Abstract
Background
Hepatitis B virus (HBV) and human immunodeficiency virus (HIV) share common risk factors. The parallel description of their frequency over time may help capture their similarities and differences.
Methods
Using data from the National Transfusion Center of Abidjan, we estimated the following over a 20-year period: (1) the prevalence of HIV and hepatitis B surface antigen (HBsAg) positivity at first contact; and (2) the incidence of HIV and HBsAg seroconversion in negative first-time blood donors.
Results
Between 1992 and 2012, 422319 donors (men [M] = 74%) provided 1063825 blood donations. For first-time donors, HIV prevalence decreased from 7.1% (M = 5.9%, women [W] =11.0%) in 1992–1994 to 1.1% (M = 0.8%, W = 2.0%) in 2010–2012. Prevalence of HBsAg positivity remained stable at 10.8% (M = 11.7%, W = 7.3%) in 1992–1994 to 11.1% (M = 12.5%, W = 7.1%) in 2010–2012. Among regular donors (N = 129256), the incidence of becoming HIV or HBsAg positive, respectively, decreased from 4.9 per 100 (M = 4.5, W = 8.6) and 7.3 per 100 person-years (M = 7.8, W = 2.3) in 1992–1994 to 0.07 (M = 0.06, W = 0.11) and 0.2 per 100 person-years (M = 0.2, W = 0.2) in 2010–2012.
Conclusions
Human immunodeficiency virus prevalence and incidence decreased dramatically over time, whereas HBV prevalence remained stable. Incidence of HBsAg seroconversion, although decreasing, still reached unexpected levels, suggesting that the risk of HBV infection in adults may be higher than expected. Hepatitis B surface antigen-negative blood-donors should be offered HBV vaccination.
Keywords: blood donors, HBV, HIV, incidence, sub-Saharan Africa
Chronic hepatitis B virus (HBV) infection accounts for approximately 0.7 million deaths per year and is the main cause of liver cirrhosis and hepatocellular carcinoma worldwide [1]. Hepatitis B virus is an important public health concern for the Western Pacific and sub-Saharan African regions, where HBV prevalence is highest [2]. The latter region is also known to have the highest endemicity of human immunodeficiency virus (HIV) infection [3].
Hepatitis B virus and HIV share several common modes of transmission, such as sexual contact, percutaneous injury, and perinatal infection [4]. Nevertheless, transmission of these viruses commonly occurs at different time-points during the lifespan of an individual residing in sub-Saharan Africa. Hepatitis B virus is generally transmitted through horizontal contact with bodily fluids in early childhood, whereas new infections among adults are rare [5]. In contrast, HIV transmission mostly occurs via sexual contact during adulthood [5, 6] and, to a lesser extent, mother-to-child transmission [7].
The major preventative measures are also quite different between the 2 viruses. Blocking transmission of HBV infection depends mostly on vaccination [8], whereas the tools for HIV prevention principally involve reducing at-risk sexual activity, HIV testing, and initiating appropriate antiretroviral treatment in HIV-infected patients [9]. However, prevention strategies of 1 virus, notably integrating expanded access to antiretroviral treatment, could have consequences for the other [10]. Long-term data on the prevalence and incidence of these infections would help provide evidence for the potential successes of preventative efforts in the past and also clarify the current needs of transmission reduction.
To date, few studies in sub-Saharan Africa have evaluated the prevalence and especially incidence of HBV infections over the last several decades and how the epidemiology of HBV infection compares with that of HIV. We used unique data collected over an extensive time frame from voluntary blood donors in Abidjan, Côte d’Ivoire to estimate the prevalence and incidence of HIV and HBV.
MATERIALS AND METHOD
Study Design
The National Blood Transfusion Center (NBTC) of Abidjan was created in 1958 and is the only institution of Côte d’Ivoire accredited for supplying blood products at the country level. Since October 1992, donor registration information and laboratory results were available in a centralized computerized database. We analyzed data recorded at the NBTC between October 1, 1992 and July 31, 2012.
Study Population
The NBTC follows a voluntary, nonremunerated blood donation system. At each contact, volunteers are screened for the following eligibility criteria: (1) apyrexia, (2) age 17 to 65 years, (3) body weight at least 50 kilograms, and (4) no contraindication for blood donation as determined by a self-questionnaire and medical examination. At the end of the self-questionnaire, persons with at least 1 contraindication (previous transfusion, history of infectious or cardiovascular diseases, previous sexually transmitted disease, engaging in at-risk behavior) were not considered eligible for blood donation. Persons who fulfill all criteria undergo blood collection totaling 7 mL of blood per kilogram. After collection, HIV, HBV, hepatitis C virus, and syphilis tests are performed on each individual blood bag. If any of the tests above are positive, the blood bag is discarded and the donor is referred for further consultation to the Centre Médical de Suivi des Donneurs de Sang, a nearby medical center qualified to provide appropriate care and treatment to HIV and/or HBV-infected individuals. If all tests are negative, the blood bag is stored for later use and the donor is allowed to return for further donation.
Each donor is allowed to provide a maximum of 4 donations per year. These procedures were not modified during the entire study period.
Laboratory Tests
Human immunodeficiency virus screening was based on 2 consecutive enzyme-linked immunosorbent assay (ELISA) tests, and hepatitis B surface antigen (HBsAg) screening was based on a single ELISA test. The list of assays used by calendar period is shown in Supplementary Appendix 1, Supplementary Table SA1. Only data on HIV and HBsAg were considered in the present analysis.
Recorded Data
As of October 1992, individual data regarding each blood donation were prospectively recorded at the NBTC in a standardized database. Recorded data included the following: (1) at first donation - age, sex, name, and anonymous ID code; (2) at first and subsequent donations - date, results of the 2 HIV and single HBsAg screening tests, and outcome after screening (ie, allowed or not allowed to provide further donations).
Statistical Analysis
For every 3-year period from 1992, we estimated (1) the prevalence of HIV and HBsAg positivity among individuals undergoing first donation and (2) the incidence of HIV and HBsAg seroconversion in repeat blood donors without evidence of infection for either virus at first donation. Period prevalence and incidence were given for the overall population, as well as stratified by sex and age group.
Prevalence of HIV and HBsAg positivity was calculated by dividing the number of persons with a positive test by the total number of persons who provided their first voluntary blood donation and were eligible to donate blood during the period. For HIV, only persons with 2 positive tests were considered positive.
Incidence rates were estimated in donors who were found HIV- and HBsAg-negative at first contact, were eligible to provide further blood donations, and returned at least once for a repeat blood donation during the study period. Rates were calculated by dividing the overall number of blood donors with a newly positive test by the overall person-time at risk. Incident infection was recorded at the exact date when the test was found positive. The overall person-time at risk was the sum of individual person-time at risk. For a given individual, the period at risk started at first donation and was censored at the date of last donation, irrespective of the result (positive or negative) of the test. Linear trends across periods were tested using Cochran Armitage tests. Statistical analyses were performed using SAS (Cary, NC) version 9.3.
RESULTS
Donors and Donations
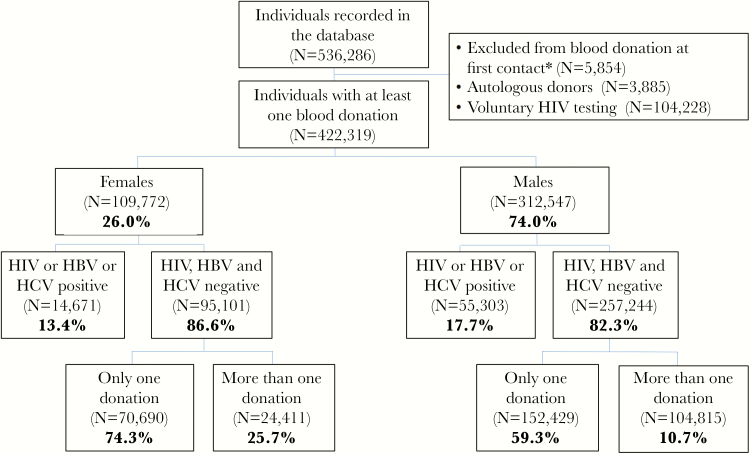
During the study period, 536286 persons were registered in the database, 113967 of whom were not included in the study because they were autologous donors (n = 3885), attended the NBTC for voluntary HIV testing but not to provide blood donation (n = 104228), or were ineligible at first donation based on answers of their questionnaire (n = 5854) (Figure 1).
Figure 1.
Flowchart of voluntary blood donors at the National Blood Transfusion Center of Abidjan, Côte d’Ivoire, 1992–2012. *Excluded from blood donation after questionnaire assessing previous at-risk behavior and medical examination. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
The remaining 422319 individuals were included in analyses. Of them, 26% were female and 74% were male. Their median age at first donation was 22 years (interquartile range, 20–28 years). A total of 1063825 donations occurred during follow-up from 291999 single donors and 130320 regular donors (Figure 1). The latter group had a mean 5.9 donations per person (standard deviation = 7.5). The total follow-up time between first and last donation in regular donors was 477301 person-years.
Seroprevalence at First Donation
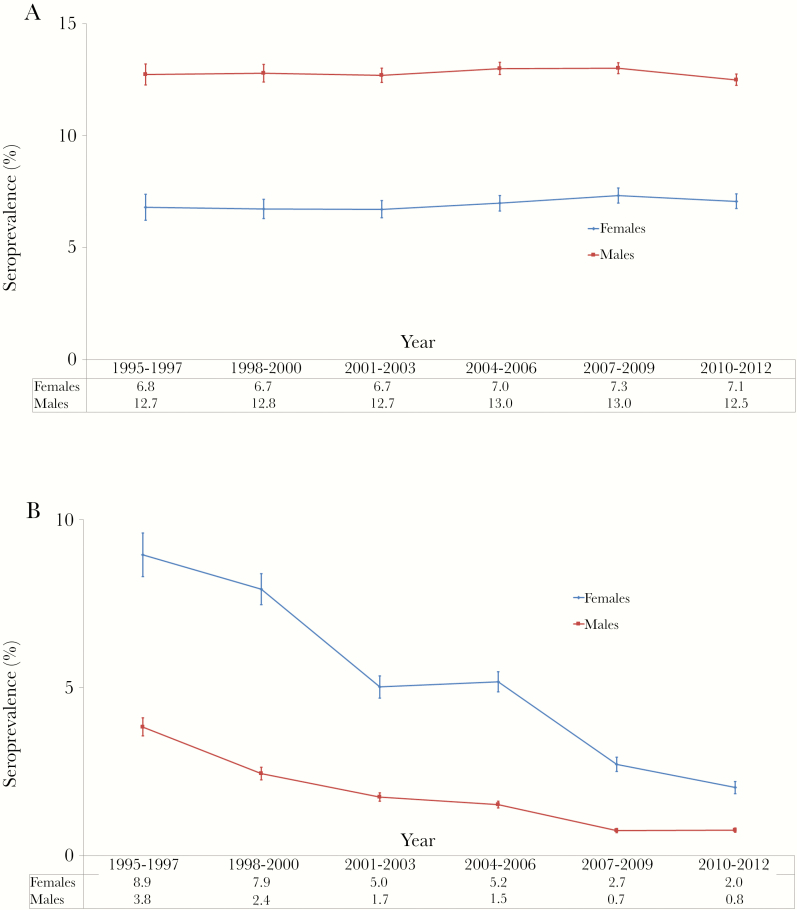
The prevalence of HBsAg positivity was 10.8% (95% confidence interval [CI], 10.4–11.1) in 1992–1994 and 11.1% (95% CI, 10.9–11.4) in 2010–2012, with no statistically significant changes observed over time (Table 1). Prevalence was higher in men than in women and remained stable over time in both genders (Figure 2A and Supplementary Appendix Table 2). In women, there were no statistical differences across age categories, whereas younger men had higher HBsAg prevalence compared with older ones (Supplementary Appendix Tables 3 and 4).
Table 1.
Seroprevalence of HBsAg and HIV at First Blood Donation, National Blood Transfusion Center, Abidjan, Côte d’Ivoire 1992–2012
| Variables | 1992–1994 | 1995–1997 | 1998–2000 | 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | P* |
|---|---|---|---|---|---|---|---|---|
| HBsAg | ||||||||
| Total screened†, N | 24 067 | 26 806 | 40 544 | 58 062 | 79 460 | 96 676 | 91 962 | NS |
| HBsAg positive†, N | 2589 | 2979 | 4400 | 6360 | 9094 | 11 300 | 10 235 | |
| Seroprevalence, % | 10.8 | 11.1 | 10.9 | 11.0 | 11.4 | 11.7 | 11.1 | |
| (95% CI) | (10.4–11.1) | (10.6–11.6) | (10.5–11.2) | (10.6–11.3) | (11.2–11.7) | (11.4–11.9) | (10.9–11.4) | |
| HIV | ||||||||
| Total screened, N | 24 700 | 27 196 | 40 816 | 58 418 | 80 031 | 98 232 | 92 926 | <.0001 |
| HIV positive, N | 1742 | 1423 | 1711 | 1577 | 1974 | 1182 | 1001 | |
| Seroprevalence, % | 7.1 | 5.2 | 4.2 | 2.7 | 2.5 | 1.2 | 1.1 | |
| (95% CI) | (6.7–7.4) | (5.0–5.5) | (4.0–4.4) | (2.6–2.8) | (2.4–2.6) | (1.1–1.3) | (1.0–1.1) | |
Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; N, number of individuals; NS, not significant.
*Significance for linear trend determined using a Pearson’s χ2 test.
†Over the entire 1992–2012 period, HBsAg testing results were available in the database for 417577 individuals, and HIV testing results were available for 422319 individuals. Therefore, 1.12% of the 422319 individuals with available HIV testing results had missing data on HBsAg testing.
Figure 2.
Seroprevalence of hepatitis B surface antigen (HBsAg) and human immunodeficiency virus (HIV) at first blood donation, National Blood Transfusion Center of Abidjan, Côte d’Ivoire, 1992–2012. Seroprevalence of HBsAg and anti-HIV antibodies in individuals presenting for their first blood donation for each 3-year period. Prevalences are stratified according to sex (A) and age groups (B). Vertical bars represent 95% confidence intervals.
The prevalence of HIV positivity significantly decreased from 7.1% (95% CI, 6.7–7.4) in 1992–1994 to 1.1% (95% CI, 1.0–1.1) in 2010–2012 (Table 1). Human immunodeficiency virus prevalence was higher in women than men, while significantly declining for both genders over time (Figure 2B and Supplementary Appendix Table 5). In men, HIV prevalence was consistently lower in younger men compared with older ones. In women, the prevalence was higher in the 26–35 years categories during the first 10 years, and then the pattern across age groups tended to resemble that of men (Supplementary Appendix Tables 6 and 7).
Incidence in Regular Blood Donors
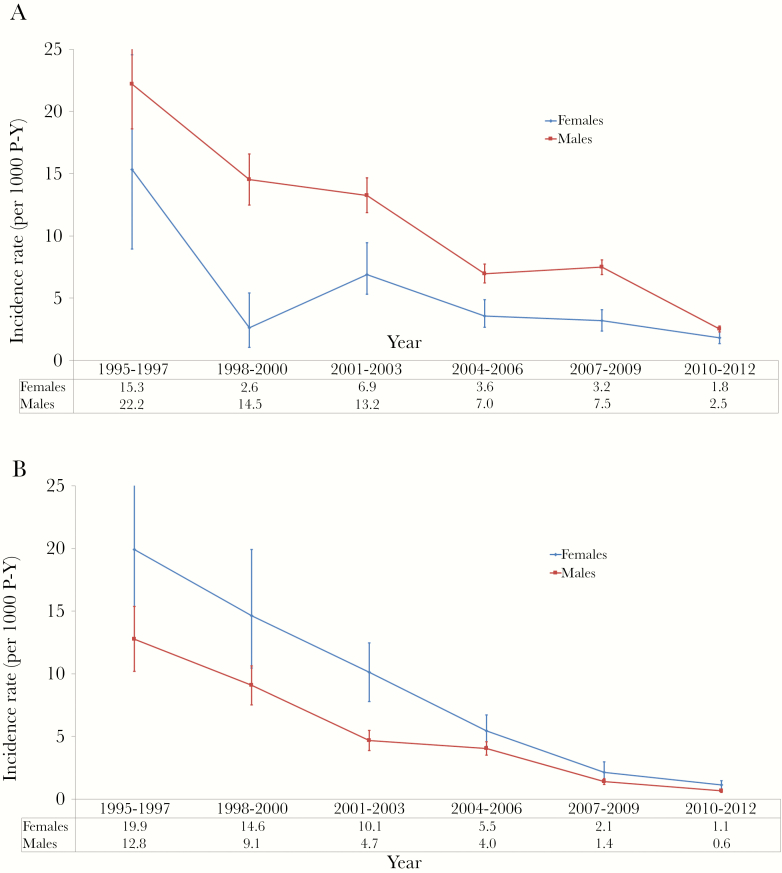
The incidence rate of becoming HBsAg positive was 7.3 per 100 person-years (95% CI, 5.9–8.8) in 1992–1994 and 0.2 per 100 person-years years (95% CI, 2.2–2.6) in 2010–2012, a decrease that was statistically significant (Table 2). Incidence rates were higher in men than women (Figure 3A and Supplementary Appendix Table 8) and higher in donors aged 17–25 compared with older (Supplementary Appendix Tables 9 and 10).
Table 2.
Incidence of HBsAg-Positive and HIV Seroconversion Among Regular Blood Donors, National Blood Transfusion Center, Abidjan, Côte d’Ivoire 1992–2012
| Variables | 1992–1994 | 1995–1997 | 1998–2000 | 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | P* |
|---|---|---|---|---|---|---|---|---|
| HBsAg | ||||||||
| Donors, N | 1822 | 4661 | 6470 | 10 579 | 17 974 | 26 509 | 51 463 | <.0001 |
| Follow-up†, P-Y | 1341 | 7685 | 16 088 | 33 090 | 60 683 | 98 844 | 221 942 | |
| Seroconversion‡, N | 98 | 163 | 202 | 394 | 381 | 667 | 538 | |
| Incidence rate/1000 P-Y | 73.1 | 21.2 | 12.6 | 11.9 | 6.3 | 6.7 | 2.4 | |
| (95% CI) | (58.6–87.6) | (18.0–24.5) | (10.8–14.3) | (10.7–13.1) | (5.6–6.9) | (6.2–7.3) | (2.2–2.6) | |
| HIV | ||||||||
| Donors, N | 2017 | 5223 | 6772 | 10 943 | 19 316 | 30 062 | 54 574 | <.0001 |
| Follow-up†, P-Y | 1453 | 8483 | 16 948 | 34 480 | 65 488 | 111 801 | 234 932 | |
| Seroconversion‡, N | 71 | 117 | 169 | 200 | 283 | 169 | 168 | |
| Incidence rate/1000 P-Y | 48.9 | 13.8 | 10.0 | 5.8 | 4.3 | 1.5 | 0.7 | |
| (95% CI) | (37.5–60.3) | (11.3–16.3) | (8.5–11.5) | (5.0–6.6) | (3.8–4.8) | (1.3–1.7) | (0.6–0.8) | |
Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; N, number of individuals; P-Y, person-years.
*Significance for linear trend determined using a Pearson’s χ2 test.
†Cumulative follow-up time between first and last donations among included individuals, in P-Y.
‡Seroconversion: individuals who had a negative test at the previous donation(s) and whose serological test became positive.
Figure 3.
Incidence of hepatitis B surface antigen (HBsAg) and human immunodeficiency virus (HIV) seroconversion among regular blood donors, National Blood Transfusion Center, Abidjan, Côte d’Ivoire, 1992 – 2012. Incidence rates of becoming HBsAg positive or seroconverting anti-HIV antibody positive in regular blood donors for each 3-year period. Incidence rates are stratified according to sex (A) and age groups (B). Vertical bars represent 95% confidence intervals. P-Y, person-years.
The incidence rate of HIV seroconversion significantly decreased from 4.9 per 100 person-years (95% CI, 3.8–6.0) in 1992–1994 to 0.07 per 100 person-years years (95% CI, 0.06–0.08) in 2010–2012 (Table 2). Incidence rates were consistently higher in women than men (Figure 3B and Supplementary Appendix Table 11), with no significant difference between age categories in both genders (Supplementary Appendix Tables 12 and 13).
Human Immunodeficiency Virus-Hepatitis B Virus Coinfection
During the entire 1992–2012 period, 46957 individuals were found to be HBsAg positive at first contact, and 2443 individuals who were initially HBsAg negative were found to be HBsAg positive at further donation.
Of the 46957 individuals who were HBsAg positive at first contact, 1019 (2%) were also HIV positive. The prevalence of HIV positivity among individuals who were HBsAg positive at first contact was 7% in 1992–1994, 5% in 1995–1997, 4% in 1998–2000, 2% in 2001–2003, 2% in 2004–2006, 1% in 2007–2009, and 1% in 2010–2012.
Of the 2443 individuals who were initially HBsAg negative and further became positive, 9 (0.4%) concomitantly became HIV positive. The prevalence of HIV positivity among donors who became HBsAg positive remained below 1% in all calendar periods.
DISCUSSION
Several methodological limitations need to be noted before interpreting the results of our study. First, we included voluntary blood donors, who are themselves known to differ from the general population in a number of characteristics [11]. At initial donation, further selection of participants occurred in either an implicit (ie, seropositive individuals aware of their ineligibility to donate) or explicit manner (ie, individuals with identified risk factors via questionnaire) [12]. Incident rate estimates also incorporated individuals with probably less frequent at-risk activity. Consequently, the data presented herein likely do not represent true prevalence and incidence rates of HIV and HBsAg-positivity in the general population of Côte d’Ivoire.
Second, because anti-hepatitis B surface (anti-HBs) antibodies were not tested, we could not assess the underlying levels of anti-HBV immunity, either from vaccination or cleared infection, and thus the risk of acquiring HBV in the group of regular blood donors. A recent survey with a limited sample size has suggested that 23% of adults in Côte d’Ivoire have anti-HBs antibody positive serology [13].
Third, neither HBV-deoxyribonucleic acid (DNA) nor anti-hepatitis B core (anti-HBc) antibodies were sampled, thereby making it impossible to establish whether patients had occult infection. Defined as active viral replication in the liver with HBsAg-negative serology [14], this form of HBV infection is commonplace in sub-Saharan Africa [15, 16]. Some of the incident HBV infections observed could in fact be patients with occult infection who residually expressed detectable levels of HBsAg in the blood [17]. This may also explain why serology compared with HBV-DNA is not highly concordant for repeat blood donors [18].
Fourth, the HBsAg assays changed over time and because there are slight differences in the performance of these tests [19], our estimates on HBsAg prevalence and incidence may have been biased.
Fifth, 1.1% of individuals who underwent HIV serotesting at first contact did not have concomitant HBsAg test results according to the database. If missing data did not occur at random, this may have also biased our HBsAg prevalence and incidence estimates.
Sixth, voluntary testing for HIV was poorly available at the beginning of the 1990s in Abidjan and then increased over time. Some people could have used blood donation as a means of testing, whereas this phenomenon could have decreased over time. This may partly explain the decrease in HIV prevalence at first contact described herein.
Finally, follow-up and blood collection terminated in individuals infected with HBV after the first HBsAg-positive test. We were unable to assess the proportion with chronic infection from a second HBsAg-positive sample within at least a 6-month period. Considering that approximately 5% of adults infected with HBV develop chronic infection [20], we would expect that the majority of incident infections were acute.
Within the context of these limitations, our article provides the longest follow-up period to date allowing a more thorough description of temporal patterns in prevalence and incidence rates of HIV and HBsAg seropositivity among blood donors from sub-Saharan Africa.
Both the prevalence of HIV in first-time blood donors and incidence of HIV in repeat donors decreased over time. Our data uphold previous accounts of decreasing HIV prevalence across many parts of the continent [21]. The observed decrease in HIV incidence could be due to lower at-risk sexual activity, specific large-scale interventions aimed at increasing condom use among casual partners, or widespread antiretroviral treatment in individuals infected with HIV [9]. The only other study to examine HIV incidence in sub-Saharan Africa with a similar design did not replicate these temporal findings [22]. Given the close resemblance of sex and age distributions, it is difficult to pinpoint any demographic phenomenon explaining these differences.
Data on HBsAg-positive serostatus suggest a different pattern. The prevalence of HBsAg positive for first-time donations remained largely stable over the 20-year period. Research in other regions of the world has in fact observed decreases in HBsAg-positive prevalence in the general population, yet stable time trends have been described in other African countries such as Ghana or Tanzania [23] or among certain blood donor groups [24–26]. Decreases in HBV prevalence in other countries have been largely attributed to successful vaccine campaigns after childbirth [27, 28]. Infant HBV vaccination has been standard in Côte d’Ivoire since 2000 [29]; therefore, we would not expect to see any effect from vaccination in our 1992–2012 estimates.
In contrast, the incidence of HBsAg positivity decreased dramatically over time. The literature on HBV infection within the continent supports that the vast majority of infections are horizontally acquired during childhood and rarely during sexual contact [30–32]. It is then surprising that significant rates of incident infections were observed during the study period. Other studies with similar designs and smaller sample sizes have also observed incidence rates ranging from 2.5 to 5.9 per 100 persons-years [24, 33], yet it is difficult to infer any time trends given their short periods of observation. Laboratory diagnostic tests have changed little over the past 2 decades, and, without strong vaccination efforts during childhood for many of the participants, it is unlikely that incidence of occult HBV infection increased over time [34]. Therefore, the decreases observed in our study may be due to declines in HBV transmission.
There are several interesting features to mention when contrasting data on HIV and HBV infection. First, despite declines in both HIV- and HBsAg-positive incidence rates over time, the HBsAg/HIV incidence ratio increased from 1.5 in 1992–1994 to 4.5 in 2010–2012. These data underscore the higher relative prominence of HBV infection, which has been emerging over the last decade as a major public health issue in sub-Saharan Africa [35]. Second, with the decrease in both HIV- and HBsAg-positive incidence rates, it could be speculated that these findings were due to reduction in commonly shared transmission factors. Whereas scaled-up antiretroviral treatment is likely to have influenced decreases in HIV incidence, it is unlikely to be the most important explanation of the trends in HBsAg. An antiretroviral therapy (ART) regimen containing the anti-HBV agents lamivudine and tenofovir has been associated with reductions in HBV transmission [10, 36]. These agents started to be commonly used in Côte d’Ivoire in 2004 and 2010, respectively. However, individuals coinfected with HIV-HBV account for a limited fraction of the overall population of adults infected with HBV. Therefore, it is unlikely that this exceedingly small fraction of HBV-infected people receiving ART explains the trends we observed. Third, the fact that HBV prevalence was highly similar to estimates from voluntary counseling centers, whereas HIV prevalence was much lower [37], suggests that self-selection before blood donation might occur less frequently with HBV than HIV.
In addition, the epidemiology of HBV and HIV infection, according to our data, starkly contrasted between genders. Human immunodeficiency virus estimates in both prevalence and incidence rate were higher in women than in men, whereas the opposite was true for HBsAg-positive serology. These findings, as well as their consistency over time, are in line with others regarding the HBV and HIV prevalence from the region [24, 25, 38–40]. This contends that gender may need to be considered when targeting testing and prevention strategies for specific infections.
CONCLUSIONS
In conclusion, HIV prevalence at first contact decreased dramatically, while HBsAg seropositivity remained stable, in this large, 2-decade study among voluntary blood donors from Côte d’Ivoire. Incidence of both infections substantially decreased among regular donors over time, possibly highlighting the successes of previous intervention efforts. Nevertheless, reasons for the relatively high incidence of HBV infection should be elucidated in further research, because it could suggest a much more frequent incidence in HBV infections among adults than previously expected. Anti-HBs antibody status should be further provided to HBsAg-negative blood donors so that they may benefit from HBV vaccination.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We also gratefully acknowledge the valuable contributions of the CNTS, Centre Médical de Suivi des Donneurs de Sang, and INSERM 1219 teams.
Finanical support. This study was funded by grants from the French National Agency for Reserch on AIDS and Viral Hepatitis, Paris, France (Grant 1220). A. B. was awarded a postdoctoral fellowship from SIDACTION.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stanaway JD, Flaxman AD, Naghavi M et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014; 384: 2053–63. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Global Health Observatory Data Repository. Available at: http://apps.who.int/gho/data/view.main.22500WHOREG?lang=en. Accessed April 29, 2017. [Google Scholar]
- 4. Lacombe K, Bottero J, Lemoine M et al. HIV/hepatitis B virus co-infection: current challenges and new strategies. J Antimicrob Chemother 2010; 65:10–7. [DOI] [PubMed] [Google Scholar]
- 5. Hadziyannis SJ. Natural history of chronic hepatitis B in Euro-Mediterranean and African countries. J Hepatol 2011; 55:183–91. [DOI] [PubMed] [Google Scholar]
- 6. de Oliveira T, Kharsany AB, Gräf T et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2017; 4:e41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med 2016; 374:761–70. [DOI] [PubMed] [Google Scholar]
- 8. WHO Publication. Hepatitis B vaccines: WHO position paper--recommendations. Vaccine 2010; 28:589–90. [DOI] [PubMed] [Google Scholar]
- 9. Mutevedzi PC, Newell ML. Review: [corrected] the changing face of the HIV epidemic in sub-Saharan Africa. Trop Med Int Health 2014; 19:1015–28. [DOI] [PubMed] [Google Scholar]
- 10. Heuft MM, Houba SM, van den Berk GE et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014; 28:999–1005. [DOI] [PubMed] [Google Scholar]
- 11. Tagny CT, Owusu-Ofori S, Mbanya D, Deneys V. The blood donor in sub-Saharan Africa: a review. Transfus Med 2010; 20:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Pillonel J, Saura C, Couroucé AM. Prevalence of HIV, HTLV, and hepatitis B and C viruses in blood donors in France, 1992-1996. Transfus Clin Biol 1998; 5:305–12. [DOI] [PubMed] [Google Scholar]
- 13. N’Dri-Yoman T, Anglaret X, Messou E et al. Occult HBV infection in untreated HIV-infected adults in Côte d’Ivoire. Antivir Ther 2010; 15:1029–34. [DOI] [PubMed] [Google Scholar]
- 14. Raimondo G, Allain JP, Brunetto MR et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008; 49:652–7. [DOI] [PubMed] [Google Scholar]
- 15. Apica BS, Seremba E, Rule J et al. High prevalence of occult hepatitis B infection in an African urban population. J Med Virol 2016; 88:674–80. [DOI] [PubMed] [Google Scholar]
- 16. Oluyinka OO, Tong HV, Bui Tien S et al. Occult hepatitis B virus infection in nigerian blood donors and hepatitis B virus transmission risks. PLoS One 2015; 10:e0131912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyd A, Maylin S, Moh R et al. Identifying patients infected with hepatitis B virus in sub-Saharan Africa: potential for misclassification. Diagn Microbiol Infect Dis 2015; 83:248–51. [DOI] [PubMed] [Google Scholar]
- 18. Lelie N, Bruhn R, Busch M et al. Detection of different categories of hepatitis B virus (HBV) infection in a multi-regional study comparing the clinical sensitivity of hepatitis B surface antigen and HBV-DNA testing. Transfusion 2017; 57:24–35. [DOI] [PubMed] [Google Scholar]
- 19. Scheiblauer H, El-Nageh M, Diaz S et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang 2010; 98:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol 2009; 19:231–4. [DOI] [PubMed] [Google Scholar]
- 21. Murray CJ, Ortblad KF, Guinovart C et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mapako T, Mvere DA, Chitiyo ME et al. Human immunodeficiency virus prevalence, incidence, and residual transmission risk in first-time and repeat blood donations in Zimbabwe: implications on blood safety. Transfusion 2013; 53:2413–21. [DOI] [PubMed] [Google Scholar]
- 23. Ott JJ, Horn J, Krause G, Mikolajczyk RT. Time trends of chronic HBV infection over prior decades - a global analysis. J Hepatol 2017; 66:48–54. [DOI] [PubMed] [Google Scholar]
- 24. Nagalo BM, Bisseye C, Sanou M et al. Seroprevalence and incidence of transfusion-transmitted infectious diseases among blood donors from regional blood transfusion centres in Burkina Faso, West Africa. Trop Med Int Health 2012; 17:247–53. [DOI] [PubMed] [Google Scholar]
- 25. Egah DZ, Banwat EB, Audu ES et al. Hepatitis B surface antigen, hepatitis C and HIV antibodies in a low-risk blood donor group, Nigeria. East Mediterr Health J 2007; 13:961–6. [PubMed] [Google Scholar]
- 26. Stokx J, Gillet P, De Weggheleire A et al. Seroprevalence of transfusion-transmissible infections and evaluation of the pre-donation screening performance at the Provincial Hospital of Tete, Mozambique. BMC Infect Dis 2011; 11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendy M, Peterson I, Hossin S et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One 2013; 8:e58029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amponsah-Dacosta E, Lebelo RL, Rakgole JN et al. Evidence for a change in the epidemiology of hepatitis B virus infection after nearly two decades of universal hepatitis B vaccination in South Africa. J Med Virol 2014; 86:918–24. [DOI] [PubMed] [Google Scholar]
- 29. Magoni M, Ekra KD, Aka LN et al. Effectiveness of hepatitis-B vaccination in Ivory Coast: the case of the Grand Bassam health district. Ann Trop Med Parasitol 2009; 103:519–27. [DOI] [PubMed] [Google Scholar]
- 30. Martinson FE, Weigle KA, Royce RA et al. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. Am J Epidemiol 1998; 147:478–87. [DOI] [PubMed] [Google Scholar]
- 31. Whittle H, Inskip H, Bradley AK et al. The pattern of childhood hepatitis B infection in two Gambian villages. J Infect Dis 1990; 161:1112–5. [DOI] [PubMed] [Google Scholar]
- 32. Abdool Karim SS, Thejpal R, Coovadia HM. Household clustering and intra-household transmission patterns of hepatitis B virus infection in South Africa. Int J Epidemiol 1991; 20:495–503. [DOI] [PubMed] [Google Scholar]
- 33. Namululi BA, Guerrieri C, Dramaix MW. Prevalence and incidence of HIV and hepatitis B among blood donors and estimated residual risk of transmission of HIV and HBV virus by blood transfusion. A study at the Provincial General Referee Hospital Bukavu, Democratic Republic of the Congo. Rev Epidemiol Sante Publique 2013; 61:139–44. [DOI] [PubMed] [Google Scholar]
- 34. Hsu HY, Chang MH, Ni YH et al. Universal infant immunization and occult hepatitis B virus infection in children and adolescents: a population-based study. Hepatology 2015; 61:1183–91. [DOI] [PubMed] [Google Scholar]
- 35. Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. J Hepatol 2017; 66:645–54. [DOI] [PubMed] [Google Scholar]
- 36. Shilaih M, Marzel A, Scherrer AU et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016; 214:599–606. [DOI] [PubMed] [Google Scholar]
- 37. Kouassi-M’Bengue A, Boni C, Ouattara D et al. Co-infection of HIV and HBV in voluntary counseling and testing center in Abidjan. Asian Pac J Trop Dis 2011; 1:275–8. doi:10.1016/S2222-1808(11)60064-9 [Google Scholar]
- 38. Lemoine M, Shimakawa Y, Njie R et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: the Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. Lancet Glob Health 2016; 4:e559–67. [DOI] [PubMed] [Google Scholar]
- 39. Hegdahl HK, Fylkesnes KM, Sandøy IF. Sex differences in HIV prevalence persist over time: evidence from 18 countries in sub-Saharan Africa. PLoS One 2016; 11:e0148502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Touré-Fall AO, Dièye TN, Sall A et al. Residual risk of transmission of HIV and HBV, in Senegalese national blood bank from 2003 to 2005. Transfus Clin Biol 2009; 16:439–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.