ABSTRACT
We report that CD47 was upregulated in different EMT-activated human breast cancer cells versus epithelial MCF7 cells. Overexpression of SNAI1 or ZEB1 in epithelial MCF7 cells activated EMT and upregulated CD47 while siRNA-mediated targeting of SNAI1 or ZEB1 in mesenchymal MDA-MB-231 cells reversed EMT and strongly decreased CD47. Mechanistically, SNAI1 and ZEB1 upregulated CD47 by binding directly to E-boxes in the human CD47 promoter. TCGA and METABRIC data sets from breast cancer patients revealed that CD47 correlated with SNAI1 and Vimentin. At functional level, different EMT-activated breast cancer cells were less efficiently phagocytosed by macrophages vs. MCF7 cells. The phagocytosis of EMT-activated cells was rescued by using CD47 blocking antibody or by genetic targeting of SNAI1, ZEB1 or CD47. These results provide a rationale for an innovative preclinical combination immunotherapy based on PD-1/PD-L1 and CD47 blockade along with EMT inhibitors in patients with highly aggressive, mesenchymal, and metastatic breast cancer.
KEYWORDS: Breast cancer, CD47, Dendritic cells, Epithelial to Mesenchymal Transition, Immune checkpoint, Macrophage, Phagocytosis and Immunotherapy, ZEB1
Introduction
“Epithelial-to-mesenchymal transition” (EMT) is a dynamic process during which both normal or neoplastic epithelial cells transform into a more mobile, invasive and aggressive mesenchymal phenotype. EMT is orchestrated by a series of master EMT-inducing transcription factors (EMT-TFs) and promotes stemness, immune evasion and drug resistance.1,2
Immune checkpoint-based cancer immunotherapy revolution has just started and it has transformed the field of onco-immunology. New exciting combination immunotherapies (including PD-1/PD-L1 antibodies) are being designed to deliver durable and synergistic survival benefits.3
CD47 is a cell surface transmembrane protein which delivers a strong “don't eat me signal” by binding to its ligands, signal regulatory protein α (SIRPα) and thrombospondin-1 (TSP-1), on the surface of macrophages and dendritic cells to block phagocytosis.4 CD47 is highly expressed on a variety of cancer cells and cancer stem cells, including both haematopoietic and solid tumors.5 Blockade of CD47 by using anti-CD47 monoclonal antibodies increased macrophage-mediated phagocytosis and elimination of various cancer cells.4 Using various syngeneic tumor models, CD47 blockade has been shown to promote their destruction in a manner largely depending on the activation of T cells.6
Human CD47-blocking monoclonal antibodies have proven incredibly efficacious in various pre-clinical models (patient tumor-derived orthotopic xenograft transplantation models) of human lymphoma, bladder cancer, colon cancer, glioblastoma, breast cancer, acute lymphocytic leukemia and acute myeloid leukemia.4 More recently, CD47-blocking immunotherapies were shown to activate macrophage-mediated destruction of human small-cell lung cancer cells.7
Despite the above mentioned recent excitement in targeting CD47 for cancer therapy, very little is known about the transcriptional regulation of the CD47 gene. CD47 expression is known to be regulated by several signaling pathways, transcription factors,8,9 and microRNA's (miRs).10 However, the expression and regulation of CD47, as well as the potential contribution of various EMT-TFs in highly metastatic and invasive mesenchymal tumors, remains unexplored.
In the present study, using multiple EMT-activated mesenchymal human breast cancer cell lines, we analyzed the expression and regulation of CD47. We showed that cells harboring an EMT-activated phenotype displayed a higher expression of CD47 by a direct binding of SNAI1 and ZEB1 to its proximal promoter. More importantly, we showed that EMT-dependent upregulation of CD47 inhibited the phagocytosis of EMT-activated mesenchymal cancer cells. Our in vitro data were supported by clinical data showing that CD47 expression correlated with SNAI1 and Vimentin expression in human breast cancer patients.
Materials and methods
Culture of tumor and human THP-1 cells
The human breast cancer cell lines (MCF7 and EMT-activated) were maintained in culture as described.11 Human monocyte THP1 cells was obtained from ATCC, and cultured at 2 × 105 cells/ml in RPMI 1640 medium. THP1 cells were differentiated into human macrophages by using 200 nM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 3–5days.
RNA isolation, SYBR-GREEN qRT-PCR (quantitative real time-polymerase chain reaction) and Western blot
RNA isolation and SYBR-GREEN qRT-PCR were performed as described.11 Expression level of 18S was used as an endogenous control. Western blotting was performed as described previously.11
Flow cytometry analysis
Flow cytometry was performed using FACS LSR-II. Data were further analyzed by FACS DIVA 7.0 or Flow Jo 7.6.5 software.11,12
Gene silencing by RNA interference
Pre-designed siRNAs against SNAI1, ZEB1, CD47 and scrambled control were purchased from Life Technologies and transfected by using Lipofectamine RNAiMAX Transfection Reagent as described earlier.11
Confocal microscopy
Confocal microscopy was performed as described.11
Statistical analysis
Data were analyzed with GraphPad Prism. Unpaired 2 tailed student's t-test was used for single comparisons. Statistically significant differences (indicated by asterisks) are shown (* = P < 0.05, ** = P < 0.005, and *** = P < 0.0005). Error bars indicate SD.
Results and discussion
CD47 is upregulated in EMT activated mesenchymal as compared with epithelial breast cancer cells
We first compared the expression of CD47 in 2 EMT-activated mesenchymal MCF7 clones (MCF7 sh-WISP2 and MCF7 1001),11 MDA-MB-231 cells and epithelial MCF7 human breast cancer cells. Western blot analysis (Figs. 1A and 1B) and confocal microscopy (Fig. 1C) showed that CD47 was significantly upregulated in MCF7 sh-WISP2, MCF7 1001 and MDA-MB-231 mesenchymal cells displaying loss of the epithelial marker E-cadherin and gain in the mesenchymal marker vimentin as compared with parental epithelial MCF7 cells. Similarly, as depicted in Figs. 1D and 1E, surface expression of CD47 analyzed by flow cytometry was significantly upregulated in MCF7 sh-WISP2, MCF7–1001 and MDA-MB-231 cells as compared with MCF7 cells. CD47 mRNA levels were also upregulated in MCF7 sh-WISP2 (more than 8-fold), MCF7 1001 (more than 6-fold) and MDA-MB-231 cells (more than 15-fold) vs. MCF7 cells (Fig. 1F).
Figure 1.
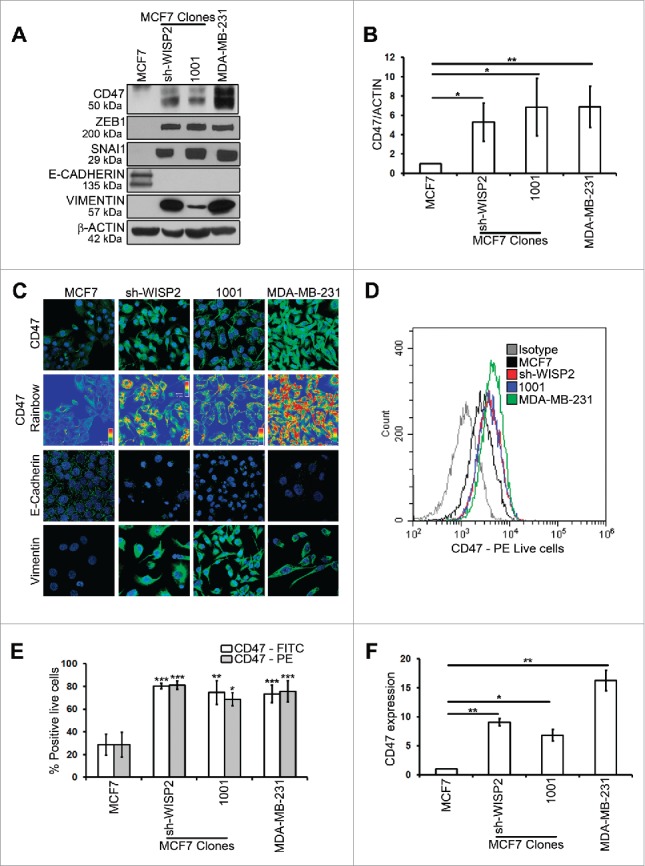
MCF7 sh-WISP2, MCF7 1001 and MDA-MB-231 mesenchymal cells selectively upregulate CD47 as compared with epithelial MCF7 cells.(A) Western blot was performed to show CD47, ZEB1, SNAI1, E-CADHERIN, VIMENTIN and β-ACTIN protein levels. (B) Densitometry was performed to compare CD47 protein levels. The experiment was repeated 5 times. (C) Confocal microscopy analysis of CD47, E-CADHERIN and VIMENTIN expression (in green) in indicated cells. CD47 Rainbow panel indicates CD47 staining intensity (blue to red corresponds to low to high intensity respectively). Nuclei were counterstained with DAPI (in blue). Magnification 40X, bar: 20 μm. The experiment was repeated 6 times. (D and E) Surface expression of CD47 (using 2 different antibodies: Human CD47 PE-conjugated Antibody and Anti-Human CD47 FITC) on live cells was evaluated by flow cytometry as compared with isotype control (gray-shaded histogram). The experiment was repeated 6 times. (F) SYBR-GREEN RT-qPCR was used to monitor CD47 mRNA expression. The experiment was performed in triplicate and repeated 3 times.
We have previously shown that TGF-β signaling is activated in MDA-MB-231 and MCF7 sh-WISP2 cells and promotes EMT.11 Consequently, we examined whether the TGF-β signaling pathway modulated CD47 expression. As shown in Fig. S1, neither of the 2 TGF-β inhibitors had any effect on CD47 expression at either the mRNA (Fig. S1A) or protein levels (Figs. S1B and S1C) in both MDA-MB-231 and MCF7 sh-WISP2 cells. These data clearly indicate that upregulated CD47 in EMT-activated mesenchymal cells is not regulated by TGF-β signaling. Different signaling pathways such as MAPK, IFN-γ and PI3K/Akt have been reported to regulate PD-L1.13 Whether these signaling pathways are involved in the up regulation of CD47 in EMT-activated cells remains to be explored.
We and others previously demonstrated that immune checkpoint ligand PD-L1 was upregulated in EMT-activated human breast cancer11 and lung cancer14 cells by a mechanism involving the miR-200/ ZEB-1 axis. Moreover, in patient-derived mesenchymal tumors, a pan-cancer EMT signature showed high expression of multiple immune checkpoints such as PD1, PD-L1, CTLA4, OX40L, and PD-L2.15
In the current study, we demonstrate for the first time that EMT-activated mesenchymal breast cancer cells overexpress CD47 as compared with epithelial breast cancer cells. Consistent with our findings, CD47 was found to be overexpressed in metastatic tumors as compared with primary tumors in melanoma patients.16
Overexpression of SNAI1 and ZEB1 in epithelial MCF7 cells activated EMT and upregulated CD47
Stress-induced expression of EMT-TFs (SNAIL, ZEB and TWIST families) results in EMT activation and cancer progression toward a metastatic phenotype.1,2 We have previously shown that SNAI1 and ZEB1 up-regulate immune checkpoint PD-L1 in EMT-activated breast cancer cells.11 Here, we investigated whether SNAI1 and ZEB1 could also regulate macrophage immune checkpoint CD47. To address this issue we used MCF7 cells stably expressing wild type (MCF7 SNAI1) or constitutively active (MCF7 SNAI1–6SA) SNAI1,11 as well as ZEB1 (MCF7 ZEB1),17 and examined EMT activation and CD47 expression.
Western blot analysis and confocal microscopy showed that along with EMT-activation, characterized by loss of E-cadherin and gain of vimentin, CD47 was upregulated in MCF7 SNAI1, MCF7 SNAI1–6SA and MCF7 ZEB1 cells vs. MCF7 cells (Figs. 2A, 2B and 2C). Similarly, surface expression of CD47, analyzed by flow cytometry was significantly upregulated in SNAI1 or ZEB1-overexpressing MCF7 cells (Figs. 2D and 2E). CD47 mRNA levels (Fig. 2F) were also strongly upregulated in MCF7 SNAI1 (more than 3-fold), MCF7 SNAI1–6SA (more than 4-fold) and MCF7 ZEB1 cells (more than 3-fold) vs. MCF7 cells. These data provide additional evidence that EMT-activation driven by overexpression of SNAI1 or ZEB1 in epithelial MCF7 cells leads to upregulation of CD47.
Figure 2.
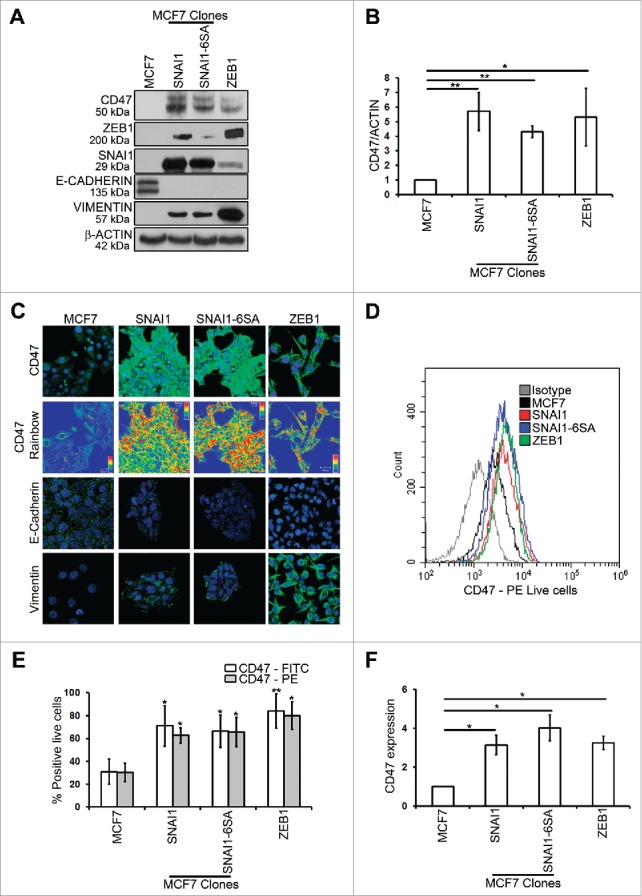
MCF7 SNAI1, MCF7 SNAI1–6SA and MCF7 ZEB1 EMT-activated cells selectively upregulate CD47 as compared with epithelial MCF7 cells.(A) Western blot was performed to show CD47, ZEB1, SNAI1, E-CADHERIN, VIMENTIN and β-ACTIN protein levels. (B) Densitometry was performed to compare CD47 protein levels. The experiment was repeated 5 times. (C) Confocal microscopy analysis of CD47, E-CADHERIN and VIMENTIN expression (in green) in indicated cells. CD47 Rainbow panel indicates CD47 staining intensity (blue to red corresponds to low to high intensity respectively). Nuclei were counterstained with DAPI (in blue). Magnification 40X, bar: 20 μm. The experiment was repeated 6 times. (D and E) Surface expression of CD47 (using 2 different antibodies: Human CD47 PE-conjugated Antibody and Anti-Human CD47 FITC) on live cells was evaluated by flow cytometry as compared with isotype control (gray-shaded histogram). The experiment was repeated 6 times. (F) SYBR-GREEN RT-qPCR was used to monitor CD47 mRNA expression. The experiment was performed in triplicate and repeated 3 times.
CD47 is downregulated by targeting EMT-transcription factors SNAI1 and ZEB1
Based on our data showing that SNAI1 and ZEB1 upregulate the expression of CD47, we performed loss of function studies of SNAI1 and ZEB1 in mesenchymal MDA-MB-231 cells expressing both SNAI1 and ZEB1. We found that siRNA targeting either SNAI1 or ZEB-1 strongly decreased CD47 at both the mRNA (Fig. 3A) and protein (Figs. 3B and 3C) levels. Similarly, as depicted in Figs. 3D and 3E, surface expression of CD47 significantly decreased in MDA-MB-231 cells after either SNAI1 or ZEB-1 silencing. It is noteworthy that in MDA-MB-231 cells, upregulation of CD47 was found to be dependent more on SNAI1 than on ZEB-1 at both the mRNA (Fig. 3A) and protein (Figs. 3B, 3C, 3D and 3E) levels. This is likely due to the fact that SNAI1 is known to occupy the ZEB1 promoter and ZEB-1 expression is controlled by SNAI1 in MDAMB-231 cells (siRNA-SNAI1 strongly decreased ZEB1, shown in Fig. 3B).18
Figure 3.
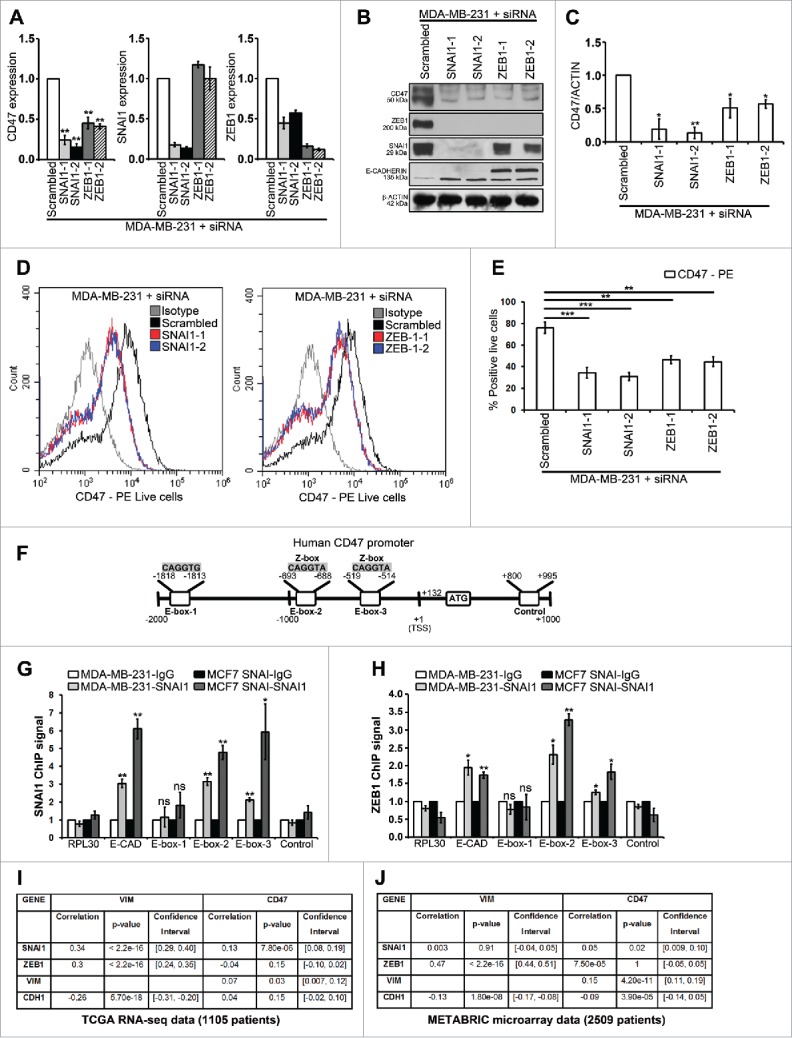
SNAI1 and ZEB-1 directly regulate CD47 in EMT-activated mesenchymal MDA-MB-231 cells and CD47 in human breast cancers is correlated with EMT marker expression.(A-C) MDA-MB-231 cells were transfected with different siRNAs targeting SNAI1, ZEB-1 or scrambled control. (A) SYBR-GREEN RT-qPCR was used to monitor CD47, SNAI1 and ZEB1 mRNA expressions levels. The experiment was performed in triplicate and repeated 3 times. (B) Western blot was performed to show CD47, ZEB1, SNAI1, E-CADHERIN and β-ACTIN protein levels. The experiment was repeated 3 times. (C) Densitometry was performed to compare CD47 protein levels. The experiment was repeated 3 times. (D and E) Surface expression of CD47 on live cells was evaluated by flow cytometry as compared with isotype control (gray-shaded histogram). The experiment was repeated 3 times. (F) Different E-boxes in the Human CD47 promoter (CD47 mRNA, NCBI Reference Sequence: NM_198793.2) are shown. (G and H) ChIP was performed on MDA-MB-231 and MCF7 SNAI1 cells by using either anti-SNAI1 antibody (G) or anti-ZEB1 antibody (H) followed by SYBR-GREEN RT-qPCR using RPL30, E-CAD (E-CADHERIN), E-box sites (E-box-1, E-box-2 and E-box-3) and control primers (a region containing no E-box). For each gene, the RT-qPCR signals were normalized to control IgG. SNAI1 ChIP signal or ZEB1 ChIP signal (fold enrichment over IgG control). Two separate experiments (in triplicate) with the same results were performed. (I and J) Correlation analysis between mRNA expression profiles of CD47 and various EMT marker genes in TCGA (I) and METABRIC (J), 2 large breast cancer data sets, was performed using Pearson's correlation test. *P < 0.05 and ***P < 0.001.
SNAI1 and ZEB1 upregulate the expression of CD47 by binding directly to E-box motifs in the human CD47 proximal promoter and CD47 correlates with EMT markers expression
To investigate the molecular mechanism underlying the regulation of CD47 by SNAI1 and ZEB1, we performed in silico analysis to assess whether CD47 is a direct target gene for SNAI1 and ZEB1. We first looked for EMT-TFs binding motifs called E-boxes (CAGGTA, CAGGTG and CACCTG) using the HOCOMOCO database. One of these E-boxes is restricted to ZEB factors (CAGGTA, called Z-box), while the 2 remaining E-boxes (CAGGTG and CACCTG) can bind ZEB and other EMT-TFs such as SNAIL.19 We successively searched for and found 3 E-boxes (Fig. 3F) in the proximal promoter of the human CD47 gene by using the Eukaryotic Promoter Database (Swiss Institute of Bioinformatics) and fuzznuc (EMBOSS explorer) software (Sequence provided in Fig. S2).
Using ChIP, we found that both SNAI1 (Fig. 3G) and ZEB1 (Fig. 3H) strongly bind to 2 E-boxes (E-box-2 and E-box-3) in both MDA-MB-231 and MCF7 SNAI1 cells. SNAI1 ChIP complexes showed a significant binding of SNAI1 to E-box-2 (3-fold) and E-box-3 (5-fold) in MDA-MB-231 cells and for MCF7 SNAI1 cells, E-box-2 (3.5-fold) and E-box-3 (6-fold) (Fig. 3G). Similarly, ZEB1 ChIP complexes showed a significant binding of ZEB1 to E-box-2 (3-fold) and E-box-3 (3.5-fold) in MDA-MB-231 cells and for MCF7 SNAI1 cells, E-box-2 (1.5-fold) and E-box-3 (2-fold) (Fig. 3H). This was comparable to SNAI1 and ZEB1 binding to the E-box in E-CADHERIN promoter, an established SNAI1 and ZEB1 target gene (Figs. 3G and 3H). The results presented in Fig. 3 clearly demonstrate that CD47 is a direct SNAI1 and ZEB1 target gene in MDA-MB-231 and MCF7 SNAI1 cells. These results are further reinforced by previous data suggesting that mouse Snail1 can bind to the mouse Cd47 promoter in a ChIP-sequencing data set in mouse MMTV-PyMT breast cancer cell line (overexpressing mouse Snail1).18 Similarly, evidence was found to show that mouse Zeb1 can bind to the mouse Cd47 promoter20 and human ZEB1 can bind to the human CD47 promoter21 in ChIP-sequencing data sets.
Despite the fact that there is a lot of excitement in drugging CD47 for cancer immunotherapy, remarkably, we know very little about CD47 gene transcriptional regulation. We and others have reported that Hypoxia-inducing factors, HIF-1α12 and HIF-2α22 can regulate immune checkpoints such as PD-L1 expression. In this regard, hypoxia via HIF-1α was also shown to regulate CD47 expression in breast cancer cells. Hypoxia-induced CD47 promoted evasion of phagocytosis and maintenance of the cancer stem cell phenotype.9 MYC regulated the antitumor immune response in both human and mouse tumors through direct regulation of both CD47 and PD-L1.8 Similarly, CD47 expression has been found to be regulated by TNF-α,23 nuclear factor kappa B,24 and miR-133a.10
We next analyzed the gene expression data from human breast cancer patients that are publicly available in The Cancer Genome Atlas (TCGA) database (RNA-seq data, 1105 patients) and the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) database (microarray data, 2509 patients) by using cBioPortal. Interestingly, our statistical analysis revealed that CD47 mRNA levels significantly correlated with 2 out of 4 EMT marker mRNAs levels (SNAI1 and VIM but not ZEB1 and CDH1) for the TCGA data set (Fig. 3I) and with 3 out of 4 EMT marker mRNAs levels (SNAI1, VIM and CDH1 but not ZEB1) for the METABRIC data set (Fig. 3J). Similar results were recently reported showing a significant increase in CD47 mRNA between a primary tumor vs. a metastatic tumor (both regional cutaneous and lymph node metastasis) by using 2 large melanoma patient cohorts.16
To our best knowledge, we provide here the first evidence that SNAI1 and ZEB1 are major regulators of CD47 mRNA and protein expression in human breast cancer cells and that endogenous SNAI1 and ZEB1 regulate the expression of CD47 by binding directly to E-box-2 and E-box-3 in the human CD47 proximal promoter. Future experiments will attempt to study whether other EMT-TFs, such as SLUG, ZEB2 or TWIST driven EMT-activation, is also able to upregulate CD47 expression.
Blocking CD47 in EMT-activated breast cancer cells restores their phagocytosis by macrophages
Based on the results described above, we next assessed the functional impact of EMT-dependent upregulation of CD47 on the phagocytosis of tumor cells. We performed in vitro phagocytosis assays on EMT-activated breast cancer cells as well as on epithelial MCF7 cells. Human macrophage THP-1-mediated Phagocytosis was significantly decreased in EMT-activated cells (MCF7 sh-WISP2, MCF7 1001, MDA-MB-231, MCF7 SNAI1, MCF7 SNAI1–6SA and MCF7 ZEB1, Fig. 4A) as compared with epithelial MCF7 cells (Fig. S3). Similarly, by using mouse macrophages and dendritic cells, EMT-activated cells (Figs. 4B and 4C) were much less phagocytosed as compared with epithelial MCF7 cells. To determine whether the decreased phagocytosis of EMT-activated breast cancer cells was mediated by CD47, we used CD47 blocking antibody and performed phagocytic assays. Our results clearly demonstrate that human macrophage THP-1-mediated phagocytosis was significantly increased after CD47 blockade in EMT-activated cells (MCF7 sh-WISP2, MCF7 1001, MDA-MB-231, MCF7 SNAI1, MCF7 SNAI1–6SA and MCF7 ZEB1, Fig. 4D) as well as in epithelial MCF7 cells. These results were further confirmed by using mouse macrophages and dendritic cells, as CD47 blockade significantly increased phagocytosis of EMT-activated cells (Figs. 4E and 4F) and MCF7 cells. It is important to note that CD47 blockade did not induce breast cancer cell death before the phagocytic assay. Our data convincingly demonstrate that EMT-activated mesenchymal breast cancer cells are less phagocytosed as compared with epithelial MCF7 cells and that blockade of CD47 efficiently increases the phagocytosis of EMT-activated mesenchymal breast cancer cells. Our results are in total agreement with a recent finding demonstrating that CD47 blocking antibody (using both anti-human CD47 and anti-mouse CD47) induced efficient mouse macrophage-mediated phagocytosis of metastatic melanoma (expressing high levels of CD47) as compared with primary melanoma cells.16
Figure 4.
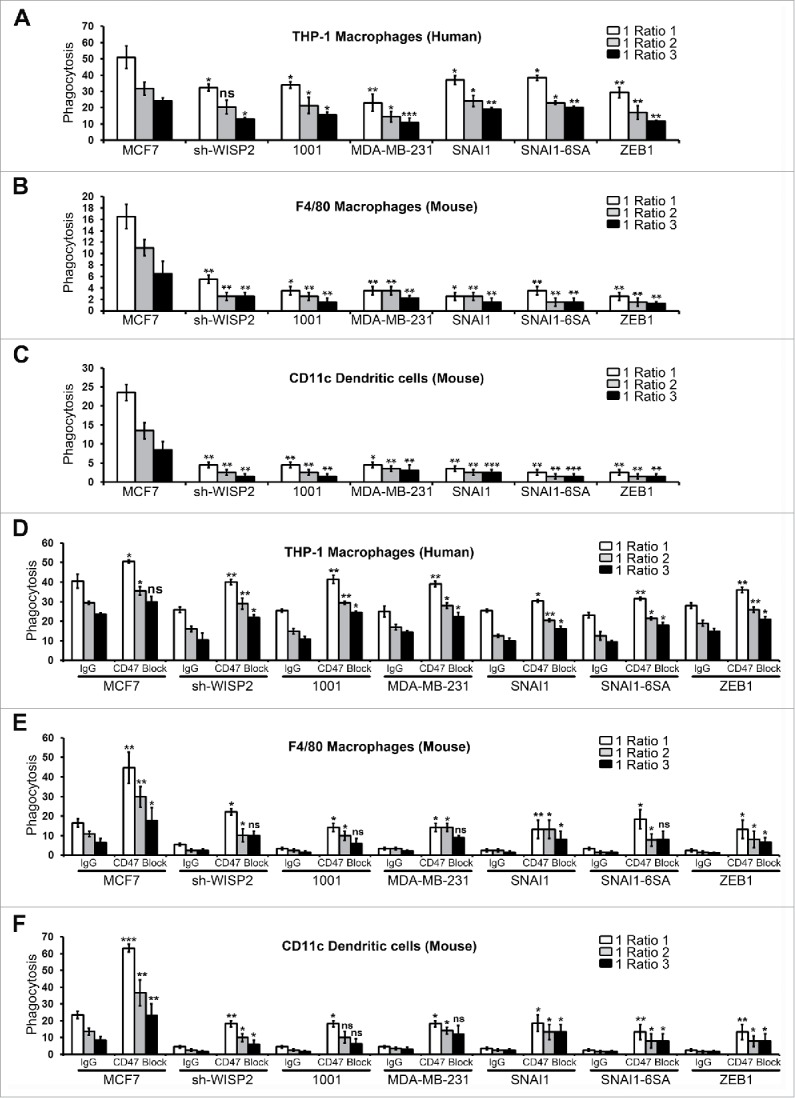
EMT upregulated CD47 inhibits the phagocytosis of EMT-activated cells and increased phagocytosis of human breast cancer cells after CD47 blockade.(A) EMT-activated cells and epithelial MCF7 cells were stained with CFSE, co-cultured with Human THP-1 derived macrophages for 2 h (stained with CellTrace™ Far Red-APC) at different target to effector (cancer: macrophage) ratios, and analyzed by flow cytometry. Phagocytosis is shown as the percentage of CFSE+APC+ phagocytosed cancer cells. The experiment was repeated 6 times. (B and C) EMT-activated cells and epithelial MCF7 cells were stained with CFSE, co-cultured with mouse macrophages or dendritic cells for 2 h at different target to effector (cancer: macrophage or dendritic cells) ratios, stained with F4/80-APC or CD11c-APC antibody and analyzed by flow cytometry. Phagocytosis is shown as the percentage of CFSE+APC+ phagocytosed cancer cells. The experiment was repeated 3 times. (D-F) Phagocytosis assay was performed with EMT-activated cells and epithelial MCF7 cells as described in 4A in the presence of CD47 blocking antibody (B6H12; e Bioscience) or matching IgG control by using Human THP-1 derived macrophages (D), mouse macrophages (E) or mouse dendritic cells (F). The experiment was repeated 3 times.
Targeting of CD47 was shown to inhibit non-small cell lung cancer25 and melanoma16 tumor cell invasion and metastasis in vitro and in vivo, but whether there is a bidirectional crosstalk between CD47 expression and EMT activation remains uninvestigated. It would be interesting to study the impact of CD47 silencing on the EMT process as well as different EMT-TFs (SNAI1, TWIST, SLUG or ZEB-1) in EMT-activated cells.
Targeting SNAI1, ZEB1 or CD47 rescues the phagocytosis of mesenchymal MDA-MB-231 cells
To investigate whether SNAI1, ZEB1 (via CD47 upregulation) or CD47 mediates protection of EMT-activated breast cancer cells, MDA-MB-231 cells were transfected with different siRNAs targeting either SNAI1, ZEB1, CD47 or scrambled control (Fig. S4) and phagocytosis assay was performed. We observed a significant increase in human macrophage THP-1-mediated phagocytosis of SNAI1, ZEB1 or CD47 silenced MDA-MB-231 mesenchymal cells (Fig. S5A). Similarly, by using mouse macrophages and dendritic cells, EMT-activated MDA-MB-231 cells (Figs. S5B and S5C) were much more phagocytosed after siRNA-mediated targeting of SNAI1, ZEB1 and CD47.
Taken together, CD47 was highly upregulated in EMT-activated mesenchymal breast cancer cells via direct binding of SNAI1 and ZEB1 to human CD47 proximal promoter and CD47 blockade efficiently increased their phagocytosis.
The immune checkpoint-based cancer immunotherapy revolution has just begun and this is high time for combination cancer immunotherapies. We need to design safe, robust, synergistic and intelligent combination immunotherapies, to maximize the benefit to a larger class of breast cancer patients.3 We have recently shown that EMT-activation increased PD-L1 and decreased CTL-mediated killing.11 Here we describe a new mechanism by which EMT-activated mesenchymal cells evade macrophage-mediated phagocytosis by upregulating CD47. Increased PD-L1 and CD47 helps EMT-activated mesenchymal cells to evade from both innate and adaptive immunity. Based on our results we propose a combinatorial regime using EMT inhibitors as adjuvants along with simultaneous blockade of macrophage CD47 and T cell PD-1/PD-L1 immune checkpoints. Such combination will lead to a concurrent activation of innate (via macrophage-induced phagocytosis) and adaptive (via activated T CD8+ cell-mediated killing) immunity, thus effectively suppressing breast cancer progression and metastasis.
Supplementary Material
Funding Statement
This work was supported by grants from: FNRS-Televie (20150327), Janssen Pharmaceutical, Fondation Cancer (20160517), Celgene and LIH (2013 11 05). RMG was supported by grants from the Dept. of Defense, DOD LC150622 and from the Veterans Administration, BX003333. We thank Cristina Maximo and Joshua Brown-Clay (LIH, Luxembourg) for their help in editing the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell 2016; 166:21-45; PMID:27368099; https://doi.org/ 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 2.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16:488-94; PMID:24875735; https://doi.org/ 10.1038/ncb2976 [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; https://doi.org/ 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 4.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al.. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109:6662-7; PMID:22451913; https://doi.org/ 10.1073/pnas.1121623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138:271-85; PMID:19632178; https://doi.org/ 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015; 21:1209-15; PMID:26322579; https://doi.org/ 10.1038/nm.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS, et al.. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 2016; 126:2610-20; PMID:27294525; https://doi.org/ 10.1172/JCI81603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al.. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016; 352:227-31; PMID:26966191; https://doi.org/ 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015; 112:E6215-23; PMID:26512116; https://doi.org/ 10.1073/pnas.1520032112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Wang Y, Li YZ. MicroRNA-133a suppresses the proliferation, migration, and invasion of laryngeal carcinoma cells by targeting CD47. Tumour Biol 2016; 37:16103-13; PMID:27730543; https://doi.org/ 10.1007/s13277-016-5451-x [DOI] [PubMed] [Google Scholar]
- 11.Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, Mami-Chouaib F, Thiery JP, Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology 2017; 6:e1263412; PMID:28197390; https://doi.org/ 10.1080/2162402X.2016.1263412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:24778419; https://doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann Oncol 2016; 27:409-16; PMID:26681673; https://doi.org/ 10.1093/annonc/mdv615 [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al.. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5:5241; PMID:25348003; https://doi.org/ 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II, et al.. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res 2016; 22:609-20; PMID:26420858; https://doi.org/ 10.1158/1078-0432.CCR-15-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngo M, Han A, Lakatos A, Sahoo D, Hachey SJ, Weiskopf K, Beck AH, Weissman IL, Boiko AD. Antibody therapy targeting CD47 and CD271 effectively suppresses melanoma metastasis in patient-derived xenografts. Cell Rep 2016; 16:1701-16; PMID:27477289; https://doi.org/ 10.1016/j.celrep.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Song L, Bai Y, Kinose F, Li J, Ohaegbulam KC, Muñoz-Antonia T, Qu X, Eschrich S, Uramoto H, et al.. ZEB1 mediates acquired resistance to the epidermal growth factor receptor-tyrosine kinase inhibitors in non-small cell lung cancer. PloS One 2016; 11:e0147344; PMID:26789630; https://doi.org/ 10.1371/journal.pone.0147344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015; 525:256-60; PMID:26331542; https://doi.org/ 10.1038/nature14897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017; 168:670-91; PMID:28187288; https://doi.org/ 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubelmann C, Schwalie PC, Raghav SK, Roder E, Delessa T, Kiehlmann E, Waszak SM, Corsinotti A, Udin G, Holcombe W, et al.. Identification of the transcription factor ZEB1 as a central component of the adipogenic gene regulatory network. Elife 2014; 3:e03346; PMID:25163748; https://doi.org/ 10.7554/eLife.03346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Shao X, Gao L, Zhang S. Systematic DNA methylation analysis of multiple cell lines reveals common and specific patterns within and across tissues of origin. Hum Mol Genet 2015; 24:4374-84; PMID:25954028; https://doi.org/ 10.1093/hmg/ddv172 [DOI] [PubMed] [Google Scholar]
- 22.Messai Y, Gad S, Noman MZ, Le Teuff G, Couve S, Janji B, et al.. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 Alpha, is regulated by von hippel-lindau gene mutation status. Eur Urol 2016; 70:623-32; PMID:26707870 [DOI] [PubMed] [Google Scholar]
- 23.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et al.. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016; 536:86-90; PMID:27437576; https://doi.org/ 10.1038/nature18935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015; 62:534-45; PMID:25902734; https://doi.org/ 10.1002/hep.27859 [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Wang J, Kong X, Li E, Liu Y, Du X, Kang Z, Tang Y, Kuang Y, Yang Z, et al.. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep 2016; 6:29719; PMID:27411490; https://doi.org/ 10.1038/srep29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


