The term ‘minimally conscious state’ (MCS) was introduced to describe complex non-reflexive behaviours in the absence of functional communication. In a critical review, Naccache argues that MCS criteria do not inform us about residual consciousness, but do inform us with certainty about the presence of a Cortically Mediated State (CMS).
Keywords: consciousness, minimally conscious state, vegetative state, functional brain imaging
Abstract
Durable impairments of consciousness are currently classified in three main neurological categories: comatose state, vegetative state (also recently coined unresponsive wakefulness syndrome) and minimally conscious state. While the introduction of minimally conscious state, in 2002, was a major progress to help clinicians recognize complex non-reflexive behaviours in the absence of functional communication, it raises several problems. The most important issue related to minimally conscious state lies in its criteria: while behavioural definition of minimally conscious state lacks any direct evidence of patient’s conscious content or conscious state, it includes the adjective ‘conscious’. I discuss this major problem in this review and propose a novel interpretation of minimally conscious state: its criteria do not inform us about the potential residual consciousness of patients, but they do inform us with certainty about the presence of a cortically mediated state. Based on this constructive criticism review, I suggest three proposals aiming at improving the way we describe the subjective and cognitive state of non-communicating patients. In particular, I present a tentative new classification of impairments of consciousness that combines behavioural evidence with functional brain imaging data, in order to probe directly and univocally residual conscious processes.
Why minimally conscious state is a problematic label
Neurological examination is a ‘bipedal’ exercise
When confronted with a patient, a neurologist has to master two methods. The first component of this ‘bipedal approach’ corresponds to the collection of spontaneous and elicited behaviours of the patient. This ‘behaviourist foot’ of the neurological examination includes the observation of spontaneous behaviours, the reflex testing stage, as well as the numerous procedures conceived to characterize various sensory-motor impairments such as, for instance, the Romberg manoeuvre designed to disentangle between vestibular, cerebellar or proprioceptive impairment in front of a gait instability. Nevertheless, while this ‘behaviourist foot’ of clinical neurology is tremendously important and the neurological examination often comes down to it for non-neurologists, it is legitimate to claim that its importance is overwhelmed by the second method a neurologist needs to master: the art of collecting relevant subjective reports from a patient: ‘What does it feel like to experience what I experience’. This ‘psychologist foot’ of the neurological examination requires much more training and effort to be acquired. I call this component of neurological examination the ‘psychologist foot’ because its value lies in the collected subjective reports. However, note that, obviously, these subjective reports are expressed and communicated through behaviours including verbal and non-verbal acts [e.g. functional communication with an ocular code in conscious but paralysed patients in the locked-in syndrome (Feldman, 1971)]. By collecting spontaneous and elicited subjective reports, the neurologist achieves a correct diagnosis in the vast majority of cases. For instance, the trained questioning of a patient suffering from headaches will be sufficient, in most cases, to distinguish between migraines, cluster headaches, essential or symptomatic facial neuralgias, tension headaches, intracranial hypertension/hypotension or meningeal syndrome. The same applies to most situations encountered in neurology: from motor impairment to memory complaints. Importantly, the neurologist also looks for the precious mismatches that may exist between those subjective reports and objective reality, as illustrated by hallucinations, asomatognosias and misindentification syndromes. Subjective reports can also be dissociated from the data gained by the neurologist himself with his ‘behaviourist foot’, such as in anosognosia: a patient with a left hemiplegia claiming that he is not paralysed. Conversive disorders may capture the inverse dissociation. In short, a neurologist works optimally when he can use this ‘bipedal approach’.
The distinction we raise between the ‘psychologist’ and ‘behaviourist’ feet of neurology is close to the seminal distinction between physical examination and patient interview in medicine. However, it is of special interest in the present case because patient’s subjective data are not only a noisy source of knowledge about his condition, but the mere object of our inquiry: is this patient still holding a conscious subjective posture?
The paradox of inferring conscious states from non-psychologically oriented behaviours
However, in some extreme clinical conditions a bipedal neurologist turns to a ‘one-legged neurologist’, unable to make use of his/her ‘psychologist foot’. Such an awkward situation corresponds to the general category of non-communicating patients including comatose, vegetative state (VS) also coined unresponsive wakefulness syndrome (UWS), and minimally conscious state (MCS) patients.
It is paradoxical to use this ‘behaviourist foot’ of neurological examination—unable to collect subjective reports—in order to infer patient’s subjective states. Indeed, being conscious can be defined as being able to formulate internal subjective reports about oneself or about anything, as verbally illustrated by typical conscious reports such a: ‘I see X, I feel Y, I’m doing Z,…’ (Dehaene and Naccache, 2001).
A few comments on consciousness defined as the ability to self-report are useful to clarify ideas developed in this article. First, it is important to keep in mind that a subjective report is not to be confounded with the behaviour used to communicate this internal process to an external examiner (Naccache and Dehaene, 2008). Moreover, a subjective report is not necessarily verbal. Defining consciousness as the ability to self-report is a theoretical view shared by many models and authors (Dennett, 1992; Weiskrantz, 1997; Rosenthal, 2005). While some authors discuss its completeness by proposing the existence of conscious but not self-reported state [see for instance the Phenomenal consciousness (P-Consciousness) theory developed by Block (1995)], there is a consensus about the unique specificity of reportability: all theories consider that self-reporting is necessarily and univocally a conscious act. Consequently, the reportability definition of consciousness is not only a theoretical construct, but proves to be very close to the neurological definition of consciousness framed by the seminal study of Plum and Posner (1972) as follows: ‘Consciousness means awareness of self and environment’. Being aware of something means being able to self-report it as a conscious content.
This paradox of using the ‘behaviourist foot’ of neurological examination in order to infer patient’s subjective states constitutes the key problem of our current definitions of conscious state of non-communicating patients. It is strengthened by the major difficulty to distinguish volitional from reflex behaviours in such non-communicating patients (Fischer and Truog, 2015). This paradox has been pointed out very early by Bernat who wrote: ‘How can we be certain that the awareness of patients in MCS is minimal? Given that the criteria for MCS measure impaired responsiveness, perhaps it would be more accurate to use the older term ‘minimally responsive’ to describe them’ (Bernat, 2002).
The power of one-legged neurology
Before moving to the proposed reinterpretation of MCS, we should still emphasize and recognize the importance of this imperfect one-legged neurology applied to non-communicating patients.
The case of comatose states constitutes a paradigmatic illustration of the power of this approach. Using a neo-jacksonian approach to probe the functioning of the CNS as a vertical and hierarchical integrated system with a gradient of resistance to ‘aggressions’ (Jackson, 1931–1932) (higher anatomical structures being less resistant than lower structures), Plum and Posner (1972) were able to classify the functional severity of comatose states according to their ordered depth: from light comatose states corresponding to ‘sleep-coma’, to functional di-encephalic states (with preservation of brainstem reflexes, reactive myosis, Cheynes-Stokes ventilation and decortication reaction to painful stimuli), then to deeper levels of vigilance impairment such as peduncular, pontic and medullar levels. This classification still inspires implicitly our current clinical scales [from the Glascow Coma Scale (GCS) (Teasdale and Jennett, 1974) to Liège-GCS (Born et al., 1985) and more recent scales such as the FOUR score (Wijdicks et al., 2005). Recent developments of this ‘behaviourist foot’ of comatose assessment even proved that it was still effective under drug anaesthesia (Sharshar et al., 2011), and that it could even reveal original behavioural patterns associated with specific outcomes, unpredicted by a strict neo-jacksonian framework (Rohaut et al., 2017a).
In the case of awake but non-communicating patients, this behavioural approach also led to significant achievements. In front of an eyes wide open patient, the ability to distinguish between behaviours that rely exclusively upon the preserved functioning of the brainstem and of the ascending reticular activating system (ARAS), and between richer behaviours that imply a substantial contribution of cortico-thalamic networks is fundamental. As early as 1972, the explicit definition of the VS (Jennett and Plum, 1972) was a substantial conceptual and clinical achievement. This definition enabled a rigorous distinction to be made between patients in the VS and patients showing richer behaviours. Since then several studies confirmed the prognosis value of this distinction, as well as the definitive poor outcome of VS in some patients such as anoxic patients remaining in this state for more than 3 months (The Multi-Society Task Force on PVS, 1994b).
In this context, the next significant progress was made by Giacino and his colleagues who defined, in 2002, the MCS as being a state in which: ‘cognitively mediated behaviour occurs inconsistently, but is reproducible or sustained long enough to be differentiated from reflexive behaviour’ (Giacino et al., 2002).
The rise of the JFK Coma Recovery Scale
A precious behavioural scale (the JFK Coma Recovery Scale or CRS) was then conceived by Kalmar and Giacino to enable the crucial distinction between VS and MCS (Kalmar and Giacino, 2005). The revised version of this scale (CRS-R) is now the gold standard and provides clinicians with a hierarchical (Gerrard et al., 2014), reliable, fast and easy-training tool to distinguish VS, from MCS and conscious (exit-MCS or EMCS) patients.
Clearly, the CRS-R increases substantially the diagnostic and prognostic performance of clinicians. The group of Laureys and colleagues reported that the rigorous and standardized use of this scale enabled to correct up to 41% of underestimation errors (Schnakers et al., 2009): patients being diagnosed as being in the VS whereas clear evidence of MCS was demonstrated by the CRS-R scores. This diagnosis power also translates into a prognosis value. Several recent studies reported that being in a MCS is associated with a better prognosis than being in the VS (Luaute et al., 2010; Noe et al., 2012; Klein et al., 2013; Faugeras et al., 2017). This prognosis value holds in terms of survival, of general outcome and of consciousness recovery. Importantly, several arguments suggest that this prognosis value cannot be solely accounted for by a self-fulfilling prophecy bias. In our own prospective study, this difference of outcome between initially MCS or VS patients persisted even when restricting the analysis to patients still alive by the end of the study (Faugeras et al., 2017).
Probing consciousness through behaviours that require conscious processing
An indirect way of probing consciousness only with the ‘behaviourist foot’ could consist in probing behavioural properties, the presence of which would unquestionably sign a conscious state (Naccache, 2006b). While this area is still discussed and under inquiry, the following properties seem to require conscious processing: (i) active maintenance of explicit mental representations in working memory for several seconds (Greenwald, 1996; Rossetti, 1998; Naccache et al., 2002); (ii) strategical processing; and (iii) spontaneous intentional behaviour (Dehaene and Naccache, 2001). Bekinschtein et al. (2009a) capitalized on the (i) working memory property, and used an eyeblink conditioning paradigm in which a tone stimulus can be paired with an air-puff delivered on the cornea. Delay conditioning, where the conditioned stimulus and the unconditioned air-puff overlap in time, does not require conscious processing of the stimuli. In contrast, trace conditioning where a temporal gap is inserted between the two stimuli seems to require conscious processing in working memory (Clark and Squire, 1998). Interestingly, they showed that some clinically-defined VS patients were able to demonstrate both delay conditioning and trace conditioning, suggesting a possible reinterpretation of their genuine status. The CRS-R does not capitalize on this potentially relevant distinction between those cortically driven behaviours that may require conscious processing on the one hand, and those that do not on the other hand. However, future developments of the ‘behaviourist foot’ may explore this approach, illustrating the unbounded territory of this incomplete but precious one-legged neurology.
The limits of one-legged neurology
In spite of its unquestionable value, the one-legged behavioural approach is by definition unable to capture explicit behavioural evidence of inner subjective states. Therefore, relying on it to diagnose MCS is problematic as we will now detail, by exploring those behaviours the presence of which enable the differentiation of MCS from the VS. We will focus our study on the CRS-R items (Table 1).
Table 1.
CRS-R is designed to recognize cortically mediated behaviours
| CRS subscales | CRS-R item | MCS, VS/UWS or EMCS item | Cortically mediated behaviour? |
|---|---|---|---|
| Auditory function | |||
| 4 | Consistent movement to command | MCS | Yes |
| 3 | Reproducible movement to command | MCS | Yes |
| 2 | Localization to sound | VS | No |
| 1 | Auditory startle | VS | No |
| 0 | None | X | X |
| Visual function | |||
| 5 | Object recognition | MCS | Yes |
| 4 | Object localization: reaching | MCS | Yes |
| 3 | Visual pursuit | MCS | Yes |
| 2 | Fixation | Debated | Debated |
| 1 | Visual startle (blink to threat) | VS | No |
| 0 | None | X | X |
| Motor function | |||
| 6 | Functional object use | EMCS | Yes |
| 5 | Automatic motor response | MCS | Yes |
| 4 | Object manipulation | MCS | Yes |
| 3 | Localization to noxious stimulation | MCS | Yes |
| 2 | Flexion withdrawal | VS | No |
| 1 | Abnormal posturing | VS | No |
| 0 | None/flaccid | X | X |
| Oromotor function | |||
| 3 | Intelligible verbalization | MCS | Yes |
| 2 | Vocalization/oral movement | Dubious | Dubious |
| 1 | Oral reflexive movement | VS | No |
| 0 | None | X | X |
| Communication | |||
| 2 | Functional: accurate | EMCS | Yes |
| 1 | Non-functional: intentional | MCS | Yes |
| 0 | None | X | X |
| Arousal | |||
| 3 | Attention | Dubious | Dubious |
| 2 | Eye opening without stimulation | VS | No |
| 1 | Eye opening with stimulation | VS | No |
| 0 | Unarousable | X | X |
Items of the CRS-R (second column) indicative of MCS are in bold (third column). The last column indicates the cortical (‘Yes’ in bold), versus subcortical (‘No’) neural basis of each of these behaviours. The three behaviours with a debated or dubious neural basis are indicated. We observe an almost perfect matching between MCS/VS items and cortical/subcortical origin of these behaviors.
EMCS = exit-MCS; X = absence of behavioural response.
From minimally conscious state to cortically mediated state
Of the 23 behaviours probed during CRS-R testing, 10 can be observed in the VS (VS items), 11 enable the diagnosis of MCS, and two are indicative of a conscious state (exit-MCS or EMCS). More precisely, an MCS item is defined as an item the presence of which is sufficient to categorize the patient as being in a MCS.
From an anatomo-functional point of view, it is striking to note an almost perfect mapping between MCS/VS items and cortical/subcortical corresponding neural circuits. In other words, while each of the 11 MCS items are known to reflect the activity of cortical networks, it is also exact that the 10 VS items are known to reflect the activity of subcortical structures including in particular medulla and brainstem regions. For instance, while the auditory startle (VS item) is a polysynaptic reflex relying on a specific cochlear nerve-cochlear nucleus-reticular-spinal pathway (Yeomans and Frankland, 1995) located outside cortical structures, smooth visual pursuit behaviour (MCS item) demonstrates the functionality of a widespread set of fronto-temporo-parietal areas (Thier and Ilg, 2005) projecting on pontic and cerebellar structures. In each of the five subscales of the CRS-R including MCS items (the arousal subscale does not include any MCS item), the sharp transition between VS and MCS items follows a similarly sharp transition between subcortical and cortical corresponding neural circuits. For instance, if we consider the auditory function subscale, startle (cochlear-reticular-spinal) and localization to sounds (olivar complex within the brainstem) are VS items and map to subcortical circuits, whereas reproducible and consistent movement to command requires the contribution of language-related cortical networks.
Within this mapping observed across the 23 items between VS/MCS labels and subcortical/cortical corresponding circuits, only two are open to discussion.
In the ‘visual function’ subscale, fixation is interpreted as a MCS item, but more recent studies questioned the specificity of this behaviour. Bruno and colleagues compared the cerebral metabolism of five VS patients (therefore without visual fixation) and of five MCS patients in whom the single MCS item was preserved visual fixation (Bruno et al., 2010). In both populations a major dysfunction in a widespread fronto-parietal network was found as compared to controls. These authors suggested that visual fixation may be a VS item. This discussion about the meaning of visual fixation is also reflected in two reference papers written by assemblies of experts that insist on consistency and on the sustained versus fleeting aspect of fixation. On the one hand, the Royal College of Physicians stated that: ‘patients’ eyes may turn fleetingly to follow a moving object’ in the VS/UWS, and that visual fixation was atypical but not incompatible with the VS/UWS (Working Party of the Royal College of Physicians, 2003). On the other hand, the Multi-Society PVS Task Force (The Multi-Society Task Force on PVS, 1994a) also considered visual fixation as a mostly VS/UWS item, but insisted that: ‘Nevertheless, one should be extremely cautious in making a diagnosis of the vegetative state when there is any degree of consistent and reproducible visual fixation’. We recently raised this issue of the unclear value of visual fixation (Rohaut et al., 2013), and mentioned that it can be observed in hemianopic ‘blindsight’ patients (Ro et al., 2004).
This last argument emphasizes the fact that fixation may rely on a subcortical pathway including the superior colliculus, with no contribution of visual cortical areas. Naro et al. (2016) probed fronto-parietal integration in three patients showing visual fixation but lacking other MCS items. While one of them did not show electrophysiological evidence of fronto-parietal integration after an associative TMS-tACS (transcranial magnetic stimulation-transcranial alternate current stimulation) protocol, the other two patients did show such evidence. They proposed to distinguish between ‘aware fixation’ and ‘unaware fixation’, but this distinction cannot be made on a pure behavioural basis and the proposed terminology (‘aware’ versus ‘unaware’) is a new illustration of the confusion between consciousness and cortical activity.
In the oromotor subscale, the ‘vocalization and oral movements’ item is not sufficient to diagnose MCS, but one may note this item spans across diverse behaviours from non-verbal vocalization to unintelligible phoneme production. In other terms this item probably merges subcortical and cortical behaviours.
The arousal subscale deserves a special attention because none of its four items enables the diagnosis of MCS. The higher item (‘attention’) is not considered specific of MCS, even if it is improbable to observe it in the VS/UWS (Chatelle et al., 2016). To score this item, the patient has to react to verbal commands in such a way that: ‘There are no more than 3 occasions across the length of the evaluation in which the patient fails to respond to a verbal prompt’. These responses do not need to be semantically congruent with the verbal commands, but to show consistency. Therefore, according to this loose criterion this item does not disentangle between behavioural responses reflecting semantic integration within the language networks, and those that may occur in the absence of cortical processing.
As an interim conclusion, the CRS-R enables us to differentiate behaviours relying on cortical activity from those that do not. This clinical tool therefore constitutes a powerful probe of cortically mediated behaviours. From this perspective—and in the strictest sense—the CRS-R enables us to recognize cortically mediated states (CMS) rather than MCS.
Presence of cortical processing does not guarantee conscious state
Once reinterpreting MCS as a CMS, the potential gap between MCS and consciousness increases because cortical activity and cortically driven behaviours are not specific to conscious states. Over the last 20 years, empirical and theoretical studies have demonstrated that conscious states do not rely on a single cortical area or network, but require, instead, a brain-scale communication that has to be sustained, complex and differentiated. These properties were emphasized by several theoretical models such as the global neuronal workspace theory of consciousness (Dehaene and Naccache, 2001; Dehaene et al., 2006, 2011), the fronto-parietal network of consciousness (Laureys and Schiff, 2012), the meso-circuit hypothesis (Schiff, 2010), or the recent TMS-EEG studies (Ferrarelli et al., 2010; Casali et al., 2013) inspired by the Integrated Information theory (Tononi, 2004).
Cortically generated behaviours can escape conscious reports
A large set of cortically generated complex behaviours escape conscious reports in conscious individuals. Desmurget and colleagues (Desmurget et al., 2009) used electrical stimulation in seven patients undergoing awake brain surgery and showed that stimulation of the premotor region triggered overt mouth and contralateral limb movements while the patients firmly denied that they had moved. Additionally, a large set of studies also demonstrated that a subliminal visual stimulus inaccessible to conscious report can induce a ballistic chain of cortical processing from early visual areas to both high-level ventral pathways and dorsal pathway regions (Dehaene and Naccache, 2001, 2006; Naccache, 2006a; Kouider and Dehaene, 2007). Crucially, this unconscious cortical processing can affect motor behaviour as illustrated by lateralized responses of primary and premotor areas using scalp event-related potentials (Eimer and Schlaghecken, 1998) and functional MRI (Dehaene et al., 1998). Note that beyond observable behaviours, many types of unconscious cognitive operations have been associated with the activity of various cortical networks.
Cortically generated behaviours can occur in unconscious individuals
One of the most demonstrative illustrations of the existence of cortically generated behaviours in unconscious individuals originates from epilepsy (Blumenfeld, 2016). In complex partial seizures (in particular in frontal lobe seizures) or in absence seizures, patients present a ‘pure’ form of unconsciousness: while their vigilance is still preserved, as indicated by spontaneous eyes-opening and preserved postural tonus, they typically lose abilities of (i) self-reporting any subjective content; (ii) engaging in intentional voluntary behaviours; and (iii) of storing this current episode in their conscious episodic memory. However, in spite of this unconscious state, many of these patients engage in repetitive and stereotypical motor behaviours. Several electrophysiological studies showed the frontal origin of many such behaviours (Bonini et al., 2014). Another illustration of such complex behaviours of cortical origin in unconscious subjects can be found in sleepwalking parasomnia (Bassetti et al., 2000; Laureys, 2005). Typically, while patients are in slow wave sleep stage and usually unconscious, they engage in behaviours such as sitting up in bed, standing, walking, cleaning, or even in more complex patterns of activities such as cooking, talking or driving. A TMS study clarified the functional involvement of cortical structures during these slow-wave sleep complex behaviours by reporting a disinhibition of cortical activity during wakefulness in these patients as compared with normal controls (Oliviero et al., 2007).
On a confusion between consciousness and cortically mediated behaviours
The absence of strict identity or ‘bijective mapping’ between cortical processing on the one hand, and consciousness on the other hand, is at the origin of the confusion related to the name of MCS.
From MCS to MCS+/MCS−
In the light of our reinterpretation of the MCS as a CMS, it becomes obvious that MCS covers a large and heterogeneous set of states that may span from unconscious patients with residual islets of cortical activity that translates into overt behaviour, to conscious but cognitively impaired patients that may be self-conscious but unable to go from preserved response to command to the functional use of a communication code, due to executive deficits (working memory, executive control). Actually, this predicted heterogeneity of MCS is already present in the literature as illustrated by the recent proposed fractionation of MCS into at least two subsets: ‘MCS plus’ (MCS+) and ‘MCS minus’ (MCS−). Bruno and colleagues (Bruno et al., 2011) distinguished patients satisfying MCS criterion according to cognitively poor items such as fixation or visual pursuit, from patients showing cognitively richer behaviours such as reliable response to verbal command in the absence of reliable functional communication (in which case patients are labelled exit-MCS, meaning conscious). They could then report the existence of higher cerebral metabolism (18F-deoxyglucose PET data) in left-sided cortical areas encompassing the language network, premotor, pre-supplementary motor, and sensorimotor cortices in the MCS+ group as compared to MCS− patients (Bruno et al., 2012).
‘Minimally conscious state’ includes the adjective ‘conscious’
The adverb ‘minimally’ in ‘minimally conscious state’ is rather dubious and introduces some perplexity for theorists, clinicians, caregivers and patients’ relatives. What is really ‘minimal’ in MCS: consciousness, state definition or stability over time … or simply our understanding? In addition to introducing some confusion, the mere presence of the adjective ‘conscious’ is often understood by families as positive evidence supporting the existence of a self-reportable subjective posture in patients. We obviously spend time and efforts with families and patients’ relatives to explain to them our large incertitude relative to conscious state in MCS, in particular when only the presence of visual fixation or visual pursuit classify him or her as MCS.
This confusion can reach physicians, caregivers and can obviously propagate through media framing of current brain imaging findings in these patients (Kitzinger and Samuel, 2013; Naccache, 2013; Samuel and Kitzinger, 2013). Once we redefine the MCS as a CMS, rather than a direct and univocal evidence of conscious processing (conscious but ‘minimal’), these problems and misunderstandings should be addressed more easily. This could be a clear starting point to then explain the much less clear issue of consciousness in the concerned patient.
Wrangling about a name: ‘vegetative state’ is less fuzzy than ‘minimally conscious state’
Ironically, while the attention of the scientific and medical community has been recently attracted to the problems raised by the terminology of ‘vegetative state’, it did not tackle so far with the caveats raised here about the ‘minimally conscious state’ expression. Indeed, the European task force on disorders of consciousness noticed two principal problems related to the use of the clinical label ‘vegetative state’ (Laureys et al., 2010). First, the adjective ‘vegetative’ often conveys a pejorative connotation originating from an incorrect etymological association with the noun ‘vegetable’ (whereas it actually derives from the vegetative functions). Second, the task force considered that the use of the name ‘state’ rather than ‘syndrome’ could play a role in the large rate of diagnostic errors reported in these patients who are often in a MCS rather than in a VS. Reminding the strict syndromic value of the gathering of elementary behavioural signs may emphasize the limitation of this incomplete description (lacking brain activity measures). Therefore, they proposed to replace VS expression by ‘unresponsive wakefulness syndrome’ (UWS). I share some of the concerns raised by my colleagues, agree with some of their arguments, and also note that given that most VS patients show cortical activity (EEG, functional MRI recordings), it is incorrect to describe their neural activity as being strictly restricted to subcortical or to vegetative nervous system structures. Neurophysiologically speaking, most VS patients are not apalic (‘without cortex’ etymologically) (Schiff et al., 2002).
However, I do not consider this proposal as a satisfactory solution, mostly because the adjective unresponsive is open to many ambiguous meanings ranging from intentional responses to reflex or automatic behavioural responses. Using the adjective ‘unresponsive’ may confuse families and relatives who often observe behavioural responses in these patients (even though only reflexive). Adopting the ‘unresponsive wakefulness’ label may even suggest to some families, relatives and caregivers of such a patient that he/she is very similar to a conscious but paralysed patient (like for instance conceiving the patient as being in the locked-in syndrome): a conscious but unresponsive person.
Proposals
In the light of this constructive criticism of the MCS label, I propose three classes of actions that could improve the way we describe subjective and cognitive state of non-communicating patients, and avoid the prejudicial confusions we addressed above.
An educational effort beyond clinical terminologies
We need to deploy efforts to train and inform colleagues, caregivers and patients’ relatives about the meaning of our explorations, and of the vocabulary we use to describe their status.
Whether or not we decide to update this vocabulary (see proposal 2.3) we have to highlight the following three principles:
Principle 1: Current definitions of MCS and VS labels simply reflect our tentative answer to the following question: do cortical networks contribute to the overt behaviour of the patient?
Principle 2: When a patient is labelled as MCS, he is more prone to be in a conscious state and to experience conscious contents, because we know cortical activity is mandatory to conscious states and conscious contents. However, there is by definition no guarantee that this is the case. This uncertainty is so clear that as soon as the patient is able to engage in a functional communication he/she is not labelled as MCS anymore, but as exit-MCS or conscious.
Principle 3: One of the reasons why an MCS patient is not necessarily conscious originates from the numerous illustrations of unconscious cortical processing and of unconscious cortically mediated behaviours, both during conscious (unreported behaviour) and unconscious states.
When targeting patients’ relatives, this educational effort can take the form of dedicated consultations during which these principles can be explained. Such dedicated meetings and follow-ups also enable us to minimize the ‘violence’ inherent to revealing information that may be incongruent with the subjective representations of the current state of the patient by his/her relatives. This is also the occasion to distinguish the diagnosis/prognosis value of our evaluations, and to inform relatives of the degree of confidence (high or low) we have in our own statements.
Restoring a bipedal neurology with functional neuro-imaging in non-communicating patients
Once we realize that MCS does not directly address the issue of consciousness, it becomes even more urgent to try to restore a bipedal neurology. In order to refurbish the missing ‘psychologist foot’ we can make use of functional brain imaging tools (mostly bedside EEG, and functional MRI) combined with cognitive neuroscience paradigms to record brain activity, and infer conscious states and/or conscious contents of the patients. Obviously, these tools are limited, imperfect and need to be improved, but they already provide valuable information to address the following three major questions that escape a strict ‘behaviourist foot’ neurology.
Inferring conscious state from functional brain imaging
One can engage the patient in an active cognitive task that cannot be performed unconsciously. If a patient’s brain shows patterns of activity observed in conscious controls performing this task, one may infer he/she is conscious. The seminal ‘imagine play tennis/navigating in your home’ task conceived by Owen and colleagues illustrates this approach (Naccache, 2006b; Owen et al., 2006).
Another approach consists of studying patterns of activity ‘at rest’ (Raichle et al., 2001). A recent set of studies (using mostly functional MRI and PET brain imaging) revealed some key neural signatures of conscious default mode networks (DMN) (Stender et al., 2014; Demertzi et al., 2015) in the averaged value of DMNs but also in its dynamics (Barttfeld et al., 2015). Two recent studies developed an EEG version of this approach and could successfully describe a set of EEG metrics specific to conscious states (Sitt et al., 2014; Chennu et al., 2017). We could demonstrate how these markers enabled the detection of conscious state in a paralysed and multisensory disconnected ‘locked-in syndrome’ patient (Rohaut et al., 2017b), and in two clinically VS patients (Faugeras et al., 2011), several days before clinical examination.
A last approach aims at perturbing brain activity with a focal and transient electromagnetic stimulation while recording how it reacts to this stimulation. While early and focal brain responses can be observed in unconscious states, late, lasting and complex responses are specific to conscious state studies (Ferrarelli et al., 2010; Rosanova et al., 2012; Casali et al., 2013).
Inferring conscious contents from functional brain imaging
It is also possible to probe some conscious contents by exposing patients to stimuli and by probing if their brains show neural signatures of conscious access, such as the typical P3b scalp EEG event-related potential (Sergent et al., 2005; Bekinschtein et al., 2009b; Faugeras et al., 2011, 2012). The ‘local global’ task does not probe directly conscious state, but probes if a patient could consciously access a specific attribute of the auditory stimuli (Naccache et al., 2015). We recently provided a proof-of-concept of the superiority of a multidimensional evaluation of cognition in non-communicating patients using EEG as compared to unidimensional paradigms (Sergent et al., 2016). Naci et al. (2014) showed patients a movie full of suspense, and could then identify those subjects who were able to consciously follow the up and downs of the narrative structure.
Probing and restoring communication with functional brain imaging
Once at least two distinct patterns of brain activity can be reliably induced by tasks/questions and identified with functional MRI/EEG, the door is open to use functional brain imaging to exchange conscious information with a patient, and to create a new channel of functional communication as illustrated by several proof-of-concept studies (Monti et al., 2010; Cruse et al., 2011; Goldfine et al., 2011).
Therefore, not only does the use of functional brain imaging in non-communicating patients provide an additional source of information, but I advocate that its role could be even more ambitious: functional brain imaging could be used to restore a bipedal neurology, and therefore re-establish a ‘psychologist foot’.
One may also note that this proposal of restoring a bipedal neurology with functional neuro-imaging may improve patients’ management (e.g. correct diagnosis errors), but also enrich neurological knowledge. Indeed, while current behavioural assessment reflects a cortical/subcortical origin of the MCS versus VS/UWS items, respectively, one may question if it is really the case, or if some VS/UWS behavioural items might actually be of cortical origin. This issue is supported both by recent reports, mentioned above, related to the difficulty of labelling an observed behaviour as volitional or reflex (Fischer and Truog, 2015), and by the discovery of patterns of cortical activity in VS/UWS patients (Laureys et al., 2004; Laureys, 2005). Therefore, by combining behaviour to functional brain imaging we may not only correct some diagnosis errors, but also ultimately discover some behavioural counterparts to unconscious cortically driven patterns of activity in VS/UWS patients. For example, one may describe a novel behavioural correlate of auditory mismatch negativity event-related potential component elicited in auditory cortices (Tiitinen et al., 1994; El Karoui et al., 2015); and see also our earlier discussion of the study by Naro et al. (2016) on visual fixation.
I close this proposal by underlining the importance of considering these functional brain imaging tools with caution regarding the complex data analysis methods that are often used, and the statistical challenges they raise (see for instance the following studies and discussions: Boly et al., 2011; King et al., 2011; Cruse et al., 2013; Goldfine et al., 2013; Naccache et al., 2015, 2016; Tzovara et al., 2015a, b; Gabriel et al., 2016). A set of basic principles have to be respected such as: standardization of methods across sites, high-replicability and high-sensitivity, as well as using a hierarchical approach (from low to high cognitive measures), and combining basic and complex data analysis methods on the same datasets (Naccache et al., 2016).
A new terminology combining clinical and functional brain imaging evidence
The terminology we use should evolve to better reflect our understanding of the state of a patient, and should combine both behavioural evidence and functional brain imaging data.
In this perspective I would advocate to replace MCS by CMS, and to refine the four major clinical states (comatose, VS/UWS, CMS and conscious states) in eight categories, according to the source of evidence used to define the clinical state (Table 2). This proposal is not to be considered as a definitive model but rather as a sketch of what our next classification should be.
Table 2.
Proposal of a new classification of impairments of consciousness
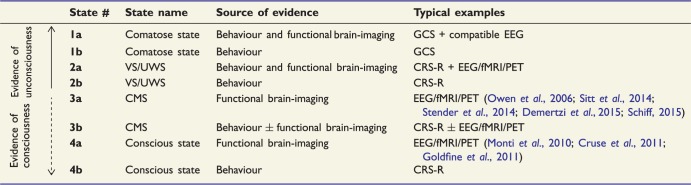 |
This classification integrates both behavioural and functional brain-imaging data, reframes MCS as CMS, and goes from unconscious to conscious states. The original labels are referred to patients described in the literature like for instance those patients able to engage in functional communication with EEG or functional MRI (fMRI) whereas they are clinically scored as VS/UWS. The scale orders states (from 1a to 4b) from the most probably unconscious ones, to the most probably conscious ones.
The categories are ordered from unconscious states (e.g. comatose) to conscious states, and follow the progression of certitude based on the availability of both behavioural and functional brain imaging data (from the most certain unconscious to the most certain conscious states).
In this classification, behavioural evidence of CMS and of consciousness is weighed higher than functional brain imaging data for three reasons. First, behavioural evidence is the gold standard and it capitalizes on firmly grounded sources such as detailed neurological examination and the current relevant scales [e.g. GCS (Teasdale and Jennett, 1974) completed by brainstem reflexes such as in the Glasgow-Liège scale (Born et al., 1982); the CRS-R but also the FOUR score scales (Wijdicks et al., 2005)]. Note that these behavioural scales require an expertise, and that subjectivity of the examiner plays a role in some aspects of the evaluation. Second, functional brain imaging tools are still under scrutiny for methodological, statistical and epistemic reasons. Third, there is a pragmatic reason to this priority of behaviour: when a patient is behaviourally in a CMS or conscious state, this can be observed and used by a much larger community of individuals (e.g. clinicians, caregivers, relatives), than when functional brain imaging measurements are needed. For the case of functional communication, once a patient is behaviourally conscious, he/she is able to communicate with many more people than when functional communication requires functional brain imaging.
Therefore CMS and conscious states that are defined only by functional brain imaging (e.g. CMS clinically VS/UWS) are ranked lower than those defined both by behavioural plus/minus functional brain imaging data (e.g. 3a < 3b, Table 2), and the reverse holds for unconscious states (comatose and VS/UWS).
Crucially this classification offers explicit status to the more and more frequent mismatches or dissociations discovered between behavioural and functional brain imaging data (Schiff, 2015).
This classification takes into account the asymmetry between the presence of signs of consciousness or CMS, and their absence. Therefore, once a patient is behaviourally in the CMS (label 3b; e.g. visual pursuit present), the addition of brain imaging evidence does not impact on the category (see the plus/minus symbol in label 3b, Table 2). Similarly, once a patient is behaviourally conscious (label 4b), no further distinction is proposed based on the availability of functional brain imaging data. At the top of this classification, the label 4b corresponds to the current exit-MCS label and opens the door to more detailed cognitive and behavioural evaluation.
In the present version of the proposed new classification, I limited access to 4a label (conscious state defined on the basis of functional brain-imaging) to cases in which one can demonstrate functional communication with the patient, because no one would question here the interpretation of functional brain imaging results: the patient is conscious (‘Typical examples’ column in Table 2).
However, some conscious but cognitively impaired patients can actually be conscious (even according to the reportability definition of consciousness), but unable to consciously access a stimulus or to mentally perform a task during a functional brain imaging test. For instance, conscious patients suffering from aphasia, from working memory impairments or from low-level perceptual deficits may fail the test. Crucially, we have to be aware of this limit and of the fuzzy frontier between 3a/b and 4a/b categories. A promising way to minimize type 2 errors (false negatives) is to use the two other approaches that have been developed with functional brain imaging (see above): probing neural signatures of conscious states and of conscious contents, respectively. As soon as we have at our disposal a measure of conscious state proven both to be present in all conscious controls (able to engage in functional communication of their subjective reports), and to be absent in all controls when we causally abolish consciousness (e.g. anaesthesia), one may theoretically use such a signature to label a patient as 4a. To my knowledge, the closest available measure of this kind is the TMS-EEG perturbational complexity index (PCI) designed by the group of Massimini (Casali et al., 2013; Casarotto et al., 2016); but see also a recent promising case report using multivariate EEG classification (Rohaut et al., 2017b).
Finally, cognitive neuroscience of disorders of consciousness may be unique to disentangle reportability theory from phenomenal consciousness theory (see above): if one could reliably observe single-subject based signature of conscious state (e.g. PCI) in the absence of single-subject based sensitive signature of conscious access (e.g. P3b observable in the absence of external stimuli), one may define the existence of ‘P-Conscious with no self-reportability’ states. Such a potential dissociation is highly difficult to test in healthy controls who are self-reporting, and who may (according to P-Consciousness theory) also be P-conscious of unreported mental representations. Inversely, if the former is systematically associated with the latter (i.e. if conscious states never dissociate from conscious self-reports), this would strengthen the reportability theory.
Obviously, additional collaborative studies are needed to refine and define those categories and to converge on functional brain imaging criterion. Note that this proposal will not magically suppress type 2 errors (i.e. missing conscious patients), but by stating the concepts more clearly, this could help to select among the non-behavioural tools those that really target conscious states and conscious contents from those that do not (e.g. teasing apart specific neural signatures of conscious access, from neural signatures of unconscious cortical processing).
As a conclusion, our progressive understanding of the complex relations prevailing between cortical activity and consciousness, requires us to update our clinical vocabulary in such extreme conditions. Conceiving ‘minimally conscious state’ as a ‘cortically mediated state’ would improve the way we think about these patients, the way we try to take care of them, and last but not least, the way we explain their states to their relatives.
Acknowledgements
I thank Dr Benjamin Rohaut and Dr Bertrand Hermann for their comments on the first version of this manuscript. I thank the three reviewers for their stimulating and constructive comments.
Funding
This work was supported by the FRM (‘Equipe FRM 2015’), by the Académie des Sciences (Prix Lamonica 2016), and by and the ‘Recovery of consciousness after severe brain injury Phase II’ grant of the James S. McDonnell Foundation.
Glossary
Abbreviations
- CMS
cortically mediated state
- CRS-R
Coma Recovery Scale-Revised
- GCS
Glasgow Coma Scale
- MCS
minimally conscious state
- UWS
unresponsive wakefulness syndrome
- VS
vegetative state
References
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci USA 2015; 112: 887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet 2000; 356: 484–5. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA 2009a; 106: 1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein TA, Shalom DE, Forcato C, Herrera M, Coleman MR, Manes FF, et al. Classical conditioning in the vegetative and minimally conscious state. Nat Neurosci 2009b; 12: 1343–9. [DOI] [PubMed] [Google Scholar]
- Bernat JL. Questions remaining about the minimally conscious state. Neurology 2002; 58: 337–8. [DOI] [PubMed] [Google Scholar]
- Block N. On a confusion about the role of consciousness. Behav Brain Sci 1995; 18: 227–87. [Google Scholar]
- Blumenfeld H. Epilepsy and consciousness. In: Laureys S, Gosseries O, Tononi G, editors. The neurology of consciousness. London: Academic Press; 2016. p. 255–70. [Google Scholar]
- Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science 2011; 332: 858–62. [DOI] [PubMed] [Google Scholar]
- Bonini F, McGonigal A, Trebuchon A, Gavaret M, Bartolomei F, Giusiano B, et al. Frontal lobe seizures: from clinical semiology to localization. Epilepsia 2014; 55: 264–77. [DOI] [PubMed] [Google Scholar]
- Born JD, Albert A, Hans P, Bonnal J. Relative prognostic value of best motor response and brain stem reflexes in patients with severe head injury. Neurosurgery 1985; 16: 595–601. [DOI] [PubMed] [Google Scholar]
- Born JD, Hans P, Dexters G, Kalangu K, Lenelle J, Milbouw G, et al. [Practical assessment of brain dysfunction in severe head trauma (author's transl)]. Neurochirurgie 1982; 28: 1–7. [PubMed] [Google Scholar]
- Bruno MA, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 2012; 259: 1087–98. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Vanhaudenhuyse A, Schnakers C, Boly M, Gosseries O, Demertzi A, et al. Visual fixation in the vegetative state: an observational case series PET study. BMC Neurol 2010; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011; 258: 1373–84. [DOI] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013; 5: 198ra105. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016; 80: 718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelle C, Bodien YG, Carlowicz C, Wannez S, Charland-Verville V, Gosseries O, et al. Detection and interpretation of impossible and improbable Coma recovery scale-revised scores. Arch Phys Med Rehabil 2016; 97: 1295–1300.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennu S, Annen J, Wannez S, Thibaut A, Chatelle C, Cassol H, et al. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 2017; 140: 2120–32. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 1998; 280: 77–81. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernandez-Espejo D, Pickard JD, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2011; 378: 2088–94. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernandez-Espejo D, Pickard JD, et al. Reanalysis of bedside detection of awareness in the vegetative state: a cohort study—authors' reply. Lancet 2013; 381: 291–2. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L. The global neuronal workspace model of conscious access: from neuronal architectures to clinical applications. In: Dehaene S, Christen Y, editors. Characterizing consciousness: from cognition to the clinic? Berlin Heidelberg: Springer; 2011. p. 55–84. [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 2006; 10: 204–11. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79: 1–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Can one suppress subliminal words? Neuron 2006; 52: 397–9. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Le Clec HG, Koechlin E, Mueller M, Dehaene-Lambertz G, et al. Imaging unconscious semantic priming. Nature 1998; 395: 597–600. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Antonopoulos G, Heine L, Voss HU, Crone JS, de Los Angeles C, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015; 138(Pt 9): 2619–31. [DOI] [PubMed] [Google Scholar]
- Dennett DC. Consciousness explained. London: Penguin; 1992. [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science 2009; 324: 811–13. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schlaghecken F. Effects of masked stimuli on motor activation: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform 1998; 24: 1737–47. [DOI] [PubMed] [Google Scholar]
- El Karoui I, King JR, Sitt J, Meyniel F, Van Gaal S, Hasboun D, et al. Event-related potential, time-frequency, and functional connectivity facets of local and global auditory novelty processing: an intracranial study in humans. Cerebral cortex 2015; 25: 4203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Valente M, Sitt J, Demeret S, Bolgert F, et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Injury 2018; 72–7. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein T, Galanaud D, Puybasset L, et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia 2012; 50: 403–18. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology 2011; 77: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MH. Physiological observations in a chronic case of locked-in syndrome. Neurology 1971; 21: 459–78. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA 2010; 107: 2681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DB, Truog RD. What is a reflex? A guide for understanding disorders of consciousness. Neurology 2015; 85: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Muzard E, Henriques J, Mignot C, Pazart L, Andre-Obadia N, et al. Replicability and impact of statistics in the detection of neural responses of consciousness. Brain 2016; 139: e30. [DOI] [PubMed] [Google Scholar]
- Gerrard P, Zafonte R, Giacino JT. Coma recovery scale-revised: evidentiary support for hierarchical grading of level of consciousness. Arch Phys Med Rehabil 2014; 95: 2335–41. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002; 58: 349–53. [DOI] [PubMed] [Google Scholar]
- Goldfine AM, Bardin JC, Noirhomme Q, Fins JJ, Schiff ND, Victor JD. Reanalysis of Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2013; 381: 289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AM, Victor JD, Conte MM, Bardin JC, Schiff ND. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol 2011; 122: 2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG. Three cognitive markers of unconscious semantic activation. Science 1996; 273: 1699–702. [DOI] [PubMed] [Google Scholar]
- Jackson JH. Selected writings of John Hughlings Jackson. Vols I, II. Edited by Taylor J. London, Hodder, 1931–1932. [Google Scholar]
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1: 734–7. [DOI] [PubMed] [Google Scholar]
- Kalmar K, Giacino JT. The JFK Coma recovery scale–revised. Neuropsychol Rehabil 2005; 15: 454–60. [DOI] [PubMed] [Google Scholar]
- King JR, Bekinschtein T, Dehaene S. Comment on preserved feedforward but impaired top-down processes in the vegetative state. Science 2011; 334: 1203; author reply 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzinger J, Samuel G. A response to Naccache’s comment on ‘Reporting consciousness in coma’. JOMEC Journal (June)2013. [Google Scholar]
- Klein AM, Howell K, Vogler J, Grill E, Straube A, Bender A. Rehabilitation outcome of unconscious traumatic brain injury patients. J Neurotrauma 2013; 30: 1476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Dehaene S. Levels of processing during non-conscious perception: a critical review of visual masking. Philos Trans R Soc Lond B Biol Sci 2007; 362: 857–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci 2005; 9: 556–9. [DOI] [PubMed] [Google Scholar]
- Laureys S, Celesia GG, Cohadon F, Lavrijsen J, Leon-Carrion J, Sannita WG, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010; 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004; 3: 537–46. [DOI] [PubMed] [Google Scholar]
- Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012; 61: 478–91. [DOI] [PubMed] [Google Scholar]
- Luaute J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010; 75: 246–52. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010; 362: 579–89. [DOI] [PubMed] [Google Scholar]
- Naccache L. Le nouvel inconscient. Freud, Christophe Colomb des neurosciences Paris: Odile Jacob; 2006a. [Google Scholar]
- Naccache L. Psychology. Is she conscious? Science 2006b; 313: 1395–6. [DOI] [PubMed] [Google Scholar]
- Naccache L. A few comments about ‘Reporting consciousness in coma’ by Samuel and Kitzinger. JOMEC Journal (June) 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends on temporal attention. Psychol Sci 2002; 13: 416–24. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S. Reportability and illusions of phenomenality in the light of the global neuronal workspace model. Behav Brain Sci 2008; 30: 518–20. [Google Scholar]
- Naccache L, King JR, Sitt J, Engemann D, El Karoui I, Rohaut B, et al. Neural detection of complex sound sequences or of statistical regularities in the absence of consciousness? Brain 2015; 138(Pt 12): e396. [DOI] [PubMed] [Google Scholar]
- Naccache L, Sitt J, King JR, Rohaut B, Faugeras F, Chennu S, et al. Reply: replicability and impact of statistics in the detection of neural responses of consciousness. Brain 2016; 139: e31. [DOI] [PubMed] [Google Scholar]
- Naci L, Cusack R, Anello M, Owen AM. A common neural code for similar conscious experiences in different individuals. Proc Natl Acad Sci USA 2014; 111: 14277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naro A, Leo A, Buda A, Manuli A, Bramanti A, Bramanti P, et al. Do you see me? The role of visual fixation in chronic disorders of consciousness differential diagnosis. Brain Res 2016; 1653: 59–66. [DOI] [PubMed] [Google Scholar]
- Noe E, Olaya J, Navarro MD, Noguera P, Colomer C, Garcia-Panach J, et al. Behavioral recovery in disorders of consciousness: a prospective study with the Spanish version of the coma recovery scale-revised. Arch Phys Med Rehabil 2012; 93: 428–33.e12. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Della Marca G, Tonali PA, Pilato F, Saturno E, Dileone M, et al. Functional involvement of cerebral cortex in adult sleepwalking. J Neurol 2007; 254: 1066–72. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006; 313: 1402. [DOI] [PubMed] [Google Scholar]
- Plum F, Posner JB. The diagnosis of stupor and coma. Contemp Neurol Ser 1972; 10: 1–286. [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Shelton D, Lee OL, Chang E. Extrageniculate mediation of unconscious vision in transcranial magnetic stimulation-induced blindsight. Proc Natl Acad Sci USA 2004; 101: 9933–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaut B, Faugeras F, Naccache L. Neurology of consciousness impairments. In: Stevens R, Sharshar T, Wes E, editors. Brain dysfunction in critical illness. Cambridge, UK: Cambridge University Press; 2013. [Google Scholar]
- Rohaut B, Porcher R, Hissem T, Heming N, Chillet P, Djedaini K, et al. Brainstem response patterns in deeply-sedated critically-ill patients predict 28-day mortality. PLoS One 2017a; 12: e0176012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaut B, Raimondo F, Galanaud D, Valente M, Sitt JD, Naccache L. Probing consciousness in a sensory-disconnected paralyzed patient. Brain Inj 2017b; 31: 1398–403. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno MA, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain 2012; 135(Pt 4): 1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DM. Consciousness and mind. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Rossetti Y. Implicit short-lived motor representations of space in brain damaged and healthy subjects. Conscious Cogn 1998; 7: 520–58. [DOI] [PubMed] [Google Scholar]
- Samuel G, Kitzinger J. Reporting consciousness in coma: media framing of neuro-scientific research, hope, and the response of families with relatives in vegetative and minimally conscious states. JOMEC Journal (3)2013. [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015; 72: 1413–15. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010; 33: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Ribary U, Moreno DR, Beattie B, Kronberg E, Blasberg R, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain 2002; 125: 1210–34. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009; 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 2005; 8: 1391–400. [DOI] [PubMed] [Google Scholar]
- Sergent C, Faugeras F, Rohaut B, Perrin F, Valente M, Tallon-Baudry C, et al. Multidimensional cognitive evaluation of patients with disorders of consciousness using EEG: a proof of concept study. Neuroimage Clin 2016; 13: 455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharshar T, Porcher R, Siami S, Rohaut B, Bailly-Salin J, Hopkinson NS, et al. Brainstem responses can predict death and delirium in sedated patients in intensive care unit. Crit Care Med 2011; 39: 1960–7. [DOI] [PubMed] [Google Scholar]
- Sitt JD, King JR, El Karoui I, Rohaut B, Faugeras F, Gramfort A, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014; 137(Pt 8): 2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender J, Gosseries O, Bruno MA, Charland-Verville V, Vanhaudenhuyse A, Demertzi A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014; 384: 514–22. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2: 81–4. [DOI] [PubMed] [Google Scholar]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. N Engl J Med 1994a; 330: 1499–508. [DOI] [PubMed] [Google Scholar]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. N Engl J Med 1994b; 330: 1572–9. [DOI] [PubMed] [Google Scholar]
- Thier P, Ilg UJ. The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol 2005; 15: 645–52. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, May P, Reinikainen K, Naatanen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature 1994; 372: 90–2. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci 2004; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzovara A, Simonin A, Oddo M, Rossetti AO, De Lucia M. Neural detection of complex sound sequences in the absence of consciousness. Brain 2015a; 138(Pt 5): 1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzovara A, Simonin A, Oddo M, Rossetti AO, De Lucia M. Reply: neural detection of complex sound sequences or of statistical regularities in the absence of consciousness? Brain 2015b; 138(Pt 12): e396. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Consciousness lost and found: a neuropsychological exploration. New York: Oxford University Press; 1997. [Google Scholar]
- Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol 2005; 58: 585–93. [DOI] [PubMed] [Google Scholar]
- Working Party of the Royal College of Physicians. The vegetative state: guidance on diagnosis and management. Clin Med 2003; 3: 249–54. doi:10.7861/clinmedicine.3-3-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 1995; 21: 301–14. [DOI] [PubMed] [Google Scholar]


