Abstract
Intratumoral androgen biosynthesis has been recognized as an essential factor of castration‐resistant prostate cancer. The present study investigated the effects of curcumin on the inhibition of intracrine androgen synthesis in prostate cancer. Human prostate cancer cell lines, LNCaP and 22Rv1 cells were incubated with or without curcumin after which cell proliferation was measured at 0, 24, 48 and 72 hours, respectively. Prostate tissues from the transgenic adenocarcinoma of the mouse prostate (TRAMP) model were obtained after 1‐month oral administration of 200 mg/kg/d curcumin. Testosterone and dihydrotestosterone concentrations in LNCaP prostate cancer cells were determined through LC‐MS/MS assay. Curcumin inhibited cell proliferation and induced apoptosis of prostate cancer cells in a dose‐dependent manner. Curcumin decreased the expression of steroidogenic acute regulatory proteins, CYP11A1 and HSD3B2 in prostate cancer cell lines, supporting the decrease of testosterone production. After 1‐month oral administration of curcumin, Aldo‐Keto reductase 1C2 (AKR1C2) expression was elevated. Simultaneously, decreased testosterone levels in the prostate tissues were observed in the TRAMP mice. Meanwhile, curcumin treatments considerably increased the expression of AKR1C2 in prostate cancer cell lines, supporting the decrease of dihydrotestosterone. Taken together, these results suggest that curcumin's natural bioactive compounds could have potent anticancer properties due to suppression of androgen production, and this could have therapeutic effects on prostate cancer.
Keywords: AKR1C2, androgen receptor, curcumin, prostate cancer, testosterone
1. INTRODUCTION
Prostate cancer is currently the second leading cause of cancer death among males. In Japan, both morbidity and mortality associated with prostate cancer are rapidly increasing. Studies have shown an increase in prostate cancer incidence in Asian men after emigration to the USA.1 These results suggest the possible role of a Western high‐fat and low‐fiber diet in prostate carcinogenesis. In addition, the Western diet does not include certain substances present in the Asian diet, such as plant‐derived antioxidants, isoflavone‐containing soy and tea polyphenols, which may protect against cancer.2 Therefore, it is hypothesized that dietary changes and pharmacologic intervention may impact prostate cancer development and progression. Indeed, increased research on functional food factors and nutrients involved in prostate cancer prevention will yield a plausible approach to measuring chemopreventive effects.
Curcumin, which is extensively used as a source of spice or pigment in curry and other foods in the Asian diet, is under clinical trial for various cancers and related diseases.3 In cultured cell analyses, curcumin has been shown to cause apoptosis and cell cycle arrest with inhibited cell growth in prostate cancer cells.4, 5, 6 Previously, we conducted a double‐blind placebo‐controlled clinical trial to evaluate the effects of soy isoflavone and curcumin on serum prostate specific antigen (PSA) levels in male patients with negative prostate cancer biopsies.7 In that clinical trial, PSA levels decreased in the patients among the group which PSA > 10 and treated with supplement containing isoflavones and curcumin (P = .01). Furthermore, curcumin treatment strongly inhibited PSA production and suppressed expression of the androgen receptor in cultured LNCaP prostate cancer cells.7 These results were used to evaluate the potential efficacy of curcumin as a prostate cancer preventive and therapeutic agent because prostate cancer has a strong association with androgens. In addition, many of curcumin's biological effects involve key components of signal transduction pathways within cancer cells, including prostate cancer cells. We previously reported that curcumin induces apoptosis through DNA damage response, which obstructs progression of various cancers.6 We performed preliminary microarray analyses to examine changes in gene expression between curcumin‐treated and curcumin‐untreated LNCaP cells. Notably, we identified an mRNA of Aldo‐Keto reductase 1C2 (AKR1C2) as being strongly induced by curcumin.8 These human aldo‐keto reductases (AKR) can convert potent sex hormones into their cognate inactive metabolites or vice versa. AKR1C2 is 1 of 4 AKR1C subfamily members that has unique substrate specificity and tissue distribution, despite sharing >84% sequence identity.9 Selective reduction occurred for the gene expression of AKR1C2 and AKR1C1, but not AKR1C3, in prostate tumor samples compared with that of their paired benign tissue.10 In prostate cells, AKR1C2 acts as a 3‐ketosteroid reductase to decrease the dihydrotestosterone (DHT) level and prevents activation of the androgen receptor. Ji et al11 suggest that selective loss of AKR1C2 in prostate cancer may promote clonal expansion of tumor cells by the enhancement of androgen‐dependent cellular proliferation through reduced DHT metabolism.
In the present study, we investigated the effect of curcumin on steroidogenesis in prostate cancer. We observed that curcumin significantly decreased testosterone and DHT levels, thereby suppressing the proliferation of LNCaP and 22Rv1 prostate cancer cells. In prostate tissues, curcumin decreased the testosterone level, which may be important for the suppression of prostate cancer tissues in vivo. Curcumin was involved in the regulation of steroidogenic enzyme expression, including AKR1C2, and suppresses prostate cancer cell growth by decreasing testosterone production, possibly by interfering with the androgen signaling pathway.
2. MATERIALS AND METHODS
2.1. Human prostate cancer cell lines and cell culture
Human prostate cancer cell lines LNCaP and 22Rv1 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), cultured in 10‐cm diameter dishes with RPMI 1640 Medium (Gibco, Gran Island, NY, USA) containing 10% (v/v) FBS (Gibco, Gran Island, NY, USA) and 1% penicillin‐streptomycin (Gibco, Gran Island, NY, USA) at 37°C under 5% CO2, and passaged with TrypLE Select (Gibco, Gran Island, NY, USA) every 3 days to maintain the cell monolayer. For investigation of testosterone and DHT levels in LNCaP cells, charcoal stripped FBS (Gibco, Gran Island, NY, USA) was used in the medium instead of FBS for 24 hours culture before curcumin treatment. LNCaP and 22Rv1 cells were characterized for androgen receptor expression. We obtained an effective preparation of curcumin (THERACURMIN),12 a nano‐particle colloidal dispersion with improved oral bioavailability (THERACURMIN, Theravalues, Tokyo, Japan). Curcumin was dissolved in ethanol at a concentration of 10 mmol/L, and was stored at −20°C until use.
2.2. Cell proliferation assay
LNCaP and 22Rv1 cells were incubated with or without curcumin, after which cell proliferation was measured by MTS assay (Promega, Madison, WI, USA). The cell proliferation of LNCaP and 22Rv1 cells were measured at the time points of 0, 24, 48 and 72 hours, respectively; responses to all treatments were assayed in quintuplicate, and results were expressed as the means of 3 separate experiments.
2.3. JC‐1 Dye to detect apoptosis
To verify the mechanism by which curcumin can induce apoptosis, we measured mitochondrial membrane potential changes in LNCaP cells. Cells were plated in 12‐well plates at a density of 1 × 105 cells/well and incubated at 37°C overnight. Cells were treated with curcumin and incubated for 6 hours. Subsequently, cells were washed with PBS 3 times and 100 μL of the JC‐1 (5,5′,6,6′‐tetrachloro‐1,1′,3,3′ tetraethylbenzimidazolylcarbocyanine iodide; Cayman Chemical, Michigan, USA). staining solution was added to each well. The cells were then incubated for 20 min in a 5% CO2 incubator at 37°C. Apoptotic cells were directly visualized with inverted fluorescence microscopy (Olympus DP72, Tokyo, Japan). In healthy cells, the dye stains the mitochondria bright red. The negative charge established by the intact mitochondrial membrane potential allows the lipophilic dye, bearing a delocalized positive charge, to enter the mitochondrial matrix and accumulate. When the critical concentration is exceeded, J‐aggregates form and become fluorescent red. In apoptotic cells, JC‐1 remains in the cytoplasm in a green fluorescent monomeric form reflecting a collapse of the mitochondrial membrane potential and an inability of JC‐1 to accumulate within the mitochondria. A viability reagent was added to wells and viability was measured after incubation for 30 minutes at 37°C. Then Caspase‐GloR 3/7 Reagent was added and luminescence was measured after 30 minutes incubation at room temperature.
2.4. Apoptosis assay for viability and caspase activation
To quantify the apoptosis induced by curcumin, we measured caspase‐3/7 activity consistent with apoptosis by ApoLive‐Glo Multiplex assay (Promega). LNCaP cells in a 96‐well plate at a density of 1 × 105 cells/mL were incubated at 37°C overnight. Cells were then incubated with curcumin at a final concentration of 0, 10, 25 and 50 μmol/L for 6 hours. As per manufacturer protocol, following incubation, 20 μL of viability reagent was added to each well and the cells were incubated for 30 m at 37°C. We measured fluorescence (relative fluorescence units [RFU]) at the wavelength of 400EX/505EM for viability using a fluorescence spectrophotometer F‐7000 (Hitachi, Tokyo, Japan). We then added 100 μL of Caspase‐Glo 3/7 Reagent to each well and incubated for 1 h at room temperature. We measured luminescence (relative luminescence units [RLU]) for apoptosis using Luminometer AB‐2350 (Atto, Tokyo, Japan).
2.5. Relative quantitation of AKR1C2 mRNA expression by RT‐PCR
Total RNA of cells were extracted with RNA‐Bee isolation reagent (Tel‐Test, Oxfordshire) according to the manufacturer's instructions. First‐strand cDNA was synthesized from total RNA with Oligo(dt)20 by SuperScript III First‐Strand Synthesis System (Gibco, Gran Island, NY, USA). RT‐PCR was carried out in a 20‐μL reaction mixture at 65°C for 5 minutes, and at 50°C for 50 minutes, using 1 μg of total RNA; 1 μL of synthesized cDNA was used for real‐time PCR using the LightCycler 480 System (Roche Molecular Systems, Mannheim, Germany). The sequences of primers for human AKR1C2 were as follows: forward 5′ GTGTGAAGCTGAATGATGGTCA 3′ and reverse 5′ TCTGATGCGCTGCTCATTGTAGCTC 3′, β‐actin: forward 5′ GACTACCTCATGAAGATCCT‐3′ and reverse 5′ GCGGATGTCCACGTCACACT 3′, respectively. Real‐time PCR was carried out in a 20‐μL reaction mixture (SYBR Green Realtime PCR Master Mix; Toyobo, Tokyo, Japan) at 95°C for 30 seconds, after which amplification was performed for 40 cycles by denaturing at 95°C for 5 seconds, annealing at 58°C for 10 seconds, and extending at 72°C for 15 seconds before continuing with melting curve analysis. All quantitative analyses were normalized to β‐actin.
2.6. Immunoblotting analysis
Subconfluent LNCaP and 22Rv1 cells were treated with curcumin for 24 h. Cells were washed twice with cold PBS and then lysed in RIPA buffer on ice for 10 min. The cell lysates were centrifuged at 10 000 g for 10 minutes at 4°C, and the supernatants were collected. Protein concentrations were measured by a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford IL, USA). Proteins were separated by SDS‐PAGE (5‐20%, SuperSep Ace, Wako, Tokyo, Japan) and transferred to a PVDF membrane (Millipore). The membrane was blocked in Blocker (Thermo Fisher Scientific, Rockford IL USA), 1% BSA in TBS (Tris‐buffered saline) for 1 hour at room temperature and then blotted with AKR1C2 (ABGENT, WuXi, China; 1:1000 dilution) rabbit polyclonal antibody, type 2 3β‐hydroxysteroid dehydrogenase (HSD3B2) (Abcam, Cambridge, UK, clone#373CT9.1.3; 1:1,000 dilution) mouse monoclonal antibody, Cytochrome P450scc (CYP11A1) (Abcam, Cambridge, UK, clone#EPR6293; 1:400 dilution) rabbit polyclonal antibody, Cytochrome P450 (17α) (CYP17A1) (Santa Cruz, Dallas, TX, USA, clone#D‐12; 1:1,000 dilution) rabbit monoclonal antibody, steroid 5α‐reductase type 1 (SRD5A1) (Proteintec, WuHan, China; 1:1,000 dilution), steroid 5α‐reductase type 2 (SRD5A2) (Abcam, Cambridge, UK, clone#EPR6181(B); 1:1000 dilution) rabbit monoclonal antibody and steroidogenic acute regulatory protein (StAR) (Abcam, Cambridge, UK, clone#EP7639; 1:3,000 dilution) rabbit monoclonal antibody, and were incubated overnight at 4°C. β‐actin antibody (Sigma‐Andrich, St. Louis, MO, USA, Clone#AC‐15; 1:10,000 dilution) was used as an internal loading control. Blots were washed 3 times (15 minutes each) with TBST (Tris‐buffered saline containing 0.1% Tween‐20). The blots were then incubated with HRP‐conjugated secondary antibody (Bio‐Rad Laboratories, Hercules, CA, USA) at a dilution of 1 in 3000 for 1 hour at room temperature and washed 3 times (15 minutes each) with TBST. Proteins were visualized with ECL Western Blotting Detection Reagents (GE Healthcare, Little Chalfont Buckinghamshire, UK) and the LAS‐3000 mini detection system (Fujifilm Medical Systems, Tokyo, Japan). An image processing program (ImageJ, NIH) was used to quantify the band density.
2.7. Immunohistochemistry
Prostate tissues excised from control and the TRAMP mice were fixed in buffered formalin and embedded in paraffin. Immunostaining was done according to standard procedures. Briefly, 4‐μm paraffin sections were deparaffinized and hydrated, and endogenous peroxidase was blocked with 3% H2O2. Antigen retrieval was done by boiling in 0.01 M citrated buffer and blocking with normal serum. Sections were incubated with primary antibody of rabbit anti‐human AKR1C2 (ABGENT, WuXi, China; 1:200 dilution) overnight at 4°C, then incubated with a second antibody (EnVision+ System Labelled Polymer‐HRP, Dako North America, CA, USA) for 30 min at room temperature. The enzyme reaction was developed with liquid chromogen‐DAB (EnVision+ Kit/HRP, Dako North America, CA, USA). Mayer's hematoxylin (MUTO PURE CHEMICALS, Tokyo, Japan) was used for nuclear counterstain according to standard protocol.
2.8. Animals
All experiments were conducted in accordance with institutional guidelines and approved by the Animal Ethics Committee (Approval No. 11‐013). All manipulations of mice were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 1 June 2006). C57BL/6 mice and heterozygous female TRAMP mice were purchased from The Jackson Laboratory. Briefly, the TRAMP model is a transgenic line of C57BL/6 mice that develop histologic prostatic intraepithelial neoplasia by 8 to 12 weeks of age, which progresses to adenocarcinoma with metastasis by 24 to 30 weeks of age. The TRAMP was established using simian virus 40 (SV40) early genes (large and small T tumor antigens) under the control of the rat probasin promoter.13
Male TRAMP mice (8 to 12 weeks old) were generated by mating TRAMP+/− females (C57BL/6 background) with non‐transgenic C57BL/6 males. The PB‐SV40 T transgene was identified using DNA extracted from tail samples and PCR primers directed at the PB‐SV40 T antigen sequence. Prostate tissues from C57BL/6 mice and TRAMP mice were obtained after 1‐month oral administration of 200 mg/kg curcumin. The prostate tissues were collected under stereoscopic microscope and stored at −80°C until analysis.
2.9. Mass‐spectrometric assay of testosterone and dihydrotestosterone
Detailed procedures are described elsewhere,14, 15 and we here briefly describe the procedures with some modifications. For extraction of steroids, cultured LNCaP cells and prostate tissues were suspended in 1 mL of distilled water and homogenized by Ultra‐Turrax homogenizer. For determination of the concentration of testosterone and DHT, the LC‐MS/MS system, which consists of an Agilent 1290 Infinity LC System (Agilent technologies, Santa Clara, CA, USA) and an API‐5000 triple stage quadrupole mass spectrometer (Applied Biosystems, USA), were employed. MS analysis was operated with electro spray ionization (ESI) in the positive‐ion mode. In the multiple reaction monitoring mode, the instrument monitored the m/z transition, from 394 to 253 for T‐picolinoyl anf from 396 to 203 for DHT‐picolinoyl.14 The limits of quantification were 1 pg for testosterone and DHT.
2.10. Statistical analysis
Data are expressed as mean ± SEM. For statistical analysis, we used 1‐way ANOVA tests (SPSS). **P < .01 was considered significant.
3. RESULTS
3.1. Growth inhibition in prostate cancer cell lines by curcumin
We examined the effects of curcumin on the proliferation of both LNCaP and 22Rv1 cells that express androgen receptors. Curcumin inhibited the growth of these cells in a dose‐dependent manner. Figure 1A shows that cell viability of LNCaP decreased by 50% when 5 μmol/L of curcumin was added to the culture medium. Higher concentrations of curcumin (25 and 50 μmol/L) could completely suppress the growth of LNCaP cells. Figure 1B also shows that 25 and 50 μmol/L of curcumin completely inhibit the growth of 22Rv1 cells.
Figure 1.
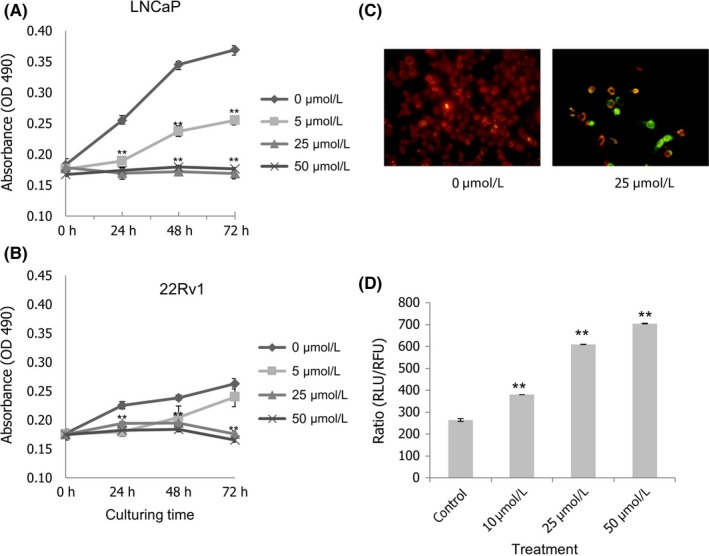
Effects of curcumin on the growth of prostate cancer cell lines. Cells were treated with different concentrations of curcumin (0 μmol/L [◆], 5 μmol/L [■], 25 μmol/L [▲] and 50 μmol/L [X]) and 3 days of MTS proliferation assays were performed. Each point represents mean ± SEM for 6 wells. Curcumin has an inhibitory effect on cell viability of both LNCaP (A) and 22Rv1 (B) cells. Statistical significance (**P < .01) was obtained for all cases of curcumin treatments (5, 25 and 50 μmol/L) compared with the control (0 μmol/L) at the time points of 24, 48 and 72 h, except for 22Rv1 with 5 μmol/L curcumin at the 72‐h time point. Induction and quantification of apoptosis after treatment of curcumin were examined. LNCaP cells were incubated with 25 μmol/L of curcumin for 6 h. Red fluorescence shows intact mitochondria in healthy cells. Green fluorescence shows that cells underwent apoptosis. JC‐1 was stained with fluorescent dye JC‐1 (C). Quantification of apoptotic cells after 6‐h treatment with curcumin (D). Error bar represents mean ± SEM from 6 wells. Vertical axis shows the ratio of caspase‐3/7 activity (relative luminescence units [RLU]) to viable cells (relative fluorescence units [RFU]). Statistical significance (**P < .01) was obtained for all cases of curcumin treatments (10, 25 and 50 μmol/L) compared with the control (0 μmol/L)
3.2. Induction of apoptosis after curcumin treatment
JC‐1, a unique cationic dye, is widely used in apoptosis studies to monitor mitochondrial health. In our study, LNCaP cells were incubated with 25 μmol/L of curcumin for 6 hours, then membrane‐permeant JC‐1 dye was used to monitor the loss of mitochondrial membrane potential.16 Figure 1C shows that fluorescent green apoptotic cells were induced after treatment with 25 μmol/L of curcumin. In addition, caspase‐3/7 activity was consistent with apoptosis and ApoLive‐Glo Multiplex Assay for quantifiable measurements of apoptosis. This resulted in a dose‐dependent decrease in viability. Caspase‐3/7 activity was normalized by viability. Figure 1D shows that curcumin treatment resulted in an increase in the ratio of caspase‐3/7 activity (RLU) to viable cells (RLU), demonstrating that curcumin‐induced apoptosis occurs in a dose‐dependent manner.
3.3. Testosterone and dihydrotestosterone levels decreased in LNCaP cells after treatment with curcumin
The levels of androgens in LNCaP decreased with 24 hours curcumin treatments in a dose‐dependent manner. Testosterone level decreased from 1.19 ng/g (control without curcumin) to 0.76 ng/g (10 μmol/L curcumin), 0.63 ng/g (25 μmol/L curcumin) and 0.33 ng/g (50 μmol/L curcumin; Figure 2A). DHT level also considerably decreased from 0.34 ng/g (control without curcumin) to 0.12 ng/g (10 μmol/L curcumin), 0.04 ng/g (25 μmol/L curcumin), and to an undetectable level (<0.03 ng/g) (50 μmol/L curcumin; Figure 2B).
Figure 2.
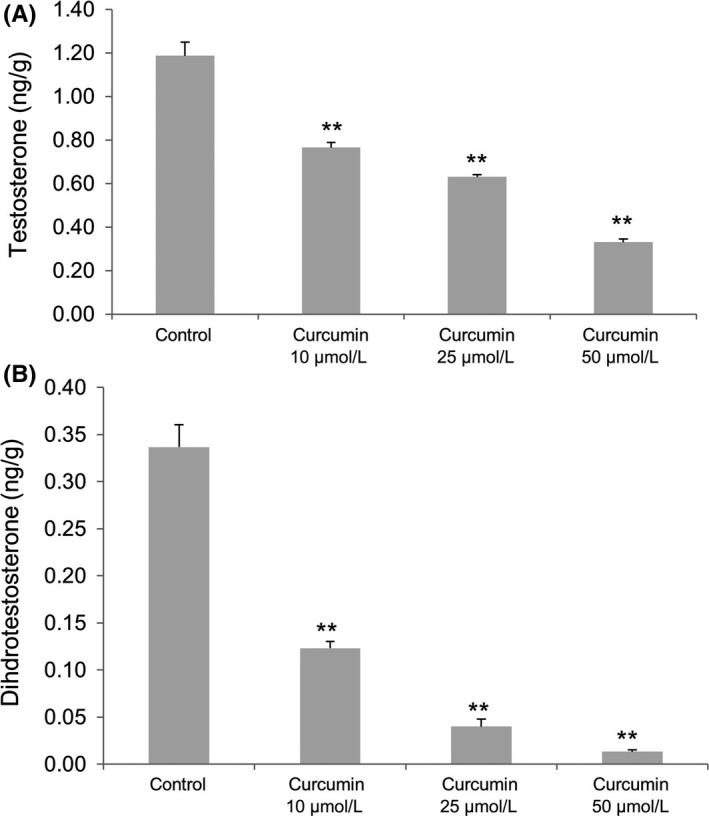
(A) Testosterone and (B) dihydrotestosterone levels in LNCaP cells after curcumin treatment at various concentrations. Statistical significance (**P < .01) was obtained for all cases of curcumin treatments (10, 25 and 50 μmol/L) compared with the control (0 μmol/L)
3.4. Curcumin upregulated AKR1C2 expression in LNCaP
AKR1C2 proteins were detected in LNCaP, 22Rv1 and DU145 cells, but not in PC3 cells (Figure 3A). AKR1C2 mRNA expression was considerably elevated, by approximately 170‐fold at 50 μmol/L curcumin, compared with control, suggesting the increase of DHT reduction (Figure 3B).
Figure 3.
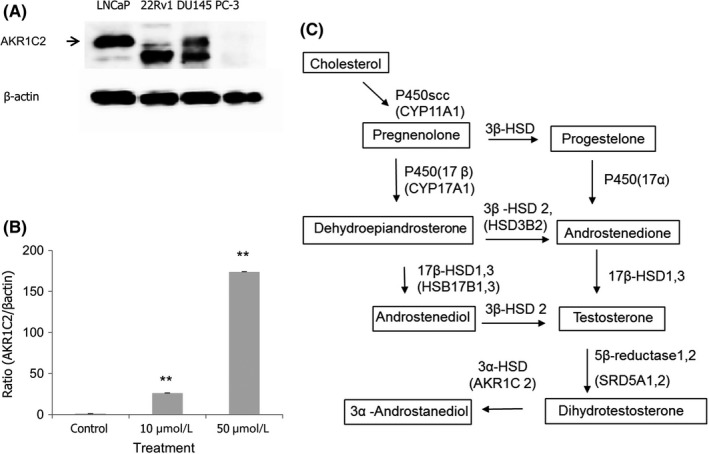
Aldo‐Keto reductase 1C2 (AKR1C2) expression in prostate cancer cell lines. (A) Expression of AKR1C2 protein shows a 37‐KDa band (arrow) in LNCaP, 22Rv1 and DU145 but not in PC‐3 cells. (B) After treatment with curcumin in LNCaP for 6 h, expression of AKR1C2 mRNA was dose‐dependently increased, approximately 170‐fold at 50 μmol/L compared with untreated cells. Values represents mean ± SEM (n = 5). All quantitation was undertaken after normalization to β‐actin. Statistical significance (**P < .01) was obtained for all cases of curcumin treatments (10 and 50 μmol/L) compared with the control (0 μmol/L). (C) Pathway of steroid metabolism in cells. Enzyme and genetic names are indicated. Steroid metabolism in the prostate tissue of transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse may be very similar to the pathway of steroid metabolism in cells
3.5. Alteration of testosterone metabolism in prostate cancer cell lines treated with curcumin
To evaluate the effect of curcumin on the proteins involved in steroidogenesis in LNCaP prostate cancer cells (Figure 3C), we examined these proteins expression using western immunoblotting after treatment with various concentrations of curcumin. StAR transports cholesterol into the mitochondria for downstream conversion into androgens.17 This is the first reaction in the process of steroidogenesis. The expression of StAR was decreased by curcumin in a dose‐dependent manner in LNCaP cells (Figure 4A). Low level expression of CYP11A1 (P450scc) was decreased after treatment of curcumin. CYP11A1 catalyzes conversion of cholesterol to pregnenolone.18 The HSD3B2 expression was decreased. CYP17A1 was expressed at a very low level but increased after treatment with curcumin. We examined the protein expression of SRD5A1 and SRD5A2, which convert testosterone to DHT. The expression of SRD5A2 was unaffected by the treatment of curcumin. The result showed that SRD5A1 expression in LNCaP cells was dose‐dependently increased with curcumin (10 to 50 μmol/L) compared with control (Figure 4A). We also performed these analyses in another androgen receptor‐positive prostate cancer cell line, 22Rv1, and similar results were obtained (Figure 4B).
Figure 4.
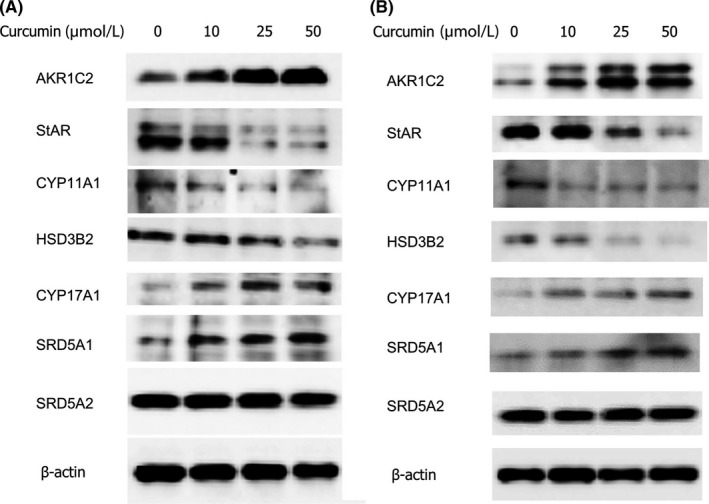
Curcumin alters the expression of enzymes involved in testosterone metabolism. Protein expression in LNCaP (A) and 22Rv1 (B) were measured by western blot after 24‐h treatment with curcumin. The protein bands from top to bottom were Aldo‐Keto reductase 1C2 (AKR1C2), steroidogenic acute regulatory protein (StAR), Cytochrome P450scc (CYP11A1), type 2 3β‐hydroxysteroid dehydrogenase (HSD3B2), Cytochrome P450(17α) (CYP17A1), steroid 5α‐reductase type 1 (SRD5A1), steroid 5α‐reductase type 2 (SRD5A2) and β‐actin. Molecular weight is 37, 30, 60, 42, 55, 28, 29 and 42 KDa, respectively. Mouse anti‐human β‐actin antibody was used as an internal control
3.6. Testosterone and dihydrotestosterone levels in mouse prostate tissues after curcumin treatment
Figure 5A shows the androgen concentrations of prostate tissue of TRAMP mice, a well‐established prostate cancer model,13 after 1‐month oral administration of 200 mg/kg/d of curcumin. Curcumin reduced the testosterone level in mouse prostate tissues of TRAMP mice compared with untreated mice (P < .01) (Figure 5A). Nevertheless, the DHT concentration was not decreased (Figure 5B).
Figure 5.
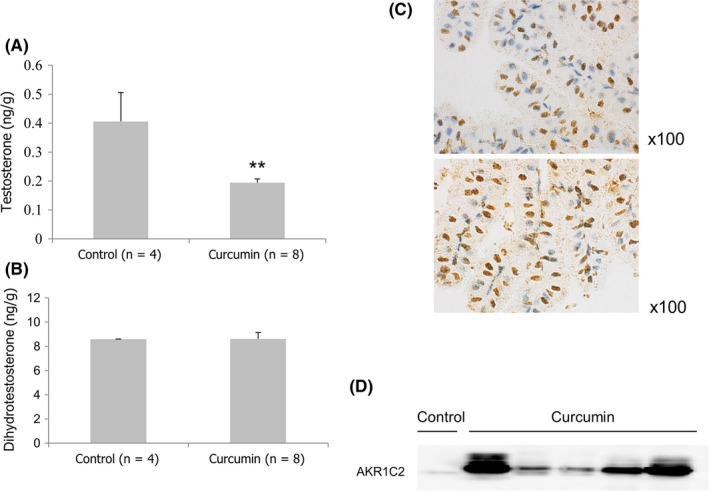
(A) Testosterone and (B) dihydrotestosterone levels in prostate tissues of transgenic adenocarcinoma of the mouse prostate (TRAMP) mice after 1‐month oral administration of curcumin. Each bar represents mean ± SEM for 4 untreated and 8 curcumin‐treated TRAMP mice, respectively. Statistical significance (**P < .01) was obtained for curcumin treatment compared with the control. (C) Immunohistochemistry showing Aldo‐Keto reductase 1C2 (AKR1C2) proteins were expressed in prostate epithelial cells of TRAMP mice. (D) Expression of AKR1C2 in individual prostate tissue of curcumin‐treated TRAMP mice compared with control
As shown in Figure 5c, AKR1C2 was expressed in prostate epithelial cells although the staining pattern was heterogeneous. The expressed protein was localized in nucleus and also weakly detected in cytoplasm of prostate epithelial cells. AKR1C2 expression was upregulated in the prostate cancer tissues of TRAMP mice after curcumin treatment for 1 month (Figure 5D). After 1‐month consumption of curcumin diets, no significant changes in body weight and prostate volume were observed between the treatment and control groups. Administration of curcumin in these mice showed no influence on the serum testosterone level (data not shown). According to recent research,19 1000 mg/d curcumin was used in clinical trials. In our study, we estimated that approximately 10 times the circulating concentration of curcumin was used in in vitro (50 μmol/L) and in vivo (200 mg/kg/d) experiments compared with this clinical trial.
4. DISCUSSION
Many prostate cancer cells undergo androgen‐dependent proliferation. Signal transduction aiding androgen and androgen receptors plays an important role, by governing propagation and differentiation of prostate cancer cells. However, there is no clear data indicating any correlation between serum testosterone levels and prostate cancer in humans.20 Multiple studies have shown that intratumoral steroidogenesis of testosterone in prostate cancer cells is active, including the case of castration‐resistant prostate cancer (CRPC).20 To date, a series of steroidogenic enzymes necessary for androgen biosynthesis from cholesterol were observed in prostate cancer (Figure 3C).21, 22, 23 Moreover, circulating cholesterol levels were directly correlated with tumor expression of CYP17A1.24 CYP17A1 is the critical enzyme required for de novo synthesis of androgens, because CYP17A1 synthesizes dehydroepiandrosterone (DHEA). These results are consistent with the hypothesis that intratumoral androgen synthesis may accelerate the growth of prostate tumors. Currently, abiraterone, an inhibitor of cytochrome CYP17A1, has yielded high durable responses in CRPC patients, by reducing testosterone levels.25 We studied the effect of curcumin on the proliferation of other cancer cell lines, such as T24 and RT4 bladder cancer cells, by MTS assay. Although at the concentration of 50 μmol/L curcumin demonstrated suppression of cell proliferation, the degree of growth suppression in bladder cancer cells was lower compared with prostate cancer cells. As we previously, reported curcumin also induces DNA damage response to suppress the cell growth, and we suppose that this signaling pathway may be involved in bladder cancer cells.6
In LNCaP, cDNA microarray analysis identified AKR1C2 as a factor in androgen metabolism and verified that curcumin increases its expression. In the prostate, DHT is predominantly metabolized by AKR1C2.9, 10 AKR1C2 is highly expressed in human prostate.26 Compared to the paired benign tissues, prostate cancer tissues showed reduced metabolism of DHT, which corresponded with a loss of AKR1C2 expression.10 Transient expression of AKR1C2 reduced DHT‐stimulated proliferation of LAPC‐4 prostate cancer cells. Cellular proliferation experiments showed that increased AKR1C2 expression reduced DHT‐stimulated cell growth, and the decrease of DHT blocked the activation of AR.11 Therefore, the induction of AKR1C2 by curcumin may reduce androgenic activities in prostate cancer cells. We observed a decrease of concentration in testosterone and DHT when AKR1C2 expression was increased by curcumin administration in LNCaP cells. We also examined the testosterone and DHT levels in LNCaP cells treated with siRNA of AKR1C2. The testosterone and DHT levels were increased from 0.51 to 1.09 pg per assay and 0.95 to 1.17 pg per assay, respectively (data not shown).
Intraprostatic androgen levels in castrated male patients with locally recurrent CRPC are further elevated relative to serum levels, while testosterone levels in tissues in metastatic CRPC may actually be higher than in the prostate prior to castration.23, 27, 28, 29 Moreover, the recent study showed that the high testosterone levels in prostate tissue had a good correlation with high Gleason scores and advanced clinical stages.30 The presence of significant amounts of testosterone in the tissues of recurrent prostate cancers may be due to endogenous synthesis of androgens from adrenal steroid precursors or cholesterol.29 It could be deduced from the current study that curcumin may reduce testosterone in these prostate cancer cells through the reduction of expressions of testosterone synthesizing proteins, because in LNCaP, the expression of StAR, CYP11A1 and HSD3B2 were significantly decreased. A decrease in testosterone concentration in the prostate was observed in both in vitro and in vivo analysis when AKR1C2 expression was increased by curcumin administration. However, the concentration of DHT, which has higher androgen activity than testosterone, remained unchanged in an in vivo model. One possible reason is that DHT synthesis through HSD17B10 may be able to convert 5α‐androstanediol into DHT in vivo.31 Further studies should be conducted in different prostate cancer mouse models.
The current study is the first report of a curcumin‐facilitated reduction in intracellular prostate testosterone. As curcumin reduces testosterone activity in LNCaP and prostate cancer model TRAMP, it may have a role in inhibiting cancer onset and growth. Collectively, our studies suggest that curcumin can directly and indirectly regulate the intracellular activity of androgen signaling, thereby providing new therapeutic targets for prostate cancer. The combination of docetaxel and curcumin in patients with CRPC was well tolerated in a phase II study; and a randomized trial is pending.32 As a new molecular targeted therapy, combination therapy of curcumin with some of the enzyme inhibitors of testosterone metabolism may allow lower doses of these inhibitors for improved efficacy and decreased side effects.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
ACKNOWLEDGMENTS
We thank Dr H. Sasamoto (Aska Pharmamedical) for steroid analysis and K. Hamamoto for animal experiments.
Ide H, Lu Y, Noguchi T, et al. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018;109:1230–1238. https://doi.org/10.1111/cas.13517
REFERENCES
- 1. Namiki M, Akaza H, Lee SE, et al. Prostate Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i70‐i75. [DOI] [PubMed] [Google Scholar]
- 2. Horie S. Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean J Urol. 2012;53:665‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prasad S, Gupta SC, Tyagi AK, et al. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053‐1064. [DOI] [PubMed] [Google Scholar]
- 4. Teiten MH, Gaascht F, Eifes S, et al. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5:61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer‐I. curcumin induces apoptosis in both androgen‐dependent and androgen‐independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84‐93. [DOI] [PubMed] [Google Scholar]
- 6. Ide H, Yu J, Lu Y, et al. Testosterone augments polyphenol‐induced DNA damage response in prostate cancer cell line, LNCaP. Cancer Sci. 2011;102:468‐471. [DOI] [PubMed] [Google Scholar]
- 7. Ide H, Tokiwa S, Sakamaki K, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate‐specific antigen. Prostate. 2010;70:1127‐1133. [DOI] [PubMed] [Google Scholar]
- 8. Ide H, Lu Y, Yamaguchi R, et al. Chemopreventive potential of curcumin in prostate cancer. American Association of Cancer Research 105th Annual Meeting. 2014; Abstract #832.
- 9. Penning TM, Burczynski ME, Jez JM, et al. Human 3alpha‐hydroxysteroid dehydrogenase isoforms (AKR1C1‐AKR1C4) of the aldo‐keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ji Q, Chang L, Van Den Berg D, et al. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate. 2003;54:275‐289. [DOI] [PubMed] [Google Scholar]
- 11. Ji Q, Chang L, Stanczyk FZ, et al. Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre‐receptor regulator of androgen receptor signaling. Cancer Res. 2007;67:1361‐1369. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660‐665. [DOI] [PubMed] [Google Scholar]
- 13. Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439‐3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hojo Y, Higo S, Ishii H, et al. Comparison between hippocampus‐synthesized and circulation‐derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106‐5112. [DOI] [PubMed] [Google Scholar]
- 15. Kato A, Hojo Y, Higo S, et al. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smiley ST, Reers M, Mottola‐Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J‐aggregate‐forming lipophilic cation JC‐1. Proc Natl Acad Sci U S A. 1991;88:3671‐3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration‐resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390‐400. [DOI] [PubMed] [Google Scholar]
- 18. Pinski J, Xiong S, Wang Q, et al. Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate. 2011;71:892‐898. [DOI] [PubMed] [Google Scholar]
- 19. Nasseri E, Mohammadi E, Tamaddoni A, et al. Benefits of curcumin supplementation on antioxidant status in beta‐thalassemia major patients: a double‐blind randomized controlled clinical trial. Ann Nutr Metab. 2017;71:136‐144. [DOI] [PubMed] [Google Scholar]
- 20. Armandari I, Hamid AR, Verhaegh G, et al. Intratumoral steroidogenesis in castration‐resistant prostate cancer: a target for therapy. Prostate Int. 2014;2:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration‐resistant prostate cancer. Cancer Res. 2008;68:6407‐6415. [DOI] [PubMed] [Google Scholar]
- 22. Dillard PR, Lin MF, Khan SA. Androgen‐independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration‐resistant tumor growth. Cancer Res. 2008;68:4447‐4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mostaghel EA, Solomon KR, Pelton K, et al. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7:e30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen‐independent prostate cancer. Cancer Res. 2006;66:2815‐2825. [DOI] [PubMed] [Google Scholar]
- 27. Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850‐3856. [DOI] [PubMed] [Google Scholar]
- 28. Mohler JL, Gregory CW, Ford OH III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440‐448. [DOI] [PubMed] [Google Scholar]
- 29. Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653‐4657. [DOI] [PubMed] [Google Scholar]
- 30. Miyoshi Y, Uemura H, Umemoto S, et al. High testosterone levels in prostate tissue obtained by needle biopsy correlate with poor‐prognosis factors in prostate cancer patients. BMC Cancer. 2014;14:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jernberg E, Thysell E, Bovinder Ylitalo E, et al. Characterization of prostate cancer bone metastases according to expression levels of steroidogenic enzymes and androgen receptor splice variants. PLoS One. 2013;8:e77407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahammedi H, Planchat E, Pouget M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration‐resistant prostate cancer: a Pilot Phase II Study. Oncology. 2016;90:69‐78. [DOI] [PubMed] [Google Scholar]


