Abstract
Chronic inflammation may be a risk factor for the development and progression of breast cancer, yet it is unknown which inflammatory biomarkers and pathways are especially relevant. The present study included 27,071 participants (mean age = 54.5 years) in the Women’s Health Study who were free of cancer and cardiovascular disease at enrollment (1992–1995), with baseline measures of 4 inflammatory biomarkers: high-sensitivity C-reactive protein, fibrinogen, N-acetyl side-chains of acute phase proteins, and soluble intercellular adhesion molecule-1. We used Cox proportional hazards regression models to evaluate associations between baseline concentrations of biomarkers and incident breast cancer, and adjusted for baseline and time-varying factors such as age and body mass index. Self-reported invasive breast cancer was confirmed against medical records for 1,497 incident cases (90% postmenopausal). We observed different patterns of risk depending on the inflammatory biomarker. There was a significant direct association between fibrinogen and breast cancer risk (for quintile 5 vs. quintile 1, adjusted hazard ratio = 1.25, 95% confidence interval: 1.03, 1.51; P for trend = 0.01). In contrast, soluble intercellular adhesion molecule-1 was inversely associated with breast cancer (for quintile 5 vs. quintile 1, adjusted hazard ratio = 0.79, 95% confidence interval: 0.66, 0.94; P for trend = 0.02). N-acetyl side-chains of acute phase proteins and high-sensitivity C-reactive protein were not associated with breast cancer. The complex association of chronic inflammation and breast cancer may be considered when formulating anti-inflammatory cancer prevention or intervention strategies.
Keywords: biomarkers, breast cancer, inflammation, prospective cohort study
Chronic inflammation is a complex biological process involved in a range of infectious and noninfectious stimuli, and functions as part of the immune system’s response to promote cell division and repair at the site of tissue injury (1, 2). At the same time, cancer cells may leverage components of the inflammatory process to stimulate angiogenesis, prevent apoptosis, and promote proliferation, migration and metastasis (1, 2). Accumulating evidence highlights the key role of individual markers of chronic inflammation in elevated breast cancer risk (3, 4), although substantial heterogeneity across studies was noted. For example, a meta-analysis reported a significant dose-response correlation for C-reactive protein (CRP) with breast cancer risk (5). Fibrinogen, however, was not found to be related to incident breast cancer within 5 years, despite a strong positive association with lung and colorectal cancers (6), and a consistent inverse association with cancer survival (7). Similarly, prior studies of inflammation and breast cancer risk have had largely inconsistent results, and few have been prospective with long-term follow-up (2).
There are a variety of molecules involved in systemic inflammation, each representing different components or pathways of an underlying response (8). High-sensitivity C-reactive protein (hsCRP) and fibrinogen are hepatic-synthesized downstream acute phase proteins that become elevated in the circulation in response to inflammation. Soluble intercellular cell adhesion molecule 1 (sICAM-1) is a circulating biomarker that mediates leukocyte adhesion and trafficking as part of the immune response and vascular inflammation. Circulating N-acetyl methyl groups reflect post-translational glycosylation of a broad range of inflammatory and immune response glycoproteins, and were recently quantified by nuclear magnetic resonance spectroscopy as GlycA (9, 10). We hypothesized that some, but not necessarily all, of the inflammatory biomarkers may be positively related to breast cancer risk, owing to the diverse pathways and underlying perturbations they represent. Thus, we evaluated breast cancer associations for 4 circulating biomarkers that represent distinct, yet correlated inflammatory processes.
In cross-sectional studies of biomarkers in patients with prevalent cancer and relatively short-term follow-up, it is not possible to determine whether inflammation is a causal component of cancer development and growth or whether it is primarily the host’s response to tumor growth. Therefore, a prospective, longitudinal design is warranted to assess the prediagnostic inflammatory status of individuals with cancer. Additionally, it is unknown whether inflammation in general is relevant to breast cancer risk, or whether specific pathways of the inflammatory process are implicated. Therefore, we examined the prospective associations of biomarkers of inflammation with incident breast cancer among 27,071 initially healthy women for whom hsCRP, fibrinogen, GlycA, and sICAM-1 were measured at baseline, prior to cancer diagnosis. Secondarily, we evaluated histological subtypes and conducted analyses stratified by breast cancer risk factors for potential effect modification.
METHODS
Study population
Our analysis included participants in the Women’s Health Study (WHS), a completed, randomized, placebo-controlled, factorial trial of low-dose aspirin, β-carotene, and vitamin E for the primary prevention of cardiovascular disease and cancer (ClinicalTrials.gov identifier: NCT00000479). The trial randomized 39,876 female US health professionals who were aged 45 years or older without a history of cancer (except nonmelanoma skin cancer) or cardiovascular disease (11, 12). The clinical trial, which ran from 1993 to 2004, continues to follow participants annually on an observational basis. Blood samples were collected voluntarily from a total of 28,345 participants prior to randomization, shipped to the laboratory on ice via overnight courier, processed, and stored at −170°C in vapor liquid nitrogen until biomarker measurements were performed. Our analyses included participants with assay data available for all 4 biomarkers of interest (96%; n = 27,071). Questionnaires captured information on demographics, health status, reproductive history, and lifestyle characteristics. Menopausal status, hysterectomy and bilateral oophorectomy status, hormone therapy use, smoking status, physical activity level, and body weight were updated every 1–3 years. Usual frequency of alcohol consumption was ascertained at baseline, and 4 other times throughout follow-up. We asked participants if they had undergone mammography for screening at years 1 and 9 during the trial period and biennially during the observational follow-up. A semiquantitative food frequency questionnaire was self-administered at baseline and 10-year follow-up to capture usual dietary intake. Written informed consent was obtained from all participants and the study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital (Boston, Massachusetts).
Laboratory measurements
Our project leveraged biomarkers assayed in the stored plasma samples for previous unrelated investigations. Assay laboratory methods have been previously described in detail (9, 13–16). Briefly, hsCRP was assayed on a Hitachi 917 auto-analyzer with a high-sensitivity immunoturbidimetric assay (Denka Seiken, Tokyo, Japan) (13). We used an immunoturbidimetric assay with internal standards to measure fibrinogen concentrations (Kamiya Biomedical, Seattle, Washington) (14). LipoScience (now LabCorp; Raleigh, North Carolina) obtained measurements of GlycA from 400 MHz plasma proton (1H) nuclear magnetic resonance spectra. Signals were quantified through deconvolution analysis from signal amplitudes that originated from N-acetyl methyl group protons of the N-acetylglucosamine moieties of specific serum proteins (9, 15). sICAM-1 was assayed with the R&D assay via a standard quantitative sandwich enzyme immunoassay technique by enzyme-linked immunosorbent assay that does not detect the modified form of sICAM-1 in black individuals (R&D Systems; Minneapolis, Minnesota) (16). CRP, fibrinogen, and sICAM-1 were not subjected to prior freeze-thaw cycles when assayed, and investigators were blinded to participant outcome status. GlycA was quantified at a later date, and most samples had undergone 1 previous freeze-thaw cycle. Experiments that subjected samples to 3 freeze-thaw cycles (−80°) had no discernable effect on GlycA levels (James Otvos, LipScience, Inc., personal communication, 2016). Laboratory inter-assay coefficients of variation for CRP, fibrinogen, GlycA, and sICAM-1were 3.0%, 1.17%, 1.9%, and 7.4%, respectively, indicating minimal concern for systematic error or laboratory drift.
Ascertainment of breast cancer cases
Participants received mailed questionnaires that inquired about any newly diagnosed endpoints every 6 months in the first year, and annually thereafter. Medical records were obtained for incident breast cancer cases, and diagnoses were confirmed by a committee of physicians on the basis of pathology or cytology reports (17). Additional information were extracted from medical records, including estrogen and progesterone receptor status, histologic grading and differentiation, tumor size, and lymph node metastases. Deaths were identified from reports by family members, postal authorities, or the National Death Index, with nearly 100% mortality follow-up (18). Only cases of confirmed invasive breast cancer that were reported up to the return of the 2013 questionnaire were included in our analysis.
Statistical analysis
We analyzed biomarkers categorically using quintiles and continuously using the natural log of concentration. We examined the possibility of a nonlinear relationship between each biomarker and the relative risk of breast cancer with nonparametric restricted cubic splines (19). Likelihood ratio tests compared models with only the linear term to models with both the linear and cubic spline terms. None of the biomarkers were significant for tests for nonlinearity; thus, continuous models per 1 standard deviation were used. Baseline characteristics were generated across quintiles of each biomarker. The Alternative Health Eating Index 2010 (aHEI-2010) dietary pattern score was calculated as previously described (20), with a higher score indicating a higher quality diet. Cox proportional hazards regression models were used to estimate the hazard ratios and 95% confidence intervals of baseline markers of inflammation with incident breast cancer risk. We excluded cases reported within the first year of follow-up to minimize the influence of undiagnosed malignancy. In addition to the age- and treatment group-adjusted model, we adjusted for breast cancer risk factors, including family history of breast cancer less than 60 years of age (yes or no), personal history of benign breast disease (yes or no), white race/ethnicity (yes or no), menopausal status (premenopausal, postmenopausal, unsure), hormone therapy use (never, past, current), type of most recent therapy among users (estrogen alone, estrogen plus progestogen, other), age at menarche (≤11 years, 12 years, 13 years, or ≥14 years), parity (as number of pregnancies lasting ≥6 months: nulliparous, 1–2, 3–4, or ≥5), age at first birth (nulliparous, <30 years, or ≥30 years), oral contraceptive use (never or ever), mammography screening (yes or no), aHEI-2010 score (quartiles), physical activity level (metabolic equivalent task-hours per week, quartiles), usual frequency of alcohol consumption (rarely/never, 1–3 drinks/month, 1–6 drinks/week, or ≥1 drink/day), smoking status (never, past, or current), and body mass index (BMI; measured as weight (kg)/height (m)2) (<18.5, 18.5–19.9, 20.0–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, or ≥35.0). Simple updating was used to update status for time-varying covariates (all except family history, race/ethnicity, age at menarche and first birth, parity, and oral contraceptive use), and missing indicator categories were used for missing covariate data. Our final multivariable model included adjustment for the other biomarker measures to estimate the independent relationship.
We performed secondary analyses by breast cancer subtypes, including fatal and nonfatal cancer, presence of lymph node metastases, hormone estrogen receptor (ER) and progesterone receptor (PR) status (positive or negative) histologic grading and differentiation (well, moderate, poor), tumor size (≤2 cm, >2 cm), and follow-up time (<10 years vs. ≥10 years). We also stratified by characteristics at the time of blood draw that may be potential effect modifiers, including age (<55 years vs. ≥55 years), menopausal status (premenopausal, postmenopausal), postmenopausal hormone use (never, past, current), BMI category (18.5–24 vs. ≥25.0), moderate alcohol consumption (<7 g/day vs. ≥7 g/day), smoking status (never, past, or current), family history of breast cancer in a relative less than 60 years old, and personal history of benign breast disease. There was no evidence for interaction by randomized treatment assignments, thus findings were pooled across intervention and control arms, and tests for interaction by follow-up time confirmed that the proportional hazards assumption was met (for the interaction of biomarker with time, P > 0.05). Statistical tests for heterogeneity were performed with −2 likelihood ratio tests that compared the multivariable models with and without inclusion of the multiplicative interaction term.
We conducted a sensitivity analyses and excluded events in the first 2 years of follow-up to further minimize the influence of undiagnosed cancer on biomarker levels, and also excluded outliers at the top and bottom 1% of distributions. We accounted for potential screening bias by restricting analyses to participants who reported regular mammography for screening purposes. Bias due to screening behavior may be of concern if factors or behaviors associated with regular mammography screening are also associated with the biomarkers of interest, and thus differentially associated with diagnosis of breast cancer.
RESULTS
Our analysis included 27,071 women with measured baseline hsCRP, fibrinogen, GlycA, and sICAM-1, with a median follow-up of 19.0 years. The biomarkers were all significantly positively correlated with each other, and ranged from r = 0.22 between fibrinogen and sICAM-1, and r = 0.62 between CRP and GlycA (Appendix Table 1). Baseline characteristics of the participants are given in Table 1, which were stratified by biomarker quintiles. Women were on average 54.5 years of age, with a mean BMI of 25.9 kg/m2. About half (54.3%) of women were postmenopausal. Higher baseline concentrations of all 4 inflammatory markers were associated with older age, postmenopausal status, higher BMI and overweight/obesity status, lower aHEI-2010 dietary quality score, less frequent alcohol consumption, less leisure-time physical activity level, and current smoking. Additionally, current hormone therapy was associated with higher hsCRP and GlycA levels, and lower fibrinogen levels. Race/ethnicity did not differ by levels of hsCRP or GlycA, but women with higher fibrinogen levels and lower sICAM-1 levels were less likely to report as white.
Table 1.
Characteristics of Participants Free of Cancer and Cardiovascular Disease at Baseline, by Baseline Concentrations of Biomarkers of Inflammation (n = 27,071), Women’s Health Study, 1993
| Characteristic | hsCRP, mg/L | Fibrinogen, mg/dL | GlycA, μmol/L | sICAM-1, ng/mL | ||||
|---|---|---|---|---|---|---|---|---|
| Q1, % (median, 0.36) | Q5, % (median, 7.74) | Q1, % (median, 271.4) | Q5, % (median, 457.9) | Q1, % (median, 289.0) | Q5, % (median, 463.0) | Q1, % (median, 267.2) | Q5, % (median, 455.8) | |
| Age, yearsa | 52.7 (6.7) | 55.3 (6.9) | 52.7 (3.1) | 56.2 (7.7) | 53.1 (6.8) | 55.2 (7.0) | 52.8 (6.3) | 55.7 (7.4) |
| BMIb | 22.9 (2.9) | 29.4 (6.0) | 23.9 (3.7) | 28.7 (6.1) | 23.4 (3.5) | 28.9 (5.8) | 24.4 (4.0) | 27.5 (5.9) |
| BMI categoryb | ||||||||
| Normal (18.5–24.9) | 79.8 | 25.8 | 69.0 | 31.0 | 75.0 | 27.3 | 65.0 | 38.7 |
| Overweight/obese (≥25.0) | 20.2 | 74.3 | 31.0 | 69.0 | 25.0 | 72.7 | 35.0 | 61.3 |
| Menopausal status | ||||||||
| Premenopausal | 44.1 | 17.2 | 37.8 | 21.4 | 37.9 | 19.7 | 38.8 | 17.9 |
| Postmenopausal | 39.8 | 61.9 | 45.6 | 61.1 | 43.9 | 60.5 | 42.2 | 62.0 |
| Unsure/perimenopause | 11.5 | 16.5 | 14.8 | 12.9 | 13.7 | 15.1 | 13.9 | 15.2 |
| Hormone therapy use | ||||||||
| Never | 66.4 | 34.5 | 45.9 | 54.5 | 58.0 | 41.1 | 52.4 | 46.4 |
| Past | 8.3 | 8.1 | 5.3 | 13.3 | 7.4 | 9.1 | 5.5 | 12.7 |
| Current | 25.2 | 57.1 | 48.7 | 31.9 | 34.4 | 49.6 | 42.0 | 40.8 |
| Hormone therapy type, among ever users | ||||||||
| Estrogen only | 78.7 | 54.6 | 67.8 | 67.6 | 73.5 | 60.5 | 71.4 | 63.2 |
| Estrogen AND progestogen | 13.6 | 38.7 | 25.1 | 24.3 | 18.7 | 32.6 | 21.7 | 29.9 |
| Oral contraceptive use | ||||||||
| Never | 27.6 | 30.0 | 23.0 | 37.6 | 26.9 | 30.9 | 24.8 | 33.6 |
| Ever | 72.1 | 69.7 | 76.6 | 62.0 | 72.7 | 68.8 | 74.8 | 66.1 |
| aHEI-2010 diet quality scorea | 54.8 (10.3) | 52.1 (10.1) | 54.5 (10.3) | 52.6 (10.2) | 55.0 (10.4) | 52.0 (10.1) | 54.8 (10.3) | 51.7 (10.2) |
| Alcohol consumption | ||||||||
| Never/rarely | 38.5 | 52.2 | 33.0 | 54.4 | 36.5 | 52.8 | 37.1 | 51.6 |
| 1–3 drinks/month | 13.4 | 13.7 | 11.6 | 13.8 | 13.0 | 14.0 | 12.7 | 12.9 |
| 1–6 drinks/week | 35.8 | 26.6 | 38.0 | 25.5 | 38.3 | 26.0 | 37.4 | 26.6 |
| ≥1 drinks/day | 12.4 | 7.5 | 17.4 | 6.3 | 12.2 | 7.2 | 12.9 | 8.9 |
| Leisure-time physical activity level, MET-hours/weeka | 17.9 (20.7) | 11.4 (15.3) | 16.9 (19.7) | 12.0 (16.2) | 18.1 (21.0) | 11.5 (15.3) | 16.7 (19.4) | 11.9 (17.3) |
| Smoking status | ||||||||
| Never | 53.9 | 50.0 | 53.2 | 47.9 | 56.1 | 48.2 | 56.9 | 38.0 |
| Past | 36.0 | 36.7 | 39.1 | 35.2 | 36.6 | 35.7 | 39.2 | 29.9 |
| Current | 10.0 | 13.2 | 7.6 | 16.8 | 7.3 | 16.0 | 3.9 | 32.0 |
| Age at menarche, years | ||||||||
| ≤11 | 21.4 | 27.9 | 22.2 | 28.1 | 20.9 | 28.5 | 21.3 | 27.7 |
| 12 | 27.0 | 28.7 | 27.7 | 27.9 | 28.4 | 29.8 | 28.9 | 27.8 |
| 13 | 30.9 | 27.4 | 30.5 | 27.5 | 31.0 | 26.4 | 31.0 | 26.6 |
| ≥14 | 20.7 | 16.0 | 19.6 | 16.5 | 19.8 | 15.3 | 18.8 | 17.9 |
| Parity | ||||||||
| Nulliparous | 14.1 | 12.2 | 11.9 | 15.6 | 14.1 | 12.4 | 13.7 | 12.3 |
| Parous (pregnancy ≥6 months) | 85.3 | 87.2 | 87.5 | 84.7 | 85.2 | 87.0 | 85.7 | 87.1 |
| Parity among parous individuals | 2.7 (1.2) | 3.0 (1.3) | 2.8 (1.2) | 3.0 (1.3) | 2.8 (1.2) | 3.0 (1.3) | 2.8 (1.2) | 3.0 (1.3) |
| Age at first birth, years | ||||||||
| <30 | 87.1 | 90.1 | 88.1 | 89.3 | 86.1 | 89.9 | 87.6 | 88.9 |
| ≥30 | 12.9 | 9.9 | 11.9 | 10.7 | 13.9 | 10.1 | 12.4 | 11.1 |
| History of benign breast disease | 40.1 | 31.6 | 40.1 | 31.7 | 39.6 | 31.1 | 39.1 | 31.5 |
| Family history of breast cancer in relative <60 years old | 6.4 | 6.0 | 5.7 | 6.5 | 5.9 | 5.9 | 6.0 | 6.4 |
| Mammography screening in past year | 59.3 | 62.7 | 64.7 | 58.3 | 62.1 | 61.5 | 63.4 | 56.6 |
| White race/ethnicity | 94.5 | 94.5 | 96.1 | 92.8 | 93.7 | 94.5 | 91.7 | 95.5 |
Abbreviations: aHEI-2010, Alternative Healthy Eating Index 2010 dietary quality score; BMI, body mass index; GlycA, N-linked glycoproteins; hsCRP, high-sensitivity C-reactive protein; MET, metabolic equivalent of tasks; Q, quintile; sICAM-1, soluble intercellular adhesion molecule-1.
a Values are presented as mean (standard deviation).
b Weight (kg)/height (m)2.
We documented 1,497 incident invasive breast cancer cases diagnosed during a mean of 9.6 years from baseline (range, 1–20 years; median follow-up 19.0 years). Most cases of breast cancer were postmenopausal (90.3%) and reported among women who had recent mammography screening prior to diagnosis (72%). Overall, 82% of cases were designated to be ER-positive, and 73% of cases were designated to be PR-positive. Notable differences in risk associations were observed for the various biomarkers (Table 2 and Appendix Table 2). Baseline concentrations of hsCRP and GlycA were not related to breast cancer risk in either the age- and treatment-adjusted models or the fully adjusted models. In comparison, higher fibrinogen concentrations were associated with elevated breast cancer risk in the fully adjusted model, which suggested a 25% greater breast cancer risk among women when comparing extreme quintiles (hazard ratio (HR) = 1.25, 95% confidence interval (CI): 1.03, 1.51; P for trend = 0.01). In contrast, sICAM-1 concentrations were inversely associated with a 21% lower breast cancer risk when comparing extreme quintiles (HR = 0.79, 95% CI: 0.66, 0.94; P for trend = 0.02). Modeling the log-transformed biomarkers continuously per each 1-standard-deviation increase gave similar results, which indicates a modest 7% greater risk with fibrinogen (HR = 1.07, 95% CI: 1.00, 1.15) and 6% lower risk with sICAM-1 (HR = 0.94, 95% CI: 0.88, 0.99) (Web Figure 1, available at https://academic.oup.com/aje).
Table 2.
Cox Proportional Hazards Models for Biomarkers of Inflammation and Risk of Incident Breast Cancer, Women’s Health Study, 1993–2013
| Biomarker and Model | Quintilea | P for Trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| CRP | |||||||||
| Model 1b | 0.92 | 0.78, 1.09 | 1.07 | 0.92, 1.26 | 1.13 | 0.97, 1.32 | 0.90 | 0.76, 1.07 | 0.4 |
| Model 2c | 0.91 | 0.77, 1.07 | 1.04 | 0.88, 1.22 | 1.08 | 0.91, 1.27 | 0.86 | 0.72, 1.04 | 0.2 |
| Model 3d | 0.91 | 0.77, 1.08 | 1.05 | 0.88, 1.25 | 1.08 | 0.90, 1.29 | 0.84 | 0.69, 1.04 | 0.1 |
| Fibrinogen | |||||||||
| Model 1b | 1.12 | 0.95, 1.32 | 1.06 | 0.90, 1.25 | 1.25 | 1.06, 1.47 | 1.12 | 0.95, 1.32 | 0.1 |
| Model 2c | 1.15 | 0.97, 1.35 | 1.09 | 0.92, 1.29 | 1.30 | 1.10, 1.53 | 1.16 | 0.97, 1.39 | 0.06 |
| Model 3d | 1.18 | 1.00, 1.39 | 1.13 | 0.95, 1.35 | 1.37 | 1.15, 1.63 | 1.25 | 1.03, 1.51 | 0.01 |
| GlycA | |||||||||
| Model 1b | 0.87 | 0.74, 1.02 | 0.96 | 0.82, 1.13 | 0.94 | 0.80, 1.10 | 0.98 | 0.84, 1.15 | 0.9 |
| Model 2c | 0.86 | 0.73, 1.01 | 0.95 | 0.81, 1.11 | 0.92 | 0.78, 1.09 | 0.96 | 0.81, 1.14 | 0.9 |
| Model 3d | 0.84 | 0.71, 0.99 | 0.92 | 0.77, 1.09 | 0.89 | 0.74, 1.07 | 0.96 | 0.79, 1.17 | 0.9 |
| sICAM-1 | |||||||||
| Model 1b | 0.89 | 0.76, 1.04 | 0.84 | 0.71, 0.98 | 0.88 | 0.75, 1.03 | 0.83 | 0.71, 0.97 | 0.04 |
| Model 2c | 0.89 | 0.76, 1.04 | 0.84 | 0.72, 0.99 | 0.87 | 0.74, 1.02 | 0.81 | 0.68, 0.96 | 0.02 |
| Model 3d | 0.88 | 0.75, 1.03 | 0.83 | 0.71, 0.98 | 0.86 | 0.73, 1.01 | 0.79 | 0.66, 0.94 | 0.02 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; GlycA, N-linked glycoproteins; HR, hazard ratio; sICAM-1, soluble intercellular adhesion molecule-1.
a Quintile 1 was the reference category.
b Adjusted for age and treatment.
c Adjusted for the variables in model 1 and family history of breast cancer in a relative younger than 60 years of age (yes or no), personal history of benign breast disease (yes or no), white race/ethnicity (yes or no), menopausal status (premenopausal, postmenopausal, or unsure), hormone therapy use (never, past, or current), type of most recent hormone therapy use among ever users (estrogen alone, estrogen plus progestogen, or other), age at menarche (≤11 years, 12 years, 13 years, r ≥14 years), parity (as number of pregnancies lasting ≥6 months: nulliparous, 1–2, 3–4, or ≥5), age at first birth (nulliparous, <30 years, or ≥30 years), oral contraceptive use (never or ever), mammography screening (yes of no), Alternative Healthy Eating Index 2010 score (quartiles), physical activity level (metabolic equivalent of tasks-hours/week, quartiles), usual frequency of alcohol consumption (rarely/never, 1–3 drinks/month, 1–6 drinks/week, or ≥1 drink/day), smoking status (never, past, or current), and body mass index (weight (kg)/height (m)2; <18.5, 18.5–19.9, 20.0–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, or ≥35.0).
d Adjusted for the variables in model 2 and the other biomarkers presented in the Table.
Secondary analyses by breast cancer subtypes are shown in Web Figure 1, which suggest that the relationship between fibrinogen and breast cancer may be more relevant for subtypes that are both estrogen ER- and PR-positive, and may not extend to metastatic and fatal subtypes, although the number of events for these categories was small. Effect estimates for sICAM-1 were largely consistent with inverse associations seen across breast cancer subtypes. The associations for both fibrinogen and sICAM-1 remained significant for events diagnosed within the first 10 years of follow-up, but not among those diagnosed 10 years or later after blood draw. Although no association was found between hsCRP and total breast cancer, heterogeneity by receptor subtype was suggested, with an elevated risk of ER-negative breast cancers in those with higher hsCRP levels. GlycA remained null across all the subtypes evaluated.
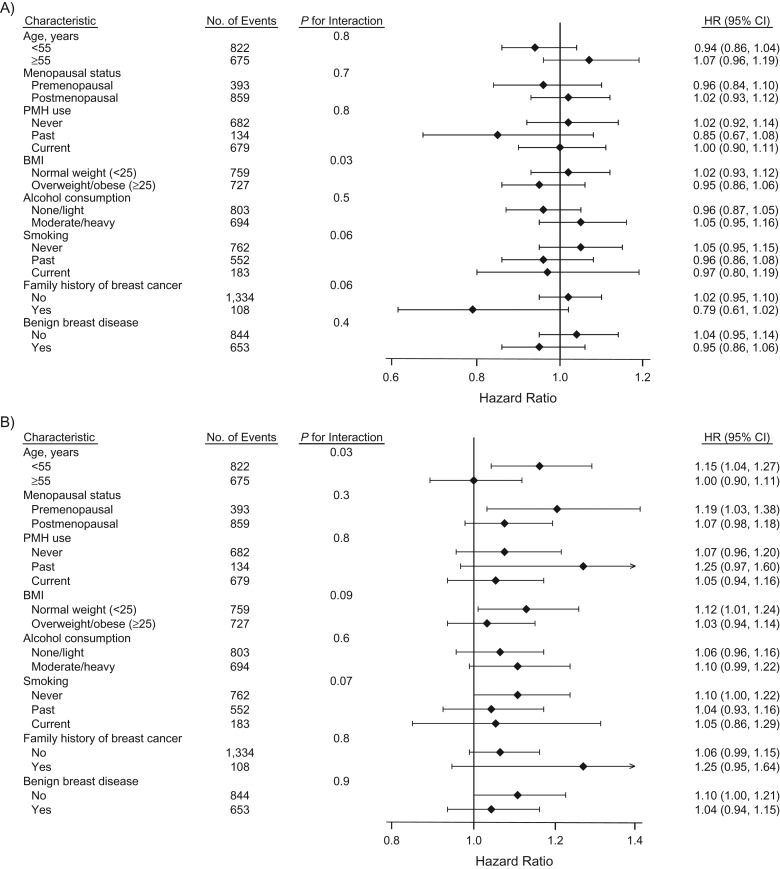
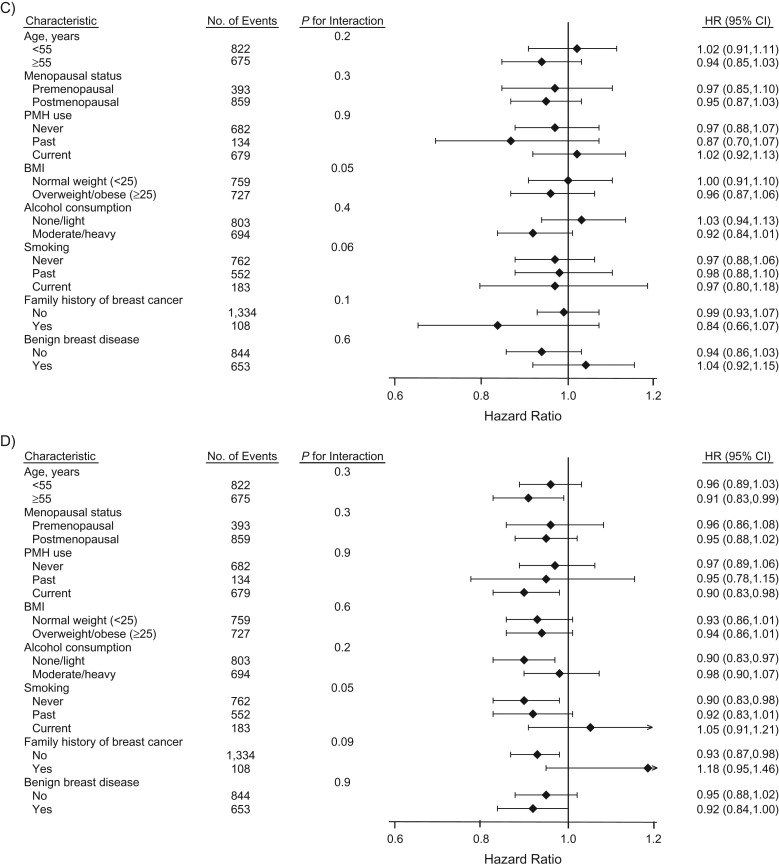
We also conducted pre-specified secondary analyses stratified by baseline risk factor status (Figure 1). There was significant heterogeneity for fibrinogen and breast cancer risk by baseline age at time of blood draw (<55 years vs. ≥55 years; P for interaction = 0.03), with an attenuation among older women. Results were similar across other baseline characteristics evaluated.
Figure 1.
Multivariable Cox proportional hazards models for biomarkers of inflammation and risk of incident breast cancer by baseline risk factor status in the Women’s Health Study, 1993–2013. Biomarkers of inflammation include: A) C-reactive protein, B) fibrinogen, C) N-linked glycoproteins, and D) soluble intercellular adhesion molecule-1. Point estimates and bars represent hazard ratios (HR) and 95% confidence intervals (CI) per each 1-standard-deviation increase in biomarker level. The multivariable model was adjusted for age, treatment randomization, family history of breast cancer in a relative younger than 60 years of age (yes or no), personal history of benign breast disease (yes or no), white race/ethnicity (yes or no), menopausal status (premenopausal, postmenopausal, or unsure), hormone therapy use (never, past, or current), type of most recent hormone therapy use among ever users (estrogen alone, estrogen plus progestogen, or other), age at menarche (≤11 years, 12 years, 13 years, or ≥14 years), parity (as number of pregnancies lasting ≥6 months: nulliparous, 1–2, 3–4, or ≥5), age at first birth (nulliparous, <30 years, or ≥30 years), oral contraceptive use (never or ever), mammography screening (yes or no), Alternative Healthy Eating Index 2010 score (quartiles), physical activity level (metabolic equivalent of task-hours per week, in quartiles), usual frequency of alcohol consumption (rarely/never, 1–3 drinks/month, 1–6 drinks/week, or ≥1 drink/day), smoking status (never, past, or current), and body mass index (BMI; measured as weight (kg)/height (m)2) (<18.5, 18.5–19.9, 20.0–22., 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, or ≥35.0), and the other biomarkers presented in the Figure. The standard deviations for log-transformed concentrations of high-sensitivity C-reactive protein, fibrinogen, N-linked glycoproteins, and soluble intercellular adhesion molecule-1 are 1.20, 0.25, 0.18, and 0.22, respectively. PMH, postmenopausal hormones.
Similar results were found when we excluded outliers and events occurring within the first 2 years (data not shown). Effect estimates were also similar when person-time was limited to participants who reported recent mammography screening (n = 1,079 cases) (per each 1-standard-deviation increase: for hsCRP, HR = 0.98, 95% CI: 0.90, 1.06; for fibrinogen, HR = 1.07, 95% CI: 0.98, 1.16; for GlycA, HR = 0.96, 95% CI: 0.89, 1.04; and for sICAM-1, HR = 0.94, 95% CI: 0.88, 1.01).
DISCUSSION
In the present study, we evaluated the relationships between baseline concentrations of 4 circulating plasma biomarkers that represented various pathways of inflammation with incident breast cancer risk in a large prospective cohort of US women, and had a median 19.0 years of follow-up. Overall, we observed that plasma markers of various inflammatory pathways were differentially associated with the risk of incident breast cancer. Specifically, we observed a significant direct relationship between fibrinogen and breast cancer risk, and a significant but modest inverse association between sICAM-1 and breast cancer risk. Secondary hypothesis-generating analyses suggest that fibrinogen may play a role in nonfatal and nonmetastatic cancers, while findings for sICAM-1 were consistent across subtypes. Further, these relationships may be more relevant for shorter to medium-term cancer risk, as the associations between fibrinogen and sICAM-1 with breast cancer were attenuated after 10 years of follow-up. Neither GlycA nor hsCRP were associated with overall breast cancer risk. Results were similar when stratified by pre- and postmenopausal status at baseline blood draw, although the majority (>90%) of cases occurred in the postmenopausal period. These findings support the complex role of chronic inflammation in breast cancer development and also indicate that some pathways may be more relevant than others.
Discrepancies across previous studies of biomarkers of inflammation and breast cancer may be partially attributable to the timing in which blood samples were drawn relative to cancer development, or to other important factors (e.g., age, menopausal status) or potential confounders. Our prospective, longitudinal study allowed us to evaluate the relationship between pre-diagnostic makers of inflammation with subsequent invasive breast cancer risk years prior to diagnosis. In a previous prospective analysis among older postmenopausal women (mean baseline age = 62.7 years) in the Women’s Health Initiative study, no association was found between fibrinogen and incident breast cancer (21), which is consistent with our stratified analysis among women 55 years of age or older. However, we did observe a significant association with breast cancer risk in women below 55 years of age at baseline (per each 1-standard-deviation increase, HR = 1.13, 95% CI: 1.03, 1.23), and the interaction by age was statistically significant (P for interaction = 0.03). Similarly, in the Copenhagen General Population Study (6), no relationship between baseline fibrinogen and long-term risk of breast cancer was found. Differences in the age distribution (median, 58 years) may not explain the discrepancy; however, ever use of hormones was much lower among the Danish population (18% vs. 50% in the WHS) and may contribute to differences in risk profiles.
The significant inverse association with sICAM-1 observed in the present study differs from previous studies. For example, the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort of France (218 cases) observed a significant 2-fold greater risk when comparing extreme quartiles of baseline sICAM-1 levels with incident breast cancer (22). Retrospective case-control studies in which breast cancer cases and healthy controls were compared have also indicated significantly higher sICAM-1 levels among cases of breast cancer (23–25). It is unclear why sICAM may be inversely related to breast cancer risk in our cohort and external analyses are needed to confirm the present finding.
Numerous studies have evaluated the relationship of hsCRP with breast cancer, which include a previous analysis among participants in the Nurses’ Health Study and WHS (18). Consistent with this updated analysis, baseline hsCRP was not associated with breast cancer risk in the WHS; however, hsCRP was modestly related to elevated breast cancer risk in the Nurses’ Health Study. Overall, our null finding is consistent with some (26, 27), but not all (6) prospective analyses of hsCRP and invasive breast cancer risk. In a recent meta-analysis, Chan et al. (5) observed a significant positive relationship between CRP and breast cancer risk overall and in postmenopausal women only. Heterogeneity in the meta-analysis was largely explained by differences in confounder adjustment, with the relationship between CRP and breast cancer largely attenuated in studies that adjusted for lifestyle factors (e.g., physical activity level, alcohol consumption, hormone therapy use). Thus, our null finding may be partially due to our more thorough confounder adjustment.
In previous prospective studies, including the WHS, investigators observed that circulating baseline plasma GlycA levels were associated with total cancer incidence (28), total cancer mortality (10), and incident colorectal cancer (29). Additionally, several protein glycan signatures have been characterized for malignant versus normal adjacent breast tissue (30) and significant differences in circulating levels comparing prevalent breast cancer cases versus healthy controls have been observed cross-sectionally, (31, 32). However, cross-sectional study design is limited in its ability to establish temporality, and it remains unknown when these characteristics emerged in relation to disease onset.
Chronic inflammation may promote the development and/or progression of cancer through a variety of mechanisms, such as stimulating angiogenesis, preventing apoptosis, and promoting proliferation, migration and metastasis (2, 4). Furthermore, these complex relationships may have a broad impact and universally affect cancer initiation and growth, or they may be pathway and tissue site-specific, with varying degrees of relevance and effect sizes across cancer sites. Elevated circulating fibrinogen among prevalent cancer cases is a consistent and independent predictor of poor outcomes for various cancer types (33–35), including breast cancer (7). Fibrinogen is a complex glycoprotein synthesized in the liver and involved in blood coagulation that is key in the inflammatory response. In vitro studies have demonstrated its role in tumor progression via enhanced cell proliferation, invasion, tumor-associated angiogenesis, and metastasis (36, 37). Studies have also shown endogenous production of fibrinogen by tumor cells (38), further supporting its importance in creating a favorable tumor microenvironment for cancer progression. sICAM-1 is expressed in many cell types and it has been shown that adhesion molecules play a role in cancer progression by enabling cancer-related processes such as cell survival and migration (39, 40). sICAM-1 serum levels have been positively correlated with advanced state and recurrent breast cancer (41, 42). Similarly, the in vitro inhibition of ICAM-1 function in breast cancer cells decreased invasive potential (43). However, contrary evidence also suggests that expression of ICAM-1 in breast cancer cells enhances immunologic surveillance, resulting in tumor suppression and the inhibition of metastasis (44). It is possible that higher sICAM-1 may reduce the risk of developing breast cancer, but if measured after the onset of cancer initiation, may reflect a response to malignancy, and thus be positively associated.
Strengths of this study include its prospective design that evaluated several biomarkers of chronic inflammation prior to breast cancer diagnosis. Additionally, our cohort accrued a large number of breast cancer cases and benefited from detailed assessment of tumor characteristics and breast cancer subtypes. The WHS has gathered comprehensive information on participant lifestyle and health status, allowing us to carefully control for a number of potential confounders. There are some limitations to note. Given the fluctuation of these markers in response to inflammatory conditions and other exogenous and endogenous exposures, additional measures of the biomarkers would have improved our estimate of individuals’ long-term exposure. However, hsCRP and GlycA, for which we did not observe significant associations, were significantly associated with other long-term outcomes in this cohort, including cardiovascular disease risk (15, 45), which indicates our ability to detect significant relationships from single baseline measurements. The latency analysis according to follow-up time indicated that the statistically significant findings for fibrinogen and sICAM-1 may be more relevant during the first 10 years of follow-up. A lack of association with longer-term follow-up may indicate that a more recent timeframe is etiologically relevant for inflammation and cancer risk. It is unlikely that this represents a reverse causation of undiagnosed cancer at baseline that led to an inflammatory response, as precautions to minimize this concern such as excluding cases in the first 2 years of follow-up and limiting analyses to include only women with regular mammography screening did not change the overall findings. Finally, the assay used to measure sICAM-1 does not detect the modified form of sICAM-1 that can be seen in black individuals. Given the relative racial/ethnic homogeneity of our cohort, these findings need to be replicated in other populations to determine their generalizability.
In summary, our findings suggest that plasma biomarkers of inflammation were inconsistently associated with breast cancer risk, which may be linked to specific inflammatory components or pathways rather than a global proinflammatory state. Understanding which biomarkers may be causal factors rather than simply surrogate markers of the response to disease presence is essential for developing effective anti-inflammatory therapies to reduce breast cancer risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Deirdre K. Tobias, Paulette D. Chandler, JoAnn E. Manson, Julie E. Buring, Lu Wang, I-Min Lee); Center for Lipid Metabolomics, Divisions of Preventive Medicine and Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Akintunde O. Akinkuolie, Patrick R. Lawler, Paul M Ridker, Samia Mora); Peter Munk Cardiac Centre, University Health Network, Toronto, Ontario, Canada (Patrick R. Lawler); Heart and Stroke Richard Lewar Centre of Excellence in Cardiovascular Research, University of Toronto, Toronto, Ontario, Canada (Patrick R. Lawler); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (JoAnn E. Manson, Julie E. Buring, Paul M Ridker, I-Min Lee); and Connors Center for Women’s Health and Gender Biology, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson).
The Women’s Health Study is supported by the National Institutes of Health (grants CA-047988, HL-043851, HL-080467, HL-099355, and UM1 CA182913). D.K.T. is supported by the National Institutes of Health (grant K01DK103720). A.O.A. and P.R.L. received support from the National Institutes of Health Institutional National Research Service Award (grant HL007575). P.D.C. received support from the American Cancer Society (grant 127524-MRSG-15-012-01-CNE). S.M. has received research support from the National Heart, Lung, and Blood Institute, The biomarker measurements were supported by the Donald W. Reynolds Foundation, the Fondation Leducq, and the American Heart Association.
P.M.R. is co-inventor on patents held by Brigham and Women’s Hospital related to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to Siemens and AstraZeneca and has served as a research consultant to Schering-Plough, Sanofi/Aventis, Isis, Siemens, and Vascular Biogenics. S.M. has received research support from AstraZeneca and Atherotec Diagnostics; served as a consultant to Pfizer, Genzyme, and Quest Diagnostics; received speaker honoraria from AstraZeneca, Abbot, and the National Lipid Association for educational (nonpromotional) activities; and received travel expense reimbursement from Pfizer. The other authors report no conflicts.
Abbreviations
- aHEI-2010
Alternative Health Eating Index 2010
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- ER
estrogen receptor
- GlycA
N-linked glycoproteins
- HR
hazard ratio
- hsCRP
high-sensitivity C-reactive protein
- PR
progesterone receptor
- sICAM-1
soluble intercellular adhesion molecule-1
- WHS
Women’s Health Study
APPENDIX
Appendix Table 1.
Pearson Correlation Matrixa of ln-Transformed Biomarkers, Women’s Health Study, 1993–2013
| Biomarker | CRP | Fibrinogen | GlycA | sICAM-1 |
|---|---|---|---|---|
| CRP | 1.00 | 0.36 | 0.62 | 0.28 |
| Fibrinogen | 1.00 | 0.39 | 0.22 | |
| GlycA | 1.00 | 0.29 | ||
| sICAM-1 | 1.00 |
Abbreviations: CRP, C-reactive protein; GlycA, N-linked glycoproteins; sICAM-1, soluble intercellular adhesion molecule-1.
a All correlations statistically significant at P < 0.05.
Appendix Table 2.
Median Biomarker Levels and Number of Incident Breast Cancer Cases by Quintile of Biomarker, Women’s Health Study, 1993–2013
| Biomarker | Quintile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| Median | No. of Events | Median | No. of Events | Median | No. of Events | Median | No. of Events | Median | No. of Events | |
| CRP, mg/L | 0.36 | 294 | 1.01 | 274 | 2.03 | 324 | 3.73 | 337 | 7.74 | 268 |
| Fibrinogen, mg/dL | 271.4 | 267 | 316.9 | 301 | 351.2 | 289 | 390.7 | 338 | 457.9 | 302 |
| GlycA, μmol/L | 289 | 315 | 335 | 275 | 370 | 313 | 406 | 293 | 463 | 301 |
| sICAM-1, ng/mL | 267.2 | 332 | 309.9 | 302 | 342.9 | 289 | 381.5 | 300 | 455.8 | 274 |
Abbreviations: CRP, C-reactive protein; GlycA, N-linked glycoproteins; sICAM-1, soluble intercellular adhesion molecule-1.
REFERENCES
- 1. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 2. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo L, Liu S, Zhang S, et al. . C-reactive protein and risk of breast cancer: a systematic review and meta-analysis. Sci Rep. 2015;5:10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen MD, Jones LJ. The role of inflammation in progression of breast cancer: friend or foe? (Review). Int J Oncol. 2015;47(3):797–805. [DOI] [PubMed] [Google Scholar]
- 5. Chan DS, Bandera EV, Greenwood DC, et al. . Circulating C-reactive protein and breast cancer risk-systematic literature review and meta-analysis of prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1439–1449. [DOI] [PubMed] [Google Scholar]
- 6. Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer. 2016;139(7):1493–1500. [DOI] [PubMed] [Google Scholar]
- 7. Perisanidis C, Psyrri A, Cohen EE, et al. . Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. [DOI] [PubMed] [Google Scholar]
- 8. Gallin JI, Snyderman R, eds. Inflammation: Basic Principles and Clinical Correlates. Philadelphia, PA: Lippincott, Williams, and Wilkins; 1999. [Google Scholar]
- 9. Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. . GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61(5):714–723. [DOI] [PubMed] [Google Scholar]
- 10. Lawler PR, Akinkuolie AO, Chandler PD, et al. . Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118(7):1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buring JE, Hennekens CH, Group ftWsHSR . The Women’s Health Study: summary of the study design. J Myocardial Ischemia. 1992;4:27–29. [Google Scholar]
- 12. Ridker PM, Cook NR, Lee IM, et al. . A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Rifai N, Rose L, et al. . Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. [DOI] [PubMed] [Google Scholar]
- 14. Whitton CM, Sands D, Hubbard AR, et al. . A collaborative study to establish the 2nd International Standard for Fibrinogen, Plasma. Thromb Haemost. 2000;84(2):258–262. [PubMed] [Google Scholar]
- 15. Akinkuolie AO, Buring JE, Ridker PM, et al. . A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3(5):e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albert MA, Glynn RJ, Buring JE, et al. . Differential effect of soluble intercellular adhesion molecule-1 on the progression of atherosclerosis as compared to arterial thrombosis: a prospective analysis of the Women’s Health Study. Atherosclerosis. 2008;197(1):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee IM, Cook NR, Manson JE, et al. . Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. 1999;91(24):2102–2106. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Lee IM, Tworoger SS, et al. . Plasma C-reactive protein and risk of breast cancer in two prospective studies and a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 20. Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabat GC, Salazar CR, Zaslavsky O, et al. . Longitudinal association of hemostatic factors with risk for cancers of the breast, colorectum, and lung among postmenopausal women. Eur J Cancer Prev. 2016;25(5):449–456. [DOI] [PubMed] [Google Scholar]
- 22. Touvier M, Fezeu L, Ahluwalia N, et al. . Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thielemann A, Baszczuk A, Kopczynski Z, et al. . The clinical usefulness of assessing the concentration of cell adhesion molecules sVCAM-1 and sICAM-1 in the serum of women with primary breast cancer. Contemp Oncol (Pozn). 2014;18(4):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tesarova P, Kalousova M, Zima T, et al. . Endotelial activation and flow-mediated vasodilation in young patients with breast cancer. Neoplasma. 2013;60(6):690–697. [DOI] [PubMed] [Google Scholar]
- 25. Regidor PA, Callies R, Regidor M, et al. . Expression of the cell adhesion molecules ICAM-1 and VCAM-1 in the cytosol of breast cancer tissue, benign breast tissue and corresponding sera. Eur J Gynaecol Oncol. 1998;19(4):377–383. [PubMed] [Google Scholar]
- 26. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217–2224. [DOI] [PubMed] [Google Scholar]
- 27. Gaudet MM, Patel AV, Teras LR, et al. . Obesity-related markers and breast cancer in CPS-II Nutrition Cohort. Int J Mol Epidemiol Genet. 2013;4(3):156–166. [PMC free article] [PubMed] [Google Scholar]
- 28. Duprez DA, Otvos J, Sanchez OA, et al. . Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem. 2016;62(7):1020–1031. [DOI] [PubMed] [Google Scholar]
- 29. Chandler PD, Akinkuolie AO, Tobias DK, et al. . Association of N-linked glycoprotein acetyls and colorectal cancer incidence and mortality. PLoS One. 2016;11(11):e0165615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Potapenko IO, Haakensen VD, Luders T, et al. . Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol Oncol. 2010;4(2):98–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abd Hamid UM, Royle L, Saldova R, et al. . A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18(12):1105–1118. [DOI] [PubMed] [Google Scholar]
- 32. Kyselova Z, Mechref Y, Kang P, et al. . Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54(7):1166–1175. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Yin W, Wang Z, et al. . Pretreatment plasma fibrinogen as an independent prognostic indicator of prostate cancer patients treated with androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2016;19(2):209–215. [DOI] [PubMed] [Google Scholar]
- 34. Selzer E, Grah A, Heiduschka G, et al. . Pre-therapeutic fibrinogen levels are of prognostic significance in locally advanced head and neck cancer. Wien Klin Wochenschr. 2016;128(9–10):320–328. [DOI] [PubMed] [Google Scholar]
- 35. Zhang SS, Lei YY, Cai XL, et al. . Preoperative serum fibrinogen is an independent prognostic factor in operable esophageal cancer. Oncotarget. 2016;7(18):25461–25469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther. 2003;3(7):1105–1120. [DOI] [PubMed] [Google Scholar]
- 37. Palumbo JS, Kombrinck KW, Drew AF, et al. . Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–3309. [PubMed] [Google Scholar]
- 38. Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. . Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6(1):176–183. [DOI] [PubMed] [Google Scholar]
- 39. Schroder C, Witzel I, Muller V, et al. . Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol. 2011;137(8):1193–1201. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed M, Kundu GC. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol Cancer. 2010;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kostler WJ, Tomek S, Brodowicz T, et al. . Soluble ICAM-1 in breast cancer: clinical significance and biological implications. Cancer Immunol Immunother. 2001;50(9):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Hanlon DM, Fitzsimons H, Lynch J, et al. . Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38(17):2252–2257. [DOI] [PubMed] [Google Scholar]
- 43. Rosette C, Roth RB, Oeth P, et al. . Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26(5):943–950. [DOI] [PubMed] [Google Scholar]
- 44. Ogawa Y, Hirakawa K, Nakata B, et al. . Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res. 1998;4(1):31–36. [PubMed] [Google Scholar]
- 45. Ridker PM, Buring JE, Shih J, et al. . Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.