Abstract
Cancer remains a leading cause of death in the USA and around the world. Although the current synthetic inhibitors used in targeted therapies have improved patient prognosis, toxicity and development of resistance to these agents remain a challenge. Plant-derived natural products and their derivatives have historically been used to treat various diseases, including cancer. Several leading chemotherapeutic agents are directly or indirectly based on botanical natural products. Beyond these important drugs, however, a number of crude herbal or botanical preparations have also shown promising utility for cancer and other disorders. One such natural resource is derived from certain plants of the family Annonaceae, which are widely distributed in tropical and subtropical regions. Among the best known of these is Annona muricata, also known as soursop, graviola or guanabana. Extracts from the fruit, bark, seeds, roots and leaves of graviola, along with several other Annonaceous species, have been extensively investigated for anticancer, anti-inflammatory and antioxidant properties. Phytochemical studies have identified the acetogenins, a class of bioactive polyketide-derived constituents, from the extracts of Annonaceous species, and dozens of these compounds are present in different parts of graviola. This review summarizes current literature on the therapeutic potential and molecular mechanism of these constituents from A.muricata against cancer and many non-malignant diseases. Based on available data, there is good evidence that these long-used plants could have both chemopreventive and therapeutic potential. Appropriate attention to safety studies will be important to assess their effectiveness on various diseases caused or promoted by inflammation.
Introduction
Cancer remains one of the most grave health threats and the leading cause of death in the USA and around the world. The International Agency for Research on Cancer (IARC) reported 14.1 and 8.2 million new cancer cases and deaths, respectively, worldwide in 2012, and are expecting 21.7 and 13 million new cancer cases and deaths, respectively, in 2030 (1). In the USA alone, 1735350 new cases are expected, with 609640 deaths in 2018 (2). Although prognosis of patients with early tumors has significantly improved, limited efficacy and associated toxicity of existing therapies have resulted in significant co-morbidities in patients with advanced tumors. Although these limitations have prompted development of synthetic compounds for molecular targeted therapies, development of resistance to these therapies has also limited their utility (3). Over the past several decades, identification of plant-based drugs has significantly contributed to anticancer drug discoveries and has resulted in one-third of anticancer drugs approved by the United States Food and Drug Administration (USFDA) (4) historically, e.g. paclitaxel, camptothecin, vincristine and their analogs. Given this history, it is critical to continue to explore and identify new medicinal plants and to determine their potential as a source of new drugs. However, many of these plant-derived pure compound drugs were selected based on high potency, target-specific cell killing and, as such, display considerable potential for adverse effects. But beyond these sources of new drug discovery, plant-based medicines have also been used for centuries to treat many ailments and diseases, and their phytochemical constituents are known to possess numerous pharmacologically useful properties. Some of these phytochemicals are well tolerated based on their historical use; they display limited-potency interactions with a broad range of cellular targets and limited overt toxicity. Annona muricata has extensive traditional use, and considerable evidence has been developed that it may be useful therapeutic agents in the battle against certain cancers.
Annona muricata (or graviola, as the fruit is known) belongs to the family of Annonaceae and is commonly known by many names, including Sour Sop, custard apple, guanabana, huanaba, guanabano, guanabana, durian benggala, nangka blanda, toge-banreisi and cachiman epineux (5). Graviola is an evergreen and fruit bearing plant native to the hottest tropical areas of North and South America. It is widely distributed in Venezuela, Central America, Peru, Columbia, Mexico, Brazil, Cuba and India (6). The tree is low-branching, hairy and willowy, with rhombus, tapered leaves which appear as soft, silky, dark green on the upper surface and lighter underneath (7). The weight (0.4–4 kg) of the fruit varies from country to country.
Graviola is very popular in the USA, sold in capsules (leaf and stem powder) and tea under various trade names (6). The fruit flesh and pulp are rich in water, carbohydrate, vitamins and salts, are ideal for drinks and juices and may also be readily eaten (Table I). In the food industry, graviola is extensively used in the preparation of jellies, jams, ice-creams, candies and nectars. Of note, during the ripening processes, graviola synthesizes a strong compound giving it a custard flavor and similar aroma to the fruit. Graviola fruit and fruit juice are often consumed to treat fevers, increase food in nursing mothers and as a mordant for gastrointestinal orders such as diarrhea, dysentery and parasite infection. The bark, leaves and roots of A.muricata have been used in various parts of the world to manage many diseases, including inflammatory conditions, rheumatism, diabetes, hypertension, insomnia, cystitis, parasitic infections and cancer (8–10) (Table II).
Table I.
Chemical composition of graviola
| S.No. | Constituents | Quantity |
|---|---|---|
| A. | Fruit flesh | |
| 1 | Water | 80% |
| 2 | Carbohydrate | 18% |
| 3 | Protein | 1% |
| 4 | Vitamin B | Traces |
| 5 | Vitamin B2 | Traces |
| 6 | Potassium | Traces |
| 7 | Dietary fiber | Traces |
| B. | Fruit pulp | |
| 1 | 2-hexenoic acid methyl ester | 23.9% |
| 2 | 2-hexenoic acid ethyl ester | 8.6% |
| 3 | 2-octenoic acid methyl ester | 5.4% |
| 4 | 2-butenoic acid methyl ester | 2.4% |
| 5 | β-caryophyllene | 12.7% |
| 6 | 1,8- cineole | 9.9% |
| 7 | linalool | 7.8% |
| 8 | α-terpineol | 2.8% |
| 9 | lialyl propionate | 2.2% |
Table II.
Country-wise use of graviola for various ailments
| S. No. | Country | Treatment |
|---|---|---|
| 1 | Peruvian Andes | Combat parasites |
| Treat diabetes | ||
| 2 | Brazilian Amazon | Liver disease |
| Rheumatism | ||
| Neuralgia | ||
| Arthritis | ||
| 3 | Eastern Andes and Jamaica | Diarrhea |
| Relaxant | ||
| Intestinal Acidity | ||
| 4 | Tropical Africa | Skin disease |
| Fever | ||
| 5 | Haiti | Fever |
| Flu | ||
| Cough | ||
| Diarrhea | ||
| Pellagra | ||
| Heart disease | ||
| 6 | Malaysia | Dermatosis |
| Rheumatism | ||
| Cough | ||
| Diarrhea | ||
| Hypertension | ||
| 7 | Trinidad | Flu |
| High blood pressure | ||
| Insomnia | ||
| Baby lactation | ||
| 8 | United States | Cancer |
| Lactation | ||
| Fungal infection | ||
| Hypertension |
The chemistry of Annona muricata
The fruit, bark, leaves and roots of A.muricata are known to be rich in flavonoids, isoquinoline alkaloids and annonaceous acetogenins (ACGs). It has been reported that the stem, leaves and seeds of graviola contain more than 70 acetogenins (11–14), derivatives of long chain C35-C37 fatty acids synthesized through the polyketide pathway. Other Annonaceous species have afforded more than 500 ACGs (15). These compounds are characterized by a C2 combination with 2-propanol to form a lactone ring, and generally by cyclization with oxygen to form one to three tetrahydrofuran or tetrahydropyran moieties (15,16), and complex diastereomeric mixtures are the result. In A.muricata, the ACGs predominantly have a single THF ring, and some non-cyclized analogs have been identified (16). The ACGs are generally waxy substances, difficult to work with because of poor water solubility (17). However, in suitable delivery systems, they readily penetrate into cells and exert biological effects. In this respect, often delivery of such compounds within the complex plant matrix (in the form of crude extract or finely ground and dispersed plant material) can aid absorption compared with the purified lipophilic compounds.
Pharmacology of graviola
A number of pharmacological activities of A.muricata in vitro as well as in vivo have been reported and are now being extensively studied. The main biological activities of the leaf, fruit, fruit-pulp and bark extract are described below.
Anticancer activity
Graviola has been found effective against many cancers in vitro (Table III). A detailed description and molecular mechanisms of the antiproliferatve effects of graviola against various cancers are described below. The active ACGs have been shown to successfully induce death in cancer cells resistant even to chemotherapeutic drugs (5).
Table III.
Mechanism of graviola-induced anticancer effects
| Cancer Type | Models used | Graviola Extract | Outcome | (Ref) |
|---|---|---|---|---|
| Pancreatic cancer | in vitro and in vivo | Finely milled graviola leaf/stem powder | Metabolic catastrophe by downregulation of HIF-1α, GLUT1, GLUT4, HK2 and LDHA; decreased cell motility and invasion by downregulating MUC4, Tumor regression | (19) |
| Colon cancer | in vitro | Leaf extract | Antiproliferative | (20) |
| in vitro | Leaf extract | Apoptotic | (38,63) | |
| in vitro | Leaf extract | Cytotoxic | (40–42) | |
| Lung cancer | in vitro | Leaf extract | Cell cycle arrest | (27) |
| Prostate cancer | in vitro | Fruit pulp extract | Antiproliferative effect by decreasing HIF- 1α expression | (30) |
| Breast cancer | in vitro | Acetogenins from Graviola fruit | Antiproliferative and apoptotic | (31) |
| in vitro and in vivo | Leaf extract | Antitumorigenic and apoptotic | (33) | |
| HNSCC | in vitro and in vivo | Fruit extract | Antitumorigenic and antiproliferative | (34) |
| in vitro | Leaf extract | Antiproliferative | (44) | |
| Hematological malignancies | in vitro | Leaf, twigs and roots | Antiproliferative, cell cycle arrest, loss of MMP | (46) |
| in vitro | Leaf extract | Apoptotic | (47) |
Pancreatic cancer
Pancreatic cancer (PC), the most lethal malignancy and the fourth cause of cancer-related deaths worldwide, has a median 5 year survival rate of only 8%. In the USA, 55440 new cases are reported, and 44330 people are expected to die of this PC in 2018 (2). Lack of early detection markers and clinical symptoms result in frequent late detection of advanced tumors that are difficult to treat and have poor patient prognosis. Late detection, resistance to available chemotherapeutic drugs, and the high aggressiveness of the disease with low patient survival argue for novel early detection markers, and for the investigation and development of chemopreventive and chemotherapeutic agents. Although many natural products have been explored for PC therapeutics, unfortunately, none have translated clinically (18). Our lab reported the antiproliferative and antitumor effects of graviola (capsules containing leaf and stem powder) in PC cells and subcutaneous xenografts; these actions involved induction of cell cycle arrest accompanied by apoptosis (19). Similar antiproliferative effects of the hexane fraction of A.muricata leaves, rich in flavonoids, were reported (20). We further reported that by downregulating mucin MUC4, graviola inhibited PC cell motility and invasion. The mucin MUC4 is a member of the family of glycoproteins, and our lab has extensively established its role in the pathogenesis of various cancers, including PC, ovarian cancers, and head and neck cancers (HNC) (21). We also reported that MUC4, by physical interaction and stabilization of the ErbB family of growth factor receptor tyrosine kinases (RTKs), modulates the PI-3K, Ras/RAF/extracellular signal-regulated kinase (ERK1/2), and STAT signaling pathways that suppress apoptosis and induce resistance to several chemotherapeutic agents (21). MUC4, by regulating Src/FAK signaling, also regulates the invasion and motility of cancer cells and is responsible for the down-regulation of MUC4/Src/FAK signaling pathway by guggulsterone, which has been shown to inhibit motility and invasion of PC cells (22). Keeping in view the significant pathological importance of MUC4 in various cancers, the downregulation of MUC4 indicates the high therapeutic potential of graviola for various tumors, especially PC.
Besides downregulating MUC4, we and others reported that graviola induces cytotoxicity by altering glucose metabolism and induction of metabolic catastrophe (19,23). In this connection, much recent appreciation has arisen for targeting cancer cell metabolism as an antitumor strategy (24). Proliferating tumor cells inexorably requires more energy, and this is normally provided by increased aerobic glycolysis or oxidative phosphorylation. Recent studies show that Kirsten rat sarcoma viral oncogene (KRAS) signaling regulates glycolysis by enhancing the expression of glucose transporter 1 (GLUT1), with several other glycolytic genes, including hexokinase 1/2 (HK1, HK2), phosphofructokinase-1 (PFKl), and lactate dehydrogenase A (LDHA) (25). Of interest, when targeting KRAS signaling, attenuated cellular metabolism was associated with antitumor effects (26). Therefore, modulating glucose metabolism by either reducing glucose uptake or modifying GLUT expression is a possible target to achieve this antiproliferative effect. We were surprised to observe that Graviola-induced metabolic catastrophe caused by down-regulating hypoxia-inducible factor-1α (HIF-1α), GLUT1, GLUT4, HK2, and LDHA expression that was associated with decreased glucose uptake and constrained adenosine triphosphate (ATP) production in PC cells (19) (Table III). A similar observation was reported by Esposti D et al. that acetogenins such as bullatacin promote cytotoxicity by manipulating metabolism and inhibiting the proton pumping function of mitochondrial complex I, thereby inhibiting ATP generation and NADH oxidation (23). These studies suggest that the constituents present in graviola extract inhibit the signaling pathways involved in PC cell growth, proliferation and metabolism.
Lung carcinoma
Lung cancer is the leading cause of cancer-related deaths worldwide and accounts for about 26% (154050) of the deaths in USA in 2018 (2). The majority of lung cancer patients succumb to the disease due to chemotherapeutic resistance. A recent in vitro study using the lung cancer cell line, A549, has shown that graviola leaf extract caused cell cycle arrest at the G0/G1 phase and induced apoptosis. The underlying molecular mechanism revealed that graviola treatment suppresses nuclear factor-κB (NF-κB) signaling, induces reactive oxygen species (ROS) production and increases the Bax/Bcl-2 ratio–mediated attenuation of mitochondrial membrane potential (MMP), cytosolic cytochrome c and caspase-3/9 activation (27) (Table III). Zhao et al. reported similar antitumor effects of graviola in lung cancer cells (28).
Prostate carcinoma
Prostate carcinoma (PCa), with over 164690 estimates and 29430 deaths in 2018, is the second leading cause of cancer-related deaths in the USA (2) and in most Western industrialized countries. Although recent improvement in early detection and new treatment options have improved quality of life, a substantial fraction of these patients develop aggressive and refractory tumors, with very poor prognosis (29). Although many phytochemicals, either as single agents or adjuvants to current therapy, have been tested for prevention or treatment of hormone-refractory PCa, no clinical success has been reported (29). In search of novel natural compounds, recent reports show that a low concentration (≥1 μg/ml) of graviola fruit pulp extract (GPE) has potent antiproliferative activity in a panel of PCa cell lines (22Rv1, LNCaP and PC3), as revealed by MTT and colony formation assay (30). They also reported the antiproliferative effects of GPE that were mediated through decreasing HIF-1α expression and inhibition of NADPH oxidase (NOX) activity (30). Further fractionation of bioactive components revealed that acetogenin derivatives, including muricins J, K and L, were responsible for antiproliferative and apoptotic effects on PCa PC-3 cells (6). There have been similar reports of antitumor effects of pure acetogenins (muricin M, N and muricenin) isolated from a bioactive ethanolic extract (EE) of graviola fruit (31).
Breast cancer
Breast cancer is the most common malignancy in women worldwide, and the second leading cause of cancer-related death in the USA (2). With over 266120 incidences, 40920 women in the USA are expected to die of breast cancer in 2018 (2). Although early stage breast cancer is treatable, no promising therapeutic options are available for advanced breast cancer. There is an immediate need for novel chemopreventive and chemotherapeutic agents to slow tumor growth and decrease associated morbidity. Although many natural products have been tested in vitro and found effective and less toxic compared with small synthetic molecules (32), translational use has always been hampered by the low clinical efficacy of these compounds. However, recent studies have shown the strong antiproliferative and antitumor potential of Graviola using breast cancer cell lines and xenograft mouse models. These studies revealed that graviola inhibited the growth of MCF-7 breast cancer cells by decreasing estrogen receptor (ER), cyclin D1 and antiapoptotic gene Bcl2 expression in cell lines and xenografts (33). Further reports showed that graviola fruit extract also inhibited the proliferation and growth of xenograft tumors of EGFR-overexpressing MDA-MB468 cells, but did not affect the growth of normal MCF-10A cells (34). In addition, butanol and aqueous extract of A.muricata was also found to inhibit proliferation of MDA-MB435S, HaCaT and T47D breast cancer cells.
Multidrug resistance (MDR) is the principal mechanism used by cancer cells to develop therapeutic resistance resulting in treatment failure and the development of refractory tumors. Of interest, ACG bullatacin was shown to be cytotoxic to the multidrug-resistant breast cancer MCF-7/ADR cell line by depleting ATP content (35). Furthermore, ACG Annosquacin B, isolated from the seeds of Annona squamosa, was shown to modulate mitogen-activated protein kinase (MAPK) signaling and induced apoptosis in MCF-7/ADR cells (36). All these studies suggest that ACGs from A.muricata offer a distinct advantage against the MDR breast tumors.
Colon carcinoma
Colorectal carcinoma (CRC) with 140250 incidences in USA is the third leading cause of cancer-associated deaths (50630) in the USA and accounts for ~8% of the total estimated deaths in 2018 (2). Therapeutic resistance and toxicity to available drugs is the greatest problem for CRC and is associated with poor patient prognosis (37). Natural products, including graviola, have played a major role in the prevention of and therapy for CRC. Current research is delineating the molecular mechanism of various extracts of A.muricata against CRC. Furthermore, leaf extract of A.muricata also showed anticancer properties in COLO-205 cell line in enhancing proapoptotic protein caspase-3 (38). Using the same extract, Zorofchian et al. showed cell cycle arrest in the G1 phase, potent inhibition of migration, invasion and apoptosis in CRC cell lines HT-29 and HCT-116 (39). Phytochemical constituents from A.muricata leaves such as annomuricin A, B, C, E and muricapentocin also showed cytotoxicity against CRC HT-29 cells (40–42). ACG desacetyl uvaricin was also shown to induce DNA damage via MAPK pathway inactivation and generation of superoxides that culminate in growth inhibition of SW480 cells (43).
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinomas (HNSCC) is the sixth most common cancer and accounts for over 600000 new cases and 350000 deaths worldwide per year. Cisplatin-based chemoradiation therapy (CRT) is the standard of care for HNSCCs. In addition to cisplatin, other anticancer drugs, including etoposide, topotecan, doxorubicin and fluorouracil, are associated with unfavorable serious side effects that significantly limit their usefulness. Despite recent improvements in disease understanding and treatment, development of resistance to the available CRTs results in poor patient prognosis for HNSCC patients. Exploration of less toxic anticancer compound is therefore urgently needed. Aqueous graviola leaf extract (AGLE) was recently shown to induce G2/M cell cycle arrest, and it also exhibits antiproliferative activity against the squamous carcinoma cell line SCC-25 (44). Cisplatin alone is not an effective drug for HNSCC, but its efficacy significantly improves with radiation therapy or in combination with other chemotherapeutic drugs, suggesting that a suboptimal dose of cisplatin administered with Graviola may show significant antitumor effects in HNSCC.
Hematological malignancies
Hematological malignancies including B-cell chronic lymphocytic leukemia (CCL), acute myeloid leukemia (AML), multiple myeloma, or non-Hodgkin’s lymphoma constitute about 4% and 9% of all the diagnosed cancers in the world and in the USA (2). However, unique therapeutic challenges are presented by the biological characteristics and heterogeneity of the disease. Many plant-based natural compounds and their derivatives including indirubin, flavopiridol, maytansinoids, meisoindigo, noscapine and bruceantin have been investigated in many clinical trials for hematologic malignancies (45). Although most of the compounds failed due to insufficient efficacy resulting in development of resistance, identification of novel natural compounds with enhanced efficacy is an urgent need. Although random screening is expensive and time-consuming, targeted investigation of traditionally used medicinal plants may accelerate identification of novel anticancer products. As noted, investigations have indeed revealed antiproliferative and apoptotic effects of graviola in human leukemia HL-60 cells (46). Mechanistic studies showed that antiproliferative and apoptotic effects were associated with cell cycle arrest and loss of MMP (46). Furthermore, the ethanolic leaf extract (ELE) and methanolic leaf extract (MLE) of A.muricata were shown to induce apoptosis in chronic myelogenous K562 leukemia cells, in CCRF-CEM and in its multidrug-resistant subline CEM/ADR5000 cells, respectively (47). These studies suggested that graviola can be used for multidrug resistant cancer phenotypes and therefore is an excellent source for the development of novel anticancer drugs for hematological malignancies also.
Cancer hallmarks and graviola
Antioxidant activity
ROS are highly reactive molecules produced by a mitochondrial respiratory chain and play important roles in cellular physiology. Many cell-scavenging systems such as Catalase, superoxide dismutases (SOD), peroxiredoxins and glutathione regulate physiological levels of ROS for normal cell function. Excessive ROS levels can severely damage the cell and induce cell death; low ROS levels have been shown to induce cell proliferation and differentiation by stimulating various signaling pathways, such as phosphoinositide-3-kinase/serine-threonine kinase (PI3K/Akt), protein kinases B/C (PKB/C), ERK1/2, p38 MAPK and Janus Kinase (JNK), that contribute to carcinogenesis (48). Although cancer cells have elevated ROS levels compared with normal cells, increased antioxidant enzyme levels and molecules protect cancer cells from ROS-induced cellular damage and death (48). Thus, it seems reasonable that preventing excessive ROS accumulation in normal cells may prevent cellular damage, whereas blocking the antioxidant defense system in cancer cells may promote cellular damage and induce cell death. Caution should be taken that cell type, environmental conditions and the amount of agent to inhibit antioxidant defense will determine cellular damage or protecting effects.
The therapeutic potential of the newly identified molecules is suggested by their differential ability to inhibit cell proliferation and induce greater cell death in cancer than in normal cells. Although naturally occurring phytochemicals have gained much attention for their remarkable role in preventing ROS-induced cellular damage (49) and protecting development of many diseases, they have also been shown to impair antioxidant defense, sensitizing tumor cells to chemo-radiation therapy (reviewed in Ref. 50). Of interest, similar differential effects of butanolic leaf extract (BLE) and aqueous leaf extract (ALE) of A.muricata have been reported. Although BLE and ALE inhibited growth of cancer cells, no effect on normal cells was observed. Furthermore, they also reported that BLE and ALE have strong antioxidative effects that protected hydrogen peroxide- (H2O2) induced DNA damage in normal cells (51). Another in vivo study reported the protective effects of ALE in diabetes-induced rats (52). Furthermore, GLE has been shown to scavenge peroxyl and nitrogen radicals effectively and reduced H2O2-induced ROS generation. They further showed upregulation of antioxidant enzymes such as SOD1 and nuclear factor erythroid 2–related factor 2 (Nrf2) expression by GLE (53). SOD1 is overexpressed in many cancers and plays a crucial role in conversion of superoxide to H2O2. Overexpression of SOD1 in turn promotes cancer cell growth; it is now therapeutically targeted in many types of cancer (54) to induce either cell death via apoptosis or oxidative stress (55). Nrf2 is a master regulator of antioxidant enzymes, including phase II detoxifying enzymes and transporters, and it protects cells from genotoxic damage by carcinogens. In addition, it also promotes survival and chemoresistance in cancer cells (56). These findings provide an opportunity to explore the possible beneficial effects of graviola while considering its anticancer and antioxidant activities.
Anti-inflammatory activity
Inflammation is an emerging field of research for intervention targets in many diseases including asthma, arthritis and Crohn’s disease, Alzheimer’s disease, cardiovascular disease, diabetes, high blood pressure and more importantly cancers. Because of the significant side effects of steroid and Nonsteroidal Anti-inflammatory Drugs (NSAIDs), there is a greater interest in natural compounds present in dietary supplements and herbal remedies, which have been used for years to minimize pain and inflammation (57). A great number of natural compounds work mechanistically by inhibiting inflammatory signaling pathways that are similarly targeted by NSAIDs. Metabolites of the arachidonic acid pathway, produced via cyclooxygenase and lipoxygenase, are involved in creating inflammation when a cell is activated by mechanical stress (e.g. trauma), and via stimulation of cytokines and growth factors (58). The mechanism of antinociception (reduced sensitivity to pain) has been shown to inhibit cyclooxygenase (COX) and lipoxygenases (LOX) through inflammatory mediators such as flavonoids present in the plant extract. Studies from various groups show that oral administration of ELE of A.muricata at different doses significantly reduced carrageenan induced edema in rat paws. Interestingly, due to this anti-inflammatory effect of ELE of A.muricata, there was a related decrease in leukocyte migration and exudate volume (24). In addition, Foong CP et al. also reported strong anti-inflammatory action of LE of Graviola (10–300 mg/kgBW) in xylene-induced ear edema mice model and Complete Freund’s adjuvant (CFA)–induced arthritis rat models (59). They further showed significant suppressing of proinflammatory cytokines including tumor necrosis factor-α (TNF-α) and IL-1 β in CFA-induced arthritis rats (59). The anti-inflammatory effects of A.muricata are thus demonstrated to occur through suppression of inflammatory mediators (10). No animal toxicity was reported in these studies.
Wound healing
Wound cure follows from communication between cytokines, growth factors, blood, cellular elements, and the extracellular matrix (60). Cytokines most importantly “endorse” healing by stimulating the assembly of constituents of the basement membrane, increasing inflammation that results in formation of granulated tissue. Wound healing is a flow of cellular, biochemical, molecular and physiological actions divided into four processes: coagulation, inflammation, proliferation and tissue makeover. At present, the search for the ideal treatment to ameliorate or accelerate wound healing continues, but with challenges. Medicinal plants have provided significant wound healing products with high safety and therapeutic potential. It has even been reported that plant extracts accelerate all four phases of wound healing (61), and in rats, research groups have shown wound healing activity of the ethyl acetate extract of A.muricata leaves against excisional wounds (61). Macroscopic and microscopic analyses demonstrated significant healing after topical administration of the extract for 15 days. Moreover, during the healing process, immunohistochemical evaluation revealed up-regulation of heat shock protein 70 (Hsp70), an essential protein for wound healing that also has a role in cell proliferation (61). The antioxidant potential of A.muricata leaves also accelerated wound healing by elevating levels of catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (Cu, Zn-SOD). Annona muricata extract also markedly reduces the lipid peroxidation marker malondialdehyde (MDA), known for its damaging effect on the fibroblasts, endothelial cells and collagen metabolism essential for wound repair (61). Under the same experimental conditions, alcoholic extract of the stem bark from the fourth day after injury onwards showed noteworthy healing capacity. The evidence is thus strong in excision models that A.muricata extract has promising wound healing abilities.
Graviola as a chemopreventive agent
The alarming incidences and the failure of the current chemotherapeutic approaches for advance stage cancers to reduce mortality is stressing for new approaches of cancer control. Although it seems difficult to treat advanced cancers by targeted therapies due to their genotypic or phenotypic heterogeneity and associated toxicity, efforts to stop the process of carcinogenesis to decrease cancer incidences seem more realistic strategy. Chemoprevention is the use of pharmacological agents that can arrest or reverse the process of carcinogenesis. Many natural compounds have been used as chemopreventive agents with capacity to intervene either at the stage of initiation, promotion or progression during carcinogenesis (62). Graviola extracts have also been used as chemopreventive agent in many carcinogen-induced mouse models as discussed below.
Colorectal tumors are thought to arise from a cluster of abnormal tube-like glands called aberrant crypt foci (ACF), lining the colon and rectum. These ACF’s are formed before colorectal polyps and are one of the earliest changes seen during colon carcinogenesis. Apart from the therapeutic potential, it was recently reported that the leaf ethyl acetate extract of graviola (250 and 500 mg/kgBW) prevented azoxymethane (AZM) -induced colonic ACF formation in rats (63). The histological analysis revealed down regulation of PCNA, BCL2 and upregulation of proapoptotic Bax protein in extract-treated animals compared with AZM-treated animals. They further observed decreased malondiadehyde expression that was associated with decreased lipid peroxidation (63). Eggadi et al. also showed the chemopreventive effects of EE of graviola in 1,2-dimethyl hydrazine (DMH) -induced colon cancer in Wistar albino rats (64). They showed that of EE (300 mg/kgBW) significantly decreased the number of DMH induced ACF’s in colorectal epithelium (64). Recently, Roduan MR et al. analyzed the chemopreventive efficacy of hexane, dichloromethane and methanol fractions from graviola leaves on 7,12-dimethylbenzeneanthracene (DMBA) and 12-0-tetradecaboylphorbol-13-acetate (TPA) promoted skin tumorigenesis using mice models (65). They observed significant decrease in the incidence of tumors, volume of the tumors and over all tumor burden compared with carcinogen-treated controls. Furthermore, they reported restoration of DMBA/TPA-induced levels of SOD (by methanolic extract), CAT and lipid peroxidation by these extracts. They further concluded that chemopreventive potential of graviola extracts may be due to their both antioxidant and cytotoxic effects (65). Similar chemopreventive effects of EE of graviola leaves (EEL) were reported by Hamizah S et al., in DMBA-induced skin papillomagenesis mice model (66). They showed that topical application of EEL (30, 100 and 300 mg/kg BW) to DBMA-treated [(100 µg/100 µl acetone/single dose followed by croton oil (1% in acetone/twice a week) for 10 weeks as cancer promoter)] mice increased the average latency period and significantly reduced skin papillomagenesis compared with DBMA-treated control animals. They further showed that EEL at 100 and 300 mg/kg BW doses completely inhibited the tumor development (66). The histopathological analysis revealed insignificant hyperplastic lesions with no kareatin pearls compared with DBMA-treated animals suggesting a strong chemopreventive effect of graviola. Adelina R et al. reported that EEL graviola (200, 400 and 800 mg/kg BW) given to the Sprague Dawley rats for 17 days resulted in p53-dependent apoptosis of DMBA-induced liver cancer cells (67). Similar chemopreventive effects of MLE of graviola were reported by Rajesh M et al., in benzo[a]pyrene (BPa)-induced lung cancers (68). BPa treatment (50 mg/kg BW) given orally twice a week for 4 successive weeks induces lung cancer by 16th week. They observed that treatment with MLE (400 mg/kg BW) started week before the BPa treatment and continued for 16 weeks (single dose/week) significantly reduced the incidence and burden of the lung tumors. They further observed normal lung gross morphology and significant decrease in the expression of serum cancer marker carcinoembryonic antigen (CEA) in MLE- and BPa-treated animals compared with only BPa-treated animals (68). Similar to the results from Roduan MR et al., they also observed decreased lipid peroxidation and increased SOD, CAT and GSH levels in MLE-pretreated animals compared with BPa only–treated animals (68), suggesting the prevention of BPa-induced free radicals and cell damage by MLE. They further reported no acute toxicity of the MLE at 2000 mg/kg BW suggesting its safe use (68). In addition, GLE has been reported to inhibit the growth of benign prostatic hyperplasia-1 cells (BPH-1) and to decrease prostate size in rats after oral administration for 60 days (8). A recent study demonstrated that EE of graviola inhibits DMBA-induced breast cell proliferation in the breast tissues of female albino mice (69). The histopathological analysis revealed that graviola prevented DMBA-induced DNA damage and prevented the development of hyperplastic epithelium which are the physiologic precursors for the development of ductal carcinoma in situ (69). All these studies suggest that graviola is a chemopreventive agent along with its therapeutic potential to inhibit the progression from premalignant lesions to frank malignancy.
Of more interest, graviola not only effects in in vitro and in vivo studies, but the consumption of boiled leaves of A.muricata in combination with the chemotherapeutic drug capecitabine (trade name Xeloda, 2500 mg PO) showed stable diseases in a 66-year-old woman with refractory metastatic breast cancer and regressed to multiple therapeutic regimens including adjuvant anthracyclines and taxanes and radiation; Femara, Tamoxifen and Faslodex; Navelbine; Abraxane, Avastin, Gemcitabine (AAG); Doxil and Xeloda (70). Although no cytotoxic effects of graviola consumption were observed even after 5 years, the authors cautioned that further clinical investigation is necessary to determine effective dose, efficacy and potential drug interactions. Another study also reported complete regression of the colon tumors with the daily ingestion of 5 g GLE along with lifestyle modifications (71). In a recent safety evaluation double blinded clinical trial (the identifier NCT02439580) in resected colorectal cancer patients, no sign of significant toxicity was observed with EE of graviola (300 mg) when given for 8 weeks (72). Furthermore, no significant difference in energy intake was observed between these two groups (73). In addition, administration of EE of graviola (180 mg) in diabetic patients along with glibenclamide for 30 days showed lower blood glucose with only 4% (2/45) patients showing burning pain in epigastrium and 6% (3/45) showed nausea (74). Although many ACG’s including isoannonacinone, annonacinone, coreximine solamin, alkaloids, reticuline and annonacin acetogenins were earlier shown to be toxic to dopaminergic cells by impairing energy production (reviewed in Ref. 75), however, further studies showed that this decreased ATP production and associated neurodegeneration did not change the mice behavior or locomotor activity (76,77). In addition, the consumption of graviola was earlier associated with the appearance of atypical Parkinson’s disease in humans (78,79); however, recent studies by l’Agence Francaise de Se′curite′ des Aliments (AVIS), based on the amount of various constituents in the graviola, pharmacokinetics studies and daily human consumption have suggested no clinical link between them (reviewed in Ref. 75). Overall, majority of the studies undertaken for dose escalation and to identify the therapeutic efficacy of various fractions of Graviola in many diseases have shown either very mild or no toxicity associated with these extracts (reviewed in Refs. 9 and 75) suggesting its safety in patients.
Other pharmacological activities of graviola
Besides showing the anticancer activities, graviola effects many other non-malignant diseases; those share many common signaling pathways with the cancer. Therefore, it is pertinent to understand the common underlying pathways effected in human diseases effected by graviola to develop a comprehensive and systemic therapeutic regime against human diseases.
Graviola against non-microbial human diseases
Chronic dysregulation of proinflammatory enzymes and cytokines that facilitate the production of prostaglandins, leukotrienes, adhesion molecules and matrix metalloproteinases results in arthritis besides cancer. All these factors are controlled by initiation of the transcription NF-κB, which controls expression of TNF-α, interleukin-1β, cyclooxygenase-2, lipoxygenase, matrix metalloproteinases or adhesion molecules, all of which mediate inflammatory events. It has been found that products derived from plants can suppress these cell-signaling intermediates and preclinical, and clinical studies demonstrate that these agents have potential for arthritis treatment. Annona muricata is already used to treat arthritis in Nigeria and Malaysia, for example. An in vivo study on different doses of the ELE from A.muricata has shown antiarthritic activity in CFA-induced arthritis in rats (59). Oral administration of the extract reduced edema and at higher doses significantly suppressed TNF-α and IL-1β expression; Annona muricata leaves added suppression of proinflammatory cytokines (59).
Liver is vitally important for metabolism and detoxification of endogenous and exogenous substances, especially drugs. However, continuous expulsion or metabolism these substances by liver cells results in generation of free radicals that are associated with membrane lipids peroxidation, hepatic cell damage (80) and sometimes cancer. Ten percent of the world’s population are affected by liver diseases, including hepatitis, cirrhosis, fibrosis, fatty liver, alcoholic liver disease and drug-induced liver disease (81). Herbal medicines have been used to treat liver disorders for centuries and at present are promising for various pathophysiological liver conditions. Annona muricata is commonly used to treat several liver disorders, particularly jaundice. A study was performed on phenyl hydrazine–induced jaundice in adult rats that were orally treated with A.muricata aqueous extract at doses of 50 and 400 mg/kg and found a substantial reduction in elevated bilirubin. Alcoholic extract of A.muricata has been evaluated for protection against carbon tetrachloride (CCl4) toxicity, and the extract was found to scavenge ROS generated during CCl4 metabolism. It has also been shown to protect against increased SGOT (serum glutamic-oxaloacetic transaminase), SGPT (serum glutamic-pyruvic transaminase), ALP (alkaline phosphatase) and liver and brain lipid peroxidation (82). Apart from the hepatoprotective property of the alcoholic extract of the leaves, the aqueous extract was also reported to protect against liver damage induced by CCl4 and acetaminophen. Results demonstrated that liver damage returned toward normal hemostasis when pretreated with different doses of the extract for 7 days. The presence of glucosides in A.muricata mechanistically reduced bilirubin levels by converting to glucuronic acid that conjugates with bilirubin for excretion. Similarly, phytochemicals in the extract might act as regulators to increase the activity of enzymes and transporters and thereby enhance the activity of the pathway involved in clearing bilirubin. These outcomes validate the traditional use of A.muricata against jaundice and display its potential hepatoprotective function.
The gastro protective activity of A.muricata leaves has been studied against gastric injury induced by ethanol, and results demonstrate that oral administration of the ethyl acetate extract at doses of 200 and 400 mg/kg displayed a significant antiulcer effect. The protective effect of the extract on rat gastric wall tissue was accompanied by an increase in enzymatic activity of antioxidants and suppression of lipid peroxidation, followed by an increase in protein expression of Hsp70 and a decrease in Bax expression. Reports have also shown that ethyl acetate and ethanol extracts from the leaves of A.muricata displayed a protective gastric effect that was same as that of omeprazole in ethanol ulcerogenesis in rats. Antioxidant compounds of A.muricata promote antiulcer potential by increasing mucosal non-protein sulfhydryl group content, with related reduction in gastric wall mucus (GWM) from excessive production of gastric acid. The antioxidant constituents present in A.muricata extract may also play a major role in gastro protection. ROS has also been found to induce oxidative damage to the gastric mucosa, but A.muricata extract restores the enzyme activity of CAT, prostaglandin E2, SOD, glutathione, nitric oxide and malondialdehyde, all of which are responsible for reduction in cellular ROS. Histopathological examination provides additional evidence revealing that the extract protects gastric tissue from hemorrhagic lesions by decreasing leukocyte infiltration and submucosal edema.
Diabetes (hyperglycemia) is considered a silent deadly disease, a consequence of high glucose levels in the blood due to lack of insulin production or excess and resistance to insulin. If untreated, diabetes causes damage to nerves, eyes and internal organs, often resulting in coronary complications and requiring limb amputation in the later stages. However, there are many natural ways to treat and control insulin levels, including plant products that contain secondary metabolites such as flavonoids, tannins and triterpenoids, which have a direct influence on glucosidase activity. Flavonoids inhibit α-glucosidase through substitution at β-ring and hydroxylation, decreasing hydrolysis of carbohydrates and in turn inhibiting glucose absorption and preventing carbohydrate metabolism. It has been reported that after a month of daily treatment, A.muricata resulted in close to normal blood glucose levels, body weight, food and water intake, lipid profile and oxidative defense (83). Studies in murine models have shown that A.muricata aqueous and methanolic leaf extracts led to decreases in concentration of blood glucose in rats with diabetes induced by streptozotocin (STZ). Moreover, the extract caused decreased levels of serum cholesterol, low-density lipoproteins, very low-density lipoprotein, cholesterol and triglycerides (83). The fruit of A.muricata has also been shown to lower the glycemic index (GI) and glycemic load (GL), confirming its antihyperglycemic potential.
In A.muricata leaf extract, alkaloids such as anomurine, reticulin, coreximine and essential oil constituents such as β-caryophyllene have been shown to lower hypertension (84). Depending on dose, the leaf extracts of A.muricata also lower blood pressure in normotensive rats without disturbing heart rate. The mechanism driving the antihypertensive activity of A.muricata was shown to be due to Ca2+ antagonism, but did not appear to be via the endothelial or nitric oxide pathways. Blockage of Ca2+ channels demonstrated that A.muricata extract can relax high K+-induced contraction (84).
Graviola against microbial and parasitic diseases
Malaria is a devastating disease, affecting tropical as well as subtropical areas (85). Antimalarial drugs have shown inefficiency due to parasite resistance (86), and thus, there is an urgent need to discover new antimalarial agents. Phytochemical compounds including alkaloids, anonaine, ACG’s and gallic acid isolated from A.muricata have been reported to possess antimalarial properties. Annona muricata leaf extract has been tested against two strains of Plasmodium falciparum, which include the Nigerian chloroquine-sensitive strain and FcM29-Cameroon. Of interest, the potent activity of this extract was found at an IC50 value of just 16 and 8 µg/ml after 72 h of treatment (87). Results also revealed that leaf extract at 20 µg/mL displayed 67% inhibition against an asynchronous F32 strain of P.falciparum. Another report found that A.muricata leaves and stems exhibited cytotoxic effects against chloroquine-sensitive (F32) and resistant (W2) P.falciparum. Compared with methanolic extract, A.muricata seed extract also displayed potent efficacy (88). Furthermore, phenolic compounds mechanistically inhibit the activity of the fatty acid biosynthesis responsible for the growth of P.falciparum. The β-ketoacyl-ACP-reductase (FabG) is non-competitively inhibited by luteolin, which subsides acetoacetyl-CoA and NADPH. Moreover, β-hydroxyacyl-ACP-dehydratase (FabZ) is competitively inhibited by luteolin and competes with substrate crotonyl-CoA. In another malarial strain, Plasmodium berghi, the aqueous A.muricata leaf extract showed significant antimalarial activity with prolonged survival and no detectable toxicity. Plasmodium berghei–infected a 4-week-old female Imprinting Control Region (ICR) mice were fed orally with ALE (100 to 1000 mg/kg) resulting in parasitemia reduction from 38.3% to 85.61%, depending on dose (89). These studies suggest that the antimalarial activity of A.muricata may contain potential molecule(s) for drug treatment development.
Antibiotics remain the most valuable drugs to combat bacterial infections, but it is well known that commonly used antibiotics have become less effective and have promoted development of multidrug resistance bacteria. There is thus an urgent need to explore new drugs with proven noteworthy roles in treatment and prevention of infection, and it has been found that A.muricata extracts possess a broad range of activity against bacteria responsible for diseases and disorders such as intractable diarrhea, urinary tract infection (UTI), skin diseases and treatment-resistant secondary pneumonia (75). Aqueous, ethanolic and methanolic extracts of A.muricata were screened for antibacterial activity against Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Streptococcus pyogenes, Bacillus subtilis, Salmonella typhimurium, Klebsiella pneumonia and Enterobacter aerogenes microorganisms. The extract exhibited antibacterial activity against S.aureus and Vibrio cholera, and EE showed potent activity against Pseudomonas aeruginosa and S.aureus (75).
Leishmaniosis and Trypanosomosis are common and deadly protozoan diseases. Resistance to available drugs is a major hindrance to treatment of protozoal diseases. Annona muricata has been screened on several pathogenic parasites for cytotoxic activity. It has been shown that ethyl acetate leaf extract of A.muricata treatment against three species of Leishmania, i.e. PH8, M2903 and PP75, and Trypanosoma cruzi resulted in important, potent antileishmania and antitrpanosom activity (88). Apart from this finding, ethyl acetate and methanolic extract have shown an appreciable antileishmanial effect against Leishmania braziliensis and Leishmania panamensis species when compared with glucantime (90). Phytochemicals including annonacinone and corossolone isolated from A.muricata seeds and tested against three species of Leishmania, i.e. donovani, mexicana and major, further showed an EC50 value of 6.72–8.00 and 16.14–18.73 µg/ml (91). The methanolic extract of A.muricata seeds also exhibited substantial antiparasitic activity against Molinema dessetae infective larva (92), and the ALE showed 89.08% and 84.91% toxicity against larvae and eggs of Haemonchus contortus, a well-known gastrointestinal parasite (93).
Conclusion and future directions
Despite significant medical and technological developments, cancer remains one of the leading causes of death in the USA and around the world. The advent of modern drug-targeted therapies, while improving patient prognosis, is associated with serious toxicities and development of resistance. Compounds derived from plants have long been used to prevent and to treat various diseases, including cancer. The present review highlights the pharmacological importance of natural products from the plant A.muricata and their efficacy as a candidate for a variety of treatments, especially for cancers.
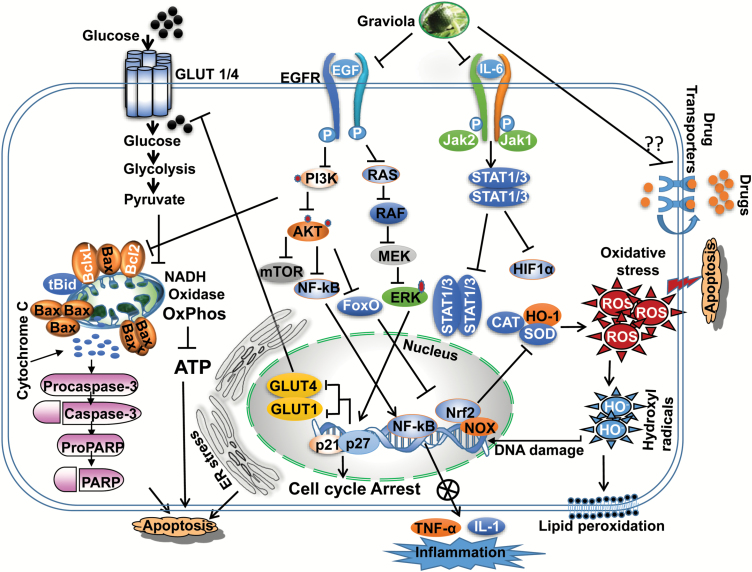
Various extracts from the plant have been used as traditional herbal medicine and have proved to possess a broad range of properties, including antioxidant, anti-inflammatory, antiarthritic, hepato-protective, gastro-protective, antidiabetic, antimalarial, antibacterial, antiprotozoan, insecticidal, larvicidal and healing of wounds. It is interesting as well that the extract of this plant provides notable protection against various malignancies, including leukemia, colon, breast, HNSCC and prostate cancers. A recent descriptive and cross-sectional questionnaire identified graviola as a most popular herbal remedy in Trinidad, used for PCa, breast and colorectal cancer patients (94). Intensive pharmacological studies identified almost 200 phytochemicals, mostly acetogenins and alkaloids from A.muricata, and few of these were shown to possess anticancer properties. Various mechanistic studies associate these with ACG’s: (i) by effecting metabolism through down-regulation of mitochondrial NADH oxidase function, inhibiting the mitochondrial complex I of the electron transport chain (95); (ii) modulating and/or down-regulating drug efflux proteins to kill multidrug-resistant tumors (30,96); (iii) targeting EGFR signaling to induce cell cycle arrest and inhibit cytotoxic cell survival and metastasis (19,34,95); and (iv) inducing endoplasmic reticulum (ERe) stress (97). These actions are summarized schematically in Figure 1. A recent molecular docking study demonstrated robust and improved interaction of acetogenin with the Bcl2 family of proteins, compared with available synthetic and natural inhibitors for their proapoptotic effects (98). This suggests the strong therapeutic efficacy of graviola in combating various malignancies.
Figure 1.
Possible anticancer mechanisms of graviola. Graviola induced apoptosis by loss of MMP and activation of caspases. Suppression of EGFR and JAK signaling leads to blockade of the PI3K, RAS and STAT pathways, respectively, culminating in decreasing cell viability and metabolic catastrophe by down-regulating HIF-1α, GLUT1, GLUT4, expression, associated with decreased glucose uptake and cell cycle arrest in human cancer cells. Graviola inhibits NF-κB mediated TNF-α and IL-1 expression to control inflammation. Graviola increases ROS generation by effecting the expression of catalase (CAT), SOD and heme-oxygenase (HO-1) expression. Graviola also kills the drug-resistant cells possible by modulating multidrug-resistant export proteins.
Toxicity and associated side effects of available chemotherapies clearly indicate the urgent need for novel drug combinations. Several recent studies show strong efficacy of combination of conventional therapy with natural compounds, compared with conventional drugs alone (reviewed in Ref. 99). These combinations work either additively or synergistically, while minimizing side effects and sensitizing tumors to CRT through modulation of multiple resistant signaling pathways (reviewed in Ref. 99). Keeping in mind the low toxicity of graviola towards normal cells and its modulation of various cancer-associated signaling pathways, a combination of CRT and graviola would likely provide the benefits of decreased dosage while selectively killing tumor cells.
Inflammation is also a prerequisite for the development of various cancers, and NF-kB regulated proinflammatory cytokines such as TNF-α and IL-1 are critical to the regulation of inflammation (100). Although graviola has been shown to inhibit cell proliferation and to decrease tumor growth, its effect on NF-kB-mediated anti-inflammatory effects can also be a beneficial and better strategy for management of solid cancers through inhibiting the site of inflammation.
Many current studies on graviola were performed using crude extracts from various parts of the plant, consisting of a mixture of phenolic and nonphenolic compounds, with amounts which may vary by geographical locations of the plant. They may thus display varied anticancer proprieties. To address this, a recent study investigated crude extracts from A.muricata collected from various locations and reported on how their differing potencies affected breast cancer cell lines. Results indeed suggested that geographical distribution affects phytochemical composition and therefore anticancer properties (101). In addition, as with many herbal medicine or supplement products, processing and formulation may vary widely in different products available in the market, making reliable results more difficult to achieve. Because of these and other factors, the antitumor effects seen in these complex mixtures may be difficult to interpret. Their overall effects can be due to interactions between a variety of constituents, or it may be the case that one key component plays the crucial role in a desired biological activity. Systematic and robust screening, therefore, is needed to identify biochemical fractionation and the physiological effects of all isolated phytochemicals, with detailed investigation of underlying mechanisms.
We conclude with the important caveat that many questions still need to be addressed in the future exploration of the potential of graviola for therapeutic utility; further identification and characterization of active constituents is crucial for the development of new pharmacological candidates to treat cancer and other diseases. It is critical to understand, for example, that earlier toxicological studies suggested that annonacin, a prototypical acetogenin and potent lipophilic inhibitor of complex I of the mitochondrial respiratory chain, could have contributed to the etiology of certain cases of Parkinson’s disease-like syndromes, and that this mechanism may be pertinent for the Annonaceae species (78,79). Studies must weigh the various therapeutic benefits (such as antimicrobial, anticancer and antiarthritis) and potential toxic effects (especially the putative Parkinson’s disease connection), and critically assess these with respect to required dosage, risk/benefit and “therapeutic window” in different indications. Although the depletion of ATP appears to be one of the major mechanisms of the anticancer actions of graviola, efforts to restore the potency of graviola may require avoiding other supplements that increase ATP production. Despite numerous studies suggestive of the potential utility of graviola for pathological conditions, it is necessary to select well-characterized and sufficiently quality-controlled products to perform well-designed, randomized, robust and systematic clinical trials to further validate its efficacy and safety.
Funding
The authors on this manuscript were supported, in parts, by the grants from National Institutes of Health (Cancer Center Support Grant P30 CA036727, UO1 CA210240, EDRN CA200466, SPORE P50 CA127297 and RO1 CA183459, UO1 CA185148 ).
Acknowledgements
We thank Dr. Adrian Koesters, Research Editor at UNMC, for her editorial contribution to the paper.
Abbreviations
- ACF
aberrant crypt foci
- ACG
annonaceous acetogenins
- ALE
aqueous leaf extract
- ATP
adenosine triphosphate
- BPa
benzo[a]pyrene
- CAT
catalase
- CRC
colorectal cancer carcinoma
- CRT
chemoradiation therapy;
- DMBA
7,12 dimethyl benzene anthracene
- EE
ethanolic extract
- EEL
ethanolic extract of graviola leaves
- ELE
ethanolic leaf extract
- GLUT
glucose transporter
- HNSCC
head and neck squamous cell carcinomas
- MLE
methanolic leaf extract
- PC
pancreatic cancer
- PCa
prostate cancer
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
References
- 1. Ferlay J., et al. (2015)Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R.L., et al. (2018)Cancer statistics, 2018. CA Cancer J. Clin., 68, 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Zubair H., et al. (2017)Cancer Chemoprevention by Phytochemicals: nature’s Healing Touch. Molecules, 22, E395. doi: 10.3390/molecules22030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newman D.J. (2008)Natural products as leads to potential drugs: an old process or the new hope for drug discovery?J. Med. Chem., 51, 2589–2599. [DOI] [PubMed] [Google Scholar]
- 5. Chang F.R., et al. (2001)Novel cytotoxic annonaceous acetogenins from Annona muricata. J. Nat. Prod., 64, 925–931. [DOI] [PubMed] [Google Scholar]
- 6. Sun S., et al. (2014)Three new anti-proliferative Annonaceous acetogenins with mono-tetrahydrofuran ring from graviola fruit (Annona muricata). Bioorg. Med. Chem. Lett., 24, 2773–2776. [DOI] [PubMed] [Google Scholar]
- 7. Ribeiro de Souza E.B., et al. (2009)Enhanced extraction yields and mobile phase separations by solvent mixtures for the analysis of metabolites in Annona muricata L. leaves. J. Sep. Sci., 32, 4176–4185. [DOI] [PubMed] [Google Scholar]
- 8. Asare G.A., et al. (2015)Antiproliferative activity of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells, and some target genes. Integr. Cancer Ther., 14, 65–74. [DOI] [PubMed] [Google Scholar]
- 9. Gavamukulya Y., et al. (2014)Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med., 7S1, S355–S363. [DOI] [PubMed] [Google Scholar]
- 10. Ishola I.O., et al. (2014)Mechanisms of analgesic and anti-inflammatory properties of Annona muricata Linn. (Annonaceae) fruit extract in rodents. J. Med. Food, 17, 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alali F.Q., et al. (1997)(2,4-cis and trans)-gigantecinone and 4-deoxygigantecin, bioactive nonadjacent bis-tetrahydrofuran annonaceous acetogenins, from Goniothalamus giganteus. J. Nat. Prod., 60, 929–933. [DOI] [PubMed] [Google Scholar]
- 12. Matsushige A., et al. (2012)Three new megastigmanes from the leaves of Annona muricata. J. Nat. Med., 66, 284–291. [DOI] [PubMed] [Google Scholar]
- 13. Nawwar M., et al. (2012)A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Arch. Pharm. Res., 35, 761–767. [DOI] [PubMed] [Google Scholar]
- 14. Yang C., et al. (2015)Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis, 36, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liaw C.C., et al. (2010)Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials. Planta Med., 76, 1390–1404. [DOI] [PubMed] [Google Scholar]
- 16. Moghadamtousi S.Z., et al. (2015)Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci., 16, 15625–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dang Y.J., et al. (2012)In situ absorption in rat intestinal tract of solid dispersion of annonaceous acetogenins. Gastroenterol. Res. Pract., 2012, 879676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stan S.D., et al. (2010)Chemoprevention strategies for pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol., 7, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres M.P., et al. (2012)Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett., 323, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosdi M.N.M., et al. (2015)Cytotoxic effect of Annona muricata Linn leaves extract on Capan-1 cells. J. Appl. Pharm. Sci., 7, 045-048.
- 21. Macha M.A., et al. (2015)Emerging potential of natural products for targeting mucins for therapy against inflammation and cancer. Cancer Treat. Rev., 41, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macha M.A., et al. (2013)Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett., 341, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Degli Esposti M., et al. (1994)Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem. J., 301, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Sousa O.V., et al. (2010)Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L. leaves in animal models. Int. J. Mol. Sci., 11, 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ying H., et al. (2012)Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viale A., et al. (2014)Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moghadamtousi S.Z., et al. (2014)Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. BMC Complement. Altern. Med., 14, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao G.X., et al. (1993)Biologically active acetogenins from stem bark of Asimina triloba. Phytochemistry, 33, 1065–1073. [DOI] [PubMed] [Google Scholar]
- 29. Kallifatidis G., et al. (2016)Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol., 40-41, 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deep G., et al. (2016)Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity. Sci. Rep., 6, 23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun S., et al. (2016)Isolation of three new annonaceous acetogenins from Graviola fruit (Annona muricata) and their anti-proliferation on human prostate cancer cell PC-3. Bioorg. Med. Chem. Lett., 26, 4382–4385. [DOI] [PubMed] [Google Scholar]
- 32. Ko E.Y., et al. (2015)Natural products for chemoprevention of breast cancer. J. Cancer Prev., 20, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko Y.M., et al. (2011)Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J. Ethnopharmacol., 137, 1283–1290. [DOI] [PubMed] [Google Scholar]
- 34. Dai Y., et al. (2011)Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr. Cancer, 63, 795–801. [DOI] [PubMed] [Google Scholar]
- 35. Oberlies N.H., et al. (1997)The Annonaceous acetogenin bullatacin is cytotoxic against multidrug-resistant human mammary adenocarcinoma cells. Cancer Lett., 115, 73–79. [DOI] [PubMed] [Google Scholar]
- 36. Yuan F., et al. (2016)Annosquacin B induces mitochondrial apoptosis in multidrug resistant human breast cancer cell line MCF-7/ADR through selectively modulating MAPKs pathways. Pharm. Biol., 54, 3040–3045. [DOI] [PubMed] [Google Scholar]
- 37. Qazi A.K., et al. (2014)Cell specific apoptosis by RLX is mediated by NFκB in human colon carcinoma HCT-116 cells. BMC Cell Biol., 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdullah M., et al. (2017)The value of caspase-3 after the application of Annona muricata leaf extract in COLO-205 colorectal cancer cell line. Gastroenterol. Res. Pract., 2017, 4357165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zorofchian Moghadamtousi S., et al. (2014)Annona muricata leaves induce G₁ cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. J. Ethnopharmacol., 156, 277–289. [DOI] [PubMed] [Google Scholar]
- 40. Kim G.S., et al. (1998)Two new mono-tetrahydrofuran ring acetogenins, annomuricin E and muricapentocin, from the leaves of Annona muricata. J. Nat. Prod., 61, 432–436. [DOI] [PubMed] [Google Scholar]
- 41. Wu F.E., et al. (1995)Two new cytotoxic monotetrahydrofuran Annonaceous acetogenins, annomuricins A and B, from the leaves of Annona muricata. J. Nat. Prod., 58, 830–836. [DOI] [PubMed] [Google Scholar]
- 42. Wu F.E., et al. (1995)New bioactive monotetrahydrofuran Annonaceous acetogenins, annomuricin C and muricatocin C, from the leaves of Annona muricata. J. Nat. Prod., 58, 909–915. [DOI] [PubMed] [Google Scholar]
- 43. Xue J.Y., et al. (2014)Desacetyluvaricin induces S phase arrest in SW480 colorectal cancer cells through superoxide overproduction. J. Cell. Biochem., 115, 464–475. [DOI] [PubMed] [Google Scholar]
- 44. Magadi V.P., et al. (2015)Evaluation of cytotoxicity of aqueous extract of Graviola leaves on squamous cell carcinoma cell-25 cell lines by 3-(4,5-dimethylthiazol-2-Yl) -2,5-diphenyltetrazolium bromide assay and determination of percentage of cell inhibition at G2M phase of cell cycle by flow cytometry: an in vitro study. Contemp. Clin. Dent., 6, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucas D.M., et al. (2010)Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets, 11, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pieme C.A., et al. (2014)Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement. Altern. Med., 14, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuete V., et al. (2016)Cytotoxicity of methanol extracts ofAnnona muricata,Passiflora edulisand nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus, 5, 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marengo B., et al. (2016)Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev., 2016, 6235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liao J.C., et al. (2012)Chemical compositions, anti-inflammatory, antiproliferative and radical-scavenging activities of Actinidia callosa var. ephippioides. Am. J. Chin. Med., 40, 1047–1062. [DOI] [PubMed] [Google Scholar]
- 50. Sznarkowska A., et al. (2017)Inhibition of cancer antioxidant defense by natural compounds. Oncotarget, 8, 15996–16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. George V.C., et al. (2015)Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J. Food Sci. Technol., 52, 2328–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ojewole J.A., et al. (2009)Hypoglycaemic effect of mollic acid glucoside, a 1alpha-hydroxycycloartenoid saponin extractive from Combretum molle R. Br. ex G. Don (Combretaceae) leaf, in rodents. J. Nat. Med., 63, 117–123. [DOI] [PubMed] [Google Scholar]
- 53. Son Y.R., et al. (2016)Bioefficacy of Graviola leaf extracts in scavenging free radicals and upregulating antioxidant genes. Food Funct., 7, 861–871. [DOI] [PubMed] [Google Scholar]
- 54. Somwar R., et al. (2011)Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. U. S. A., 108, 16375–16380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang P., et al. (2000)Superoxide dismutase as a target for the selective killing of cancer cells. Nature, 407, 390–395. [DOI] [PubMed] [Google Scholar]
- 56. Ikeda H., et al. (2004)Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem. J., 380(Pt 2), 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reynolds J.F., et al. (1995)Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S. Afr. Med. J., 85, 517–522. [PubMed] [Google Scholar]
- 58.Martel-Pelletier, J., et al. (2003) Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Annals of the rheumatic diseases, 62, 501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Foong C.P., et al. (2012)Evaluation of anti-inflammatory activities of ethanolic extract of Annona muricata leaves. Rev. bras. farmacogn., 22, 1301–1307. [Google Scholar]
- 60. Dorai A.A. (2012)Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg., 45, 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moghadamtousi, S.Z. et al. (2015) Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int. J. Surg. 18, 110-117. [DOI] [PubMed] [Google Scholar]
- 62. Wattenberg L.W. (1990)Inhibition of carcinogenesis by minor anutrient constituents of the diet. Proc. Nutr. Soc., 49, 173–183. [DOI] [PubMed] [Google Scholar]
- 63. Zorofchian Moghadamtousi S., et al. (2015)The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of Acetogenin Annomuricin E in HT-29 cells: a bioassay-guided approach. PLoS One, 10, e0122288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eggadi V., et al. (2014)Evaluation of anticancer activity of Annona muricata in 1, 2-dimethyl hydrazine induced colon cancer. World Appl. Sci. J, 32, 444–450. [Google Scholar]
- 65. Md Roduan M.R., et al. (2017)Annona muricata leaves extracts prevent DMBA/TPA-induced skin tumorigenesis via modulating antioxidants enzymes system in ICR mice. Biomed. Pharmacother., 94, 481–488. [DOI] [PubMed] [Google Scholar]
- 66. Hamizah S., et al. (2012)Chemopreventive potential of Annona muricata L leaves on chemically-induced skin papillomagenesis in mice. Asian Pac. J. Cancer Prev., 13, 2533–2539. [DOI] [PubMed] [Google Scholar]
- 67. Adelina R., et al. (2013)Annona muricata’s leaves ethanolic extract enhance p53 expression in rat hepatic cells induced by Dimethylbenz (a) Anthracene (DMBA). Indones. J. Cancer Chemoprev., 4, 483-487. [Google Scholar]
- 68. Rajesh V., et al. (2015)Antiproliferative and chemopreventive effect of Annona muricata Linn. on Ehrlich ascites carcinoma and Benzo[a]pyrene induced lung carcinoma. Orient. Pharm. Exp. Med., 15, 239–256. [Google Scholar]
- 69. Minari J., et al. (2014)Chemopreventive effect of Annona muricata on DMBA-induced cell proliferation in the breast tissues of female albino mice. Egypt. J. Med. Hum. Genet., 15, 327–334. [Google Scholar]
- 70. Hansra D.M., et al. (2014)Patient with metastatic breast cancer achieves stable disease for 5 years on graviola and xeloda after progressing on multiple lines of therapy. Breast Cancer Res. Treat., 3, 84. [Google Scholar]
- 71. Yap S. (2013)Colon cancer reversed by phyto-nutritional therapy: a case study. Int. J. Biotechnol. Wellness Ind., 2, 132–139. [Google Scholar]
- 72. Indrawati L., et al. (2017)Safety of Annona muricata extract supplementation for colorectal cancer patients. Indones. J. Gastroenterol. Hepatol. Dig. Endoscopy, 17, 170–175. [Google Scholar]
- 73. Indrawati L., et al. (2017)The effect of an Annona muricata leaf extract on nutritional status and cytotoxicity in colorectal cancer: a randomized controlled trial. Asia Pac. J. Clin. Nutr., 26, 606–612. doi:10.1016/j.arabjc.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 74. Arroyo J., et al. (2009)Hypoglycemic effect adjuvant extract ethanolic leaf Annona muricata L. (guanábana), in patients with diabetes type 2 in treatment of glibenclamide. An. Fac. Med., 70, 163–167. [Google Scholar]
- 75. Coria-Téllez A.V., et al. (2016)Annona muricata: a comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. [Google Scholar]
- 76. Champy P., et al. (2004)Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem., 88, 63–69. [DOI] [PubMed] [Google Scholar]
- 77. Lannuzel A., et al. (2002)Toxicity of Annonaceae for dopaminergic neurons: potential role in atypical parkinsonism in Guadeloupe. Mov. Disord., 17, 84–90. [DOI] [PubMed] [Google Scholar]
- 78. Escobar-Khondiker M., et al. (2007)Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J. Neurosci., 27, 7827–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Höllerhage M., et al. (2015)Neurotoxicity of dietary supplements from Annonaceae species. Int. J. Toxicol., 34, 543–550. [DOI] [PubMed] [Google Scholar]
- 80. Kohen R., et al. (2002)Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol., 30, 620–650. [DOI] [PubMed] [Google Scholar]
- 81. Zhang A., et al. (2013)Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem., 63, 570–577. [DOI] [PubMed] [Google Scholar]
- 82. Padma P., et al. (1999)Hepatoprotective activity of Annona muricata Linn. and Polyalthia cerasoides bedd. Anc. Sci. Life, 19, 7–10. [PMC free article] [PubMed] [Google Scholar]
- 83. Florence N.T., et al. (2014)Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol., 151, 784–790. [DOI] [PubMed] [Google Scholar]
- 84. Nwokocha C.R., et al. (2012)Possible mechanisms of action of the hypotensive effect of Annona muricata (soursop) in normotensive Sprague-Dawley rats. Pharm. Biol., 50, 1436–1441. [DOI] [PubMed] [Google Scholar]
- 85. Snow R.W., et al. (1999)A preliminary continental risk map for malaria mortality among African children. Parasitol. Today, 15, 99–104. [DOI] [PubMed] [Google Scholar]
- 86. Winstanley P.A. (2000)Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol. Today, 16, 146–153. [DOI] [PubMed] [Google Scholar]
- 87. Ménan H., et al. (2006)Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J. Ethnopharmacol., 105, 131–136. [DOI] [PubMed] [Google Scholar]
- 88. Osorio E., et al. (2007)Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol., 111, 630–635. [DOI] [PubMed] [Google Scholar]
- 89. Somsak V., et al. (2016)In vivo antimalarial activity of annona muricata leaf extract in mice infected with plasmodium berghei. J. Pathog., 2016, 3264070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jaramillo M.C., et al. (2000)Cytotoxicity and antileishmanial activity of Annona muricata pericarp. Fitoterapia, 71, 183–186. [DOI] [PubMed] [Google Scholar]
- 91. Vila-Nova N.S., et al. (2013)Different susceptibilities of Leishmania spp. promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol., 133, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bories C., et al. (1991)Antiparasitic activity of Annona muricata and Annona cherimolia seeds. Planta Med., 57, 434–436. [DOI] [PubMed] [Google Scholar]
- 93. Ferreira L.E., et al. (2013)In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol., 134, 327–332. [DOI] [PubMed] [Google Scholar]
- 94. Clement Y.N., et al. (2016)Herbal remedies and functional foods used by cancer patients attending specialty oncology clinics in Trinidad. BMC Complement. Altern. Med., 16, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McLaughlin J.L. (2008)Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. J. Nat. Prod., 71, 1311–1321. [DOI] [PubMed] [Google Scholar]
- 96. Alali F.Q., et al. (1999)Annonaceous acetogenins: recent progress. J. Nat. Prod., 62, 504–540. [DOI] [PubMed] [Google Scholar]
- 97. Liu N., et al. (2016)Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J. Ethnopharmacol., 189, 210–217. [DOI] [PubMed] [Google Scholar]
- 98. Antony P., et al. (2016)Acetogenins from Annona muricata as potential inhibitors of antiapoptotic proteins: a molecular modeling study. Drug Des. Devel. Ther., 10, 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Redondo-Blanco S., et al. (2017)New Insights toward colorectal cancer chemotherapy using natural bioactive compounds. Front. Pharmacol., 8, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lewandowska H., et al. (2016)The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem., 32, 1–19. [DOI] [PubMed] [Google Scholar]
- 101. Syed Najmuddin S.U., et al. (2016)Anti-cancer effect of Annona muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med., 16, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]