Significance
The cellular stress response and circadian clock system are fundamental functions in homeostatic regulation in almost all organisms. However, whether these two mechanisms are interlocked with each other, and the key molecule that links cellular stress and the circadian clock, remain unclear. Here we identify ASK family kinases that are essential for the circadian clock to respond to cellular stress, and report that Ask1 transcription is rhythmically controlled by the circadian clock. Moreover, LC-MS/MS–based proteomic analysis provides insight into a molecular mechanism in which dephosphorylation-triggered changes to the ASK complex mediate cellular stress to the circadian clock. From the perspective of cell signaling, our present findings expand previously reported roles of stress signaling toward regulation of the circadian clock.
Keywords: circadian, cellular stress, redox, apoptosis signal-regulating kinase, phosphorylation
Abstract
Daily rhythms of behaviors and physiologies are generated by the circadian clock, which is composed of clock genes and the encoded proteins forming transcriptional/translational feedback loops (TTFLs). The circadian clock is a self-sustained oscillator and flexibly responds to various time cues to synchronize with environmental 24-h cycles. However, the key molecule that transmits cellular stress to the circadian clockwork is unknown. Here we identified apoptosis signal-regulating kinase (ASK), a member of the MAPKKK family, as an essential mediator determining the circadian period and phase of cultured cells in response to osmotic changes of the medium. The physiological impact of ASK signaling was demonstrated by a response of the clock to changes in intracellular redox states. Intriguingly, the TTFLs drive rhythmic expression of Ask genes, indicating ASK-mediated association of the TTFLs with intracellular redox. In behavioral analysis, Ask1, Ask2, and Ask3 triple-KO mice exhibited compromised light responses of the circadian period and phase in their activity rhythms. LC-MS/MS–based proteomic analysis identified a series of ASK-dependent and osmotic stress-responsive phosphorylations of proteins, among which CLOCK, a key component of the molecular clockwork, was phosphorylated at Thr843 or Ser845 in the carboxyl-terminal region. These findings reveal the ASK-dependent stress response as an underlying mechanism of circadian clock flexibility.
Circadian rhythms of gene expression and physiological functions are generated by a cell-autonomous time-measuring system termed the circadian clock, which is driven by transcriptional/translational feedback loops (TTFLs) composed of a subset of clock genes (1, 2). The circadian clock is robust in oscillation under constant conditions, but can respond flexibly to a variety of signals and adjust its period and phase to environmental 24-h cycles (2–4). It is well known that a series of protein kinases are required for the two defining properties of the circadian clock, i.e., robustness and flexibility (3, 5, 6). In mammals, a central clock resides in the hypothalamic suprachiasmatic nucleus (SCN) and is synchronized to 24-h light–dark cycles (2, 3). In contrast, peripheral clocks are distributed among most of the peripheral tissues and even in cultured cells, and are controlled by various nonphotic stimuli (7–10). These stimuli potentially evoke a cellular stress response through protein kinase signaling (11). The cellular stress response and the circadian clock system are the most fundamental functions conserved in almost all organisms for adaptation to changes in environmental conditions. However, whether the circadian clock responds to cellular stress through protein kinase signaling and, if so, the key molecule(s) transmitting the cellular stress to the circadian clockwork, remain elusive.
Results
Apoptosis Signal-Regulating Kinases Are Responsible for Osmolarity-Dependent Period Change.
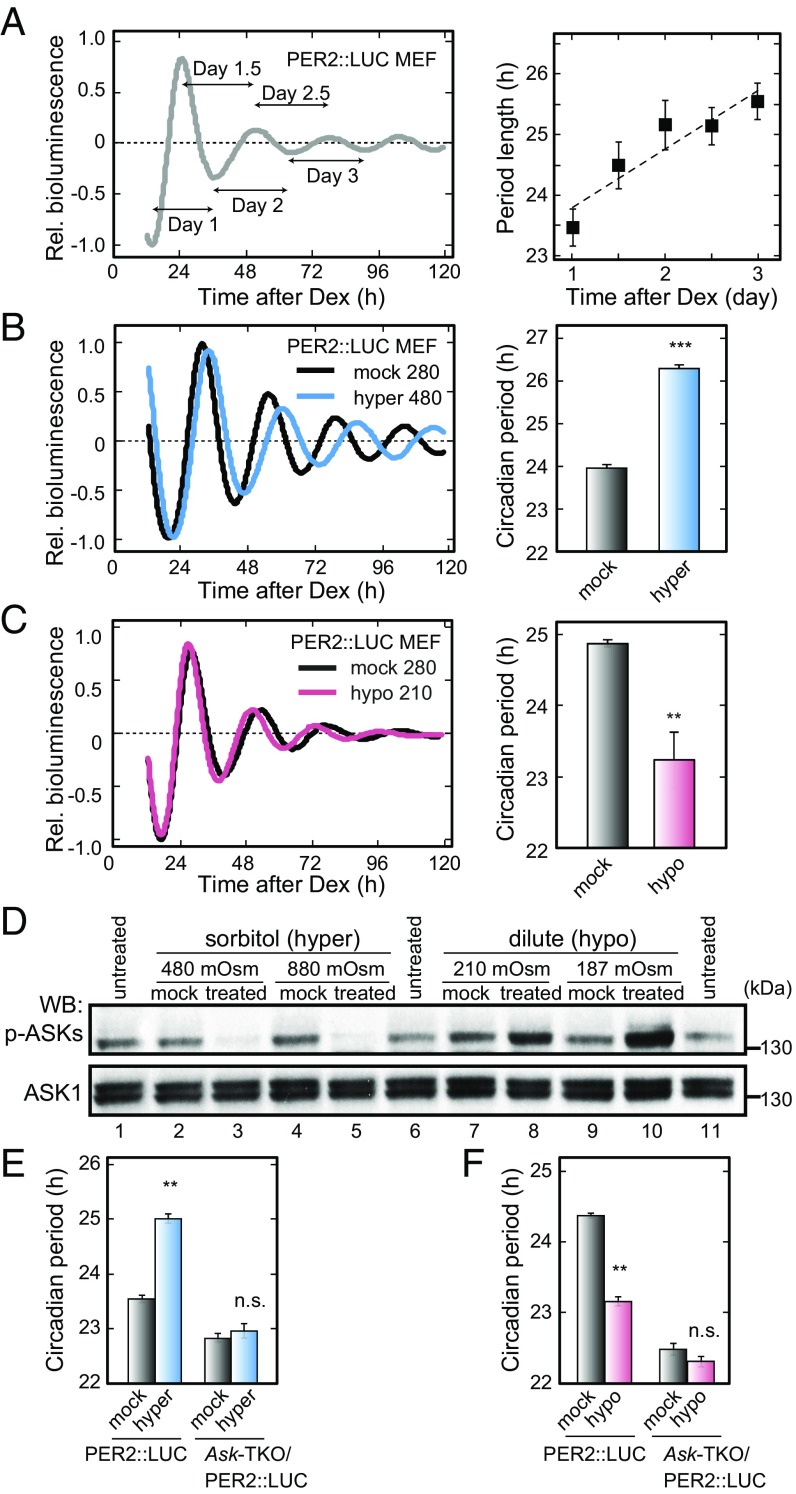
We first noticed that the circadian period of cellular rhythms in culture is lengthened day by day not only in PER2::LUC mouse embryonic fibroblasts (MEFs) (Fig. 1A) but also in NIH 3T3 and U2OS cells (Fig. S1 A and B) after synchronization with dexamethasone (Dex) pulse treatment. When the media were collected at day 7 and reused for monitoring the circadian rhythms of freshly plated PER2::LUC MEFs (Fig. S1C), the period was significantly longer than that recorded in the fresh media (25.13 ± 0.09 h vs. 23.91 ± 0.06 h) (Fig. S1D). This period-lengthening effect of the reused media was blunted when the reused media were diluted with distilled water to isotonicity (Fig. S1 C and D), suggesting that the osmolarity of the culture media determines the cellular circadian period. Indeed, the circadian period was markedly lengthened when the cells were chronically exposed to hypertonic media by adding final concentrations of 200 mM sorbitol (Hyper 480; Fig. 1B and Fig. S1 E and F) or 100 mM NaCl (Fig. S1G) to the fresh media (isotonic, approximately 280 mOsm). In contrast, the period was shortened when the cells were exposed to hypotonic media (Hypo 210; Fig. 1C and Fig. S1H). Taken together, these results indicate that the oscillation speed of the cellular clock is regulated bidirectionally by the increases and decreases in extracellular osmolarity that evoke cellular stress responses (12–14).
Fig. 1.
ASK family members are essential for the osmotic stress response of the circadian period of cellular rhythms. (A) The change in the circadian period length in PER2::LUC MEFs. The period length was calculated (n = 22) as a period between the trough (or peak) of the day and the next trough (or peak) as shown at the left. (B, C, E, and F) The effects of chronic hypertonic (B and E; final 200 mM sorbitol to approximately 480 mOsm) and chronic hypotonic (C and F; diluted by distilled water to approximately 210 mOsm) treatment on the circadian period of the cellular rhythms in PER2::LUC MEFs (B and C) and Ask-TKO/PER2::LUC MEFs (E and F). Data are reported as mean ± SEM. n = 4 (B and C) or n = 3 (E and F). Student’s t test, **P < 0.01; ***P < 0.001; not significant (n.s.), P ≥ 0.05 vs. mock treatment. (D) Immunoblot analysis of the phosphorylation state of ASKs in response to the hyperosmotic (final 200 or 600 mM sorbitol to approximately 480 or 880 mOsm) and hypoosmotic (diluted by distilled water to approximately 210 or 187 mOsm) stress. HEK293A cells were collected 30 min after treatment with the indicated stimuli.
In many cases, cellular stress activates a variety of mitogen-activated protein kinase kinase kinases (MAPKKKs), which trigger the MAPK cascade conserved from yeast to humans (15). Among the MAPKKKs, apoptosis signal-regulating kinases (ASKs) ASK1, ASK2, and ASK3 play pivotal roles in response to various stress stimuli, such as oxidative stress and ultraviolet radiation (16). Notably, phosphorylation levels of cellular ASKs are increased in hypotonic media and decreased in hypertonic media (Fig. 1D), while their phosphorylation in the activation loop causes phosphorylation signaling of the downstream MAPK pathway (17).
We hypothesized that ASKs are key players in the bidirectional clock responses to the osmolarity changes. To test this hypothesis, triple-KO (TKO) mice deficient for Ask1 (18), Ask2 (19), and Ask3 (17) were generated (Ask-TKO) and further crossed with PER2::LUC knockin mice (20) to prepare MEFs. The circadian period of the PER2::LUC rhythms in control MEFs was gradually lengthened during the culture, whereas the period of Ask-TKO MEFs was kept mostly constant during the long culture time (Fig. S2 A–C). The period changes by the chronic hypertonic (200 mM sorbitol) or hypotonic treatment were also blunted in the Ask-TKO MEFs (Fig. 1 E and F and Fig. S2 D and E). These results demonstrate that ASKs are indispensable for the osmotic stress response of the oscillation speed of the cellular clock.
ASKs Are Essential for the Osmotic Stress-Dependent Circadian Phase Shift.
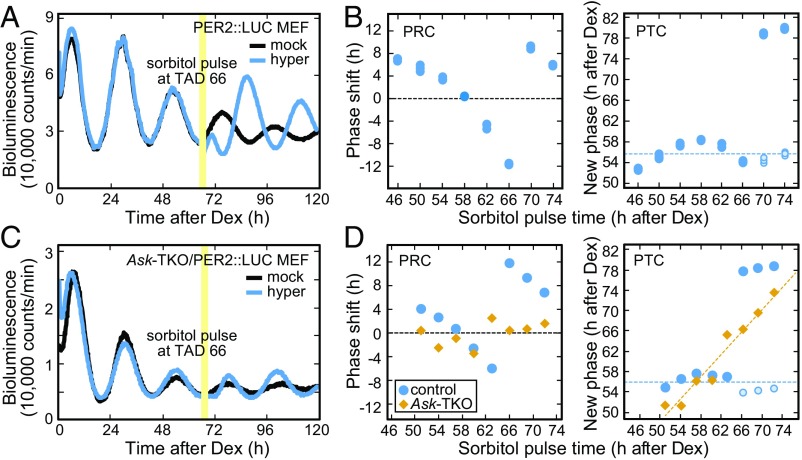
We found that osmotic stress regulates not only the circadian period, but also the circadian phase as a time cue in a manner similar to photic stimuli (21–23). As illustrated in Fig. S3A, when the MEFs were exposed to a 30-min sorbitol pulse (hyperosmotic stress) at 66 h after the Dex synchronization [time after Dex (TAD), 66 h], the phase of the cellular rhythms was markedly shifted compared with that after the mock treatment (Fig. 2A). The phase response curve (PRC) and phase transition curve (PTC) after the hypertonic pulse treatment demonstrated type 0 resetting, which is characterized by phase shifts of the oscillator to a common new phase after the sorbitol pulse given at various time of the day (Fig. 2B). Remarkably, no significant phase shift was observed in the Ask-TKO MEFs when they received the hypertonic pulse at any time of day (Fig. 2 C and D), while the Dex pulse synchronized the mutant MEFs in a manner indistinguishable from the control PER2::LUC MEFs (Fig. S3B). These results reveal that hyperosmotic stress resets the cellular clock through ASK signaling, which is dispensable for Dex-induced resetting.
Fig. 2.
Acute changes of osmolarity reset the circadian phase of the cellular clock through ASK signaling. The effect of a 30-min hypertonic pulse (final 600 mM sorbitol to approximately 880 mOsm; Fig. S3A) on the circadian phase of the cellular rhythms in PER2::LUC MEFs (A and B) and Ask-TKO/PER2::LUC MEFs (C and D). (B and D) PRCs and PTCs for the rhythms of PER2::LUC MEFs and PER2::LUC/Ask-TKO MEFs in response to the hyperosmotic pulses; n = 3 (B) or n = 1 (D). The pale-blue circles in the PTC panels indicate the extrapolated new phases by subtracting intrinsic periods from the actual data.
Molecularly, we found rapid induction of Dec1, Dec2, and E4bp4 mRNA levels within 30 min after the 30-min sorbitol pulse treatment (Fig. S4A). The induction of these clock(-related) genes was abrogated by treatment with a transcription inhibitor, actinomycin D, but not by a protein synthesis inhibitor, cycloheximide, indicating immediate early responses of Dec1, Dec2, and E4bp4 to the hyperosmotic stress (Fig. S4B). The hyperosmolarity-induced transcriptions of the three genes were blunted in the Ask-TKO MEFs (Fig. S4C), suggesting that the immediate early responses of these genes may be involved in ASK-dependent clock resetting.
Ask1 and Ask2 Expression Is Rhythmically Controlled by the Circadian Clock.
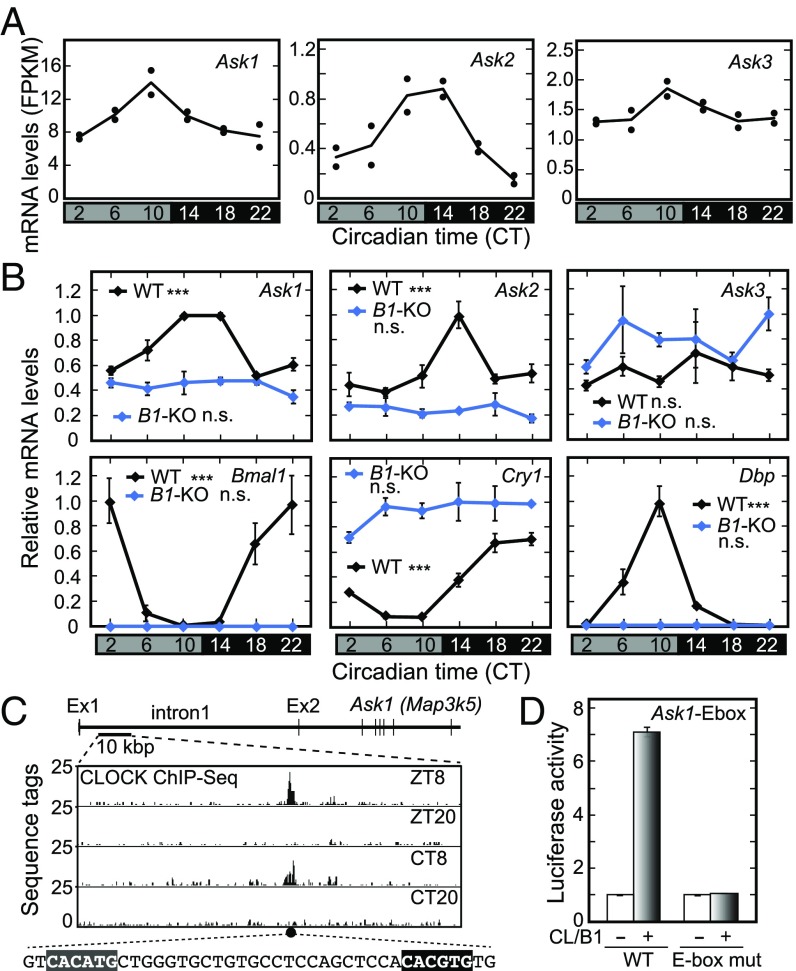
Our previously reported RNA-Seq data revealed potential circadian changes of Ask1 and Ask2 mRNAs in the mouse liver (24) (Fig. 3A). Here we performed quantitative RT-PCR (qRT-PCR) analysis and found circadian rhythms of Ask1 and Ask2 expression in the liver of mice reared in the constant dark (DD) condition (Fig. 3B). Ask1 and Ask2 transcripts were kept at low levels throughout the day in Bmal1-KO livers, in which expression of clock-controlled genes, such as Bmal1, Cry1, and Dbp, became arrhythmic (Fig. 3B). Intriguingly, our CLOCK ChIP-Seq data in the mouse livers (25) showed rhythmic binding of CLOCK to the first intron region of Ask1 (Map3k5) loci, where we found canonical (CACGTG) and noncanonical (CACATG) E-box sequences (25) in the CLOCK-binding peak region (Fig. 3C). In a promoter analysis using a luciferase reporter, CLOCK and BMAL1 activated transcription of Ask1 from the first intronic region, and introduction of mutations in the E-box sequences caused marked reduction of transcriptional activation (Fig. 3D). Collectively, these data indicate that ASK signaling regulates the TTFLs (Figs. 1 and 2) and, vice versa, Ask (Ask1) expression is controlled by the TTFLs.
Fig. 3.
E-box–dependent circadian expression of Ask family members. (A) Temporal profiles of mRNA levels of Ask1, Ask2, and Ask3 in our previous RNA-Seq analysis of the mouse liver (24). (B) Temporal expression profiles of Asks and typical clock genes in the liver, determined by RT-PCR analysis in WT (black) and Bmal1-KO (blue) mice. n = 3. One-way ANOVA, ***P < 0.001; not significant (n.s.), P ≥ 0.05. (C) CLOCK ChIP-Seq data at the Ask1 locus from our previous study (25). Canonical (black) and noncanonical E-box sequences (gray) near the CLOCK-binding peak region are highlighted. CT, circadian time; ZT, zeitgeber time. (D) CLOCK/BMAL1 (CL/B1)-dependent transactivation through the E-box sequences in the Ask1 intron 1, determined by a dual luciferase reporter assay in HEK293T17 cells. Reporter vectors including WT (CACATG and CACGTG) or E-box mutation (ACCAGT and ACCGGT) sequences were used. Data are mean ± SEM. n = 3.
Physiological Roles of ASK Signaling in Clock Input.
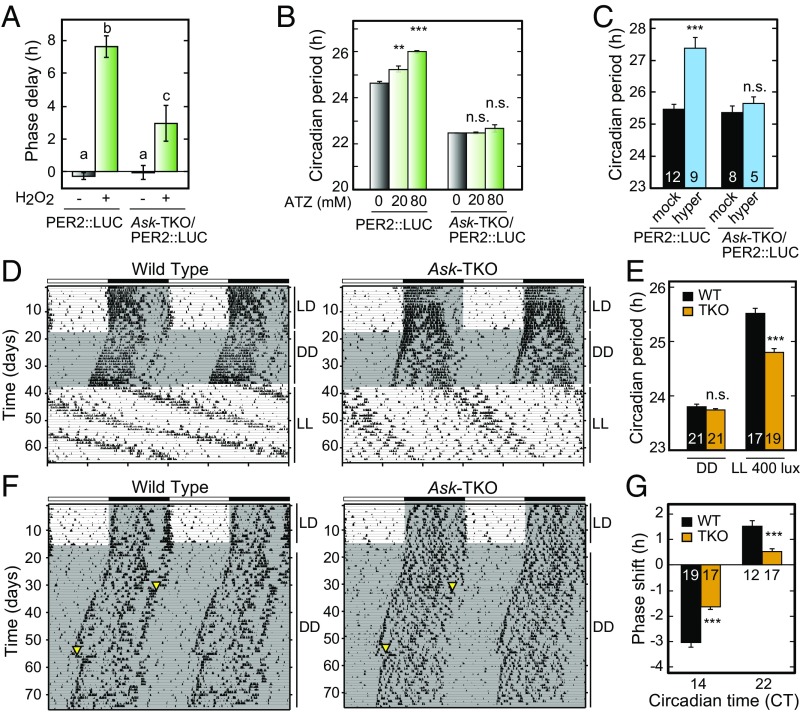
ASKs are phosphorylated in response to reactive oxygen species (ROS) (16), and we verified that ASKs are phosphorylated by H2O2 treatment (Fig. S5A). A 30-min pulse treatment of PER2::LUC MEFs with 0.4 mM H2O2 at TAD 66 h caused a remarkable phase shift of cellular rhythms, and the phase shift was significantly attenuated in the Ask-TKO MEFs (Fig. 4A and Fig. S5B). Then PER2::LUC MEFs were exposed to an intrinsic oxidative stress by treatment with 3-amino-1, 2, 4-aminotriazole (ATZ), a cell-permeable inhibitor of catalase that is a key enzyme catalyzing the conversion of H2O2 to water and oxygen. We found that the circadian period of the cellular rhythm was lengthened by the chronic ATZ treatment, and the period-lengthening effect was significantly attenuated in Ask-TKO MEFs (Fig. 4B and Fig. S5C). Taken together, these results indicate that the intracellular redox state provides input to the TTFLs through ASK signaling (Fig. S5D).
Fig. 4.
In vivo roles of ASK signaling in regulating circadian phase and period. (A) The effect of a 30-min H2O2 (final concentration 0.4 mM) pulse at TAD 66 h on the circadian phase of the cellular rhythms in PER2::LUC and Ask-TKO/PER2::LUC MEFs. Data are mean ± SEM. n = 3. Tukey’s test, P < 0.01. (B) The effect of chronic ATZ treatment on the circadian period of the cellular rhythms in PER2::LUC and Ask-TKO/PER2::LUC MEFs. Data are mean ± SEM. n = 4. Student’s t test, **P < 0.01; ***P < 0.001; not significant (n.s.), P ≥ 0.05 vs. mock treatment. (C) The effect of chronic hypertonic treatment (final 200 mM sorbitol to approximately 480 mOsm) on the circadian period in 200-µm-thick SCN sections from PER2::LUC and Ask-TKO/PER2::LUC mice. The numbers in the bars indicate the sample sizes. Data are mean ± SEM. Student’s t test, **P < 0.01 vs. mock treatment. (D and F) Double-plotted wheel-running activity rhythms of representative WT (Left) and Ask-TKO mice (Right). (E) Circadian free-running periods of the behavioral rhythms under constant dark (DD) and constant light (LL; approximately 400 lux) conditions in WT and Ask-TKO mice. (G) Phase responses to brief light pulses (approximately 400 lux for 30 min; yellow triangles in F) at CT14 and CT22. Phase-advance and phase-delay shifts are expressed in positive and negative values, respectively. (E and G) The numbers in the bars indicate the sample sizes. Data are mean ± SEM. Student’s t test, ***P < 0.001; n.s., P ≥ 0.05 vs. WT.
These effects of stress signals on the circadian period and phase of the cellular clock are reminiscent of photic input to the central clock in the SCN (22, 23). Physiological relevance of the cellular stress to the photic regulation of the clock system has been implicated by pioneering studies on melatonin secretion rhythms in cultured chick pineal cells, which were phase-shifted by hypertonic and hypotonic stimuli in a manner very similar to the effects of a light pulse and a dark pulse, respectively (26, 27). We asked whether the SCN clock is also sensitive to the osmotic stress, and if so, whether ASK signaling is involved in the photic input to the SCN in vivo. We found lengthening of the circadian period of the PER2::LUC SCN slice on chronic exposure to the hypertonic media containing 200 mM sorbitol (Fig. 4C and Fig. S6A), with the period-lengthening effect markedly attenuated in the Ask-TKO SCN (Fig. 4C and Fig. S6A). To evaluate the in vivo roles of ASK signaling, we monitored wheel running rhythms of Ask-TKO mice, and found that the mutant mice exhibited robust daily behavioral rhythms under the 12-h light/12-h dark (LD) condition (Fig. 4D and Fig. S6B). Ask deficiency had no significant effect on temporal profiles of the clock(-related) genes at protein (Fig. S6C) and mRNA (Fig. S6D) levels in the mouse liver under the LD condition. When transferred to DD, Ask-TKO mice showed a stable period of the wheel running rhythms indistinguishable from that of WT (Fig. 4 D and E). Striking phenotypes of Ask deficiency were identified under a constant light (LL) condition, in which the period of the wheel running rhythms of WT mice became longer than that in DD (Fig. 4 D and E; 23.80 ± 0.05 h in DD and 25.51 ± 0.10 h in 400-lux LL), depending on the light intensity of LL (Fig. S6E). This property is recognized as Aschoff’s rule: the higher the light intensity in LL, the longer the circadian period in nocturnal animals (21–23, 28).
Intriguingly, this period-lengthening effect of LL was significantly reduced in the Ask-TKO mice, which showed a shorter period compared with WT mice under LL (Fig. 4 D and E and Fig. S6E). The circadian period of Ask1 single KO in LL was significantly shorter than that in WT mice but longer than that in Ask-TKO mice, suggesting that each ASK member contributes cooperatively to circadian behavioral rhythms (Fig. S6F). In phase-response analysis of the behavioral rhythms by a 30-min light pulse, we found significant effects of Ask deficiency on both the phase delay (WT: −3.03 ± 0.21 h, KO: −1.64 ± 0.10 h) by the light at circadian time (CT) 14 and the phase advance (WT: 1.53 ± 0.22 h, KO: 0.54 ± 0.11 h) at CT22 (Fig. 4 F and G). These results demonstrate that ASKs play pivotal roles not only in the cultured cells, but also in the central clock in the SCN for regulation of the light-regulated circadian period and phase.
Molecular Mechanism for ASK-Dependent Clock Input.
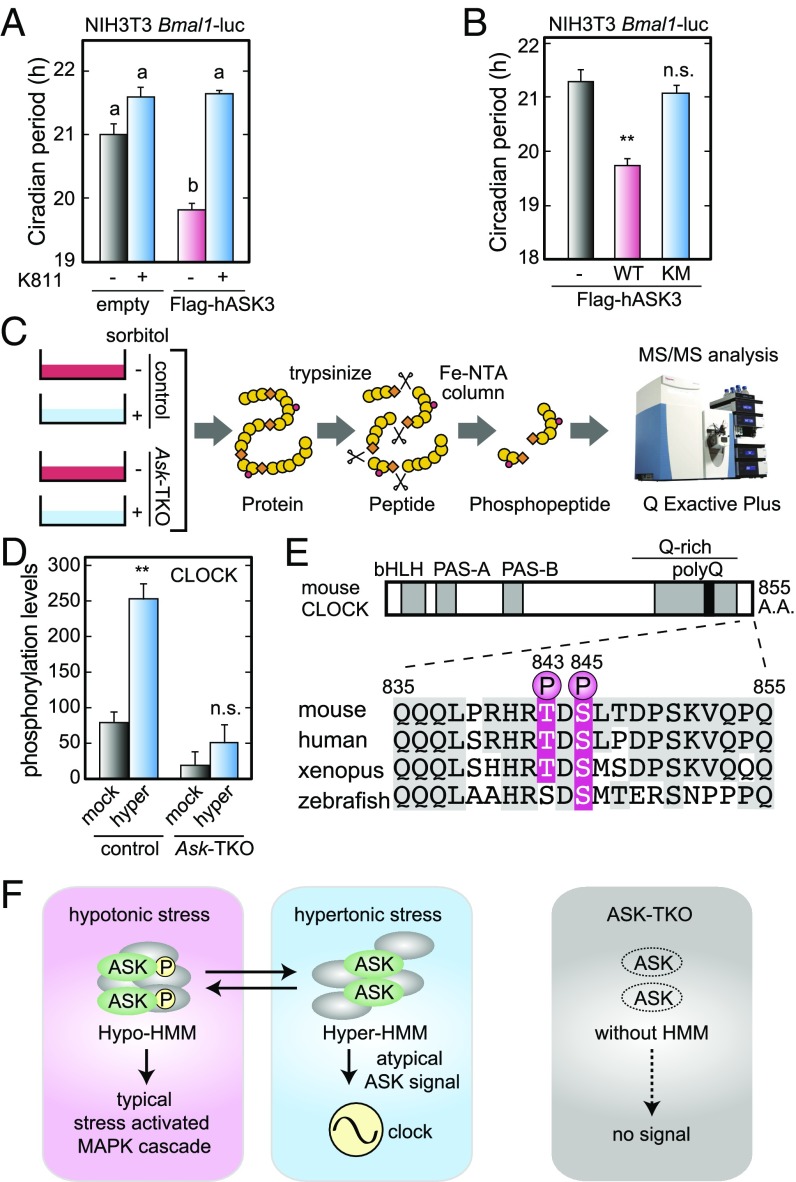
To understand the molecular mechanisms of how ASKs mediate the clock input, we investigated correlations between the kinase activities of ASKs and the cellular circadian period. The circadian period in NIH 3T3 cells was markedly shortened by overexpression of ASK3 (Fig. 5A), which is autophosphorylated in the transfected cells (17). Importantly, the period-shortening effect was significantly suppressed by chronic treatment of an ASK inhibitor, K811 (29) (Fig. 5A), and overexpression of ASK3 KM, a kinase-dead mutant (17), had no obvious effect on the circadian period (Fig. 5B). These results demonstrate that the kinase activity of ASK3 is important in determining the cellular circadian period. In the MAPK cascade, c-Jun N-terminal kinase (JNK) and p38 are widely known as major stress-activated kinases (17, 30, 31). In our experimental condition, however, hypertonic treatment resulted in the dephosphorylation of endogenous ASKs (Fig. 1D) and phosphorylation (activation) of endogenous JNK and p38 (Fig. S7 A and B) (14, 17). Furthermore, a JNK inhibitor, SP600125, and/or a p38 inhibitor, SB203580, had no obvious effect on the hyperosmolarity-induced resetting (Fig. S7 C–E), indicating atypical signaling downstream of ASKs as the clock input. In addition, the hyperosmotic stress caused ASK dephosphorylation and input to the clock, whereas the clock input was abolished by Ask deficiency (Figs. 1 and 2). Based on the apparent inconsistency, we now propose a model in which ASK dephosphorylation triggers atypical ASK signaling in terms of clock input (Fig. 5F). This model is based on the previous observation that ASK1 forms a high molecular mass (HMM) complex (>1,500 kDa) including ASK1 regulatory proteins, which are changed in response to the phosphorylation levels of ASK1 (32).
Fig. 5.
The molecular mechanisms of how ASKs mediate the stress signal to the circadian clock. (A and B) The effect of ASK3 overexpression and ASK inhibitor K811 on the circadian period of the cellular rhythms in NIH 3T3 cells. NIH 3T3 cells were transiently transfected with a Bmal1-luc reporter with Flag-ASK3 (WT) or Flag-ASK3 (KM) expression vectors. After synchronization with Dex, bioluminescence rhythms of the transfected cells in the presence or absence of K811 (5 μM) were measured. Data are mean ± SEM. (A) n = 3. Tukey’s test, P < 0.05. (B) n = 4. Student’s t test, **P < 0.01; not significant (n.s.), P ≥ 0.05 vs. empty. (C) The experimental procedures for the LC-MS/MS–based proteomic analysis. ASK-TKO (or control) MEFs were harvested at 15 min after a 30-min pulse treatment with 600 mM sorbitol (or mock) before the LC-MS/MS analysis. (D) ASK-dependent phosphorylation of CLOCK protein at Thr843 or Ser845 in the carboxyl-terminal region in response to hyperosmotic stress. Data are mean ± SEM. n = 3. Student’s t test, **P < 0.01; n.s., P ≥ 0.05 vs. mock. (E) Comparison of amino acid sequences in the carboxyl-terminal region of CLOCK among four animal species. Identical amino acid residues to those of mouse CLOCK are highlighted by gray backgrounds. (F) A model for ASK signaling in the clock input. In this model, hyperosmotic stress-induced dephosphorylation of ASKs triggers the stress signaling to the circadian clockwork by forming a hyper-HMM complex. In ASK-TKO cells (Right), both the formation of HMM complex and the MAPK cascade with the downstream signaling are completely lost.
To reinforce this model, we searched for ASK-dependent and hyperosmotic stress-induced changes in protein phosphorylation by performing LC-MS/MS–based proteomic analysis (Fig. 5C). We identified 2,821 phosphopeptides (Dataset S1), among which the amounts of 65 peptides were significantly changed by the hyperosmotic treatment in control MEFs but were kept constant in the Ask-TKO MEFs (Fig. S8 and Dataset S2). Importantly, 21 of the 65 phosphopeptides were up-regulated by the osmotic treatment in control MEFs but were constantly low in the Ask-TKO MEFs (Fig. S9), and this group included a phosphopeptide derived from CLOCK protein. The MS/MS spectrum identified Thr843 or Ser845 as an ASK-dependent phosphorylation site in the carboxyl-terminal region of CLOCK (Fig. 5 D and E). These data raise the possibility that the ASK-dependent and hyperosmotic stress-induced phosphorylation of CLOCK is a key step for clock input.
Based on our collective findings, we conclude that the cellular stress regulates the period and phase of the circadian clock through ASK signaling and phosphorylation/dephosphorylation of their downstream targets.
Discussion
In the present study, we have demonstrated that osmotic stress has prominent effects on the period and phase of the mammalian circadian clock, and that these effects were completely blocked by deficiencies of ASK family members (Figs. 1 and 2). Similarly, extracellular and intracellular oxidative stress, akin to osmotic stress, provides input to the circadian clockwork through ASK signaling (Fig. 4 and Fig. S5). Our behavioral analysis revealed in vivo roles of ASK signaling to regulate the oscillation speed and phase in response to light (Fig. 4 and Fig. S6). It is probable that the cellular stress acts as a time cue through a mechanism mediated by ASK signaling and that cellular stress and light may share, at least in part, a common input pathway to the circadian clockwork.
We previously reported a strong impact of ASK3 on water/salt reabsorption in renal tubular epithelial cells directly exposed to massive osmotic changes in body fluids (17). Osmotic stress is regulated rhythmically, peaking at night in the inner medulla of the kidney (33), and such a rhythm of cellular stress may be physiologically important for the peripheral clock. The circadian clock is known to control many renal functions, and dysfunction of the clock is associated with several diseases, including hypertension and type II diabetes (34).
Osmotic stress is known to stimulate stress-activated protein kinases, JNK and p38, downstream of the MAPK cascade (14, 16). We previously found that JNKs phosphorylate BMAL1 on exposure to hyperosmotic stress and regulate the circadian clock by controlling the oscillation speed and phase in response to light (28). In the present study, however, no remarkable alterations were observed by inhibition of JNK and p38 in the phase responses to acute hyperosmotic stimuli (Fig. S7 C–E). Therefore, hyperosmotic stress-induced phosphorylation/activation of p38/JNK is not involved in ASK-mediated clock input; rather, we propose that an HMM complex is important for the clock input (Fig. 5F).
Changes in the ASK HMM components in response to hyperosmotic stress should trigger atypical ASK signaling accompanied by dephosphorylation of ASKs. As evidence supporting this model, we found increases or decreases in ASK-dependent protein phosphorylation in response to hyperosmotic stress (Figs. S8 and S9 and Dataset S2), and identified T843 or S845 as the phosphorylation site of CLOCK protein (Fig. 5 D and E). It is notable that a phosphopeptide database (www.phosphosite.org) includes a human pS836-CLOCK peptide corresponding to mouse pS845 as an in vivo phosphorylation site induced by cold ischemia in tumors (35). In this model (Fig. 5F), we consider that a balance between phosphorylated and dephosphorylated forms of ASKs should determine the circadian period under various osmotic conditions. Even in the isotonic condition, clock input signal from intermediately dephosphorylated ASKs lengthens the circadian period, and the hypoosmotic treatment causes ASK phosphorylation, which further weakens the period-lengthening signal (Fig. 1F). In the Ask-TKO MEFs, the period-lengthening signal is completely lost, resulting in its short period phenotype (Fig. 1F). The model may need refinement when we take into account the fact that ASK1, ASK2, and ASK3 form a heteromeric complex (16), because various cellular stress signals received differently by the isoforms may be integrated into a change in the components of the HMM complex.
Aerobic organisms use oxygen to obtain energy efficiently, and as a result, they are inevitably exposed to oxidative stress, such as H2O2 (36). The intracellular level of the redox-related metabolite NAD+ and redox state of the antioxidant enzyme peroxiredoxin (PRX) are reported to have daily rhythms in mouse liver (37, 38). We emphasize that the PRX redox rhythms also have been identified in human red blood cells, in which transcription is arrested (39). The identification of the redox rhythms has led to a new idea of a redox oscillator (37, 39), in parallel with the TTFL oscillator. Nevertheless, the mechanism of coupling between the redox rhythms and the TTFLs remains largely unknown. The present study indicates that ASKs mediate redox signaling to the TTFLs (Fig. 4 A and B and Fig. S5), and, vice versa, Ask expression rhythms are controlled by the TTFLs (Fig. 3). The expression rhythms of Ask1 and Ask2 in the mouse liver exhibited peaks at CT10–14 (Fig. 3 A and B), a time of peak intracellular oxidation levels, as measured by PRX redox and NAD+/NADH levels (37–39). These lines of evidence support the fascinating idea that the TTFLs and intracellular redox state are interlocked with each other via ASK signaling (Fig. S5D).
Materials and Methods
Further elaboration of the methods used in this study is provided in SI Materials and Methods.
Real-Time Monitoring of Cellular Rhythms and Osmotic Stress Treatments.
Real-time monitoring of the cellular bioluminescence rhythms was performed as described previously (10) with minor modifications. In brief, cells were treated with 0.1 µM (final concentration) dexamethasone for 2 h, after which the media were replaced by recording media. The bioluminescence signals were continuously recorded for 5–10 d at 37 °C in air with a Kronos AB-2500 or AB-2550 (Atto), LumiCycle (Actimetrics), or CL24A (Churitsu) luminometer. For chronic hypertonic stimuli, NaCl (final concentration 100 mM) or sorbitol (final concentration 200 mM) was added to the cultured media. For pulse hypertonic stimuli, the cultured media (2.5 mL) were removed, and 0.88 mL of the used media was rapidly returned back to the original wells after mixing with 0.12 mL of 5 M sorbitol in the fresh media (final concentration 600 mM). After a 30-min incubation, the hypertonic media were replaced by the residual used media that had been kept at 37 °C (Fig. S3A). For hypotonic stimuli, the cultured media were diluted by mixing with distilled water (hypo) or 140 mM NaCl (mock).
Mice and Behavioral Rhythms.
The mice used in this study (C57BL/6 background) were handled according to approved Institutional Animal Care and Use Committees of The University of Tokyo (14-2). Ask-TKO mice were generated by crossing Ask1-KO (18), Ask2-KO (19), and Ask3-KO (17) mice. Behavioral rhythms of mice were recorded as described previously (24), with minor modifications. In brief, male mice were individually housed in a polycarbonate cage equipped with a running wheel in a light-tight chamber. The locomotor activity rhythms of mice were measured by wheel revolutions in 5-min bins and analyzed using ClockLab software (Actimetrics). The circadian periods were analyzed by a χ2 periodogram with a P value < 0.001, based on locomotor activity at days 4–17 after the start of each condition.
LC-MS/MS–Based Phosphoproteomic Analysis.
Enzymatic digestion by trypsin was performed according to a phase-transfer surfactant (PTS) protocol (40) with modifications. The peptides were desalted using a MonoSpin C18 column (GL Sciences) and applied to a high-select Fe-NTA phosphopeptide enrichment kit (Thermo Fisher Scientific). The enriched phosphopeptides were subjected to LC-MS/MS analyses using a mass spectrometer (Q Exactive Plus; Thermo Fisher Scientific) equipped with a nano ultra-HPLC system (Dionex Ultimate 3000; Thermo Fisher Scientific). The raw spectra were extracted using Proteome Discoverer 2.2 (Thermo Fisher Scientific) and searched against the mouse SwissProt database (v2017-06-07), with phosphorylation (+79.966 Da) at Ser, Thr, and Tyr set as a dynamic (nonfixed) modification for peptide. The amount of each peptide was semiquantified using peak area with the Precursor Ions Quantifier mode in Proteome Discoverer 2.2.
Supplementary Material
Acknowledgments
We thank Dr. Hiroshi Kiyota, Dr. Kuniyoshi Niwa, and Miho Yoshimura for their help with experiments, and Hiroto Fukuwa and Hiroaki Wakatsuki for mouse breeding. K.I. is supported by a Japan Society for the Promotion of Science research fellowship for young scientists. This work was partially supported by Grants-in-Aid for Scientific Research (to H.Y., K.H., I.N., H.I., and Y.F.) from the Ministry of Education, Culture, Sports, Science and Technology–Japan and by PRIME (to H.Y.) and JP17gm5010001 (to H.I.) from the Japan Agency for Medical Research and Development.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719298115/-/DCSupplemental.
References
- 1.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zool Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 4.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 6.Hirano A, Fu YH, Ptáček LJ. The intricate dance of post-translational modifications in the rhythm of life. Nat Struct Mol Biol. 2016;23:1053–1060. doi: 10.1038/nsmb.3326. [DOI] [PubMed] [Google Scholar]
- 7.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 8.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 9.Asher G, Sassone-Corsi P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Kon N, et al. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol. 2008;10:1463–1469. doi: 10.1038/ncb1806. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 12.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Naguro I, Ichijo H, Watanabe K. Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim Biophys Acta. 2016;1860:2037–2052. doi: 10.1016/j.bbagen.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Schliess F, Reinehr R, Häussinger D. Osmosensing and signaling in the regulation of mammalian cell function. FEBS J. 2007;274:5799–5803. doi: 10.1111/j.1742-4658.2007.06100.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- 17.Naguro I, et al. ASK3 responds to osmotic stress and regulates blood pressure by suppressing WNK1-SPAK/OSR1 signaling in the kidney. Nat Commun. 2012;3:1285–1295. doi: 10.1038/ncomms2283. [DOI] [PubMed] [Google Scholar]
- 18.Tobiume K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iriyama T, et al. ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis. EMBO J. 2009;28:843–853. doi: 10.1038/emboj.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Daan S. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms. 2000;15:195–207. doi: 10.1177/074873040001500301. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- 24.Terajima H, et al. ADARB1 catalyzes circadian A-to-I editing and regulates RNA rhythm. Nat Genet. 2017;49:146–151. doi: 10.1038/ng.3731. [DOI] [PubMed] [Google Scholar]
- 25.Yoshitane H, et al. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol Cell Biol. 2014;34:1776–1787. doi: 10.1128/MCB.01465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zatz M, Wang HM. High salt mimics effects of light pulses on circadian pacemaker in cultured chick pineal cells. Am J Physiol. 1991;260:R769–R776. doi: 10.1152/ajpregu.1991.260.4.R769. [DOI] [PubMed] [Google Scholar]
- 27.Zatz M, Wang HM. Low salt mimics effects of dark pulses on circadian pacemaker in cultured chick pineal cells. Am J Physiol. 1991;261:R1424–R1430. doi: 10.1152/ajpregu.1991.261.6.R1424. [DOI] [PubMed] [Google Scholar]
- 28.Yoshitane H, et al. JNK regulates the photic response of the mammalian circadian clock. EMBO Rep. 2012;13:455–461. doi: 10.1038/embor.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa Y, et al. Apoptosis signal-regulating kinase-1 inhibitor as a potent therapeutic drug for the treatment of gastric cancer. Cancer Sci. 2012;103:2181–2185. doi: 10.1111/cas.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichijo H, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 31.Takeda K, et al. Apoptosis signal-regulating kinase (ASK) 2 functions as a mitogen-activated protein kinase kinase kinase in a heteromeric complex with ASK1. J Biol Chem. 2007;282:7522–7531. doi: 10.1074/jbc.M607177200. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi T, et al. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- 33.Hara M, et al. Robust circadian clock oscillation and osmotic rhythms in inner medulla reflecting cortico-medullary osmotic gradient rhythm in rodent kidney. Sci Rep. 2017;7:7306. doi: 10.1038/s41598-017-07767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonny O, Firsov D. Circadian regulation of renal function and potential role in hypertension. Curr Opin Nephrol Hypertens. 2013;22:439–444. doi: 10.1097/MNH.0b013e32836213b8. [DOI] [PubMed] [Google Scholar]
- 35.Mertins P, et al. Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol Cell Proteomics. 2014;13:1690–1704. doi: 10.1074/mcp.M113.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 37.Edgar RS, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peek CB, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda T, Tomita M, Ishihama Y. Phase transfer surfactant-aided trypsin digestion for membrane proteome analysis. J Proteome Res. 2008;7:731–740. doi: 10.1021/pr700658q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.