Abstract
Lyme neuroborreliosis, caused by the gram-negative bacterium Borrelia burgdorferi, may affect the central and/or peripheral nervous systems. In previous studies, we showed that human oligodendrocytes exposed to the bacteria undergo apoptosis in an inflammatory environment, and that inflammatory pathways trigger cell-death pathways. We further demonstrated that several receptor tyrosine kinases were involved in triggering downstream effects, leading to inflammation and apoptosis. Toll-like receptors TLR2 and TLR5, which are commonly studied receptors in Lyme disease, only had a minimal role in inflammatory processes. To delineate the role of other TLRs, if any, real-time RT-PCR array experiments were carried out as an initial screen. Along with several inflammatory genes, TLR7 mRNA was upregulated in cells exposed to B. burgdorferi. Further analysis by immunohistochemistry showed that the TLR7 protein is present in readily detectable amounts, although no discernible differences could be seen between medium and B. burgdorferi-exposed cells by this technique. Nevertheless, use of specific inhibitors and siRNA showed that TLR7 is involved in inducing IL-6 and CCL2 in a dose dependent manner, and likely CXCL8. Triggering an intracellular receptor such as TLR7, which senses RNA, in typically non-phagocytic oligodendrocytes indicates either a niche for the bacterium inside the cell or novel uptake of nucleic acids to initiate inflammatory responses.
Keywords: Toll-like receptor, oligodendrocytes, innate immunity, Lyme neuroborreliosis
1. Introduction
Lyme neuroborreliosis (LNB), a form of Lyme disease caused by the spirochete Borrelia burgdorferi, affects approximately 10–15% of patients infected with this bacterium. Early stage LNB is characterized by meningitis, meningoradiculitis, neuritis or myelitis, and can progress to late stage as mono or polyneuropathy, radiculopathy, progressive Lyme encephalitis or cerebral vasculitis [12]. However, the pathogenesis of LNB is incompletely understood. In our rhesus macaque model of acute LNB, intrathecal inoculation with B. burgdorferi resulted in production of inflammatory mediators in the serum and cerebrospinal fluid (CSF), CSF pleocytosis, along with other signs of acute LNB in humans, such as leptomeningitis, radiculitis, and inflammatory lesions in dorsal root ganglia (DRG) [22]. Importantly, B. burgdorferi infection also resulted in the apoptosis of neurons and glia in the DRG. Subsequent studies, that were performed in vitro, ex vivo or in vivo, demonstrated neuronal and glial cell apoptosis in response to B. burgdorferi, and inflammation as a causative factor of neural cell death [20, 21, 23, 25, 26]. However, while oligodendrocytes and Schwann cells undergo apoptosis in the presence of B. burgdorferi alone, in an inflammatory environment, microglia are required for CNS neurons to undergo cell death when exposed to the bacteria in vitro [11, 20, 25]. Presumably, the intense inflammatory environment created by microglia is required to induce apoptosis of by-standing CNS neurons. Mechanistic studies have shown that the MEK/ERK pathway plays a predominant role in mediating inflammation in multiple glial cells. It also activated the mitochondrial p53 pathway to mediate apoptosis in human oligodendrocytes, indicating yet again that inflammatory pathways lead to apoptotic cell death [16]. While TLRs such as TLR2 and 5 were demonstrated to trigger downstream inflammation in microglia, in oligodendrocytes, receptor tyrosine kinases such as epidermal growth factor/fibroblast growth factor/platelet-derived growth factor had a much greater role in inflammatory mediator production as well as apoptosis [15, 17]. TLR2 and TLR5 had only a minimal role in inflammation output. TLR2, particularly, seemed to be part of inhibitory pathways in oligodendrocytes [17]. Subsequently, efforts were undertaken to determine the role of other TLRs, if any, in mediating chemokine/cytokine production from oligodendrocytes. An initial screen using a TLR PCR array indicated that intracellular receptors such as TLR7 might play a role. As B. burgdorferi is largely extra-cellular, and oligodendrocytes are generally not considered phagocytic, we decided to assess whether this receptor had a concrete role in B. burgdorferi-mediated inflammation. If so, it would indicate that novel mechanisms might be at play in this interaction.
2. Materials and Methods
2.1. B. burgdorferi culture
B. burgdorferi strain B31, clone 5A19, carrying the full complement of plasmids [19] was used throughout the study, and cultured according to previously published protocols [14, 16, 17]. Briefly, bacteria were routinely cultured in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma Aldrich, St. Louis-MO) supplemented with amphotericin (0.25 μg/mL), phosphomycin (193 μg/mL) and rifampicin (45.4 μg/mL), for about 5–6 days, under microaerophilic conditions. Bacterial concentration was determined using a dark-field microscope, and the required numbers of bacteria were harvested at 2095 × g for 30 minutes at room temperature (RT) without brakes. The bacterial pellet was resuspended in experimental medium containing DMEM-high glucose (Invitrogen/Life Technologies, Inc., Grand Island-NY) and 100 nM phorbol myristate acetate (PMA) (Sigma Aldrich, St. Louis-MO) to the same concentration prior to centrifugation, and diluted further to the required multiplicity of infection (MOI).
2.2. Cell culture
The human oligodendrocyte cell line MO3.13 was purchased from CELLutions Biosystems Inc., Ontario, Canada, and cultured according to the manufacturer’s protocol. These cells were created by fusion of human oligodendrocytes with rhabdomyosarcoma cells, generating a cell line with oligodendrocyte cell characteristics. Cells were routinely grown in DMEM (high glucose) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37°C, 5% CO2, for 4 days in tissue culture flasks, and then seeded on 6-well plates (0.8 × 104/well), 2-well chamber slides (0.6 × 104/well) or T-75 flasks (1 × 105/flask) as required. After 3 days in growth medium, cells were allowed to differentiate into mature oligodendrocytes by replacing the culture medium with DMEM high glucose devoid of serum, but supplemented with 100 nM PMA and 1% P/S (differentiation medium). After 3 days in differentiation medium, experiments were carried out. For all experiments, differentiation medium without P/S (experimental medium) was used.
2.3. Real-time RT-PCR
MO3.13 cells seeded in T-75 flasks were treated with either medium or B. burgdorferi (10:1 MOI) for 6 h, after which RNA was extracted using RNeasy® mini kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. NanoDrop® ND-1000 (NanoDrop Technologies Inc., Wilmington, DE) was used to quantify RNA concentration, and 1 μg of RNA was used for cDNA synthesis (RT2 First strand kit), which was aliquoted on RT2 Profiler PCR array plates (human TLR signaling pathway) using RT2 SYBR® Green ROX™ qPCR mastermix (Qiagen, Germantown, MD). The real time PCR reactions were run on Applied Biosystems 7900HT cycler and data captured using SDS 2.4 software. All protocols were carried out according to the manufacturer’s handbook. The resulting raw data were analyzed on SABiosciences web-based software (www.SABiosciences.com/pcrarraydataanalysis.php) to determine the fold change in gene expression in cells exposed to B. burgdorferi over medium control. Fold changes (upregulation) over 1.2 fold were considered for further review. Ct values > 34 were considered as too low an expression and not included for analysis.
2.4. TLR7 inhibition assay
Infection assays were carried out with B. burgdorferi in the presence or absence of a TLR7/9 inhibitor. At 2 h prior to the addition of bacteria, 1 μM Dual iODN (inhibitory oligodeoxynucleotide that inhibits TLR7/9) was added followed by B. burgdorferi (10:1 MOI). At 48h after the addition of bacteria, supernatants were collected after centrifugation at 2095 × g, 10 minutes, 4°C to remove bacteria and cellular debris, aliquoted and stored at −20°C until analysis by enzyme-linked immuno sorbent assay (ELISA) for chemokines and cytokines. A neutral ODN (1 μM) was used as a control for Dual iODN-mediated effects (both obtained from Enzo lifesciences). Imiquimod (5 μg/mL Imgenex, San Diego, CA) was used as a positive control for TLR7 effects, and medium-only wells as a negative control for B. burgdorferi.
2.5. RNAi
siRNA technology was used to determine the role of oligodendrocyte TLR7 in inducing inflammation in response to B. burgdorferi. The experimental protocol was carried out according to Parthasarathy and Philipp, 2017 [17]. Briefly, transfection complexes were generated by incubating (12.5–50 nM) TLR7 siRNA and (2 μL) HiPerfect transfection reagent for 30 minutes at RT in experimental medium. After removing the differentiation medium from the culture wells, 200 μL of the transfection complex was added, immediately followed by 800 μL of experimental medium to prevent drying of monolayer. Cells were incubated with siRNA transfection complexes for 24 h at 37°C, 5% CO2, followed by addition of B. burgdorferi (10:1 MOI), medium or positive control Imiquimod (5 μg/mL) for additional 48 h. At the end of the time period, supernatants were collected as before and analyzed for chemokine and cytokine expression. A non-specific control siRNA was used as negative control for all experiments. TLR7 and control siRNA were obtained from Santa Cruz Biotechnology, Dallas, TX. HiPerfect transfection reagent was from Qiagen, Valencia, CA.
2.6. ELISAs for chemokines and cytokines
The cell supernatants from various experimental conditions were analyzed for specific chemokines and cytokines using custom Multiplex ELISA kits obtained from EMD Millipore (Billerica, MA) and carried out at the Pathogen Detection and Quantification Core, Tulane National Primate Research Center, according to the manufacturer’s protocols.
2.7. Immunohistochemistry
Mature MO3.13 cells growing in 2-well chamber slides were immunostained for TLR7 in the presence or absence of B. burgdorferi (10:1 MOI). After a 48 h exposure, the supernatants were removed, and cells were processed for immunofluorescence as described previously [17]. A rabbit polyclonal TLR7 antibody (1:50, Santa Cruz Biotechnology, Dallas, TX) was used as a primary antibody, while a corresponding goat-anti rabbit antibody conjugated to Alexa 488 (1:800; Invitrogen) was used as a secondary. In separate wells, cells were stained with myelin basic protein (MBP) antibody (1:100; EMD Millipore) as a positive control for cell type, while a negative control with only the secondary antibody was also carried out (not shown). Slides were mounted with an anti-quenching reagent, covered with coverslips and visualized for TLR7 expression patterns.
2.8. Microscopy
The cells were visualized for TLR7 expression using a Leica DMRE fluorescent microscope (Leica microsystems, Buffalo Grove-IL) and Lumecor SOLA GUI software (Lumencor, Beaverton-OR). Images were obtained using Nuance Multispectral Imaging System (CRi, PerkinElmer, Waltham-MA). Adobe® Photoshop CS6 software was used to assemble the images.
2.9. Statistics
The Student’s t-test was used to determine the statistical significance of an experimental result, with each analysis using duplicate values. The results were considered significantly different if the probability values (p) were < 0.05.
3. Results
3.1. TLR7 and other inflammatory pathway genes are upregulated in MO3.13 cells exposed to B. burgdorferi
As TLR2 and TLR5, two very prominent immune mediators with respect to B. burgdorferi, played only a minor role in induction of immune responses in MO3.13 oligodendrocytes [17], a TLR PCR array was employed as a screening technique to determine if other TLRs were upregulated and thus possibly involved in induction of immune responses. An initial screening during B. burgdorferi exposure for 6 h, using real time (RT)-PCR, indicated that several genes were significantly upregulated (Table 1). However, while CXCL10 (IP-10) and IL-1α transcripts were upregulated, we did not see a significant secretion of these mediators in the supernatants of these cells at the protein level [16]. NFkB1(p50), NFkBIA (IkBα), and REL are associated with the NFkB pathway. We have shown in the same previous study [16] that while NFkB p65 had an inconclusive role in immune response, IKK (α,β) had a role in the induction of CCL2 and IL-6. TBK1 is a non-canonical IkB kinase and an upstream activator of transcription factor IRF3, generally associated with intracellular receptors such as TLR3 [10]. IRF3 is normally indicated for the induction of Type I interferon (IFN) secretion. However, the secretion of IFNα2 into the supernatants of MO3.13 cells is inconclusive at this point (data not shown), and other Type I IFNs have not been evaluated as yet. Interestingly, TLR7, another intracellular receptor was also significantly upregulated in response to B. burgdorferi. Both TLR3 and TLR7 recognize RNA, with the former being responsive to double stranded RNA (dsRNA) and the latter to single stranded RNA (ssRNA) [6]. HSPA1A, which codes for the 70 KDa heat shock protein (Hsp70), is associated with chaperone functions, inflammation, and apoptosis [8], and was also upregulated in response to B. burgdorferi exposure. Other genes that were upregulated, but not significantly, were IL-1β, CXCL8, TNFα, LTA (Lymphotoxin A/TNFβ) and LY96 (Lymphocyte antigen 96/MD2) (not shown). We decided to further investigate the role of TLR7, of which little is known with respect to oligodendrocytes and B. burgdorferi.
Table 1.
Genes significantly upregulated in MO3.13 cells exposed to B. burgdorferi for 6 h, over medium control
| Symbol | Gene Description (Gene name) | Fold changea |
|---|---|---|
| CXCL10 | Chemokine (C-X-C motif) ligand 10 (IP-10) | 3.67 (± 0.698)* |
| HSPA1A | Heat shock 70kDa protein 1A (HSP70-1A) | 1.57 (± 0.171)* |
| IL1A | Interleukin 1, alpha (IL-1α) | 2.69 (± 0.765)* |
| IRF3 | Interferon regulatory factor 3 | 1.28 (± 0.089)* |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NFKB-p50) | 1.25 (± 0.020)** |
| NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IKBA/MAD-3/NFKBI) | 1.33 (± 0.010)*** |
| REL | V-rel reticuloendotheliosis viral oncogene homolog, avian (C-Rel) | 1.37 (±0.015)*** |
| TBK1 | TANK-binding kinase 1 (NAK/T2K) | 1.28 (± 0.040)** |
| TLR7 | Toll-like receptor 7 | 2.42 (±0.094)** |
Average fold change (± standard deviation) from two independent experiments is indicated.
p < 0.05,
p < 0.01,
p < 0.001
3.2. Expression of TLR7 protein in MO3.13 cells
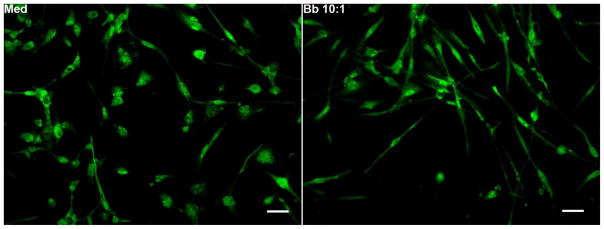
As more than a 2-fold increase in TLR7 mRNA was observed in cells exposed to B. burgdorferi, we decided to look at the expression level of TLR7 through immunofluorescence. TLR7 protein expression was detected throughout the cell, as seen in Fig. 1. However, no difference in the level of protein expression was discernible between medium-conditioned cells and cells exposed to the bacterium, as measured through this technique. Nevertheless, the data shows that the receptor is present in fair amounts in oligodendrocytes.
Fig. 1. Immunofluorescent staining of TLR7 in MO3.13 cells in response to B. burgdorferi.
Differentiated MO3.13 cells grown in chamber slides were exposed to either medium alone (Med) or B. burgdorferi (Bb, MOI 10:1) for 48 h, and assessed for TLR7 expression, as described in Materials and Methods. Bar represents 50 nm. A representative photograph from 2 independent experiments is shown.
3.3. TLR7 plays a role in inducing chemokines/cytokines from human oligodendrocytes in response to B. burgdorferi
In many of our previous studies we showed that inflammation as caused by B. burgdorferi plays an important role in disease pathogenesis. As TLR7 mRNA was upregulated in response to B. burgdorferi, we next evaluated whether TLR7 was involved in inducing inflammation mediated by oligodendrocytes. We used both a TLR7/9 inhibitor and TLR7 siRNA for this purpose. As seen in Table 2, in the presence of the inhibitor, CXCL8, IL-6 and CXCL(1,2,3) were significantly downregulated for both B. burgdorferi and TLR7 agonist (Imiquimod) treatments. CCL5 was additionally downregulated significantly with respect to Imiquimod. CCL2 levels were significantly upregulated in the presence of Imiquimod and the inhibitor, while the effect was not significant with B. burgdorferi treatment, although they both showed the same trend.
Table 2.
Average fold changea in chemokine/cytokine levels in human oligodendrocytes in the presence of TLR7/9 inhibitor or TLR7 siRNA at 48h.
| Chemokine-Cytokine/Treatment | CCL2 | CXCL8 | IL-6 | CXCL(1,2,3) | CCL5 |
|---|---|---|---|---|---|
| Bb + 1 μM TLR7/9 inhibitor | 0.574 | 1.147 | 1.542 | 1.139 | 0.866 |
| (±0.192) | (±0.191) | (±0.181) | (±0.184) | (±0.195) | |
| Imiquimod + 1 μM TLR7/9 inhibitor | 0.856 | 1.266 | 1.646 | 1.651 | 1.442 |
| (±0.103) | (±0.173) | (±0.403) | (±0.253) | (±0.110) | |
| Bb + 12.5 nM TLR7 siRNA | 1.353 | 1.609 | 1.119 | 0.952 | 0.781 |
| (±0.172) | (±0.317) | (±0.138) | (±0.015) | (±0.085) | |
| Bb + 25 nM TLR7 siRNA | 1.372 | 1.081 | 1.249 | 0.966 | 0.760 |
| (±0.135) | (±0.118) | (±0.097) | (±0.044) | (±0.097) | |
| Bb + 50 nM TLR7 siRNA | 1.539 | 1.102* | 1.573 | 1.023 | 0.914 |
| (±0.202) | (±0.239) | (±0.238) | (±0.070) | (±0.028) | |
| Imiquimod + 12.5 nM TLR7 siRNA | 1.235 | 1.181 | 1.404 | 1.733 | 1.030 |
| (±0.127) | (±0.082) | (±0.118) | (±0.491) | (±0.138) | |
| Imiquimod + 25 nM TLR7 siRNA | 1.174 | 1.217* | 1.336* | 1.218* | 0.833 |
| (±0.202) | (±0.363) | (±0.452) | (±0.427) | (±0.078) | |
| Imiquimod + 50 nM TLR7 siRNA | 2.058 | 1.437 | 1.813 | 1.330 | 1.044 |
| (±0.341) | (±0.074) | (±0.238) | (±0.255) | (±0.173) |
Fold change for each experiment was calculated as [Treatment + Neutral ODN/Treatment + Dual iODN] for inhibitor experiments and [Treatment + Control siRNA/Treatment + TLR7 siRNA] for the RNAi experiments. For the inhibitor experiments, average fold change values were calculated from the mean of three experiments for B. burgdorferi (MOI 10:1) and two to three experiments for Imiquimod (5μg/mL). For the siRNA experiments, average fold change values were calculated from the mean of three to five independent experiments for B. burgdorferi (MOI 10:1), two to three experiments for Imiquimod (5μg/mL). Standard error of the mean is indicated within parenthesis. Fold change values > 1 indicate a downregulation of chemokine/cytokine levels and values < 1 indicate an increase in those levels. Values in italics or those underlined indicate statistically significant upregulation or downregulation respectively, in a majority (2/2, 2/3 or 3/5) of experiments.
indicates inconclusive results.
As the inhibitor was not specific to TLR7 alone, and to obtain additional lines of evidence for the role of the receptor, we used TLR7 siRNA. As seen in Table 2, the effect was dose dependent. At 12.5 nM siRNA concentration, and in the presence of the bacterium, CCL2, CXCL8 and IL-6 were significantly downregulated, while CCL5 was significantly upregulated. At 50 nM concentration however, only CCL2 and IL-6 were significantly downregulated. In the presence of the positive control Imiquimod and 12. 5 nM siRNA, only CXCL(1,2,3) was significantly downregulated, but at 50nM siRNA concentration, CCL2, CXCL8, IL-6 and CCL5 were all significantly downregulated. Thus, stark differences in effects were seen between 12.5 and 50 nM concentrations of siRNA, with the 25 nM concentration showing transitioning effects between the two. Production of IL-6 was reduced by both the TLR7/9 inhibitor and TLR7 siRNA in the presence of B. burgdorferi, indicating that this cytokine is definitely induced in response to TLR7 activation, with the additional likely induction of CCL2, and possibly also CXCL8.
4. Discussion
Toll-like receptors have been shown to play an important role in Lyme disease pathogenesis. Intracellular TLRs, particularly TLR7, TLR8, and TLR9 are involved in inflammation mediated by phagocytic immune cells such as peripheral blood mononuclear cells and monocytes, in response to B. burgdorferi [2, 18]. TLR7 and TLR8 recognize ssRNA and nucleoside analogs, while TLR9 recognizes CpG DNA in the endosomes of cells [6]. Since TLRs other than the predominantly studied TLR2 have also been implicated in Lyme disease pathogenesis, we decided to explore the roles of these other receptors, if any, in oligodendrocyte mediated inflammation. A real time RT-PCR TLR array (TLR1-10) was employed to screen for these receptors’ activation upon bacterial addition. A number of inflammatory genes were upregulated (> 1.2 fold) including TLR7 (Table 1). However, a few of these (IP-10, IL-1β) were already determined to be not significantly secreted in the supernatants [16] in response to B. burgdorferi. The roles of other molecules, e.g. NFKB1, NFKBIA, REL, which are part of the NFkB pathway, have also been determined [16]. As oligodendrocytes are not traditionally phagocytic, upregulation of RNA-sensing intracellular receptors such as TLR7 indicated that this receptor’s activation might not be associated only with traditional phagocytic cells as reported previously, and hence we decided to explore the role of this receptor further. Curiously, TBK1 and IRF3 are associated with activation of another RNA sensing (dsRNA) intracellular receptor, TLR3, and await further study, as does HSPA1A. It is also worth mentioning that upregulation of chemokine/cytokine mRNA and secretion of the corresponding protein into the supernatant do not always correlate. This indicates either differential transcriptional/translational control, or that intracellular cytokine/chemokine levels will also need to be examined in the future.
In microglia, upon addition of B. burgdorferi, dramatic upregulation of TLRs were observed through immunohistochemistry [1]. As TLR7 mRNA was upregulated in response to B. burgdorferi, we decided to determine the corresponding protein levels by the same technique. However, no obvious differences were seen in protein expression between cells exposed to medium and B. burgdorferi, although TLR7 expression levels were qualitatively highly visible in both cases (Fig. 1). It is possible that this technique is not sensitive enough to evince such differences in these cells and need a more sensitive assay such as a Western blot.
We next attempted to evaluate the role of TLR7 in B. burgdorferi-mediated inflammation by inhibition of signaling and silencing the transcript levels. Imiquimod, a TLR7 agonist was used as a positive control. It should be noted that TLR7 activation by B. burgdorferi is likely through RNA sensing, while that of Imiquimod, an imidazoquinoline amine, is as a nucleoside analog. It has been shown that RNA and imidazoquinolines are engaged by distinct sites on TLR7/8, leading to different signaling events [3]. In the presence of B. burgdorferi and 1 μM TLR7/9 inhibitor, CXCL8, IL-6 and CXCL (1,2,3) levels were downregulated compared to the levels induced in the presence of the bacterium and a control ODN that has no activity (neutral ODN)). This indicated that TLR7/9 played a role in immune modulation in response to B. burgdorferi. As the inhibitor was not specific to TLR7, TLR7 siRNA was also employed, and it showed a dose dependent response. At lower siRNA concentrations (12.5 nM), it significantly downregulated CCL2, CXCL8, IL-6 and significantly upregulated CCL5, while at higher concentrations (50 nM), it significantly affected CCL-2 and IL-6 levels with respect to B. burgdorferi. Taken together, these results indicate that TLR7 activation by B. burgdorferi affects IL-6 and CCL2 induction in a dose dependent manner, and likely also the induction of CXCL8. The contrasting effects on CCL2 and CXCL (1,2,3) is likely due to TLR9 mediated effects, wherein activation of TLR9 inhibits CCL2 production while increasing CXCL(1,2,3) levels. With respect to Imiquimod, both the inhibitor and siRNA addition downregulated CXCL8, IL-6 and CCL5 levels. While CXCL(1,2,3) was also downregulated, it occurred in a dose dependent manner. Similar to the effect on CCL2 with B. burgdorferi, the contrasting effect of inhibitor and siRNA is likely through TLR9. This study also shows that apart from TLR7, other intracellular TLR receptors such as TLR3 and TLR9 might play a role in innate immunity of oligodendrocytes towards B. burgdorferi.
With respect to the roles of individual chemokines/cytokines, IL-6 has been shown to stimulate hepatocytes, differentiate B-cells, aid in development of effector T-cells, proliferation of non-immune cells and others [27]. CCL2 is involved in recruitment of monocytes and T-cells, while CXCL8 has a similar role towards neutrophils, and likely other white blood cells [29]. IL-6 and CCL2 can have pro or anti-inflammatory roles depending on the tissue/disease context, while CXCL8 is generally pro-inflammatory [24]. Since inflammation induces apoptosis in LNB, all three are likely to be pro-inflammatory in oligodendrocytes, as suppression of all three mediators through inhibition of receptor tyrosine kinases downregulated oligodendrocyte apoptosis [17].
Oligodendrocytes are not considered to be professional phagocytic cells like macrophages or microglia, although there is some evidence of their phagocytic abilities. In one study, apoptotic lymphocytes were demonstrated to be phagocytosed by these cells [13]. And there is evidence of B. burgdorferi internalization in neuroglial cells [7]. Therefore, it possible that there is limited phagocytosis by these cells, that enables the bacterial RNA to be sensed by TLR7 receptors. Alternatively, B. burgdorferi has been shown to produce outer membrane vesicles [28] that can package nucleic acids [4]. These outer membrane vesicles can then be trafficked internally by endocytosis, triggering intracellular TLR activation [9]. In preliminary studies, addition of cytochalasin D, which affects most endocytic pathways by depolymerizing F-actin [5], downregulated CXCL8, CXCL(1,2,3), CCL2 and IL-6 in a dose dependent manner in the presence of B. burgdorferi (Parthasarathy and Philipp, unpublished results), indicating the importance of these pathways in oligodendrocyte innate immunity. We show here that intracellular TLR pathways such as TLR7 may play a role in innate immune responses in cells other than professional phagocytes.
4.1. Conclusions
B. burgdorferi is a versatile pathogen that utilizes its repertoire of available molecular patterns to induce inflammation in a cumulative manner. In the absence of traditional receptor activation such as TLR2, other novel receptors such as receptor tyrosine kinases and intracellular TLRs are utilized to possibly cause disease.
Highlights.
Inflammation has a causal role in B. burgdorferi mediated pathogenesis
Intracellular TLR7 contributes to B. burgdorferi induced immunity in oligodendrocytes
This indicates a novel possible niche for bacteria, or uptake of ligands by novel pathways
Acknowledgments
This study was supported by the National Institute of Neurologic Disorders and Stroke through grant NS048952, and by the National Center for Research Resources/Office of Research Infrastructure Programs of the National Institutes of Health through grant P51RR000164/P51OD011104. We thank the TNPRC Pathogen Detection and Quantification Core Laboratory for help with the multiplex ELISA assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun. 2008;76:4385–4395. doi: 10.1128/IAI.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervantes JL, La Vake CJ, Weinerman B, Luu S, O’Connell C, Verardi PH, Salazar JC. Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. J Leukoc Biol. 2013;94:1231–1241. doi: 10.1189/jlb.0413206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colak E, Leslie A, Zausmer K, Khatamzas E, Kubarenko AV, Pichulik T, Klimosch SN, Mayer A, Siggs O, Hector A, Fischer R, Klesser B, Rautanen A, Frank M, Hill AV, Manoury B, Beutler B, Hartl D, Simmons A, Weber AN. RNA and imidazoquinolines are sensed by distinct TLR7/8 ectodomain sites resulting in functionally disparate signaling events. J Immunol. 2014;192:5963–5973. doi: 10.4049/jimmunol.1303058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorward DW, Garon CF. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl Environ Microbiol. 1990;56:1960–1962. doi: 10.1128/aem.56.6.1960-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta D, Donaldson JG. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell Logist. 2012;2:203–208. doi: 10.4161/cl.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BL, Barton GM. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014;24:360–369. doi: 10.1016/j.tcb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livengood JA, Gilmore RD., Jr Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006;8:2832–2840. doi: 10.1016/j.micinf.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Martinez de Toda I, De la Fuente M. The role of Hsp70 in oxi-inflamm-aging and its use as a potential biomarker of lifespan. Biogerontology. 2015;16:709–721. doi: 10.1007/s10522-015-9607-7. [DOI] [PubMed] [Google Scholar]
- 9.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muvaffak A, Pan Q, Yan H, Fernandez R, Lim J, Dolinski B, Nguyen TT, Strack P, Wu S, Chung R, Zhang W, Hulton C, Ripley S, Hirsch H, Nagashima K, Wong KK, Janne PA, Seidel-Dugan C, Zawel L, Kirschmeier PT, Middleton RE, Morris EJ, Wang Y. Evaluating TBK1 as a therapeutic target in cancers with activated IRF3. Molecular cancer research: MCR. 2014;12:1055–1066. doi: 10.1158/1541-7786.MCR-13-0642. [DOI] [PubMed] [Google Scholar]
- 11.Myers TA, Kaushal D, Philipp MT. Microglia are mediators of Borrelia burgdorferi-induced apoptosis in SH-SY5Y neuronal cells. PLoS Pathog. 2009;5:e1000659. doi: 10.1371/journal.ppat.1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I S. European Federation of Neurological. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010;17:8–16. e11–14. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen KB, Pender MP. Phagocytosis of apoptotic lymphocytes by oligodendrocytes in experimental autoimmune encephalomyelitis. Acta Neuropathol. 1998;95:40–46. doi: 10.1007/s004010050763. [DOI] [PubMed] [Google Scholar]
- 14.Parthasarathy G, Fevrier HB, Philipp MT. Non-viable Borrelia burgdorferi induce inflammatory mediators and apoptosis in human oligodendrocytes. Neurosci Lett. 2013;556:200–203. doi: 10.1016/j.neulet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parthasarathy G, Philipp MT. Inflammatory mediator release from primary rhesus microglia in response to Borrelia burgdorferi results from the activation of several receptors and pathways. J Neuroinflammation. 2015;12:60. doi: 10.1186/s12974-015-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parthasarathy G, Philipp MT. The MEK/ERK pathway is the primary conduit for Borrelia burgdorferi-induced inflammation and P53-mediated apoptosis in oligodendrocytes. Apoptosis. 2014;19:76–89. doi: 10.1007/s10495-013-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parthasarathy G, Philipp MT. Receptor tyrosine kinases play a significant role in human oligodendrocyte inflammation and cell death associated with the Lyme disease bacterium Borrelia burgdorferi. J Neuroinflammation. 2017;14:110. doi: 10.1186/s12974-017-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009;183:5279–5292. doi: 10.4049/jimmunol.0901390. [DOI] [PubMed] [Google Scholar]
- 19.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, Philipp MT. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol. 2008;173:1415–1427. doi: 10.2353/ajpath.2008.080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, Martin DS, Jacobs MB, Didier PJ, Philipp MT. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramesh G, Didier PJ, England JD, Santana-Gould L, Doyle-Meyers LA, Martin DS, Jacobs MB, Philipp MT. Inflammation in the pathogenesis of lyme neuroborreliosis. Am J Pathol. 2015;185:1344–1360. doi: 10.1016/j.ajpath.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators of inflammation. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh G, Meisner OC, Philipp MT. Anti-inflammatory effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in neuronal cultures of dorsal root ganglia and myelinating cells of the peripheral nervous system. J Neuroinflammation. 2015;12:240. doi: 10.1186/s12974-015-0461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramesh G, Santana-Gould L, Inglis FM, England JD, Philipp MT. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J Neuroinflammation. 2013;10:88. doi: 10.1186/1742-2094-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun. 2012;80:359–368. doi: 10.1128/IAI.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et biophysica acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]