Abstract
Age is an independent risk factor of multiple organ failure in patients with sepsis. However, the age-related mechanisms of injury are not known. AMPK is a crucial regulator of energy homeostasis, which controls mitochondrial biogenesis by activation of peroxisome proliferator-activated receptor-γ coactivator-α (PGC-1α) and disposal of defective organelles by autophagy. We investigated whether AMPK dysregulation might contribute to age-dependent liver injury in young (2–3 mo) and mature male mice (11–13 mo) subjected to sepsis. Liver damage was higher in mature mice than in young mice and was associated with impairment of hepatocyte mitochondrial function, structure, and biogenesis and reduced autophagy. At molecular analysis, there was a time-dependent nuclear translocation of the active phosphorylated catalytic subunits AMPKα1/α2 and PGC-1α in young, but not in mature, mice after sepsis. Treatment with the AMPK activator 5-amino-4-imidazolecarboxamide riboside-1-β-d-ribofuranoside (AICAR) improved liver mitochondrial structure in both age groups compared with vehicle. In loss-of-function studies, young knockout mice with systemic deficiency of AMPKα1 exhibited greater liver injury than did wild-type mice after sepsis. Our study suggests that AMPK is important for liver metabolic recovery during sepsis. Although its function may diminish with age, pharmacological activation of AMPK may be of therapeutic benefit.—Inata, Y., Kikuchi, S., Samraj, R. S., Hake, P. W., O’Connor, M., Ledford, J. R., O’Connor, J., Lahni, P., Wolfe, V., Piraino, G., Zingarelli, B. Autophagy and mitochondrial biogenesis impairment contribute to age-dependent liver injury in experimental sepsis: dysregulation of AMP-activated protein kinase pathway.
Keywords: AICAR, cecal ligation and puncture, MODS, PGC-1α
Sepsis is a life-threatening organ dysfunction caused by dysregulated host responses to infection (1). Although comorbidities contribute to the clinical variability, age is an independent risk factor of increased mortality to sepsis (2, 3). Compared with patients younger than 50 yr of age, large age-related differences are observed in early mortality during hospitalization and in 90 d mortality among the adult population (4, 5). Despite these clinical observations, the molecular mechanisms that link age to enhanced susceptibility to sepsis are not known.
Persistent multiple organ dysfunction syndrome is the most common pattern before death (1, 6). Cellular energetic failure secondary to mitochondrial damage has been suggested to contribute to the development of the syndrome (7, 8).
AMPK is a crucial sensor of energy status and contributes to several metabolic processes for energy homeostasis. This kinase consists of a catalytic α-subunits (-α1 and -α2) and regulating subunits-2β and -γ, which are activated by low levels of ATP and high levels of AMP (9). A key component of the metabolic effects of AMPK is the activation of peroxisome proliferator-activated receptor-γ coactivator-α (PGC-1α), a transcriptional coactivator, which modulates mitochondrial biogenesis leading to improved cellular energy utilization (10, 11). AMPK also contributes to activation of autophagy, a highly conserved catabolic process that degrades and recycles dysfunctional cytoplasmic constituents, including damaged mitochondria, to ensure metabolic homeostasis (9, 12).
We investigated the age-dependent molecular mechanisms of the sepsis-induced liver dysmetabolism and the inability to recover in adult mature mice (11–13 mo old), compared with young mice (2–3 mo old). We demonstrated that AMPK-dependent pathways of autophagy and mitochondrial biogenesis were impaired in mature mice subjected to polymicrobial sepsis and correlated with increased liver injury when compared to young mice. Pharmacological activation of AMPK by 5-amino-4-imidazolecarboxamide riboside-1-β-d-ribofuranoside (AICAR), an AMP analog (13), exerted beneficial effects in both young and mature mice. For functional characterization of the biologic role of AMPK, we also used young knockout (KO) animals deficient of the α1-catalytic subunit when compared to wild-type (WT) littermates (14). In these loss-of-function studies, we demonstrated that AMPKα1 gene deletion in young septic mice (2–3 mo old) was associated with increased susceptibility to sepsis-induced liver injury. Thus, our data suggest that AMPK is an important modulator of the metabolic response in sepsis; however, its function declines with age. Nevertheless, activation of the residual AMPK has therapeutic potentials in adults.
MATERIALS AND METHODS
Murine model of polymicrobial sepsis
The investigation conformed to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA] and was approved by the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital. C57/BL6 mice were used at age between 2 and 3 mo (young group) or between 11 and 13 mo (mature group). Ampkα1+/+ WT and Ampkα1−/− KO mice on a C57BL/6×129/Sv mixed background were used at between 2 and 3 mo of age (14). Mice of male gender only were chosen for the experimentation to avoid interference of female hormonal fluctuations in sepsis responses during the estrous cycle in the young groups and during the decline of fertility in the mature groups (15). Mice were anesthetized with pentobarbital (40 mg/kg, i.p.), and polymicrobial sepsis was induced by cecal ligation and puncture (CLP) (16). To achieve a similar magnitude of mortality between the 2 groups of ages, we used a severe model of CLP (22-gauge needle) without antibiotics, which causes ∼80% mortality within 48 h (17). After the abdomen was opened, the cecum was exteriorized, ligated, and punctured twice with a 22-gauge needle. The cecum was then returned to the peritoneal cavity, and the abdominal incision was closed. The mice were then resuscitated subcutaneously with 35 ml/kg normal saline solution. In pharmacological studies, the C57BL/6 young and mature mice were assigned to 2 treatment groups: the vehicle-treated group received distilled water (200 µl/mouse); the AICAR-treated group received the AMPK activator AICAR (500 mg/kg, i.p.) at 1 and 6 h after CLP. Mice were euthanized at 3, 6, and 18 h after CLP. Blood and liver were collected for biochemical assays. In survival studies, Ampkα1+/+ WT and Ampkα1−/− KO mice (n = 15 in each group) were monitored for mortality rates for 168 h.
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was measured as an indicator of neutrophil infiltration in liver tissue (18). Tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7.0) and centrifuged for 30 min at 4000 g at 4°C. An aliquot of the supernatant was allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and hydrogen peroxide (0.1 mM). The rate of change in absorbance was measured by spectrophotometry at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μM of hydrogen peroxide at 37°C and expressed in units per 100 mg of tissue.
Histopathologic analysis
Livers were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin and evaluated by 2 independent observers blinded to the treatment groups. Liver injury was analyzed by a semiquantitative score based on the following histologic features: necrosis, sinusoid congestion and edema, lipid vacuoles, and infiltration of red blood and inflammatory cells. A score of 0 represented normal findings and scores of 1, 2, 3, and 4 represented minimal (<25% liver involvement), mild (25–50% liver involvement), significant (50–75% liver involvement), and severe (>75% liver involvement) injury, respectively. The 4 variables were summed to represent the total liver injury score (range, 0–16).
Plasma alanine aminotransferase and aspartate aminotransferase
Plasma levels of aminotransferase (ALT) and aspartate aminotransferase (AST), as indices of liver function, were evaluated by enzymatic assay kits (Sekisui Diagnostics, Charlottetown, PEI, Canada), using the protocols recommended by the manufacturer.
Measurement of ATP levels
Homogenates were obtained from fresh livers and were deproteinized with perchloric acid by a Deproteinization Sample Preparation Kit (BioVision, San Francisco, CA, USA). Liver ATP levels were measured with an ATP Fluorometric Assay Kit (BioVision). ATP levels were expressed as micromoles per gram tissue weight.
Mitochondrial isolation and citrate synthase activity
Mitochondria were isolated from fresh liver tissue in a Dounce homogenizer (Bellco Glass, Vineland, NJ, USA) and differential centrifugation using a Thermo Scientific Mitochondria Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA). Mitochondrial proteins were determined by the Bradford protein assay. Citrate synthase activity was measured as index of total viable mitochondria (19) with a Citrate Synthase Assay Kit (Millipore-Sigma, Billerica, MA, USA) by spectrophotometry and was expressed in nanomoles per milligram protein per minute.
Gene array analysis
Total RNA was extracted from liver tissues using the RNeasy Purification Kit; Qiagen (Germantown, MD, USA). An iScript Advanced cDNA synthesis kit for was used to synthesize cDNA from total RNA (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR was performed to evaluate the expression of 84 genes involved in mitochondrial biogenesis and function (PrimePCR Pathway, Mitochondria; Bio-Rad) with SYBR Green PCR SuperMix (Bio-Rad). Relative quantities of mRNA were calculated with 18S rRNA as an internal reference. The relative expression levels of the target genes were calculated after normalization against a reference gene (Gadph).
Transmission electron microscopy
Liver samples were fixed in 3% glutaraldehyde, postfixed in 1% osmium tetroxide in sodium phosphate buffer, and cut on an ultramicrotome. Sections were stained with 2% uranyl acetate and lead citrate and viewed and photographed on Hitachi H-7650 transmission electron microscope at 120 kV. The total number of mitochondria and autophagosomes, and the presence of abnormal or enlarged mitochondria with loose matrix, fragmented cristae, and membranes were determined in 9 consecutive cells in 4 different sections for each animal by using ImageJ (NIH) analysis (20).
Cytosol and nuclear protein extraction
Livers were homogenized in a buffer containing 0.32 M sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM, 2-ME, 20 µM leupeptin, 0.15 µM pepstatin A, 0.2 mM PMSF, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. Samples were centrifuged at 1000 g for 10 min at 4°C and the supernatants collected as cytosol extracts. The pellets were then solubilized in Triton buffer [1% Triton X-100, 250 mM NaCl, 50 mM Tris HCl (pH 7.5), 3 mM EGTA, 3 mM EDTA, 0.1 mM PMSF, 0.1 mM sodium orthovanadate, 10% glycerol, 2 mM p-nitrophenyl phosphate, 0.5% NP-40, and 46 µM aprotinin]. The lysates were centrifuged at 15,000 g for 30 min at 4°C, and the supernatant was collected as nuclear extracts.
Western blot analysis
Immunoblot analyses were used to quantify cytosol and nuclear content of AMPKα1/α2 and the phosphorylated active forms AMPKα1/α2, PGC1-α, cytosol content of acetyl-CoA carboxylase (ACC), and the phosphorylated form of ACC, as downstream targets of AMPK, and cytosol content of beclin-1, light-chain (LC)3B-I, and LC3B-II proteins, as markers of autophagy (21). Cytosol and nuclear extracts were boiled in equal volumes of NuPAGE LDS Sample Buffer (Thermo Fisher Scientific) and 25–40 μg of protein loaded per lane on a 16% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblot analysis of AMPKα1/α2, PGC1-α, and LC3B-I and -II, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline and probed with primary antibodies. Membranes were washed in Tris-buffered saline with 0.1% Tween 20 and incubated with secondary peroxidase-conjugated antibody and reprobed with primary antibody against β-actin, to ensure equal loading samples of both cytosol and nuclear proteins (22). Immunoreaction was visualized by chemiluminescence. Densitometric analysis was performed with Quantity One (Bio-Rad Laboratories, Des Plaines, IL, USA). For immunoblot analysis of ACC and beclin-1, the membranes were blocked with Odyssey blocking buffer and incubated with specific primary antibodies; β-actin was concomitantly probed as loading control. Membranes were washed in PBS with 0.1% Tween 20 and incubated with LI-COR secondary antibodies. The Odyssey scanner (Li-Cor Biosciences, Lincoln, NE, USA) was used for detection.
Materials
AICAR was obtained from L. C. Laboratories (Woburn, MA, USA) and was reconstituted in sterile distilled water. The primary antibodies directed at ACC, pACC, AMPKα1/α2, pAMPKα1/α2, beclin-1, and LC3B-I and -II were obtained from Cell Signaling Technology (Danvers, MA, USA); the primary antibody directed at PGC-1α, was obtained from Abcam (Cambridge, MA, USA). The secondary antibodies and β-actin were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). All other chemicals were obtained from Millipore-Sigma.
Data analysis
Data are means ± sem or median with 25th and 75th percentiles of n observations (n = 5–19 animals for each group). The results were examined by ANOVA, followed by the Student-Newman-Keuls’s correction post hoc Student’s t test with SigmaStat for Windows v.3.10 (Systat Software, San Jose, CA, USA). If the data failed to follow a normal distribution, a Mann-Whitney rank sum test or an ANOVA on ranks test was performed. Survival rate (n = 15 animals for each group) was analyzed using χ2 test. A value of P < 0.05 was considered significant.
RESULTS
Age-dependent liver histologic changes
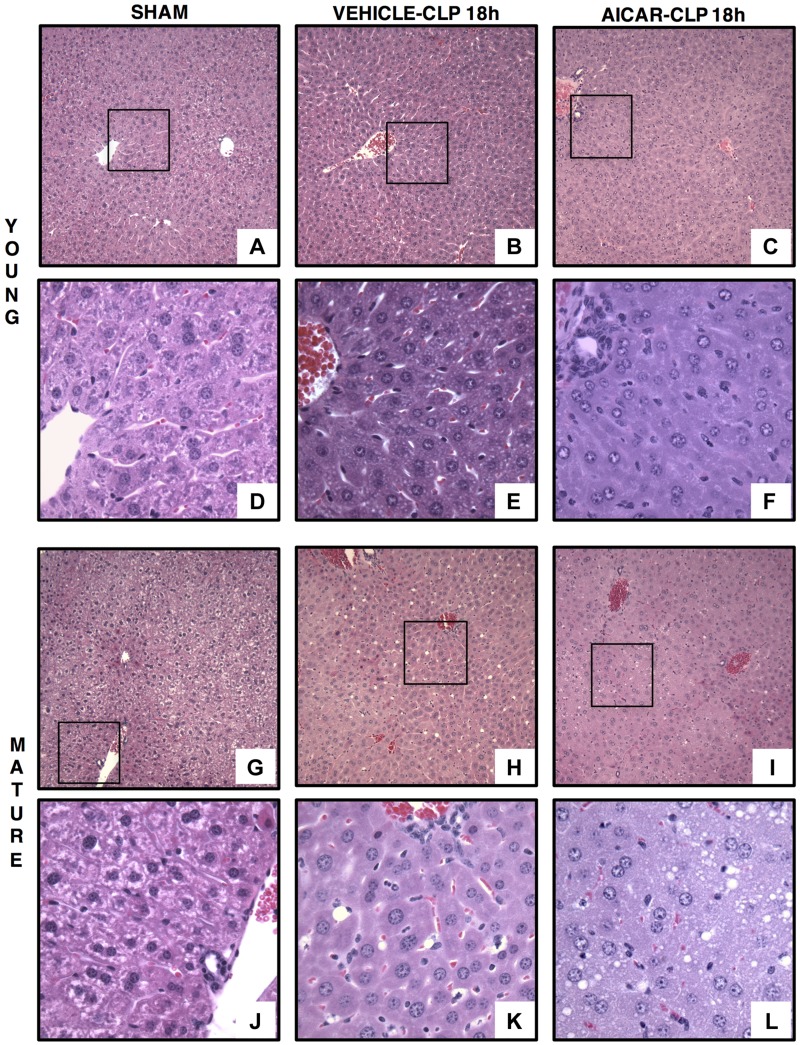
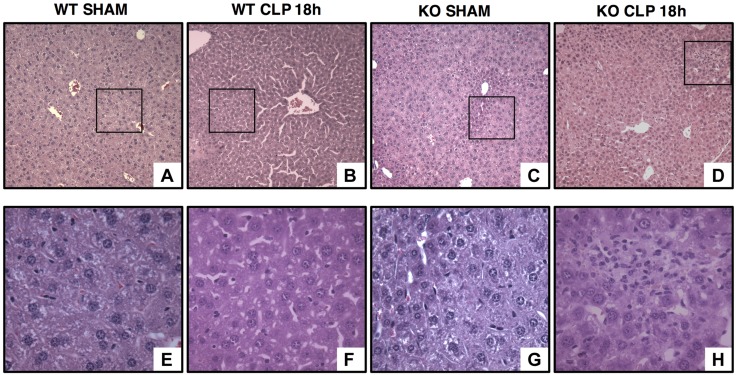
At 18 h after resuscitation, histologic analysis revealed that vehicle-treated young mice experienced very mild or no liver injury mostly characterized by intercellular edema. On the contrary, mature adult mice exhibited severe liver injury, which was characterized by infiltration of inflammatory cells, congestion of sinusoids and steatotic damage with macrovacuoles diffusely distributed and displacing the hepatocyte nucleus toward the periphery. AICAR treatment decreased liver injury in both young and mature adult mice. In AICAR-treated mice the extent of steatosis was moderate and was characterized by lipid vacuoles of smaller size when compared with vehicle treatment (Figs. 1 and 2A). To further define the biologic role of AMPK in liver injury, in additional studies we used young AMPKα1-deficient mice. In these loss-of-function studies, young AMPKα1 KO mice exhibited more severe liver injury than WT littermates (Figs. 3 and 4A).
Figure 1.
Representative histology photomicrographs of liver sections. Normal liver architecture in control young [A, D (inset)] and mature [G, J (inset)] mice. Liver damage in vehicle-treated young (B) and mature (H) mice at 18 h after CLP with edema, inflammatory cell infiltration, and lipid vacuoles [E, K (insets)]. Amelioration of liver architecture in AICAR-treated young mice [(C, F (inset)]. Amelioration of liver architecture in AICAR-treated mature mice (I) with presence of small lipid vacuoles (L, inset) (n = 4–6 different tissue sections in each experimental group showed similar patterns). Original magnification: ×100 (A–C, G–I); ×400 (D–F, J–L).
Figure 2.
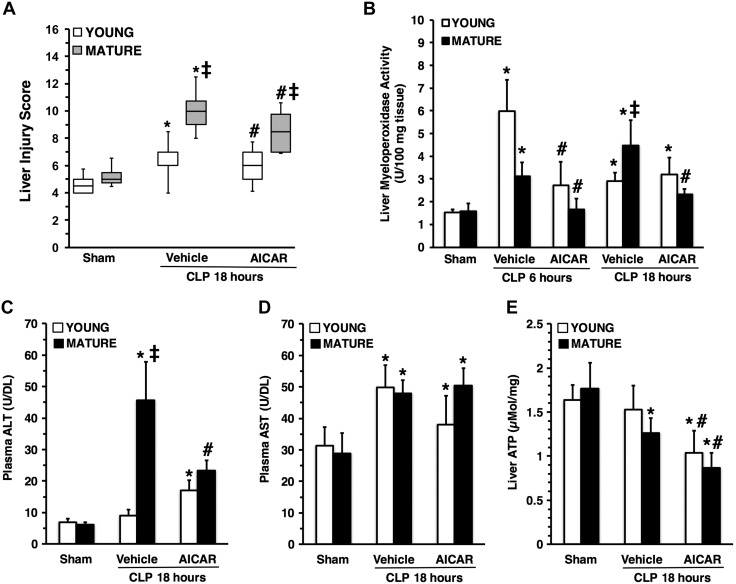
Liver injury score (A), liver myeloperoxidase activity (B), plasma ALT levels (C), plasma AST levels (D) and liver ATP content (E) in young and mature mice subjected to CLP. Data are means ± sem (n = 5–19 mice for each group). Vehicle (distilled water) or AICAR (500 mg/kg) was administered i.p. at 1 and 6 h after CLP. *P < 0.05 vs. sham-treated mice of the same age; #P < 0.05 vs. vehicle-treated group of the same age; ‡P < 0.05 vs. young group.
Figure 3.
Representative histology photomicrographs of liver sections of young AMPKα1 WT and KO mice. Normal liver architecture in young WT (A, inset shown in E) and KO mice (C, inset shown in G). Liver damage in young WT (B) and KO (D) mice after sepsis with edema, necrosis, and inflammatory cell infiltration [F, H (insets)] (n = 4 different tissue sections in each experimental group showing a similar pattern). Original magnification: ×100 (A–D); ×400 (E–H).
Figure 4.
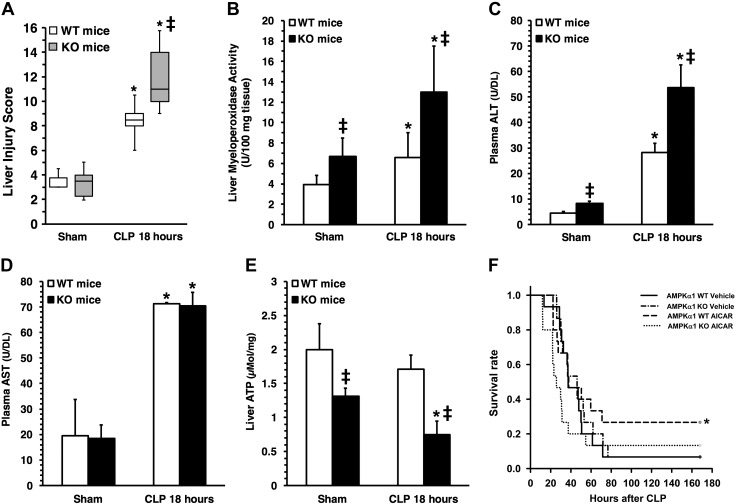
Liver injury score (A), liver MPO activity (B), plasma ALT levels (C), plasma AST levels (D), and liver ATP content (E) in young AMPKα1 WT and KO mice subjected to CLP. Data represent means ± sem (n = 5–9 mice for each group). *P < 0.05 vs. sham-treated mice; ‡P < 0.05 vs. WT mice. Survival rate of young AMPKα1 WT and KO mice subjected to CLP. Curves represent rate of 15 mice for each group. *P < 0.05 vs. vehicle-treated mice by χ2 test.
Age-dependent neutrophil infiltration in liver
To confirm the histologic findings of liver neutrophil infiltration we measured the activity of MPO, a neutrophil lysosomal enzyme. Vehicle-treated young and mature mice exhibited a similar increase in MPO activity in the liver at the early phase of sepsis (at 6 h after CLP) when compared to their age-matched control mice. However, neutrophil infiltration persisted at a higher level in vehicle-treated mature mice than in young mice, where the cell influx subsided at 18 h, probably reflecting an inability of mature mice to turn off the inflammatory process. Treatment with AICAR reduced MPO activity in both young and mature mice when compared with vehicle treatment (Fig. 2B). In loss-of-function studies, MPO activity was significantly higher in young AMPKα1 KO mice than in WT mice both in basal conditions and after sepsis (Fig. 4B).
Age-dependent liver injury
To further quantify liver injury, we measured plasma levels of ALT and AST. Levels of ALT were remarkably higher in vehicle-treated mature mice than young mice at 18 h after CLP. Treatment with AICAR reduced ALT levels in mature mice when compared to vehicle treatment (Fig. 2C). Elevation of AST levels was similar between the 2 age groups after CLP and was not affected by AICAR treatment (Fig. 2D). In loss-of-function studies, ALT levels were significantly higher in young AMPKα1 KO mice than in WT mice, in both basal conditions and after sepsis (Fig. 4C), whereas AST levels were similar between the 2 genotypes (Fig. 4D).
Age-dependent changes of liver ATP and AMPKα1-dependent effects of AICAR on survival
To determine whether sepsis affects energy homeostasis, we measured liver content of ATP. Basal levels of ATP were similar in young and mature control mice (Fig. 2E). At 18 h after CLP, ATP levels significantly decreased in vehicle-treated mature, but not young mice, when compared to baseline levels. Treatment with AICAR further reduced ATP levels in both age groups when compared with vehicle treatment (Fig. 2E). In loss-of-function studies, young AMPKα1 KO mice had lower ATP levels than WT in basal conditions. Young AMPKα1 KO, but not WT mice, experienced a significant decrease in ATP levels after sepsis when compared to sham-treated animals (Fig. 4E). To confirm the protective role of AMPKα1 in sepsis, we performed additional survival studies. In vehicle-treated groups, survival rate and time were similar in AMPKα1 WT and KO mice after sepsis. Treatment with AICAR improved the survival rate in AMPKα1 WT, but not in KO, mice (Fig. 4F).
Age-dependent changes in mitochondria gene expression profile in liver during sepsis
Because mitochondria are the main organelles for energy production through oxidative phosphorylation and they maintain cell viability by regulation of apoptotic proteins, we hypothesized that age-dependent liver injury after sepsis correlates with alteration of transcription associated with the mitochondrial quality program. Expression of 84 selected genes for mitochondria structure and function was evaluated at 3 h after CLP (Table 1). When compared to age-matched control mice, vehicle-treated young mice subjected to sepsis exhibited a >2-fold increase in genes involved with antioxidative (Sod2) and antiapoptotic defense mechanisms (Bcl2l1 and Pmaip1); on the contrary, they exhibited a marked down-regulation of several solute carriers and mitochondrial membrane translocases. Similarly, vehicle-treated mature mice exhibited down-regulation of the solute carriers Slc25a30 and Slc25a27 and the apoptotic protein Bbc3, and up-regulation of the antioxidant Sod2 gene and the antiapoptotic Bcl2l1 gene when compared to age-matched control mice. AICAR treatment increased gene expression of superoxide dismutases and transport carriers of metabolites and intermediates for ATP production, including voltage-dependent anion channels and membrane translocases in mature mice. AICAR treatment decreased gene expression in young mice when compared with vehicle treatment, suggesting restoration of transcription to basal levels.
TABLE 1.
Fold change of expression of genes for mitochondrial structure and function at 3 h after CLP
| Mice | Treatment | Gene symbol | Gene name | Function | Fold change | Regulation |
|---|---|---|---|---|---|---|
| Young | CLP+vehicle | Slc25a27 | Solute carrier family 25, member 27 | Small molecule transport | −4.7 | Down-regulated |
| Bbc3 | BCL2 binding component 3 | Proapoptosis | −3.7 | Down-regulated | ||
| Slc25a30 | Solute carrier family 25, member 30 | Small molecule transport | −3.7 | Down-regulated | ||
| Slc25a14 | Solute carrier family 25, member 14 | Small molecule transport | −3.7 | Down-regulated | ||
| Cpt2 | Carnitine palmitoyltransferase 2 | Long-chain fatty acid oxidation | −2.3 | Down-regulated | ||
| Taz | Tafazzin | Inner membrane translocation | −1.9 | Down-regulated | ||
| Tomm40l | Translocase of outer mitochondrial membrane 40 homolog-like | Outer membrane translocation | −1.8 | Down-regulated | ||
| Rhot2 | Ras homolog family member T2 | Mitochondrial localization | −1.8 | Down-regulated | ||
| Slc25a4 | Solute carrier family 25, member 4 | Small molecule transport | −1.8 | Down-regulated | ||
| Slc25a24 | Solute carrier family 25, member 24 | Small molecule transport | −1.8 | Down-regulated | ||
| Pmaip1 | Phorbol-12-myristate-13-acetate-induced protein 1 | Proapoptosis | 2.4 | Up-regulated | ||
| Sod2 | Superoxide dismutase 2 | Antioxidant | 2.9 | Up-regulated | ||
| Bcl2l1 | BCL2-like 1 | Antiapoptosis | 4.3 | Up-regulated | ||
| CLP+AICAR | Slc25a2 | Solute carrier family 25, member 2 | Small molecule transport | 1.8 | Up-regulated | |
| Bcl2l1 | BCL2-like 1 | Antiapoptosis | 2.4 | Up-regulated | ||
| Mature | CLP+vehicle | Slc25a30 | Solute carrier family 25, member 30 | Small molecule transport | −3.6 | Down-regulated |
| Slc25a27 | Solute carrier family 25, member 27 | Small molecule transport | −2.4 | Down-regulated | ||
| Bbc3 | BCL2 binding component 3 | Proapoptosis | −2.4 | Down-regulated | ||
| Sod2 | Superoxide dismutase 2 | Antioxidant | 2.2 | Up-regulated | ||
| Bcl2l1 | BCL2-like 1 | Antiapoptosis | 2.9 | Up-regulated | ||
| CLP+AICAR | Sod1 | Superoxide dismutase 1 | Antioxidant | 1.5 | Up-regulated | |
| Timm23 | Translocase of inner mitochondrial membrane 23 | Inner membrane translocation | 1.6 | Up-regulated | ||
| Hsp90aa1 | Heat shock protein 90, α, class A member 1 | Chaperone | 1.6 | Up-regulated | ||
| Timm10b | Translocase of inner mitochondrial membrane 10B | Inner membrane translocation | 1.7 | Up-regulated | ||
| Slc25a37 | Solute carrier family 25, member 37 | Small molecule transport | 1.7 | Up-regulated | ||
| Timm8a1 | Translocase of inner mitochondrial membrane 8A1 | Inner membrane translocation | 1.8 | Up-regulated | ||
| Uxt | Ubiquitously expressed transcript | Mitochondrial localization | 2.0 | Up-regulated | ||
| Sod2 | Superoxide dismutase 2 | Antioxidant | 2.1 | Up-regulated | ||
| Slc25a25 | Solute carrier family 25, member 25 | Small molecule transport | 2.9 | Up-regulated |
Data are for young and mature mice treated with vehicle or AICAR (500 mg/kg, i.p.) vs. baseline expression of age-matched control mice. The regulation threshold was set at >1.5-fold change vs. control age-matched mice. The significance threshold was set at P < 0.05
Age-dependent changes of mitochondrial ultrastructure in liver during sepsis
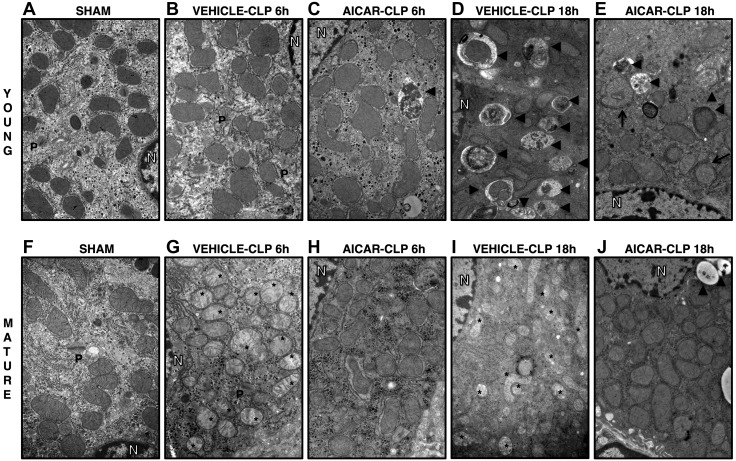
TEM analysis showed mild mitochondria damage in vehicle-treated young mice at 18 h after CLP, characterized by a few swollen mitochondria (Fig. 5). Young mice also exhibited massive autophagy with a 4-fold increase in the number of autophagosomes and autolysosomes with sequestrated materials when compared to basal sham-treatment levels. On the contrary, in vehicle-treated mature mice, marked structural damage of mitochondria was evident as early as 6 h, increased further at 18 h after CLP, and was characterized by the presence of swollen organelles with distorted cristae, translucent matrix, and disrupted membrane (Figs. 5 and 6A, B). Fewer autophagic vacuoles were observed in vehicle-treated mature mice than in young mice. In AICAR-treated groups of both ages, most mitochondria presented normal ultrastructure, and the number of damaged organelles was like age-matched sham-treatment levels. AICAR treatment increased autophagy in mature animals; whereas in young animals, it normalized autophagy to basal levels.
Figure 5.
TEM of hepatocytes in sham-treated young (A) and mature (F) mice, in vehicle-treated young (B), and mature (G) mice at 6 h after CLP, in AICAR-treated young (C) and mature (H) mice at 6 h after CLP, in vehicle-treated young (D) and mature (I) mice at 18 h after CLP, in AICAR-treated young (E) and mature (J) mice at 18 h after CLP. Asterisks, damaged mitochondria presenting translucent matrix, disrupted membrane, and cristae; arrowheads, autophagic vesicles. N, nucleus; P, peroxisomes.
Figure 6.
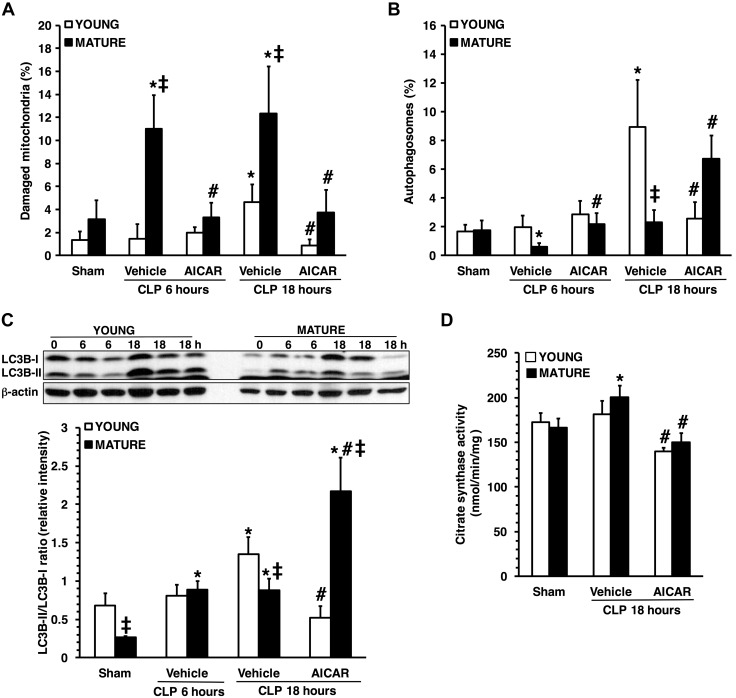
Quantification of damaged mitochondria (A) and autophagosomes (B) in hepatocytes in young and mature mice subjected to CLP. Damaged mitochondria and autophagosomes were determined by image-analysis software and expressed as a percentage of the total number of mitochondria in 9 consecutive cells. Data are means ± sem (n = 3–4 mice for each group. Western blot analysis of LC3B-I and -II and β-actin (used as the loading control) in liver cytosol extracts and image analyses of LC3B-II:LC3B-I ratio, as determined by densitometry (C). Data are means ± sem and are expressed as ratio of relative intensity units (n = 4–6 animals for each group). Citrate synthase activity in liver mitochondrial extracts (D). Data are means ± sem (n = 4–11 animals for each group). *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. vehicle-treated group of the same age; ‡P < 0.05 vs. young group.
Age-dependent impairment of liver autophagy
We quantified the autophagic process by measuring the conversion of LC3B-I to -II, a marker for autophagosome formation (19). Under basal conditions the LC3B-I:LC3B-II ratio was similar between the 2 age groups; however, there was a reduced expression of both LC3B-I and -II in livers of control mature mice when compared with those of young mice (Fig. 6C). At 18 h after sepsis, LC3B-II conversion was significantly lower in vehicle-treated mature mice than in young mice, confirming the limited number of autophagic vacuoles seen in TEM analysis. AICAR treatment increased LC3B-II conversion in mature animals; whereas in young animals, it normalized the LC3B-I:LC3B-II ratio to basal levels (Fig. 6C).
To confirm the effect of AICAR on autophagy and mitochondrial mass, we measured the amount of citrate synthase, a mitochondrial protein of the outer membrane (17). At 18 h after CLP, citrate synthase activity significantly rose above baseline levels in vehicle-treated mature mice and was higher than in young mice, suggesting an increase in mitochondrial mass, probably because of accumulation of damaged organelles. Treatment with AICAR restored citrate synthase in both age groups to baseline levels, indicating proper mitochondrial protein degradation (Fig. 6D).
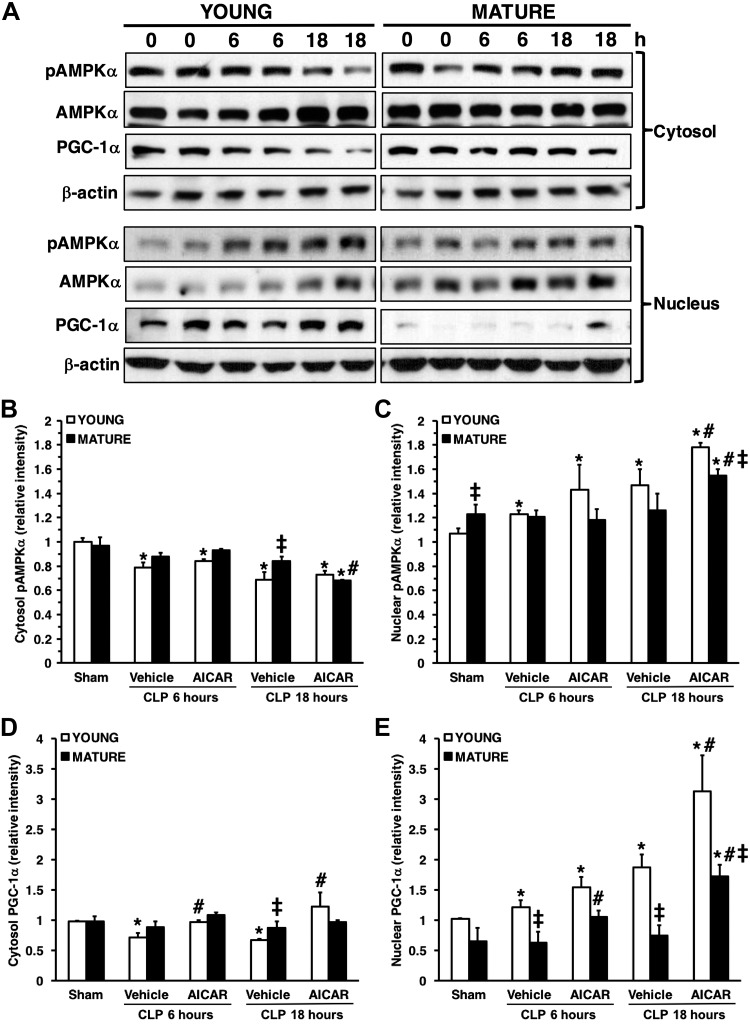
Age-dependent dysregulation of AMPKα1/α2 activation
To evaluate AMPK activity, we measured the expression of the phosphorylated form of both catalytic subunits-α1 and -α2. In basal conditions, the cytosolic pAMPKα1/α2 expression was similar in young and mature mice; however, the nuclear pAMPKα1/α2 expression of mature control mice was significantly higher than in the control young mice, suggesting a distinct age-dependent intracellular localization of the kinase. After sepsis, the cytosol content of pAMPKα1/α2 decreased in a time-dependent manner in vehicle-treated young, but not in mature mice (Fig. 7). There was an increase in nuclear pAMPKα1/α2, which was particularly high at 18 h after sepsis in young mice, but not in mature mice. AICAR treatment increased the nuclear pAMPKα1/α2 in young mice without affecting cytosolic levels when compared to vehicle treatment. AICAR treatment in mature animals markedly down-regulated levels of pAMPKα1/α2 in the cytosol and increased its nuclear translocation. However, levels of pAMPKα1/α2 of AICAR-treated mature mice were significantly lower than AICAR-treated young mice.
Figure 7.
A) Western blot analysis of pAMPKα1/α2, AMPKα1/α2, PGC-1α, and β-actin (used as the loading control) in liver cytosol and nuclear extracts. Image analyses of cytosol pAMPKα1/α2 (B), nuclear pAMPKα1/α2 (C), cytosol PGC-1α (D), and nuclear PGC-1α (E), as determined by densitometry. Data are means ± sem of 4–6 animals for each group and are expressed as relative intensity units. Expression of pAMPKα1/α2 was normalized toward total AMPKα1/α2 and β-actin. Expression of PGC-1α was normalized toward β-actin. *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. vehicle-treated group of the same age; ‡P < 0.05 vs. young group.
Age-dependent nuclear translocation of PGC-1α
To examine the differential role of AMPK cellular compartmentalization and function, we evaluated the liver expression of PGC-1α, a downstream molecule of AMPK and regulator of mitochondrial biogenesis (11) (Fig. 7). Vehicle-treated young mice exhibited a time-dependent decrease in cytosolic levels of PGC-1α that correlated with an increase in nuclear levels at 18 h after CLP. On the contrary, PGC-1α was retained in the cytosol, whereas its nuclear expression was markedly down-regulated in vehicle-treated mature animals after sepsis, suggesting an impairment of nuclear translocation. AICAR treatment increased PGC-1α nuclear expression in both age groups. However, levels of PGC1α of AICAR-treated mature mice were still significantly lower than those in AICAR-treated young mice.
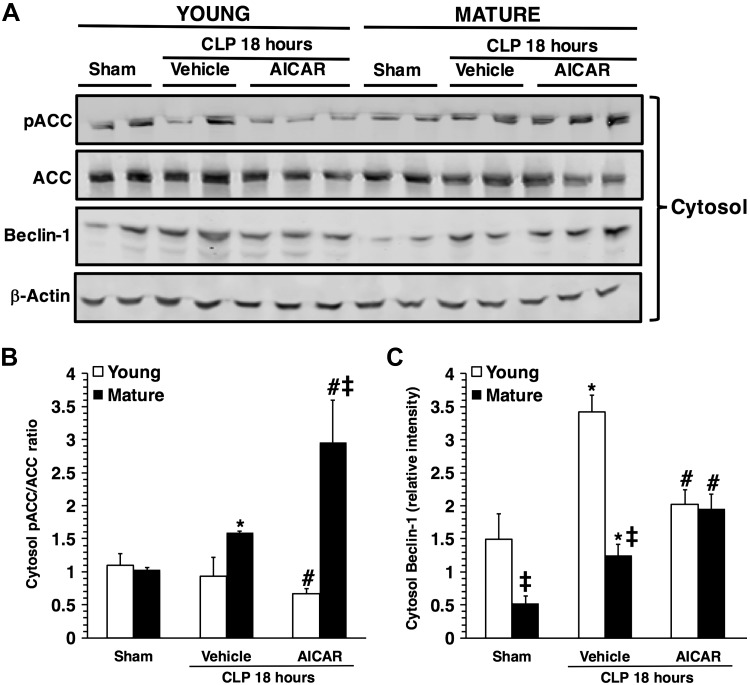
Age-dependent cytosolic phosphorylation of ACC
We then investigated the cytosolic phosphorylation of ACC, AMPK downstream molecule involved in fatty acid oxidation. Total expression of ACC was similar in both young and mature control mice in basal conditions and did not change after sepsis in vehicle-treated mice. After sepsis, cytosol content of pACC and, consequently, the pACC:ACC ratio significantly increased in vehicle-treated mature, but not in young, mice when compared to basal control levels (Fig. 8). Treatment with AICAR caused a significant reduction in total ACC content in livers of young septic mice, but only a nonsignificant trend toward a decrease in mature mice, when compared to basal levels of age-matched control mice. When the pACC:ACC ratio was analyzed, treatment with AICAR caused a significant decrease in ACC phosphorylation in young septic mice, whereas it caused a significant increase in mature mice, when compared to vehicle treatment.
Figure 8.
A) Western blot analysis of pACC, ACC, beclin-1 and β-actin (used as the loading control) in liver cytosol extracts. Image analyses of pACC/ACC ratio (B) and beclin-1 expression (C), as determined by densitometry. Data are means ± sem of 4–6 animals for each group and are expressed as relative intensity units. Expression of pACC was normalized toward total ACC and β-actin. Expression of beclin-1 was normalized toward β-actin. *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. vehicle-treated group of the same age; ‡P < 0.05 vs. young group.
Age-dependent cytosolic expression of beclin-1
Because AMPK also regulates content of the autophagy-related proteins (9), we evaluated cytosolic expression of beclin-1. At basal conditions beclin-1 expression was lower in control mature mice than young mice. After sepsis, beclin-1 significantly increased in both vehicle-treated young and mature mice when compared to age-matched control mice; however, levels of beclin-1 were significantly higher in vehicle-treated young, when compared to mature, mice (Fig. 8). Treatment with AICAR caused a significant decrease in beclin-1 expression in young septic mice, whereas it caused a significant increase in mature mice, when compared to vehicle treatment.
DISCUSSION
In this study, we demonstrated that the metabolic and inflammatory features of sepsis-induced liver injury are age dependent; mature mice exhibited an exuberant neutrophil infiltration and signs of necrosis and steatosis associated with a higher mitochondrial structural derangement. Furthermore, we identified AMPK as a crucial age-dependent regulator of hepatic mitochondrial homeostasis and a potential therapeutic target to ameliorate organ damage.
AMPK is the master regulator of diverse metabolic events to maintain energy homeostasis. The protein consists of a catalytic α-subunit and 2 regulatory β- and γ-subunits. Two isoforms have been identified of the α- and β-subunit and 3 isoforms of the γ-subunit. Both AMPKα1- and α2-containing complexes are present in the liver in equal distribution (23,–26). Phosphorylation of the catalytic α-subunits by upstream kinases is essential for AMPK activation (9). We demonstrated for the first time that phosphorylation of AMPKα1/α2 had a distinct age-dependent cellular localization during sepsis. In young mice, activation of AMPKα1/α2 was mostly confined to the nucleus and paralleled with nuclear translocation of PGC-1α, raising the possibility that AMPK regulates gene expression in response to sepsis. However, the most intriguing finding was that, with mature adult age, there was no cytosolic or nuclear activation of AMPKα1/α2 during sepsis, with consequent impairment of PGC-1α nuclear translocation. Baseline nuclear levels of AMPKα1/α2 were higher in mature mice than in younger mice. Despite this high baseline, AMPK was not responsive to sepsis. Our data are consistent with previous findings in mouse livers, which exhibited baseline elevations in pAMPK levels with age, but an absent response to hypoxia (27). It is possible, therefore, that the baseline increase in AMPK activity in aging reflects defensive metabolic processes related to age-related oxidative damage, causing an inability to respond to acute stressors. In line with this age-dependent dysregulation of AMPK activation, treatment with AICAR did not cause any further increase in AMPKα1/α2 in mature mice, but induced only a peculiar shuttling of this kinase into the nucleus from the cytosol. This age-dependent dysregulation of AMPK also explains the more severe mitochondrial damage in hepatocytes and the significant decrease in ATP production in vehicle-treated mature animals when compared to young mice. Our study, therefore, suggests that, during sepsis, AMPK is activated in the liver as a mechanism to maintain metabolic demands. Subcellular localization of AMPK could have important functional consequences by affecting nuclear substrates such as the PGC-1α, the master regulator of mitochondrial biogenesis. However, these compensatory mechanisms are lacking in aging animals. Nevertheless, our findings show that pharmacological activation of AMPK can still ameliorate liver injury in mature animals by inducing a robust gene transcription of mitochondrial structural and transport proteins, and metabolic enzymes (Fig. 9).
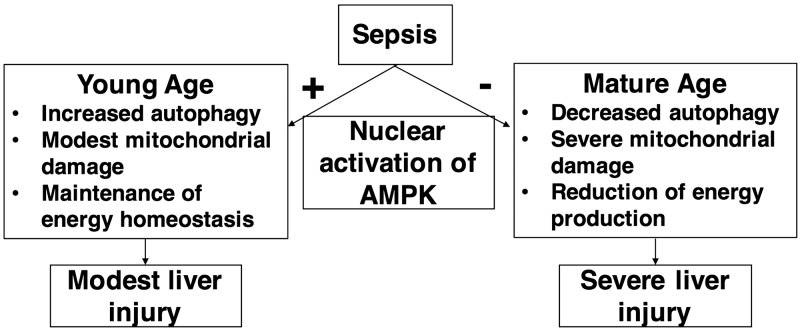
Figure 9.
A proposed overview of AMPK-dependent regulation of sepsis-induced liver injury in young and mature mice. During sepsis, AMPK is activated in the nucleus in young mice as a compensatory mechanism to maintain energy homeostasis and limit mitochondrial damage by increased autophagy. On the contrary, nuclear down-regulation of AMPK in mature mice is associated with energy failure and extensive mitochondrial damage with impaired autophagy resulting in severe liver damage.
AMPK has been shown to promote mitochondrial fatty acid oxidation by phosphorylating and thereby inhibiting ACC, which plays a pivotal role in hepatic lipid metabolism (9, 28). In our study, consistent with the nuclear activation of AMPK, there was no cytosolic phosphorylation of ACC in vehicle-treated young mice after sepsis, whereas treatment with AICAR caused a significant reduction of phosphorylation of ACC. ACC phosphorylation increased in livers of vehicle-treated mature mice and was further augmented in AICAR-treated mature mice after sepsis, but it did not correlate with cytosolic activation of AMPK. Because other protein kinases can phosphorylate ACC (29), these findings suggest that, with mature age, regulation of energy metabolism is dependent on pathways distinct from AMPK, further confirming peculiar age-dependent intracellular compartmentalization of metabolic functions.
The transaminases ALT and AST represent important links between carbohydrate and protein metabolism in the liver (30). In our study, ALT was markedly high in vehicle-treated mature, but not in young, mice after sepsis. AICAR treatment significantly reduced ALT levels in mature mice. We did not find any age-dependent differences in AST levels, which were unaffected by AICAR treatment. Because this transaminase is also important in other metabolically active organs (31), we cannot rule out whether AST levels may reflect heart and skeletal muscle injury. Nevertheless, these data further support the evidence of age-dependent metabolic disturbances in response to sepsis.
In support to the age-dependent mitochondrial damage we observed that liver ATP synthesis was significantly depressed in mature, but not young, animals after sepsis, indicating a compromise of oxidative metabolism. AICAR treatment caused a paradoxical decrease in liver ATP content in both age groups. The decline in ATP levels after acute treatment with AICAR is consistent with several reports demonstrating that the initial metabolic effect of AMPK activators is to inhibit mitochondrial respiration, thus decreasing ATP production (32, 33). Upon cellular uptake, AICAR is converted to 5-aminoimidazole-4-carboxamide ribonucleotide, which mimics the effects of AMP. This conversion utilizes ATP (13). Furthermore, in isolated mitochondria 5-aminoimidazole-4-carboxamide ribonucleotide appears to impair complex I–dependent respiration (34). Conversely, it must be noted that AMPK activation does not affect mitochondrial oxidative capacity in the short term, but stimulates mitochondrial biogenesis via PGC1-α activation (9,–11). Therefore, although we did not measure ATP levels beyond 18 h after CLP, it is possible that energy production is re-established at a later time after AICAR treatment when the mitochondrial pool is fully functioning.
The generation of KO mice with genetic deficiency in the catalytic subunit-α1 or -α2 has allowed identification of the regulatory role for AMPK in energy homeostasis. However, double-KO mice are not viable (26). To prove the hepatoprotective role of AMPK during sepsis, we also demonstrated that genetic deficiency of the α1 isoform of AMPK in young KO mice caused more severe liver damage, which correlated well with hepatic energy failure when compared to the age-matched WT littermates. Thus, these data further confirm that metabolic instability consequent to AMPK impairment is a likely contributor to sepsis-induced liver dysfunction. Moreover, our data demonstrated that activation of AMPKα1 is a requisite for the mechanism of action of AICAR, given that genetic deficiency of AMPKα1 in young KO mice abolished the beneficial effects of AICAR on survival.
AMPK induces autophagy by inhibiting the mammalian target of rapamycin pathway (9, 12) and by controlling gene expression of autophagy-related proteins (35). Autophagy is a critical step in maintaining mitochondrial quality control by selective removal of damaged organelles. Studies in liver autopsy samples of nonsurviving septic patients have shown that formation of autophagolysosomes is associated with mitochondrial injury (36). However, whether autophagy is an adaptive response or another mechanism of cell death in sepsis has not been fully explored. There is experimental evidence that disruption of autophagy is associated with cell degeneration. Using a young rat model, Chien et al. (37) demonstrated that liver autophagy occurs early during sepsis, but declines at later time points, when it was associated with liver dysfunction. In contrast, induction of autophagy affords beneficial effects in liver and kidney ischemia and reperfusion injury in young rodents (38,–40). However, the beneficial effects of autophagy activation have never been tested in adult and aging models of sepsis. In our model, mature mice exhibited an impairment of damaged mitochondria disposal in the liver as estimated by the analysis of several indices of autophagy, including expression of beclin-1, which is involved in the initial phase of the phagosome membrane formation, and LC3B-I conjugation to form LC3B-II, which is the final step of the autophagosome formation (21). In our studies, although beclin-1 expression increased after sepsis in both vehicle-treated age groups, levels of beclin-1 were significantly higher in vehicle-treated young mice than in mature mice. Treatment with AICAR further increased beclin-1 expression in mature mice, whereas it reduced it in young mice, thus supporting TEM findings of age-dependent distinct pattern of autophagosome formation and LC3B protein conjugation. Notably, presence of damaged organelles after sepsis in mature mice at TEM also corresponded with increase of citrate synthase, which is routinely used as a marker of mitochondrial density (19), thus suggesting engulfment of mitochondria. AICAR improved autophagy and caused a decrease in mitochondrial engulfment in both age groups. Taken together, our data suggest that AMPK activation improves organ metabolic recovery also through regulation of efficient removal of damaged mitochondria in hepatocytes. However, we must note that multiple types of cells, including Kupffer cells, sinusoidal endothelial cells, and stellate cells in the liver contribute to immune responses by bacterial phagocytosis and clearance (41). Furthermore, activation of Kupffer cells has been reported to contribute to oxidative stress and to generate proinflammatory cytokines, which in turn may disrupt lipid metabolism (42). Therefore, it is possible that AICAR may exert beneficial effects by activation of AMPK in Kupffer cells and other liver cell types in addition to hepatocytes; however, this has yet to be investigated.
In our study, activation of autophagy by AICAR treatment correlated with nuclear, but not cytosolic, activation of AMPK, thus suggesting that, during sepsis, the kinase is involved in autophagy control, most probably, through nuclear transcriptional changes. Additional studies are needed to further define the nuclear specific roles of AMPK on transcription factors.
In summary, our data suggest that, during sepsis, liver injury is age-dependent and is associated with a dysregulation of AMPK activation and its downstream metabolic pathways. Despite these age-dependent changes AICAR treatment exerts hepatoprotective effects by autophagy and mitochondrial quality control. Whether AMPK activation may serve as a novel therapeutic approach of sepsis in human application warrants further investigation.
ACKNOWLEDGMENTS
The authors thank Dr. Benoit Viollet (INSERM and Cochin Institute, University Paris Descartes, Paris, France) for providing AMPK-α1 WT and KO mice. This work was supported by the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant R01 GM-067202 (to B.Z.) and, in part, by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-078392 to the Digestive Research Core Center (Integrative Morphology Core). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- AICAR
5-amino-4-imidazolecarboxamide riboside-1-β-d-ribofuranoside
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CLP
cecal ligation and puncture
- KO
knockout
- LC3B
light-chain 3B
- MODS
multiple organ dysfunction syndrome
- MPO
myeloperoxidase
- PGC-1α
peroxisome proliferator-activated receptor-γ co-activator α
- TEM
transmission electron microscopy
- WT
wild type
AUTHOR CONTRIBUTIONS
Y. Inata, S. Kikuchi, and B. Zingarelli designed the research; P. W. Hake, J. R. Ledford, and V. Wolfe maintained the animal colony; Y. Inata, S. Kikuchi, R. S. Samraj, P. W. Hake, M. O’Connor, J. R. Ledford, J. O’Connor, P. Lahni, V. Wolfe, G. Piraino, and B. Zingarelli performed the research; Y. Inata, S. Kikuchi, R. S. Samraj, G. Piraino, and B. Zingarelli analyzed the data; and Y. Inata, G. Piraino, and B. Zingarelli wrote the manuscript.
REFERENCES
- 1.Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., Rubenfeld G. D., van der Poll T., Vincent J. L., Angus D. C. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt E. B., Eldadah B., Nayfield S., Hadley E., Angus D. C. (2010) Toward an integrated research agenda for critical illness in aging. Am. J. Respir. Crit. Care Med. 182, 995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GenIMS Investigators (2010) The effects of age on inflammatory and coagulation-fibrinolysis response in patients hospitalized for pneumonia. PLoS One 5, e13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin G. S., Mannino D. M., Moss M. (2006) The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 34, 15–21 [DOI] [PubMed] [Google Scholar]
- 6.Vincent J. L., Nelson D. R., Williams M. D. (2011) Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit. Care Med. 39, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 7.Donnino M. W., Cocchi M. N., Salciccioli J. D., Kim D., Naini A. B., Buettner C., Akuthota P. (2011) Coenzyme Q10 levels are low and may be associated with the inflammatory cascade in septic shock. Crit. Care 15, R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brealey D., Brand M., Hargreaves I., Heales S., Land J., Smolenski R., Davies N. A., Cooper C. E., Singer M. (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360, 219–223 [DOI] [PubMed] [Google Scholar]
- 9.Steinberg G. R., Kemp B. E. (2009) AMPK in health and disease. Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 10.Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 104, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohas L. M., St-Pierre J., Uldry M., Jäger S., Handschin C., Spiegelman B. M. (2007) A fundamental system of cellular energy homeostasis regulated by PGC-1α. Proc. Natl. Acad. Sci. USA 104, 7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson A. B., Gottlieb R. A. (2008) Recycle or die: the role of autophagy in cardioprotection. J. Mol. Cell. Cardiol. 44, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. (1995) 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen S. B., Viollet B., Andreelli F., Frøsig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. (2004) Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 279, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 15.Angele M. K., Pratschke S., Hubbard W. J., Chaudry I. H. (2014) Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botez G., Piraino G., Hake P. W., Ledford J. R., O’Connor M., Cook J. A., Zingarelli B. (2015) Age-dependent therapeutic effects of liver X receptor-α activation in murine polymicrobial sepsis. Innate Immun. 21, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullane K. M., Kraemer R., Smith B. (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 14, 157–167 [DOI] [PubMed] [Google Scholar]
- 19.Kramer K. A., Oglesbee D., Hartman S. J., Huey J., Anderson B., Magera M. J., Matern D., Rinaldo P., Robinson B. H., Cameron J. M., Hahn S. H. (2005) Automated spectrophotometric analysis of mitochondrial respiratory chain complex enzyme activities in cultured skin fibroblasts. Clin. Chem. 51, 2110–2116 [DOI] [PubMed] [Google Scholar]
- 20.Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth S., Glick D., Macleod K. F. (2010) Autophagy: assays and artifacts. J. Pathol. 221, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettinger B. T., Gilbert D. M., Amberg D. C. (2004) Actin up in the nucleus. Nat. Rev. Mol. Cell Biol. 5, 410–415 [DOI] [PubMed] [Google Scholar]
- 23.Stapleton D., Mitchelhill K. I., Gao G., Widmer J., Michell B. J., Teh T., House C. M., Fernandez C. S., Cox T., Witters L. A., Kemp B. E. (1996) Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 271, 611–614 [DOI] [PubMed] [Google Scholar]
- 24.Stapleton D., Woollatt E., Mitchelhill K. I., Nicholl J. K., Fernandez C. S., Michell B. J., Witters L. A., Power D. A., Sutherland G. R., Kemp B. E. (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett. 409, 452–456 [DOI] [PubMed] [Google Scholar]
- 25.Cheung P. C., Salt I. P., Davies S. P., Hardie D. G., Carling D. (2000) Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 346, 659–669 [PMC free article] [PubMed] [Google Scholar]
- 26.Viollet B., Athea Y., Mounier R., Guigas B., Zarrinpashneh E., Horman S., Lantier L., Hebrard S., Devin-Leclerc J., Beauloye C., Foretz M., Andreelli F., Ventura-Clapier R., Bertrand L. (2009) AMPK: lessons from transgenic and knockout animals. Front. Biosci. (Landmark Ed.) 14, 19–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulligan J. D., Gonzalez A. A., Kumar R., Davis A. J., Saupe K. W. (2005) Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J. Gerontol. A Biol. Sci. Med. Sci. 60, 21–27 [DOI] [PubMed] [Google Scholar]
- 28.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. (2006) Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol. 574, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownsey R. W., Boone A. N., Elliott J. E., Kulpa J. E., Lee W. M. (2006) Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 34, 223–227 [DOI] [PubMed] [Google Scholar]
- 30.Botros M., Sikaris K. A. (2013) The de ritis ratio: the test of time. Clin. Biochem. Rev. 34, 117–130 [PMC free article] [PubMed] [Google Scholar]
- 31.Collier J., Bassendine M. (2002) How to respond to abnormal liver function tests. Clin. Med. (Lond.) 2, 406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen M. R., Doran E., Halestrap A. P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 [PMC free article] [PubMed] [Google Scholar]
- 33.El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 [DOI] [PubMed] [Google Scholar]
- 34.Guigas B., Taleux N., Foretz M., Detaille D., Andreelli F., Viollet B., Hue L. (2007) AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem. J. 404, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihaylova M. M., Shaw R. J. (2011) The AMPK signaling pathway coordinates cell growth, autophagy, and metabolism. Nat. Cell Biol. 13, 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe E., Muenzer J. T., Hawkins W. G., Davis C. G., Dixon D. J., McDunn J. E., Brackett D. J., Lerner M. R., Swanson P. E., Hotchkiss R. S. (2009) Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab. Invest. 89, 549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chien W. S., Chen Y. H., Chiang P. C., Hsiao H. W., Chuang S. M., Lue S. I., Hsu C. (2011) Suppression of autophagy in rat liver at late stage of polymicrobial sepsis. Shock 35, 506–511 [DOI] [PubMed] [Google Scholar]
- 38.Liu A., Fang H., Dahmen U., Dirsch O. (2013) Chronic lithium treatment protects against liver ischemia/reperfusion injury in rats. Liver Transpl. 19, 762–772 [DOI] [PubMed] [Google Scholar]
- 39.Sun K., Xie X., Liu Y., Han Z., Zhao X., Cai N., Zhang S., Song J., Wei L. (2013) Autophagy lessens ischemic liver injury by reducing oxidative damage. Cell Biosci. 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parajuli N., MacMillan-Crow L. A. (2013) Role of reduced manganese superoxide dismutase in ischemia-reperfusion injury: a possible trigger for autophagy and mitochondrial biogenesis? Am. J. Physiol. Renal Physiol. 304, F257–F267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Protzer U., Maini M. K., Knolle P. A. (2012) Living in the liver: hepatic infections. Nat. Rev. Immunol. 12, 201–213 [DOI] [PubMed] [Google Scholar]
- 42.Nguyen-Lefebvre A. T., Horuzsko A. (2015) Kupffer cell metabolism and function. J. Enzymol. Metab. 1, 101 [PMC free article] [PubMed] [Google Scholar]