Abstract
Background
Structural imaging has not been used previously to predict the effect of treatment in primary progressive aphasia (PPA).
Aims
This study examined relationships between baseline brain volume and the effects of phonological and orthographic treatments for anomia in PPA. It was predicted that lower baseline volume would be associated with lower post-treatment naming accuracy for treated items and smaller generalization effects.
Methods & Procedures
Twenty-one individuals with PPA participated. The treatment stimuli consisted of nouns that were consistently named correctly at baseline (Prophylaxis items) and/or nouns that were consistently named incorrectly at baseline (Remediation items). All 21 participants had Prophylaxis items, while 10 participants had Remediation items. Naming accuracy for untrained and trained items (Exemplar set 1) was measured. In addition, stimulus generalization was examined by having participants name an alternative exemplar of each untrained and trained item (Exemplar set 2). Correlational analyses focused on the relationships between naming accuracy and volume of regions previously identified as having a role in naming and semantic processing.
Outcomes & Results
Unexpectedly, there were no significant correlations between baseline volume and post-treatment accuracy for treated items. However, baseline volume within the left temporal pole was positively correlated with post-treatment accuracy for Untrained Exemplar set 2 Prophylaxis items, while baseline volume in the left inferior temporal gyrus was positively correlated with post-treatment accuracy for Untrained Exemplar set 1 Remediation items.
Conclusions
These findings suggest that lower volume in the left temporal pole is associated with decline for Untrained items, while lower volume in the left inferior temporal gyrus is associated with a lack of improvement for Untrained items. Possible explanations for the different patterns observed across Exemplar sets are discussed.
Keywords: treatment, primary progressive aphasia, anomia, MRI, temporal lobe
Introduction
Primary progressive aphasia (PPA) is a clinical syndrome characterized by progressive language impairment (Mesulam, 1982; Gorno-Tempini et al., 2011). Other aspects of cognition, such as episodic memory and visuospatial skills, are relatively preserved during the initial phases of the illness. Anomia is a prominent feature of PPA (Westbury & Bub, 1997).
Three variants of PPA have been identified: semantic, logopenic, and nonfluent/agrammatic (Gorno-Tempini et al., 2011). The semantic variant (svPPA) has been associated with bilateral atrophy of the anterior temporal lobe, typically with greater atrophy in the left hemisphere (Gorno-Tempini et al., 2004, 2011; Mummery et al., 2000). The resulting deficits include impaired confrontation naming and single-word comprehension deficits. Impaired object knowledge, surface dyslexia, or surface dysgraphia may also be present, while repetition and speech production are typically spared. svPPA has been associated with TDP-43 positive frontotemporal lobar degeneration (FTLD; Mesulam et al., 2014).
The logopenic variant (lvPPA) has been associated with atrophy of the left inferior parietal lobe and the left posterior superior temporal lobe (Gorno-Tempini et al., 2004; Josephs et al., 2013; Rohrer et al., 2010). The resulting deficits include impaired single-word retrieval and impaired repetition of sentences and phrases. Phonological speech errors may also occur. Single-word comprehension, object knowledge, motor speech, and grammar are typically spared. lvPPA has been associated with an atypical form of Alzheimer’s Disease (Mesulam et al., 2008; Mesulam et al., 2014; Rabinovici et al., 2008).
The nonfluent/agrammatic variant (nfvPPA) has been associated with atrophy in several left hemisphere areas, including the inferior frontal gyrus, insula, and premotor and supplementary motor areas (Grossman et al., 1996; Josephs et al., 2006; Nestor et al., 2003). The resulting language deficits include effortful, halting speech with apraxia, and/or agrammatic language production. Impaired comprehension of syntactically complex sentences may also be present, while single-word comprehension and object knowledge are typically spared. nfvPPA has been associated with tau-positive inclusions in FTLD, progressive supranuclear palsy, or corticobasal degneration (Hodges et al., 2004; Knibb et al., 2006; Mesulam et al., 2008, 2014).
Semantic deficits and semantic paraphasic errors occur in svPPA, suggesting that anomia is caused by degraded semantic representations or difficulty accessing the phonological representation from the semantic representation (Hodges, Patterson, & Tyler, 1994; Mesulam et al., 2009; Neary et al., 1998). In contrast, phonemic paraphasic errors are more likely to occur in lvPPA (Gorno-Tempini et al., 2008; Henry & Gorno-Tempini, 2010) and nfvPPA (Ash et al., 2010, 2013), suggesting that the problem in these two subtypes is at the level of the phonological representation itself.
A number of studies have focused on the treatment of anomia in svPPA, and these studies have typically found that anomia treatment has a positive effect (see reviews by Cathery-Goulart et al., 2013; Croot, Nickels, Laurence, & Manning, 2009; Jokel, Graham, Rochon, & Leonard, 2014). Treatment approaches have included semantic, phonological, and orthographic interventions, as well as hybrid treatments. While treatment is effective in this subtype, anomia treatment effects do not typically generalize to untreated items (item generalization) or untrained tasks (task generalization; see Jokel et al., 2014). However, generalization to alternative exemplars of trained items (stimulus generalization) has been observed in a few case studies (Green Heredia, Sage, Lambon Ralph, & Berthier, 2009; Jokel, Rochon, & Anderson, 2010; Mayberry, Sage, Ehsan, & Lambon Ralph, 2011). Furthermore, Jokel and Anderson (2012) found generalization to a category fluency task, while Savage, Piguet, and Hodges (2014) found generalization to several untrained tasks, including video description, comprehension of verbal instructions, and word-picture matching. Finally, Henry et al. (2013) found item generalization, and another study found both item generalization and improved discourse production in three individuals with svPPA (Beales, Cartwright, Whitworth, & Panegyres, 2016; the treated items in this study included nouns, verbs, and adjectives).
Six studies have investigated treatment for anomia in lvPPA (Beales et al., 2016; Beeson et al., 2011; Croot et al., 2015; Henry et al., 2013; Meyer, Snider, Eckmann, & Friedman, 2015; Newhart et al., 2009; some of these studies also treated one or more participants with a different subtype of PPA). All of these studies have found positive treatment effects in lvPPA. Treatment types have included combined phonological/orthographic (Croot et al., 2015; Newhart et al., 2009), semantic/orthographic (Beeson et al., 2011), and semantic/phonological/orthographic (Beales et al., 2016; Henry et al., 2013) interventions, while Meyer et al. (2015) utilized separate phonological and orthographic treatments. Four of these studies found item generalization (Beales et al., 2016; Beeson et al., 2011; Henry, et al., 2013; Newhart et al., 2009), while Meyer et al. (2015) found cross-language transfer within confrontation naming and naming to definition tasks.
Three studies have investigated treatment for anomia for nouns in nfvPPA (Croot et al., 2015; Jokel, Cupit, Rochon, & Leonard, 2009; Marcotte & Ansaldo, 2010; Croot et al. also treated a participant with lvPPA). In a study that included two participants and a phonological/orthographic treatment, improvement in naming and generalization to a sentence production task were found in both participants (Jokel et al., 2009). In a study that utilized a phonological/orthographic treatment, improvement for trained items (primarily nouns) and stimulus generalization were found in a participant with nfvPPA (Croot et al., 2015). In a case study that utilized a semantic treatment, improvement for treated nouns and verbs was found (Marcotte & Ansaldo, 2010).
Several studies have utilized structural imaging to examine associations between regional brain atrophy and naming impairment in PPA (Amici et al., 2007; Mesulam et al., 2013; Migliaccio et al., 2016; Race et al., 2013). These studies have found that atrophy in multiple areas of the left temporal lobe is associated with naming impairment, including atrophy in the anterior temporal lobe (Amici et al., 2007; Mesulam et al., 2013; Migliaccio et al., 2016), the superior temporal gyrus (Migliaccio et al., 2016), the middle temporal gyrus (Amici et al., 2007; Migliaccio et al., 2016), the inferior temporal gyrus (Amici et al., 2007; Migliaccio et al., 2016; Race et al., 2013), and the fusiform gyrus (Amici et al., 2007).
However, we are not aware of any studies that have used imaging to predict the effect of treatment in PPA. In the current study, relationships between baseline volume in particular brain regions and post-treatment naming accuracy were examined in PPA. It was predicted that lower left temporal volume would be associated with smaller treatment effects, including smaller stimulus and item generalization effects, because patients with more severe semantic deficits (typically associated with atrophy in left temporal regions; Binney et al., 2010; Gorno-Tempini et al., 2004; Mesulam et al., 2009; Migliaccio et al., 2016; Mummery et al., 2000; Rogalski et al., 2011; Rogers et al., 2006) might be less likely to respond to treatment or less likely to show generalization (Jokel et al., 2014; Newhart et al., 2009).
We utilized two types of treatment for anomia: a treatment that focuses on phonology, and an orthographic treatment that includes reading and writing tasks. In the Phonological Treatment Condition, an auditorily-presented word occurs in conjunction with the corresponding picture, and the participant repeats the word. The goal of this treatment is to strengthen the phonological representations of the treated words, thereby bolstering their production. The orthographic treatment capitalizes on the fact that in the early stages of PPA, oral reading deficits are absent or relatively mild (Westbury & Bub, 1997; some individuals with PPA have surface alexia, but low frequency exception words were not included in our study). In the Orthographic Treatment Condition, the written word occurs in conjunction with the corresponding picture, and the participant reads the word out loud and copies it. The goal of this treatment is to strengthen the orthographic representations of the treated words, thereby bolstering the orthographic route to word production (see Meyer, Tippett, & Friedman, 2016).
Method
Participants
MRI data were collected from an unimpaired control group, consisting of 11 participants with a mean age of 62.7 (SD = 8.4). There were 7 males and 4 females.
Twenty-one individuals with PPA participated, including 9 with lvPPA, 5 with svPPA, and 7 with nfvPPA.1 Demographic information for participants with PPA is presented in Table 1. The inclusion criteria were a clinical diagnosis of PPA, English fluency since childhood, at least 10 years of education, age of at least 40 years, and no history of other neurological or psychiatric disorders.
Table 1.
Demographic Information and Baseline Assessment Results for Participants with PPA
| lvPPA (N = 9) | svPPA (N = 5) | nfvPPA (N = 7) | Normative Data | |
|---|---|---|---|---|
|
|
|
|
|
|
| Age at Baseline | 69.1 (2.3) | 65.6 (5.3) | 68.0 (12.2) | |
| Education, years | 17.4 (1.5) | 17.2 (1.8) | 15.3 (2.5) | |
| Sex | 7 F, 2 M | 2 F, 3 M | 2 F, 5 M | |
| MoCA/30 | 14.8 (5.8) a, b | 14.4 (8.2)a | 21.1 (4.5)a | 27 (2) |
| Boston Naming Test, T-Score | 10.9 (6.4) a, b | 7.7 (2.7)a, b | 30.1 (13.3)a | 50 (10) |
| P&PT, 3 Pictures/52 | 48.2 (3.3)a | 32.6 (12.3)a, b, c | 48.7 (2.4)a | 51.2 (range = 49–52) |
| Word-Picture Matching/48 | 46.9 (2.0) | 34.6 (11.7) | 47.1 (1.2) | 47.7 (0.5) |
| Northwestern Anagram Test/10 | 6.6 (1.7)a | 5.6 (3.8) | 5.1 (3.0)a | 8.5 (2.4) |
| BDAE Articulatory Agility/7 | 6.8 (0.4) | 7.0 (0) | 5.3 (1.7)d | NA |
| BDAE Phrase Length/7 | 7.0 (0) | 6.8 (0.4) | 5.3 (2.0) | NA |
| BDAE Embedded Sentences/10 | 6.6 (2.7)a | 6.4 (3.8) | 8.7 (1.4) | 9.5 (0.9) |
| BDAE Sentence Repetition/10 | 5.0 (2.3)a | 6.8 (4.0) | 6.6 (2.9)a | 9.8 (0.6) |
| Pseudoword Repetition/10 | 5.3 (3.7)a | 6.8 (3.0) | 6.0 (3.4) | 8.9 (1.0) |
| Reading, Irregular minus Regular | −2.1 (2.0)b, e | −4.8 (2.6)b, e | 0.3 (1.1) | −0.4 (0.7) |
Note. Means are reported, with SDs in parentheses, except where noted. BDAE = Boston Diagnostic Aphasia Examination; MoCA = Montreal Cognitive Assessment; NA = not available; P&PT = Pyramids and Palm Trees.
less than the group mean from published norms, one-sample t-test.
less than nfvPPA, independent-samples t-test.
less than lvPPA, independent-samples t-test.
less than svPPA, independent-samples t-test.
less than unimpaired controls, independent-samples t-test [controls: N = 18 (10 F, 8 M), mean age = 63.1 (9.6), mean education = 16.7 (2.2)].
Subtyping was based on the international criteria (Gorno-Tempini et al., 2011). Two neurologists and one clinical neuropsychologist independently reviewed each participant’s baseline assessment results and medical history, including the results of prior language and neuropsychological testing. The subtype raters also viewed videos of the participant performing language tasks, including the Cookie Theft narrative (Goodglass, Kaplan, & Barresi, 2001) and the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001). When videos were unavailable, the raters listened to audio recordings. Disagreements between the raters were resolved through additional review of these materials and discussion between the raters.
Treatment Stimuli
For each participant, up to 120 items were selected from a set of 294 nouns. For each selected item, there were three different picture exemplars. Oral naming accuracy for Exemplar set 1 was tested twice during the baseline evaluation. This exemplar set was utilized during treatment, and naming accuracy for this set was tested during the post-treatment assessment. Oral naming accuracy for Exemplar set 2 was tested once at baseline. Exemplar set 2 was not utilized during treatment, but it was used to assess stimulus generalization during post-treatment testing. Pictures from Exemplar set 3 were only used as foils during treatment. Exemplar sets 1 and 2 have high name agreement, as determined by norming conducted with unimpaired controls (see Meyer, Tippett, et al., 2016). See Figure 1 for an example item.
Figure 1.
Example stimulus item (elephant). Exemplar 1 (left); Exemplar 2 (right).
Trained and Untrained items were selected during the baseline evaluation. Each selected item was either named correctly by the participant during all three of the baseline oral naming tests (Prophylaxis Items), or it was named incorrectly during all three of these tests (Remediation Items). Ten participants had both Prophylaxis and Remediation items, while the remaining 11 participants only had Prophylaxis items. All of the selected words were read and repeated accurately at baseline. The selected items were divided into three sets (Phonological Treatment Condition, Orthographic Treatment Condition, and the Untrained Condition), and they were matched across sets for frequency (Baayen, Piepenbrock, & Gulikers, 1995), semantic category, and length (number of syllables, phonemes, and letters).
Participants typically had 40 items per treatment condition, resulting in 80 trained items per session. For five participants (LV6, LV7, LV8, SV2, and NFV1), filler items were included in order to reach 40 items per condition. The range was 1 to 11 filler items per condition (each participant had an equal number of fillers across treatment conditions). The fillers were selected from the items that could not be matched (on frequency, semantic category, or length) across the treatment conditions, and they were not included in the statistical analyses.
Procedure
Baseline evaluation
The baseline evaluation occurred over the course of six sessions, with one or two sessions per week. During these sessions, participants completed a battery of language and cognitive tests, including the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), the BNT, the 3-Picture version of the Pyramids and Palm Trees test (P&PT; Howard & Patterson, 1992), Word-Picture Matching (Rogers & Friedman, 2008), subject and object Wh-questions from the Northwestern Anagram Test (NAT; Weintraub et al., 2008), selected subtests from the Boston Diagnostic Aphasia Examination (BDAE; Goodglass et al., 2001), repetition of 5-syllable pseudowords (Meyer, Snider, Campbell, & Friedman, 2015), and the reading of irregular and regular words. The reading task was developed at the Center for Aphasia Research and Rehabilitation at Georgetown University Medical Center. The baseline assessment results are presented in Table 1. The one-sample t-test was utilized to compare each subgroup with published norms. The normative data for each test are cited above, except for the BNT; the Heaton norms (Heaton, Avitable, Grant, & Matthews, 1999) were utilized for this test. The independent-samples t-test was used for subgroup comparisons, and it was used to compare each subgroup’s performance on the reading task with a group of unimpaired controls. All tests were two-tailed, with an alpha of .05.
Treatment
Orthographic Treatment involved the pairing of a pictured noun and the corresponding written word, which was read orally and then copied by the participant. In the Phonological Treatment, a pictured noun was paired with a symbol string and the corresponding auditory word, which was repeated by the participant. See Meyer, Tippett, et al. (2016) for additional details.
In the first month of treatment, two sessions were conducted each week. These sessions included a spaced retrieval recognition task to aid in stimulus encoding. Home practice sessions occurred over the subsequent five months. During this time period, each participant used training cards to perform the treatment tasks with a caregiver three times per week. Three individuals participated remotely (see Meyer, Getz, et al., 2016), and the experimenter conducted all sessions with these participants, including home practice. For all participants, one treatment session was also conducted by the experimenter each month to help ensure that the participant was performing the tasks correctly and to help the participant remain engaged in the study.
Post-Treatment Evaluation
One month after the six-month period of treatment and practice sessions was completed, the post-treatment evaluation began. During this evaluation, naming accuracy for untrained and trained items (Exemplar set 1) was measured. In addition, stimulus generalization was examined by having participants name an alternative exemplar of each untrained and trained item (Exemplar set 2).
Imaging Analysis
Scans were obtained during the baseline evaluation. The structural scans consisted of T1-WIs (TE=6ms/TR=300ms, matrix:256x256mm; FOV:212x212, 1.1mm thickness). They were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using Statistical Parametric Mapping (SPM8). The skull-stripped brain was defined by masking the original images with the region of interest (ROI) defined by adding these segmentation maps. Our image quantification was based on the large deformation diffeomorphic metric mapping (LDDMM). Using DiffeoMap, the images were first linear normalized and then non-linear normalized by LDDMM. In addition, we superimposed an “anatomical parcellation” that divides the brain into about 211 three-dimensional areas, in an approach herein called “atlas-based” (see Faria et al., 2010; Oishi et al., 2009). The atlas integrates information from various white matter structures, based on Diffusion Tensor Images (DTI), and the gray matter structures, based on T1-WI. LDDMM registers each patient’s data into our atlas coordinates. When the registration is completed, the atlas can be “inversely” warped to the subject’s original space, defining the 211 brain regions automatically in each participant.
Statistical Analysis
SPSS 23 (IBM) was used for statistical analyses.
Group-Level Treatment Results
For each participant, naming accuracy at one month post-treatment was calculated within each treatment condition (Untrained Condition, Phonological Treatment Condition, or Orthographic Treatment Condition). For each of the four combinations of item type (Prophylaxis or Remediation) and Exemplar set (1 or 2), a one-way repeated-measures analysis of variance (ANOVA) was used to examine the effect of treatment condition. The Greenhouse-Geisser correction was utilized when Mauchly’s Test indicated that sphericity was not present.
Baseline Volume
The initial statistical analyses focused on 8 left-hemisphere ROIs. These ROIs were selected because they are prominent areas of atrophy in PPA (Gorno-Tempini et al., 2011; Mesulam et al., 2012). The ROIs included the following areas: inferior frontal gyrus, supramarginal gyrus, angular gyrus, temporal pole, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, and the fusiform gyrus. These areas have also been implicated in language in studies of stroke and unimpaired controls (Binder et al., 1997; Binney et al., 2010; Hickok & Poeppel, 2004, 2007; Indefrey, 2011; Indefrey & Levelt, 2004; Rogers et al., 2006). Each ROI consisted of the cortex and the underlying white matter.
The independent-samples t-test was then used to compare volume for each ROI between the PPA and control groups. We corrected for multiple comparisons, using Bonferroni correction for 8 comparisons and an alpha level of .05. The corrected alpha was .0063.
Correlations between Baseline Brain Volume and Post-Treatment Naming Accuracy
Correlations were computed between each of the ROIs and post-treatment naming accuracy. However, we focused on the left temporal pole and left inferior temporal gyrus (ITG) for this analysis because these were the only two ROIs that had significantly lower volume than controls in the initial analysis. We were also interested in these two ROIs because they have been shown to be critical for semantic processing (Binney et al., 2010; Migliaccio et al., 2016; Mummery et al., 2000; Rogalski et al., 2011; Rogers et al., 2006) and/or naming (Race et al., 2013), which may influence response to treatment (Jokel et al., 2014; Newhart et al., 2009).
The volume of each area was transformed into a z-score based on data from the control participants. For each combination of item type (Prophylaxis or Remediation), treatment condition (Untrained, Phonological, or Orthographic), and stimulus set (Exemplar set 1 or Exemplar set 2), naming accuracy was calculated. We then used Pearson correlations to examine the relationships between baseline volume within each of the ROIs and post-treatment naming accuracy. Our primary analysis included only the left temporal pole and ITG, for the reasons stated above. We corrected for multiple comparisons, using Bonferroni correction for 24 comparisons and an alpha level of .05. The corrected alpha was .0021. We secondarily evaluated the correlations between naming accuracy and the other ROIs.
Results
Group-Level Treatment Results
Prophylaxis Items (items consistently named correctly at baseline)
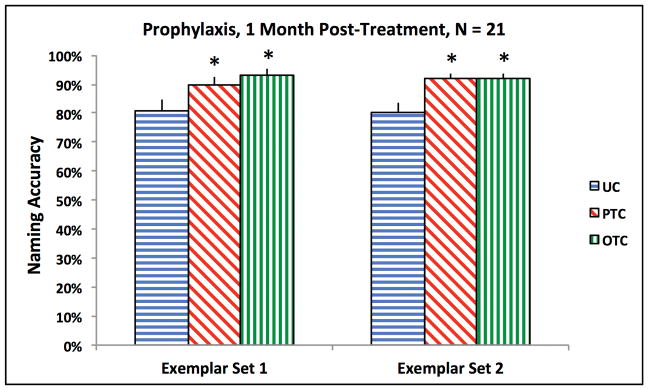
All participants had Prophylaxis items. The results are plotted in Figure 2. The effect of treatment condition was significant for the oral naming of Exemplar set 1 [F(2, 40) = 10.05, p < .001] and Exemplar set 2 [F(1.4, 27.7) = 19.06, p < .001]. For both exemplar sets, naming accuracy within each treatment condition was significantly greater than in the Untrained Condition [Exemplar set 1, Phonological Treatment: t(20) = 3.51, p = .002; Exemplar set 1, Orthographic Treatment: t(20) = 3.56, p = .002; Exemplar set 2, Phonological Treatment: t(20) = 4.74, p < .001; Exemplar set 2, Orthographic Treatment: t(20) = 4.54, p < .001].
Figure 2.
Group-level naming accuracy for Prophylaxis items. The bars represent the standard error. Each asterisk denotes a significant difference, compared to UC. UC = Untrained Condition; PTC = Phonological Treatment Condition; OTC = Orthographic Treatment Condition.
Remediation Items (items consistently named incorrectly at baseline)
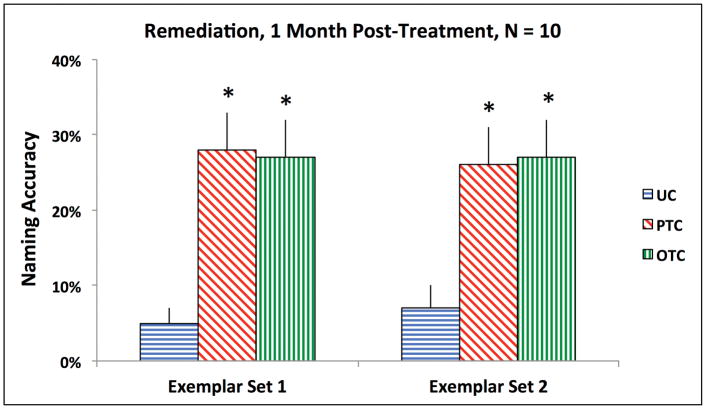
Ten participants had Remediation items. Five of these participants have lvPPA (LV1, LV3, LV4, LV6, and LV9), and five have svPPA. The results are plotted in Figure 3. The effect of treatment condition was significant for the oral naming of Exemplar set 1 [F(2, 18) = 11.22, p = .001] and Exemplar set 2 [F(2, 18) = 8.31, p = .003]. For both exemplar sets, naming accuracy in both treatment conditions was significantly greater than in the Untrained Condition [Exemplar set 1, Phonological Treatment: t(9) = 4.22, p = .002; Exemplar set 1, Orthographic Treatment: t(9) = 3.71, p = .005; Exemplar set 2, Phonological Treatment: t(9) = 4.31, p = .002; Exemplar set 2, Orthographic Treatment: t(9) = 3.17, p = .011].
Figure 3.
Group-level naming accuracy for Remediation items. The bars represent the standard error. Each asterisk denotes a significant difference, compared to UC. UC = Untrained Condition; PTC = Phonological Treatment Condition; OTC = Orthographic Treatment Condition.
Areas of Atrophy at Baseline (low brain volume relative to age-matched controls)
Two of the 8 ROIs within the left hemisphere had significantly lower volume in the PPA group, compared to the controls. The two ROIs were the temporal pole [t(30) = 3.48, p = .002; Controls: M = 9796 mm3, SD = 1159; PPA: M = 7536 mm3, SD = 1973] and the ITG [t(30) = 3.60, p = .001; Controls: M = 15517 mm3, SD = 1756; PPA: M = 12282 mm3, SD = 2681].
No other comparisons were significant (see Supplemental Table 1). The comparison that was closest to being significant was for the middle temporal gyrus [t(30) = 2.80, p = .009; Controls: M = 22533 mm3, SD = 3061; PPA: M = 18805 mm3, SD = 3816].
Correlations between Baseline Brain Volume and Post-Treatment Naming Accuracy
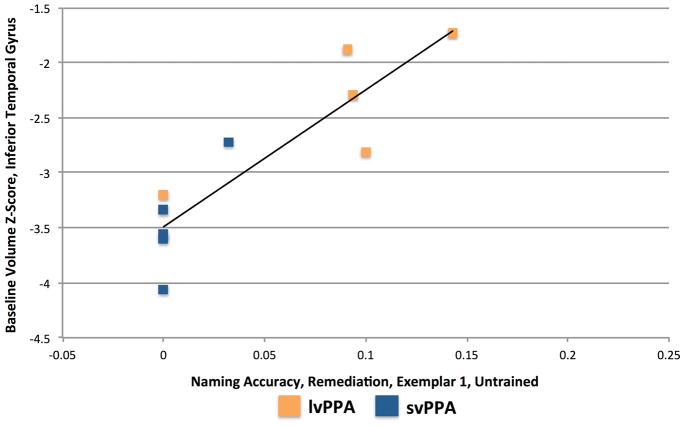
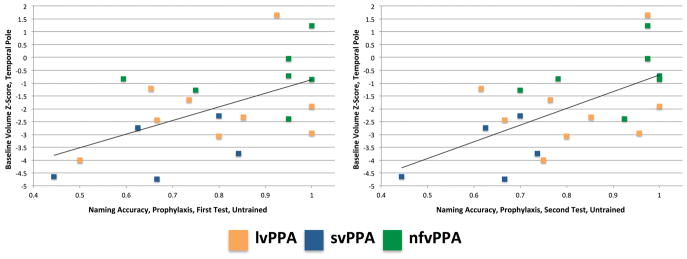
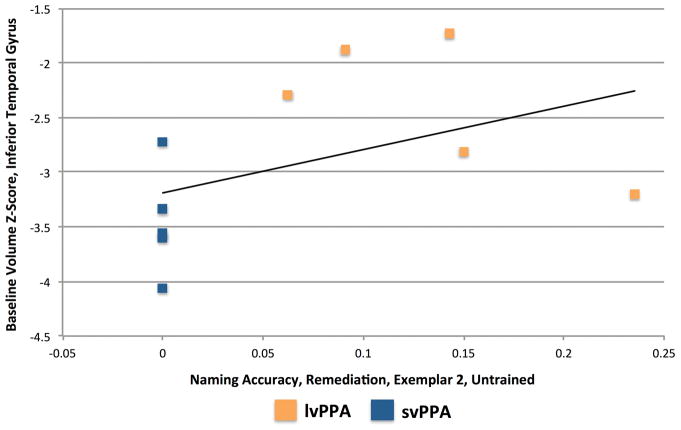
The correlations for the left temporal pole and left ITG (our primary analysis) are presented in Table 2. Two correlations were significant. For Prophylaxis items, accuracy for Exemplar set 2 in the Untrained Condition was correlated with volume in the left temporal pole [r(19) = .641, p = .0017; see Figure 4]. For Remediation items, accuracy for Exemplar set 1 in the Untrained Condition was correlated with volume in the left ITG [r(8) = .888, p = .0006; see Figure 5].
Table 2.
Correlations between Baseline Volume and Post-Treatment Naming Accuracy
| Temporal Pole | Inferior Temporal Gyrus | |||
|---|---|---|---|---|
|
|
|
|||
| r | p | r | p | |
|
|
|
|
|
|
| Prophylaxis, UC, Exemplar 1 | .500 | .021 | .374 | .095 |
| Prophylaxis, UC, Exemplar 2 | .641 | .0017** | .524 | .015 |
| Prophylaxis, PTC, Exemplar 1 | .431 | .051 | .291 | .201 |
| Prophylaxis, PTC, Exemplar 2 | .400 | .072 | .289 | .204 |
| Prophylaxis, OTC, Exemplar 1 | .304 | .181 | .093 | .688 |
| Prophylaxis, OTC, Exemplar 2 | .486 | .026 | .364 | .105 |
| Remediation, UC, Exemplar 1 | .540 | .107 | .888 | .0006** |
| Remediation, UC, Exemplar 2 | .438 | .205 | .432 | .213 |
| Remediation, PTC, Exemplar 1 | .010 | .979 | −.277 | .439 |
| Remediation, PTC, Exemplar 2 | .062 | .866 | −.124 | .733 |
| Remediation, OTC, Exemplar 1 | −.811 | .004 | −.757 | .011 |
| Remediation, OTC, Exemplar 2 | −.559 | .093 | −.575 | .082 |
Note. N = 21 participants for Prophylaxis items; N = 10 participants for Remediation items. Asterisks denote a significant correlation. The corrected alpha was .0021 (Bonferroni correction for 24 comparisons). OTC = Orthographic Treatment Condition; PTC = Phonological Treatment Condition; UC = Untrained Condition.
Figure 4.
Relationship between baseline volume in the left Temporal Pole and post-treatment naming accuracy for Exemplar set 2 Prophylaxis items in the Untrained Condition.
Figure 5.
Relationship between baseline volume in the left Inferior Temporal Gyrus and post-treatment naming accuracy for Exemplar set 1 Remediation items in the Untrained Condition.
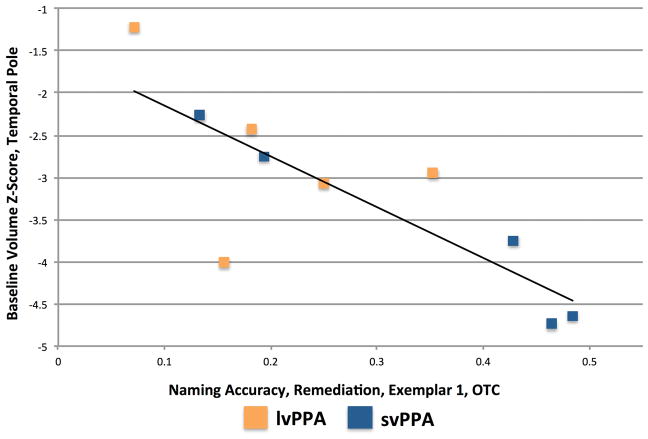
No other correlations involving these two ROIs were significant. There was a trend toward a significant negative correlation between volume in the left temporal pole and accuracy for Exemplar set 1 Remediation items in the Orthographic Treatment Condition [r(8) = −.811, p = .0044; see Figure 6]. Correlations between baseline volume in the other ROIs and naming accuracy are reported in Supplemental Table 2.
Figure 6.
Relationship between baseline volume in the left Temporal Pole and post-treatment naming accuracy for Exemplar set 1 Remediation items in the Orthographic Treatment Condition.
Discussion
In this study, relationships between post-treatment naming accuracy and baseline brain volume were examined in a group of participants with PPA. Correlational analyses focused on two left temporal regions (temporal pole and ITG) that had significantly lower baseline volume, compared to age-matched control participants. Both of these ROIs have been associated with naming (Amici et al., 2007; Brambati et al., 2006; Mesulam et al., 2013; Migliaccio et al., 2016; Race et al., 2013) and semantic processing (Binney et al., 2010; Migliaccio et al., 2016; Mummery et al., 2000; Rogalski et al., 2011; Rogers et al., 2006).
It was predicted that lower baseline volume in the ROIs would be associated with lower post-treatment naming accuracy, including smaller stimulus and item generalization effects. Naming accuracy was calculated for each combination of item type (Prophylaxis or Remediation), treatment condition (Untrained, Phonological, or Orthographic), and stimulus set (Exemplar set 1 or Exemplar set 2).
Unexpectedly, there were no significant correlations between baseline volume and post-treatment accuracy for treated items. However, baseline volume within each ROI was positively correlated with accuracy for Untrained items. The presence of a significant relationship between volume and naming accuracy depended on the brain area, the type of item, and the stimulus set. Lower baseline volume in the left temporal pole was associated with lower post-treatment naming accuracy for Untrained Exemplar set 2 Prophylaxis items (which, by definition, were consistently named correctly at baseline). This association suggests that the noun-naming abilities of individuals with lower volume in this area may be declining more rapidly. This interpretation is consistent with the strong association between svPPA and atrophy in the left temporal pole (Gorno-Tempini et al., 2004; Leyton et al., 2016; Mesulam et al., 2009; Mummery et al., 2000). Furthermore, individuals with lvPPA may also develop atrophy in the left temporal pole (Leyton et al., 2016). In contrast, nfvPPA is not typically associated with atrophy in the temporal pole (Gorno-Tempini et al., 2004; Leyton, Britton, Hodges, Halliday, & Kril, 2016), and anomia for nouns emerges later in nfvPPA (Hillis, Oh, & Ken, 2004; Hillis, Tuffiash, & Caramazza, 2002).
One way to evaluate this interpretation would be to examine decline on a standardized measure of naming, such as the BNT. However, if a 2 SD cutoff is applied to the atrophy measure, individuals with left temporal pole atrophy were near floor on the BNT, with a baseline raw score of 16.1 (SD = 10.7) and T-score of 9.9 (SD = 4.7). In contrast, participants above this cutoff had a baseline raw score of 40.2 (SD = 13.0) and T-score of 23.9 (SD = 15.4). Thus, the participants without atrophy in the temporal pole had more room to decline on the BNT. Numerically, participants without atrophy in this area showed a slightly larger mean decline on the BNT (7.2 for those without atrophy vs. 4.2 for those with atrophy), although the relationship between temporal pole volume and decline on the BNT was not significant [r(19) = .213, p = .354].
Another interpretation of the correlation between temporal pole volume and accuracy for Untrained Exemplar set 2 Prophylaxis items is that individuals with greater atrophy in this area are less likely to generalize to Untrained items. This interpretation is consistent with findings from other studies of anomia treatment in svPPA (Jokel et al., 2014; Newhart et al., 2009), although two studies have found item generalization in this subtype (Beales et al., 2016; Henry et al., 2013). Both of these studies utilized semantic/phonological/orthographic hybrid treatments, while the current study focused on separate phonological and orthographic treatments. Therefore, item generalization may be more likely to occur with a semantic or hybrid treatment.
It is unclear why the correlation between left temporal pole volume and naming accuracy for Untrained Exemplar set 1 Prophylaxis items was not also significant. One possibility is that the post-treatment testing order played a role. Fourteen out of 21 participants were tested on Exemplar set 1 before Exemplar set 2 (each set was tested in a different session). Thus, the first testing session provided an opportunity for retrieval practice (Friedman, Sullivan, Snider, Luta, & Jones, in press; Middleton, Schwartz, Rawson, & Garvey, 2015; Roediger & Butler, 2011). This opportunity followed a seven-month period during which testing of these items did not occur. Participants with greater temporal pole volume may have been more likely to benefit from retrieval practice during the initial post-treatment testing session. When post-treatment accuracy and baseline temporal pole volume are plotted by testing session (see Figure 7), the pattern is similar across the two sessions, but the correlation is stronger for the second session (First Session: r(19) = .539, p = .0117; Second Session: r(19) = .610, p = .0033).
Figure 7.
Relationship between baseline volume in the left Temporal Pole and post-treatment naming accuracy for Untrained Prophylaxis items. First session (left); second session (right).
In the left ITG, greater baseline volume was associated with greater post-treatment naming accuracy for Exemplar set 1 Remediation items in the Untrained Condition. This finding provides additional evidence that individuals with greater temporal atrophy are less likely to generalize to Untrained items. A different possibility is that participants with lvPPA, who tend to have less atrophy in the ITG (compared to those with svPPA), have greater variability in lexical access, which results in sporadic naming accuracy for a portion of the items that were incorrect at baseline. This possibility can be evaluated by examining the pattern for the two sets of Exemplars. As can be seen in Figures 5 and 8, participants with svPPA were clustered at or near 0% accuracy for both sets, while 4 participants with lvPPA had scores between 5% and 15% on the two sets, and one participant with lvPPA showed an increase from 0% for Exemplar set 1 to 24% for Exemplar set 2. For the participants in the 5% to 15% range, two showed partial or full overlap between the Untrained items that were named correctly on the two tests, while the other two participants showed no overlap between these items. Thus, 2 out of 5 participants with lvPPA showed relatively consistent improvement for specific Untrained items, suggesting that item generalization occurred, while the other 3 showed inconsistent improvement for Untrained items, which may be indicative of variability in lexical access.
Figure 8.
Relationship between baseline volume in the left Inferior Temporal Gyrus and post-treatment naming accuracy for Exemplar set 2 Remediation items in the Untrained Condition.
While there were no significant correlations between volume and naming accuracy for treated items, there was a trend toward a negative correlation between volume in the left temporal pole and accuracy for Exemplar set 1 Remediation items in the Orthographic Treatment Condition (see Figure 6). Thus, individuals with greater atrophy in the left temporal pole may demonstrate a larger response to orthographic treatment. This pattern would be consistent with studies that have found positive effects of orthographic treatment in svPPA (Green Heredia et al., 2009; Mayberry et al., 2011). By definition, the Remediation items were consistently named incorrectly at baseline, suggesting that the semantic representations or semantic-phonological connections for these items were already damaged at baseline. The goal of the Orthographic Treatment Condition is to strengthen orthographic representations, thereby bolstering the alternative, orthographic route from the semantic representations to the phonological representations (see Meyer, Tippett, et al., 2016). The trend toward a negative correlation between temporal pole volume and accuracy in the Orthographic Treatment Condition suggests that this treatment may have had the intended result, facilitating access to the remediation items’ phonological representations via the alternative route.
Although our focus on areas with significant baseline atrophy has the advantage of restricting the ROIs, it does not allow for examination of potential correlations involving other areas that may play a role in naming, such as the superior temporal gyrus (Migliaccio et al., 2016) or the middle temporal gyrus (Amici et al., 2007; Migliaccio et al., 2016). If greater statistical power had been present, correlations between post-treatment naming accuracy and baseline volume within the other six ROIs may have been significant. These correlations are presented in Supplemental Table 2. The correlation between accuracy for Untrained Exemplar set 2 Prophylaxis items and volume in the middle temporal gyrus (MTG) had the lowest p-value (.0035). The MTG is a common area of atrophy in svPPA (Gorno-Tempini et al., 2004; Mesulam et al., 2009; Mummery et al., 2000) and lvPPA (Leyton et al., 2016; Migliaccio et al., 2016; Rohrer et al., 2010). The MTG is hypothesized to link lexical information with semantic information (Hickok & Poeppel, 2004, 2007; Indefrey, 2011; Indefrey & Levelt, 2004; Migliaccio et al., 2016), possibly in conjunction with the ITG (Hickok & Poeppel, 2004, 2007). Thus, if it were significant, this correlation would be consistent with the other findings from the current study. In order to fully evaluate the role played by each left temporal area in naming, greater statistical power will be needed in future studies.
Another potential limitation is that separate correlations within each PPA subgroup were not examined, due to a lack of power for such analyses. However, PPA group-level correlational analyses may be more representative, given that an individual participant may have atrophy in areas that are not considered to be typical for that individual’s subtype (Rogalski et al., 2011). The different patterns observed across Exemplar sets are another potential limitation of the current study. Finally, the current study did not address the question of whether additional atrophy during the treatment period is correlated with anomia treatment effects. We will address this question in a future study.
In conclusion, the findings of this study suggest that lower volume in temporal areas is associated with greater decline and less improvement for Untrained items. Specifically, lower temporal pole volume is associated with greater decline for Untrained Prophylaxis items, which may be due to a larger decrease in noun-naming ability during the treatment period, less generalization to Untrained items, or a combination of the two. Lower volume in the ITG is associated with less improvement for Untrained Remediation items, which may be due to reduced generalization to Untrained items, reduced variability in lexical access, or a combination of the two.
Supplementary Material
Acknowledgments
This study was supported by the NIDCD under grant numbers R01DC011317 and R01DC011317-01AS1.
Footnotes
Treatment results from 16 of the current study’s participants were included in Meyer, Getz, Brennan, Hu, and Friedman (2016); LV8, LV9, SV5, NFV6, and NFV7 were not included. In addition, treatment results from 8 of the current study’s participants with lvPPA and all of the participants with svPPA were included in Meyer, Tippett, et al. (2016); LV9 was not included. One participant (identified as LV3 in the former study and LV4 in the latter study) was not included in the current study because he was not eligible to be scanned.
References
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and Behavioral Neurology. 2007;20:203–211. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Ash S, Evans E, O’Shea J, Powers J, Boller A, Weinberg D, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81:329–336. doi: 10.1212/WNL.0b013e31829c5d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, et al. Speech errors in progressive non-fluent aphasia. Brain & Language. 2010;113:13–20. doi: 10.1016/j.bandl.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database (Release 2) [CD-ROM] Philadelphia: Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Beales A, Cartwright J, Whitworth A, Panegyres PK. Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology. 2016 doi: 10.3109/17549507.2016.1151936. [DOI] [PubMed]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, Rapcsak SZ. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience. 2011;45:724–736. doi: 10.1007/s12031-011-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, Lambon Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRi, rTMS, and semantic dementia. Cerebral Cortex. 2010;20:2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, et al. The anatomy of category-specific object naming in neurodegenerative diseases. Journal of Cognitive Neuroscience. 2006;18:1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Cathery-Goulart MT, DaSilveira AD, Machado TH, Mansur LL, DeMattos Pimenta Parente MA, Senaha MLH, et al. Nonpharmacological interventions for cognitive impairments following primary progressive aphasia: A systematic review of the literature. Dementia and Neuropsychologia. 2013;7:122–131. doi: 10.1590/S1980-57642013DN70100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croot K, Nickels L, Laurence F, Manning M. Impairment- and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology. 2009;23:125–160. [Google Scholar]
- Croot K, Taylor C, Abel S, Jones K, Krein L, Hameister I, et al. Measuring gains in connected speech following treatment for word retrieval: a study with two participants with primary progressive aphasia. Aphasiology. 2015 doi: 10.1080/02687038.2014.975181. [DOI] [Google Scholar]
- Faria A, Zhang J, Oishi K, Li X, Jiang H, Akhter K, et al. Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. NeuroImage. 2010;52:415–428. doi: 10.1016/j.neuroimage.2010.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RB, Sullivan KL, Snider SF, Luta G, Jones KT. Leveraging the test effect to improve maintenance of the gains achieved through cognitive rehabilitation. Neuropsychology. doi: 10.1037/neu0000318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3. Austin: Pro-Ed; 2001. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green Heredia C, Sage K, Lambon Ralph MA, Berthier ML. Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology. 2009;23:192–209. [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding XS, et al. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience. 1996;8:135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Avitable N, Grant I, Matthews CG. Further crossvalidation of regression-based neuropsychological norms with an update for the Boston Naming Test. Journal of Clinical and Experimental Neuropsychology. 1999;21:572–582. doi: 10.1076/jcen.21.4.572.882. [DOI] [PubMed] [Google Scholar]
- Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Current Opinion in Neurology. 2010;23:633–637. doi: 10.1097/WCO.0b013e32833fb93e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Rising K, DeMarco AT, Miller BL, Gorno-Tempini ML, Beeson PM. Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain & Language. 2013;127:145–156. doi: 10.1016/j.bandl.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Annals of Neurology. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Tyler LK. Loss of semantic memory: Implications for the modularity of mind. Cognitive Neuropsychology. 1994;11:505–542. [Google Scholar]
- Howard D, Patterson K. The pyramids and palm trees test: A test of semantic access from words and pictures. Bury St. Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology. 2011;2:1–16. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jokel R, Anderson ND. Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychological Rehabilitation. 2012;22:187–214. doi: 10.1080/09602011.2011.639626. [DOI] [PubMed] [Google Scholar]
- Jokel R, Cupit J, Rochon E, Leonard C. Re-learning lost vocabulary in non-fluent progressive aphasia with Mosstalk Words. Aphasiology. 2009;22:175–191. [Google Scholar]
- Jokel R, Graham NL, Rochon E, Leonard C. Word retrieval therapies in primary progressive aphasia. Aphasiology. 2014;28:1038–1068. [Google Scholar]
- Jokel R, Rochon E, Anderson ND. Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation. 2010;20:16–41. doi: 10.1080/09602010902879859. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW, Murray ME, Senjem ML, Parisi JE, Petersen RC, et al. Quantitative neurofibrillary tangle density and brain volumetric MRI analyses in Alzheimer’s disease presenting as logopenic progressive aphasia. Brain and Language. 2013;127:127–134. doi: 10.1016/j.bandl.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Philadelphia: Lippincott, Williams, & Wilkins; 2001. [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Britton AK, Hodges JR, Halliday GM, Kril JJ. Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiology of Aging. 2016;38:82–92. doi: 10.1016/j.neurobiolaging.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Marcotte K, Ansaldo AI. The neural correlates of semantic feature analysis in chronic aphasia: discordant patterns according to the etiology. Seminars in Speech and Language. 2010;31:52–63. doi: 10.1055/s-0029-1244953. [DOI] [PubMed] [Google Scholar]
- Mayberry EJ, Sage K, Ehsan S, Lambon Ralph MA. Relearning in semantic dementia reflects contributions from both medial temporal lobe episodic and degraded neocortical semantic systems: Evidence in support of the complementary learning systems theory. Neuropsychologia. 2011;49:3591–3598. doi: 10.1016/j.neuropsychologia.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132:2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan D, Hu T, Friedman RB. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. 2016;30:483–507. doi: 10.1080/02687038.2015.1081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Campbell RE, Friedman RB. Phonological short-term memory in logopenic variant primary progressive aphasia and mild Alzheimer’s disease. Cortex. 2015;71:183–189. doi: 10.1016/j.cortex.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Eckmann CB, Friedman RB. Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology. 2015;29:1062–1081. doi: 10.1080/02687038.2015.1028327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, Friedman RB. Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation. 2016 doi: 10.1080/09602011.2016.1148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EL, Schwartz MF, Rawson KA, Garvey K. Test-enhanced learning versus errorless learning in aphasia rehabilitation: Testing competing psychological principles. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41:1253–1261. doi: 10.1037/xlm0000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Boutet C, Valabregue R, Ferrieux S, Nogues M, Lehericy S, et al. The brain network of naming: A lesson from primary progressive aphasia. PLoS ONE. 2016;11(2):e0148707. doi: 10.1371/journal.pone.0148707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centered on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Newhart M, Davis C, Kannan V, Heidler-Gary J, Cloutman L, Hillis AE. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23:823–834. [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer’s disease participants. NeuroImage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Aβ Amyloid and Glucose Metabolism in Three Variants of Primary Progressive Aphasia. Annals of Neurology. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race DS, Tsapkini K, Crinion J, Newhart M, Davis C, Gomez Y, et al. An area essential for linking word meanings to word forms: Evidence from primary progressive aphasia. Brain & Language. 2013;127:167–176. doi: 10.1016/j.bandl.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, Butler AC. The critical role of retrieval practice in long-term retention. Trends in Cognitive Sciences. 2011;15:20–27. doi: 10.1016/j.tics.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. Journal of Neuroscience. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Friedman RB. The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia. 2008;46:12–21. doi: 10.1016/j.neuropsychologia.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, et al. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive Affective and Behavioral Neuroscience. 2006;6:201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage. 2010;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S, Piguet O, Hodges JR. Giving words new life: Generalisation of word retraining outcomes in semantic dementia. Journal of Alzheimer’s Disease. 2014;40:309–317. doi: 10.3233/JAD-131826. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern Anagram Test: Measuring sentence production in Primary Progressive Aphasia. American Journal of Alzheimer’s Disease & Other Dementias. 2009;24:408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbury C, Bub D. Primary progressive aphasia: A review of 112 cases. Brain and Language. 1997;60:381–406. doi: 10.1006/brln.1997.1840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.