Supplemental Digital Content is available in the text.
Abstract
Background:
The addition of platelet-rich plasma (PRP) to adipose tissue may improve fat graft survival, although graft retention rates vary markedly between studies. To what extent this outcome heterogeneity reflects differing methodological factors remains unknown. This systematic review aims to synthesize and critically review methodological approaches to autologous PRP and fat cotransplantation in both human and animal studies.
Methods:
In accordance with PRISMA guidelines, Ovid MEDLINE, Scopus, and Cochrane Library databases were searched from inception to April 2017. Data were extracted from all in vivo studies involving autologous PRP and fat cotransplantation. A secondary aim was to assess reporting of technical detail; authors were not contacted to provide missing data.
Results:
From 335 articles, 23 studies were included in the qualitative synthesis. Some 21 were performed in humans and 2 in rabbits. Six studies were randomized control trials; the remainder reported on observational data. Methods of PRP extraction and activation varied markedly between studies. Fat graft preparation was comparatively more consistent. Methods of PRP and fat mixing differed significantly, especially with regards to relative volume/volume ratios.
Conclusions:
Our study represents the first systematic review of methodological factors in autologous PRP and fat cotransplantation. It demonstrates that technical factors in graft preparation and administration vary significantly between in vivo studies. Such methodological heterogeneity may explain observed differences in experimental and clinical outcomes. Reporting of key procedural information is inconsistent and often inadequate. These issues make meaningful evaluation of the PRP-enhanced fat grafting literature difficult and may limit its translation into clinical practice.
INTRODUCTION
Autologous fat grafting is used extensively in reconstructive and aesthetic plastic surgery for the correction of soft-tissue volume defects. The popularity of structural free fat transfer comes from the fact that it is nonimmunogenic, versatile, and easily harvested with low donor-site morbidity.1
More recently, there has been growing interest in the regenerative potential of free fat transfer.2,3 Multipotent precursor cells transplanted within the fat stromal compartment—termed adipocyte-derived stem cells (ASCs)—are able to differentiate into various cell lineages4 and secreting soluble mediators with angiogenic, immunosuppressive, and antiinflammatory properties.5 Several preliminary studies have demonstrated that autologous free fat transfer may significantly enhance healing of diabetic foot ulcers,6 pressure sores,7 and radiotherapy scars.8
However, long-term fat graft retention is highly variable with resorption rates in the order of 25% to 80%.9–11 Fat necrosis and subsequent graft resorption is therefore a significant problem. Various technical factors related to fat preparation and administration have been implicated in graft failure.12–15 Similarly, adequate neovasculascularization appears to be an equally important prognostic factor in graft survival. Adipocytes poorly tolerate ischemic conditions,16 and early angiogenesis is essential for successful transplantation.17–19
Platelet-rich plasma (PRP) is an autologous source of concentrated platelets, growth factors, and cytokines used widely in regenerative medicine.20–24 Its therapeutic efficacy derives from the supraphysiological release of several growth factors and bioactive proteins from platelet α granules.17,25 These mediators—including platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor β, and insulin-like growth factor—have 2 primary effects: they promote the recruitment and activation of cells responsible for tissue repair, and they encourage new blood vessel formation.22
Independently, both PRP and fat transplantation seem to have a role in regenerative medicine; however, the addition of PRP to fat transplants may synergistically enhance graft survival by a number of mechanisms. First, the secretion of proangiogenic factors by PRP may improve early graft vascularization and minimize ischemic injury26,27; second, the release of antiinflammatory cytokines is thought to have a role in preventing graft degeneration27; and third, PRP-secreted factors have been shown to enhance the differentiation of preadipocytes into their mature form.28,29
The majority of human and animal studies suggest that PRP and fat cotransplantation increase graft survival rates. However, the rate of graft resorption varies markedly between studies and, in particular, between research groups. Furthermore, several human30–32 and animal33 studies report that the addition of PRP to fat transplants confers no survival advantage when compared with free fat transfer alone. To what extent these variable outcomes reflect differing methodological factors remains unknown.
For PRP alone, different study methodologies produce context-dependent results.34–36 PRP preparation techniques, composition, mechanism of activation, and outcome measurements vary significantly.23 This inconsistency is further confounded by poor reporting of key technical factors.24,37,38 Together, these issues limit the ability to draw overall conclusions about the efficacy of PRP in each setting and hinder its translation into clinical practice. Similarly, methodological heterogeneity in the harvesting and processing of fat transplants affects both experimental39–42 and clinical15 outcomes.
It is therefore reasonable to expect that methodological factors in PRP-enriched fat grafting will be important determinants of transplant survival rates. As in individual PRP and fat transfer studies, there is—as yet—no standardized transplantation protocol for their combined use.24,43,44 Preliminary evidence from both in vitro28,45 and animal studies46,47 supports the hypothesis that technical factors significantly influence PRP–fat cotransplantation outcomes.
This systematic review aims to synthesize and critically review the various methodological approaches to autologous PRP and fat cotransplantation in both human and animal studies. In particular, it seeks to assess to what extent technical factors in PRP and fat processing vary between comparable studies.
METHODS
The objective of this systematic review is to critically evaluate the literature on PRP-enriched fat grafting in accordance with PRISMA guidelines.48 It aims to provide a descriptive synthesis and critical appraisal of technical factors in autologous PRP and fat cotransplantation.
Search Methods
Bibliographic databases (Ovid MEDLINE, Scopus, and The Cochrane Library) were searched for relevant articles from inception to April 2017. Both “free-text terms” and “MeSH term” searches were run sequentially to capture all papers in which PRP was coadministered with fat. Variations on the following keywords were combined using the matrix of Boolean operators detailed in Supplemental Digital Content 1: “platelet-rich plasma,” “PRP,” “platelet concentrate(s),” “fat graft(s),” “fat transfer,” “fat injection(s),” “mixed,” “method(s),” “extraction,” “preparation,” “activated,” “human,” “animal,” “autologous.” English language studies from any country of origin were eligible for inclusion. (See appendix, Supplemental Digital Content 1, which displays the electronic database search matrix, http://links.lww.com/PRSGO/A622.)
After merging database search results and discarding duplicate entries, titles and abstracts were screened to eliminate studies unrelated to the current research objective. The remaining articles were read in full to assess their eligibility for qualitative synthesis. Additional articles were identified by routinely screening the reference lists of all included studies. All authors agreed upon the final list of included studies; any disagreement between authors was resolved by discussion and consensus.
Study Selection Criteria
A full set of inclusion and exclusion criteria were prespecified and agreed upon by all authors in advance. This study protocol was not eligible for registration on the PROSPERO database49 as it constitutes a review of methodological factors only.
All in vivo studies in which autologous PRP was mixed or coadministered with autologous fat grafts were eligible for inclusion. Both preclinical and clinical trials performed for any surgical or medical indication were included. All methods of fat grafting (such as lipofilling, contouring, and solid tissue transplant) were deemed eligible. No differentiation was made between pure PRP and leucocyte-rich PRP.50 All experimental study designs (irrespective of number of fat grafts or length of follow-up) were included.
In vitro studies only (without a concurrent in vivo human or animal model) were excluded. Studies in which PRP or fat grafting had been performed separately (ie, without mixing or coadministration) were excluded. Animal studies using a pooled isogenous source of PRP were ineligible for inclusion. Studies reporting solely on platelet-rich fibrin (even if added to fat grafts) were excluded. Similarly, those studies involving only secondarily extracted fat products (ie, the stromal vascular fraction or isolated ASCs) were discarded. Case reports, letters, editorials, book chapters, and reviews were also excluded.
During the shortlisting process, articles were screened for reporting of technical and methodological factors related to PRP and/or fat processing and coadministration. Articles with either a comprehensive or partial description of their methods were included; studies with no methods were excluded. One of the secondary aims of this study was to assess whether authors were reporting sufficient technical detail in their published manuscripts; therefore, no additional contact was made with authors to provide missing data.
Data Collection
All data were extracted by a single author (JL) and recorded on a predesigned bespoke electronic form before being independently verified by a second author (OJS).
Data collected were divided into the following subgroups:
baseline study data (title, author(s), year, journal, country, in vivo model)
experimental details (study design, number of fat graft procedures, length of follow-up)
PRP extraction (whole blood extraction methodology, PRP processing steps, PRP activation details, mean platelet counts, PRP storage)
fat extraction (method of fat harvest, donor-recipient site combination, fat processing methods, fat storage)
PRP–fat mixing (method and timing of PRP–fat mixing, use of prosurvival adjuncts).
No assumptions were made about the methods used if they were not explicitly reported. If authors referenced supplemental materials, appendices, or other articles in their methods, these were reviewed for relevant data extraction. Methodological factors not reported either directly or indirectly were listed as “unspecified.”
In studies with multiple different experimental protocols, data were extracted from those related to the current research objective only.
Quality Assessment and Statistical Analysis
All studies fulfilling the inclusion criteria were eligible for inclusion, and low-quality studies (eg, those performing poorly according to GRADE criteria51) were not discarded.
The purpose of this study was to provide a descriptive summary and critical review of variation in experimental technical factors—no attempt to account for risk of bias either in or between studies has been made.
Formal evaluation of experimental and clinical outcomes was beyond the scope of the current research objective; therefore, we have made no attempt to perform pooled statistical comparisons or meta-analyses. Owing to significant study design heterogeneity, such quantitative comparisons would be fundamentally misleading. Where appropriate, we have indicated which studies demonstrate improved graft retention rates with autologous PRP and fat cotransplantation and which do not.
RESULTS
Study Selection
The electronic database search strategy returned 335 results. After the elimination of 58 duplicates and the inclusion of 7 additional records, 286 titles and abstracts were screened against the predefined research objectives. At this stage, 203 articles were excluded; 83 papers were read in full to assess their eligibility for inclusion.
During this process, 60 manuscripts were eliminated for the following reasons: either PRP (n = 21) or fat (n = 10) was used in isolation; transplants were obtained from nonautologous sources (n = 8); reviews, letters, and abstracts (n = 15); no reporting of methods (n = 2); single case reports (n = 4); in vitro experimental studies only (n = 3), or the use of cultured ASCs only (n = 1).
In total, data were extracted from a final shortlist of 23 articles included in the qualitative synthesis. A comprehensive study attrition diagram is provided in Fig. 1.
Fig. 1.
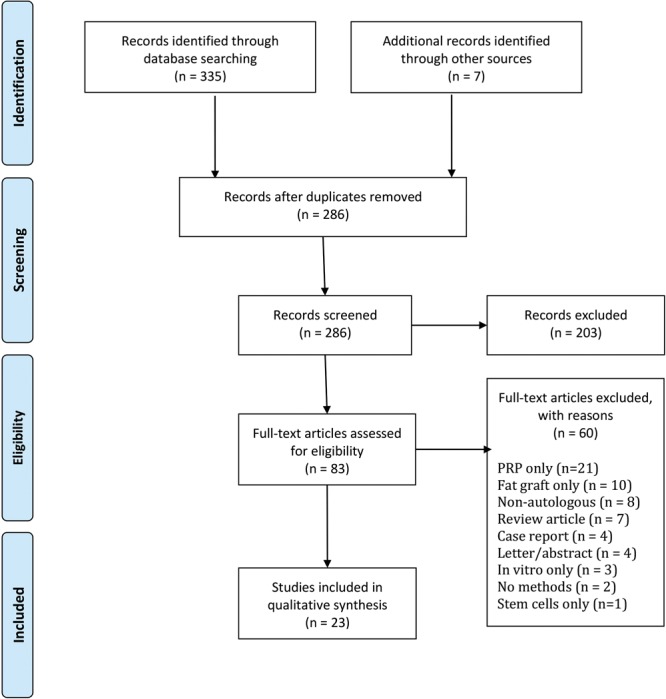
Study attrition chart.
Study Characteristics
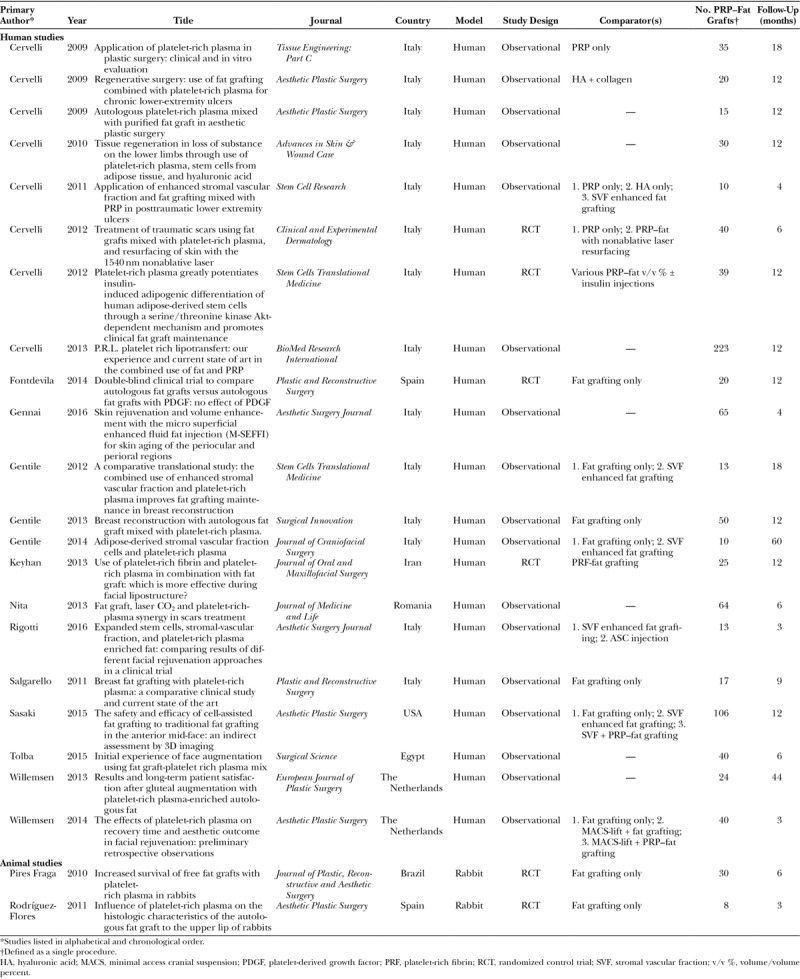
Of the 23 shortlisted studies, 21 were performed in humans and 2 in rabbits. The majority of human studies were presented as observational case series, although many included a comparator control arm of some description; only one52 was explicitly described as a case-control study. Four human studies used a randomized control trial design, as did both animal experiments.
All included studies were published since 2009. Studies performed in 4 different continents were included; 11 included articles were published by 2 interrelated Italian research groups.29,53–62
Data were extracted from only those experimental protocols where PRP was combined with fat for cotransplantation. The number of grafts fulfilling the inclusion criteria and length of follow-up are provided in Table 1.
Table 1.
Characteristics of Included Studies
PRP Extraction
Methods of PRP extraction varied significantly, particularly between research groups (Table 2). The majority of human studies employed a commercially available PRP extraction system—in these 17 studies, 5 different manufacturer devices were used, each with their own protocol and PRP product characteristics.35,36,63 Only 4 human trials (and both animal studies) did not use a commercial device to produce PRP.
Table 2.
PRP Extraction Methods
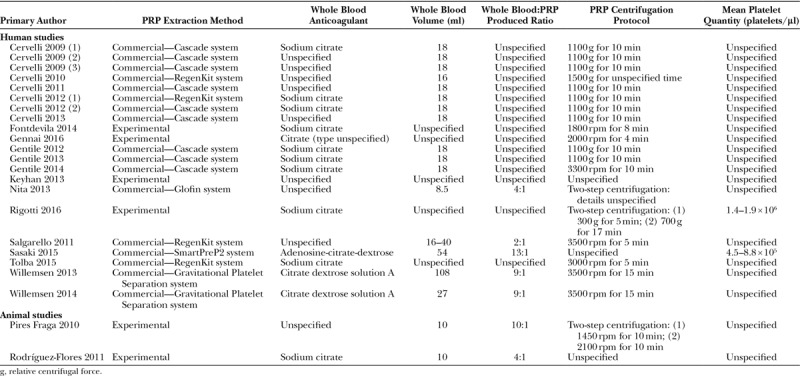
Most studies used a citrate-based anticoagulant to obtain whole blood; 9 studies did not report on their choice of anticoagulant. Where citrate was used, reporting on its strength and volume/volume (v/v) ratio to blood was inconsistent and often omitted (data not shown).
The volume of whole blood collected varied between studies and depended largely on the anticipated volume of PRP required. However, 16 studies did not specify what volume of PRP was produced for a given volume of blood; in the 9 studies where these data were reported, the ratio of whole blood to PRP varied from 2:131 to 13:1.52 Overall, only 4 studies52,64–66 provided comprehensive whole blood collection details.
Reporting of PRP centrifugation protocols was generally better, with only 5 studies providing inadequate methodological detail.52,54,64,67,68 Most studies used a single centrifugation spin, although the relative centrifugal forces used and the length of time doing so varied between different study designs. Interestingly, this was not necessarily a consequence of using different commercial PRP extraction systems—for example, 4 studies using a commercial RegenKit device employed different centrifugation parameters.31,54,56,69 Only 2 studies quantified mean PRP platelet concentrations32,69 before fat grafting; none of the other studies provided either absolute PRP concentrations or change from baseline data.
PRP Activation
Most studies included a PRP activation step (Table 3)—2 human studies did not use activated PRP52,70 and 2 studies did not report on whether or not activated PRP was used.57,69
Table 3.
PRP Activation Methods
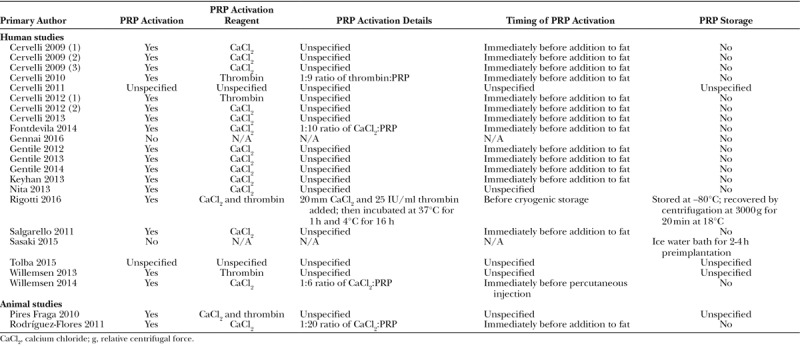
Studies using commercial devices typically followed manufacturer instructions for activation using calcium chloride, autologous thrombin, or both. However, of the 17 studies using commercial PRP systems, only 2 provided methodological detail within their published manuscript.54,66 Of the 5 studies using a bespoke extraction method in which activated PRP was used, 2 did not describe the steps involved.67,71
In general, authors added the activation reagent to PRP immediately before admixing with fat. Where PRP was injected into the area of fat grafting in vivo, activation was performed either immediately before percutaneous injection66 or not at all.52
The majority of studies extracted PRP at the point of use and did not store PRP in either its active or inactive form for any significant length of time. Where PRP was stored for a short period during fat harvest, it appears that this occurred at room temperature—only one author described maintaining PRP in an ice water bath before implantation.52
One study with an optimized experimental PRP extraction protocol activated PRP with calcium chloride and thrombin before cryogenic storage at −80°C.52 To complete the freeze-thaw cycle, the authors then recentrifuged the product at 18°C to achieve a platelet concentration of 1.4–1.9 × 106 platelets/μl.
Fat Preparation
Fat preparation methods were more uniform between studies (Table 4). All human studies used a version of the Coleman liposuction technique,72 excluding one70 in which fat was obtained using microsuperficial enhanced fluid fat injection. Abdominal fat harvest (either with or without additional donor sites) was common to all human studies excluding one66 where only the thigh was used. In the rabbit animal study using liposuction harvest, adipose tissue was obtained from the groin fat pad.64 With the exception of the other animal study—in which adipose tissue was harvested from the scapular region and grafted to the ear using an open surgical approach71—all studies transplanted fat and PRP via percutaneous injection.
Table 4.
Fat Preparation Methods
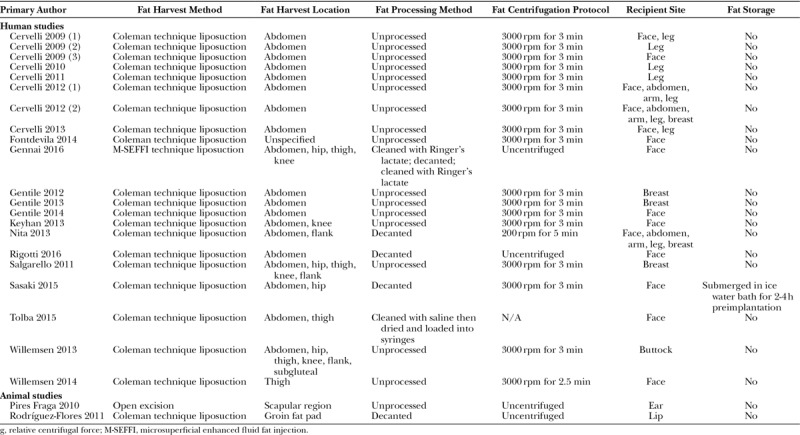
Several authors commented on a decantation step before fat centrifugation.32,52,64,68 Centrifugation was typically performed for 3 minutes at 3000 rpm. Only 2 studies did not include a fat centrifugation step—in both cases, the authors used either saline69 or Ringer’s lactate70 to clean the tissue before transplantation. Only one study52 described routine fat storage—in this case using an ice bath for between 2 and 4 hours to cool fat before implantation, although no rationale was provided for this decision.
Recipient sites varied according to the indication. Most study designs involved a single recipient site for fat grafting (eg, for gluteal augmentation65 or facial lipostructure32,52,67); other study designs were based around an indication for fat grafting, irrespective of target site (eg, for scar improvement56,68); 2 studies compiled data from unrelated patient series.59,60
PRP–Fat Grafting
Most studies admixed PRP and fat in sterile syringes immediately before cotransplantation. Three studies52,66,68 transplanted PRP and fat separately, injecting PRP into the fat compartment after lipofilling. One animal study71 soaked each 0.8 g adipose tissue fragment in 1 ml of activated PRP (for an unspecified length of time) before transplantation.
Relative PRP and fat volumes varied significantly between studies (Table 5). Several studies used a 1:1 ratio of PRP to fat,32,64,71 whereas others used as little as 0.1 ml of PRP per ml of fat.31,65–67 Many study designs used a range of PRP volumes for a given quantity of fat (eg, between 0.2 and 0.5 ml of PRP per ml of fat29,53,56,57,60–62). Only 3 of these studies provided a rationale for this: 2 were attempting to identify the optimal in vivo v/v concentration29,60 and 1 selected PRP volume according to the size of the recipient site.61
Table 5.
PRP and Fat Cotransplantation Methods
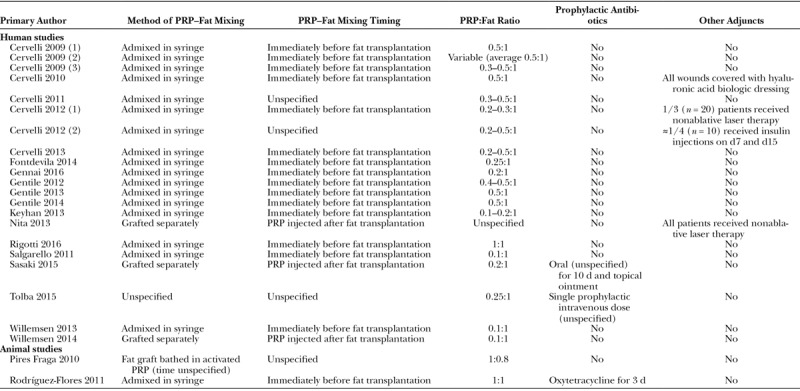
Three studies (2 in humans52,69 and 1 in rabbits64) routinely used prophylactic antibiotics. Several studies used additional regenerative adjuncts for either some or all of their grafts, including nonablative laser therapy,56,68 hyaluronic acid–medicated biologic dressings,54 and delayed percutaneous graft site insulin injections.29
DISCUSSION
Our article represents the first systematic review of methodological factors in autologous PRP and fat cotransplantation. It demonstrates that technical factors in graft preparation and administration vary significantly between in vivo studies. It also highlights that reporting of key procedural information is inconsistent and, in many cases, inadequate.
Evidence from both the fat12,14,73 and PRP35,36,63 literature suggests that methodological factors are critical determinants of experimental and clinical outcomes. It is therefore reasonable to assume that these procedural elements will be equally important in PRP and fat cotransplantation. Most studies included in this systematic review found that the addition of PRP to fat improves graft retention rates29,52–62,64–71; however, 3 articles30–32 report no such survival advantage. It is possible that this variability is at least partially explained by differing study methodologies.
The lack of consistent reporting of essential technical factors further compounds the interpretation of such outcome heterogeneity. Without a minimum description of the qualitative and quantitative characteristics of the PRP–fat transplant used, it is impossible to draw meaningful cross-comparisons between studies.23 The absence of sufficient experimental methodological detail or standardized preparation protocol may limit the translation of PRP-enhanced fat grafting into clinical practice.
For PRP alone, various authors have proposed descriptive classification taxonomies based upon either preparation methods23 or final product constituents.50 The absence of an accepted methodology for the preparation and activation of PRP reflects ongoing disagreement in the preclinical literature.44 Several experimental studies have attempted to optimize this process,25,74 but consensus has yet to be reached. The growing use of commercial devices—each with their own unique PRP product characteristics34,35,63,75—further compounds the issue.
Comparatively, the preparation of adipose tissue for structural fat grafting is less controversial, and most included studies employed a version of the Coleman liposuction technique.72 However, greater variability is seen in the v/v mixing ratio of PRP to fat, with up to 10-fold differences in substrate concentrations used. Again, this reflects a lack of consensus in the preclinical literature. Two studies found that a PRP volume fraction of 5% provided the optimal environment for ASC proliferation in vitro28,45 (with paradoxical inhibition of ASC proliferation at higher concentrations28). However, these findings have not been replicated elsewhere: 1 study found that 20% PRP v/v promoted maximal graft viability,47 whereas a different research group identified a dose-dependent effect for concentrations up to 50%.29 This inconsistency likely reflects intertrial heterogeneity of the PRP product used.47 It is also worth considering that, in most clinical scenarios, the most appropriate v/v concentration will be derived from the minimally effective ratio of PRP to fat. In the case of large volume fat transplantation, higher ratios (for example, like those approaching 1:1 in the 2 included animal studies64,71) would otherwise require the use of an unfeasibly large volume of whole blood.
This systematic review provides a comprehensive descriptive analysis of the current methodological approaches to in vivo autologous PRP and fat cotransplantation. It is based upon a robust, multiperson search strategy with additional article retrieval from reference lists, but it remains possible that potentially eligible studies were not identified. During the protocol stage, we designed a set of inclusion criteria to address our current research objective; studies using nonautologous and isogenous sources of PRP were excluded, as were trials using second-generation PRP products (such as platelet-rich fibrin). Similarly, experimental approaches using secondarily extracted fat components only (including the stromal vascular fraction and isolated ASCs) were deemed beyond the scope of this review. Finally, we have focused on critically reviewing methodological factors in PRP and fat cotransplantation but have not evaluated other areas that could influence experimental outcome (including outcome measure selection or indication for intervention).
CONCLUSIONS
Methodological factors in autologous PRP-enhanced fat cotransplantation vary significantly between in vivo studies. This is confounded by inconsistent and inadequate reporting of essential procedural information and combined PRP–fat transplant characteristics. The absence of sufficient methodological detail makes meaningful evaluation of the PRP-enhanced fat grafting literature difficult.
PRP and fat cotransplantation is an innovative and potentially transformative therapeutic strategy in regenerative medicine. However, a failure to address the fundamental issues identified in this review may limit its translation into clinical practice.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was kindly paid for by University College London as part of their open access policy.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28:111–119.. [PubMed] [Google Scholar]
- 2.Pu LLQ, Yoshimura K, Coleman SR. Fat grafting: current concept, clinical application, and regenerative potential, part 1. Clin Plast Surg. 2015;42(2):ix–x.. [DOI] [PubMed] [Google Scholar]
- 3.Pu LL, Yoshimura K, Coleman SR. Fat grafting: current concept, clinical application, and regenerative potential, part 2. Preface. Clin Plast Surg. 2015;42:xiii–xxiv.. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228.. [DOI] [PubMed] [Google Scholar]
- 5.Varghese J, Griffin M, Mosahebi A, et al. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stasch T, Hoehne J, Huynh T, et al. Débridement and autologous lipotransfer for chronic ulceration of the diabetic foot and lower limb improves wound healing. Plast Reconstr Surg. 2015;136:1357–1366.. [DOI] [PubMed] [Google Scholar]
- 7.Marangi GF, Pallara T, Cagli B, et al. Treatment of early-stage pressure ulcers by using autologous adipose tissue grafts. Plast Surg Int. 2014;2014:817283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez Santamaría J, Masiá Gridilla J, Pamias Romero J, et al. Fat grafting is a feasible technique for the sequelae of head and neck cancer treatment. J Craniomaxillofac Surg. 2017;45:93–98.. [DOI] [PubMed] [Google Scholar]
- 9.Sinna R, Delay E, Garson S, et al. Breast fat grafting (lipomodelling) after extended latissimus dorsi flap breast reconstruction: a preliminary report of 200 consecutive cases. J Plast Reconstr Aesthet Surg. 2010;63:1769–1777.. [DOI] [PubMed] [Google Scholar]
- 10.Missana MC, Laurent I, Barreau L, et al. Autologous fat transfer in reconstructive breast surgery: indications, technique and results. Eur J Surg Oncol. 2007;33:685–690.. [DOI] [PubMed] [Google Scholar]
- 11.Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29:360–376.. [DOI] [PubMed] [Google Scholar]
- 12.Bertossi D, Zancanaro C, Trevisiol L, et al. Lipofilling of the lips: ultrastructural evaluation by transmission electron microscopy of injected adipose tissue. Arch Facial Plast Surg. 2003;5:392–398.. [DOI] [PubMed] [Google Scholar]
- 13.Stallworth CL, Wang TD. Fat grafting of the midface. Facial Plast Surg. 2010;26:369–375.. [DOI] [PubMed] [Google Scholar]
- 14.Pu LLQ. Towards more rationalized approach to autologous fat grafting. J Plast Reconstr Aesthetic Surg. 2012;65:413–419.. [DOI] [PubMed] [Google Scholar]
- 15.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381.. [DOI] [PubMed] [Google Scholar]
- 16.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 1997;4:211–232.. [DOI] [PubMed] [Google Scholar]
- 17.Pallua N, Pulsfort AK, Suschek C, et al. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg. 2009;123:826–833.. [DOI] [PubMed] [Google Scholar]
- 18.Carpaneda CA, Ribeiro MT. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg. 1994;18:17–19.. [DOI] [PubMed] [Google Scholar]
- 19.Baran CN, Celebioğlu S, Sensöz O, et al. The behavior of fat grafts in recipient areas with enhanced vascularity. Plast Reconstr Surg. 2002;109:1646–1651.; 1652. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;2016:CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272.. [DOI] [PubMed] [Google Scholar]
- 22.Cohn CS, Lockhart E. Autologous platelet-rich plasma: evidence for clinical use. Curr Opin Hematol. 2015;22:527–532.. [DOI] [PubMed] [Google Scholar]
- 23.Frautschi RS, Hashem AM, Halasa B, et al. Current evidence for clinical efficacy of platelet rich plasma in aesthetic surgery: a systematic review. Aesthetic Surg J. 2016;37:sjw178. [DOI] [PubMed] [Google Scholar]
- 24.Sommeling CE, Heyneman A, Hoeksema H, et al. The use of platelet-rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2013;66:301–311.. [DOI] [PubMed] [Google Scholar]
- 25.Amable PR, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanton MW, Hadad I, Johnstone BH, et al. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg. 2009;123:56S–64S.. [DOI] [PubMed] [Google Scholar]
- 27.Seyhan N, Alhan D, Ural AU, et al. The effect of combined use of platelet-rich plasma and adipose-derived stem cells on fat graft survival. Ann Plast Surg. 2015;74:615–620.. [DOI] [PubMed] [Google Scholar]
- 28.Kakudo N, Minakata T, Mitsui T, et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–1360.. [DOI] [PubMed] [Google Scholar]
- 29.Cervelli V, Scioli MG, Gentile P, et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med. 2012;1:206–220.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontdevila J, Guisantes E, Martínez E, et al. Double-blind clinical trial to compare autologous fat grafts versus autologous fat grafts with PDGF: no effect of PDGF. Plast Reconstr Surg. 2014;134:219e–230e.. [DOI] [PubMed] [Google Scholar]
- 31.Salgarello M, Visconti G, Rusciani A. Breast fat grafting with platelet-rich plasma: a comparative clinical study and current state of the art. Plast Reconstr Surg. 2011;127:2176–2185.. [DOI] [PubMed] [Google Scholar]
- 32.Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, et al. Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36:261–270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Por YC, Yeow VK, Louri N, et al. Platelet-rich plasma has no effect on increasing free fat graft survival in the nude mouse. J Plast Reconstr Aesthet Surg. 2009;62:1030–1034.. [DOI] [PubMed] [Google Scholar]
- 34.Chandler WL. Microparticle counts in platelet-rich and platelet-free plasma, effect of centrifugation and sample-processing protocols. Blood Coagul Fibrinolysis 2013;24:125–132.. [DOI] [PubMed] [Google Scholar]
- 35.Everts PA, Brown Mahoney C, Hoffmann JJ, et al. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors 2006;24:165–171.. [DOI] [PubMed] [Google Scholar]
- 36.Leitner GC, Gruber R, Neumüller J, et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135–139.. [DOI] [PubMed] [Google Scholar]
- 37.Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 38.Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53–S59.. [DOI] [PubMed] [Google Scholar]
- 39.Hoareau L, Bencharif K, Girard AC, et al. Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiency. J Plast Reconstr Aesthet Surg. 2013;66:712–719.. [DOI] [PubMed] [Google Scholar]
- 40.Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010;126:1500–1505.. [DOI] [PubMed] [Google Scholar]
- 41.Minn KW, Min KH, Chang H, et al. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg. 2010;34:626–631.. [DOI] [PubMed] [Google Scholar]
- 42.Paik KJ, Zielins ER, Atashroo DA, et al. Studies in fat grafting: part V. Cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstr Surg. 2015;136:67–75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serra-Mestre JM, Serra-Renom JM, Martinez L, et al. Platelet-rich plasma mixed-fat grafting: a reasonable prosurvival strategy for fat grafts? Aesthetic Plast Surg. 2014;38:1041–1049.. [DOI] [PubMed] [Google Scholar]
- 44.Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20:267–276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao HT, James IB, Marra KG, et al. The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng Part A. 2015;21:2714–2722.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K, Li F, Li J, et al. Increased survival of human free fat grafts with varying densities of human adipose-derived stem cells and platelet-rich plasma. J Tissue Eng Regen Med. 2017;11:209–219.. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Guo W, Li K, et al. Improved fat graft survival by different volume fractions of platelet-rich plasma and adipose-derived stem cells. Aesthet Surg J. 2015;35:319–333.. [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;7:1–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158–167.. [DOI] [PubMed] [Google Scholar]
- 51.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki GH. The safety and efficacy of cell-assisted fat grafting to traditional fat grafting in the anterior mid-face: an indirect assessment by 3D imaging. Aesthetic Plast Surg. 2015;39:833–846.. [DOI] [PubMed] [Google Scholar]
- 53.Gentile P, Orlandi A, Scioli MG, et al. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1:341–351.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cervelli V, De Angelis B, Lucarini L, et al. Tissue regeneration in loss of substance on the lower limbs through use of platelet-rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care 2010;23:262–272.. [DOI] [PubMed] [Google Scholar]
- 55.Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg. 2014;25:267–272.. [DOI] [PubMed] [Google Scholar]
- 56.Cervelli V, Nicoli F, Spallone D, et al. Treatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol. 2012;37:55–61.. [DOI] [PubMed] [Google Scholar]
- 57.Cervelli V, Gentile P, De Angelis B, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res. 2011;6:103–111.. [DOI] [PubMed] [Google Scholar]
- 58.Gentile P, Di Pasquali C, Bocchini I, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov. 2013;20:370–376.. [DOI] [PubMed] [Google Scholar]
- 59.Cervelli V, Gentile P, Scioli MG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625–634.. [DOI] [PubMed] [Google Scholar]
- 60.Cervelli V, Bocchini I, Di Pasquali C, et al. P.R.L. platelet rich lipotransfert: our experience and current state of art in the combined use of fat and PRP. Biomed Res Int. 2013;2013:434191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cervelli V, Gentile P, Grimaldi M. Regenerative surgery: use of fat grafting combined with platelet-rich plasma for chronic lower-extremity ulcers. Aesthetic Plast Surg. 2009;33:340–345.. [DOI] [PubMed] [Google Scholar]
- 62.Cervelli V, Palla L, Pascali M, et al. Autologous platelet-rich plasma mixed with purified fat graft in aesthetic plastic surgery. Aesthetic Plast Surg. 2009;33:716–721.. [DOI] [PubMed] [Google Scholar]
- 63.Castillo TN, Pouliot MA, Kim HJ, et al. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271.. [DOI] [PubMed] [Google Scholar]
- 64.Rodríguez-Flores J, Palomar-Gallego MA, Enguita-Valls AB, et al. Influence of platelet-rich plasma on the histologic characteristics of the autologous fat graft to the upper lip of rabbits. Aesthetic Plast Surg. 2011;35:480–486.. [DOI] [PubMed] [Google Scholar]
- 65.Willemsen JC, Lindenblatt N, Stevens HP. Results and long-term patient satisfaction after gluteal augmentation with platelet-rich plasma-enriched autologous fat. Eur J Plast Surg. 2013;36:777–782.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willemsen JC, van der Lei B, Vermeulen KM, et al. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014;38:1057–1063.. [DOI] [PubMed] [Google Scholar]
- 67.Keyhan SO, Hemmat S, Badri AA, et al. Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: which is more effective during facial lipostructure? J Oral Maxillofac Surg. 2013;71:610–621.. [DOI] [PubMed] [Google Scholar]
- 68.Nita AC, Orzan OA, Filipescu M, et al. Fat graft, laser CO2 and platelet-rich-plasma synergy in scars treatment. J Med Life 2013;6:430–433.. [PMC free article] [PubMed] [Google Scholar]
- 69.Tolba AM, Nasr M. Initial experience of face augmentation using fat graft-platelet rich plasma mix. Surg Sci. 2015;6:489–498.. [Google Scholar]
- 70.Gennai A, Zambelli A, Repaci E, et al. Skin rejuvenation and volume enhancement with the micro superficial enhanced fluid fat injection (M-SEFFI) for skin aging of the periocular and perioral regions. Aesthetic Surg J. 2016;37:14–23.. [DOI] [PubMed] [Google Scholar]
- 71.Pires Fraga MF, Nishio RT, Ishikawa RS, et al. Increased survival of free fat grafts with platelet-rich plasma in rabbits. J Plast Reconstr Aesthet Surg. 2010;63:e818–e822.. [DOI] [PubMed] [Google Scholar]
- 72.Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33:567–577.. [DOI] [PubMed] [Google Scholar]
- 73.Condé-Green A, Wu I, Graham I, et al. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: a pilot study. Aesthet Surg J. 2013;33:713–721.. [DOI] [PubMed] [Google Scholar]
- 74.Franco D, Franco T, Schettino AM, et al. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012;36:1254–1259.. [DOI] [PubMed] [Google Scholar]
- 75.Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–316.. [DOI] [PubMed] [Google Scholar]


