex or gonads that synthesize cholesterol for local use, the liver is considered as the key player in the process of maintaining the whole body’s cholesterol homeostasis.
Because cholesterol and cholesterol ester are hydrophobic molecules, these lipids are transported in the blood by lipoproteins. Dietary cholesterol absorbed by enterocytes is mostly converted to cholesterol ester by the acyl-CoA:cholesterol acyltransferase enzyme and incorporated into the triacylglycerol-enriched core of chylomicron particles. These particles are secreted into the lymph and transported throughout the blood where their triacylglycerol content is hydrolyzed by the circulating lipoprotein lipase. The resulting chylomicron remnants, whose hydrophobic core is enriched with cholesterol ester particles, are rapidly captured and internalized by hepatocytes.
Forward and reverse cholesterol transport
In hepatocytes, the acyl-CoA:cholesterol acyltransferase catalyzes the esterification of newly synthetized cholesterol with a fatty acid to produce cholesterol esters. These molecules are incorporated into triacylglycerol-enriched particles called very-low–density lipoproteins (VLDLs). VLDLs are secreted by the liver into the bloodstream, and, similar to chylomicrons, are acted upon by the lipoprotein lipase, which results in the production of intermediate-density lipoproteins and, eventually, mature low-density lipoproteins (LDLs) enriched with cholesterol ester. LDLs are internalized by cells of peripheral tissues, thereby delivering cholesterol and fatty acids in a process called forward cholesterol transport (5).
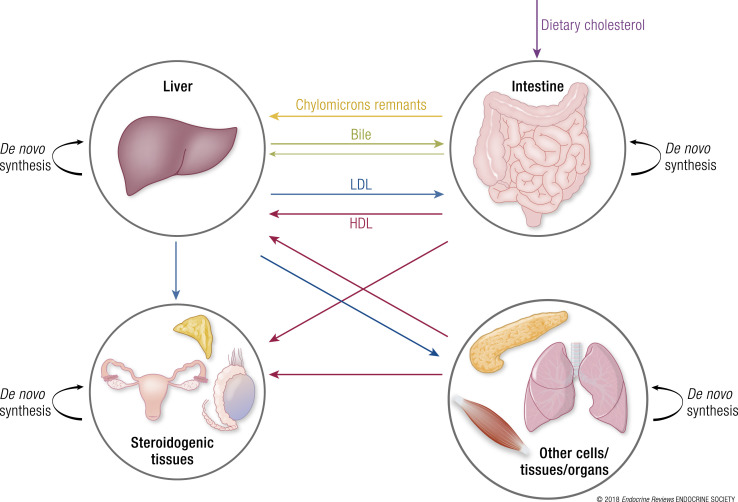
Conversely, reverse cholesterol transport refers to the process by which the excess of cholesterol is extracted from peripheral cells and carried to steroidogenic tissues (e.g., adrenal cortex or gonads) for steroid hormone production or to the liver for elimination. This transport is mediated by high-density lipoproteins (HDLs) in a process called reverse cholesterol transport. During this process, the cholesterol ester transfer protein may transfer cholesterol esters from HDLs to VLDLs and intermediate-density lipoproteins. Once HDLs reach their target tissues, particles bind to the plasma membrane scavenger receptor class B type I receptor that facilitates selective transfer of cholesterol esters from HDLs into the cytoplasm of target cells. In the liver, a fraction of cholesterol acquired by the reverse cholesterol transport pathway serves as the substrate for the production of bile acids. The remaining fraction is secreted along with the bile acids into the intestine in the form of bile and is excreted from the body. Therefore, cellular, tissue, and whole-body cholesterol homeostasis is a dynamic process regulated by complex pathways that function in an orchestrated manner. This results in a complete balance between cholesterol absorption, cholesterol synthesis, cholesterol transport between tissues, and eventually cholesterol excretion in healthy individuals (5) (Fig. 1).
Figure 1.
Major sources of cholesterol and cholesterol trafficking in the organism. In mammals, cholesterol sources include diet or de novo biosynthesis. Dietary cholesterol (purple arrow) is absorbed by enterocytes and mostly transferred to the liver via chylomicron remnants (yellow arrow). The liver supplies most organs with cholesterol via receptor-mediated endocytosis of LDL-derived cholesterol in a process called forward cholesterol transport (blue arrows). Additionally, most cells are fully capable of de novo cholesterol synthesis to meet their cellular cholesterol requirements (black arrows). Cellular excess of cholesterol is transported in the blood via HDLs to the liver and steroidogenic tissues in a process called reverse cholesterol transport (pink arrows). Ultimately, the liver secretes the excess of cholesterol in the intestine via the bile where it is reabsorbed by enterocytes or excreted in feces (green arrows).
Role of NPC1 and Niemann–Pick type C2 proteins in intracellular LDL-derived cholesterol transport
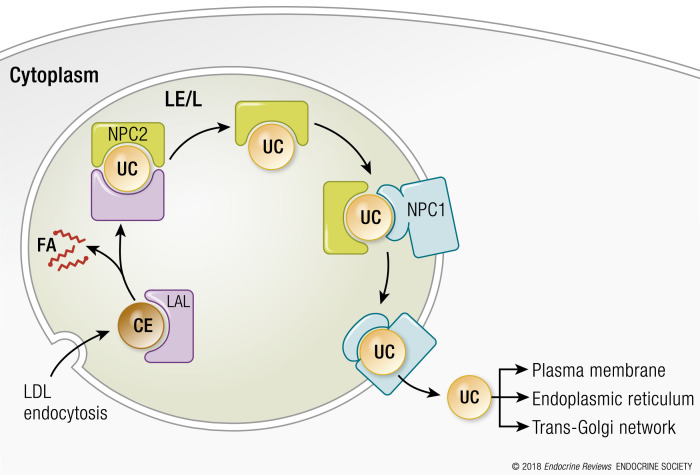
Receptor-mediated and bulk phase endocytosis of LDLs is the terminal step of forward cholesterol transport. The binding of apolipoprotein B100 present on the surface of LDLs to the LDL plasma membrane receptor (LDLR) stimulates the internalization of LDL lipoproteins into clathrin-coated vesicles that fuse with acidified early endosomes. The eventual fusion of early endosomes with late endosomes/lysosomes containing an assortment of hydrolytic enzymes results in the accumulation of degradation products poised for entry into the cytoplasm. The transport of LDL-derived cholesterol and fatty acids from late endosomes/lysosomes to the cytoplasm and other cellular compartments is mediated by the NPC1 and Niemann–Pick type C2 (NPC2) proteins (6). In brief, NPC2 is a soluble protein located in the lumen of the late endosomes/lysosomes that binds and transfers cholesterol to NPC1 (7). NPC1, which is associated with the limiting membrane of late endosomes/lysosomes, regulates the efflux of cholesterol and fatty acids from these vesicles (6) (Fig. 2). It is thought that different proteins interact with NPC1 to accept cholesterol and facilitate the transport of these lipids to different cellular compartments, including the trans-Golgi network, plasma membrane, and endoplasmic reticulum (8–11).
Figure 2.
Proposed mechanism of cholesterol efflux from lysosomes. The endocytosis of LDLs into the cell and resulting accumulation of cholesterol ester in late endosomes/lysosomes (LE/L) initiates a series of events that culminates in cholesterol efflux from these compartments. In brief, lysosomal acid lipase (LAL) catalyzes the hydrolysis of cholesterol esters (CE) to produce unesterified cholesterol (UC) and fatty acids (FA). The NPC2 protein binds UC (but not FAs) and transports cholesterol to the NPC1 protein, which is bound by the amino terminal domain (NTD). Through a mechanism that remains undefined, the NTD transfers UC to a different region of the NPC1 protein called the sterol-sensing domain that is thought to be responsible for cholesterol efflux from lysosomes.
Obesity and Complex Metabolic Diseases in the Genomic Era
During the last two decades, researchers have made several important strides in the discovery of new obesity predisposing genes. These advances were the result of a sequential development of new and more powerful genetic tools. For instance, most genes involved in Mendelian disorders were discovered and validated using different natural or genetically engineered mice models, positional cloning, homozygosity mapping, and linkage analysis (12). These types of tools allowed the discovery the NPC1 gene that causes NPC1 disease (13), and the LEP gene whose mutations are associated with monogenic forms of obesity (14). Nevertheless, the above-mentioned tools have a very limited power when applied to the discovery of genes involved in complex metabolic traits (12).
Complex traits result from the interplay of numerous genetic variants and environmental factors. Examples of complex traits include body mass index (BMI), plasma levels of fasting glucose, fasting insulin, HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), and triglycerides. Metabolic diseases such as common obesity, type 2 diabetes (T2D), metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) are also categorized as complex disorders. These traits are modestly to highly heritable, with a heritability ranging between 26% and 80% (15–23).
The second major stride in the discovery of obesity genes resulted from the development of genome-wide association studies (GWASs). As they are more suited to detect modest genetic effects, GWASs helped discover numerous unsuspected polygenic variants, as well as equally unsuspected physiological and physiopathological mechanisms. Examples of major metabolic genes/loci identified by GWASs include the β cell function–associated HHEX/IDE locus and the zinc transporter SLC30A8 for T2D, the gene encoding for the kinase TRIB1 for blood lipid levels (24), and the trans-acting fat mass gene FTO and its subsequently identified cluster of genes (IRX3, IRX5, and RPGRIP1L) for obesity (25–28).
NPC1 is another example of an obesity gene identified by GWASs. Indeed, although the gene itself and its function were already known and studied in the context of the Mendelian NPC1 disease, the association between NPC1 and polygenic obesity was first identified by a GWAS published in 2009 and performed in adult and children participants of European descent (25). Since this publication, NPC1 became the subject of several post-GWAS studies where several common NPC1 single nucleotide polymorphisms (SNPs) have been significantly associated with other metabolic traits in both NPC1+/+ and NPC1+/− human and mouse subjects (29). One follow-up study also investigated the correlation between the severity of NPC1 mutations and the obesity phenotype by sequencing and analyzing rare variants of NPC1+/− participants (29). The results of all of these studies are detailed in “Beyond NPC1 Disease: Further Evidence of an Effect of NPC1 Variants on Metabolic Phenotypes” below.
NPC1 Disease
NPC1 gene and protein
The human NPC1 gene and mouse ortholog (Npc1) encoding for the NPC1 protein are localized to a syntenic region on chromosome 18 (chromosomes 18q11.2 and 18A1, respectively) (30, 31). According to the Ensembl database (http://www.ensembl.org) (32), up to 14 transcripts translated into six protein isoforms could be encoded by the human NPC1 gene (Ensembl version ENSG00000141458.11). The 1278–amino acid protein is, however, considered as the principal isoform (APPRIS P1). According to the same database, the Npc1 gene encodes a total of five transcripts and a single protein isoform (1277 amino acids) in mice (Ensembl version ENSMUSG00000024413.9). NPC1 is ubiquitously expressed, but the highest levels of expression are detected in the liver, adrenal glands, and lungs (33). Results from the GTEx Consortium (https://www.gtexportal.org, query date: 20 October 2017) indicate that NPC1’s most significant expression quantitative trait loci (P < 3.5 × 10−32) are located within 0.5 Mb upstream and downstream of the gene itself, and they are highly active in the skin and subcutaneous adipose tissue (34). The expression profile of NPC1 in different tissues, its regulation, and the function of the other NPC1 isoforms in humans remain poorly described in the literature.
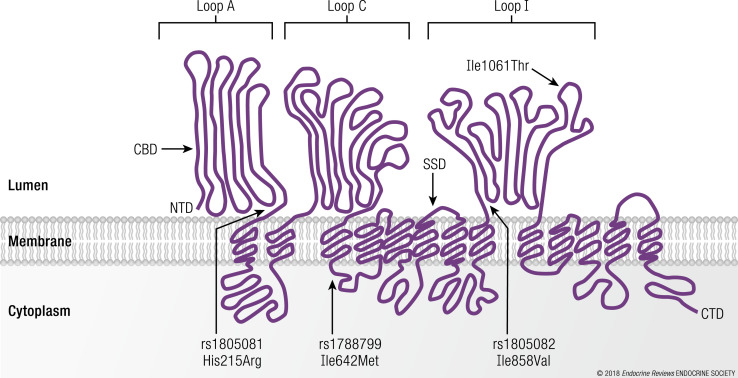
The principal NPC1 protein has 13 transmembrane domains and several interesting structural motifs that provide insight into its function (Fig. 3) (35). Crystal and cryo-electron microscopy structures of the human NPC1 have recently been described (36–39). In brief, the N-terminal domain oriented within the lumen of vesicles possesses an unconventional cholesterol-binding domain that accepts cholesterol from the NPC2 protein (40, 41). An additional cholesterol-binding domain, referred to as a sterol-sensing domain, is present between the fourth to seventh transmembrane domains and is homologous to three other proteins (HMG-CoA reductase, SCAP, and Patched) that also have an important role in regulating cholesterol metabolism (42). A conserved caveolin-binding motif responsible for transferring cholesterol and fatty acids to caveolin that transports these lipids to other cellular compartments is located next to the sterol-sending domain and positioned within the fourth cytoplasmic loop (43). Finally, a dileucine motif that facilitates the trafficking of NPC1 to late endosomes/lysosomes is positioned near the carboxyl terminus and within the cytoplasm (44).
Figure 3.
The human NPC1 protein and its major structural domains. NPC1 reference SNPs (rs1805082/rs1788799/rs1805081) and their respective encoded NPC1 protein variants (His215Arg/Ile642Met/Ile858Val) are indicated using a schematic representation of the human NPC1 protein. The NPC1 protein variant (Ile1061Thr) location (responsible for most cases of NPC1 disease) is provided. CBD, cholesterol binding domain; CTD, carboxyl terminal domain; NTD, amino terminal domain; SSD, sterol-sensing domain.
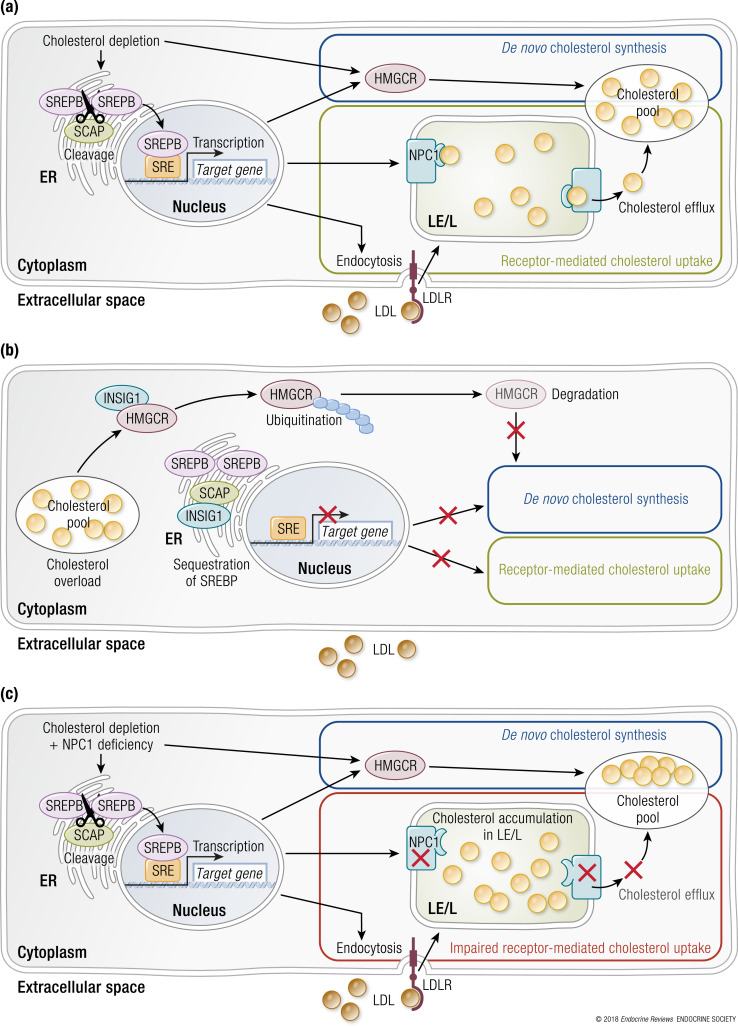
In addition to cholesterol and fatty acids transfer, NPC1 plays a central role in regulating cellular lipid homeostasis by promoting feedback inhibition of the sterol regulatory element–binding protein (SREBP) pathway (45) (Fig. 4) and feed-forward activation of the liver X receptor (LXR) pathway. NPC1 also downregulates the activity of mTORC1, a kinase involved in cell growth and sterol biosynthesis by increasing the expression of SREBP1c and SREBP2 (46–48). Collectively, these transcription factors and signaling proteins regulate the expression of several hundred genes encoding proteins that participate in diverse metabolic pathways.
Figure 4.
The SREBP pathway regulates cellular cholesterol metabolism. (a) In sterol-depleted cells, the relative absence of cholesterol and oxysterols located in the endoplasmic reticulum prevents feedback inhibition of the SREBP pathway by inactivating INSIG (retention protein) and activating SCAP (escort protein). SREBP1 and SREBP2 proteins are transported to the Golgi apparatus for proteolytic processing by proteases. The newly generated mature SREBP1 and SREBP2 are then capable of translocating into the nucleus and serving as transcription factors. They increase expression of genes encoding proteins that regulate cellular cholesterol metabolism (e.g., LDLR, NPC1, HMG-CoA synthase, and HMG-CoA reductase). (b) In sterol-enriched cells, the relative abundance of cholesterol and oxysterols located in the endoplasmic reticulum promotes feedback inhibition of the SREBP pathway by activating INSIG and inactivating SCAP to prevent transport of SREBP1 and SREBP2 to the Golgi apparatus for proteolytic processing. (c) In NPC1-deficient cells, cholesterol accumulates in the late endosomes/lysosomes. Because cholesterol is unable to gain access to the endoplasmic reticulum to activate INSIG and inactivate SCAP, impaired feedback inhibition of the SREBP pathway results in increased cholesterol synthesis and receptor-mediated endocytosis of LDLs although the cell has an abundance of cholesterol. ER, endoplasmic reticulum; HMGCR, HMG-CoA reductase; LDLR, LDL receptor; LE/L, late endosome/lysosome; SCAP, SREBP cleavage-activating protein; SRE, sterol regulatory element.
Update on NPC1 disease
NPC1 loss-of-function mutations cause NPC1 disease (OMIM no. 257220), a rare autosomal recessive lipid storage disorder, biochemically characterized by an accumulation of cholesterol and other lipids in late endosomes/lysosomes (13). Patients with NPC1 disease display a large spectrum of clinical phenotypes. Their life expectancy can vary from a few days to >60 years. However, the vast majority of patients die between 10 and 25 years from complications related to neurologic degeneration and lung failure (49). The prevalence of NPC disease was estimated to range between 1/120,000 to 1/150,000 cases in Western Europe (50). However, a more recent study reports a higher worldwide incidence rate for NPC1 diseases (~1 in 92,104 conceptions), whereas the incidence of the late-onset forms is thought to range between 1/19,000 and 1/36,000 (51).
Approximately 95% of the cases display mutations in the NPC1 gene, whereas the remaining cases result from mutations in NPC2 (49, 52). Founder mutations in NPC1 have been identified in French Acadians from Nova Scotia (Canada), Hispanics from New Mexico, and Greeks from the Aegean Sea Island (53–55). According to the Niemann–Pick C Disease Database (56), 289 NPC1 disease causal mutations have been identified in patients from around the world.
Clinical features
The symptoms associated with NPC disease are heterogeneous and vary according to the age of onset (early infantile, infantile, juvenile, or adult forms), as well as between individuals of the same age group, which makes clinical diagnosis difficult. Although this disease affects all organs, the accumulation of cholesterol is more pronounced in the viscera and nervous system, giving rise to systemic, neurologic, and psychiatric symptoms: fetal ascites or severe neonatal liver disease and/or lung failure, infantile hypotonia and developmental delay, vertical supranuclear gaze palsy beginning in childhood, followed by progressive ataxia, dysarthria, dystonia, seizures and gelastic cataplexy, psychiatric presentations, mimicking depression, dementia, or schizophrenia, with subtle neurologic signs, beginning in adolescence or adulthood. Enlargement of the liver or spleen can be observed, particularly in early childhood. Dysarthria and dysphagia can become disabling, making oral feeding impossible. Death from inhalation pneumonia usually occurs in the late second or third decade. The different symptoms and the chronology of their appearance have been extensively reviewed elsewhere (49, 57–59).
Magnetic resonance imaging of the brain is usually normal until the late stages of the illness, but atrophy of the superior and anterior cerebellar vermis, thinning of the corpus callosum, and mild cerebral atrophy appear sometimes, as well as secondary demyelination in the periatrial white matter. Quantitative magnetic resonance imaging studies in adults with NPC disease showed also gray and white matter abnormalities, resulting in a reduction in total cerebellar volume, correlating to ataxia and ocular motor function but not to duration and severity of disease (60). Moreover, one study of magnetic resonance spectroscopy reported improvement in magnetic resonance spectroscopy parameters with miglustat (inhibitor of glucosylceramide synthase) therapy (61).
Testing strategies
Owing to the inconsistency and the lack of specificity of disease symptoms between cases, suspicion indexes and diagnosis recommendations have been developed to assist clinicians in diagnosing patients with NPC disease (62, 63). One of the first and most commonly used NPC laboratory diagnosis tests was filipin staining. For this test, a skin biopsy is collected from individuals, and fibroblasts are grown in culture. Fixed cells are incubated in buffer containing filipin, a dye that binds cholesterol. The observation of an abnormal cholesterol accumulation in cells (fluoresces) under ultraviolet light usually confirms the diagnosis (64, 65) (Fig. 5). The detection of excessive cholesterol accumulation using filipin staining in blood smear or with alternative reagents has also been proposed (65–67). The identification of NPC1 or NPC2 gene mutations must be performed for patients with highly suspected NPC (based on suspicion indexes or filipin staining) through several DNA sequencing techniques. Detection of the NPC causing mutations in NPC patients also facilitates the screening of NPC1 mutations in high-risk relatives. Genetic testing should also include analysis of at least one of the parents to better interpret the pathogenicity of the mutation. Prenatal or preimplantation genetic diagnosis requires prior identification of the disease-causing mutations in the family (57).
Figure 5.
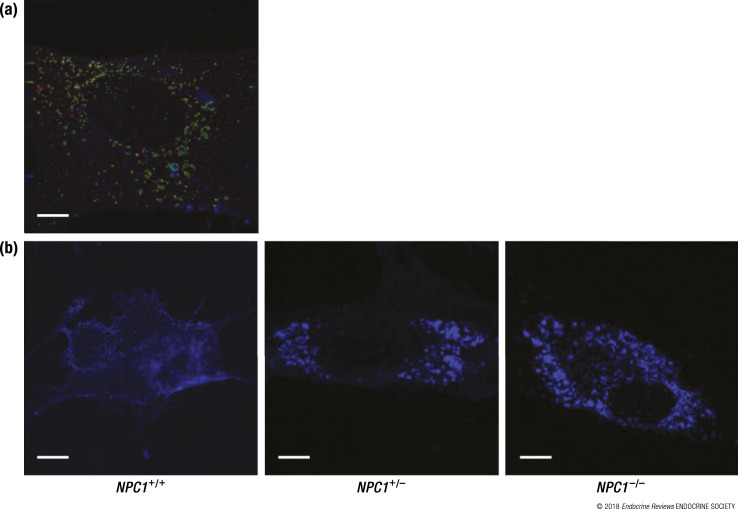
Fluorescence microscopy of human NPC1+/+, NPC1+/−, and NPC1−/− fibroblasts. (a) Normal human fibroblasts (NPC1+/+) were grown in basic media and triple stained for NPC1 (red), late endosomes/lysosomes (green), and cholesterol (blue) followed by visualization using deconvolution fluorescence microscopy. (b) Normal human fibroblasts (NPC1+/+), NPC1 heterozygous fibroblasts (NPC1+/−), and NPC1 homozygous fibroblasts (NPC1−/−) were grown in lipoprotein-deficient media enriched with LDL and stained for cholesterol (blue), followed by visualization using conventional fluorescence microscopy. Scale bars, 10 μm.
Once NPC-causing mutations have been identified in different subjects, genotype/phenotype correlation tests can be performed to explain the differences in the severity of clinical and biochemical phenotypes observed between cases. For instance, it has been shown that nonsense mutations or nonsynonymous mutations in the sterol-sensing domain of NPC1 correlate with severe infantile neurologic forms, whereas mutations affecting the cysteine-rich luminal loop are associated to the “variant” biochemical phenotype (i.e., moderate/inconclusive filipin test) (68–70). However, genotype/phenotype correlation studies are limited for the following reasons: the number of patients is low; a lot of the affected individuals are compound heterozygotes; some biochemical phenotyping methods used (e.g., filipin test) are not precise; and the clinical phenotyping is usually poor (70).
Several NPC disease biomarkers (e.g., 7-ketocholesterol, cholestane-3β,5α,6β-triol, lysosphingomyelin isoforms, bile acid metabolites) have recently been identified and could facilitate the diagnosis of this disease (71–76). Rapid methods for the detection of these biomarkers have been developed but have not been implemented in the clinical settings yet (77–79). The latest NPC diagnosis tests were recently reviewed by Vanier et al. (80).
Although current diagnostic tests have their limitations, they can lead to a diagnosis in most cases when used in combination. Following clinical orientation, a panel of experts has presently proposed to first assess plasma biomarkers, followed by genetic testing in case of an NPC profile. A filipin test should be mandatory only in the cases in which molecular results remain inconclusive. Mutation analysis is essential in all cases as ultimate confirmation of the diagnosis (80).
Current therapy management
Currently, no effective treatment exists for NPC disease. Based on results from a randomized controlled clinical trial (81) and long-term extension studies (82), N-butyldeoxynojirimycin (Miglustat), which is an inhibitor of glucosylceramide synthase, is the only drug approved for the treatment of progressive neurologic manifestations in pediatric and adult patients with NPC in Europe and some other countries, but not in the United States (83).
Additionally, symptomatic therapy is usually recommended and consists of the management of seizures, dystonia, and cataplexy, a nocturnal sedative to help disordered sleep, and physical therapy to maintain mobility. Secondary complications are prevented by chest physical therapy with bronchodilation and antibiotic therapy. Regular bowel programs are also set to prevent severe constipation. Swallowing is also monitored to allow placement of a gastrostomy tube when aspiration or nutritional compromise is imminent. Drugs that cause excessive salivation or that may exacerbate seizures by interacting with antiepileptic drugs are avoided as well as alcohol and other drugs that may exacerbate ataxia (57).
Metabolic phenotypes of NPC patients
Besides the systemic and neurologic symptoms that characterize NPC disease, patients develop abnormal metabolic phenotypes during the progression of the disorder. NPC patients typically lose weight shortly after onset of neurodegenerative symptoms. Studies have reported abnormal plasma blood profiles for NPC1 patients, whereby total cholesterol, LDL-C, and HDL-C levels are significantly decreased. In contrast, total triglycerides levels are significantly increased compared with sex- and age-matched healthy controls (84, 85). Finally, for reasons that remain undefined, transient hepatomegaly and possible progression to hepatic fibrosis and cirrhosis may be an early manifestation of this disease for many NPC patients (86).
Animal models of NPC1 disease
Phenotypes associated with loss-of-function mutations in NPC1 orthologs have been described for different in vivo models, including yeast (87), zebrafish (88), Drosophila (89), dogs (90), cats (91–94), and mice. In these unicellular and complex models, the hallmark of deficient NPC1 protein function is an accumulation of cellular cholesterol and fatty acids.
To date, five NPC1 disease mouse models have been identified with either spontaneous or generated mutations and have been used to investigate the pathophysiology of this complex disorder. The mouse models are denoted as Npc1spm (95), Npc1nih (96), Npc1nmf164 (97), Npc1pf/pf (41), Npc1tm(I1061T)Dso (98), and NPCimagine (99). The affected mice display phenotypes similar to humans, including cholesterol accumulation in cells, shortened lifespan, hepatomegaly, ataxia, Purkinje cell loss, neurodegeneration, and other signs of neurologic impairment. Transgenic Npc1−/− mouse models exclusively expressing the NPC1 protein in the whole brain, a subtype of brain cells, or in the viscera as well as rescue models for these organs have been described (100–104). Conversely, wild-type mice (Npc1+/+) with tissue-specific knockdown of the Npc1 gene in liver or cerebellar Purkinje cells have also been phenotyped (105, 106). Taken together, these models have led to a better understanding of NPC1 protein function.
Metabolic phenotypes of NPC1 disease mouse models
An absence of NPC1 protein function in mouse brain is responsible for weight loss in advanced stages of the disease (107). With respect to plasma glucose and lipid levels, Npc1−/− mice have impaired insulin signaling, increased glucose levels, increased total cholesterol levels, and larger and more heterogeneous HDL particles compared with Npc1+/+ mice when fed a basic diet, the phenotypes of which are more pronounced when fed a high-cholesterol diet (33, 108–110). In contrast, total triacylglycerol levels are reduced in Npc1−/− mouse serum (111). Npc1−/− hepatocytes have also been shown to secrete increased amounts of VLDLs possessing an increased cholesterol ester/triacylglycerol ratio compared with Npc1+/+ hepatocytes (112). However, some of these results (increased total cholesterol and reduced triacylglycerol) should be considered with caution, because they are discordant with the ones described in human NPC1−/− subjects and could be due to limited statistical power in some studies.
Conversely, the accumulation of cholesterol in the liver of Npc1−/− mice is a major disease phenotype in mice and replicates what was described in NPC1 patients. Because of this accumulation, Npc1−/− mice tend to develop liver steatosis and show signs of liver inflammation, damage, and fibrosis (45, 113).
Collectively, these studies describing phenotypes associated with NPC in both mice and humans underline the plausible role of NPC1 in the clustering of cardiometabolic traits. However, to better understand the function of NPC1, it is important not to restrict the research initiative to the study of compound heterozygous or homozygous affected NPC patients, and to study the full spectrum of genetic variations that could affect the gene. In the following section, we describe the effect of common genetic variants that have a less severe impact than NPC1-causing mutations (e.g., SNPs), as well as in heterozygous mice that have only one NPC1 mutation.
Beyond NPC1 Disease: Further Evidence of an Effect of NPC1 Variants on Metabolic Phenotypes
The most striking phenotypes associated with the development of NPC1 disease are the neurologic manifestations. Hence, it is expected that NPC1 gene variants and SNPs should also be associated with neurologic diseases. Consistently, two studies report an association of NPC1 common variants with Alzheimer disease (114, 115) and dementia (116). Nevertheless, most of the studies exploring the effect of NPC1 variants in a context other than NPC disease are related to metabolic traits and disorders, which is the focus of this review.
Evidence in humans
Recently, the contribution of two NPC1 common nonsynonymous SNPs (rs1805081 and rs1805082) to polygenic obesity in European populations has placed NPC1 on the list of genes associated with this common disease (25). This association was detected through GWASs, currently one of the most powerful approaches in generating new biological hypotheses. The nonsynonymous coding rs1805081 (H215R) SNP (minor allele frequency ∼38% in samples of European ancestry of the 1000 Genomes Project) was associated with an increased risk of adult morbid obesity (P = 7.7 × 10−8) (25). The rs1805082 SNP is also nonsynonymous (I858V) and displays strong linkage disequilibrium (r2 = 1) with rs1805081 in Europeans (25). Both SNPs are predicted to have a benign effect on NPC1 protein function according to SIFT (http://sift.jcvi.org/) (117) and Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) (118) in silico tools. However, these computation predictions must be confirmed by functional studies performed in both cellular and animal models using site-directed mutagenesis. The association between rs1805081 and adult obesity has been replicated in a consistent direction in several independent European adult cohorts (119–122). In the Genetic Investigation of ANthropometric Traits (GIANT) consortium (https://www.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium), the NPC1 rs1805081 “A” obesity allele was associated with an increased risk for overweight [odds ratio (OR) = 1.02, P = 0.01], obesity class I (OR = 1.04, P = 5 × 10−4), obesity class II (OR = 1.05, P = 0.04), and obesity class III (OR = 1.07, P = 0.01) (122). More recently, Sanger and whole-exome sequencing also showed that risk alleles in both rare NPC1 mutations and in common NPC1 SNPs were more frequently observed in extremely obese cases than in lean controls (29, 123). In contrast to what was observed in European studies, the association between NPC1 rs1805081 and adult obesity was not replicated in East Asian and Latino American populations (124, 125). The absence of association between NPC1 rs1805081 and childhood obesity described in the initial GWAS was confirmed in European and non-European replication studies (126, 127). If the association between rs1805081 and adult BMI has been inconsistent in single study reports of European populations (120, 121, 128, 129), a convincing association was observed in the large-scale GIANT meta-analysis (β = 0.016, P = 6.5 × 10−7, N = 319,533) (130) (Table 1) (22, 122, 130–135). The association between NPC1 rs1805081 and adult BMI was not replicated in Asian populations (136, 137). Similarly, no association between NPC1 rs1805081 and BMI was observed in young European and non-European populations (25, 126, 138). Finally, an association between the NPC1 rs1805081 A obesity risk allele and increased body fat mass was reported in three independent European adult populations (β ≥ 0.013, P ≤ 0.04) (121, 128, 139).
Table 1.
Results of the Association of NPC1 rs1805082, rs1788799, and rs1805081 With T2D, Obesity, and Lipid-Related Traits in the MAGIC, DIAGRAM, GIANT, GIANT EXTREME, and GLGC Consortia
| Traits | rs1805082 (I 858V) | rs1788799 (M642I) |
rs1805081 (H215R) |
Consortium | n Total or n Cases/n Controls | Refs. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Allele | β or OR | 95% CI or SE | P Value | Effect Allele | β or OR | 95% CI or SE | P Value | Effect Allele | β or OR | 95% CI or SE | P Value | ||||
| Fasting glucose | A | 0.009 | 0.003 | 0.004 | G | 0.005 | 0.003 | 0.09 | A | 0.010 | 0.002 | 6 × 10−6 | MAGIC | 133,010,a 58,074b | (131, 132) |
| Fasting glucose adj BMI | A | 0.004 | 0.003 | 0.20 | G | 0.002 | 0.003 | 0.61 | A | 0.005 | 0.003 | 0.10 | MAGIC | 58,074 | (131) |
| 2-h glucose | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | A | 0.003 | 0.011 | 0.80 | MAGIC | 42,854 | (132) |
| 2-h glucose adj BMI | A | −0.01 | 0.018 | 0.54 | G | −0.02 | 0.02 | 0.35 | A | −0.010 | 0.019 | 0.58 | MAGIC | 15,234 | (133) |
| Fasting insulin | A | 0.009 | 0.003 | 0.004 | G | 0.005 | 0.003 | 0.09 | A | 0.010 | 0.002 | 7 × 10−5 | MAGIC | 108,557,a 51,750b | (131, 132) |
| Fasting insulin adj BMI | A | 0.004 | 0.003 | 0.20 | G | 0.002 | 0.003 | 0.61 | A | 0.004 | 0.002 | 0.06 | MAGIC | 108,557,a 51,750b | (131, 132) |
| HOMA-B | A | −9 × 10−4 | 0.003 | 0.78 | G | 0.003 | 0.003 | 0.39 | A | −0.001 | 0.003 | 0.87 | MAGIC | 46,186 | (134) |
| HOMA-IR | A | 0.006 | 0.004 | 0.14 | G | 0.009 | 0.004 | 0.03 | A | 0.009 | 0.004 | 0.03 | MAGIC | 46,186 | (134) |
| T2D | A | 1.03 | 1.01–1.05 | 0.006 | G | 1.03 | 1.01–1.06 | 0.02 | A | 1.02 | 1.00–1.04 | 0.07 | DIAGRAM | 22,669/58,119 | (135) |
| T2D adj BMI | A | 1.03 | 1.00–1.07 | 0.06 | G | 1.03 | 0.99–1.07 | 0.14 | A | 1.01 | 0.98–1.05 | 0.44 | DIAGRAM | 9580/53,810 | (135) |
| BMI | A | 0.016 | 0.003 | 1.4 × 10−7 | G | 0.165 | 0.003 | 1.9 × 10−7 | A | 0.016 | 0.003 | 6.5 × 10−7 | GIANT | ≥319,533 | (130) |
| Overweight | A | 1.03 | 1.01–1.05 | 0.003 | G | 1.02 | 1.01–1.04 | 0.01 | A | 1.03 | 1.01–1.04 | 0.01 | GIANT | ≥92.588/≥65.782c | (122) |
| Obesity class I | A | 1.05 | 1.03–1.08 | 3 × 10−5 | G | 1.04 | 1.02–1.07 | 7 × 10−4 | A | 1.04 | 1.02–1.07 | 5 × 10−4 | GIANT | ≥32.668/≥65.575c | (122) |
| Obesity class II | A | 1.04 | 1.00–1.08 | 0.04 | G | 1.048 | 1.00–1.08 | 0.05 | A | 1.04 | 1.00–1.08 | 0.04 | GIANT | ≥9.839/≥62.111c | (122) |
| Obesity class III | A | 1.03 | 1.03–1.10 | 0.005 | G | 1.10 | 1.03–1.18 | 0.008 | A | 1.09 | 1.02–1.17 | 0.01 | GIANT | ≥2.673/≥41.495c | (122) |
| Total cholesterol | A | 0.005 | 0.0035 | 0.12 | n.d. | n.d. | n.d. | n.d. | A | 0.004 | 0.004 | 0.15 | GLGC | 188.577 | (24) |
| HDL-C | A | -0.01 | 0.003 | 0.007 | n.d. | n.d. | n.d. | n.d. | A | -0.01 | 0.003 | 0.004 | GLGC | 188.577 | (24) |
| LDL-C | A | 0.006 | 0.004 | 0.07 | n.d. | n.d. | n.d. | n.d. | A | 0.009 | 0.004 | 0.01 | GLGC | 188.577 | (24) |
| Triglycerides | A | 0.006 | 0.003 | 0.03 | n.d. | n.d. | n.d. | n.d. | A | 0.004 | 0.003 | 0.165 | GLGC | 188.577 | (24) |
Significant results are represented in bold type.
Abbreviations: adj, adjusted; CI, confidence interval; DIAGRAM, DIAbetes Genetics Replication and Meta‐analysis; GIANT, Genetic Investigation of ANthropometric Traits; GLGC, Global Lipids Genetics Consortium; HOMA-B, homeostatic model assessment of β-cell function; MAGIC, Meta-Analyses of Glucose and Insulin-related traits Consortium; n.d., not determined; SE, standard error.
Applies to the rs1805081.
Applies to the rs1805082 and rs1788799.
Number of cases and controls varies between SNPs.
The study of the effect of NPC1 mutations at the heterozygous state can also be informative, as carriers of a single NPC1 mutation sometimes display partial manifestations of NPC disease (140–143). In a recent publication, Liu et al. (29) examined and described obesity-related phenotypes in a limited number of parents of NPC cases (NPC1 loss-of-function mutation carriers NPC1+/− participants). In this study, haploinsufficient males had a significantly higher BMI than did their matched controls or than population-based controls. After investigating the severity of several NPC1 rare mutations identified in severely obese subjects, Liu et al. (29) also demonstrated that heterozygous carriers of a single rare (minor allele frequency < 1%) and severe NPC1 mutation (resulting in >50% loss in cholesterol transport ability) had a 4.8-fold higher risk to develop morbid adult obesity, higher waist circumferences, waist-to-hip ratios, and an increased trend of visceral and subcutaneous fat than did carriers of a rare NPC1 mutation that has a mild/no damaging effect (29).
In addition to obesity, two studies in European, non-European, adult, and child populations suggest an association between rs1805081 and glycemic parameters, such as fasting insulin (β for the A allele = −0.1, P = 0.001), insulin sensitivity index (β = −0.06, P = 0.004), and the homeostatic model assessment of insulin resistance (HOMA-IR; β = 0.03, P = 0.02) (126, 144). The NPC1 rs1805081 A allele was associated with an increased risk of T2D in 18,014 adult Danes (OR = 1.09, P = 0.03) (120). This association was no longer significant after adjusting for BMI (120). Interestingly, when tested among Arabs, no association was observed between the NPC1 rs1805081 SNP and T2D whereas another functional polymorphism of NPC1, the rs1788799/M642I SNP, was nominally associated with T2D in the same study (145). Further in silico tests performed in SIFT and Polyphen-2 revealed that the effect of this amino acid change resulting from the rs1788799 SNP is also likely to have only benign consequences on NPC1 protein function. Because rs1805081, rs1805082, and rs1788799 SNPs are in variable linkage disequilibrium in diverse ethnic groups (Supplemental Table 1), the genetic association with metabolic traits may find its origin in a cumulative deleterious functional effect of a coding variant cluster. Our analysis of data from the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC; http://www.magicinvestigators.org/) confirms that the obesity risk alleles of rs1805081, rs1805082, and/or rs1788799 SNPs are associated with higher fasting glucose levels, fasting insulin, HOMA-IR, and homeostatic model assessment of β-cell function (Table 1) (131–134). The obesity risk alleles of rs1805082 and rs1788799 SNPs have also been associated with an increased risk of T2D in the DIAbetes Genetics Replication And Meta‐analysis (DIAGRAM) consortium (http://diagram-consortium.org; OR = 1.03 for the three SNPs) (135). These associations with T2D were no longer significant after adjusting for BMI, thereby suggesting, at least in part, a BMI-dependent effect of NPC1 on predisposition to T2D (135).
Two studies have reported an association between NPC1 and blood lipid levels in Europeans. Borderline associations were found between the rs1805081 SNP and total cholesterol, but not LDL-C (111, 120). Moreover, rs1805081 and rs1805082 obesity risk alleles were significantly associated with lower HDL-C (β = −0.01, 0.004 ≤ P ≤ 0.007 for both SNPs) and higher LDL-C (β = 0.009, P = 0.01 for rs1805081) and triglycerides (β = 0.006, P = 0.03 for rs1805082) in the Global Lipids Genetics Consortium (GLGC; http://csg.sph.umich.edu/abecasis/public/lipids2013/; Table 1) (24). Modestly powered studies did not show evidence of any association between NPC1 SNPs and blood lipids in non-European populations (126, 145).
Data from mice studies
The association between NPC1 gene variants and anthropometric, glycemic, and lipid traits observed in humans has been elegantly complemented by mouse models. The expression of Npc1 was negatively correlated with body fat percentage in mice from 100 different strains (146). In the same study, SNPs at the Npc1 locus were significantly associated with body fat percentage increase after 8 weeks of exposure to a high-fat/high-sucrose diet through GWASs (146). This is an excellent illustration of scientific convergence that has led independent researchers to link NPC1 and metabolism using hypothesis generating approaches in both humans and rodents. Interestingly, no association between SNPs of the Npc1 locus and body fat percentage changes was observed when mice were fed with a chow diet only (146), which suggests a gene–diet interaction. Other evidence linking Npc1 genetic variations to altered metabolic traits and demonstrating a gene–diet interaction in rodents arose from the study of Npc1 heterozygous knockout (Npc1+/−) mice. These Npc1 haploinsufficient mice have a similar or slightly increased body weight (0% to 16% increase) compared with control mice (Npc1+/+) when fed a chow diet (147–151). However, the Npc1+/− mice displayed a significantly increased body weight (8% to 37.4% depending on genetic background of the mice) compared with Npc1+/+ mice when fed a high-fat diet, thereby consistent with a gene–diet interaction (147–149, 151, 152).
“Two studies have reported an association between NPC1 and blood lipid levels in Europeans.”
In addition to the high-fat diet, the effect of Npc1 haploinsufficiency on body weight is also modulated by the age of mice, so that the difference in body weight between Npc1+/− and Npc1+/+ fed a high-fat diet is significantly increased for mature adult mice (13 to 30 weeks of age) but not for younger mice (147, 151). These data fully support the age-dependent effect on obesity observed in humans (25). In the case of NPC disease, the accumulation of cholesterol increases over time, which is associated with altered expression in a number of genes and metabolic pathways that parallels the progression of the disease (72, 153). Hence, it is not surprising that the deleterious effect of NPC1 genetic variations increases with age in humans and mice (25, 147).
Similar to what was observed in humans, the study of Npc1+/− mice also showed a gene by sex interaction where only Npc1+/− male mice fed with a high-fat diet showed a significant increase in body weight (29). Studies have also determined that Npc1+/− mice fed a high-fat diet are more glucose intolerant and have a significantly increased impaired fasting glycaemia, higher insulin, total cholesterol, HDL-C, and LDL-C levels, as well as higher liver weights compared with Npc1+/+ mice fed the same diet (148, 151, 154). Moreover, fasting blood glucose and HDL/LDL-associated triglycerides were also significantly increased among Npc1+/− mice as compared with Npc1+/+ mice, independently of diet (148, 151).
The levels of Npc1 protein were decreased in liver of Npc1+/− mice compared with Npc1+/+ mice fed a high-fat diet (151). Additionally, differences in the liver lipid content between Npc1+/+ and Npc1+/− mice have also been described. Npc1+/− mice tend to accumulate more cholesterol in the liver and develop hepatic steatosis marked by an increased content of triacylglycerol and fatty acids at a relatively early age (before onset of weight gain or IR development) when fed either a low-fat or a high-fat diet (148–151).
Based on data from GWASs performed in humans and mice, as well as from candidate gene studies using various Npc1 haploinsufficient mouse models, a global scheme emerges in which NPC1 modulates the clustering of cardiometabolic traits, including obesity, T2D, dyslipidemia, and NAFLD.
Possible Mechanisms Linking NPC1 to Metabolic Diseases
Prior to suggesting any possible mechanism linking NPC1 to metabolism, it is important to ensure that the gene of interest is directly causing the observed phenotypes. In this review, we consider NPC1 as the causal gene and exclude the possibility that other genes in its vicinity (e.g., RIOK3 and ANKRD29) might be responsible for the association with metabolic phenotypes for the following reasons: 1) The SNPs reaching GWAS significance when testing the association with obesity are restricted to a linkage disequilibrium block that includes NPC1 and C18orf8 only. 2) No biological evidence relates the other genes surrounding NPC1 to energy metabolism. 3) The genetic changes responsible for the loss of function of NPC1 in halploinsufficient subjects and knockout mice described in this review are specific to the NPC1 gene and do not affect other genes. Because NPC1 and C18orf8 are overlapping in humans, it is impossible to trace back the association signal to one of these two genes based on human genetic studies only. However, the fact that C18orf8’s mouse homolog (3110002H16Rik) is further upstream of the Npc1 gene and does not overlap with Npc1’s coding region strongly suggests that NPC1 is indeed responsible for the metabolic phenotypes observed in both mice and humans.
Few studies have investigated the mechanisms underlying the Npc1 gene–diet interaction and its effect on body weight. The metabolic phenotyping of wild-type and Npc1 haploinsufficient mice revealed no differences in nutritional energy consumed, energy expenditure (measured by oxygen consumption, carbon dioxide elimination, locomotor activity, heat production), intestinal fat absorption, and fecal lipid content (and composition) when fed a high-fat diet (148, 151). Although the exact mechanism remains unknown, these data suggest that NPC1 does not primarily influence the metabolism and weight gain via neuronal/behavioral mechanisms, but instead by influencing peripheral tissues and organs.
Influence of NPC1 on metabolism via steroid hormones
Glucocorticoids (cortisol) and sex hormones (estradiol, progesterone, and testosterone) are synthetized from cholesterol and have been shown to influence metabolism. We hypothesize that NPC1 genetic variations that impact the expression/function of the encoded NPC1 protein also affect steroidogenesis, which will consequently modulate various metabolic traits.
Glucocorticoids
Effect of glucocorticoids on metabolism.
Cortisol (in humans) and corticosterone (in rodents) are produced as a result of the activation of the hypothalamic–pituitary–adrenal (HPA) axis in response to stress (155, 156). Their effect on metabolism varies according to the duration of the stressful events. In response to acute stress, glucocorticoids induce hepatic glucose production and influence cardiac frequency and blood pressure as part of the fight or flight reaction. In parallel, vegetative functions such as eating or reproduction are inhibited (155, 156). The effect of glucocorticoids on metabolism during the acute stress is rapid and extensive; however, this reaction is also brief and does not significantly impact energy homeostasis over the long term. Alternatively, chronic stress reactions result in the activation of the HPA axis as well as other secondary stress management structures such as the brain reward system, which can increase the palatability of food and induce behavior changes such as binge eating (156–158). These secondary stress management systems escape the control of the energy homoeostasis system and create a disturbed metabolic state causing various metabolic and cardiovascular diseases (155). The impact of long-term exposure to glucocorticoids on metabolism is illustrated from an extreme point of view by the cases of patients affected with Cushing syndrome or undergoing long-term corticotherapy, who tend to develop central obesity, fatty liver, hypertension, dyslipidemia, hypertriglyceridemia, IR, and T2D.
The increase in plasma glucocorticoid levels modulates the expression of various proteins implicated in the regulation of the energy metabolism (159, 160), including pro-opiomelanocortin in the hypothalamus (161, 162), ghrelin in the stomach (163) and leptin in adipocytes (164–165). Interestingly, the highest concentration of glucocorticoid receptors in adipose tissue has been detected within visceral fat (167), whose expansion is associated with the deleterious metabolic phenotypes, including insulin resistance, dyslipidemia, and NAFLD. Finally, studies indicate that glucocorticoids influence the expression of several genes/proteins implicated in lipid and glucose metabolism, resulting in the inhibition of the glucose transporter 4 in adipose and skeletal muscles (168). In the liver, glucocorticoids increase the activity of phosphoenolpyruvate carboxykinase that regulates both the fatty acid/triacylglycerol cycle through glyceroneogenesis, mobilization of hepatic glucose through gluconeogenesis, and insulin secretion from the pancreas.
Effect of NPC1 gene variations on glucocorticoid levels.
LDL-derived cholesterol, the main substrate of NPC1, accounts for about a fourth of the total cholesterol in the adrenal cortex, a proportion that is higher than in other steroidogenic tissues (169). Because the adrenal cortex requires a relatively large amount of LDL-derived cholesterol to synthesize glucocorticoids, this tissue may be more susceptible to altered cellular cholesterol homeostasis induced by NPC1 genetic variations.
For evidence in mice, although Npc1−/− mice display no major disturbances in their circadian rhythm (170), their levels of corticosterone are significantly higher than in Npc1+/+ mice (169). Additionally, our studies indicate that the difference in the concentration of corticosterone between Npc1+/+ and Npc1+/− mice when fed a low-fat diet is not significant, whereas Npc1+/− mice fed a high-fat diet have significantly increased concentrations of cortisol compared with Npc1+/+ mice fed the same diet (151). These observations reinforce the gene–diet interactions we have previously described and support our hypothesis that the effect of NPC1 genetic variations on metabolism might be mediated by variations in plasma cortisol and corticosterone concentrations.
Several mechanisms may explain why an increase, rather than a decrease, of corticosterone is observed in the context of NPC1 deficiency. First, although the transport of cholesterol from late endosomes/lysosomes to the endoplasmic reticulum or plasma membrane is impaired in cells of Npc1−/− mice, the transport of cholesterol from late endosomes/lysosomes to the mitochondria for initiation of cortisol synthesis is not mediated by the NPC1 protein and is consequently not impaired as a result of NPC1 deficiency (171). Second, the numerous disabilities experienced during progression of NPC1 disease may stimulate the activation of stress pathways, thereby explaining the increased amount of glucocorticoids in affected individuals (169). Thus, an accumulation of cholesterol in late endosomes/lysosomes due to NPC1 deficiency could paradoxically be associated with increased transport of cholesterol to mitochondria, resulting in higher levels of cholesterol in the inner matrix of this organelle. This may increase mitochondrial stress and explain the increased synthesis of cortisol.
For evidence from human studies, no NPC1 SNP was significantly associated with variations in plasma or saliva levels of cortisol at a GWAS significance threshold (P < 5 × 10−8) (172–174). However, these results are not considered conclusive, as the studies typically had a small size and, hence, a reduced power to detect modest genetic effects.
The effect of NPC1 genetic variations metabolism via glucocorticoids is suspected to be modulated by various environmental conditions in both mice and humans. This hypothesis is supported by studies indicating that an exposition to high-fat diet downregulates the expression of Npc1 in mice (a biological interaction) and enhances the hyperglycemic and diabetes-inducing effect of glucocorticoids (175–178). This hypothesis is also consistent with the observation that the effect of chronic stress on the development of obesity and other metabolic disease phenotypes in mice is enhanced in a context of a high-fat/high-sugar diet (179).
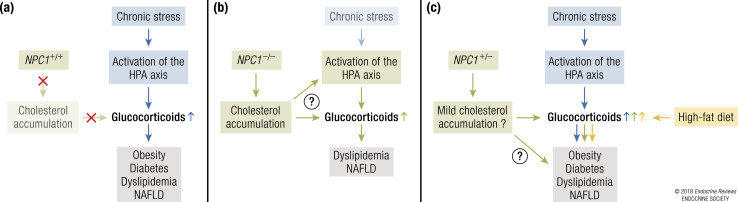
The model by which NPC1 could modulate the risk of metabolic disorders via glucocorticoids is represented in Fig. 6.
Figure 6.
Mechanistic model of the effect of NPC1 on metabolic disorders via glucocorticoids. (a) In healthy individuals, chronic stress results in increased levels of glucocorticoids, leading to weight gain and development of metabolic disorders. Because NPC1 is fully functional in these individuals, its effect on glucocorticoid levels is negligible. (b) In NPC1 patients, cholesterol accumulation increases glucocorticoid levels (either directly or by activating the HPA axis). This increase in glucocorticoids could be implicated in onset of some metabolic phenotypes observed in NPC1 patients. (c) In heterozygous carriers of NPC1 loss-of-function mutations or individuals with possibly deleterious NPC1 common gene variants, the combination of environmental factors such as chronic stress and/or high-fat diets/sedentary lifestyles may lead to higher levels of glucocorticoids and risk of metabolic disorders.
Sex steroid hormones
Effect of sex steroid hormones on metabolism.
Many studies report the effects of sex steroid hormones on metabolism. With respect to estrogens, food intake largely varies across the estrous cycle and during pregnancy in women and mice (180). Decreased levels of estradiol after ovariectomy or menopause are associated with weight gain, reduced energy expenditure, increase in adipocytes size, adipose tissue inflammation, and hepatic steatosis. Conversely, lower food intake and increased energy expenditure are observed after estradiol replacement therapy in the context of ovariectomy or menopause. Additionally, mice with global or brain-specific inactivation of estrogen receptor α are obese, hyperphagic, and develop metabolic syndrome [reviewed in (181, 182)]. Estrogens also modulate blood lipid levels. For instance, the beneficial effect of hormonal contraceptive therapy on HDL, LDL, total cholesterol, triacylglycerol levels, metabolic syndrome, and NAFLD in women has long been known (183). Conversely, ovariectomy or menopause are accompanied with increased levels of LDL, total cholesterol and triacylglycerol, and a higher risk of T2D, obesity, and NAFLD (184). Hence, a new estrogen-based therapy that successfully reverts metabolic syndrome and avoids the adverse effects associated with estrogen supplementation (cancer and disturbed reproduction) has recently been tested in mice (185).
In addition to being the primary reproductive steroid hormone in males (androgenic effect), testosterone has a strong anabolic effect and regulates energy metabolism. Testosterone deficiency is associated with reduced food intake, increased body fat mass and central obesity, reduced insulin sensitivity, elevated triacylglycerol and cholesterol, low HDL-C, hepatic steatosis, impaired glucose tolerance, T2D, and cardiovascular diseases in men and rodents [reviewed in (182, 186, 187)]. Conversely, testosterone replacement therapy improves IR found in these conditions, in addition to reducing body fat mass (in particular visceral adiposity) and improving glycemic control, cholesterol, and triacylglycerol levels (182, 186, 185).
The sex hormone–binding globulin (SHBG), which binds androgens and estrogens with high affinity, regulates the bioavailability of these sex hormones and therefore their effects. Among other factors, the expression of SHBG is regulated by sex steroid hormone levels themselves. It is upregulated by estrogens and downregulated by androgens. In humans, SHBG concentrations have been negatively associated with obesity T2D and fatty liver (188, 189).
Effect of NPC1 gene variations on sex steroid hormones levels.
For evidence in mice, ~2.5% and 6% of the cellular cholesterol in testis and ovaries is provided by LDLs (169). Nevertheless, the NPC1 gene is important for the normal development of reproductive functions. This is illustrated by the fact that both Npc1−/− affected males and females are sterile and display important histological abnormalities in gonads (108, 190). We hypothesize that NPC1 deficiency could affect the synthesis and plasma concentrations of sex steroid hormones and subsequently influence metabolism though them.
Studies have shown that plasma concentrations of progesterone are not significantly different between Npc1+/+ and Npc1−/− female mice (169, 191). In contrast, estradiol levels are reduced in the brain and ovaries of these mice (190, 192). The administration of 17β-estradiol to these mice delays the onset of neurologic symptoms (192) and increases the number and size of follicles in ovaries (190, 192). Additionally, synthesis of allopregnanolone, a neurologic steroid hormone derived from estradiol, is also impaired in Npc1−/− mice, and the treatment with allopregnanolone and/or its carrier, the cyclodextrin, significantly improves their neurologic phenotypes, which reinforces the notion that NPC1 deficiency adversely effects steroid hormones synthesis (193, 194).
In Npc1−/− male mice, plasma concentrations of testosterone are lower than in their Npc1+/+ counterparts (108, 195), although this difference was not observed in all studies (169). Additionally, a recent study showed that, when fed a high-fat diet, the body weight, fat mass, and adipocyte size of Npc1+/− male mice were significantly higher in their Npc1+/+ male counterparts (29). The same difference was not observed in Npc1+/− and Npc1+/+ female mice, which resulted in a significant gene–sex interaction.
For evidence in humans, several GWASs did not identify NPC1 as a significant contributor to variations in the levels of sex steroid hormones (testosterone or estradiol) (196–200). These negative results do not invalidate our sex steroid hormones hypothesis, especially considering the small sample size of these studies, and hence their lack of power to detect modest genetic effects. Additionally, diet, an important modulator of the effect of NPC1 genetic variations, was not taken into account in these studies, which could have weakened the association results. NPC1 SNPs have not been shown to influence the levels of SHBG either (196, 197, 199, 201, 202). Nevertheless, a gene–sex interaction was recently described in a study where the BMI of NPC1+/− men participants was significantly higher than in NPC1+/+ men controls. This difference was not significant in women, which mirrors the gene–sex interaction observed in mice (29).
Our hypothesis that the NPC1 gene might influence metabolism by modulating sex steroid hormone production in both mouse and humans is also supported by two other indirect observations. First, dietary fatty acids have been shown to regulate expression of both NPC1 and the synthesis of these steroid hormones (176, 203), which together explain the biological gene–environmental interaction that results when Npc1+/− mice are fed a high-fat diet. Second, it is tempting to speculate that the association between NPC1 SNPs and obesity in adults but not in children or young mice is due to the higher production and/or long-term impregnation of tissues with sex steroid hormones, a phenomenon observed in adolescents and adults only. However, all the obesity GWASs performed in children so far typically had smaller samples sizes than the ones performed in adults. Hence, the difference in the effect of NPC1 in adults and children should be investigated more thoroughly to exclude the possibility of a false-negative result caused by a lack of power.
In summary, NPC1 deficiency and haploinsufficiency have been associated with disturbed steroid hormones levels (corticosterone, estradiol, and testosterone) in mice. However, the number of studies reporting these associations is still limited. Further studies are needed to firmly establish the causal relationship between NPC1 genetic variations, steroid hormones levels, and metabolic disorders in humans and mice.
Influence of NPC1 on metabolism via blood and peripheral tissues lipids
Although the accumulation of cholesterol is the most prominent biochemical phenotype in NPC1 disease, the homeostasis of all lipids and lipoproteins is disturbed by NPC1 genetic variations. This disturbance is primarily due to the accumulation of not only cholesterol, but also other lipids in late endosomes/lysosomes. Additionally, because of the strong interplay between the metabolism of cholesterol and fatty acids, the disturbance of one of these two metabolisms impacts the other. For instance, the synthesis of cholesterol requires the use of fatty acids derived from triacylglycerol. As a result, LDL-derived cholesterol deficiency is associated with a higher de novo cholesterol synthesis and, consequently, a reduced triacylglycerol production and a disturbed cholesterol/triacylglycerol ratio and concentration of circulating lipoproteins (112, 169).
To date, an increasing number of studies associate abnormal lipoproteins levels to the development of metabolic disorders. For instance, low HDL levels predict the development of insulin resistance, T2D, and fatty liver disease in humans (204, 205), whereas the increase in HDL concentrations in vivo by reconstituted HDL injections is associated with improvement in glucose metabolism (206). By inducing reverse cholesterol transport and removing lipids from the liver, the adipose tissue, and the muscles, HDL particles reduce the inflammation and improve insulin sensitivity in those organs. In the pancreas, cholesterol accumulation is known to cause endoplasmic reticulum stress and to impair insulin secretion and induce lipotoxicity and β-cell failure (207–211). Hence, by depleting the pancreas of its excess cholesterol and lipids, HDL lipoproteins help restore a normal insulin secretion and rescue β-cells from death. The impact of HDL concentrations on glucose metabolism has recently been reviewed by Drew et al. (206).
The hypothesis that NPC1 might influence glucose, lipid, and liver metabolism by modulating blood lipid levels is supported by the fact that the regulation of both intracellular NPC1 and blood lipids levels is modulated by dietary fats and metabolic status (176, 212). Additionally, different genomic expression analyses show that NPC1 deficiency is associated with an important dysregulation of the expression of major genes implicated in intracellular or plasma blood lipids metabolism (e.g., LXRβ, SREBP1, LDLR, NPC2, peroxisome proliferator-activated receptor γ, adiponectin receptor, ATP-binding cassette subfamily A member 1, ATP-binding cassette subfamily G members 5 and 8, lipoprotein lipase, hepatic lipase, endothelial lipase) (72, 151, 153, 213, 214). Accordingly, Npc1+/− mice fed a high-fat diet also had increased expression of genes encoding proteins of the PGC-1α/β, LXRα/β, and SREBP-1 glycolysis and lipogenesis pathways compared with Npc1+/+ mice fed the same diet (151). Our hypothesis is also supported by the observation that plasma lipoprotein levels vary according to NPC1 genotype in a non-NPC disease context (human NPC1 SNPs and mouse Npc1 haploinsufficiency) as previously mentioned (see “Beyond NPC1 Disease: Further Evidence of an Effect of NPC1 Variants on Metabolic Phenotypes,” earlier). We have also recently demonstrated that Npc1 gene dosage influenced lipid levels in peripheral tissues that regulate blood lipids, including the liver (151). In adipocytes, we also showed that Npc1 haploinsufficient mice had decreased activation of hormone sensitive lipase, of triacylglycerol lipolysis, and of glycerol mobilization, compared with Npc1+/+ mice when fed a high-fat diet (151). When studying fibroblasts, Npc1+/− cells displayed a higher capacity to store neutral lipids (e.g., triacylglycerol and cholesterol) than Npc1+/+ fibroblasts (151).
In brief, the association between NPC1 genetic variations (deficiency, haploinsufficiency, SNPs) and changes in plasma, as well as peripheral tissue lipids, has been established in different human and mouse studies. Nevertheless, whether a disturbed blood lipid profile is the main cause of the metabolic abnormalities associated with NPC1 variants still needs to further investigation.
Other possible mechanisms
Another potential mechanism by which NPC1 deficiency could favor the development of metabolic disorders is through oxidative stress, a well-known biological marker of NPC1 disease (215). Previous studies have shown that reactive oxygen species and cholesterol oxidative product levels are higher in NPC1 diseases than in other metabolic disorders also characterized by increased oxidative stress, such as obesity, T2D, or the metabolic syndrome (71, 216). Our results also show that Npc1+/− cultured fibroblasts have a higher basal oxidative metabolism of exogenous fatty acids (associated with a reduced oxidative metabolism of endogenous fatty acids) than do Npc1+/+ cells. This suggests that the mitochondrial function and the oxidative metabolism are also disturbed in haploinsufficient individuals (151). Although it may not be the only cause of these metabolic disorders, the disturbance of the oxidative metabolism induced by NPC1 variants may predispose to these diseases. In this case, by reducing the efficiency of cholesterol turnover in cells, even modestly, NPC1 variants could eventually precipitate the onset of this disease in predisposed organisms.
NPC1 indirectly regulates the activity of mTORC1, a kinase involved in fatty acids and sterols biosynthesis and cell growth (46, 47). Additionally, proteins involved in mTORC1’s signaling are associated with different obesity and insulin resistance phenotypes in response to dietary fat (217). This suggests that the effect of NPC1 genetic variations and dietary fat on energy metabolism could be partly mediated by mTORC1 signaling.
“This suggests that the effect of NPC1 genetic variations and dietary fat on energy metabolism could be partly mediated by mTORC1 signaling.”
Finally, NPC1 inactivation has been associated with the accumulation of metabolites such as sphingolipids (218, 219), phospholipids, and glycerophospholipids (219), as well as certain lipid-soluble vitamins, including vitamin E (220). NPC1 deficiency also results in altered transport of copper (221), defective autophagy (222), inflammation and activation of immune response systems (72, 153, 213, 214, 223), and disturbed Ca2+ signaling (214, 224). Hence, one cannot exclude the possibility that NPC1’s effect is pleiotropic, and that its impact on metabolic diseases results from several of the above-mentioned mechanisms.
Future Directions
Mice studies
Because the expression of NPC1 is ubiquitous, identifying the organs most likely affected by NPC1 genetic variations responsible for the onset of metabolic abnormalities should be investigated by developing more mice with Npc1 tissue-specific expression. For example, whether the effect of NPC1 gene variants are mediated through either altered adipose lipid metabolism will require generating tissue-specific knockdown of the Npc1 gene in specific tissues. Moreover, one can envision additional genetic manipulations using mouse models to examine whether Npc1 overexpression results in a lean phenotype and thereby resistance to metabolic diseases (225). To date, no mouse model of global Npc1 overexpression has been described.
Human studies
The NPC1 gene variants rs1805081, rs1805082, and rs1788799 are in stronger linkage disequilibrium in Europeans (Supplemental Table 1). Hence, genetic and functional studies of this haplotype could be performed to reveal a possible cumulative deleterious effect of each variant within the gene cluster. The variable frequency of the rs1805082–rs1788799–rs1805081 A-G-A risk haplotype may explain the genetic heterogeneity observed between NPC1 and metabolic parameters in diverse ethnic groups.
Npc1 affected mice are sterile (226). In humans, most of the NPC patients develop the symptoms and impairments associated with the disease before puberty and die at an early age. The reproductive capacity in the adult forms of NPC1 disease, which represents a minority of the total diagnosed cases (e.g., only 10% in France) (227), is poorly described in literature. Hence, although it has not been precisely estimated, the fitness (reproductive success) of NPC1 patients is thought to be very low to null. The frequency of NPC1 disease causing mutations should therefore remain low in populations as a consequence of purifying selection at each generation (228). However, although they are subject to negative selection, these deleterious mutations do not totally disappear in the population because the heterozygotes still transmit them to the next generation (228). According to the “thrifty genotype” hypothesis, the carriers of NPC1 mutations in a heterozygous state, if they harbor a similar phenotype than Npc1+/− mice, may have undergone positive selection during historical periods of erratic food supply because of their higher capacity to store fat. This potential balanced pattern of natural selection makes the NPC1 gene an interesting candidate for evolutionary genetic studies. The fact that heterozygous individuals harbor higher adiposity than homozygous NPC1 wild-type (29) or homozygous mutated individuals (who typically lose weight after the beginning of the neurodegenerative symptoms) may be considered as a case of human overdominance for adiposity. If the overdominance pattern at the NPC1 locus is formally demonstrated, this gene will join the list of rare examples described in humans to date.
Knowing that most of the deleterious phenotypes associated to NPC1 inactivation in mice are mainly observed under some specific dietary conditions (high-fat, lithogenic, or high-fat/high-sucrose diets) (147–149, 154), the genotype–diet interactions on metabolic traits in humans should be explored more deeply in large observational/interventional studies or in families of NPC1 disease affected individuals. As most of the gene–environment interactions described in human populations are likely to be false-positive reports, testing a diet–genotype interaction supported by prior work in mouse models will strengthen the level of evidence.
Finally, as both NPC1 and NPC2 are implicated in cholesterol and fatty acid export from late endosomes/lysosomes, and mutations in both of their respective genes cause NPC with undistinguishable clinical phenotypes, the study of the effect of NPC2 genetic variants on metabolic disorders could be worth investigating.
Complementary human and mice studies
The robust association between NPC1 SNPs and adult obesity that was initially detected in a GWAS has been confirmed by different replication studies (25, 119–122). However, the associations of NPC1 variants with BMI, T2D, lipids levels, and NAFLD still need to be confirmed in other large cohorts and extended to different ethnic groups. Meta-analysis of independent studies and consortia could help reveal more conclusive associations. In mice, deep phenotyping of metabolic parameters for different Npc1 genotypes (haploinsufficiency, total NPC1 deficiency, knock-in mice) in different genetic backgrounds may provide new information.
Metabolic traits tend to cluster together. As NPC1 genetic variants are associated with obesity, the association with other metabolic disorders could develop independently or as a consequence of excess adiposity. Hence, the order of appearance of these diseases according to NPC1 genotype should be determined in longitudinal human and mouse studies.
NPC1 SNPs did not reach GWAS significance level in the modestly powered GWASs for sex steroid hormone performed so far (196–200). Hence, to ascertain whether a modification of steroid hormone levels induced by NPC1 gene variants mediates the onset of metabolic diseases, measures of these biomarkers in mice (Npc1−/− and Npc1+/− models) as well as in larger human studies (including in NPC1+/− samples) should be investigated. The measure of the anthropometric and metabolic response to intravenous injections of steroids in wild-type, heterozygotes, and homozygous Npc1−/− mice could also provide information concerning the role of these molecules.
Finally, a major next step in the study of NPC1 would be to apply the targeted transethnic sequencing and fine-mapping of the NPC1 locus in large multiethnic cohorts as well as in large groups of NPC patients and their respective family members. This should enable the identification of new, low-frequency, and potentially deleterious mutations implicated in the development of obesity. As the number of large whole-genome and whole-exome sequencing efforts increases (229–232), data from large sequencing projects should be jointly used to increase statistical power and specifically analyze rare variants of promising candidate genes such as NPC1. The sequencing, fine mapping, and prioritization of genetic variants should be followed by functional characterization of the suspected causal variants by in silico, in vitro, and in vivo experiments. The different approaches used in post-GWAS studies are reviewed elsewhere (233, 234). Although it has already been successfully used to the study of some NPC1 mutations (29, 98), the application of this approach should be extended to all identified and prioritized NPC1 variants in the future to improve clinical risk stratification and advance the field of personalized medicine.
Clinical relevance and future therapies
NPC1 disease
A number of new approaches and molecules have been shown to reduce the accumulation of cholesterol in vitro in NPC1 disease. However, just a few of these have proven to be effective in vivo. Histone deacetylase inhibitor molecules (e.g., vorinostat) and the 2-hydroxylpropyl cyclodextrin (HP-β-CD), which solubilize agent lipophilic compounds, can both significantly normalize cholesterol utilization, reduce cholesterol load within tissues, and delay weight loss, motor function impairment, neurodegeneration, and disease progression in mice (194, 235–238). Clinical trials with histone deacetylase inhibitors [clinicaltrials.gov (http://clinicaltrials.gov) reference no. NCT02124083] and HP-β-CD (reference nos. NCT01747135, NCT02534844, NCT02912793, NCT02939547) are in progress. For instance, the open-label, dose-escalation phase 1-2a study (NCT02534844) recently reported that patients with NPC disease treated with intrathecal HP-β-CD display a slower disease progression with an acceptable safety profile (239). These encouraging results support the initiation of further clinical studies using HP-β-CD.
It is tempting to speculate that new treatments for NPC1 disease could be designed using the latest available gene therapy tools, such as adenoviral vectors, for a systemic delivery a functional NPC1 gene copies in NPC1−/− patients (240). This approach yielded promising results both in vitro and in vivo (241–243) but has not been implemented in humans yet. Another interesting gene therapy approach would be to use the CRISPR/Cas9 technology for mutation correction at targeted sites (244). Examples of application of this method are limited but encouraging. For instance, the CRISPR/Cas9 technique successfully corrected STAT3 mutation in neonatal diabetes mellitus in cultured cells (245).
Obesity and its complications
To date, >170 loci have been associated with BMI, fat distribution, and/or obesity, most of which have been identified by large genome-wide association meta-analyses efforts (122, 130, 246). However, so far, only a minority of these loci have been investigated further. In this context, NPC1 is one of the few GWAS-identified genes that was the subject of major follow-up studies (29, 148, 149, 151). Because it influences a wide range of metabolic traits, and as some drugs targeting the NPC1 protein are already available (238, 239), NPC1 is one of the very few genes whose study holds considerable promise of a successful application in therapeutic management of human obesity and its complications.
It is very hard to speculate on whether the use of current NPC1 disease treatments will have any effect on metabolic traits (e.g., BMI- and T2D-related phenotypes, blood lipids) of noncarriers of deleterious NPC1 mutations. This is especially true when considering that the obesity-associated SNPs identified so far have a modest effect size (122, 130). However, the use of the available NPC1 disease medications such as Miglustat and HP-β-CD on NPC1 haploinsufficient subjects is likely to improve their metabolic profile, because these participants lack half of the protein’s activity, develop obesity (29), and display some mild NPC1 disease phenotypes (136, 247). Hence, the currently available NPC1 disease medications should be tested in Npc1+/− mice. If they are proven to be beneficial in both Npc1+/− mice and NPC1+/− subjects, the application of sequencing techniques will become essential to identify NPC1 mutation carriers in the general population and provide effective, precise, and personalized treatments based on the genetic profile of each individual.
Conclusion
In this review, we have summarized evidence linking NPC1 gene variants to metabolic disorders in mice and humans (Supplemental Table 2). We also propose different mechanisms (steroid and/or lipid homeostasis) by which NPC1 gene variants could predispose to these metabolic diseases (Fig. 7). This review illustrates the synergy of using genetic approaches (hypothesis free and hypothesis driven) in humans and mice to discover new mechanisms explaining the etiology of complex disorders such as obesity, T2D, dyslipidemia, and NAFLD. However, some of the explicative models we propose remain speculative, and considerable work remains to be performed to refine the understanding of mechanisms linking NPC1 to metabolism. We hope that this review, as well as the promising new areas of research investigation that have been proposed, will ultimately improve the health of NPC patients and the general population.
Figure 7.
Possible mechanisms linking NPC1 genetic variations to metabolic diseases. NPC1 gene mutations cause NPC1 disease, whereas less severe NPC1 common gene variations cause common metabolic disorders. By modulating the concentration of steroid hormones, as well as blood and peripheral tissues lipid levels (or by other unknown mechanisms), NPC1 genetic variations can also lead various organs, including the brain, pancreas, muscles, adipose tissue, and liver, to modulate body metabolism and energy homeostasis in people not affected with NPC1 disease. This effect on metabolism can eventually result in the onset of metabolic disorders such as obesity, T2D, dyslipidemia, or NAFLD depending on the NPC1 genotype and the environment.
Supplementary Material
Acknowledgments
Figures were produced using templates from the Servier Medical Art image bank. We thank the Global Lipids Genetics Consortium (GLGC), Genetic Investigation of ANthropometric Traits (GIANT), Meta-Analyses of Glucose and Insulin-related Traits Consortium (MAGIC), DIAbetes Genetics Replication and Meta‐analysis (DIAGRAM), and the Genotype-Tissue Expression (GTEx) Project for making their data publicly available.
Financial Support:This work was supported by grants from La Société Francophone du Diabète (to A.L. and M.P.), the National Center for Research Resources (to W.G.), the National Center for Advancing Translational Sciences (CTSC 8UL1TR000041), National Institutes of Health (DHHS/NIH/NIDDK 071544) (to W.G.), the Tohono O'odham Nation, private donations for the investigation of genetic and metabolic diseases (to W.S.G.), as well as by the Canada Research Chair in Genetics of Obesity (to D.M.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- BMI
body mass index
- CoA
coenzyme A
- GWAS
genome-wide association study
- HDL
high-density lipoprotein
- HDL-C
HDL cholesterol
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- HOMA-IR
homeostatic model assessment of insulin resistance
- HPA
hypothalamic–pituitary–adrenal
- HP-β-CD
2-hydroxylpropyl cyclodextrin
- IR
insulin resistance
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol
- LDLR
low-density lipoprotein plasma membrane receptor
- LXR
liver X receptor
- NAFLD
nonalcoholic fatty liver disease
- NPC
Niemann–Pick type C
- NPC1
Niemann–Pick type C1
- NPC2
Niemann–Pick type C2
- OR
odds ratio
- SHBG
sex hormone–binding globulin
- SNP
single nucleotide polymorphism
- SREBP
sterol regulatory element–binding protein
- T2D
type 2 diabetes
- VLDL
very-low–density lipoprotein
References
- 1. Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290(5497):1721–1726. [DOI] [PubMed] [Google Scholar]
- 2. Sturley SL, Patterson MC, Pentchev P. Unraveling the sterol-trafficking defect in Niemann-Pick C disease. Proc Natl Acad Sci USA. 2009;106(7):2093–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol. 2014;7(2):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sehayek E, Nath C, Heinemann T, McGee M, Seidman CE, Samuel P, Breslow JL. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. J Lipid Res. 1998;39(12):2415–2422. [PubMed] [Google Scholar]
- 5. Harvey R, Ferrier D.. Biochemistry. 5th ed Philadelphia, PA; Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 6. Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290(5500):2295–2298. [DOI] [PubMed] [Google Scholar]
- 7. Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105(40):15287–15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garver WS, Krishnan K, Gallagos JR, Michikawa M, Francis GA, Heidenreich RA. Niemann-Pick C1 protein regulates cholesterol transport to the trans-Golgi network and plasma membrane caveolae. J Lipid Res. 2002;43(4):579–589. [PubMed] [Google Scholar]
- 9. Luo J, Jiang L, Yang H, Song BL. Routes and mechanisms of post-endosomal cholesterol trafficking: a story that never ends. Traffic. 2017;18(4):209–217. [DOI] [PubMed] [Google Scholar]
- 10. Pfisterer SG, Peränen J, Ikonen E. LDL-cholesterol transport to the endoplasmic reticulum: current concepts. Curr Opin Lipidol. 2016;27(3):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Pfeffer SR. Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. eLife. 2016;5:e21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanier MT. Niemann-Pick diseases. Handb Clin Neurol. 2013;113:1717–1721. [DOI] [PubMed] [Google Scholar]
- 14. Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond). 2016;130(12):943–986. [DOI] [PubMed] [Google Scholar]
- 15. Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne). 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87(2):398–404. [DOI] [PubMed] [Google Scholar]
- 17. Wagenknecht LE, Scherzinger AL, Stamm ER, Hanley AJ, Norris JM, Chen YD, Bryer-Ash M, Haffner SM, Rotter JI. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring). 2009;17(6):1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Dongen J, Willemsen G, Chen WM, de Geus EJ, Boomsma DI. Heritability of metabolic syndrome traits in a large population-based sample. J Lipid Res. 2013;54(10):2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zarkesh M, Daneshpour MS, Faam B, Fallah MS, Hosseinzadeh N, Guity K, Hosseinpanah F, Momenan AA, Azizi F. Heritability of the metabolic syndrome and its components in the Tehran Lipid and Glucose Study (TLGS). Genet Res. 2012;94(6):331–337. [DOI] [PubMed] [Google Scholar]
- 20. Mathias RA, Deepa M, Deepa R, Wilson AF, Mohan V. Heritability of quantitative traits associated with type 2 diabetes mellitus in large multiplex families from South India. Metabolism. 2009;58(10):1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Persky L, Lockhart JJ, Karp R, Helal M, Hakki S. The overlooked, retained double J stent. Urology. 1990;36(6):519–521. [DOI] [PubMed] [Google Scholar]
- 22. Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O’Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS, Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB; NASH CRN; GIANT Consortium; MAGIC Investigators; GOLD Consortium . Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7(3):e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Browning SR, Browning BL. Identity-by-descent-based heritability analysis in the Northern Finland Birth Cohort. Hum Genet. 2013;132(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium . Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyre D, Delplanque J, Chèvre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proença C, Gaget S, Körner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen AL, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AI, Montpetit A, Polychronakos C, Weill J, Coin LJ, Asher J, Elliott P, Järvelin MR, Visvikis-Siest S, Balkau B, Sladek R, Balding D, Walley A, Dina C, Froguel P. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41(2):157–159. [DOI] [PubMed] [Google Scholar]
- 26. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. [DOI] [PubMed] [Google Scholar]
- 27. Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, Abdennur NA, Liu J, Svensson PA, Hsu YH, Drucker DJ, Mellgren G, Hui CC, Hauner H, Kellis M. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;373(10):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stratigopoulos G, Martin Carli JF, O’Day DR, Wang L, Leduc CA, Lanzano P, Chung WK, Rosenbaum M, Egli D, Doherty DA, Leibel RL. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19(5):767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu R, Zou Y, Hong J, Cao M, Cui B, Zhang H, Chen M, Shi J, Ning T, Zhao S, Liu W, Xiong H, Wei C, Qiu Z, Gu W, Zhang Y, Li W, Miao L, Sun Y, Yang M, Wang R, Ma Q, Xu M, Xu Y, Wang T, Chan KK, Zuo X, Chen H, Qi L, Lai S, Duan S, Song B, Bi Y, Liu S, Wang W, Ning G, Wang J. Rare loss-of-function variants in NPC1 predispose to human obesity. Diabetes. 2017;66(4):935–947. [DOI] [PubMed] [Google Scholar]
- 30. Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF III, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–231. [DOI] [PubMed] [Google Scholar]
- 31. Erickson RP, Aviles RA, Zhang J, Kozloski MA, Garver WS, Heidenreich RA. High-resolution mapping of the spm (Niemann-Pick Type C) locus on mouse chromosome 18. Mamm Genome. 1997;8(5):355–356. [DOI] [PubMed] [Google Scholar]
- 32. Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Kähäri AK, Keenan S, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Overduin B, Parker A, Patricio M, Perry E, Pignatelli M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Aken BL, Birney E, Harrow J, Kinsella R, Muffato M, Ruffier M, Searle SM, Spudich G, Trevanion SJ, Yates A, Zerbino DR, Flicek P. Ensembl 2015. Nucleic Acids Res. 2015;43(Database issue):D662–D669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramirez CM, Liu B, Aqul A, Taylor AM, Repa JJ, Turley SD, Dietschy JM. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J Lipid Res. 2011;52(4):688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. GTEx Consortium Laboratory, Data Analysis and Coordinating Center (LDACC)—Analysis Working Group; Statistical Methods groups—Analysis Working Group; Enhancing GTEx (eGTEx) groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA; Biospecimen Collection Source Site—NDRI; Biospecimen Collection Source Site—RPCI; Biospecimen Core Resource—VARI; Brain Bank Repository—University of Miami Brain Endowment Bank; Leidos Biomedical—Project Management; ELSI Study; Genome Browser Data Integration and Visualization—EBI; Genome Browser Data Integration and Visualization—UCSC Genomics Institute, University of California Santa Cruz; Lead analysts: Laboratory, Data Analysis and Coordinating Center (LDACC); NIH program management; Biospecimen collection; Pathology; QTL manuscript working group: Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott C, Ioannou YA. The NPC1 protein: structure implies function. Biochim Biophys Acta 2004;1685(1–3):8–13. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Saha P, Li J, Blobel G, Pfeffer SR. Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc Natl Acad Sci USA. 2016;113(36):10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong X, Qian H, Zhou X, Wu J, Wan T, Cao P, Huang W, Zhao X, Wang X, Wang P, Shi Y, Gao GF, Zhou Q, Yan N. Structural Insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell. 2016;165(6):1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Wang J, Coutavas E, Shi H, Hao Q, Blobel G. Structure of human Niemann-Pick C1 protein. Proc Natl Acad Sci USA. 2016;113(29):8212–8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao Y, Ren J, Harlos K, Stuart DI. Structure of glycosylated NPC1 luminal domain C reveals insights into NPC2 and Ebola virus interactions. FEBS Lett. 2016;590(5):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie X, Brown MS, Shelton JM, Richardson JA, Goldstein JL, Liang G. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc Natl Acad Sci USA. 2011;108(37):15330–15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohgane K, Karaki F, Dodo K, Hashimoto Y. Discovery of oxysterol-derived pharmacological chaperones for NPC1: implication for the existence of second sterol-binding site. Chem Biol. 2013;20(3):391–402. [DOI] [PubMed] [Google Scholar]
- 43. Jelinek D, Heidenreich RA, Orlando RA, Garver WS. The Niemann-Pick C1 and caveolin-1 proteins interact to modulate efflux of low density lipoprotein-derived cholesterol from late endocytic compartments. J Mol Biochem. 2014;3(1):14–26. [PMC free article] [PubMed] [Google Scholar]
- 44. Scott C, Higgins ME, Davies JP, Ioannou YA. Targeting of NPC1 to late endosomes involves multiple signals, including one residing within the putative sterol-sensing domain. J Biol Chem. 2004;279(46):48214–48223. [DOI] [PubMed] [Google Scholar]
- 45. Garver WS, Jelinek D, Oyarzo JN, Flynn J, Zuckerman M, Krishnan K, Chung BH, Heidenreich RA. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J Cell Biochem. 2007;101(2):498–516. [DOI] [PubMed] [Google Scholar]
- 46. Castellano BM, Thelen AM, Moldavski O, Feltes M, van der Welle RE, Mydock-McGrane L, Jiang X, van Eijkeren RJ, Davis OB, Louie SM, Perera RM, Covey DF, Nomura DK, Ory DS, Zoncu R. Lysosomal cholesterol activates mTORC1 via an SLC38A9–Niemann-Pick C1 signaling complex. Science. 2017;355(6331):1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eid W, Dauner K, Courtney KC, Gagnon A, Parks RJ, Sorisky A, Zha X. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc Natl Acad Sci USA. 2017;114(30):7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64(4):269–281. [DOI] [PubMed] [Google Scholar]
- 51. Wassif CA, Cross JL, Iben J, Sanchez-Pulido L, Cougnoux A, Platt FM, Ory DS, Ponting CP, Bailey-Wilson JE, Biesecker LG, Porter FD. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med. 2016;18(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vanier MT. [Niemann-Pick C disease: history, current research topics, biological and molecular diagnosis]. Arch Pediatr. 2010;17(Suppl 2):S41–S44. [DOI] [PubMed] [Google Scholar]
- 53. Greer WL, Riddell DC, Murty S, Gillan TL, Girouard GS, Sparrow SM, Tatlidil C, Dobson MJ, Neumann PE. Linkage disequilibrium mapping of the Nova Scotia variant of Niemann-Pick disease. Clin Genet. 1999;55(4):248–255. [DOI] [PubMed] [Google Scholar]
- 54. Millat G, Marçais C, Rafi MA, Yamamoto T, Morris JA, Pentchev PG, Ohno K, Wenger DA, Vanier MT. Niemann-Pick C1 disease: the I1061T substitution is a frequent mutant allele in patients of Western European descent and correlates with a classic juvenile phenotype. Am J Hum Genet. 1999;65(5):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mavridou I, Cozar M, Douzgou S, Xaidara A, Lianou D, Vanier MT, Dimitriou E, Grinberg D, Vilageliu L, Michelakakis H. Niemann-Pick type C disease: a novel NPC1 mutation segregating in a Greek island. Clin Genet. 2014;85(6):543–547. [DOI] [PubMed] [Google Scholar]
- 56. Runz H, Dolle D, Schlitter AM, Zschocke J. NPC-db, a Niemann-Pick type C disease gene variation database. Hum Mutat. 2008;29(3):345–350. [DOI] [PubMed] [Google Scholar]
- 57. Patterson M. Niemann-Pick disease type C In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, eds. GeneReviews. Seattle, WA: University of Washington; 1993–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1296/. [PubMed] [Google Scholar]
- 58. Mengel E, Klünemann HH, Lourenço CM, Hendriksz CJ, Sedel F, Walterfang M, Kolb SA. Niemann-Pick disease type C symptomatology: an expert-based clinical description. Orphanet J Rare Dis. 2013;8(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Di Lazzaro V, Marano M, Florio L, De Santis S. Niemann–Pick type C: focus on the adolescent/adult onset form. Int J Neurosci. 2016;126(11):963–971. [DOI] [PubMed] [Google Scholar]
- 60. Walterfang M, Abel LA, Desmond P, Fahey MC, Bowman EA, Velakoulis D. Cerebellar volume correlates with saccadic gain and ataxia in adult Niemann-Pick type C. Mol Genet Metab. 2013;108(1):85–89. [DOI] [PubMed] [Google Scholar]
- 61. Galanaud D, Tourbah A, Lehéricy S, Leveque N, Heron B, Billette de Villemeur T, Guffon N, Feillet F, Baumann N, Vanier MT, Sedel F. 24 month-treatment with miglustat of three patients with Niemann-Pick disease type C: follow up using brain spectroscopy. Mol Genet Metab. 2009;96(2):55–58. [DOI] [PubMed] [Google Scholar]
- 62. Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F; NP-C Guidelines Working Group . Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012;106(3):330–344. [DOI] [PubMed] [Google Scholar]
- 63. Wijburg FA, Sedel F, Pineda M, Hendriksz CJ, Fahey M, Walterfang M, Patterson MC, Wraith JE, Kolb SA. Development of a suspicion index to aid diagnosis of Niemann-Pick disease type C. Neurology. 2012;78(20):1560–1567. [DOI] [PubMed] [Google Scholar]
- 64. Vanier MT, Rodriguez-Lafrasse C, Rousson R, Gazzah N, Juge MC, Pentchev PG, Revol A, Louisot P; Type C Niemann-Pick disease: spectrum of phenotypic variation in disruption of intracellular LDL-derived cholesterol processing. Biochim Biophys Acta. 1991;1096(4):328–337. [DOI] [PubMed] [Google Scholar]
- 65. Vanier MT, Latour P. Laboratory diagnosis of Niemann-Pick disease type C: the filipin staining test. Methods Cell Biol. 2015;126:357–375. [DOI] [PubMed] [Google Scholar]
- 66. Takamura A, Sakai N, Shinpoo M, Noguchi A, Takahashi T, Matsuda S, Yamamoto M, Narita A, Ohno K, Ohashi T, Ida H, Eto Y. The useful preliminary diagnosis of Niemann-Pick disease type C by filipin test in blood smear. Mol Genet Metab. 2013;110(3):401–404. [DOI] [PubMed] [Google Scholar]
- 67. Kwiatkowska K, Marszałek-Sadowska E, Traczyk G, Koprowski P, Musielak M, Lugowska A, Kulma M, Grzelczyk A, Sobota A. Visualization of cholesterol deposits in lysosomes of Niemann-Pick type C fibroblasts using recombinant perfringolysin O. Orphanet J Rare Dis. 2014;9(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fernandez-Valero EM, Ballart A, Iturriaga C, Lluch M, Macias J, Vanier MT, Pineda M, Coll MJ. Identification of 25 new mutations in 40 unrelated Spanish Niemann-Pick type C patients: genotype-phenotype correlations. Clin Genet. 2005;68(3):245–254. [DOI] [PubMed] [Google Scholar]
- 69. Millat G, Chikh K, Naureckiene S, Sleat DE, Fensom AH, Higaki K, Elleder M, Lobel P, Vanier MT. Niemann-Pick disease type C: spectrum of HE1 mutations and genotype/phenotype correlations in the NPC2 group. Am J Hum Genet. 2001;69(5):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Millat G, Marçais C, Tomasetto C, Chikh K, Fensom AH, Harzer K, Wenger DA, Ohno K, Vanier MT. Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am J Hum Genet. 2001;68(6):1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, Olzeski D, Sidhu R, Dietzen DJ, Fu R, Wassif CA, Yanjanin NM, Marso SP, House J, Vite C, Schaffer JE, Ory DS. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2(56):56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cluzeau CV, Watkins-Chow DE, Fu R, Borate B, Yanjanin N, Dail MK, Davidson CD, Walkley SU, Ory DS, Wassif CA, Pavan WJ, Porter FD. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick disease, type C1. Hum Mol Genet. 2012;21(16):3632–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fan M, Sidhu R, Fujiwara H, Tortelli B, Zhang J, Davidson C, Walkley SU, Bagel JH, Vite C, Yanjanin NM, Porter FD, Schaffer JE, Ory DS. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J Lipid Res. 2013;54(10):2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pajares S, Arias A, García-Villoria J, Macías-Vidal J, Ros E, de las Heras J, Girós M, Coll MJ, Ribes A. Cholestane-3β,5α,6β-triol: high levels in Niemann-Pick type C, cerebrotendinous xanthomatosis, and lysosomal acid lipase deficiency. J Lipid Res. 2015;56(10):1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reunert J, Fobker M, Kannenberg F, Du Chesne I, Plate M, Wellhausen J, Rust S, Marquardt T. Rapid diagnosis of 83 patients with Niemann Pick type C disease and related cholesterol transport disorders by cholestantriol screening. EBioMedicine. 2015;4:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Giese AK, Mascher H, Grittner U, Eichler S, Kramp G, Lukas J, te Vruchte D, Al Eisa N, Cortina-Borja M, Porter FD, Platt FM, Rolfs A. A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet J Rare Dis. 2015;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J, Dietzen DJ, Schaffer JE, Ory DS. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res. 2011;52(7):1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boenzi S, Deodato F, Taurisano R, Martinelli D, Verrigni D, Carrozzo R, Bertini E, Pastore A, Dionisi-Vici C, Johnson DW. A new simple and rapid LC-ESI-MS/MS method for quantification of plasma oxysterols as dimethylaminobutyrate esters. Its successful use for the diagnosis of Niemann-Pick type C disease. Clin Chim Acta. 2014;437:93–100. [DOI] [PubMed] [Google Scholar]
- 79. Lin N, Zhang H, Qiu W, Ye J, Han L, Wang Y, Gu X. Determination of 7-ketocholesterol in plasma by LC-MS for rapid diagnosis of acid SMase-deficient Niemann-Pick disease. J Lipid Res. 2014;55(2):338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vanier MT, Gissen P, Bauer P, Coll MJ, Burlina A, Hendriksz CJ, Latour P, Goizet C, Welford RW, Marquardt T, Kolb SA. Diagnostic tests for Niemann-Pick disease type C (NP-C): a critical review. Mol Genet Metab. 2016;118(4):244–254. [DOI] [PubMed] [Google Scholar]
- 81. Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6(9):765–772. [DOI] [PubMed] [Google Scholar]
- 82. Patterson MC, Vecchio D, Jacklin E, Abel L, Chadha-Boreham H, Luzy C, Giorgino R, Wraith JE. Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol. 2010;25(3):300–305. [DOI] [PubMed] [Google Scholar]
- 83. Patterson MC, Mengel E, Vanier MT, Schwierin B, Muller A, Cornelisse P, Pineda M; NPC Registry investigators . Stable or improved neurological manifestations during miglustat therapy in patients from the international disease registry for Niemann-Pick disease type C: an observational cohort study. Orphanet J Rare Dis. 2015;10(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garver WS, Jelinek D, Meaney FJ, Flynn J, Pettit KM, Shepherd G, Heidenreich RA, Vockley CM, Castro G, Francis GA. The National Niemann-Pick Type C1 Disease Database: correlation of lipid profiles, mutations, and biochemical phenotypes. J Lipid Res. 2010;51(2):406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tängemo C, Weber D, Theiss S, Mengel E, Runz H. Niemann-Pick type C disease: characterizing lipid levels in patients with variant lysosomal cholesterol storage. J Lipid Res. 2011;52(4):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD. Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J Pediatr. 1993;123(2):242–247. [DOI] [PubMed] [Google Scholar]
- 87. Zhang S, Ren J, Li H, Zhang Q, Armstrong JS, Munn AL, Yang H. Ncr1p, the yeast ortholog of mammalian Niemann Pick C1 protein, is dispensable for endocytic transport. Traffic. 2004;5(12):1017–1030. [DOI] [PubMed] [Google Scholar]
- 88. Schwend T, Loucks EJ, Snyder D, Ahlgren SC. Requirement of Npc1 and availability of cholesterol for early embryonic cell movements in zebrafish. J Lipid Res. 2011;52(7):1328–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development. 2005;132(22):5115–5124. [DOI] [PubMed] [Google Scholar]
- 90. Kuwamura M, Awakura T, Shimada A, Umemura T, Kagota K, Kawamura N, Naiki M; Type C Niemann-Pick disease in a boxer dog. Acta Neuropathol. 1993;85(3):345–348. [DOI] [PubMed] [Google Scholar]
- 91. Vite CH, Ding W, Bryan C, O’Donnell P, Cullen K, Aleman D, Haskins ME, Van Winkle T. Clinical, electrophysiological, and serum biochemical measures of progressive neurological and hepatic dysfunction in feline Niemann-Pick type C disease. Pediatr Res. 2008;64(5):544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garver WS, Somers K, Krishnan K, Mitchell T, Heidenreich RA, Thrall MA. The Niemann-Pick C1 protein in feline fibroblasts. Mol Genet Metab. 2002;76(1):31–36. [DOI] [PubMed] [Google Scholar]
- 93. Mauler DA, Gandolfi B, Reinero CR, O'Brien DP, Spooner JL, Lyons LA; and 99 Lives Consortium. Precision medicine in cats: novel Niemann-Pick type C1 diagnosed by whole-genome sequencing. J Vet Intern Med. 2017;31(2):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Somers KL, Royals MA, Carstea ED, Rafi MA, Wenger DA, Thrall MA. Mutation analysis of feline Niemann-Pick C1 disease. Mol Genet Metab. 2003;79(2):99–103. [DOI] [PubMed] [Google Scholar]
- 95. Miyawaki S, Mitsuoka S, Sakiyama T, Kitagawa T. Sphingomyelinosis, a new mutation in the mouse: a model of Niemann-Pick disease in humans. J Hered. 1982;73(4):257–263. [DOI] [PubMed] [Google Scholar]
- 96. Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277(5323):232–235. [DOI] [PubMed] [Google Scholar]
- 97. Maue RA, Burgess RW, Wang B, Wooley CM, Seburn KL, Vanier MT, Rogers MA, Chang CC, Chang TY, Harris BT, Graber DJ, Penatti CA, Porter DM, Szwergold BS, Henderson LP, Totenhagen JW, Trouard TP, Borbon IA, Erickson RP. A novel mouse model of Niemann–Pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations. Hum Mol Genet. 2012;21(4):730–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Praggastis M, Tortelli B, Zhang J, Fujiwara H, Sidhu R, Chacko A, Chen Z, Chung C, Lieberman AP, Sikora J, Davidson C, Walkley SU, Pipalia NH, Maxfield FR, Schaffer JE, Ory DS. A murine Niemann-Pick C1 I1061T knock-in model recapitulates the pathological features of the most prevalent human disease allele. J Neurosci. 2015;35(21):8091–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gómez-Grau M, Albaigès J, Casas J, Auladell C, Dierssen M, Vilageliu L, Grinberg D. New murine Niemann-Pick type C models bearing a pseudoexon-generating mutation recapitulate the main neurobehavioural and molecular features of the disease. Sci Rep. 2017;7:41931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu T, Shakkottai VG, Chung C, Lieberman AP. Temporal and cell-specific deletion establishes that neuronal Npc1 deficiency is sufficient to mediate neurodegeneration. Hum Mol Genet. 2011;20(22):4440–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kapur R, Donohue C, Jelinek D, Erickson RP. Amelioration of enteric neuropathology in a mouse model of Niemann-Pick C by Npc1 expression in enteric glia. J Neurosci Res. 2009;87(13):2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lopez ME, Klein AD, Dimbil UJ, Scott MP. Anatomically defined neuron-based rescue of neurodegenerative Niemann-Pick type C disorder. J Neurosci. 2011;31(12):4367–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Loftus SK, Erickson RP, Walkley SU, Bryant MA, Incao A, Heidenreich RA, Pavan WJ. Rescue of neurodegeneration in Niemann–Pick C mice by a prion-promoter-driven Npc1 cDNA transgene. Hum Mol Genet. 2002;11(24):3107–3114. [DOI] [PubMed] [Google Scholar]
- 104. Bosch M, Fajardo A, Alcalá-Vida R, Fernández-Vidal A, Tebar F, Enrich C, Cardellach F, Pérez-Navarro E, Pol A. Hepatic primary and secondary cholesterol deposition and damage in Niemann-Pick disease. Am J Pathol. 2016;186(3):517–523. [DOI] [PubMed] [Google Scholar]
- 105. Elrick MJ, Pacheco CD, Yu T, Dadgar N, Shakkottai VG, Ware C, Paulson HL, Lieberman AP. Conditional Niemann-Pick C mice demonstrate cell autonomous Purkinje cell neurodegeneration. Hum Mol Genet. 2010;19(5):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rimkunas VM, Graham MJ, Crooke RM, Liscum L. In vivo antisense oligonucleotide reduction of NPC1 expression as a novel mouse model for Niemann Pick type C- associated liver disease. Hepatology. 2008;47(5):1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lopez ME, Klein AD, Hong J, Dimbil UJ, Scott MP. Neuronal and epithelial cell rescue resolves chronic systemic inflammation in the lipid storage disorder Niemann-Pick C. Hum Mol Genet. 2012;21(13):2946–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Akpovi CD, Murphy BD, Erickson RP, Pelletier RM. Dysregulation of testicular cholesterol metabolism following spontaneous mutation of the Niemann-Pick C1 gene in mice. Biol Reprod. 2014;91(2):42. [DOI] [PubMed] [Google Scholar]
- 109. Amigo L, Mendoza H, Castro J, Quiñones V, Miquel JF, Zanlungo S. Relevance of Niemann-Pick type C1 protein expression in controlling plasma cholesterol and biliary lipid secretion in mice. Hepatology. 2002;36(4 Pt 1):819–828. [DOI] [PubMed] [Google Scholar]
- 110. Ong QR, Lim ML, Chua CC, Cheung NS, Wong BS. Impaired insulin signaling in an animal model of Niemann-Pick type C disease. Biochem Biophys Res Commun. 2012;424(3):482–487. [DOI] [PubMed] [Google Scholar]
- 111. Uronen RL, Lundmark P, Orho-Melander M, Jauhiainen M, Larsson K, Siegbahn A, Wallentin L, Zethelius B, Melander O, Syvänen AC, Ikonen E. Niemann-Pick C1 modulates hepatic triglyceride metabolism and its genetic variation contributes to serum triglyceride levels. Arterioscler Thromb Vasc Biol. 2010;30(8):1614–1620. [DOI] [PubMed] [Google Scholar]
- 112. Kulinski A, Vance JE. Lipid homeostasis and lipoprotein secretion in Niemann-Pick C1-deficient hepatocytes. J Biol Chem. 2007;282(3):1627–1637. [DOI] [PubMed] [Google Scholar]
- 113. Sayre NL, Rimkunas VM, Graham MJ, Crooke RM, Liscum L. Recovery from liver disease in a Niemann-Pick type C mouse model. J Lipid Res. 2010;51(8):2372–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang P, Sun YM, Liu ZJ, Tao QQ, Li HL, Lu SJ, Wu ZY. Association study of ABCA7 and NPC1 polymorphisms with Alzheimer’s disease in Chinese Han ethnic population. Psychiatr Genet. 2013;23(6):268. [DOI] [PubMed] [Google Scholar]
- 115. Erickson RP, Larson-Thomé K, Weberg L, Szybinska A, Mossakowska M, Styczynska M, Barcikowska M, Kuznicki J. Variation in NPC1, the gene encoding Niemann–Pick C1, a protein involved in intracellular cholesterol transport, is associated with Alzheimer disease and/or aging in the Polish population. Neurosci Lett. 2008;447(2–3):153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cupidi C, Frangipane F, Gallo M, Clodomiro A, Colao R, Bernardi L, Anfossi M, Conidi ME, Vasso F, Curcio SA, Mirabelli M, Smirne N, Torchia G, Muraca MG, Puccio G, Di Lorenzo R, Zampieri S, Romanello M, Dardis A, Maletta RG, Bruni AC. Role of Niemann-Pick type C disease mutations in dementia. J Alzheimers Dis. 2017;55(3):1249–1259. [DOI] [PubMed] [Google Scholar]
- 117. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protocol Hum Genet 2013;Chapter 7:Unit 7.20. [DOI] [PMC free article] [PubMed]
- 119. Cotsapas C, Speliotes EK, Hatoum IJ, Greenawalt DM, Dobrin R, Lum PY, Suver C, Chudin E, Kemp D, Reitman M, Voight BF, Neale BM, Schadt EE, Hirschhorn JN, Kaplan LM, Daly MJ; GIANT Consortium . Common body mass index-associated variants confer risk of extreme obesity. Hum Mol Genet. 2009;18(18):3502–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sandholt CH, Vestmar MA, Bille DS, Borglykke A, Almind K, Hansen L, Sandbæk A, Lauritzen T, Witte D, Jørgensen T, Pedersen O, Hansen T. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS One. 2011;6(9):e23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rukh G, Sonestedt E, Melander O, Hedblad B, Wirfält E, Ericson U, Orho-Melander M. Genetic susceptibility to obesity and diet intakes: association and interaction analyses in the Malmö Diet and Cancer Study. Genes Nutr. 2013;8(6):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, Justice AE, Monda KL, Croteau-Chonka DC, Day FR, Esko T, Fall T, Ferreira T, Gentilini D, Jackson AU, Luan J, Randall JC, Vedantam S, Willer CJ, Winkler TW, Wood AR, Workalemahu T, Hu YJ, Lee SH, Liang L, Lin DY, Min JL, Neale BM, Thorleifsson G, Yang J, Albrecht E, Amin N, Bragg-Gresham JL, Cadby G, den Heijer M, Eklund N, Fischer K, Goel A, Hottenga JJ, Huffman JE, Jarick I, Johansson Å, Johnson T, Kanoni S, Kleber ME, König IR, Kristiansson K, Kutalik Z, Lamina C, Lecoeur C, Li G, Mangino M, McArdle WL, Medina-Gomez C, Müller-Nurasyid M, Ngwa JS, Nolte IM, Paternoster L, Pechlivanis S, Perola M, Peters MJ, Preuss M, Rose LM, Shi J, Shungin D, Smith AV, Strawbridge RJ, Surakka I, Teumer A, Trip MD, Tyrer J, Van Vliet-Ostaptchouk JV, Vandenput L, Waite LL, Zhao JH, Absher D, Asselbergs FW, Atalay M, Attwood AP, Balmforth AJ, Basart H, Beilby J, Bonnycastle LL, Brambilla P, Bruinenberg M, Campbell H, Chasman DI, Chines PS, Collins FS, Connell JM, Cookson WO, de Faire U, de Vegt F, Dei M, Dimitriou M, Edkins S, Estrada K, Evans DM, Farrall M, Ferrario MM, Ferrières J, Franke L, Frau F, Gejman PV, Grallert H, Grönberg H, Gudnason V, Hall AS, Hall P, Hartikainen AL, Hayward C, Heard-Costa NL, Heath AC, Hebebrand J, Homuth G, Hu FB, Hunt SE, Hyppönen E, Iribarren C, Jacobs KB, Jansson JO, Jula A, Kähönen M, Kathiresan S, Kee F, Khaw KT, Kivimäki M, Koenig W, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Laitinen JH, Lakka TA, Langenberg C, Launer LJ, Lind L, Lindström J, Liu J, Liuzzi A, Lokki ML, Lorentzon M, Madden PA, Magnusson PK, Manunta P, Marek D, März W, Mateo Leach I, McKnight B, Medland SE, Mihailov E, Milani L, Montgomery GW, Mooser V, Mühleisen TW, Munroe PB, Musk AW, Narisu N, Navis G, Nicholson G, Nohr EA, Ong KK, Oostra BA, Palmer CN, Palotie A, Peden JF, Pedersen N, Peters A, Polasek O, Pouta A, Pramstaller PP, Prokopenko I, Pütter C, Radhakrishnan A, Raitakari O, Rendon A, Rivadeneira F, Rudan I, Saaristo TE, Sambrook JG, Sanders AR, Sanna S, Saramies J, Schipf S, Schreiber S, Schunkert H, Shin SY, Signorini S, Sinisalo J, Skrobek B, Soranzo N, Stančáková A, Stark K, Stephens JC, Stirrups K, Stolk RP, Stumvoll M, Swift AJ, Theodoraki EV, Thorand B, Tregouet DA, Tremoli E, Van der Klauw MM, van Meurs JB, Vermeulen SH, Viikari J, Virtamo J, Vitart V, Waeber G, Wang Z, Widén E, Wild SH, Willemsen G, Winkelmann BR, Witteman JC, Wolffenbuttel BH, Wong A, Wright AF, Zillikens MC, Amouyel P, Boehm BO, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Cupples LA, Cusi D, Dedoussis GV, Erdmann J, Eriksson JG, Franks PW, Froguel P, Gieger C, Gyllensten U, Hamsten A, Harris TB, Hengstenberg C, Hicks AA, Hingorani A, Hinney A, Hofman A, Hovingh KG, Hveem K, Illig T, Jarvelin MR, Jöckel KH, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kuh D, Laakso M, Lehtimäki T, Levinson DF, Martin NG, Metspalu A, Morris AD, Nieminen MS, Njølstad I, Ohlsson C, Oldehinkel AJ, Ouwehand WH, Palmer LJ, Penninx B, Power C, Province MA, Psaty BM, Qi L, Rauramaa R, Ridker PM, Ripatti S, Salomaa V, Samani NJ, Snieder H, Sørensen TI, Spector TD, Stefansson K, Tönjes A, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Vollenweider P, Wallaschofski H, Wareham NJ, Watkins H, Wichmann HE, Wilson JF, Abecasis GR, Assimes TL, Barroso I, Boehnke M, Borecki IB, Deloukas P, Fox CS, Frayling T, Groop LC, Haritunian T, Heid IM, Hunter D, Kaplan RC, Karpe F, Moffatt MF, Mohlke KL, O’Connell JR, Pawitan Y, Schadt EE, Schlessinger D, Steinthorsdottir V, Strachan DP, Thorsteinsdottir U, van Duijn CM, Visscher PM, Di Blasio AM, Hirschhorn JN, Lindgren CM, Morris AP, Meyre D, Scherag A, McCarthy MI, Speliotes EK, North KE, Loos RJ, Ingelsson E. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mariman EC, Bouwman FG, Aller EE, van Baak MA, Wang P. Extreme obesity is associated with variation in genes related to the circadian rhythm of food intake and hypothalamic signaling. Physiol Genomics. 2015;47(6):225–231. [DOI] [PubMed] [Google Scholar]
- 124. Hotta K, Nakamura M, Nakamura T, Matsuo T, Nakata Y, Kamohara S, Miyatake N, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Masuzaki H, Yoneda M, Nakajima A, Funahashi T, Miyazaki S, Tokunaga K, Kawamoto M, Ueno T, Hamaguchi K, Tanaka K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Nakao K, Sakata T, Matsuzawa Y, Kamatani N, Nakamura Y. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J Hum Genet. 2009;54(12):727–731. [DOI] [PubMed] [Google Scholar]
- 125. León-Mimila P, Villamil-Ramírez H, Villalobos-Comparán M, Villarreal-Molina T, Romero-Hidalgo S, López-Contreras B, Gutiérrez-Vidal R, Vega-Badillo J, Jacobo-Albavera L, Posadas-Romeros C, Canizalez-Román A, Río-Navarro BD, Campos-Pérez F, Acuña-Alonzo V, Aguilar-Salinas C, Canizales-Quinteros S. Contribution of common genetic variants to obesity and obesity-related traits in Mexican children and adults. PLoS One. 2013;8(8):e70640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mejía-Benítez A, Klünder-Klünder M, Yengo L, Meyre D, Aradillas C, Cruz E, Pérez-Luque E, Malacara JM, Garay ME, Peralta-Romero J, Flores-Huerta S, García-Mena J, Froguel P, Cruz M, Bonnefond A. Analysis of the contribution of FTO, NPC1, ENPP1, NEGR1, GNPDA2 and MC4R genes to obesity in Mexican children. BMC Med Genet. 2013;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, Henning E, Blackburn H, Loos RJ, Wareham NJ, O’Rahilly S, Hurles ME, Barroso I, Farooqi IS. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet. 2013;45(5):513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. den Hoed M, Luan J, Langenberg C, Cooper C, Sayer AA, Jameson K, Kumari M, Kivimaki M, Hingorani AD, Grøntved A, Khaw KT, Ekelund U, Wareham NJ, Loos RJ. Evaluation of common genetic variants identified by GWAS for early onset and morbid obesity in population-based samples. Int J Obes. 2013;37(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Knüppel S, Rohde K, Meidtner K, Drogan D, Holzhütter HG, Boeing H, Fisher E. Evaluation of 41 candidate gene variants for obesity in the EPIC-Potsdam cohort by multi-locus stepwise regression. PLoS One. 2013;8(7):e68941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ’t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC/ICBP/MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, Rybin D, Liu CT, Bielak LF, Prokopenko I, Amin N, Barnes D, Cadby G, Hottenga JJ, Ingelsson E, Jackson AU, Johnson T, Kanoni S, Ladenvall C, Lagou V, Lahti J, Lecoeur C, Liu Y, Martinez-Larrad MT, Montasser ME, Navarro P, Perry JR, Rasmussen-Torvik LJ, Salo P, Sattar N, Shungin D, Strawbridge RJ, Tanaka T, van Duijn CM, An P, de Andrade M, Andrews JS, Aspelund T, Atalay M, Aulchenko Y, Balkau B, Bandinelli S, Beckmann JS, Beilby JP, Bellis C, Bergman RN, Blangero J, Boban M, Boehnke M, Boerwinkle E, Bonnycastle LL, Boomsma DI, Borecki IB, Böttcher Y, Bouchard C, Brunner E, Budimir D, Campbell H, Carlson O, Chines PS, Clarke R, Collins FS, Corbatón-Anchuelo A, Couper D, de Faire U, Dedoussis GV, Deloukas P, Dimitriou M, Egan JM, Eiriksdottir G, Erdos MR, Eriksson JG, Eury E, Ferrucci L, Ford I, Forouhi NG, Fox CS, Franzosi MG, Franks PW, Frayling TM, Froguel P, Galan P, de Geus E, Gigante B, Glazer NL, Goel A, Groop L, Gudnason V, Hallmans G, Hamsten A, Hansson O, Harris TB, Hayward C, Heath S, Hercberg S, Hicks AA, Hingorani A, Hofman A, Hui J, Hung J, Jarvelin MR, Jhun MA, Johnson PC, Jukema JW, Jula A, Kao WH, Kaprio J, Kardia SL, Keinanen-Kiukaanniemi S, Kivimaki M, Kolcic I, Kovacs P, Kumari M, Kuusisto J, Kyvik KO, Laakso M, Lakka T, Lannfelt L, Lathrop GM, Launer LJ, Leander K, Li G, Lind L, Lindstrom J, Lobbens S, Loos RJ, Luan J, Lyssenko V, Mägi R, Magnusson PK, Marmot M, Meneton P, Mohlke KL, Mooser V, Morken MA, Miljkovic I, Narisu N, O’Connell J, Ong KK, Oostra BA, Palmer LJ, Palotie A, Pankow JS, Peden JF, Pedersen NL, Pehlic M, Peltonen L, Penninx B, Pericic M, Perola M, Perusse L, Peyser PA, Polasek O, Pramstaller PP, Province MA, Räikkönen K, Rauramaa R, Rehnberg E, Rice K, Rotter JI, Rudan I, Ruokonen A, Saaristo T, Sabater-Lleal M, Salomaa V, Savage DB, Saxena R, Schwarz P, Seedorf U, Sennblad B, Serrano-Rios M, Shuldiner AR, Sijbrands EJ, Siscovick DS, Smit JH, Small KS, Smith NL, Smith AV, Stančáková A, Stirrups K, Stumvoll M, Sun YV, Swift AJ, Tönjes A, Tuomilehto J, Trompet S, Uitterlinden AG, Uusitupa M, Vikström M, Vitart V, Vohl MC, Voight BF, Vollenweider P, Waeber G, Waterworth DM, Watkins H, Wheeler E, Widen E, Wild SH, Willems SM, Willemsen G, Wilson JF, Witteman JC, Wright AF, Yaghootkar H, Zelenika D, Zemunik T, Zgaga L, Wareham NJ, McCarthy MI, Barroso I, Watanabe RM, Florez JC, Dupuis J, Meigs JB, Langenberg C; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Multiple Tissue Human Expression Resource (MUTHER) Consortium . A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Mägi R, Strawbridge RJ, Rehnberg E, Gustafsson S, Kanoni S, Rasmussen-Torvik LJ, Yengo L, Lecoeur C, Shungin D, Sanna S, Sidore C, Johnson PC, Jukema JW, Johnson T, Mahajan A, Verweij N, Thorleifsson G, Hottenga JJ, Shah S, Smith AV, Sennblad B, Gieger C, Salo P, Perola M, Timpson NJ, Evans DM, Pourcain BS, Wu Y, Andrews JS, Hui J, Bielak LF, Zhao W, Horikoshi M, Navarro P, Isaacs A, O’Connell JR, Stirrups K, Vitart V, Hayward C, Esko T, Mihailov E, Fraser RM, Fall T, Voight BF, Raychaudhuri S, Chen H, Lindgren CM, Morris AP, Rayner NW, Robertson N, Rybin D, Liu CT, Beckmann JS, Willems SM, Chines PS, Jackson AU, Kang HM, Stringham HM, Song K, Tanaka T, Peden JF, Goel A, Hicks AA, An P, Müller-Nurasyid M, Franco-Cereceda A, Folkersen L, Marullo L, Jansen H, Oldehinkel AJ, Bruinenberg M, Pankow JS, North KE, Forouhi NG, Loos RJ, Edkins S, Varga TV, Hallmans G, Oksa H, Antonella M, Nagaraja R, Trompet S, Ford I, Bakker SJ, Kong A, Kumari M, Gigante B, Herder C, Munroe PB, Caulfield M, Antti J, Mangino M, Small K, Miljkovic I, Liu Y, Atalay M, Kiess W, James AL, Rivadeneira F, Uitterlinden AG, Palmer CN, Doney AS, Willemsen G, Smit JH, Campbell S, Polasek O, Bonnycastle LL, Hercberg S, Dimitriou M, Bolton JL, Fowkes GR, Kovacs P, Lindström J, Zemunik T, Bandinelli S, Wild SH, Basart HV, Rathmann W, Grallert H, Maerz W, Kleber ME, Boehm BO, Peters A, Pramstaller PP, Province MA, Borecki IB, Hastie ND, Rudan I, Campbell H, Watkins H, Farrall M, Stumvoll M, Ferrucci L, Waterworth DM, Bergman RN, Collins FS, Tuomilehto J, Watanabe RM, de Geus EJ, Penninx BW, Hofman A, Oostra BA, Psaty BM, Vollenweider P, Wilson JF, Wright AF, Hovingh GK, Metspalu A, Uusitupa M, Magnusson PK, Kyvik KO, Kaprio J, Price JF, Dedoussis GV, Deloukas P, Meneton P, Lind L, Boehnke M, Shuldiner AR, van Duijn CM, Morris AD, Toenjes A, Peyser PA, Beilby JP, Körner A, Kuusisto J, Laakso M, Bornstein SR, Schwarz PE, Lakka TA, Rauramaa R, Adair LS, Smith GD, Spector TD, Illig T, de Faire U, Hamsten A, Gudnason V, Kivimaki M, Hingorani A, Keinanen-Kiukaanniemi SM, Saaristo TE, Boomsma DI, Stefansson K, van der Harst P, Dupuis J, Pedersen NL, Sattar N, Harris TB, Cucca F, Ripatti S, Salomaa V, Mohlke KL, Balkau B, Froguel P, Pouta A, Jarvelin MR, Wareham NJ, Bouatia-Naji N, McCarthy MI, Franks PW, Meigs JB, Teslovich TM, Florez JC, Langenberg C, Ingelsson E, Prokopenko I, Barroso I; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko I, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O’Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Köttgen A, Le Bacquer O, Pattou F, Taneera J, Steinthorsdottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proença C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, J Crawford G, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jørgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Lévy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparsø T, Swift AJ, Syddall H, Thorleifsson G, Tönjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvänen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JI, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy MI, Boehnke M, Barroso I, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM; GIANT consortium; MAGIC investigators . Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dorajoo R, Blakemore AI, Sim X, Ong RT, Ng DP, Seielstad M, Wong TY, Saw SM, Froguel P, Liu J, Tai ES. Replication of 13 obesity loci among Singaporean Chinese, Malay and Asian-Indian populations. Int J Obes. 2012;36(1):159–163. [DOI] [PubMed] [Google Scholar]
- 137. Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, Qi L, Chen CH, Delahanty RJ, Okada Y, Tabara Y, Gu D, Zhu D, Haiman CA, Mo Z, Gao YT, Saw SM, Go MJ, Takeuchi F, Chang LC, Kokubo Y, Liang J, Hao M, Le Marchand L, Zhang Y, Hu Y, Wong TY, Long J, Han BG, Kubo M, Yamamoto K, Su MH, Miki T, Henderson BE, Song H, Tan A, He J, Ng DP, Cai Q, Tsunoda T, Tsai FJ, Iwai N, Chen GK, Shi J, Xu J, Sim X, Xiang YB, Maeda S, Ong RT, Li C, Nakamura Y, Aung T, Kamatani N, Liu JJ, Lu W, Yokota M, Seielstad M, Fann CS, Wu JY, Lee JY, Hu FB, Tanaka T, Tai ES, Shu XO; Genetic Investigation of ANthropometric Traits (GIANT) Consortium . Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. 2012;44(3):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Graff M, Ngwa JS, Workalemahu T, Homuth G, Schipf S, Teumer A, Völzke H, Wallaschofski H, Abecasis GR, Edward L, Francesco C, Sanna S, Scheet P, Schlessinger D, Sidore C, Xiao X, Wang Z, Chanock SJ, Jacobs KB, Hayes RB, Hu F, Van Dam RM, Crout RJ, Marazita ML, Shaffer JR, Atwood LD, Fox CS, Heard-Costa NL, White C, Choh AC, Czerwinski SA, Demerath EW, Dyer TD, Towne B, Amin N, Oostra BA, Van Duijn CM, Zillikens MC, Esko T, Nelis M, Nikopensius T, Metspalu A, Strachan DP, Monda K, Qi L, North KE, Cupples LA, Gordon-Larsen P, Berndt SI, Berndt SI; GIANT Consortium . Genome-wide analysis of BMI in adolescents and young adults reveals additional insight into the effects of genetic loci over the life course. Hum Mol Genet. 2013;22(17):3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kilpeläinen TO, Zillikens MC, Stančákova A, Finucane FM, Ried JS, Langenberg C, Zhang W, Beckmann JS, Luan J, Vandenput L, Styrkarsdottir U, Zhou Y, Smith AV, Zhao JH, Amin N, Vedantam S, Shin SY, Haritunians T, Fu M, Feitosa MF, Kumari M, Halldorsson BV, Tikkanen E, Mangino M, Hayward C, Song C, Arnold AM, Aulchenko YS, Oostra BA, Campbell H, Cupples LA, Davis KE, Döring A, Eiriksdottir G, Estrada K, Fernández-Real JM, Garcia M, Gieger C, Glazer NL, Guiducci C, Hofman A, Humphries SE, Isomaa B, Jacobs LC, Jula A, Karasik D, Karlsson MK, Khaw KT, Kim LJ, Kivimäki M, Klopp N, Kühnel B, Kuusisto J, Liu Y, Ljunggren O, Lorentzon M, Luben RN, McKnight B, Mellström D, Mitchell BD, Mooser V, Moreno JM, Männistö S, O’Connell JR, Pascoe L, Peltonen L, Peral B, Perola M, Psaty BM, Salomaa V, Savage DB, Semple RK, Skaric-Juric T, Sigurdsson G, Song KS, Spector TD, Syvänen AC, Talmud PJ, Thorleifsson G, Thorsteinsdottir U, Uitterlinden AG, van Duijn CM, Vidal-Puig A, Wild SH, Wright AF, Clegg DJ, Schadt E, Wilson JF, Rudan I, Ripatti S, Borecki IB, Shuldiner AR, Ingelsson E, Jansson JO, Kaplan RC, Gudnason V, Harris TB, Groop L, Kiel DP, Rivadeneira F, Walker M, Barroso I, Vollenweider P, Waeber G, Chambers JC, Kooner JS, Soranzo N, Hirschhorn JN, Stefansson K, Wichmann HE, Ohlsson C, O’Rahilly S, Wareham NJ, Speliotes EK, Fox CS, Laakso M, Loos RJ. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet. 2011;43(8):753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Harzer K, Beck-Wödl S, Bauer P. Niemann-pick disease type C: new aspects in a long published family—partial manifestations in heterozygotes. JIMD Rep. 2014;12:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Josephs KA, Matsumoto JY, Lindor NM. Heterozygous Niemann-Pick disease type C presenting with tremor. Neurology. 2004;63(11):2189–2190. [DOI] [PubMed] [Google Scholar]
- 142. Kluenemann HH, Nutt JG, Davis MY, Bird TD. Parkinsonism syndrome in heterozygotes for Niemann–Pick C1. J Neurol Sci. 2013;335(1-2):219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hung YH, Walterfang M, Churilov L, Bray L, Jacobson LH, Barnham KJ, Jones NC, O’Brien TJ, Velakoulis D, Bush AI. Neurological dysfunction in early maturity of a model for Niemann–Pick C1 carrier status. Neurotherapeutics. 2016;13(3):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Robiou-du-Pont S, Bonnefond A, Yengo L, Vaillant E, Lobbens S, Durand E, Weill J, Lantieri O, Balkau B, Charpentier G, Marre M, Froguel P, Meyre D. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. Int J Obes. 2013;37(7):980–985. [DOI] [PubMed] [Google Scholar]
- 145. Al-Daghri NM, Cagliani R, Forni D, Alokail MS, Pozzoli U, Alkharfy KM, Sabico S, Clerici M, Sironi M. Mammalian NPC1 genes may undergo positive selection and human polymorphisms associate with type 2 diabetes. BMC Med. 2012;10(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ, Mehrabian M, Ursell LK, He A, Castellani LW, Zinker B, Kirby M, Drake TA, Drevon CA, Knight R, Gargalovic P, Kirchgessner T, Eskin E, Lusis AJ. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17(1):141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jelinek D, Heidenreich RA, Erickson RP, Garver WS. Decreased Npc1 gene dosage in mice is associated with weight gain. Obesity (Silver Spring). 2010;18(7):1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jelinek D, Millward V, Birdi A, Trouard TP, Heidenreich RA, Garver WS. Npc1 haploinsufficiency promotes weight gain and metabolic features associated with insulin resistance. Hum Mol Genet. 2011;20(2):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jelinek D, Heidenreich RA, Garver WS. The Niemann-Pick C1 gene interacts with a high-fat diet and modifying genes to promote weight gain. Am J Med Genet A. 2011;155A(9):2317–2319. [DOI] [PubMed] [Google Scholar]
- 150. Borbon I, Campbell E, Ke W, Erickson RP. The role of decreased levels of Niemann-Pick C1 intracellular cholesterol transport on obesity is reversed in the C57BL/6J, metabolic syndrome mouse strain: a metabolic or an inflammatory effect? J Appl Genet. 2012;53(3):323–330. [DOI] [PubMed] [Google Scholar]
- 151. Castillo JJ, Jelinek D, Wei H, Gannon NP, Vaughan RA, Horwood LJ, Meaney FJ, Garcia-Smith R, Trujillo KA, Heidenreich RA, Meyre D, Orlando RA, LeBoeuf RC, Garver WS. The Niemann-Pick C1 gene interacts with a high-fat diet to promote weight gain through differential regulation of central energy metabolism pathways. Am J Physiol Endocrinol Metab. 2017;313(2):E183–E194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jelinek D, Castillo JJ, Heidenreich RA, Garver WS. The C57BL/6J Niemann-Pick C1 mouse model with decreased gene dosage is susceptible to increased weight gain when fed a high-fat diet: confirmation of a gene-diet interaction. Gene. 2015;568(1):112–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Alam MS, Getz M, Safeukui I, Yi S, Tamez P, Shin J, Velázquez P, Haldar K. Genomic expression analyses reveal lysosomal, innate immunity proteins, as disease correlates in murine models of a lysosomal storage disorder. PLoS One. 2012;7(10):e48273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jelinek D, Castillo JJ, Garver WS. The C57BL/6J Niemann–Pick C1 mouse model with decreased gene dosage has impaired glucose tolerance independent of body weight. Gene. 2013;527(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. [DOI] [PubMed] [Google Scholar]
- 156. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA. 2010;107(47):20529–20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121(7):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rose AJ, Herzig S. Metabolic control through glucocorticoid hormones: an update. Mol Cell Endocrinol. 2013;380(1-2):65–78. [DOI] [PubMed] [Google Scholar]
- 160. Rose AJ, Vegiopoulos A, Herzig S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: insights from genetic manipulations. J Steroid Biochem Mol Biol. 2010;122(1-3):10–20. [DOI] [PubMed] [Google Scholar]
- 161. Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol. 1989;9(12):5305–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Drouin J, Nemer M, Charron J, Gagner JP, Jeannotte L, Sun YL, Therrien M, Tremblay Y. Tissue-specific activity of the pro-opiomelanocortin (POMC) gene and repression by glucocorticoids. Genome. 1989;31(2):510–519. [DOI] [PubMed] [Google Scholar]
- 163. Otto B, Tschop M, Heldwein W, Pfeiffer AF, Diederich S. Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur J Endocrinol. 2004;151(1):113–117. [DOI] [PubMed] [Google Scholar]
- 164. Kinouchi R, Matsuzaki T, Iwasa T, Gereltsetseg G, Nakazawa H, Kunimi K, Kuwahara A, Yasui T, Irahara M. Prepubertal exposure to glucocorticoid delays puberty independent of the hypothalamic Kiss1-GnRH system in female rats. Int J Dev Neurosci. 2012;30(7):596–601. [DOI] [PubMed] [Google Scholar]
- 165. Miell JP, Englaro P, Blum WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res 1996;28(12):f–707. [DOI] [PubMed] [Google Scholar]
- 166. Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes. 1997;46(4):717–719. [DOI] [PubMed] [Google Scholar]
- 167. Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16(10):924–936. [DOI] [PubMed] [Google Scholar]
- 168. Weinstein SP, Paquin T, Pritsker A, Haber RS. Glucocorticoid-induced insulin resistance: dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin- and non-insulin-related stimuli. Diabetes. 1995;44(4):441–445. [DOI] [PubMed] [Google Scholar]
- 169. Xie C, Richardson JA, Turley SD, Dietschy JM. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J Lipid Res. 2006;47(5):953–963. [DOI] [PubMed] [Google Scholar]
- 170. Richardson K, Livieratos A, Dumbill R, Hughes S, Ang G, Smith DA, Morris L, Brown LA, Peirson SN, Platt FM, Davies KE, Oliver PL. Circadian profiling in two mouse models of lysosomal storage disorders; Niemann Pick type-C and Sandhoff disease. Behav Brain Res. 2016;297:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Charman M, Kennedy BE, Osborne N, Karten B. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J Lipid Res. 2010;51(5):1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, Anderson A, Huffman J, Wilson JF, Campbell H, Rudan I, Wright A, Hastie N, Wild SH, Velders FP, Hofman A, Uitterlinden AG, Lahti J, Räikkönen K, Kajantie E, Widen E, Palotie A, Eriksson JG, Kaakinen M, Järvelin MR, Timpson NJ, Davey Smith G, Ring SM, Evans DM, St Pourcain B, Tanaka T, Milaneschi Y, Bandinelli S, Ferrucci L, van der Harst P, Rosmalen JG, Bakker SJ, Verweij N, Dullaart RP, Mahajan A, Lindgren CM, Morris A, Lind L, Ingelsson E, Anderson LN, Pennell CE, Lye SJ, Matthews SG, Eriksson J, Mellstrom D, Ohlsson C, Price JF, Strachan MW, Reynolds RM, Tiemeier H, Walker BR; CORtisol NETwork (CORNET) Consortium . Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10(7):e1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Neumann A, Direk N, Crawford AA, Mirza S, Adams H, Bolton J, Hayward C, Strachan DP, Payne EK, Smith JA, Milaneschi Y, Penninx B, Hottenga JJ, de Geus E, Oldehinkel AJ, van der Most PJ, de Rijke Y, Walker BR, Tiemeier H. The low single nucleotide polymorphism heritability of plasma and saliva cortisol levels. Psychoneuroendocrinology. 2017;85:88–95. [DOI] [PubMed] [Google Scholar]
- 174. Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, Van Duijn CM, Walker BR, Tiemeier H. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36(7):1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Martens MJ, Rutters F, Lemmens SG, Born JM, Westerterp-Plantenga MS. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol Behav. 2010;101(5):563–567. [DOI] [PubMed] [Google Scholar]
- 176. Jelinek D, Castillo JJ, Richardson LM, Luo L, Heidenreich RA, Garver WS. The Niemann-Pick C1 gene is downregulated in livers of C57BL/6J mice by dietary fatty acids, but not dietary cholesterol, through feedback inhibition of the SREBP pathway. J Nutr. 2012;142(11):1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. de Oliveira C, de Mattos AB, Biz C, Oyama LM, Ribeiro EB, do Nascimento CM. High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations. Lipids Health Dis. 2011;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Shpilberg Y, Beaudry JL, D’Souza A, Campbell JE, Peckett A, Riddell MC. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis Model Mech. 2012;5(5):671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnanský R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obes Res. 2004;12(4):716–724. [DOI] [PubMed] [Google Scholar]
- 181. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9(10):584–597. [DOI] [PubMed] [Google Scholar]
- 183. Stokes T, Wynn V. Serum-lipids in women on oral contraceptives. Lancet. 1971;2(7726):677–681. [DOI] [PubMed] [Google Scholar]
- 184. Berenson AB, Rahman M, Wilkinson G. Effect of injectable and oral contraceptives on serum lipids. Obstet Gynecol. 2009;114(4):786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Finan B, Yang B, Ottaway N, Stemmer K, Müller TD, Yi CX, Habegger K, Schriever SC, García-Cáceres C, Kabra DG, Hembree J, Holland J, Raver C, Seeley RJ, Hans W, Irmler M, Beckers J, de Angelis MH, Tiano JP, Mauvais-Jarvis F, Perez-Tilve D, Pfluger P, Zhang L, Gelfanov V, DiMarchi RD, Tschöp MH. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18(12):1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47–R71. [DOI] [PubMed] [Google Scholar]
- 187. Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26(7):376–383. [DOI] [PubMed] [Google Scholar]
- 189. Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gévry NY, Lopes FL, Ledoux S, Murphy BD. Aberrant intracellular cholesterol transport disrupts pituitary and ovarian function. Mol Endocrinol. 2004;18(7):1778–1786. [DOI] [PubMed] [Google Scholar]
- 191. Erickson RP. Current controversies in Niemann-Pick C1 disease: steroids or gangliosides; neurons or neurons and glia. J Appl Genet. 2013;54(2):215–224. [DOI] [PubMed] [Google Scholar]
- 192. Chen G, Li HM, Chen YR, Gu XS, Duan S. Decreased estradiol release from astrocytes contributes to the neurodegeneration in a mouse model of Niemann-Pick disease type C. Glia. 2007;55(15):1509–1518. [DOI] [PubMed] [Google Scholar]
- 193. Griffin LD, Gong W, Verot L, Mellon SH. Niemann–Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10(7):704–711. [DOI] [PubMed] [Google Scholar]
- 194. Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4(9):e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Roff CF, Strauss JF III, Goldin E, Jaffe H, Patterson MC, Agritellis GC, Hibbs AM, Garfield M, Brady RO, Pentchev PG. The murine Niemann-Pick type C lesion affects testosterone production. Endocrinology. 1993;133(6):2913–2923. [DOI] [PubMed] [Google Scholar]
- 196. Ruth KS, Campbell PJ, Chew S, Lim EM, Hadlow N, Stuckey BG, Brown SJ, Feenstra B, Joseph J, Surdulescu GL, Zheng HF, Richards JB, Murray A, Spector TD, Wilson SG, Perry JR. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur J Hum Genet. 2016;24(2):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chen Z, Tao S, Gao Y, Zhang J, Hu Y, Mo L, Kim ST, Yang X, Tan A, Zhang H, Qin X, Li L, Wu Y, Zhang S, Zheng SL, Xu J, Mo Z, Sun J. Genome-wide association study of sex hormones, gonadotropins and sex hormone-binding protein in Chinese men. J Med Genet. 2013;50(12):794–801. [DOI] [PubMed] [Google Scholar]
- 198. Jin G, Sun J, Kim ST, Feng J, Wang Z, Tao S, Chen Z, Purcell L, Smith S, Isaacs WB, Rittmaster RS, Zheng SL, Condreay LD, Xu J. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Hum Mol Genet. 2012;21(23):5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Prescott J, Thompson DJ, Kraft P, Chanock SJ, Audley T, Brown J, Leyland J, Folkerd E, Doody D, Hankinson SE, Hunter DJ, Jacobs KB, Dowsett M, Cox DG, Easton DF, De Vivo I. Genome-wide association study of circulating estradiol, testosterone, and sex hormone-binding globulin in postmenopausal women. PLoS One. 2012;7(6):e37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Petersen AK, Eriksson J, Lehtimäki T, Huhtaniemi IT, Hammond GL, Maggio M, Coviello AD, Ferrucci L, Heier M, Hofman A, Holliday KL, Jansson JO, Kähönen M, Karasik D, Karlsson MK, Kiel DP, Liu Y, Ljunggren O, Lorentzon M, Lyytikäinen LP, Meitinger T, Mellström D, Melzer D, Miljkovic I, Nauck M, Nilsson M, Penninx B, Pye SR, Vasan RS, Reincke M, Rivadeneira F, Tajar A, Teumer A, Uitterlinden AG, Ulloor J, Viikari J, Völker U, Völzke H, Wichmann HE, Wu TS, Zhuang WV, Ziv E, Wu FC, Raitakari O, Eriksson A, Bidlingmaier M, Harris TB, Murray A, de Jong FH, Murabito JM, Bhasin S, Vandenput L, Haring R; EMAS Study Group . Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7(10):e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR, Paolisso G, Rafiq S, Simon-Sanchez J, Lango H, Scholz S, Weedon MN, Arepalli S, Rice N, Washecka N, Hurst A, Britton A, Henley W, van de Leemput J, Li R, Newman AB, Tranah G, Harris T, Panicker V, Dayan C, Bennett A, McCarthy MI, Ruokonen A, Jarvelin MR, Guralnik J, Bandinelli S, Frayling TM, Singleton A, Ferrucci L. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4(5):e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimäki T, Keildson S, Lunetta KL, He C, Fornage M, Lagou V, Mangino M, Onland-Moret NC, Chen B, Eriksson J, Garcia M, Liu YM, Koster A, Lohman K, Lyytikäinen LP, Petersen AK, Prescott J, Stolk L, Vandenput L, Wood AR, Zhuang WV, Ruokonen A, Hartikainen AL, Pouta A, Bandinelli S, Biffar R, Brabant G, Cox DG, Chen Y, Cummings S, Ferrucci L, Gunter MJ, Hankinson SE, Martikainen H, Hofman A, Homuth G, Illig T, Jansson JO, Johnson AD, Karasik D, Karlsson M, Kettunen J, Kiel DP, Kraft P, Liu J, Ljunggren Ö, Lorentzon M, Maggio M, Markus MR, Mellström D, Miljkovic I, Mirel D, Nelson S, Morin Papunen L, Peeters PH, Prokopenko I, Raffel L, Reincke M, Reiner AP, Rexrode K, Rivadeneira F, Schwartz SM, Siscovick D, Soranzo N, Stöckl D, Tworoger S, Uitterlinden AG, van Gils CH, Vasan RS, Wichmann HE, Zhai G, Bhasin S, Bidlingmaier M, Chanock SJ, De Vivo I, Harris TB, Hunter DJ, Kähönen M, Liu S, Ouyang P, Spector TD, van der Schouw YT, Viikari J, Wallaschofski H, McCarthy MI, Frayling TM, Murray A, Franks S, Järvelin MR, de Jong FH, Raitakari O, Teumer A, Ohlsson C, Murabito JM, Perry JR. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8(7):e1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, Tsubakio M, Takemura T, Ezaki H, Hayashi N, Takehara T. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301(6):G1031–G1043. [DOI] [PubMed] [Google Scholar]
- 204. Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–2898. [DOI] [PubMed] [Google Scholar]
- 205. Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev. 2011;33(1):46–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119(15):2103–2111. [DOI] [PubMed] [Google Scholar]
- 207. Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic β-cell dysfunction. Diabetes. 2007;56(9):2328–2338. [DOI] [PubMed] [Google Scholar]
- 208. Ishikawa M, Iwasaki Y, Yatoh S, Kato T, Kumadaki S, Inoue N, Yamamoto T, Matsuzaka T, Nakagawa Y, Yahagi N, Kobayashi K, Takahashi A, Yamada N, Shimano H. Cholesterol accumulation and diabetes in pancreatic β-cell-specific SREBP-2 transgenic mice: a new model for lipotoxicity. J Lipid Res. 2008;49(12):2524–2534. [DOI] [PubMed] [Google Scholar]
- 209. Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res. 2013;52(1):175–191. [DOI] [PubMed] [Google Scholar]
- 210. Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(3):193–205. [DOI] [PubMed] [Google Scholar]
- 211. Taubes G. Insulin resistance. Prosperity’s plague. Science. 2009;325(5938):256–260. [DOI] [PubMed] [Google Scholar]
- 212. Jelinek D, Castillo JJ, Arora SL, Richardson LM, Garver WS. A high-fat diet supplemented with fish oil improves metabolic features associated with type 2 diabetes. Nutrition. 2013;29(9):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. De Windt A, Rai M, Kytömäki L, Thelen KM, Lütjohann D, Bernier L, Davignon J, Soini J, Pandolfo M, Laaksonen R. Gene set enrichment analyses revealed several affected pathways in Niemann-Pick disease type C fibroblasts. DNA Cell Biol. 2007;26(9):665–671. [DOI] [PubMed] [Google Scholar]
- 214. Reddy JV, Ganley IG, Pfeffer SR. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS One. 2006;1(1):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Vázquez MC, Balboa E, Alvarez AR, Zanlungo S. Oxidative stress: a pathogenic mechanism for Niemann-Pick type C disease. Oxid Med Cell Longev 2012;2012:205713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Torres S, Matías N, Baulies A, Nuñez S, Alarcon-Vila C, Martinez L, Nuño N, Fernandez A, Caballeria J, Levade T, Gonzalez-Franquesa A, Garcia-Rovés P, Balboa E, Zanlungo S, Fabrías G, Casas J, Enrich C, Garcia-Ruiz C, Fernández-Checa JC. Mitochondrial GSH replenishment as a potential therapeutic approach for Niemann Pick type C disease. Redox Biol. 2017;11:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. [DOI] [PubMed] [Google Scholar]
- 218. Zhou S, Davidson C, McGlynn R, Stephney G, Dobrenis K, Vanier MT, Walkley SU. Endosomal/lysosomal processing of gangliosides affects neuronal cholesterol sequestration in Niemann-Pick disease type C. Am J Pathol. 2011;179(2):890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tharkeshwar AK, Trekker J, Vermeire W, Pauwels J, Sannerud R, Priestman DA, Te Vruchte D, Vints K, Baatsen P, Decuypere JP, Lu H, Martin S, Vangheluwe P, Swinnen JV, Lagae L, Impens F, Platt FM, Gevaert K, Annaert W. A novel approach to analyze lysosomal dysfunctions through subcellular proteomics and lipidomics: the case of NPC1 deficiency. Sci Rep. 2017;7:41408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11(4):419–428. [DOI] [PubMed] [Google Scholar]
- 221. Yanagimoto C, Harada M, Kumemura H, Abe M, Koga H, Sakata M, Kawaguchi T, Terada K, Hanada S, Taniguchi E, Ninomiya H, Ueno T, Sugiyama T, Sata M. Copper incorporation into ceruloplasmin is regulated by Niemann-Pick C1 protein. Hepatol Res. 2011;41(5):484–491. [DOI] [PubMed] [Google Scholar]
- 222. Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AH, Cassady JP, Cohen MA, Chakraborty S, Wang H, Spooner E, Ploegh H, Gsponer J, Korolchuk VI, Jaenisch R. Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell Reports. 2013;5(5):1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Platt N, Speak AO, Colaco A, Gray J, Smith DA, Williams IM, Wallom KL, Platt FM. Immune dysfunction in Niemann-Pick disease type C. J Neurochem. 2016;136(Suppl 1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sáez PJ, Orellana JA, Vega-Riveros N, Figueroa VA, Hernández DE, Castro JF, Klein AD, Jiang JX, Zanlungo S, Sáez JC. Disruption in connexin-based communication is associated with intracellular Ca2+ signal alterations in astrocytes from Niemann-Pick type C mice. PLoS One. 2013;8(8):e71361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–898. [DOI] [PubMed] [Google Scholar]
- 226. Donohue C, Marion S, Erickson RP. Expression of Npc1 in glial cells corrects sterility in Npc1−/− mice. J Appl Genet. 2009;50(4):385–390. [DOI] [PubMed] [Google Scholar]
- 227. Sévin M, Lesca G, Baumann N, Millat G, Lyon-Caen O, Vanier MT, Sedel F. The adult form of Niemann–Pick disease type C. Brain. 2007;130(Pt 1):120–133. [DOI] [PubMed] [Google Scholar]
- 228. Futuyma DJ. Evolution. 3rd ed Chicago, IL; Sinauer Associates; 2013. [Google Scholar]
- 229. Tachmazidou I, Süveges D, Min JL, Ritchie GRS, Steinberg J, Walter K, Iotchkova V, Schwartzentruber J, Huang J, Memari Y, McCarthy S, Crawford AA, Bombieri C, Cocca M, Farmaki AE, Gaunt TR, Jousilahti P, Kooijman MN, Lehne B, Malerba G, Männistö S, Matchan A, Medina-Gomez C, Metrustry SJ, Nag A, Ntalla I, Paternoster L, Rayner NW, Sala C, Scott WR, Shihab HA, Southam L, St Pourcain B, Traglia M, Trajanoska K, Zaza G, Zhang W, Artigas MS, Bansal N, Benn M, Chen Z, Danecek P, Lin WY, Locke A, Luan J, Manning AK, Mulas A, Sidore C, Tybjaerg-Hansen A, Varbo A, Zoledziewska M, Finan C, Hatzikotoulas K, Hendricks AE, Kemp JP, Moayyeri A, Panoutsopoulou K, Szpak M, Wilson SG, Boehnke M, Cucca F, Di Angelantonio E, Langenberg C, Lindgren C, McCarthy MI, Morris AP, Nordestgaard BG, Scott RA, Tobin MD, Wareham NJ, Burton P, Chambers JC, Smith GD, Dedoussis G, Felix JF, Franco OH, Gambaro G, Gasparini P, Hammond CJ, Hofman A, Jaddoe VWV, Kleber M, Kooner JS, Perola M, Relton C, Ring SM, Rivadeneira F, Salomaa V, Spector TD, Stegle O, Toniolo D, Uitterlinden AG, Barroso I, Greenwood CMT, Perry JRB, Walker BR, Butterworth AS, Xue Y, Durbin R, Small KS, Soranzo N, Timpson NJ, Zeggini E; SpiroMeta Consortium; GoT2D Consortium; arcOGEN Consortium; Understanding Society Scientific Group; UK10K Consortium . Whole-genome sequencing coupled to imputation discovers genetic signals for anthropometric traits. Am J Hum Genet. 2017;100(6):865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JRB, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Tajes JF, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, van de Bunt M, Pearson RD, Kumar A, Müller-Nurasyid M, Grarup N, Stringham HM, Gamazon ER, Lee J, Chen Y, Scott RA, Below JE, Chen P, Huang J, Go MJ, Stitzel ML, Pasko D, Parker SCJ, Varga TV, Green T, Beer NL, Day-Williams AG, Ferreira T, Fingerlin T, Horikoshi M, Hu C, Huh I, Ikram MK, Kim BJ, Kim Y, Kim YJ, Kwon MS, Lee J, Lee S, Lin KH, Maxwell TJ, Nagai Y, Wang X, Welch RP, Yoon J, Zhang W, Barzilai N, Voight BF, Han BG, Jenkinson CP, Kuulasmaa T, Kuusisto J, Manning A, Ng MCY, Palmer ND, Balkau B, Stančáková A, Abboud HE, Boeing H, Giedraitis V, Prabhakaran D, Gottesman O, Scott J, Carey J, Kwan P, Grant G, Smith JD, Neale BM, Purcell S, Butterworth AS, Howson JMM, Lee HM, Lu Y, Kwak SH, Zhao W, Danesh J, Lam VKL, Park KS, Saleheen D, So WY, Tam CHT, Afzal U, Aguilar D, Arya R, Aung T, Chan E, Navarro C, Cheng CY, Palli D, Correa A, Curran JE, Rybin D, Farook VS, Fowler SP, Freedman BI, Griswold M, Hale DE, Hicks PJ, Khor CC, Kumar S, Lehne B, Thuillier D, Lim WY, Liu J, van der Schouw YT, Loh M, Musani SK, Puppala S, Scott WR, Yengo L, Tan ST, Taylor HA Jr, Thameem F, Wilson G Sr, Wong TY, Njølstad PR, Levy JC, Mangino M, Bonnycastle LL, Schwarzmayr T, Fadista J, Surdulescu GL, Herder C, Groves CJ, Wieland T, Bork-Jensen J, Brandslund I, Christensen C, Koistinen HA, Doney ASF, Kinnunen L, Esko T, Farmer AJ, Hakaste L, Hodgkiss D, Kravic J, Lyssenko V, Hollensted M, Jørgensen ME, Jørgensen T, Ladenvall C, Justesen JM, Käräjämäki A, Kriebel J, Rathmann W, Lannfelt L, Lauritzen T, Narisu N, Linneberg A, Melander O, Milani L, Neville M, Orho-Melander M, Qi L, Qi Q, Roden M, Rolandsson O, Swift A, Rosengren AH, Stirrups K, Wood AR, Mihailov E, Blancher C, Carneiro MO, Maguire J, Poplin R, Shakir K, Fennell T, DePristo M, de Angelis MH, Deloukas P, Gjesing AP, Jun G, Nilsson P, Murphy J, Onofrio R, Thorand B, Hansen T, Meisinger C, Hu FB, Isomaa B, Karpe F, Liang L, Peters A, Huth C, O’Rahilly SP, Palmer CNA, Pedersen O, Rauramaa R, Tuomilehto J, Salomaa V, Watanabe RM, Syvänen AC, Bergman RN, Bharadwaj D, Bottinger EP, Cho YS, Chandak GR, Chan JCN, Chia KS, Daly MJ, Ebrahim SB, Langenberg C, Elliott P, Jablonski KA, Lehman DM, Jia W, Ma RCW, Pollin TI, Sandhu M, Tandon N, Froguel P, Barroso I, Teo YY, Zeggini E, Loos RJF, Small KS, Ried JS, DeFronzo RA, Grallert H, Glaser B, Metspalu A, Wareham NJ, Walker M, Banks E, Gieger C, Ingelsson E, Im HK, Illig T, Franks PW, Buck G, Trakalo J, Buck D, Prokopenko I, Mägi R, Lind L, Farjoun Y, Owen KR, Gloyn AL, Strauch K, Tuomi T, Kooner JS, Lee JY, Park T, Donnelly P, Morris AD, Hattersley AT, Bowden DW, Collins FS, Atzmon G, Chambers JC, Spector TD, Laakso M, Strom TM, Bell GI, Blangero J, Duggirala R, Tai ES, McVean G, Hanis CL, Wilson JG, Seielstad M, Frayling TM, Meigs JB, Cox NJ, Sladek R, Lander ES, Gabriel S, Burtt NP, Mohlke KL, Meitinger T, Groop L, Abecasis G, Florez JC, Scott LJ, Morris AP, Kang HM, Boehnke M, Altshuler D, McCarthy MI. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Hendricks AE, Bochukova EG, Marenne G, Keogh JM, Atanassova N, Bounds R, Wheeler E, Mistry V, Henning E, Körner A, Muddyman D, McCarthy S, Hinney A, Hebebrand J, Scott RA, Langenberg C, Wareham NJ, Surendran P, Howson JM, Butterworth AS, Danesh J, Nordestgaard BG, Nielsen SF, Afzal S, Papadia S, Ashford S, Garg S, Millhauser GL, Palomino RI, Kwasniewska A, Tachmazidou I, O’Rahilly S, Zeggini E, Barroso I, Farooqi IS; Understanding Society Scientific Group; EPIC-CVD Consortium; UK10K Consortium . Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci Rep. 2017;7(1):4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Gudbjartsson DF, Helgason H, Gudjonsson SA, Zink F, Oddson A, Gylfason A, Besenbacher S, Magnusson G, Halldorsson BV, Hjartarson E, Sigurdsson GT, Stacey SN, Frigge ML, Holm H, Saemundsdottir J, Helgadottir HT, Johannsdottir H, Sigfusson G, Thorgeirsson G, Sverrisson JT, Gretarsdottir S, Walters GB, Rafnar T, Thjodleifsson B, Bjornsson ES, Olafsson S, Thorarinsdottir H, Steingrimsdottir T, Gudmundsdottir TS, Theodors A, Jonasson JG, Sigurdsson A, Bjornsdottir G, Jonsson JJ, Thorarensen O, Ludvigsson P, Gudbjartsson H, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Arnar DO, Magnusson OT, Kong A, Masson G, Thorsteinsdottir U, Helgason A, Sulem P, Stefansson K. Large-scale whole-genome sequencing of the Icelandic population. Nat Genet. 2015;47(5):435–444. [DOI] [PubMed] [Google Scholar]
- 233. Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93(5):779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sandholt CH, Hansen T, Pedersen O. Beyond the fourth wave of genome-wide obesity association studies. Nutr Diabetes. 2012;2(7):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Lachmann RH, te Vruchte D, Lloyd-Evans E, Reinkensmeier G, Sillence DJ, Fernandez-Guillen L, Dwek RA, Butters TD, Cox TM, Platt FM. Treatment with miglustat reverses the lipid-trafficking defect in Niemann-Pick disease type C. Neurobiol Dis. 2004;16(3):654–658. [DOI] [PubMed] [Google Scholar]
- 236. Cruz-Pardos S, Garcia-Poza P, Sanchez-Garcia FJ. [Treatment with cyclodextrin for Niemann Pick s disease]. Farm Hosp. 2013;37(3):271–272. [DOI] [PubMed] [Google Scholar]
- 237. Marshall CA, Borbon IA, Erickson RP. Relative efficacy of nicotinamide treatment of a mouse model of infantile Niemann-Pick C1 disease. J Appl Genet. 2017;58(1):99–102. [DOI] [PubMed] [Google Scholar]
- 238. Alam MS, Getz M, Haldar K. Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann-Pick type C disease in a mouse model. Sci Transl Med. 2016;8(326):326ra23. [DOI] [PubMed] [Google Scholar]
- 239. Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, Rao R, Soldatos A, Sidhu R, Walters KA, Xu X, Thurm A, Solomon B, Pavan WJ, Machielse BN, Kao M, Silber SA, McKew JC, Brewer CC, Vite CH, Walkley SU, Austin CP, Porter FD. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet. 2017;390(10104):1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4(2):43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Paul CA, Reid PC, Boegle AK, Karten B, Zhang M, Jiang ZG, Franz D, Lin L, Chang TY, Vance JE, Blanchette-Mackie J, Maue RA. Adenovirus expressing an NPC1-GFP fusion gene corrects neuronal and nonneuronal defects associated with Niemann Pick type C disease. J Neurosci Res. 2005;81(5):706–719. [DOI] [PubMed] [Google Scholar]
- 242. Xie C, Gong XM, Luo J, Li BL, Song BL. AAV9-NPC1 significantly ameliorates Purkinje cell death and behavioral abnormalities in mouse NPC disease. J Lipid Res. 2017;58(3):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Chandler RJ, Williams IM, Gibson AL, Davidson CD, Incao AA, Hubbard BT, Porter FD, Pavan WJ, Venditti CP. Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1. Hum Mol Genet. 2017;26(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23(R1):R40–R46. [DOI] [PubMed] [Google Scholar]
- 245. Saarimäki-Vire J, Balboa D, Russell MA, Saarikettu J, Kinnunen M, Keskitalo S, Malhi A, Valensisi C, Andrus C, Eurola S, Grym H, Ustinov J, Wartiovaara K, Hawkins RD, Silvennoinen O, Varjosalo M, Morgan NG, Otonkoski T. An activating STAT3 mutation causes neonatal diabetes through premature induction of pancreatic differentiation. Cell Reports. 2017;19(2):281–294. [DOI] [PubMed] [Google Scholar]
- 246. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JMW, Buchkovich ML, Heard-Costa NL, Roman TS, Drong AW, Song C, Gustafsson S, Day FR, Esko T, Fall T, Kutalik Z, Luan J, Randall JC, Scherag A, Vedantam S, Wood AR, Chen J, Fehrmann R, Karjalainen J, Kahali B, Liu CT, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bragg-Gresham JL, Buyske S, Demirkan A, Ehret GB, Feitosa MF, Goel A, Jackson AU, Johnson T, Kleber ME, Kristiansson K, Mangino M, Leach IM, Medina-Gomez C, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Stančáková A, Sung YJ, Tanaka T, Teumer A, Van Vliet-Ostaptchouk JV, Yengo L, Zhang W, Albrecht E, Ärnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Böhringer S, Bonnet F, Böttcher Y, Bruinenberg M, Carba DB, Caspersen IH, Clarke R, Daw EW, Deelen J, Deelman E, Delgado G, Doney AS, Eklund N, Erdos MR, Estrada K, Eury E, Friedrich N, Garcia ME, Giedraitis V, Gigante B, Go AS, Golay A, Grallert H, Grammer TB, Gräßler J, Grewal J, Groves CJ, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heikkilä K, Herzig KH, Helmer Q, Hillege HL, Holmen O, Hunt SC, Isaacs A, Ittermann T, James AL, Johansson I, Juliusdottir T, Kalafati IP, Kinnunen L, Koenig W, Kooner IK, Kratzer W, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Mach F, Magnusson PK, Mahajan A, McArdle WL, Menni C, Merger S, Mihailov E, Milani L, Mills R, Moayyeri A, Monda KL, Mooijaart SP, Mühleisen TW, Mulas A, Müller G, Müller-Nurasyid M, Nagaraja R, Nalls MA, Narisu N, Glorioso N, Nolte IM, Olden M, Rayner NW, Renstrom F, Ried JS, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Sennblad B, Seufferlein T, Sitlani CM, Smith AV, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tayo BO, Thorand B, Thorleifsson G, Tomaschitz A, Troffa C, van Oort FV, Verweij N, Vonk JM, Waite LL, Wennauer R, Wilsgaard T, Wojczynski MK, Wong A, Zhang Q, Zhao JH, Brennan EP, Choi M, Eriksson P, Folkersen L, Franco-Cereceda A, Gharavi AG, Hedman ÅK, Hivert MF, Huang J, Kanoni S, Karpe F, Keildson S, Kiryluk K, Liang L, Lifton RP, Ma B, McKnight AJ, McPherson R, Metspalu A, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Olsson C, Perry JR, Reinmaa E, Salem RM, Sandholm N, Schadt EE, Scott RA, Stolk L, Vallejo EE, Westra HJ, Zondervan KT, Amouyel P, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Brown MJ, Burnier M, Campbell H, Chakravarti A, Chines PS, Claudi-Boehm S, Collins FS, Crawford DC, Danesh J, de Faire U, de Geus EJ, Dörr M, Erbel R, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gieger C, Gudnason V, Haiman CA, Harris TB, Hattersley AT, Heliövaara M, Hicks AA, Hingorani AD, Hoffmann W, Hofman A, Homuth G, Humphries SE, Hyppönen E, Illig T, Jarvelin MR, Johansen B, Jousilahti P, Jula AM, Kaprio J, Kee F, Keinanen-Kiukaanniemi SM, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Musk AW, Möhlenkamp S, Morris AD, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Palmer LJ, Penninx BW, Peters A, Pramstaller PP, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Shuldiner AR, Staessen JA, Steinthorsdottir V, Stolk RP, Strauch K, Tönjes A, Tremblay A, Tremoli E, Vohl MC, Völker U, Vollenweider P, Wilson JF, Witteman JC, Adair LS, Bochud M, Boehm BO, Bornstein SR, Bouchard C, Cauchi S, Caulfield MJ, Chambers JC, Chasman DI, Cooper RS, Dedoussis G, Ferrucci L, Froguel P, Grabe HJ, Hamsten A, Hui J, Hveem K, Jöckel KH, Kivimaki M, Kuh D, Laakso M, Liu Y, März W, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sinisalo J, Slagboom PE, Snieder H, Spector TD, Stefansson K, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Veronesi G, Walker M, Wareham NJ, Watkins H, Wichmann HE, Abecasis GR, Assimes TL, Berndt SI, Boehnke M, Borecki IB, Deloukas P, Franke L, Frayling TM, Groop LC, Hunter DJ, Kaplan RC, O’Connell JR, Qi L, Schlessinger D, Strachan DP, Thorsteinsdottir U, van Duijn CM, Willer CJ, Visscher PM, Yang J, Hirschhorn JN, Zillikens MC, McCarthy MI, Speliotes EK, North KE, Fox CS, Barroso I, Franks PW, Ingelsson E, Heid IM, Loos RJ, Cupples LA, Morris AP, Lindgren CM, Mohlke KL; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Zhang H, Wang Y, Lin N, Yang R, Qiu W, Han L, Ye J, Gu X. Diagnosis of Niemann-Pick disease type C with 7-ketocholesterol screening followed by NPC1/NPC2 gene mutation confirmation in Chinese patients. Orphanet J Rare Dis. 2014;9(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.