Abstract
Background
Racial disparities in prostate cancer survival (PCS) narrowed in the prostate-specific antigen (PSA) era, suggesting screening may induce more equitable outcomes. However, effects of lead time and overdiagnosis can inflate survival even without real screening benefit.
Methods
Simulation model of PCS in the early PSA era (1991–2000). The modeled survival starts with baseline survival in the pre-PSA era (1975–1990) and adds lead times and overdiagnosis using estimates from published studies. We quantify (1) discrepancies between modeled and observed PCS in the PSA era and (2) residual period effects on PCS given specified values for screening benefit.
Results
Lead time and overdiagnosis explain more of the improvement in PCS for older ages at diagnosis (46% (95% CI 44–50%) for blacks and 51% (CI 50–52%) for all races ages 50–54 versus 98% (CI 97–99%) for blacks and 100% for all races ages 75–79). They also explain more of the narrowing in PCS disparities for older ages (33% (CI 31–43%) for ages 50–54 versus 74% (CI 71–81%) for ages 75–79). The period effects amount to 27–40% (blacks) and 26–38% (all races) reductions in the risk of prostate cancer death depending on the screening benefit.
Conclusion
Real improvements in survival disparities in the PSA era are smaller than those observed and reflect similar reductions in the risk of prostate cancer death among blacks and all races. Understanding screening artifacts is necessary for valid interpretation of observed survival trends.
Keywords: disparities, lead time, overdiagnosis, prostatic neoplasms, prostate-specific antigen, race, screening, survival
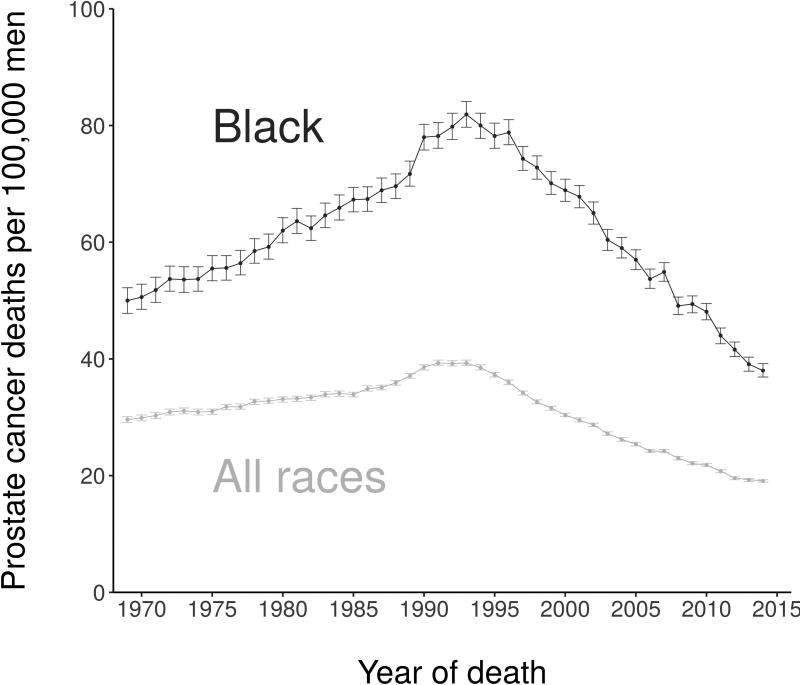
There are substantial racial disparities in prostate cancer in the US, with black men having mortality rates approximately double those in white men.1 Since the widespread adoption of prostate-specific antigen (PSA) screening for prostate cancer in the early 1990s, there has been a clear narrowing of the disparities in mortality (Figure 1). Powell and colleagues2 noted this trend and offered it as evidence in favor of early and aggressive screening among black men. However, it is well known that screening induces artifactual effects that inflate observed survival even in the absence of any screening benefit. Therefore, the objective of this study is to determine the extent to which changes in survival disparities during the PSA era reflect real survival improvement beyond artifacts of screening.
Figure 1.
Observed prostate cancer mortality rates for blacks and all races, 1969–2014
The term “artifacts of screening” refers to the predictable effects of screening on survival that are known to occur even in the absence of benefit. These include lead time, the time by which screening advances diagnosis, and overdiagnosis, the detection of cases that wouldn’t have been diagnosed within their lifetimes in the absence of screening. Retrospective studies of screening uptake in the US have shown rapid and widespread adoption of screening in both blacks and all races.3 “Real survival improvement” refers to the true reduction in prostate cancer mortality after excluding these artifacts and may be attributed to true benefit of screening or other changes in disease management in preventing or delaying prostate cancer mortality.
We first determine the portion of the improvement in disease-specific survival explained by lead time and overdiagnosis, which we previously estimated separately for blacks and all races.4, 5 We then analyze the unexplained portion of the observed improvement to make inferences about patterns of real survival improvement among blacks and all races during the PSA era.
Methods
Overview
Utilization of the PSA test for prostate cancer screening in the US began in the late 1980s and increased rapidly after 1990.3 We consider the years prior to and including 1990 as the pre-PSA era and the years from 1991 onwards as the post-PSA or the PSA era. Our analysis uses three types of net (in the absence of other-cause death) survival curves. The observed pre-PSA curve is the survival among Surveillance, Epidemiology, and End Results (SEER) prostate cancer cases diagnosed in calendar years 1975–1990, and the observed post-PSA curve is the survival among SEER prostate cancer cases diagnosed in calendar years 1991–2000. The modeled post-PSA curve is a projection of PSA-era survival that results from adding the artifacts of screening to the observed pre-PSA curve. By comparing the modeled curve against the pre- and post-PSA curves, we calculate the proportion of the change in 10-year survival from the pre-PSA era to the post-PSA era that is solely due to artifact. When this proportion is less than 1, we analyze implications for real survival improvements.
Data
We use SEER*Stat6 to calculate 10-year net disease-specific survival for prostate cancer cases diagnosed in the core 9 registries by race group (blacks and all races), 5-year age group from ages 50–54 to 75–79 years at diagnosis, and diagnosis in the pre-PSA and post-PSA years.
We source estimates of lead time, fractions of cases detected by screening, and fractions of overdiagnosed cases from the Fred Hutch model of prostate cancer in the US population.7, 8 The model produces individual-level disease histories with and without screening for blacks and all races,4, 5 which can be used to produce the requisite inputs for deriving the modeled curves.
Deriving modeled post-PSA curves
In the pre-PSA era, all cases are clinically diagnosed, meaning they are diagnosed without screening. In contrast, the post-PSA curve represents a combination of screen- and non-screen-detected cases. The screen-detected cases are themselves a combination of overdiagnosed and non-overdiagnosed cases. Overdiagnosed cases cannot die of disease; their disease-specific survival is 100% by definition. It is impossible to know whether an individual case has been overdiagnosed, though associations with patient and tumor features have been identified.9, 10 Non-overdiagnosed cases have a survival time that is the sum of the lead time and a post-lead-time survival time that begins when they would have been diagnosed without screening. If baseline survival does not change over time, the modeled post-PSA survival time is:
| (1) |
where TPRE is the observed pre-PSA era survival time, P is the fraction of cases that are screen detected, Q is the fraction of screen-detected cases that are overdiagnosed during the PSA era, and L is the lead time. The first term on the right-hand side of (1) represents the non-screen-detected fraction, and the second represents the screen-detected fraction.
We simulate times TPSA by generating times TPRE based on SEER data and estimates of P, Q, and L from the Fred Hutch model. We present two types of estimates. The first uses only the estimated mean lead times from this model and samples from exponential distributions to simulate values for L. The second samples from the full distribution of lead times output by the Fred Hutch model. Details of this process are provided in Section 1, Simulation modeling to project effects of lead time and overdiagnosis, of the Supplementary Methods. Sample sizes are set to observed numbers of men in each race and age group at diagnosis in the pre-PSA era, and 95% confidence intervals show associated uncertainty in estimated survival.
Quantifying survival improvement and survival disparity explained by artifact
For each race and age group at diagnosis, the proportion ρ of the survival improvement in the PSA era explained by artifacts of screening is:
where A is the observed 10-year survival in the pre-PSA era, B is the modeled 10-year survival in the PSA era, and C is the observed 10-year survival in the PSA era. In general, B will lie between A and C. The proportion ρ reflects the improvement explained by artifacts of screening as a fraction of the entire survival improvement between eras. If B is close to C, most of the survival improvement is explained by artifact, and the proportion explained is close to 1; but if B is close to A, the proportion explained is close to 0.
Additionally, for each age group at diagnosis, the proportion ρ′ of the improvement in the survival disparity in the PSA era explained by artifacts of screening is given by the same ratio but where A is the difference in observed 10-year survival between blacks and all races in the pre-PSA era, B is the difference in modeled 10-year survival between blacks and all races in the PSA era, and C is the difference in observed 10-year survival between blacks and all races in the PSA era.
Sensitivity analysis
It is clear that ρ and ρ′ may be sensitive to the input values for lead times and frequencies of cases detected by screening and overdiagnosed cases. To investigate this sensitivity, we re-calculate ρ and ρ′ varying these inputs over a range that is consistent with a prior study.11 Specifically, we simultaneously increase or decrease mean lead times and overdiagnosis frequencies within each age group by 20%, generate the resulting modeled 10-year survival (or difference in 10-year survival between blacks and all races) in the PSA-era, and recalculate ρ and ρ′.
Inferring real survival improvement for all cases and for cases detected by screening
When the estimated ρ is less than 1, we infer there is a real survival improvement in the PSA era compared with the pre-PSA era. We partition the real survival improvement into a period effect that applies to all prostate cancer cases and an effect associated with screen detection. Specifically, the period effect δ is a relative risk that acts on both instances of TPRE in (1), while the screen-detection effect μ is a relative risk that acts only on the second instance of TPRE (i.e., on screen-detected cases). We estimate δ given a range of fixed values for μ that includes estimates of PSA screening benefit from published trials. A lower limit of 0.5 is motivated by the Göteborg Randomized Prostate Cancer Screening Trial12, 13 and an upper limit of 1.0 is motivated by the Prostate, Lung, Colorectal, and Ovarian cancer screening trial.14, 15 Details and outputs of the estimation procedure can be found in Section 2, Inferring Survival Improvement, of the Supplementary Methods.
Results
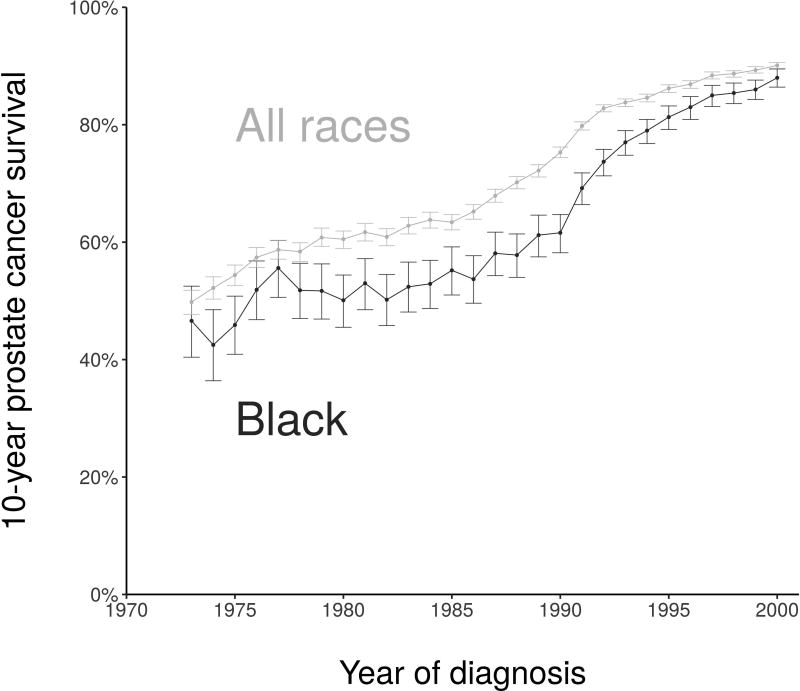
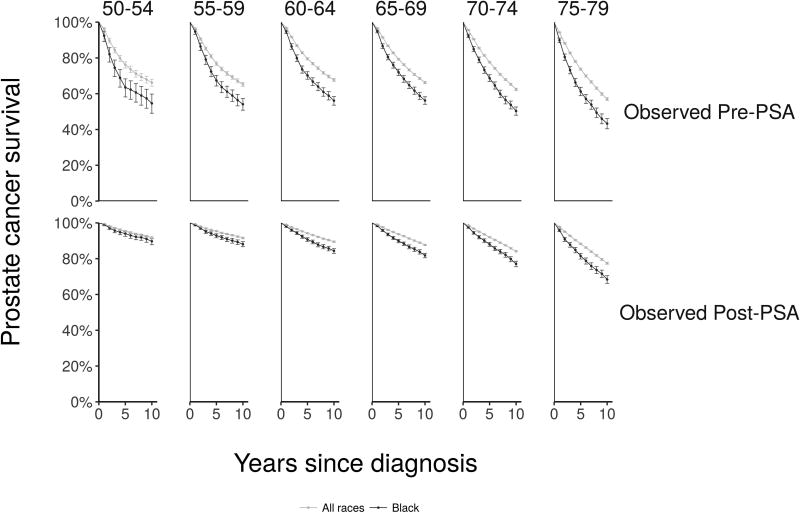
Figure 2 shows 10-year disease-specific survival and 95% confidence intervals16 for prostate cancer cases detected in SEER by race group. Observed disease-specific survival curves are shown in Figure 3 separately for the pre-PSA and PSA eras. Figures 2 and 3 demonstrate the narrowing of survival differences between blacks and all races diagnosed with prostate cancer after the introduction of PSA screening.
Figure 2.
Observed 10-year net disease-specific survival by year of diagnosis, 1973–2000
Figure 3.
Observed disease-specific survival curves by age group at diagnosis (columns) and years since diagnosis in the pre-PSA era (upper row) and PSA era (lower row)
Table 1 provides the fraction of cases detected by screening in the post-PSA era, the fraction of screen-detected cases overdiagnosed, and the mean lead times produced by the Fred Hutch model for blacks and all races. The full lead-time distributions produced by the Fred Hutch model are shown in Figure 1 of the Supplementary Methods. The fraction screen detected is similar across age groups and is generally slightly lower for blacks than for all races. The fraction overdiagnosed ranges from approximately 10% for men diagnosed at ages 50–54 to almost 60% for men diagnosed at ages 75–79. The mean lead time among non-overdiagnosed cases decreases with age because these cases must have a date of diagnosis without screening that precedes their date of other-cause death. Both overdiagnosis frequencies and mean lead times are similar for blacks and all races. This is consistent with two other models of prostate cancer natural history previously calibrated to SEER incidence data.4, 5
Table 1.
Observed 10-year disease-specific survival in the pre-PSA era and key results from Fred Hutch model used to model post-PSA survival. The percent of cases detected by screening is among all cases in the PSA era; the percent of cases overdiagnosed is among all screen-detected cases; mean lead time is the average lead time among all cases who would have been clinically diagnosed within their lifetimes in the absence of screening.
| Blacks | All Races | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | 10-year PCS, % |
Cases detected by screening, % |
Cases over- diagnosed, % |
Mean lead time, y |
10-year PCS, % |
Cases detected by screening, % |
Cases over- diagnosed, % |
Mean lead time, y |
| 50–54 | 54.6 | 54.4 | 12.3 | 8.9 | 66.2 | 55.8 | 10.0 | 9.4 |
| 55–59 | 54.1 | 61.1 | 17.3 | 8.9 | 65.2 | 63.2 | 19.6 | 9.5 |
| 60–64 | 56.1 | 57.1 | 29.1 | 8.7 | 67.7 | 63.7 | 27.4 | 8.4 |
| 65–69 | 56.2 | 59.5 | 38.3 | 7.2 | 66.2 | 65.6 | 37.1 | 7.5 |
| 70–74 | 50.3 | 58.9 | 46.1 | 6.3 | 62.4 | 66.6 | 48.3 | 6.6 |
| 75–79 | 43.3 | 56.3 | 57.8 | 5.4 | 57.0 | 63.5 | 58.9 | 5.5 |
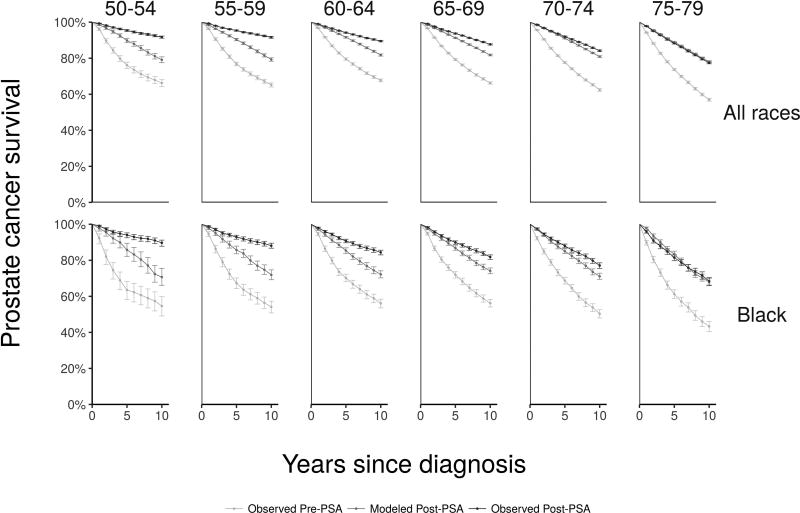
Figure 4 shows observed pre-PSA, modeled post-PSA, and observed post-PSA survival curves by race and age group. We find that artifacts of screening explain most of the observed survival improvement for older men; modeled post-PSA curves approach observed post-PSA curves. However, artifacts of screening explain much less of the observed survival improvement for younger men.
Figure 4.
Observed pre-PSA survival, modeled survival incorporating artifacts of screening, and observed post-PSA survival age group at diagnosis (columns) for all races (upper row) and blacks (lower row)
Table 2 gives the proportion of the improvement in 10-year survival explained by artifacts of screening, and Table 3 provides the proportion of the improvement in 10-year survival disparity explained by artifacts of screening. Screening artifacts explain less of the survival improvement and less of the change in survival disparity among younger than among older men. A similar pattern is observed under one-way sensitivity analysis (Table 1 of the Supplementary Methods). In all settings, the proportion explained by artifacts remains considerably lower for younger men. Relaxing the exponential assumption and using the full range of lead times from the Fred Hutch model rather than only the mean produces similar results, as shown in Section 3, Results Relaxing Exponential Assumptions, of the Supplementary Methods.
Table 2.
Observed 10-year disease-specific survival in the pre-PSA and PSA eras, modeled 10-year survival in the PSA era, and proportion of improvement explained by artifacts of screening.
| Age | Black | All races | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-PSA survival, % |
Modeled post-PSA survival, % |
Post-PSA survival, % |
Proportion of improvement explained by artifacts of screening (ρ) |
Pre-PSA survival, % |
Modeled post- PSA survival, % |
Post-PSA survival, % |
Proportion of improvement explained by artifacts of screening (ρ) |
|
| 50–54 | 54.6 | 70.7 | 89.7 | 0.46 (0.44–0.50) | 66.2 | 79.1 | 91.7 | 0.51 (0.50–0.52) |
| 55–59 | 54.1 | 71.9 | 88.1 | 0.52 (0.51–0.54) | 65.2 | 79.3 | 91.6 | 0.53 (0.53–0.54) |
| 60–64 | 56.1 | 72.2 | 84.4 | 0.57 (0.57–0.58) | 67.7 | 81.8 | 89.5 | 0.65 (0.64–0.65) |
| 65–69 | 56.2 | 74.1 | 81.8 | 0.70 (0.70–0.71) | 66.2 | 81.8 | 87.7 | 0.72 (0.72–0.73) |
| 70–74 | 50.3 | 71.1 | 77.1 | 0.78 (0.78–0.78) | 62.4 | 80.9 | 84.2 | 0.85 (0.85–0.85) |
| 75–79 | 43.3 | 67.9 | 68.4 | 0.98 (0.97–0.99) | 57.0 | 78.1 | 77.4 | 1.03 (1.03–1.03) |
Table 3.
Observed 10-year disease-specific survival disparities in the pre-PSA and PSA eras, modeled 10-year survival disparities in the PSA era, and proportion of change in disparity explained by artifacts of screening.
| Age | Pre-PSA disparity, % difference |
Modeled disparity, % difference |
Post-PSA disparity, % difference |
Proportion of change in disparity explained by artifacts of screening (ρ′) |
|---|---|---|---|---|
| 50–54 | 11.6 | 8.5 | 2.0 | 0.35 (0.01–0.58) |
| 55–59 | 11.1 | 7.4 | 3.5 | 0.36 (0.11–0.55) |
| 60–64 | 11.6 | 9.6 | 5.1 | 0.48 (0.28–0.66) |
| 65–69 | 10.0 | 7.6 | 5.9 | 0.55 (0.25–0.80) |
| 70–74 | 12.1 | 9.8 | 7.1 | 0.44 (0.14–0.69) |
| 75–79 | 13.7 | 10.2 | 9.0 | 0.69 (0.31–1.04) |
Tables 2 and 3 also indicate that there are real survival improvements beyond those explained by artifacts of screening. Table 4 presents the best-fitting values for the period effect (δ) across the values for the assumed effect of screen detection (μ) for men age 50–79 years; results for individual age groups are provided in Table 4 of the Supplementary Methods. The magnitude of the period effect depends on the specified effect associated with screen detection. If it is believed, for example, that screen detection is not efficacious, then the period effect l amounts to 38% (all races) to 40% (blacks) reduction in the risk of prostate cancer death. However, if screen detection is as efficacious as suggested by the European Randomized Study of Screening for Prostate Cancer (21% reduction in mortality risk17), then the period effect amounts to a 34% (all races) to 35% (blacks) reduction in the risk of prostate cancer death. In general, provided the effect of screen detection is similar among blacks and all races, the period effect is also similar. Table 4 of the Supplementary Methods shows that this finding persists within individual age groups. The estimated period effect is largest among younger men and is slightly stronger for blacks than all races for most assumed values for the screen-detection effect.
Table 4.
Estimated values for real survival improvement (expressed as a relative hazard of disease-specific death following the point of clinical diagnosis) due to a period effect corresponding to specified values of real survival improvement (also expressed as a relative hazard of disease-specific death) associated with screen detection.
| Specified survival improvement associated with screen detection (μ) |
Estimated value for real survival improvement due to a period effect (δ) |
|
|---|---|---|
| Blacks | All Races | |
| 0.5 | 0.73 | 0.74 |
| 0.6 | 0.70 | 0.71 |
| 0.7 | 0.67 | 0.68 |
| 0.8 | 0.65 | 0.66 |
| 0.9 | 0.62 | 0.64 |
| 1.0 | 0.60 | 0.62 |
Discussion
Since the advent of PSA screening, the profile of prostate cancer in the US has changed dramatically. Reductions in disease-specific mortality have been accompanied by a narrowing of racial disparities in disease-specific survival, leading to some optimism about the ability of screening to reduce established inequities in the burden of the disease.2 The present study has explored the extent to which these apparent trends in disparities are real. We find that a sizeable portion of these trends are not, in fact, real; rather, they are attributable to artifacts of screening, particularly in older men. Indeed, among the oldest men in our analysis, artifacts of screening explain 69% of the narrowing in 10-year survival disparities. For younger men, artifacts of screening explain only 35% of the narrowing in 10-year survival disparities.
Our results show that overall there has been a real and clinically significant survival improvement during the PSA era among prostate cancer cases, including non-screen-detected cases. Indeed, we can identify a period effect reflecting real survival improvement across a range of plausible effects of early detection. The period effect amounts to a reduction in the risk of disease-specific death that ranges 27–40% for blacks and 26–38% for all races. This period effect is likely attributable to a series of factors, including treatment availability, advances in cancer therapy both in the primary and salvage settings, and earlier identification of disease recurrence after diagnosis. Data on treatment trends indicate widespread adoption of primary prostate surgery in the late 1980s and early 1990s18 and increased use of adjuvant hormonal blockade with primary radiation for high-risk, localized disease in the 1990s.19 Both of these treatments have been associated with significant benefit in randomized trials.20, 21 Further, use of PSA monitoring after primary therapy has led to earlier detection and treatment of recurrence, which has been associated with significant benefit.22, 23
Limitations
Our approach relies on prostate cancer incidence and survival data from the SEER database. Although concerns about representativeness have been raised,24 this database is widely recognized as a high-quality source of information about national cancer trends. Further, our analysis incorporates estimates of the frequency of screen-detected cases, the frequency of overdiagnosed cases, and the lead time among those not overdiagnosed based on a model of prostate cancer natural history in the US. Inaccuracies in these estimates could translate into biases in our results, but sensitivity analysis shows our conclusions to be quite robust.
Our approach also makes several simplifying assumptions. First, in the absence of screening benefit, we assume that the distribution of disease-specific survival following clinical diagnosis is the same for cases not screen detected and for (non-overdiagnosed) cases detected by screening. Further, we assume that both survival distributions are well approximated by the observed, pre-PSA survival. When we estimate the real survival improvement during the PSA era, we assume that the period effect and the effect of early detection are independent.
An additional limitation is that when we report confidence intervals for the proportion of the improvement and change in disparity attributable to artifacts of screening we do not account for uncertainty in inputs from the Fred Hutch model, namely, the fraction of men diagnosed by screening, the fraction overdiagnosed, or the mean lead times. Finally, our primary results are based on 10-year survival rather than on the entire survival curve. Results may be mildly sensitive to the specific point selected, but we have found that choosing a different point, such as 5-year survival (Table 2 of the Supplementary Methods), does not materially affect our conclusions. We do not anticipate our primary conclusions changing with longer follow up.
In conclusion, we do not dispute that long-standing racial disparities in prostate cancer survival may be narrowing. However, the real survival improvement during the PSA era is more modest than that observed. Understanding the age- and race-specific patterns of artifacts of screening on disease-specific survival trends is a necessary prerequisite if we are to use these trends in developing prostate cancer screening policies that are appropriate for black men and for the general population.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant number U01 CA199338).
We thank Dr. Isaac Powell for comments on an earlier draft of this paper.
Footnotes
Disclaimer: The study sponsor had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest: None
Author contributions: Conceptualization: R. Etzioni
Methodology: D. Kaur, E. Ulloa-Pérez, R. Gulati, R. Etzioni
Software: D. Kaur, E. Ulloa-Pérez, R. Gulati
Validation: D. Kaur, E. Ulloa-Pérez, R. Gulati
Formal Analysis: D. Kaur, R. Gulati, R. Etzioni
Investigation: N/A
Resources: N/A
Data Curation: D. Kaur, E. Ulloa-Pérez, R. Gulati
Writing – Original Draft Preparation: D. Kaur, R. Etzioni
Writing – Review & Editing: D. Kaur, E. Ulloa-Pérez, R. Gulati, R. Etzioni
Visualization: D. Kaur, E. Ulloa-Pérez, R. Gulati
Supervision: R. Etzioni
Project Administration: R. Gulati
Funding Acquisition: R. Etzioni
References
- 1.Stanford JL, Stephenson RA, Coyle LM, et al. Prostate Cancer Trends, 1973 – 1995. Bethesda, MD: SEER Program, National Cancer Institute; 1999. [Google Scholar]
- 2.Powell IJ, Vigneau FD, Bock CH, Ruterbusch J, Heilbrun LK. Reducing prostate cancer racial disparity: evidence for aggressive early prostate cancer PSA testing of African American men. Cancer Epidemiology, Biomarkers and Prevention. 2014;23:1505–1511. doi: 10.1158/1055-9965.EPI-13-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–1886. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 4.Gulati R, Wever EM, Tsodikov A, et al. What if I don't treat my PSA-detected prostate cancer? Answers from three natural history models. Cancer Epidemiology, Biomarkers and Prevention. 2011;20:740–750. doi: 10.1158/1055-9965.EPI-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in black men? Answers from three natural history models. Cancer. 2017 doi: 10.1002/cncr.30687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; Nov, 2012. ( www.seer.cancer.gov) Sub (1973–2010), released April 2013, based on the November 2011 submission. [Google Scholar]
- 7.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11:707–719. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Annals of Internal Medicine. 2013;158:145–153. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati R, Inoue LY, Gore JL, Katcher J, Etzioni R. Individualized Estimates of Overdiagnosis in Screen-Detected Prostate Cancer. Journal of the National Cancer Institute. 2014;106:djt367. doi: 10.1093/jnci/djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers AJ, Sjoberg DD, Ulmert D, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014;12:26. doi: 10.1186/1741-7015-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugosson J. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397–1405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 14.Andriole GL, Crawford ED, Grubb RLr, et al. Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriole GL. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J. Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical Methods in Medical Research. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 17.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 18.Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer. 2012;118:5955–5963. doi: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. Journal of Clinical Oncology. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. New England Journal of Medicine. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 21.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 22.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange JM, Trock BJ, Gulati R, Etzioni R. A Framework for Treatment Decision Making at Prostate Cancer Recurrence. Medical Decision Making. 2017 doi: 10.1177/0272989X17711913. 272989X17711913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey CM, McMillen MM, Cowan CD, Horm JW, Kessler LG. Representativeness of the surveillance, epidemiology, and end results program data: recent trends in cancer mortality rates. Journal of the National Cancer Institute. 1992;84:872–877. doi: 10.1093/jnci/84.11.872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.