Abstract
Current methods for examining antibody trafficking are either non-quantitative such as immunocytochemistry or require antibody labeling with tracers. We have developed a multiplexed quantitative method for antibody ‘tracking’ in endosomal compartments of brain endothelial cells. Rat brain endothelial cells were co-incubated with blood-brain barrier (BBB)-crossing FC5, monovalent FC5Fc or bivalent FC5Fc fusion antibodies and control antibodies. Endosomes were separated using sucrose-density gradient ultracentrifugation and analyzed using multiplexed mass spectrometry to simultaneously quantify endosomal markers, receptor-mediated transcytosis (RMT) receptors and the co-incubated antibodies in each fraction. The quantitation showed that markers of early endosomes were enriched in high-density fractions (HDF), whereas markers of late endosomes and lysosomes were enriched in low-density fractions (LDF). RMT receptors, including transferrin receptor, showed a profile similar to that of early endosome markers. The in vitro BBB transcytosis rates of antibodies were directly proportional to their partition into early endosome fractions of brain endothelial cells. Addition of the Fc domain resulted in facilitated antibody ‘redistribution’ from LDF into HDF and additionally into multivesicular bodies (MVB). Sorting of various FC5 antibody formats away from late endosomes and lysosomes and into early endosomes and a subset of MVB results in increased antibody transcytosis at the abluminal side of the BBB.
Keywords: Intracellular trafficking, blood–brain barrier, mass spectrometry, selected reaction monitoring
Introduction
The principal obstacle in developing therapeutic antibodies that target the central nervous system (CNS) is their insufficient penetration through the blood–brain barrier (BBB). The tight junctions of BBB endothelial cells (BEC) prevent the free diffusion of hydrophilic molecules greater than 400 Da from the blood into the brain parenchyma.1,2 Some naturally occurring BBB receptors for circulating protein ligands, including transferrin receptor (TfR), insulin receptor and low density lipoprotein receptor-related protein (LRP1),3 undergo receptor-mediated transcytosis (RMT) and recycling; the RMT process results in a portion of the bound ligand being released at the abluminal side of the BBB into brain parenchymal space. Antibody or peptide ligands developed against these receptors have been exploited to ‘transport’ linked therapeutic ‘cargo’ including macromolecules across the BBB. Several antibodies have been developed as molecular Trojan horses for brain delivery of macromolecules, including various antibody formats against TfR,4–7 humanized IgG against human insulin receptor (Insr),8,9 antibodies against the heavy subunit of the large neutral amino-acid transporter (Lat1; Cd98)10 and species cross-reactive camelid single-domain antibody, FC5.11–13 Notably, FC5 has been shown to bind a glycosylated epitope of an antigen, putatively identified as Tmem30a, enriched in BEC, and to transmigrate across BEC monolayer via a saturable, directional, energy- and microtubule-dependent pathway, triggered by the antibody internalization via clathrin-coated vesicles.14 Furthermore, FC5 has been shown to deliver a payload, including peptides and antibodies, to their CNS targets.11,13
Recent experience with antibodies against TfR as BBB-delivery carriers revealed that antibody characteristics, including affinity and valency, can dictate mechanisms of internalization, intracellular sorting and degradation in BEC and ultimately its transcytosis into the brain parenchyma. For example, decreasing antibody affinity5,15 and using a mono-valent anti-TfR antibody reduced intracellular degradation in BEC and enhanced transcytosis.7 These modifications also improved the circulating half-life of bi-specific antibodies and enhanced the pharmacodynamic effect of therapeutic cargos, anti-BACE-15,15 and anti-β-amyloid7 antibodies. Lowering the affinity of TfR antibody binding at acidic pH (∼5.7) found in the endocytic compartments, resulted in improved antibody release at the abluminal side in in vitro BBB model.16 The TfR antibodies used in studies above were not raised against the same TfR epitope and it therefore remains uncertain whether the observations can be generalized for all TfR antibodies developed as BBB carriers. In contrast to TfR antibodies, BBB-crossing sdAb FC5 demonstrated improved transcytosis across the BBB in vitro and in vivo in a higher affinity bi-valent- compared to mono-valent Fc fusion.11 In this study, we interrogated mechanisms of endocytic sorting of mono- and bi-valent FC5 formats which regulate in their transcytosis across BEC.
Current methods for examining antibody trafficking are semi-quantitative and/or require antibody labeling with fluorescent tracers; with these methods, the intracellular compartments are typically characterized using a singular marker (e.g. Rab5a for early endosomes or Lamp1 for lysosomes) and some morphological features of intracellular vesicles.17 Improved quantitative measurements of intracellular sorting and trafficking of antibodies targeting RMT receptors are necessary to understand antibody–ligand interactions which accelerate their directional transcytosis and abluminal cargo release and diminish their intracellular degradation. We describe the application of a label-free, quantitative method for multiplexed simultaneous quantification of antibodies and multiple markers of endosomal fractions using targeted mass spectrometry. The method was used to quantify various FC5 formats in brain endothelial cell endosomal compartments and their transcytosis across the BBB. The results show that bivalent display of FC5 and the presence of Fc fragment enhance both antibody sorting into early endosomes and a subset of multivesicular bodies (MVBs), and its release at the abluminal side of the BBB. From the larger panel of antibodies examined, it was concluded that the ability of the antibody to transcytose BBB endothelium inversely correlates with the proportion of the antibody sorted into late endosomes.
Materials and methods
Antibodies tested in this study
The camelid single-domain antibodies (VHH) antibodies examined in this study are as follows: A20.1, a VHH against C. Difficile toxin A (non-mammalian target);18 EG2, a VHH against epidermal growth factor receptor Egfr;19 FC5, a BBB-crossing VHH.20 The antibodies were used as single domains (VHHs) or fused to N-terminus of human Fc in either bi-valent (Bi-FC5Fc and Bi-A20.1Fc) or mono-valent (Mono-FC5Fc) format.11 The antibody structure and characteristics are detailed in Supplementary Table 1. All antibodies were produced and purified as described in respective citations.
In vitro BBB model
An immortalized adult rat brain microvascular endothelial cell-line, SV-ARBEC, established at National Research Council of Canada by SV-40 transfection of primary rat BEC isolated from 24 to 30 days old Sprague-Dawley rats21 were used for endosome isolation (see below) and for in vitro BBB permeability assays, using recently described protocols.11,22
For endosomal fractionation, SV-ARBEC were grown to confluence on rat tail collagen type I-coated plastic dishes, as described previously.21 BEC grown on the collagen-coated plastic secrete and deposit other basement membrane components and establish polarity of transporter and receptor expression on their luminal and abluminal membranes.23,24
For transport experiments, SV-ARBEC were seeded at 80,000 cells/membrane on rat-tail collagen coated 0.83 cm2 Falcon transparent PET membrane inserts with 1 μm pore size (VWR International, Mississauga, Ontario) in 1 mL SV-ARBEC feeding media without phenol red. The inserts were placed in the wells of a 12-well tissue culture plate containing 2 mL of 50:50 (v/v) mixture of SV-ARBEC media without phenol red and with rat astrocyte-conditioned media to generate an in vitro model of the BBB as described previously.21 Upon culturing, a barrier phenotype develops restricting the passage of molecules between chambers; paracellular permeability for radioactively labeled [3H]-sucrose was monitored as described previously,21 and the cultures used only when Pe[sucrose] was between 0.4 and 0.6 [×10−3] cm/min. The model has been shown to restrict paracellular passage of peptides, antibody fragments and antibodies.11,13 Transport experiments were performed as described22 by adding an equimolar mixture of antibodies to the top chamber and by collecting a 100 µl aliquot from the bottom chamber at 15, 30, 60 and 90 min for simultaneous quantification of the antibodies using the SRM method. The apparent permeability coefficient Papp was calculated as described previously.25
Endosome isolation
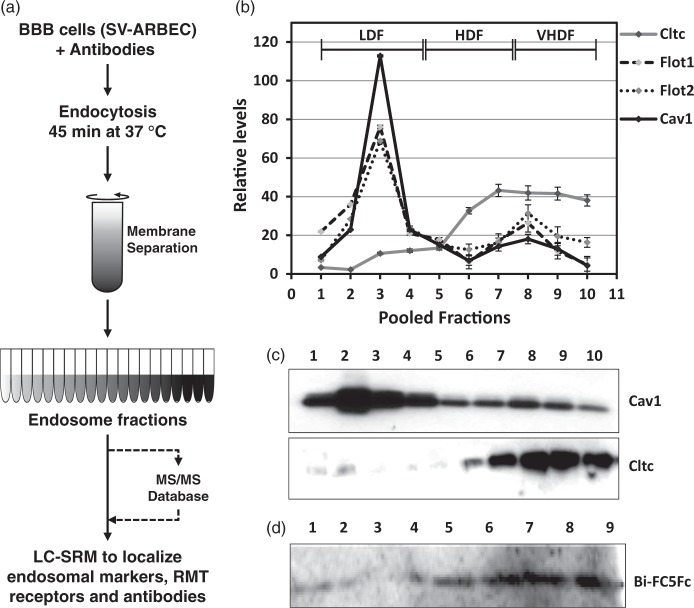
Four 150 mm confluent dishes of SV-ARBECs were washed with HBSS (ThermoFisher, Waltham, MA) and incubated with 5 µg/ml of appropriate antibodies for 45 min to trigger the RMT pathway. At the end of incubation, cells were washed twice with HBSS and scraped in ice-cold Buffer A (250 mM sucrose, 20 mM tricine, 1 mM EDTA) at 4℃. The suspension was homogenized using loose Dounce homogenizer (20 strokes) on ice. The homogenate was centrifuged at 1000 × g (Eppendorf 5417R) for 10 min at 4℃ and the supernatant (postnuclear fraction) was transferred to a fresh tube. The pellet was re-homogenized and re-centrifuged, and the resulting supernatant added to postnuclear fraction. The fraction was overlaid on 23 ml of 30% Percoll, diluted in Buffer A and centrifuged at 84,000 × g for 30 min at 4℃ in Optima TLX ultracentrifuge with 60 Ti rotor (Beckman Coulter, Mississauga, Canada). Plasma membrane (opaque-white top layer) was collected and transferred to a fresh ultracentrifuge tube, to which 1.84 ml of Buffer B (50% Optiprep, 250 mM sucrose, 120 mM Tricine, 6 mM EDTA) and 0.16 ml of Buffer A was added. The layer was overlaid with 3.5 ml of 20% and 3.5 ml of 10% Optiprep. The gradient was centrifuged at 100,000 × g (Beckman) for 90 min in a SW40 rotor at 4℃. The separation was split into top and bottom parts and transferred to separate tubes. Each one was mixed with 4 ml of Buffer B and overlaid with 2 ml of 5% Optiprep. The gradient was centrifuged at 100,000 × g (Beckman) for 18 h in an SW40 rotor at 4℃. A total of 20 equal fractions were manually collected and prepared for mass spectrometry. Generic cell fractionation procedure is shown in Figure 1(a).
Figure 1.
BBB endosome isolation and SRM analysis. (a) Workflow outlining the method for isolation of endosomes for analysis with LC-SRM. Details are described in Methods section. (b) Relative levels clathrin (Cltc), caveolin-1 (Cav1) and flotilin-1 (Flot1) and -2 (Flot2), markers various types of endocytic vesicles (clathrin-coated pits, caveolae and clathrin-independent endocytic vesicles, respectively) in endosomal fractions of BBB SV-ARBEC cells exposed to 5 mg/ml of Bi-FC5Fc for 45 min. The levels are determined using multiplexed LC-SRM. Shown are relative abundance (mean ± SD) of protein-specific peptides values from three endosome preparations. Fractions 1–4 are designated low-density fractions (LDFs); fractions 4–7 high-density fractions (HDFs); fractions 7–10 vHDFs. (c) Levels of clathrin and caveolin-1 in the same endosomal fractions detected by Western blot. (d) Levels of bivalent BBB-crossing antibody Bi-FC5Fc in the same endosomal fractions detected by Western blot.
Western blot
Primary rabbit polyclonal antibodies used for Western blotting were anti-VHH (Biogen Idec, Cambridge MA, USA), anti-caveolin-1 (N-20) sc-894 (Santa Cruz, CA, USA), anti-Eea1 ab2900 (Abcam, ON, Canada), anti-Lamp2 ab203224 (Abcam), anti-M6pr ab124767 (Abcam) or anti-clathrin HC (H-300) sc-9069 (Santa Cruz) antibodies.
Each endosome fraction was boiled for 5–10 min in Laemmli buffer (BioRad, Hercules, CA, USA), containing fresh 5% beta-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). Protein extracts were resolved on a 12% discontinuous SDS-PAGE and either silver stained or electrophoretically transferred onto nitrocellulose membranes (Millipore, Nepean, Canada). Membranes were blocked in 5% non-fat dry milk powder in TBST buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 0.02% Tween-20) for 2 h. Primary rabbit antibody was diluted at 1:1000 in 2.5% milk in TBST and incubated with the membranes for 18 h at 4℃. Membranes were washed four times in TBST and then incubated for 1 h with goat anti-rabbit-HRP (Sigma-Aldrich), diluted 1:8000 in TBST. Membranes were washed four times with TBST and then developed by ECL Plus Chemiluminescent Substrate (GE Healthcare).
Sample preparation for mass spectrometry
Filtered-aided sample preparation method was used to prepare the samples for mass spectrometry.26 Briefly, each endosome fraction was reduced in 3.5% SDS, 100 mM Tris-HCl, 100 mM DTT by boiling for 10 min. A 6.6-volume of Urea solution (8 M Urea, 100 mM Tris-HCl, pH 8.5) was added to the sample and they were transferred to pre-wetted Amicon-30 spin columns (Millipore, Billerica, MA, USA) and spun as per manufacturer’s instructions. The proteins were washed three times with the Urea solution, alkylated (10 mM iodoacetamide, 30–60 min at room temperature (RT) in dark), and then washed four times with the Urea solution and four times with 50 mM ammonium bicarbonate. The samples were digested using trypsin at 37℃ and the peptides were eluted for SRM analysis.
Mass spectrometry and selected reaction monitoring (nanoLC-SRM)
All samples were analyzed on a reversed-phase nanoAcquity UPLC (Waters, Milford, MA) coupled to LTQ XL ETD or LTQ Orbitrap ETD mass spectrometer (ThermoFisher, Waltham, MA). The analysis involved injection and loading of the desired aliquot of the sample onto a 300 µm I.D. × 0.5 mm 3µm PepMaps® C18 trap (ThermoFisher) followed by eluting onto a 100 µm I.D. × 10 cm 1.7 µm BEH130C18 nanoLC column (Waters) using a gradient from 0% to 20% acetonitrile (in 0.1% formic) in 1 min, 20%–46% in 60 min, and 46%–95% in 1 min at a flow rate of 400 nL/min. The eluting peptides were ionized into the mass spectrometer by electrospray ionization (ESI) for MS/MS using collision induced dissociation (CID) for fragmentation of the peptide ions. Data were acquired on ions with mass/charge (m/z) values between 400 and 2000 with 1.0 s scan duration and 0.1 s interscan interval. To develop the SRM assay for proteins, samples (pure antibodies and endosome fractions) were first analyzed by nanoLC-MS/MS using data-dependent acquisition to identify ionizible peptides of antibodies and of known RMT receptors and markers of early endosomes, late endosomes and lysosomes (Supplementary Table 2). Once these spectra were validated as unique signatures, multiplexed methods with these signatures were created for SRM analysis to perform targeted quantification of multiple proteins in each fraction. SRM analyses were carried out as previously described.22
Co-localization of antibodies with markers of endosomes and lysosomes by fluorescence microscopy
SV-ARBEC cells were grown to semi-confluence (60,000 cell/coverslip) on glass coverslips coated with the rat tail collagen I in a 24-well plate for two days. Cells were then transduced overnight with 25 µl/coverslip (∼40 particles/cell) of either BacMam 2.0 Early Endosomes-RFP (Rab5-RFP) or Late Endosomes-RFP (Rab7-RFP) (all Life Sciences, Burlington, ON). Cells were washed two times in DMEM (Wisent Bioproducts, St-Bruno, QC) then incubated with neutralized near-infrared fluorescent probe Cy5.5 (Life Sciences, Burlington, ON) diluted in DMEM, or with 5 µg of various antibodies labeled with Cy5.5 at 37℃ for 30 min. Cells were then washed three times in DMEM and two times in PBS. Coverslips were fixed in 4% Formaldehyde in PBS for 10 min at RT, washed three times in PBS and permeabilized in 0.1% TritonX-100 for 3 min. After washing in PBS, cells were stained with 1:2000 Alexa Fluor 488 Phalloidin (Life Sciences, Burlington, ON) for 5 min at RT to label F-actin filaments. After washing in PBS, coverslips were mounted in Dako Fluorescent Mounting Medium (Dako, Burlington, ON) spiked with 2 ug/mL of Hoechst33342 (Life Sciences, Burlington, ON) to stain cell nuclei and were then observed under Olympus 1 × 81 fluorescent microscope (60 × oil objective, NA 1.42).
CSF levels of antibodies
The results for CSF levels of antibodies shown in this manuscript for correlation purposes were compiled from data published by us previously.11,22 All animal procedures were approved by the National Research Council’s Animal Care Committee and were in compliance with the Canadian Council of Animal Care guidelines.
In brief, male Wistar rats aged 8–10 weeks (weight range, 230–250 g) were used for the cannulation of cisterna magna. The technique for multiple sampling of cisterna magna CSF was as described previously.11 All compounds were administered via the tail vein. For sample collection, rats were briefly and lightly anesthetized with 3% isoflurane, and 5 µl of CSF was collected from the collecting portion of the cannula by inserting a 30 Ga needle attached to an insulin syringe. Blood samples were taken from the tail vein as described elsewhere27 using a polymer gel tubes (Becton, Dickinson and Company Franklin Lakes, NJ USA) at the same time points as CSF sampling. Levels of antibodies in serum and CSF were determined by SRM as described before.11,22
Results
BBB endosome isolation and SRM analysis
We first examined whether various types of endosomes can be isolated from BBB cells and analyzed using mass spectrometry. Immortalized rat brain endothelial cells (SV-ARBEC), in the absence and the presence of endocytosis ‘trigger’ (e.g. FC5) were used to develop an endosome separation method, involving total membrane isolation followed by two-step sucrose-density gradient separation (Figure 1(a)). To demonstrate successful endosome separation, each fraction was analyzed using a Western blot for various markers and by a highly sensitive multiplexed nanoLC-SRM assay11,22 to allow simultaneous quantification of various endosome membrane markers and known RMT receptors listed in Supplementary Table 2.
The enrichment of key protein markers of coated pit- and caveolae-dependent and independent endocytosis (clathrin, caveolin-1 and flotilin-1 and -2, respectively), was tracked in each fraction. A strong enrichment of caveolin-1 in low-density fractions (LDFs 1-4) and clathrin in high-density fractions (HDFs 5-7) and very-high density fractions (VHDFs 8-10) of SV-ARBEC was observed by both nanoLC-SRM (Figure 1(b)) and Western blot (Figure 1(c)). Flotilin-1 and -2 were predominantly enriched in LDFs, although they were also found at lower levels in HDFs and VHDFs (Figure 1(b)). Clathrin and caveolin-1 distribution/enrichment in SV-ARBEC cellular fractions was similar at resting state and after the addition of endocytosis-triggering antibody, Bi-FC5Fc (Supplementary Figure 1). After the exposure of cells to Bi-FC5Fc (5 µg/ml; 45 min), internalized fraction of the antibody co-localized predominantly with the clathrin-containing fractions, consistent with the previous report.14
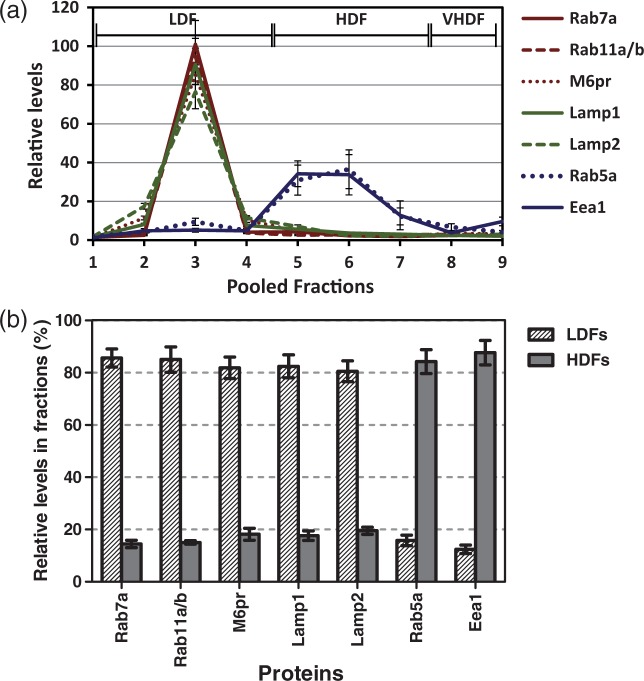
Analyses of early endosome (Rab5a), late endosome (Rab7a) and lysosome (Lamp2) markers in SV-ARBEC fractions at resting state showed a variable distribution across various subcellular fractions (Supplementary Figure 1). After the addition of endocytosis-stimulating antibody (Bi-FC5Fc), a clear re-distribution and separation of these markers between LDFs and HDFs was observed; detailed analyses of these fractions showed that Lamp1/2, M6pr, Rab7a and Rab11a/b were highly enriched (80%) in LDFs (2–4), whereas markers of early endosomes Eea1 and Rab5a were enriched (>70%) in HDFs (4–7) (Figure 2(a) and (b)). The enrichment of M6pr and Lamp2 in LDFs and Eaa1 in HDFs was also confirmed by Western blot analyses (Supplementary Figure 2(a)). Notably Cav-1 was also enriched in LDFs, typically peaking in fraction #2 in different fractionation attempts (Supplementary Figure 2(b)), whereas clathrin-enriched fractions only partially overlapped with Eaa1 and Rab5a fractions (HDFs 4-7) and also included VHDFs (8–10).
Figure 2.
(a) Relative levels of markers of late endosomes (Rab7a, Rab11a/b), lysosomes (Lamp1/2, M6pr) and early endosomes (Eea1, Rab5a) among endosome fractions of BBB cells, measured using LC-SRM. Peptides identified for Rab11 are common between Rab11a and Rab11b. M6pr is cation-dependent mannose-6-phosphate receptor. Shown are relative abundance (mean ± SD) of protein-specific peptides values from at least three endosome preparations. (b) Percentage of the same makers in LDFs and HDFs relative to total fractions as analyzed by LC-SRM. Shown are mean ± SD from at least three independent endosome preparations.
Traffic of VHHs through BBB endosomes
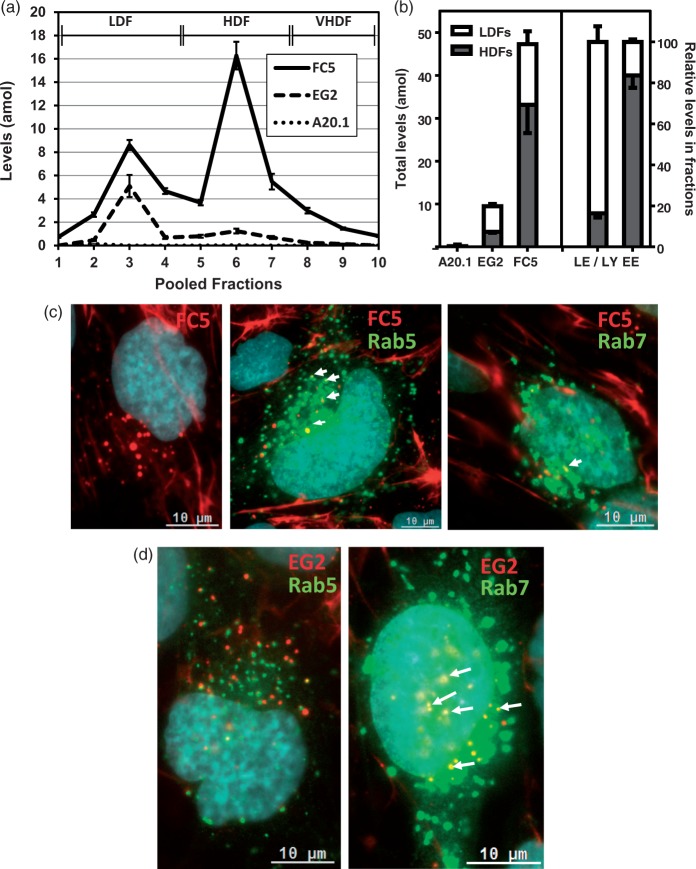
We have previously shown that FC5 VHH can cross the BBB in in vitro and in vivo models.22 The role of endosomal sorting mechanisms in BBB transcytosis was first examined with three VHHs: BBB-crossing FC5, Egfr-binding EG2, and A20.1, which has no mammalian target. SV-ARBEC were co-incubated with all three VHHs for 45 min, and endosomal fractions were examined using multiplexed nanoLC-SRM to simultaneously quantify endosomal markers, RMT receptors and co-incubated antibodies in each fraction. A20.1 was not detectable in any of the fractions (Figure 3(a)), consistent with it being a non-internalizing antibody.22 EG2 showed 75%:25%, whereas FC5 showed 30%:70% distribution between LDFs and HDFs, respectively (Figure 3(a) and (b)). Overall, FC5 showed 5-fold higher levels in the SV-ARBEC endosomes compared to EG2. Similar results were obtained when antibodies were separately incubated and analyzed by SRM (not shown). Interestingly, a putative FC5 receptor, Tmem30a, appeared in HDFs of FC5-stimulated cells (Supplementary Figure 1), suggesting its ‘removal’ from plasma membrane and co-localization in the same endocytic compartments with FC5. Internalization and tracking of Cy5.5-labeled FC5 and EG2 antibodies in SV-ARBEC cells transduced with RFP-containing markers of early (Rab5) and late (Rab7) endosomes demonstrated FC5-Cy5.5 co-localization with both compartments (Figure 3(c), upper panels), whereas EG2-Cy5.5 co-localized only with late-endosomal marker (Rab7) (Figure 3(c), lower panels).
Figure 3.
LC-SRM quantification and fluorescent tracking of VHH levels in SV-ARBEC endosomes. Cells were exposed to a mixture of VHHs, each at 5 µg/ml, for 45 min, prior to endosome fractionation as described in Materials and Methods. (a) Absolute levels of FC5, EG2 and A20.1 in endosome fractions of BBB cells measured using LC-SRM. Absolute levels are presented in attomoles (amol) corrected to 1 µg of total endosomal protein from at least three endosome preparations (mean ± SD). Fractions corresponding to LDFs and HDFs are indicated. (b) Levels of VHHs in LDFs and HDFs as determined by LC-SRM. Total levels of the three VHHs are shown on the left and the mean percentage of the markers of late endosomes/lysosomes (LE/LY) and early endosomes (EE) are shown on the right. Shown are mean ± SD from three independent endosome preparations. LE/LY markers include M6pr, Rab 7,11 and Lamp 1, 2. EE markers include Rab 5 a, Eea1. (c) Internalization of Cy5.5-labeled FC5 into non-transduced SV-ARBEC cells (left panel) and its co-localization (white arrows) with endosome markers in RFP-Rab5 (middle panel) or RFP-Rab7 (right panel) transduced SV-ARBEC. (d) Internalization and co-localization (white arrows) of Cy5.5-labeled EG2 with endosome markers in RFP-Rab5 (left panel) and RFP-Rab7 (right panel) transduced BEC. Cells were transduced and internalization studies performed as described in Materials and Methods. Micrographs are representative of three independent experiments.
The results imply that, while both FC5 and EG2 (but not A20.1) internalize into SV-ARBEC, FC5 endocytosis is more efficient and leads to preferential sorting of FC5 into early endosome compartment, while EG2 is directed into late endosomes and lysosomes.
Traffic of Fc-fused FC5 through BBB endosomes
We next compared the differences in BEC endosomal sorting among A20.1 - and FC5- fusions with human Fc. Both mono-valent and bi-valent FC5 fusions with the human Fc were engineered and produced as described before.11
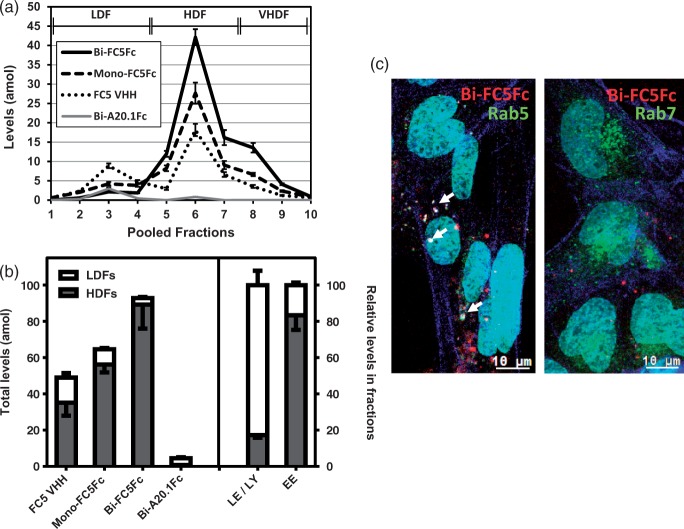
SV-ARBEC were separately incubated with each of the antibodies (FC5 VHH, Mono-FC5Fc, Bi-FC5Fc or control Bi-A20.1Fc) and endosomes were examined using multiplexed nanoLC-SRM. As seen in Figure 4(a), Bi-A20.1Fc showed a minimal internalization, was detectable in LDFs, and was below limits of detection in HDFs. Although all three FC5-containing antibodies had higher levels in HDFs than in LDFs, there was a progressive increase in relative amounts in the early endosome fractions from 70% to 85% to 95% for FC5 VHH, Mono-FC5Fc and Bi-FC5Fc, respectively (Figure 4(b)); the levels in the LDFs were respectively lower.
Figure 4.
LC-SRM quantification and fluorescent tracking of VHH-Fc fusion proteins in SV-ARBEC endosomes. (a) Absolute levels of FC5 VHH, Mono-FC5Fc, Bi-FC5Fc and Bi-A20.1Fc in endosome fractions of SV-ARBEC cells incubated separately with each of the antibodies (5 µg/ml, 45 min) and measured using LC-SRM. Absolute levels are presented in attomoles (amol) corrected to 1 µg of total endosomal protein from at least three endosome preparations (mean ± SD). Fractions corresponding to LDFs and HDFs are also indicated. (b) Levels in LDFs and HDFs as determined by LC-SRM. Total levels of the three antibodies are shown on the left and the mean percentage of the markers of late endosomes/lysosomes (LE/LY) and early endosomes (EE) are shown on the right. Shown are mean ± SD from three independent endosome preparations. LE/LY markers include M6pr, Rab 7,11 and Lamp 1, 2. EE markers include Rab 5a, Eea1. (c) Co-localization (white; indicated by white arrows) of Cy5.5-labeled Bi-FC5Fc (red) with endosome markers in RFP-Rab5 (left panel) and RFP-Rab7 (right panel) (both in green) – transduced BEC. Actin filaments labeled with Alexa Fluor 488 Phalloidin are shown in blue. Nuclei are labeled with Hoechst (shown in turquoise). Cells were transduced and internalization studies performed as described in Materials and Methods. Micrographs are representative of three independent experiments.
Similarly, Bi-FC5Fc-Cy5.5 fluorescence in cells transduced with RFP-Rab5 and RFP-Rab7 showed an exclusive co-localization with the early endosomal marker Rab5 (Figure 4(c); arrows).
We next followed the endosomal distribution of a putative FC5 receptor, Tmem30a,28 as well as two other receptors implicated in RMT, TfR and LRP1. Whereas Tmem30a was not detectable in intracellular fractions of SV-ARBEC at resting state (Supplementary Figure 1(a)), it showed a strong enrichment in HDFs 5–7 (Supplementary Figure 1(c)) upon stimulation with Bi-FC5Fc. TfR and LRP1 showed a similar high enrichment in HDFs (Supplementary Figure 1(c)); relative levels for all receptors examined were 15–20% in LDFs, and 80–85% in HDFs. This suggests that the putative FC5 receptor Tmem30a and other endocytosing receptors are likely present in the same membrane domains that internalize via clathrin-coated pits.
Markers of MVBs
In addition to high enrichment in HDFs 5-7, the profile of endosomal traffic of Bi-FC5Fc, and to a lesser degree Mono-FC5Fc showed an additional distinct peak in VHDFs 8–10 (Figure 4(a)). We postulated that these VHDFs may contain MVBs, known intermediary endosomal structures that either transition to late endosomes and lysosomes29–31 (degradative MVBs) or participate in shedding extracellular microvesicles32 (EMVs or exocytosing MVBs). To verify the composition of VHDFs, we examined MVB-associated proteins Rab27a, Pdcd61p and Cd82 in each BEC fraction. While a major proportion of these proteins were localized to LDFs 1–4, likely degradative MVBs destined to fusion with late endosomes, about 35–45% of Rab27a, Pdcd61p and Cd82 proteins were also found in the VHDFs 8–10 and had a distinct profile from that of early endosome markers (Figure 5), which are enriched in HDFs 5–7. Furthermore, the profile of MVB markers in VHDFs coincided with the ‘third’ peak of Bi-FC5Fc profile observed in these fractions.
Figure 5.
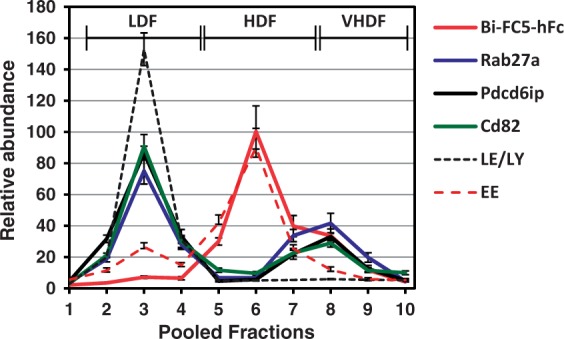
Relative levels of Bi-FC5Fc and multivesicular body (MVB)-associated proteins Rab27a, Pdcd6ip and Cd82 among endosome fractions of SV-ARBEC cells incubated with Bi-FC5Fc (5 µg/ml, 45 min). Also shown are average profiles (mean ± SD from three independent endosome preparations) of the markers of late endosomes/lysosomes (LE/LY) and early endosomes (EE) described in Figure 1.
Antibody BBB transmigration in vitro and in vivo: Endosome sorting vs. BBB transcytosis
In vitro BBB (SV-ARBEC) model21 was used to compare transcytosis of various domain and Fc-fused antibodies – depicted schematically in Figure 6(a) – across endothelial monolayer. The levels of antibodies were also measured in the CSF after an intravenous administration in rat as described.11,13,22
Figure 6.
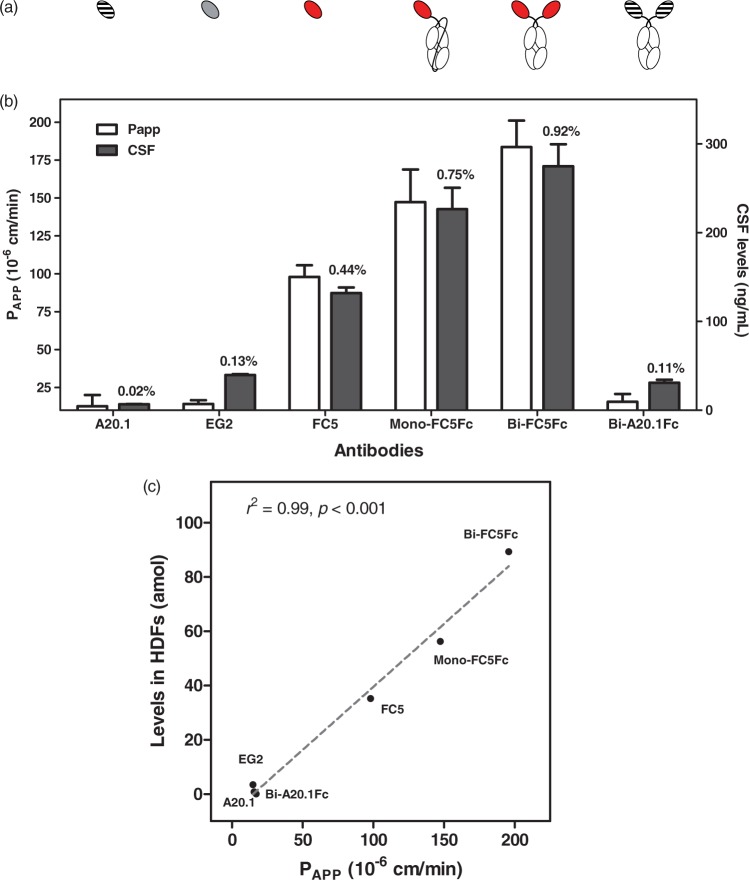
In vitro and in vivo BBB crossing of examined antibodies. (a) Structural representation of tested antibodies. (b) Apparent permeability coefficient (Papp, left axis) and CSF levels (right axis) of the tested antibodies. Papp values were derived from in vitro BBB assay as described in Materials and Methods. CSF levels were measured after iv. administration of a single 7 mg/kg dose of each antibody to rats. CSF levels of single-domain antibodies (A20.1, EG2, FC5) and Fc fusion molecules (Mono-FC5Fc, Bi-FC5Fc) were determined 30 min and 24 h after iv injection, respectively (combined data from Haqqani et al.22 and Farrington et al.11). CSF/serum ratios are indicated above the corresponding bars. Results are mean ± SD from at least four experiments. (C) Correlation between the in vitro BBB permeability coefficient (Papp) and levels of six antibodies examined in this study in HDFs of BEC endosomes.
FC5 showed a significantly higher apparent permeably coefficient (PApp) than either EG2 VHH (an internalizing antibody to Egfr) or A20.1 VHH (a non-internalizing antibody to C. Difficile toxin A)11,22 (Figure 6(a), left axis corresponding to PApp values). Similarly, FC5 showed a significantly higher level than either A20.1 or EG2 in CSF of systemically dosed rats (Figure 6(a), right axis corresponding to CSF levels).22
FC5 expressed in fusion with the human Fc fragment in either monovalent (Mono-FC5Fc) or bi-valent (Bi-FC5Fc) format demonstrated improved in vitro Papp (Figure 6(b)), enhanced CSF levels (Figure 6(b), data from Farrington et al.11) and improved pharmacodynamics potency11 compared to monomeric FC5 VHH. Bi-FC5Fc demonstrated advantage over Mono-FC5Fc in all assays above (Figure 6(b)11). Both prolonged circulation half-life due to FcRn-mediated recycling and bi-valent FC5 display have been shown to play a role in improved in vivo CNS exposure.11 In contrast, bivalent A20.1Fc showed no apparent crossing of in vitro BBB and very low levels in CSF (Figure 6(b)).
Next, levels of antibodies (A20.1, EG2, FC5, Bi-A20.1Fc, Mono-FC5Fc, Bi-FC5Fc) in SV-ARBEC endosomes were compared with their BBB-crossing ability (in vitro and in vivo). There was a strong correlation (R2 = 0.99, P < 0.001, N = 6) between antibody levels in HDFs and their in vitro transcytosis across SV-ARBEC (Figure 6(c)). In addition, the in vivo CSF levels of these antibodies also correlated with their HDF levels (not shown). In contrast, direct correlations between LDF levels and transcytosis of these antibodies both in vitro and in vivo were weak and not significant (data not shown).
Discussion
RMT is a principal route for directional delivery of antibodies from the circulation, across BEC and into brain parenchyma. A potential to deliver antibody therapeutics across the BBB, demonstrated recently with several BBB carrier antibodies, including TfR antibodies5–7 and FC5,11,13 has generated a renewed debate on mechanisms of internalization, intracellular traffic and polarized abluminal release of antibodies developed to cross the BBB via RMT. This study tracked the endocytic sorting and transcytosis of various internalizing and BBB-crossing antibody formats in BEC using a novel, quantitative mass spectrometry approach. We demonstrated a quantitative correlation between antibody sorting into early endosomes and a subset of MVBs, and the efficiency of their BBB transcytosis in vitro and in vivo.
In vitro studies have implicated at least two different types of internalizing vesicles in RMT, clathrin-coated pits and caveolae. BBB-crossing TfR antibodies33 and sdAb FC514 internalize into BEC via clathrin-dependent endocytosis. The role of caveolae in BBB RMT remains unclear; LDL has been shown to cross in vitro BBB model via a caveolae-dependent pathway which includes multivesicular organelles related to caveosome structures.34 An up-regulation of caveolae-mediated bulk-phase transcytosis has been observed in animals with the knock-down of DHA transporter mfsd2a.35 The third, clathrin- and caveolin-independent endocytosis pathway mediated by assembly of flotillin-1 and -2 in specific, laterally mobile membrane microdomains has been implicated in cholera toxin B and GPI-anchored protein endocytosis,36–38 but has not been studied as potential transcytosis route across the BBB. The exposure of BEC to FC5 resulted in FC5 co-localization with clathrin in HDFs and VHDFs, containing markers of early endosomes and MVBs, respectively. In contrast, caveolin-1 and flotilins were predominantly enriched in LDFs, also containing markers of late endosomes and lysosomes.
To examine how the intracellular fate of antibodies internalized into BEC affect their ability to transcytose the barrier in vitro and in vivo, antibodies and multiple endosomal markers were tracked in 20 cellular fractions using multiplexed quantitative SRM. The results were supported with immunofluorescence co-localization studies. A20.1, which does not have a target expressed in BEC, did not internalize and showed a minimal crossing of the BBB in vitro and in vivo.22 EG2 underwent Egfr-mediated internalization and trafficked into late endosomes/lysosomes, showing slightly higher BBB crossing in vitro and in vivo22 compared to A20.1. FC5, which engages glycosylated epitope on the luminal side of BEC,28 internalized into BEC, distributed 70%:30% between early endosomes and late endosomes, respectively, and was efficiently released on the abluminal side of the BEC monolayer.
To further examine whether the addition of Fc fragment and valency of FC5 binding to its receptor affect its endosomal sorting and transcytosis across the BBB, three FC5 variants, a monomeric FC5 VHH, monovalent- and bi-valent FC5 fusion to human Fc were compared. An Fc domain of IgG confers prolonged circulatory half-life to monoclonal antibodies through the binding, internalization and recycling in endothelial cells mediated by neonatal Fc receptor (FcRn).39 FcRn rescues IgG from intracellular lysosomal degradation by recycling it from the sorting endosome to the cell surface.40 The rat FcRn affinities to rat and human IgG have been shown to have highly comparable Kd values, with virtually identical off rates.41
Addition of Fc fragment to non-internalizing A20.1 resulted in detectable intracellular levels of A20.1Fc, mostly in late endosome/lysosome fractions; since A20.1 has no mammalian target, cellular internalization of A20.1Fc was interpreted as FcRn-mediated. Similarly, A20.1Fc showed slightly higher Papp values in vitro and measurable CSF levels in vivo compared to A20.1, suggesting that Fc fragment may contribute to low, but detectable non-specific penetration of antibodies into the brain described in several studies.5,6,13 A recent study42 using high-resolution quantitative microscopy showed that lysosomal degradation of low levels of IgGs internalized into BEC from systemic circulations may be further limiting the antibody access to the brain.
Compared to the monomeric BBB-crossing antibody FC5, both Mono-FC5Fc and Bi-FC5Fc demonstrated a significantly improved BBB transcytosis in vitro and in vivo.11 The examination of intracellular sorting of these variants showed a higher proportion of Fc-containing FC5 variants in early endosomes. Therefore, Fc fragment may facilitate sorting of antibodies targeting BBB RMT receptors away from degradative pathways thereby increasing their abluminal release. A bi-valent FC5 receptor engagement with Bi-FC5Fc contributed further to this lysosomal ‘exit’, resulting in 90% of antibody being sorted into early endosomes, as well as into VHDFs containing markers of recycling and exocytosing MVBs, Rab27a, Pdc6ip, and CD82.43–45 This is in contrast to TfR antibodies, where mono-valent target engagement was found to stimulate transcytosis by avoiding receptor cross-linking and lysosome degradation.7,16 Tmem30a,28 a putative FC5 receptor, distributed in the same fractions as FC5; other known RMT receptors, TfR and LRP1, showed a predominant (70%) distribution in early endosomes and partial (30%) distribution in late endosomes.
Overall, a very strong correlation was established between the amount of internalizing antibody sorted into early endosomes and its ability to externalize at the abluminal side of BEC in vitro (this study) and its measured exposure in the CSF.11 The temporal dynamics of the appearance of FC5-containing constructs in the CSF11 correlates with their enhanced levels in the brain parenchyma,13 and their ability to enable central pharmacological responses of neuropeptide11 or antibody13 ‘payloads’ that do not cross the BBB. Based on these observations, we could speculate that similar trafficking mechanisms observed in cultured BEC determine the fate – between degradation and facilitated transcytosis into brain parenchyma – of FC5-containing antibodies in vivo.
Whereas the molecular mechanisms of endocytosis and recycling triggered by specific receptor-ligand interactions, notably transferrin binding to TfR, have been studied extensively in various cell types,42,46 the intracellular sorting of antibodies that leads to their polarized transport and transcytosis across brain endothelium is less well understood.47 Some specific pathways favoring transcytosis have started to emerge from this study; in particular, it appears that a subset of MVBs bearing markers of exocytosing MVBs may be an important pathway leading to abluminal exocytosis of the BBB-crossing FC5 antibody. MVBs were initially regarded as purely prelysosomal structures along the degradative endosomal pathway of internalized proteins.48 MVBs are now known to be involved in numerous endocytic and trafficking functions; after sorting in the MVB, cargo can be routed back to the plasma membrane, moved to internal vesicles for extracellular release (as EMVs), or retained within the membrane of internal vesicles for eventual lysosomal degradation.48,49 The current study shows the association of BBB-crossing antibody formats with known markers of MVBs; interestingly only 40% of the overall content of these biomarkers was associated with the unique, very high density population of vesicles that also contained BBB-crossing antibodies; the remaining 60% of these biomarkers was associated with late endosomes. The authors postulate that the exocytosing MVBs and late endosomes – precursors to degradative MVBs, are generated from a common precursor vesicle in which unique receptor-associated molecular chaperons determine the ‘directional’ fate of receptor-bound antibody. The endosomal vesicular trafficking is a dynamic process that, after being triggered by the endocytosing receptor-ligand interaction, generates many transitional sorting vesicles that were not fully resolved by the fractionation method used. Further improvement of our ability to molecularly characterize the subsets of transitional endosomal vesicles will be required to fully understand the regulation of receptor-specific sorting pathways.
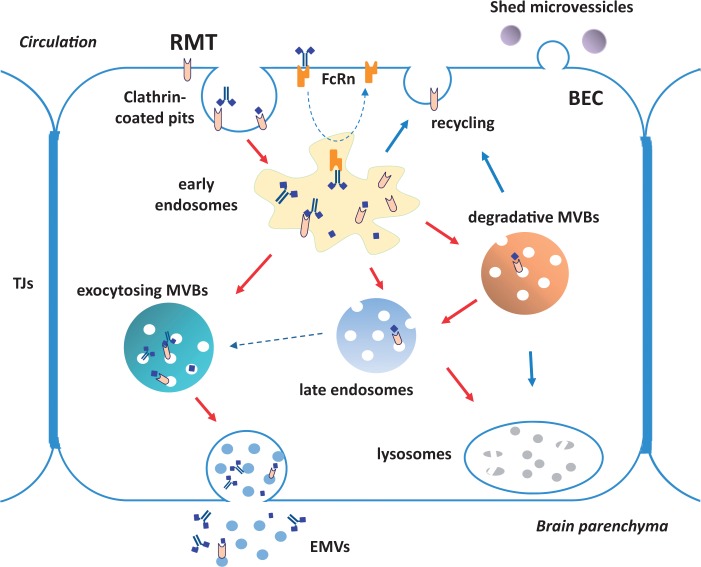
EMVs are secreted vesicles arising from the fusion of MVBs with the plasma membrane.32 Polarized signaling potentially directs MVBs to a specific point in the plasma membrane to mediate a focal delivery of EMVs.50,51 We have recently shown that FC5 is found in EMVs shed from human BEC exposed to the antibody.22 The exposure of BEC to FC5 tripled the amount of secreted EMVs and increased the association of putative FC5 receptor Tmem30a with the EMVs. Furthermore, other known RMT receptors, including TfR, IR, and LRP1 were also found to be associated with BEC EMVs. This led us to hypothesize that EMVs might function as transcytosing vesicle involved in release of RMT receptor ligands on the abluminal side of the BBB. For the BBB-crossing FC5, we suggest a pathway of transcytosis (shown in Figure 7) that involves preferential receptor-specific sorting into early endosomes and, further rerouting into exocytosing MVBs. Since Bi-FC5Fc shows different endocytic sorting profile from that of FC5, we can only postulate that it might also use EMV route for abluminal externalization. It remains to be studied whether potential EMV pathway for transcytosis is generic or RMT-receptor-specific and how it can be modulated to enhance antibody delivery.
Figure 7.
A schematic diagram of the proposed mechanisms/pathways of intracellular sorting and trafficking of the BBB-crossing antibody FC5. FC5 (monomeric or Fc fusion) internalizes into BEC via clathrin-coated vesicles and is initially sorted to early endosomes. FC5 fusion to Fc fragment (mono- or bi-valent) also triggers FcRn-mediated recycling and further re-directs the antibody away from lysosomal degradation. At least partial dissociation of FC5 from its receptor likely occurs in early endosomes under acidic pH, and the receptor is recycled back to plasma membrane. Monomeric FC5 is partially sorted to late endosomes and likely via degradative MVBs into lysosomes; however, a major portion of monomeric FC5 and virtually all of internalized bi-valent FC5Fc fusion are ‘retained’ in early endosomes and are released on the abluminal side of BEC, either as free antibody or via EMVs.
This study demonstrates that the efficiency of antibody transcytosis across the BBB may be increased by deploying antibody engineering strategies that direct the antibody into specific endocytic sorting pathways; these strategies are likely receptor specific, and may involve its mono- bi- or multi-valent engagement, modulating pH sensitivity of antibody-receptor interactions or mobilization of chaperones (such as FcRn) that support antibody exit from lysosomes.
Supplementary Material
Acknowledgements
The authors thank Mr. L. Tessier and Ms. Anna Robotham for their technical assistance with mass spectrometry.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
ASH and DBS designed the study. CED performed cellular fractionation, sample cleanup, Western blots, and SRM. TLT and WD performed sample cleanup and SRM. EwaB performed cell culturing, BBB assay and immunochemistry. EricB, GKF, WS and JE prepared/purified the antibodies. ASH and DBS supervised study, interpreted the data, and contributed in the writing and revision of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron 2002; 36: 555–558. [DOI] [PubMed] [Google Scholar]
- 3.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res 2007; 24: 1759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 1991; 259: 66–70. [PubMed] [Google Scholar]
- 5.Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 2011; 3: 84ra44. [DOI] [PubMed] [Google Scholar]
- 6.Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 2014; 6: 261ra154. [DOI] [PubMed] [Google Scholar]
- 7.Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014; 81: 49–60. [DOI] [PubMed] [Google Scholar]
- 8.Coloma MJ, Lee HJ, Kurihara A, et al. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res 2000; 17: 266–274. [DOI] [PubMed] [Google Scholar]
- 9.Boado RJ, Hui EK-W, Lu JZ, et al. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol 2010; 146: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuchero YJY, Chen X, Bien-Ly N, et al. Discovery of novel blood-brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 2016; 89: 70–82. [DOI] [PubMed] [Google Scholar]
- 11.Farrington GK, Caram-Salas N, Haqqani AS, et al. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J 2014; 28: 4764–4778. [DOI] [PubMed] [Google Scholar]
- 12.Stanimirovic D, Kemmerich K, Haqqani AS, et al. Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv Pharmacol 2014; 71: 301–335. [DOI] [PubMed] [Google Scholar]
- 13.Webster CI, Caram-Salas N, Haqqani AS, et al. Brain penetration, target engagement, and disposition of the blood-brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J 2016; 30: 1927–1940. [DOI] [PubMed] [Google Scholar]
- 14.Abulrob A, Sprong H, Van Bergen en Henegouwen P, et al. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem 2005; 95: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 15.Bien-Ly N, Yu YJ, Bumbaca D, et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med 2014; 211: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sade H, Baumgartner C, Hugenmatter A, et al. a human blood-brain barrier transcytosis assay reveals antibody transcytosis influenced by pH-dependent receptor binding. PLoS One 2014; 9: e96340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aloisi AL, Bucci C. Rab GTPases-cargo direct interactions: fine modulators of intracellular trafficking. Histol Histopathol 2013; 28: 839–849. [DOI] [PubMed] [Google Scholar]
- 18.Hussack G, Arbabi-Ghahroudi M, van Faassen H, et al. Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J Biol Chem 2011; 286: 8961–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal U, Trojahn U, Albaghdadi H, et al. Kinetic analysis of novel mono- and multivalent VHH-fragments and their application for molecular imaging of brain tumours. Br J Pharmacol 2010; 160: 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanha J, Dubuc G, Hirama T, et al. Selection by phage display of llama conventional V(H) fragments with heavy chain antibody V(H)H properties. J Immunol Meth 2002; 263: 97–109. [DOI] [PubMed] [Google Scholar]
- 21.Garberg P, Ball M, Borg N, et al. In vitro models for the blood-brain barrier. Toxicol Vitr 2005; 19: 299–334. [DOI] [PubMed] [Google Scholar]
- 22.Haqqani AS, Caram-Salas N, Ding W, et al. Multiplexed evaluation of serum and CSF pharmacokinetics of brain-targeting single-domain antibodies using a NanoLC-SRM-ILIS method. Mol Pharm 2013; 10: 1542–1556. [DOI] [PubMed] [Google Scholar]
- 23.Redzic ZB, Biringer J, Barnes K, et al. Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem 2005; 94: 1420–1426. [DOI] [PubMed] [Google Scholar]
- 24.Haqqani AS, Hill JJ, Mullen J, et al. Methods to study glycoproteins at the blood-brain barrier using mass spectrometry. Methods Mol Biol 2011; 686: 337–353. [DOI] [PubMed] [Google Scholar]
- 25.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 1991; 175: 880–885. [DOI] [PubMed] [Google Scholar]
- 26.Wiśniewski JR, Zougman A, Nagaraj N, et al. Universal sample preparation method for proteome analysis. Nat Meth 2009; 6: 359–362. [DOI] [PubMed] [Google Scholar]
- 27.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim 2000; 34: 372–378. [DOI] [PubMed] [Google Scholar]
- 28.Abulrob A, Stanimirovic D and Muruganandam A. Blood-brain barrier epitopes and uses thereof. Patent application 12/890,079, USA, 2007.
- 29.Mueller SC, Hubbard AL. Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: receptor-positive and receptor-negative endosomes. J Cell Biol 1986; 102: 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller K, Beardmore J, Kanety H, et al. Localization of the epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J Cell Biol 1986; 102: 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belcher JD, Hamilton RL, Brady SE, et al. Isolation and characterization of three endosomal fractions from the liver of estradiol-treated rats. Proc Natl Acad Sci U S A 1987; 84: 6785–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nat Cell Biol 2012; 14: 654–655. [DOI] [PubMed] [Google Scholar]
- 33.Qian ZM, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 2002; 54: 561–587. [DOI] [PubMed] [Google Scholar]
- 34.Candela P, Gosselet F, Miller F, et al. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium 2008; 15: 254–264. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014; 509: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol 2006; 8: 46–54. [DOI] [PubMed] [Google Scholar]
- 37.Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci 2009; 122: 1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frick M, Bright NA, Riento K, et al. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol 2007; 17: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 39.Giragossian C, Clark T, Piché-Nicholas N, et al. Neonatal Fc receptor and its role in the absorption, distribution, metabolism and excretion of immunoglobulin G-based biotherapeutics. Curr Drug Metab 2013; 14: 764–790. [DOI] [PubMed] [Google Scholar]
- 40.Lencer WI, Blumberg RS. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol 2005; 15: 5–9. [DOI] [PubMed] [Google Scholar]
- 41.Vaughn DE, Bjorkman PJ. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry 1997; 36: 9374–9380. [DOI] [PubMed] [Google Scholar]
- 42.Villaseñor R, Ozmen L, Messaddeq N, et al. Trafficking of endogenous immunoglobulins by endothelial cells at the blood-brain barrier. Sci Rep 2016; 6: 25658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12: 19–30. [DOI] [PubMed] [Google Scholar]
- 44.Katoh K, Shibata H, Suzuki H, et al. The ALG-2-interacting protein alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem 2003; 278: 39104–39113. [DOI] [PubMed] [Google Scholar]
- 45.van den Hoorn T, Paul P, Janssen L, et al. Dynamics within tetraspanin pairs affect MHC class II expression. J Cell Sci 2012; 125: 328–339. [DOI] [PubMed] [Google Scholar]
- 46.Urich E, Schmucki R, Ruderisch N, et al. Cargo delivery into the brain by in vivo identified transport peptides. Sci Rep 2015; 5: 14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Bock M, Van Haver V, Vandenbroucke RE, et al. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016; 64: 1097–1123. [DOI] [PubMed] [Google Scholar]
- 48.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 49.Von Bartheld CS, Altick AL. Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 2011; 93: 313–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escudero CA, Lazo OM, Galleguillos C, et al. The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J Cell Sci 2014; 127: 1966–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.