Abstract
Group 2 innate lymphoid cells (ILC2s) represent a rapid source of type 2 cytokines, such as IL-5 and IL-13, and play an important role in orchestrating type 2 immune response. Adenosine is an endogenous purine nucleoside, a catabolite of ATP that binds and activates ≥1 of 4 transmembrane G protein–coupled cell-surface adenosine receptors (ARs)—A1, A2A, A2B, and A3. Here, we studied the role of ARs in the regulation of cytokine production by ILC2s. We found that A2BARs suppress the production of both IL-5 and IL-13 by ILC2s, whereas A2AARs augment IL-5 production and fail to affect IL-13 release. Combined stimulation of all ARs led to the suppression of both IL-5 and IL-13 production, which indicated that A2BARs dominate A2AARs. Both pre- and post-transcriptional processes may be involved in the AR modulation of ILC2 IL-5 and IL-13 production. Thus, we identify adenosine as a novel negative regulator of ILC2 activation.—Csóka, B., Németh, Z. H., Duerr, C. U., Fritz, J. H., Pacher, P., Haskó, G. Adenosine receptors differentially regulate type 2 cytokine production by IL-33–activated bone marrow cells, ILC2s, and macrophages.
Keywords: A2AAR, A2BAR, ILC2s, helminth infection, allergic inflammation
Innate lymphoid cells (ILCs) are a subset of innate immune cells that are characterized by their lack of specific antigen receptors and cell lineage markers (1). ILCs are present at mucosal and barrier surfaces in smaller numbers than other tissue-resident lymphocytes, yet they produce large amounts of cytokines early upon stimulation (1). Among ILCs, group 2 ILCs (ILC2s) respond to epithelial or endothelial cell-derived cytokines, such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), by proliferating and producing copious amounts of type 2 cytokines, such as IL-5, IL-6, IL-9, IL-13, and amphiregulin, which mediate the effector pathways that are vital for antihelminth immune responses and the development of allergic inflammation (2). In contrast to ILC2-activating epithelial- or endothelial-derived cytokines, other cytokines, including type I and type II IFNs (3,–6), IL-27 (3, 4, 7) and the TNF family cytokine TL1A (8, 9), that are produced by the innate and adaptive immune systems can suppress ILC2 responses. In addition to cytokines, cell-surface molecules, such as inducible T-cell costimulator (ICOS) (5, 10) and programmed death-1 (11), eicosanoids, including leukotrienes (12) and prostaglandin D2 (13), and hormones, including androgens (14) and corticosteroids (15), have all been shown to regulate ILC2s.
The purine nucleoside, adenosine, is present in the extracellular space of tissues at low concentrations under normal conditions, but its levels increase dramatically in response to various forms of stress, damage, hypoxia, and pathologic conditions, including inflammation (16,–19). Extracellular adenosine has been shown to regulate a wide range of physiologic processes via activation of 4 GPCRs—the A1, A2A, A2B, and A3 adenosine receptors (ARs) (16, 17). ARs are found on virtually all cell types that are involved in both innate and adaptive immunity, where they regulate a vast array of cell functions, including proliferation, cytokine production, costimulation, and pathogen killing (16, 17). In the innate immune system, AR activation has been shown to decrease the production of proinflammatory cytokines by macrophages and dendritic cells as well as to promote alternative macrophage activation (20,–26). ARs can also modulate adaptive immunity (27, 28) in which they inhibit T helper (Th)1 and Th2 cell development and promote Th17 and regulatory T (Treg) cell development and function (29,–32).
Effects of AR activation on ILC function are incompletely understood. A2ARs suppress NK cell (group 1 ILCs) function by interfering with the process of granule exocytosis and by reducing the ability of NK cells to adhere to neoplastic cells as well as reducing cytokine production (33, 34); however, it is unknown whether ARs can regulate ILC2 function. In this study, we report that ILC2s express ARs and that adenosine decreases the release of IL-5 and IL-13 by activated ILC2s, effects that are mainly a result of A2BAR activation.
MATERIALS AND METHODS
Mice
C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA, USA). A2AAR−/− and A2BAR−/− mice (C57BL/6J background) (21) were bred and maintained in the specific pathogen–free animal facility in the Comparative Medicine Resources Center at the New Jersey Medical School (Newark, NJ, USA). Adult age-matched male mice were used for all experiments. These studies were performed in accordance with the guidelines for laboratory animal research outlined by the Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee at New Jersey Medical School.
Reagents
IL-2, IL-7, IL-25, and TSLP were purchased from PeproTech (Rocky Hill, NJ, USA). IL-33 was purchased from PeproTech or R&D Systems (Minneapolis, MN, USA). The selective A1AR agonist, 2-chloro-N6-cyclopentyladenosine; A2AAR agonist, 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid (CGS21680); A2BAR agonist, 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide (BAY606583); A3AR agonist, 1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl-β-d-ribofuranuronamide; and nonselective AR agonist, 1-(6-smino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide (NECA) were purchased from Tocris Cookson (Ellisville, MO, USA).
Isolation of cells from mouse tissue
Bone marrow cells were isolated from the femur and tibia by flushing the marrow with RPMI 1640 wash media with a 26-G5/8 needle attached to a 5-ml syringe. Marrow was filtered through a 70-µm cell strainer and pooled, after which red blood cells were lysed with red blood cell buffer (Sigma-Aldrich, St. Louis, MO, USA) (3). After washing, isolated cells were used for cell sorting or plated in flat-bottomed 96- or 6-well plates and cultured in RPMI 1640 medium that was supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 5% penicillin-streptomycin solution. For the isolation of cells from lung tissue, lungs were perfused with 10 ml PBS, then removed, cut, and digested with 0.5 mg/L collagenase IV (Thermo Fisher Scientific, Waltham, MA, USA) and 0.02 mg/ml DNase I (Roche, Basel, Switzerland) in PBS for 45 min at 37°C. After digestion, tissue was homogenized with a 10-ml syringe with an 18-G11/2 needle, filtered with a 70-µm cell strainer, and washed with fluorescence-activated cell sorting (FACS) buffer (2% FBS in PBS). Isolated cells were stained for cell sorting or analysis by flow cytometry.
Isolation and differentiation of bone marrow–derived macrophages
Bone marrow–derived macrophages (BMDMs) were grown in DMEM that was supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1.5 mg/ml sodium bicarbonate in a humidified atmosphere of 95% air and 5% CO2. BMDMs were isolated from femurs and tibias of wild type, A2AAR, or A2BAR knockout mice as previously described (35). In brief, bone marrows were triturated with a 26-G5/8 needle and the resultant cell suspension was passed through a 70-μm nylon mesh cell strainer in DMEM that contained 50 ng/ml M-CSF (PeproTech). Cells were then cultured for 7 d, and culture medium was changed at d 3. Thereafter, BMDMs were scraped in ice-cold 0.1% EDTA-PBS solution and cells were counted and placed in cell culture plates.
Staining of cell suspensions
Cells were isolated as previously described and nonspecific binding to FcRs was blocked with low endotoxin, azide-free (LEAF)-purified CD16/32 Ab (BioLegend, San Diego, CA, USA) according to manufacturer instructions. Surface staining of cells was performed in FACS buffer (2% FBS in PBS) for 30 min on ice, and a Live/Dead Fixable Violet Dead Cell Stain Kit (Thermo Fisher Scientific) was used to identify dead cells, according to the manufacturer’s instructions. For intracellular IL-5 and IL-13 staining, cells were fixed by using a Foxp3 staining buffer set (Thermo Fisher Scientific) according to the manufacturer’s instructions. For ILC2 sorting, cells were stained for lineage markers [CD3ε (145-2C11), CD5 (53-7.3), CD11b (M1/70), CD11c (N418), CD19 (eBio1D3), NK1.1 (PK136), TCR γδ (eBioGL3), and B220 (RA3-6B2)] and further stained for CD45 (30-F11), CD25 (PC61.5), and CD127 (SB/199). After lymphocyte and singlet gates, ILC2s were sorted as Lin−CD45+CD25+CD127+ cells by using a BD FACSAria II SORP cell sorter (BD, Brea, CA, USA).
Expansion of bone marrow ILC2s
Bone marrow ILC2s were isolated and sorted similar to a previously described method (3) (Supplemental Fig. 1). Sorted cells were plated in round-bottomed 96-well plates in RPMI 1640 medium that was supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin, and 80 µM 2-ME. Expansion was induced by incubating cells with IL-2, IL-7, IL-25, IL-33 (each at 50 ng/ml), and TSLP (20 ng/ml). After 15 d of expansion, cells were rested for 3 d in complete medium that contained IL-2 and IL-7 (10 ng/ml) before being used.
Cytokine quantification
IL-5 and IL-13 were quantified from the supernatants of cells (2.5 × 105) by using DuoSet ELISA kits from R&D Systems.
Real-time PCR
Total RNA was isolated from cultured cells by using Direct-zol RNA MicroPrep (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Primer sequences for quantitative RT-PCR were as follows: IL-5: (forward) 5′-TGAGACGATGAGGCTTCCTG-3′, (reverse) 5′-CCACACTTCTCTTTTTGGCGG-3′; and IL-13: forward: 5′-CCCTCAGCCATGAAATAACT-3′, reverse: 5′-GCGTAACAGGCCATTCTTCC-3′. RT-PCR reactions were carried out by using Power SYBR Green Master Mix (Applied Biosystems) in an Applied Biosystems 7500 Real-Time PCR system. Results were normalized to constitutive rRNA values.
RESULTS
Effect of AR activation on ILC2 cytokine production in bone marrow cultures
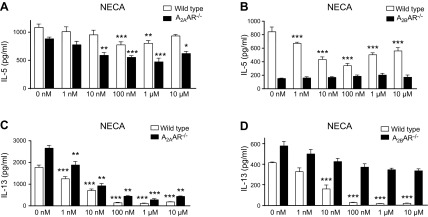
The combination of IL-7 and IL-33 triggers IL-5 and IL-13 secretion from bone marrow cultures, where ILC2 is the sole source of both IL-5 and IL-13 (3, 36); therefore, we first used bone marrow cell cultures to study the effect of adenosine signaling on cytokine production by ILC2s, as this system more accurately reflects ILC2 function in the natural environment of ILC2s compared with isolated cells. As expected on the basis of previous studies by our group (3, 36), bone marrow cell cultures released large amounts of IL-5 and IL-13 after stimulation with IL-7 and IL-33 (data not shown). In studies that investigated the effect of AR activation in cellular systems, the nonselective, stable AR agonist, NECA, is often used as a first approach, as it activates all 4 ARs—similar to the endogenous agonist, adenosine—but unlike adenosine, it is not degraded by adenosine deaminase, which is present in the medium (21). Our data indicated that NECA decreased IL-5 production in a biphasic manner, where NECA had a more pronounced effect at lower concentrations and a less powerful effect at higher concentrations (Fig. 1A, B). In contrast, NECA strongly suppressed IL-13 production, and this suppression at NECA concentrations >100 nM was almost complete (Fig. 1C, D).
Figure 1.
Effect of AR stimulation on IL-7 + IL-33–stimulated IL-5 and IL-13 production in bone marrow. Bone marrow cells from wild-type, A2AAR−/− (A, C), and A2BAR−/− (B, D) mice were cultured in medium that was supplemented with 10 ng/ml IL-7 and IL-33 and incubated with increasing concentrations of NECA for 48 h. IL-5 and IL-13 levels were measured in the supernatant by ELISA. Graph bars denote means ± sem calculated from 6 wells (2.5 × 105 cells/well) and are representative of 2–4 separate experiments (1-way ANOVA followed by Tukey’s multiple comparison test). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with IL-7 + IL-33 stimulation).
A2AARs and A2BARs differentially regulate IL-5 and IL-13 production
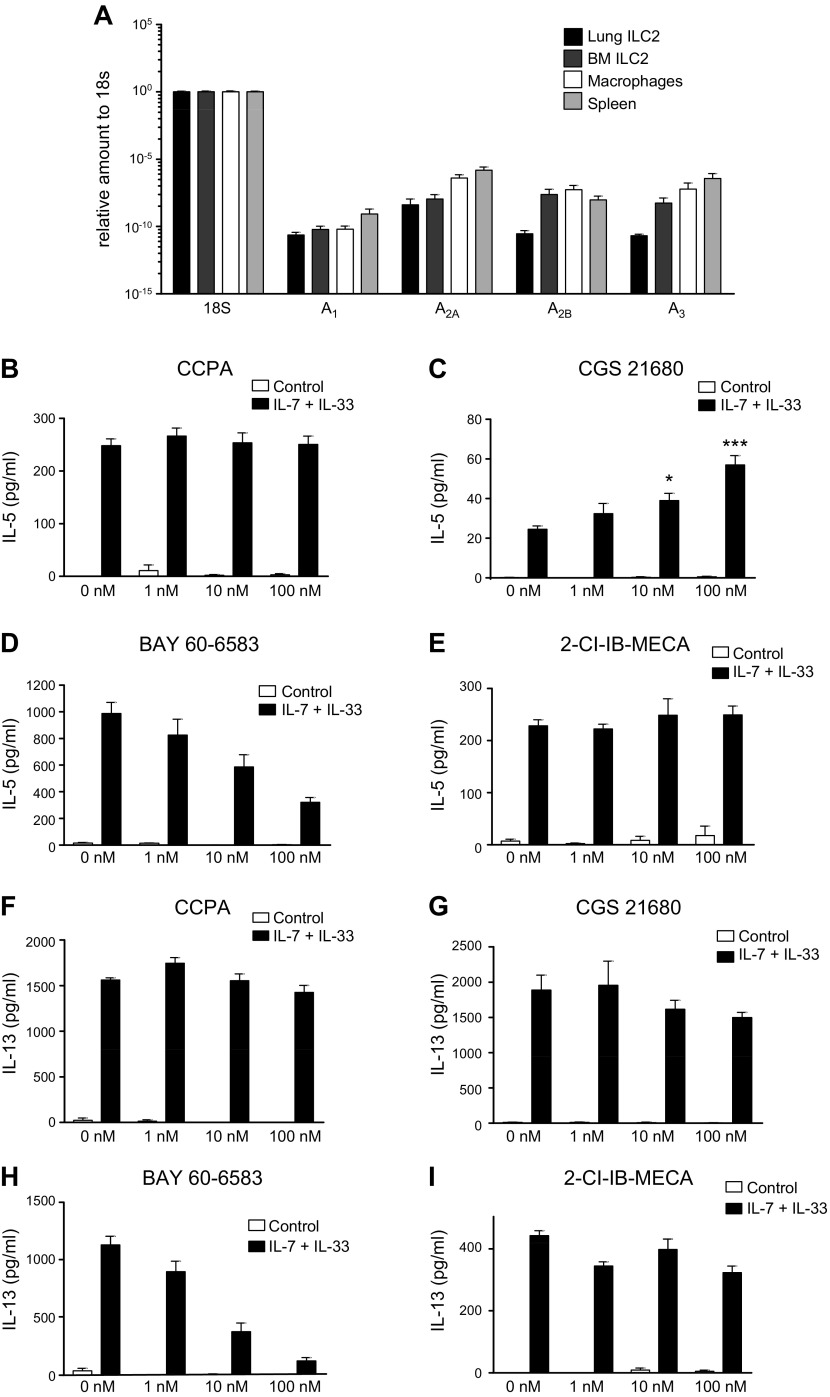
We sorted naive ILC2s from both lung and bone marrow of mice (Supplemental Fig. 1) and analyzed the expression of A1AR, A2AAR, A2BAR, and A3AR. As shown in Fig. 2A, ILC2s expressed all 4 ARs.
Figure 2.
A) AR expression levels in purified ILC2 cells. mRNA from purified lung and bone marrow (BM) ILC2s from C57/BL6 mice were isolated alongside macrophages and total spleen mRNA for comparison. Expression levels were measured by RT-PCR. Graph bars denote means ± sem calculated from 3–6 independent experiments. B–I) Effect of selective AR agonists on IL-7 + IL-33–stimulated IL-5 and IL-13 production in BM. BM cells were cultured in medium only or medium that was supplemented with 10 ng/ml IL-7 and IL-33, and were incubated with a selective agonist for each AR—2-chloro-N6-cyclopentyladenosine (CCPA; A1AR), CGS21680 (A2AAR), BAY 60-6583 (A2BAR), and 2-Cl-1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl-β-D-ribofuranuronamide (IB-MECA; A3AR)—for 48 h. IL-5 (B–E) and IL-13 (F–I) levels were measured in the supernatant by ELISA. Graph bars denote means ± sem calculated from 6 wells and are representative of 2–3 independent experiments (1-way ANOVA followed by Tukey’s multiple comparison test). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with IL-7 + IL-33 stimulation).
As A2AAR and A2BAR are the major receptors that regulate the immune system (16, 20), we next asked which of these two receptors mediated the NECA decrease of IL-5 and IL-13 by stimulated bone marrow cell cultures. Of interest, NECA not only maintained its inhibitory effect on IL-5 production in A2AAR−/− bone marrow cells, but its suppressive effect was even more pronounced in A2AAR−/− cells than in wild-type (C57BL/6J) cells (Fig. 1A). In contrast, NECA failed to inhibit IL-5 production by A2BAR−/− cells (Fig. 1B). In addition, NECA maintained its inhibitory effect on IL-13 production by A2AAR cells (Fig. 1C), but failed to suppress IL-13 production by cells from A2BAR−/− mice (Fig. 1D).
We used selective agonists of various ARs to further study the receptors that are involved. 2-Chloro-N6-cyclopentyladenosine and 1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl-β-d-ribofuranuronamide failed to affect cytokine production, excluding roles for A1ARs and A3ARs, respectively (Fig. 2B, E, F, I). CGS21680 increased and BAY60-6583 decreased IL-5 production (Fig. 2C, D). CGS21680 failed to affect IL-13 production, whereas BAY60-6583 strongly suppressed it (Fig. 2G, H).
Altogether, these data indicate that whereas A2AARs increase and A2BARs decrease IL-5 production, A2BARs decrease IL-13 production.
AR activation differentially regulates ILC2 cytokine mRNA levels in bone marrow cultures
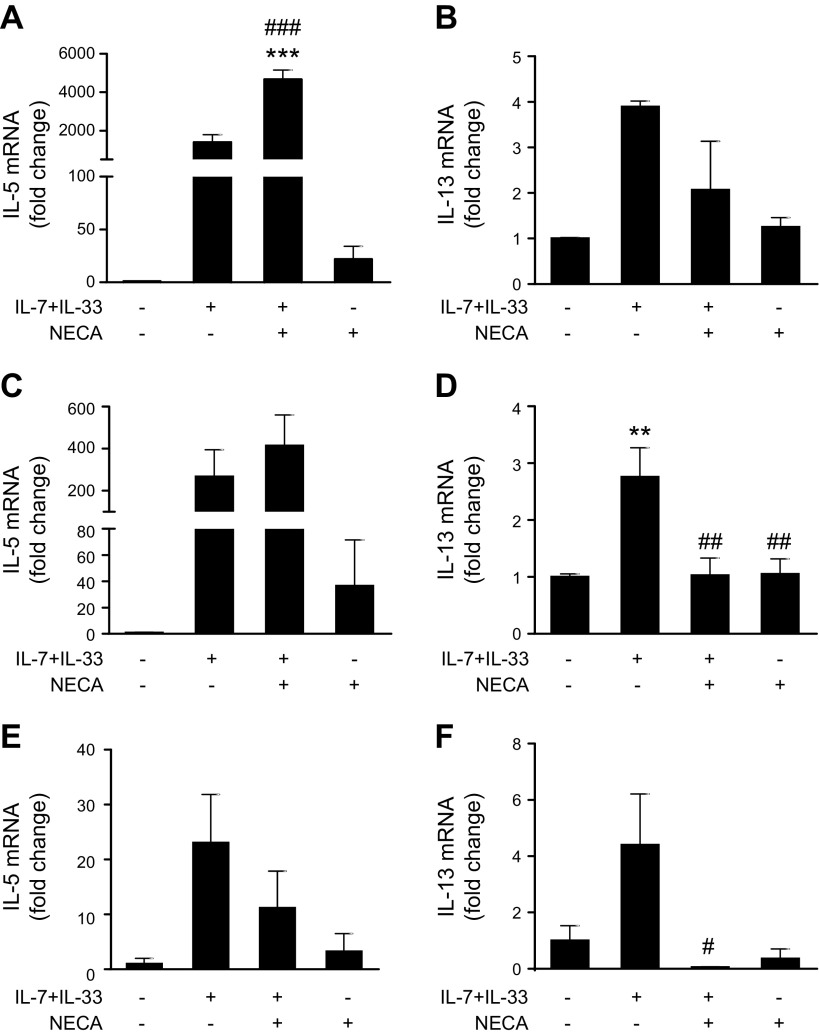
Stimulation with IL-7 and IL-33 increased the mRNA level of both IL-5 and IL-13 (Fig. 3). NECA increased IL-5 mRNA at the early 6-h (Fig. 3A) time point and had no effect later (Fig. 3C, E). In contrast, NECA decreased IL-13 mRNA at 24 h (Fig. 3D) and 48 h (Fig. 3E).
Figure 3.
Effect of AR stimulation on IL-7 + IL-33–stimulated IL-5 and IL-13 mRNA in bone marrow. Bone marrow cells were cultured in media only or media supplemented with 10 ng/ml IL-7 and IL-33, and were incubated with 1 μM NECA for 6 h (A, B), 24 h (C, D), and 48 h (E, F). Expression levels of IL-5 and IL-13 were measured by quantitative RT-PCR. Graph bars denote means ± sem calculated from 3 wells representative of 3 independent experiments (1-way ANOVA followed by Tukey’s multiple comparison test). **P < 0.01, ***P < 0.001 (compared with control conditions); #P < 0.05, ##P < 0.01, ###P < 0.001 (compared with IL-7 + IL-33 stimulation).
Effect of AR activation on secreted and intracellular IL-5 and IL-13 in purified ILC2s isolated from bone marrow
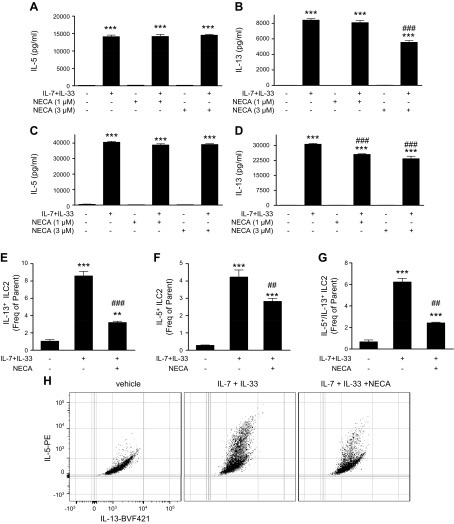
To further study the effect of AR stimulation on ILC2 cytokine production, we isolated pure bone marrow ILC2s and expanded them in vitro. These purified ILC2s increased their IL-5 and IL-13 secretion upon stimulation with IL-7 and IL-33 (Fig. 4). NECA had no effect on secreted IL-5 by purified, stimulated ILC2s (Fig. 4A, C), but induced a significant decrease of IL-13 secretion (Fig. 4B, D). In addition, NECA decreased both intracellular IL-5 and IL-13, where NECA more effectively decreased IL-13 than IL-5 (Fig. 4E–H). The overall proportion of ILC2s that produce both IL-5 and IL-13 was also reduced by NECA (Fig. 4G, H).
Figure 4.
Effect of AR activation on IL-7 + IL-33–stimulated cytokine production by purified bone marrow ILC2s. A–D) Secreted IL-5 and IL-13 in stimulated ILC2s. Purified ILC2s were cultured in media only or media supplemented with 10 ng/ml IL-7 and IL-33, and were incubated with NECA for 48 h. IL-5 (A, C) and IL-13 (B, D) levels were measured in the supernatant by ELISA. Graph bars denote means ± sem calculated from 5 wells representative of 4 independent experiments (1-way ANOVA followed by Tukey’s multiple comparison test). E–H) Intracellular IL-5 and IL-13 in stimulated ILC2s. Cells were stimulated as above and incubated with 1 μM NECA for 5 d, then intracellular IL-5 and IL-13 levels were assessed by flow cytometry after single cell and live/dead gating. Intracellular IL-13 (E) and IL-5 (F), and cells that are positive for both IL-13 and IL-5 (G). Graph bars denote means ± sem calculated from 3 wells representative of 3 independent experiments. Panel H shows a representative dot blot of the flow results (1-way ANOVA and Tukey’s multiple comparison test). **P < 0.01, ***P < 0.001 (compared with control conditions); ##P < 0.01, ###P < 0.001 (compared with IL-7 + IL-33 stimulation).
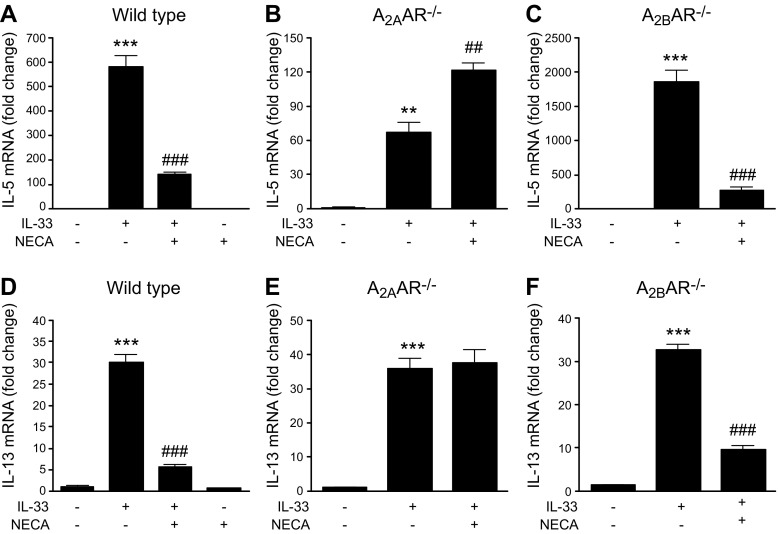
A2AAR activation inhibits IL-33–induced IL-5 and IL-13 mRNA expression by BMDMs
Previous studies have shown that BMDMs produce IL-5 and IL-13 upon IL-33 stimulation (37); therefore, we obtained BMDMs from wild-type, A2AAR−/−, and A2BAR−/− mice and assessed the effect of AR stimulation on IL-33–elicited IL-5 and IL-13 expression. As shown in Fig. 5A, D, NECA inhibited the IL-33–induced mRNA expression of IL-5 and IL-13, and the inhibitory effect of NECA disappeared in A2AAR−/− BMDMs (Fig. 5B, E). Furthermore, A2AAR deficiency increased the level of IL-33–induced IL-5 mRNA (Fig. 5B). In contrast, the inhibitory effect of NECA on IL-33–induced IL-5 and IL-13 mRNA expression was comparable both in wild-type and A2BAR−/− BMDMs (Fig. 5C, F).
Figure 5.
Effect of AR receptor activation on IL-33–induced IL-5 and IL-13 mRNA expression by BMDMs. A–C) IL-5 mRNA expression by wild-type (A), A2AAR−/− (B), and A2BAR−/− (C) BMDMs. D–F) IL-13 mRNA expression by wild-type (D), A2AAR−/− (E), and A2BAR−/− (F) BMDMs. BMDMs were activated with 20 ng/ml IL-33 in the absence or presence of 1 μM NECA for 72 h. Expression levels of IL-5 and IL-13 were measured by quantitative RT-PCR. Graph bars denote means ± sem calculated from 3 wells (1-way ANOVA followed by Tukey’s multiple comparison test). **P < 0.01, ***P < 0.001 (compared with control conditions); ##P < 0.01, ###P < 0.001 (compared with IL-33 stimulation).
DISCUSSION
Our findings reveal that ILC2s express all 4 ARs, and that adenosine signaling differentially regulates IL-5 and IL-13 production by ILC2s and BMDMs. AR signaling has a less pronounced suppressive effect on IL-5 than on IL-13 production in both IL-7 plus IL-33–stimulated bone marrow cultures and pure ILC2s. In bone marrow cells, the differential regulation of IL-5 and IL-13 production is a result of the fact that whereas the suppressive effect A2BARs on IL-5 production is moderated by an increasing effect of A2AARs, A2BARs decrease IL-13 production unopposed by A2AARs. In contrast, A2AARs decrease IL-5 and IL-13 mRNA expression in BMDMs, and A2BARs do not regulate cytokine production in these cells. Since we were not able to detect the release of IL-5 and IL-13 protein in the supernatant, this fact further underlines the notion that ILC2s are the main producers of IL-5 and IL-13 in response to IL-33 in the bone marrow.
The intracellular mechanisms that lead to the AR suppression of IL-5 and IL-13 also differ. AR signaling increases IL-5 mRNA levels in bone marrow cells early on, but fails to affect them later, which indicates that the overall suppressive effect of AR signaling may be mediated by a post-transcriptional effect. In contrast, AR signaling suppresses IL-13 mRNA levels. Similar differential regulation by pre- and post-transcriptional processes of cytokine induction by AR signaling has been observed previously in macrophages (38).
Of note, pure ILC2s are less responsive to the suppressive effects of AR signaling than ILC2s in the bone marrow. This may be a result of differences in how AR signaling interferes with transcriptional and post-transcriptional processes in pure ILC2s compared with bone marrow ILC2s in their natural environment. That is, whereas AR activation decreased both intracellular IL-5 and IL-13 in pure ILC2s, secreted IL-5 was not affected and IL-13 was suppressed only slightly. In addition, the differential responsiveness of bone marrow ILC2s and pure ILC2s may be explained by the fact that other cell types in the bone marrow modify the responsiveness of ILC2s to AR stimulation. For instance, Treg cells have been shown to modulate ILC responses (39), and the effect of AR signaling on Treg functions is well known (40); thus, Treg cells may mediate some of the effect of AR stimulation in ILC2s. Finally, it is also possible that ILC2s alter their responsiveness to AR stimulation during ex vivo expansion.
In summary, this is the first study to our knowledge to demonstrate that AR signaling has an overall suppressive effect on IL-5 and IL-13 production by ILC2s. These data indicate that ILC2s are similar to their Th2 lymphocyte counterparts in that AR signaling suppresses their IL-5 and IL-13 production (29). In contrast, nonlymphocytic cells, such as mast cells (41) and other myeloid cells (42), increase their IL-5 and IL-13 production upon AR signaling. This differential responsiveness of lymphocytes vs. nonlymphocytic cells may explain the sometimes contrasting roles of ARs in regulating type 2 immune responses in in vivo models of inflammation (42,–46).
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Joana Vindeirinho and Gábor Törő (both from Rutgers New Jersey Medical School) for technical assistance with the experiments. This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant R01-GM066189 (to G.H.), NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK113790 (to G.H.), and the Intramural Research Program of the NIH National Institute on Alcohol Abuse and Alcoholism (to P.P.). Work in the laboratory of J.H.F. is supported by a foundation grant from the Canadian Institutes of Health Research (CIHR), and a Leaders Opportunity Fund infrastructure grant from the Canadian Foundation of Innovation. J.H.F. is supported by a CIHR New Investigator Award, and by a Junior 1 and 2 Investigator Award by the Fonds de Recherche du Québec–Santé (FRQS). C.U.D. is supported by a fellowship of CIHR and the German National Academy of Sciences Leopoldina. J.H.F. and C.U.D. are supported through the American Association of Immunologists Careers in Immunology Fellowship Program. Sorting was performed on the BDFACSAria II, which was obtained through NIH Shared Instrument Grant 1S10-RR027022. The authors declare no conflicts of interest.
Glossary
- AR
adenosine receptor
- BAY606583
2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide
- BMDM
bone marrow–derived macrophage
- CGS21680
4-[2-[[6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- ILC
innate lymphoid cell
- ILC2
group 2 innate lymphoid cell
- NECA
1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide
- Th
T helper
- Treg
regulatory T
- TSLP
thymic stromal lymphopoietin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Csóka and G Haskó designed the research and analyzed data; Z. H. Németh and P. Pacher contributed vital new reagents; B. Csóka, Z. H. Németh, and C. U. Duerr performed the research; B. Csóka, J. H. Fritz, and G. Haskó wrote the paper; C. U. Duerr, J. H Fritz, and G. Haskó reviewed and edited the manuscript; and G. Haskó is the guarantor of the manuscript.
REFERENCES
- 1.Artis D., Spits H. (2015) The biology of innate lymphoid cells. Nature 517, 293–301 [DOI] [PubMed] [Google Scholar]
- 2.Licona-Limón P., Kim L. K., Palm N. W., Flavell R. A. (2013) TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 14, 536–542 [DOI] [PubMed] [Google Scholar]
- 3.Duerr C. U., McCarthy C. D., Mindt B. C., Rubio M., Meli A. P., Pothlichet J., Eva M. M., Gauchat J. F., Qureshi S. T., Mazer B. D., Mossman K. L., Malo D., Gamero A. M., Vidal S. M., King I. L., Sarfati M., Fritz J. H. (2016) Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat. Immunol. 17, 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moro K., Kabata H., Tanabe M., Koga S., Takeno N., Mochizuki M., Fukunaga K., Asano K., Betsuyaku T., Koyasu S. (2016) Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. 17, 76–86 [DOI] [PubMed] [Google Scholar]
- 5.Maazi H., Banie H., Aleman Muench G. R., Patel N., Wang B., Sankaranarayanan I., Bhargava V., Sato T., Lewis G., Cesaroni M., Karras J., Das A., Soroosh P., Akbari O. (2017) Activated plasmacytoid dendritic cells regulate type 2 innate lymphoid cell-mediated airway hyperreactivity. [E-pub ahead of print] J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2017.04.043 [DOI] [PubMed] [Google Scholar]
- 6.Molofsky A. B., Van Gool F., Liang H. E., Van Dyken S. J., Nussbaum J. C., Lee J., Bluestone J. A., Locksley R. M. (2015) Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 43, 161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mchedlidze T., Kindermann M., Neves A. T., Voehringer D., Neurath M. F., Wirtz S. (2016) IL-27 suppresses type 2 immune responses in vivo via direct effects on group 2 innate lymphoid cells. Mucosal Immunol. 9, 1384–1394 [DOI] [PubMed] [Google Scholar]
- 8.Meylan F., Hawley E. T., Barron L., Barlow J. L., Penumetcha P., Pelletier M., Sciumè G., Richard A. C., Hayes E. T., Gomez-Rodriguez J., Chen X., Paul W. E., Wynn T. A., McKenzie A. N., Siegel R. M. (2014) The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 7, 958–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X., Pappu R., Ramirez-Carrozzi V., Ota N., Caplazi P., Zhang J., Yan D., Xu M., Lee W. P., Grogan J. L. (2014) TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 7, 730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paclik D., Stehle C., Lahmann A., Hutloff A., Romagnani C. (2015) ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur. J. Immunol. 45, 2766–2772 [DOI] [PubMed] [Google Scholar]
- 11.Taylor S., Huang Y., Mallett G., Stathopoulou C., Felizardo T. C., Sun M. A., Martin E. L., Zhu N., Woodward E. L., Elias M. S., Scott J., Reynolds N. J., Paul W. E., Fowler D. H., Amarnath S. (2017) PD-1 regulates KLRG1(+) group 2 innate lymphoid cells. J. Exp. Med. 214, 1663–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Moltke J., O’Leary C. E., Barrett N. A., Kanaoka Y., Austen K. F., Locksley R. M. (2017) Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med. 214, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojno E. D., Monticelli L. A., Tran S. V., Alenghat T., Osborne L. C., Thome J. J., Willis C., Budelsky A., Farber D. L., Artis D. (2015) The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 8, 1313–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laffont S., Blanquart E., Savignac M., Cénac C., Laverny G., Metzger D., Girard J. P., Belz G. T., Pelletier L., Seillet C., Guéry J. C. (2017) Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 214, 1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walford H. H., Lund S. J., Baum R. E., White A. A., Bergeron C. M., Husseman J., Bethel K. J., Scott D. R., Khorram N., Miller M., Broide D. H., Doherty T. A. (2014) Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin. Immunol. 155, 126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskó G., Linden J., Cronstein B., Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonioli L., Blandizzi C., Pacher P., Haskó G. (2013) Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857 [DOI] [PubMed] [Google Scholar]
- 18.Sitkovsky M. V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C., Ohta A., Thiel M. (2004) Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 [DOI] [PubMed] [Google Scholar]
- 19.Sitkovsky M., Lukashev D. (2005) Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 5, 712–721 [DOI] [PubMed] [Google Scholar]
- 20.Haskó G., Cronstein B. N. (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 25, 33–39 [DOI] [PubMed] [Google Scholar]
- 21.Csóka B., Selmeczy Z., Koscsó B., Németh Z. H., Pacher P., Murray P. J., Kepka-Lenhart D., Morris S. M. Jr., Gause W. C., Leibovich S. J., Haskó G. (2012) Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novitskiy S. V., Ryzhov S., Zaynagetdinov R., Goldstein A. E., Huang Y., Tikhomirov O. Y., Blackburn M. R., Biaggioni I., Carbone D. P., Feoktistov I., Dikov M. M. (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112, 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panther E., Corinti S., Idzko M., Herouy Y., Napp M., la Sala A., Girolomoni G., Norgauer J. (2003) Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101, 3985–3990 [DOI] [PubMed] [Google Scholar]
- 24.Koscsó B., Csóka B., Kókai E., Németh Z. H., Pacher P., Virág L., Leibovich S. J., Haskó G. (2013) Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J. Leukoc. Biol. 94, 1309–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskó G., Szabó C., Németh Z. H., Kvetan V., Pastores S. M., Vizi E. S. (1996) Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 157, 4634–4640 [PubMed] [Google Scholar]
- 26.Haskó G., Kuhel D. G., Chen J. F., Schwarzschild M. A., Deitch E. A., Mabley J. G., Marton A., Szabó C. (2000) Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14, 2065–2074 [DOI] [PubMed] [Google Scholar]
- 27.Koshiba M., Kojima H., Huang S., Apasov S., Sitkovsky M. V. (1997) Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J. Biol. Chem. 272, 25881–25889 [DOI] [PubMed] [Google Scholar]
- 28.Huang S., Apasov S., Koshiba M., Sitkovsky M. (1997) Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90, 1600–1610 [PubMed] [Google Scholar]
- 29.Csóka B., Himer L., Selmeczy Z., Vizi E. S., Pacher P., Ledent C., Deitch E. A., Spolarics Z., Németh Z. H., Haskó G. (2008) Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 22, 3491–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson J. M., Kurtz C. C., Black S. G., Ross W. G., Alam M. S., Linden J., Ernst P. B. (2011) The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J. Immunol. 186, 6746–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deaglio S., Dwyer K. M., Gao W., Friedman D., Usheva A., Erat A., Chen J. F., Enjyoji K., Linden J., Oukka M., Kuchroo V. K., Strom T. B., Robson S. C. (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sitkovsky M. V. (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 30, 102–108 [DOI] [PubMed] [Google Scholar]
- 33.Raskovalova T., Huang X., Sitkovsky M., Zacharia L. C., Jackson E. K., Gorelik E. (2005) Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J. Immunol. 175, 4383–4391 [DOI] [PubMed] [Google Scholar]
- 34.Wallace K. L., Linden J. (2010) Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood 116, 5010–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csóka B., Koscsó B., Töro G., Kókai E., Virág L., Németh Z. H., Pacher P., Bai P., Haskó G. (2014) A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 63, 850–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brickshawana A., Shapiro V. S., Kita H., Pease L. R. (2011) Lineage-Sca1+c-Kit-CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J. Immunol. 187, 5795–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z., Grinchuk V., Urban J. F. Jr., Bohl J., Sun R., Notari L., Yan S., Ramalingam T., Keegan A. D., Wynn T. A., Shea-Donohue T., Zhao A. (2013) Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS One 8, e59441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Németh Z. H., Lutz C. S., Csóka B., Deitch E. A., Leibovich S. J., Gause W. C., Tone M., Pacher P., Vizi E. S., Haskó G. (2005) Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J. Immunol. 175, 8260–8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnamoorthy N., Burkett P. R., Dalli J., Abdulnour R. E., Colas R., Ramon S., Phipps R. P., Petasis N. A., Kuchroo V. K., Serhan C. N., Levy B. D. (2015) Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 194, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernst P. B., Garrison J. C., Thompson L. F. (2010) Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J. Immunol. 185, 1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryzhov S., Goldstein A. E., Matafonov A., Zeng D., Biaggioni I., Feoktistov I. (2004) Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J. Immunol. 172, 7726–7733 [DOI] [PubMed] [Google Scholar]
- 42.Belikoff B. G., Vaickus L. J., Sitkovsky M., Remick D. G. (2012) A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. J. Immunol. 189, 3707–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haskó G., Csóka B., Németh Z. H., Vizi E. S., Pacher P. (2009) A2B adenosine receptors in immunity and inflammation. Trends Immunol. 30, 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun C. X., Zhong H., Mohsenin A., Morschl E., Chunn J. L., Molina J. G., Belardinelli L., Zeng D., Blackburn M. R. (2006) Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 116, 2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel N., Wu W., Mishra P. K., Chen F., Millman A., Csóka B., Koscsó B., Eltzschig H. K., Haskó G., Gause W. C. (2014) A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe 15, 339–350 [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y., Mohsenin A., Morschl E., Young H. W., Molina J. G., Ma W., Sun C. X., Martinez-Valdez H., Blackburn M. R. (2009) Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J. Immunol. 182, 8037–8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.