ILC2 in disease
Keywords: allergies, ILC2, innate immunity, type-2 inflammation
Abstract
Group 2 innate lymphoid cells (ILC2) are now recognized as an important innate source of type-2 effector cytokines. Although initially associated with mucosal tissues, it is clear that ILC2 are present in diverse anatomical locations. The function of ILC2 at these sites is equally varied, and although ILC2 represent a relatively minor population, they are fundamentally important regulators of innate and adaptive immune processes. As such, there is much interest to understand the role of ILC2 in diseases with a type-2 inflammatory component. This review explores the known roles of ILC2 in disease, and the diseases that show associations or other strong evidence for the involvement of ILC2.
Introduction
Group 2 innate lymphoid cells (ILC2) are key mediators of type-2 immune responses and comprise a potent cellular source of the canonical type-2 effector cytokines IL-5 and IL-13 (reviewed in ( 1 )). ILC2 were initially noted as non-T-non-B cells that shared similar effector functions as CD4 + T helper 2 (T h2 ) cells ( 2–4 ). Given their low cell numbers compared with CD4 + T cells in most tissues of naive animals, and the absence of a known lineage-specific cell surface marker, ILC2 continued to fly under the radar until being characterized independently by several groups in 2010 ( 5–7 ). These, and subsequent, studies established that ILC2 comprise a distinct lineage of innate lymphocytes that, independently of T h2 cells, can mount potent type-2 immune responses ( 1 , 8 ).
Importantly, ILC2 are not activated by direct interactions with antigens though a T-cell receptor (TCR) or a BCR, or by antibody-mediated activation. Furthermore, ILC2 are not known to express pathogen-associated molecular pattern receptors. Instead, in addition to the common γ-chain (CD132; a subunit of the heteromeric receptors for e.g. IL-2, IL-4, IL-7 and IL-9), ILC2 express CD25 (another IL-2 receptor subunit) and CD127 (the other IL-7 receptor subunit), as well as receptors for cytokines that are rapidly released by damaged or stimulated epithelial cells including ST2 (an IL-33 receptor subunit), IL-17RB (a receptor subunit e.g. IL-25) and TSLPR (which, along with CD127, comprises the receptor for thymic stromal lymphopoietin) ( 1 ). Thus, tissue-resident ILC2 act as sentinels and translate epithelium-derived signals into an amplified innate type-2 response. In the gut, this response is critical for anti-helminth immunity, where ILC2-derived IL-5 and IL-13 potentiate eosinophilic inflammation, goblet cell hyperplasia and mucus secretion.
Following their discovery in gut-associated tissues, it has become evident that ILC2 may be involved in other innate type-2 immune processes. To date, ILC2 have been implicated or associated with an increasing list of diseases ( Table 1 ). Importantly, ILC2 also function in other type-2 cytokine-driven processes such as lipid metabolism and thermogenesis in adipose tissue ( 9–12 ), eosinophil homeostasis ( 13 , 14 ) and the development of alternatively activated macrophages ( 13 ). Furthermore, innate IL-5-producing cells are present in the uterus, heart, kidney and brain of naive mice ( 14 ), suggesting potential roles of ILC2 in these organs.
Table 1.
ILC2 involvement or association with disease or homeostatic processes
| Location | Disease or process | Model or infection |
|---|---|---|
| GI tract | Helmith infection | Nippostrongylus brasiliensis |
| Schistosome spp. | ||
| Bacterial infection | Bacillus anthracis | |
| Colitis | Oxazolone induced colitis | |
| Adipose tissue | Lipid metabolism | |
| Lung | Allergic inflammation | House dust mite |
| Ragweed | ||
| Alternaria alternate | ||
| Chitin | ||
| Recombinant cytokines (IL-33, IL-25, TL1A) | ||
| Protease-allergen (papain, Aspergillus spp .) | ||
| Ovalbumin | ||
| Fungal asthma exacerbation | Alternaria alternata | |
| Viral asthma exacerbation | Influenza A | |
| Rhinovirus | ||
| Viral infections | Influenza A, H1N1 | |
| Pulmonary fibrosis | Schistosoma mansoni egg | |
| Bleomycin induced fibrosis | ||
| COPD | Cigarette smoke + Influenza A | |
| Nose | Chronic rhinosinusitis | |
| Allergic rhinitis | ||
| Skin | Allergic dermatitis | House dust mite |
| Calcipotriol | ||
| Recombinant cytokine (IL-2-Ab complex) | ||
| Liver | Fibrosis | Thioacetamide induced fibrosis |
| Cholestasis-dependent fibrosis | ||
| Schistosoma mansoni | ||
| Biliary repair | Recombinant cytokines (IL-33) | |
| Viral hepatitis | Adenovirus | |
| Biliary carcinogenesis | Recombinant cytokines (IL-33) | |
| CNS | Multiple sclerosis | Experimental autoimmune encephalitis |
| Cerebral malaria | Plasmodium spp. | |
| Aorta | Atherosclerosis |
CNS, central nervous system; COPD, chronic obstructive pulmonary disease.
This review addresses the known or hypothesized roles, and mechanisms of ILC2 in disease processes, broadly divided by organ system. This is preceded by an overview of the regulation of ILC2 function.
Regulation and function
ILC2 are part of a larger ID2-transcription-factor-dependent innate lymphoid cell (ILC) family, which also includes ILC1, ILC3 and NK cells ( 8 ). ILC2 differentiate from a common ILC precursor found in the bone marrow ( 15 , 16 ) via immature ILC2 ( 17 , 18 ). The transcription factors RORα ( 18 , 19 ), GFI-1 ( 20 ), GATA3 ( 17 , 21 ), TCF1 ( 22 ) and Bcl11b ( 23 , 24 ) are essential for ILC2 development and function. Notably, GATA3 is also required for ILC3 differentiation ( 25 ).
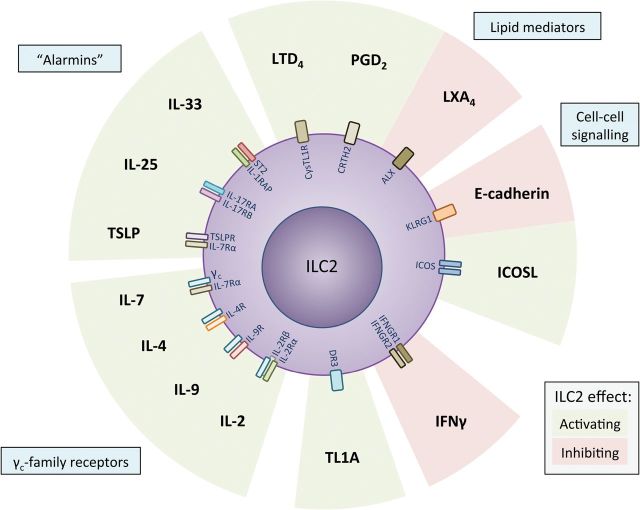
The upstream type-2-associated cytokines IL-33, IL-25 and TSLP are collectively essential for ILC2 activation but individually have different importance depending on factors such as tissue location ( 1 , 26 ). Nevertheless, other soluble and cell-to-cell signals also contribute to ILC2 regulation ( Figs 1 and 2 ), and our understanding of their function in the context of the overall immune response remains incomplete.
Fig. 1.
Regulation of ILC2 by soluble-molecules and cell-to-cell receptors. ILC2 are primarily regulated by the ‘alarmins’ IL-25, IL-33 and TSLP but are also influenced strongly by other cytokines, lipid mediators, or cell-surface molecules. TSLP, thymic stromal lymphopoietin; DR3, death receptor 3—tumor necrosis factor receptor superfamily member 25; LTD 2 , Leukotriene D 2 ; LXA 4 , Lipoxin A 4 ; PGD 2 , prostaglandin D 2 .
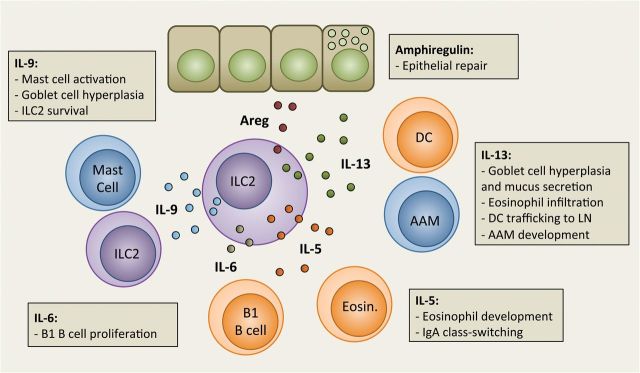
Fig. 2.
Effector functions of ILC2-derived cytokines. Activated ILC2 are a source of effector cytokines, which are important regulators of inflammation and homeostasis. ILC2 can also produce IL-4 when stimulated with prostaglandin D 2 . AAM, alternatively activated macrophage; Areg, amphiregulin; DC, dendritic cell; Eosin., eosinophil.
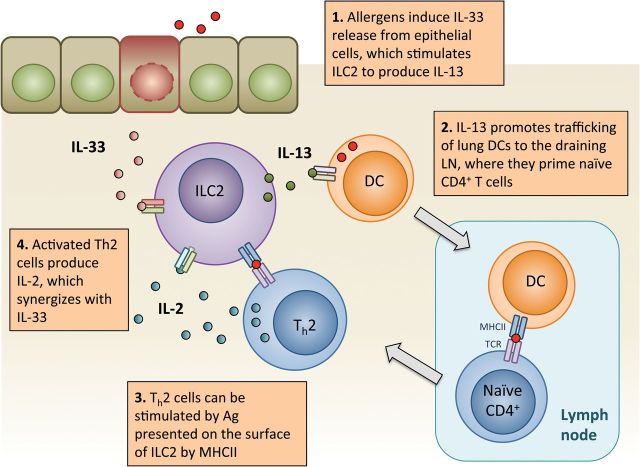
Using models of ILC2-specific depletion or deficiency, it is becoming clear that ILC2 can instruct the adaptive immune response (e.g. see Fig. 3 ) ( 27–31 ). ILC2-derived IL-13 is necessary for efficient trafficking of dendritic cells (DC) to the draining lymph node during allergic lung inflammation, thus indirectly influencing T h2 cell priming ( 29 ). ILC2 can also express MHC class II, OX40L, CD80 and CD86, and can directly activate CD4 + T cells ( 27 , 28 , 31 ). Conversely, activated CD4 + T-cell-derived IL-2 can synergize with IL-33 to stimulate ILC2 ( 27 , 28 ). In addition to T cells, ILC3 are also a potential source of IL-2 as suggested by Il2 -fate reporter analysis ( 32 ). Moreover, IL-2 is required for IL-9 production from ILC2 ( 33 ). IL-9, also produced by T h 9 cells, enhances ILC2 survival by upregulating BCL-3 ( 34 ). Furthermore, basophil-derived, and perhaps T h2 -cell-derived, IL-4 also promotes ILC2 activation ( 35 , 36 ).
Fig. 3.
ILC2 interaction with the adaptive immune system in allergic lung inflammation. Allergen exposure to the airways results in a rapid release of alarmins, including IL-33, which activate lung-resident ILC2. DCs are also activated and require ILC2-derived IL-13 to efficiently traffic to the draining LN, where they cross-present antigen to naive CD4 + T cells, resulting in T h2 cell priming. Antigen-specific T h2 cells exit the LN and can be (hypothetically) locally activated by MHCII + ILC2 presenting allergen-peptide, resulting in IL-2 production and release. IL-2 and IL-33 potently synergize to further activate ILC2. DC, dendritic cell; LN, lymph node.
Cell-to-cell signaling through ILC2-cell-surface receptors including ICOS (binds ICOS-L) and KLRG1 (binds cadherins) also influences ILC2 activation and survival ( 37 , 38 ). Moreover, prostaglandins and eicosanoids produced by myeloid cells regulate ILC2 function. The prostaglandin D 2 (PGD 2 ) receptor, CRTH2, is expressed on circulating human ILC2 and regulates their migration and accumulation in inflamed lung tissue ( 39 , 40 ), as well as their production of IL-13 ( 41 ). ILC2 also express the leukotriene D 4 receptor, CysLT1R, which stimulates the production of IL-4 in addition to IL-5 and IL-13 ( 42 ). In addition, ILC2 express DR3, the receptor for the TNF-family cytokine TL1A, which confers stimulatory signals ( 43 , 44 ).
A recent report also suggests that ILC2 activation is dampened by T regulatory (T reg ) cells ( 45 ). Conversely, ILC2 also promote Treg cell maintenance, whereas IFN-γ can directly represses IL-33-mediated activation of ILC ( 46 ). Moreover, nutritional status also likely influences ILC2 biology, as vitamins A and D are known to skew the ILC3/ILC2 balance in the intestines ( 47 , 48 ). Thus, it is clear that ILC2 are tightly regulated by their environment and function as a potent inducer of innate and adaptive immune processes.
ILC2 in diseases of the gastrointestinal tract
Helminth infection
Type-2 immunity is essential for anti-helminth responses, involving both innate and adaptive immune processes ( 49 ). ILC2 were first identified in the intestine, mesenteric lymph nodes and fat-associated lymphoid clusters (FALC) as a lineage – CD127 + CD25 + ST2 + IL-17RB + innate lymphoid population that required IL-25 and/or IL-33 signaling for activation in response to Nippostrongylus brasiliensis (Nb) infection ( 5–7 ). ILC2 are a critical innate source of IL-13 that is important for efficient helminth expulsion in the absence of adaptive immunity. In sensitized animals, both ILC2 and T h2 cells cooperate to eliminate Nb-larvae in the lungs upon re-infection, preventing their maturation and trafficking to the stomach and intestines ( 50 ). Furthermore, ILC2-derived IL-9, acting via autocrine and paracrine mechanisms, is critical for epithelial repair and efficient helminth expulsion following Nb infection ( 34 ). Besides inducing inflammation, ILC2 are also an important cellular source of amphiregulin ( 51 ), a growth factor critical in wound repair processes and anti-helminth immunity ( 49 ). Moreover, ILC2 appear to be the major source of amphiregulin following Nb infection ( 34 ).
Human lineage – CD127 + CD161 + CRTH2 + ST2 + ILC2 were identified in embryonic intestinal tissue ( 52 ). Although human and murine ILC2 are similarly regulated, little is known about the role of human ILC2 in parasite infection as of yet. Although one study reported increased blood ILC in patients infected with filarial worms ( 53 ), more recently blood ILC2 proportions were found to be reduced during Schistosome infection ( 54 ). As circulating ILC2 numbers do not accurately reflect activation and expansion in affected tissues ( 7 ), further studies are needed to reveal the role of ILC2 in human helminthiasis.
Colitis
Although ILC3 are essential for intestinal homeostasis, and both ILC1 and ILC3 have been implicated in inflammatory bowel diseases, the role of ILC2 in these processes remains uncertain ( 55 ). To date, IL-13 is known to mediate colitis after oxazolone treatment, partially driven by IL-25-dependent activation of ILC2 ( 56 ). Crohn’s disease patients also have increased numbers of intestinal IL-13 + ILC, suggesting a possible role for ILC2 in pathogenesis ( 57 ).
Other diseases
The potential function of ILC2 in other diseases of the gastrointestinal (GI) tract is of keen interest. FALC-resident ILC2 can interact with IgA-secreting B-1 cells, which are essential for the clearance of GI pathogens ( 5 ). Recently, a model of Bacillus anthracis infection in mice reported a concomitant reduction in B-1 cells and ILC2 and impairment in ILC2 function compared with uninfected animals ( 58 ). Furthermore, IL-33, IL-25 and TSLP have been linked with food allergies ( 59–61 ) and eosinophilic esophagitis ( 62 ). Nevertheless, it is important to recognize the contribution of other innate immune cells such as eosinophils, basophils and mast cells, which are also known to respond to epithelium-derived cytokines and mediate type-2 inflammation. It is likely that the relative contributions of different cells in inflammatory processes are highly tissue dependent.
ILC2 in diseases of the airways
Allergic lung disease
Type-2 immunity plays a central role in allergic lung disease ( 63 ). Furthermore, genome-wide association studies of asthmatic patients have identified numerous components of the type-2 inflammatory process, including ILC2-associated genes such as RORA , IL33 , IL1RL1 (which encodes ST2) and IL13 ( 64 ). In mice, ILC2 are found in naive lungs, where they are critical for mounting an innate type-2 response to inhaled allergens ( 65 ) and are the primary source of IL-5 and IL-13 in response to allergen-induced release of IL-33, TSLP or IL-25 ( 65–67 ). Although these three cytokines all influence ILC2 activation, purified naive lung ILC2 require IL-33 for type-2 cytokine production ( 65 ). Similar regulatory requirements are noted in human ILC2 ( 38 , 52 ). Likewise, IL-33 is a more potent inducer of ILC2 than IL-25 is, in ragweed-challenged mice or mice challenged with Alternaria alternata allergen ( 68 ).
Multiple other studies indicate that IL-33 is a key activator of lung ILC2 in the context of innate type-2 lung inflammation ( 69–71 ). IL-25, in contrast, appears to play a more prominent role in gut ILC2 activation ( 6 ), which coincides with reduced ST2 expression on enteric ILC2 ( 18 ). Interestingly, intranasal administration of recombinant IL-25 promotes the early emergence of an IL17RB + KLRG1 hi population in the lung ( 72 ), which, like enteric ILC2, are ST2 – and CD127 low . However, IL-25-elicited KLRG1 hi lung ILC2 may convert into ST2 + ILC2, suggesting that the tissue-specific microenvironment may strongly influence ILC2 regulation.
ILC2 are a potent innate source of type-2 cytokines in allergic lung inflammation. Naive lung ILC2 are primed to secrete IL-5, as indicated by elevated Il5 transcript ( 65 ) and constitutive Il5 -reporter expression ( 14 ). ILC2-derived IL-5 is important for eosinophilic homeostasis, whereas the local production of IL-13 and the eosinophil-recruiting chemokine eotaxin is essential for eosinophilic lung inflammation following allergen exposure ( 14 ). ILC2 are the major source of IL-13 during acute allergic inflammation, which induces goblet-cell hyperplasia, mucus overproduction and smooth-muscle contraction, all of which adversely affect airflow and lung function ( 73 ). In addition, ILC2 play a significant role in promoting airway hyper-reactivity, mediated by IL-25, IL-33 and ICOS signaling ( 37 , 68 , 74–76 ). In addition, ILC2-derived IL-13 promotes lymph-node trafficking of lung DC in a protease-allergen model of lung inflammation, thus linking the innate type-2 response to allergen-sensitization of T h2 cells ( 29 ). Using Rorasg/sg bone-marrow-transplanted mice, which lack ILC2 but can generate normal T h2 cells, it was shown that inhaled allergens failed to induce efficient T h2 cell priming, resulting in diminished IgE production and diminished type-2 inflammation ( 18 , 29 , 30 ).
ILC2 are also a critical early source of IL-9 in allergen-induced airway inflammation ( 33 ). Besides autocrine regulation of ILC2 survival, IL-9 supports goblet-cell hyperplasia and mast-cell proliferation in the lungs of helminth-infected mice, suggesting an analogous function in atopic airway disease ( 34 , 77 ). Mast cells were observed in proximity to lung CD117 + CD161 + ILC 2 in healthy subjects and can enhance ILC2 activation directly via the production of PGD 2 or perhaps indirectly by releasing proteases that cleave IL-33 into a more bioactive isoform ( 78 ). Moreover, mast cells are also a direct source of IL-33 upon antigen-specific IgE-mediated activation ( 79 ).
The relative contribution of ILC2 versus T h2 cells toward pathology after allergen re-exposure in a sensitized host is an important question when considering the potential impact of ILC2-targeted therapies. Several reports have shown that the magnitude of ILC2-mediated inflammation is contingent on the adaptive immune system ( 6 , 29 , 50 , 80 ). Rag2–/– mice (which are deficient in T and B cells) can mount an ILC2-driven type-2 response to allergen but, unlike wild-type mice, do not generate an amplified immune reaction after re-challenge with the same allergen ( 29 ). Upon allergen re-challenge in primed wild-type mice, T h2 cells comprise an important cellular source of type-2 cytokines, consistent with immune memory and T h2 cell differentiation.
Nevertheless, type-2-cytokine-producing ILC2 also expand greatly in number after re-challenge with a protease allergen (papain)-sensitized animals or after repeated challenges with house dust mite (HDM) extract ( 29 , 67 ). Moreover, ILC2 performed an important, but secondary, role to T h2 cells, in mediating the recall response to inhaled ovalbumin antigen ( 80 ). However, another study suggests that ILC2 play a more prominent role in an HDM model of chronic asthma ( 81 ). Nevertheless, these studies suggest collaboration between ILC2 and T h2 cells as producers of effector type-2 cytokines in the context of allergic airway inflammation.
To date, ILC2 have been identified in the lungs of human fetal tissue, and in the bronchoalveolar lavage and lungs of healthy subjects ( 51 , 52 ). Subsequently, it was reported that asthma patients have more circulating ILC2 ( 82 ). Conversely, others have observed similar numbers of circulating ILC2 in severe or mild asthma patients and healthy subjects but instead noted reduced NK cells in the blood of asthma patients ( 41 ). Interestingly, this study also shows that ILC2 activation can be dampened by lipoxin A 4 , a pro-resolvin molecule that binds to ALX/FPR2 receptors expressed on ILC2. Another study demonstrated that corticosteroids, commonly used to treat asthma, inhibit murine ILC2 activation by IL-33 ( 83 ). The same study also shows that TSLP counters the effect of corticosteroids on ILC2. Clearly, further translational work is needed to establish the role of lung ILC2 in humans.
Alternatively, ILC2 are hypothesized to play a role in asthma exacerbations. The hypothesis that susceptible individuals (perhaps because of elevated production of alarmins such as IL-33, elevated numbers of ILC2 or an elevated propensity for ILC2 stimulation) may be more prone to ILC2 activation is attractive because of their relatively indiscriminant activation by alarmin release upon cellular damage or stress ( 1 ). In support of this, mice with allergic lung inflammation had increased numbers of IL-33-producing type-2 pneumocytes, which may imply a higher potential for ILC2 activation ( 84 ). TSLP is also mainly produced by type-2 pneumocytes in response to chitin or helminth exposure ( 85 ). Moreover, it is clear that ILC2 are activated upon influenza infection in an IL-33-dependent manner ( 51 , 52 , 86 ). Influenza and also rhinovirus infections are known to cause asthma exacerbations in humans ( 63 , 87 ). Indeed, IL-33 and IL-25 are induced upon rhinovirus infection, and type-2 effector cytokines are elevated in infected asthmatic patients, thus suggesting a possible role for ILC2 ( 88 , 89 ).
Asthma exacerbations can also occur after exposure to fungal antigens. In mice, intranasal Alternaria alternata exposure caused IL-33-dependent exacerbated disease in animals sensitized with an alternative antigen ( 90 ). Overall, it appears that ILC2 are activated in response to a wide range of stimuli.
Rhinosinusitis
Type-2 inflammation is associated with chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP), an endotype of CRS that can be further subdivided based on eosinophilic infiltration ( 91 ). CRSwNP affects the nose and paranasal sinuses, with clinical symptoms including nasal congestion/obstruction and fatigue. Initially, ILC2 enrichment was demonstrated in the polyps of CRS patients ( 52 ). These and subsequent data from the same group showed that nasal polyp ILC2 are a potent source of type-2 cytokines in response to IL-33, IL-25 and TSLP ( 21 ). The association between ILC2 and CRSwNP was further demonstrated in a number of subsequent studies ( 92–95 ). Additional correlations were found between elevated polyp ILC2 numbers and increased local and circulating eosinophil numbers ( 92 , 95 ). Allergic rhinitis, which over time can develop into CRS, is also associated with ILC2, as suggested by increased circulating ILC2 after allergen challenge in sensitized patients ( 96 ). Moreover, nasal-mucosal ILC2 from CRSwNP patients produced IL-13 when stimulated with IL-33, and IL33 transcript was elevated in nasal epithelium after exposure to fungal allergen ( 94 ). Interestingly, corticosteroid treatment of CRSwNP patients led to a reduction in ILC2 number ( 95 ). The same study also showed that corticosteroids induced apoptosis of lung ILC2 in mice. Therefore, it is evident that ILC2 are present in the nasal mucosa and are likely associated with type-2 inflammation in CRSwNP.
Lung infections
Lung ILC2 were first identified in influenza models of lung inflammation ( 51 , 75 ). Importantly, Monticelli et al . ( 51 ) reported that ILC2 play a central role in tissue repair following infection by their production of amphiregulin. Moreover, the cellular source of IL-33 may be different during viral infections and allergic inflammation. In addition to epithelial cells, alveolar macrophages and invariant NK T cells were reported as potential sources of IL-33 in influenza-infected lungs ( 75 , 86 ).
Furthermore, a recent study found that cigarette smoke enhanced epithelial IL-33 production while also altering the expression of its receptor, ST2, on the surface of ILC2 and other immune cells ( 97 ). Interestingly, cigarette smoke exposure resulted in a dampened ILC2 response and an exacerbated type-1 response upon influenza infection, thus proposing another protective role of ILC2 in the lung. However, although ILC2 may be beneficially involved in the wound-healing response, the dysregulation of this pathway can lead to fibrosis. ILC2-derived IL-13 is responsible for collagen deposition in the lungs of mice treated with Schistosoma mansoni eggs ( 98 ). In another study, bleomycin-induced lung fibrosis was found to be mediated in part by IL-33 ( 99 ).
Thus, it appears that ILC2 are a piece in a complex puzzle of the lung immune system and have roles in multiple seemingly unrelated immune processes. Recently, the influence of infections, microbiota and allergen exposures on the postnatal development of the immune system has been investigated. Early-life viral infections have been associated with the development of asthma. In mice, rhinovirus infection of neonates elicits amplified ILC2 activation compared with adult mice ( 74 ), although this may be due to reduced lung T reg cells, whose emergence after birth is dependent on microbial colonization of the lung ( 100 ). Further studies are required to delineate the role of ILC2 on the lung immune system in health and disease.
ILC2 in diseases of the skin
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by increased concentrations of type-2 cytokines in skin lesions. Furthermore, samples of AD lesions exhibit higher expression of IL1RL1 (ST2), AREG , IL17RB , TSLPR and RORA compared with healthy subjects, suggesting an enrichment of ILC2 in disease ( 38 ). Skin-resident ILC2 were first characterized in mice as lineage – ST2 + CD25 + CD127 + CD90 + ICOS + cells ( 101 ) and were subsequently also distinguished from ILC2 in other anatomical sites by their expression of CD103, an integrin expressed by other skin-resident leukocytes ( 102 ).
While relatively abundant during homeostasis, skin ILC2 numbers expanded after administration of a topical vitamin D analog (calcipotriol), allergen (HDM) or in response to IL-2 signaling ( 38 , 101 , 102 ). Kim et al . ( 101 ) reported that skin ILC2 activation is critically dependent on TSLP signaling. More recently, mice with skin-specific overexpression of IL-33 were shown to develop spontaneous dermatitis with increased ILC2 numbers ( 103 ). Therefore, although skin ILC2 can respond to IL-25, IL-33 and TSLP as shown by impaired calcipotriol-induced skin inflammation in mice with knockouts of individual receptors ( 38 ), it remains unclear if one of these cytokines plays a more prominent role in AD. Nevertheless, using ILC-deficient Rorasg/sg bone-marrow-transplanted mice, or by antibody-mediated ILC2 depletion, it is clear that ILC2 are critical mediators of the acute type-2 inflammatory response in the skin ( 38 , 101 ).
Human skin ILC2 were identified as lineage – cells, which expressed CD127, CRTH2, ST2, CD161, CD25, ICOS and c-Kit. Whereas skin ILC2 were present in healthy subjects, their numbers were elevated in lesional biopsies or blood samples taken from AD patients ( 38 , 101 ). Furthermore, human skin ILC2 activation was suppressed by E-cadherin binding to KLRG1 expressed on the surface of activated ILC2 ( 38 ). Thus, human ILC2 are present in the skin during homeostasis and are a potent innate source of type-2 cytokines upon stimulation. As mouse studies also show that the adaptive immune system is necessary for efficient ILC2 responses in the skin ( 101 ), it is possible that AD in humans involves both T cells and ILC2. Interestingly, basophil-derived IL-4 can enhance ILC2 function and mast cells are found in proximity to skin ILC2, hinting at possible interactions between these cells ( 35 , 102 ). Taken together, ILC2 appear to play a prominent role in driving skin type-2 inflammation; however, further work is needed to elucidate the exact role and regulation of ILC2 in the skin.
ILC2 in other anatomical sites
Although ILC2 have a distinct role at anatomical barrier sites, it is becoming clear that they are present in numerous other tissues. Indeed, many diseases driven by type-2 inflammation, or its upstream cytokines such as IL-33, IL-25 and TSLP, may involve ILC2. Liver fibrosis is associated with increased serum IL-33, and it has now been shown that hepatic ILC2 are important for IL-33-driven IL-13 production ( 104 , 105 ). IL-13 in turn induces cholangiocyte hyperplasia and hepatic stellate-cell activation, which are associated with biliary atresia and hepatic fibrosis, respectively ( 104 , 105 ). However, these processes likely also serve a protective role as demonstrated by the increased susceptibility to virus-induced tissue damage when this pathway is blocked ( 104 ). Indeed, IL-33 is also increased in models of viral hepatitis, and activated hepatic ILC2 may also modulate the inflammatory response as indicated by their ability to limit cytokine production from macrophages and T cells ( 106 ).
ILC2 have also been identified in the brain and are implicated in polarizing the immune environment in cerebral malaria ( 107 ). Mice infected with Plasmodium spp. benefited from adoptively transferred ILC2, although other IL-33 responsive cells including T reg cells also contributed to the protection against experimental cerebral malaria. ILC2 in the nervous system may also play a role in multiple sclerosis. ILC2 were found to accumulate in the brain and draining lymph nodes of mice that were resistant to experimental autoimmune encephalitis (EAE) ( 108 ). The authors hypothesize that ILC2 skew the immune environment toward a protective T h2 -dominated response. This argument is supported by increased disease in ST2 –/– animals; and IL-33 administration is protective in EAE ( 109–111 ).
ILC2 are also reported in the aorta, where they are hypothesized to interact with B-1a cells and help regulate production of natural IgM antibody to low-density lipoprotein, which protects against atherosclerosis ( 112 ). Moreover, IL-25 administration has been shown to reduce atherosclerosis in mice, possibly through interactions between ILC2 and B-1 cells ( 113 ).
Not much is known about the role of ILC2 in cancer, although Li et al . ( 105 ) report that IL-33 facilitates biliary carcinogenesis. Furthermore, it is uncertain if some leukemias can arise from ILC2. Nevertheless, it is becoming clear that many of our current treatments influence the biology of ILC2. Severe epithelial damage caused by graft-versus-host disease (GVHD) in allogeneic hematopoietic stem-cell-transplanted (HSCT) patients may influence, or be influenced by, ILC2. A recent study indicated that ILC (including ILC2) reconstitution after HSCT was slow compared with other blood lineages and that more rapid recovery of the ILC compartment coincided with reduced GVHD ( 114 ).
Another form of immunotherapy involves treatment with IL-2, which has been approved for some forms of cancer and as anti-autoimmune therapy, but has numerous side effects including eosinophilia. We now understand that IL-2 is a potent in vivo activator of ILC2 ( 27 , 32 , 102 ), which a recent study indicates that it promotes eosinophilia via ILC2-derived IL-5 ( 115 ). As illustrated by the discovery of ILC2 in numerous non-barrier tissues, it is important to consider their function in an increasing number of organs and diseases.
Conclusion
It appears that ILC2 have a more complex role in health and disease than originally envisioned. Although initially perceived to be a remnant of the innate immune system, holding the fort until the adaptive immune system comes to the rescue, it is increasingly evident that ILC2 may be a critical component in many disease, and homeostatic, processes. A body of evidence suggests that ILC2 function is tightly controlled by the microenvironment, and therefore, it is likely that ILC2 act in a tissue-specific manner. On this, we layer the plasticity of the immune response, which underscores the importance of delineating the function and regulation of ILC2 throughout the immune response in order to devise effective therapeutic strategies.
More specifically, further studies are needed that dissect the regulation of ILC2 by the increasingly diverse activation and/or inhibitory signals. Moreover, it is important to understand the kinetics of ILC2 responses, and their development or recruitment into inflamed tissues. In relation to this aim, more research is needed into ILC2 development in the bone marrow, or maturation in the periphery, which may identify more ILC2-restricted targetable molecules. Already RORα is known to be essential for efficient ILC2 development specifically, although recent studies show its expression in other ILC lineages too. Nevertheless, mutant Rorasg/sg bone-marrow-transplanted mice appear to have relatively normal ILC3 numbers. The use of RORα small-molecule antagonists or agonists should be evaluated, although it is important to consider that RORα function is also essential for neuronal development, which may inhibit its usefulness as a drug target. However, already there are many targeted therapies that may (amongst effects on other immune cells) influence ILC2 in various stages of development or clinical use (reviewed in ( 116 )). Lastly, it is important to translate our understanding of mouse ILC2 biology to the human system. Although this is difficult, novel tools such as the humanized immune system mouse, xenotransplants or human-tissue explant cultures may provide answers.
Funding
Canadian Institutes of Health Research—Banting Postdoctoral Fellowship (to T.Y.F.H.).
Acknowledgements
We thank S. Scanlon for critical reading.
Conflict of interest statement: The author declared no conflict of interests.
References
- 1. McKenzie A. N. Spits H. and Eberl G . 2014. . Innate lymphoid cells in inflammation and immunity . Immunity 41 : 366 . [DOI] [PubMed] [Google Scholar]
- 2. Voehringer D. Reese T. A. Huang X. Shinkai K. and Locksley R. M . 2006. . Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system . J. Exp. Med . 203 : 1435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fort M. M., Cheung J., Yen D., et al. 2001. . IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo . Immunity 15 : 985 . [DOI] [PubMed] [Google Scholar]
- 4. Fallon P. G., Ballantyne S. J., Mangan N. E., et al. 2006. . Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion . J. Exp. Med . 203 : 1105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moro K., Yamada T., Tanabe M., et al. 2010. . Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells . Nature 463 : 540 . [DOI] [PubMed] [Google Scholar]
- 6. Neill D. R., Wong S. H., Bellosi A., et al. 2010. . Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity . Nature 464 : 1367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Price A. E., Liang H. E., Sullivan B. M., et al. 2010. . Systemically dispersed innate IL-13-expressing cells in type 2 immunity . Proc. Natl Acad. Sci. USA 107 : 11489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spits H., Artis D., Colonna M., et al. 2013. . Innate lymphoid cells—a proposal for uniform nomenclature . Nat. Rev. Immunol . 13 : 145 . [DOI] [PubMed] [Google Scholar]
- 9. Brestoff J. R., Kim B. S., Saenz S. A., et al. 2015. . Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity . Nature 519 : 242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hams E. Locksley R. M. McKenzie A. N. and Fallon P. G . 2013. . Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice . J. Immunol . 191 : 5349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee M. W., Odegaard J. I., Mukundan L., et al. 2015. . Activated type 2 innate lymphoid cells regulate beige fat biogenesis . Cell 160 : 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanya K. J., Jacobi D., Liu S., et al. 2013. . Direct control of hepatic glucose production by interleukin-13 in mice . J. Clin. Invest . 123 : 261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molofsky A. B., Nussbaum J. C., Liang H. E., et al. 2013. . Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages . J. Exp. Med . 210 : 535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nussbaum J. C., Van Dyken S. J., von Moltke J., et al. 2013. . Type 2 innate lymphoid cells control eosinophil homeostasis . Nature 502 : 245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Constantinides M. G. McDonald B. D. Verhoef P. A. and Bendelac A . 2014. . A committed precursor to innate lymphoid cells . Nature 508 : 397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klose C. S., Flach M., Mohle L., et al. 2014. . Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages . Cell 157 : 340 . [DOI] [PubMed] [Google Scholar]
- 17. Hoyler T., Klose C. S., Souabni A., et al. 2012. . The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells . Immunity 37 : 634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halim T. Y. MacLaren A. Romanish M. T. Gold M. J. McNagny K. M. and Takei F . 2012. . Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation . Immunity 37 : 463 . [DOI] [PubMed] [Google Scholar]
- 19. Wong S. H., Walker J. A., Jolin H. E., et al. 2012. . Transcription factor RORalpha is critical for nuocyte development . Nat. Immunol . 13 : 229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spooner C. J., Lesch J., Yan D., et al. 2013. . Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1 . Nat. Immunol . 14 : 1229 . [DOI] [PubMed] [Google Scholar]
- 21. Mjosberg J., Bernink J., Golebski K., et al. 2012. . The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells . Immunity 37 : 649 . [DOI] [PubMed] [Google Scholar]
- 22. Yang Q., Monticelli L. A., Saenz S. A., et al. 2013. . T cell factor 1 is required for group 2 innate lymphoid cell generation . Immunity 38 : 694 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu Y., Wang C., Clare S., et al. 2015. . The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development . J. Exp. Med . 212 : 865 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker J. A., Oliphant C. J., Englezakis A., et al. 2015. . Bcl11b is essential for group 2 innate lymphoid cell development . J. Exp. Med . 212 : 875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serafini N., Klein Wolterink R. G., Satoh-Takayama N., et al. 2014. . Gata3 drives development of RORgammat+ group 3 innate lymphoid cells . J. Exp. Med . 211 : 199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Dyken S. J., Mohapatra A., Nussbaum J. C., et al. 2014. . Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells . Immunity 40 : 414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliphant C. J., Hwang Y. Y., Walker J. A., et al. 2014. . MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion . Immunity 41 : 283 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirchandani A. S., Besnard A. G., Yip E., et al. 2014. . Type 2 innate lymphoid cells drive CD4+ Th2 cell responses . J. Immunol . 192 : 2442 . [DOI] [PubMed] [Google Scholar]
- 29. Halim T. Y., Steer C. A., Matha L., et al. 2014. . Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation . Immunity 40 : 425 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gold M. J., Antignano F., Halim T. Y., et al. 2014. . Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures . J. Allergy Clin. Immunol . 133 : 1142 . [DOI] [PubMed] [Google Scholar]
- 31. Drake L. Y. Iijima K. and Kita H . 2014. . Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice . Allergy 69 : 1300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roediger B., Kyle R., Tay S. S., et al. 2015. . IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation . J. Allergy Clin. Immunol . doi:10.1016/j.jaci.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 33. Wilhelm C., Hirota K., Stieglitz B., et al. 2011. . An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation . Nat. Immunol . 12 : 1071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turner J. E., Morrison P. J., Wilhelm C., et al. 2013. . IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation . J. Exp. Med . 210 : 2951 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim B. S., Wang K., Siracusa M. C., et al. 2014. . Basophils promote innate lymphoid cell responses in inflamed skin . J. Immunol . 193 : 3717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motomura Y., Morita H., Moro K., et al. 2014. . Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation . Immunity 40 : 758 . [DOI] [PubMed] [Google Scholar]
- 37. Maazi H., Patel N., Sankaranarayanan I., et al. 2015. . ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity . Immunity 42 : 538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salimi M., Barlow J. L., Saunders S. P., et al. 2013. . A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis . J. Exp. Med . 210 : 2939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tait Wojno E. D., Monticelli L. A., Tran S. V., et al. 2015. . The prostaglandin D receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung . Mucosal Immunol . doi:10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xue L., Salimi M., Panse I., et al. 2014. . Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells . J. Allergy Clin. Immunol . 133 : 1184 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barnig C., Cernadas M., Dutile S., et al. 2013. . Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma . Sci. Transl. Med . 5 : 174ra26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doherty T. A. Khorram N. Lund S. Mehta A. K. Croft M. and Broide D. H . 2013. . Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production . J. Allergy Clin. Immunol . 132 : 205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meylan F., Hawley E. T., Barron L., et al. 2014. . The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells . Mucosal Immunol . 7 : 958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu X., Pappu R., Ramirez-Carrozzi V., et al. 2014. . TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers . Mucosal Immunol . 7 : 730 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krishnamoorthy N., Burkett P. R., Dalli J., et al. 2015. . Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation . J. Immunol . 194 : 863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Molofsky A. B., Van Gool F., Liang H. E., et al. 2015. . Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation . Immunity 43:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spencer S. P., Wilhelm C., Yang Q., et al. 2014. . Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity . Science 343 : 432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruiter B. Patil S. U. and Shreffler W. G . 2015. . Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells . Clin. Exp. Allergy 45 : 1214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Allen J. E. and Sutherland T. E . 2014. . Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin . Semin. Immunol . 26 : 329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bouchery T., Kyle R., Camberis M., et al. 2015. . ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms . Nat. Commun . 6 : 6970 . [DOI] [PubMed] [Google Scholar]
- 51. Monticelli L. A., Sonnenberg G. F., Abt M. C., et al. 2011. . Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus . Nat. Immunol . 12 : 1045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mjosberg J. M., Trifari S., Crellin N. K., et al. 2011. . Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161 . Nat. Immunol . 12 : 1055 . [DOI] [PubMed] [Google Scholar]
- 53. Boyd A. Ribeiro J. M. and Nutman T. B . 2014. . Human CD117 (cKit)+ innate lymphoid cells have a discrete transcriptional profile at homeostasis and are expanded during filarial infection . PLoS One 9 : e108649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nausch N. Appleby L. J. Sparks A. M. Midzi N. Mduluza T. and Mutapi F . 2015. . Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment . PLoS Negl. Trop. Dis . 9 : e0003627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sonnenberg G. F . 2014. . Regulation of intestinal health and disease by innate lymphoid cells . Int. Immunol . 26 : 501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Camelo A., Barlow J. L., Drynan L. F., et al. 2012. . Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13 . J. Gastroenterol . 47 : 1198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bailey J. R., Bland P. W., Tarlton J. F., et al. 2012. . IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One 7 : e52332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sahay B. Owen J. L. Zadeh M. Yang T. Lightfoot Y. L. Abed F. and Mohamadzadeh M . 2014. . Impaired colonic B-cell responses by gastrointestinal Bacillus anthracis infection . J. Infect. Dis . 210 : 1499 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blázquez A. B. Mayer L. and Berin M. C . 2010. . Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance . Gastroenterology 139 : 1301 . [DOI] [PubMed] [Google Scholar]
- 60. Chu D. K., Llop-Guevara A., Walker T. D., et al. 2013. . IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization . J. Allergy Clin. Immunol . 131 : 187 . [DOI] [PubMed] [Google Scholar]
- 61. Herberth G., Daegelmann C., Roder S., et al. 2010. . IL-17E but not IL-17A is associated with allergic sensitization: results from the LISA study . Pediatr. Allergy Immunol . 21 : 1086 . [DOI] [PubMed] [Google Scholar]
- 62. Noti M., Wojno E. D., Kim B. S., et al. 2013. . Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis . Nat. Med . 19 : 1005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lambrecht B. N. and Hammad H . 2014. . The immunology of asthma . Nat. Immunol . 16 : 45 . [DOI] [PubMed] [Google Scholar]
- 64. Moffatt M. F., Gut I. G., Demenais F., et al. 2010. . A large-scale, consortium-based genomewide association study of asthma . N. Engl. J. Med . 363 : 1211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Halim T. Y. Krauss R. H. Sun A. C. and Takei F . 2012. . Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation . Immunity 36 : 451 . [DOI] [PubMed] [Google Scholar]
- 66. Barlow J. L. Bellosi A. Hardman C. S. Drynan L. F. Wong S. H. Cruickshank J. P. and McKenzie A. N . 2012. . Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity . J. Allergy Clin. Immunol . 129 : 191 . [DOI] [PubMed] [Google Scholar]
- 67. Klein Wolterink R. G. Kleinjan A. van Nimwegen M. Bergen I. de Bruijn M. Levani Y. and Hendriks R. W . 2012. . Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma . Eur. J. Immunol . 42 : 1106 . [DOI] [PubMed] [Google Scholar]
- 68. Barlow J. L., Peel S., Fox J., et al. 2013. . IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction . J. Allergy Clin. Immunol . 132 : 933 . [DOI] [PubMed] [Google Scholar]
- 69. Salmond R. J. Mirchandani A. S. Besnard A. G. Bain C. C. Thomson N. C. and Liew F. Y . 2012. . IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin . J. Allergy Clin. Immunol . 130 : 1159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bartemes K. R. Iijima K. Kobayashi T. Kephart G. M. McKenzie A. N. and Kita H . 2012. . IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs . J. Immunol . 188 : 1503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hung L. Y. Lewkowich I. P. Dawson L. A. Downey J. Yang Y. Smith D. E. and Herbert D. R . 2013. . IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms . Proc. Natl Acad. Sci. USA 110 : 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang Y., Guo L., Qiu J., et al. 2015. . IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells . Nat. Immunol . 16 : 161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wills-Karp M . 2004. . Interleukin-13 in asthma pathogenesis . Immunol. Rev . 202 : 175 . [DOI] [PubMed] [Google Scholar]
- 74. Hong J. Y., Bentley J. K., Chung Y., et al. 2014. . Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells . J. Allergy Clin. Immunol . 134 : 429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chang Y. J., Kim H. Y., Albacker L. A., et al. 2011. . Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity . Nat. Immunol . 12 : 631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim H. Y., Chang Y. J., Subramanian S., et al. 2012. . Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity . J. Allergy Clin. Immunol . 129 : 216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Townsend J. M. Fallon G. P. Matthews J. D. Smith P. Jolin E. H. and McKenzie N. A . 2000. . IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development . Immunity 13 : 573 . [DOI] [PubMed] [Google Scholar]
- 78. Lefrancais E. Duval A. Mirey E. Roga S. Espinosa E. Cayrol C. and Girard J. P . 2014. . Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells . Proc. Natl Acad. Sci. USA 111 : 15502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hsu C. L. Neilsen C. V. and Bryce P. J . 2010. . IL-33 is produced by mast cells and regulates IgE-dependent inflammation . PLoS One 5 : e11944 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu B. Lee J. B. Chen C. Y. Hershey G. K. and Wang Y. H . 2015. . Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia . J. Immunol . 194 : 3583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Christianson C. A., Goplen N. P., Zafar I., et al. 2015. . Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33 . J Allergy Clin Immunol . 136:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bartemes K. R. Kephart G. M. Fox S. J. and Kita H . 2014. . Enhanced innate type 2 immune response in peripheral blood from patients with asthma . J. Allergy Clin. Immunol . 134 : 671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kabata H., Moro K., Fukunaga K., et al. 2013. . Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation . Nat. Commun . 4 : 2675 . [DOI] [PubMed] [Google Scholar]
- 84. Hardman C. S. Panova V. and McKenzie A. N . 2013. . IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation . Eur. J. Immunol . 43 : 488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mohapatra A. Van Dyken S. J. Schneider C. Nussbaum J. C. Liang H. E. and Locksley R. M . 2015. . Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis . Mucosal Immunol . doi:10.1038/mi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gorski S. A. Hahn Y. S. and Braciale T. J . 2013. . Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection . PLoS Pathog . 9 : e1003615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnston S. L., Pattemore P. K., Sanderson G., et al. 1995. . Community study of role of viral infections in exacerbations of asthma in 9–11 year old children . BMJ 310 : 1225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jackson D. J., Makrinioti H., Rana B. M., et al. 2014. . IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo . Am. J. Respir. Crit. Care Med . 190 : 1373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beale J., Jayaraman A., Jackson D. J., et al. 2014. . Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation . Sci. Transl. Med . 6 : 256ra134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Snelgrove R. J. Gregory L. G. Peiro T. Akthar S. Campbell G. A. Walker S. A. and Lloyd C. M . 2014. . Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations . J. Allergy Clin. Immunol . 134 : 583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Akdis C. A., Bachert C., Cingi C., et al. 2013. . Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology . J. Allergy Clin. Immunol . 131 : 1479 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ho J. Bailey M. Zaunders J. Mrad N. Sacks R. Sewell W. and Harvey R. J . 2015. . Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia . Clin. Exp. Allergy 45 : 394 . [DOI] [PubMed] [Google Scholar]
- 93. Miljkovic D. Bassiouni A. Cooksley C. Ou J. Hauben E. Wormald P. J. and Vreugde S . 2014. . Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis . Allergy 69 : 1154 . [DOI] [PubMed] [Google Scholar]
- 94. Shaw J. L., Fakhri S., Citardi M. J., et al. 2013. . IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps . Am. J. Respir. Crit. Care Med . 188 : 432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Walford H. H., Lund S. J., Baum R. E., et al. 2014. . Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness . Clin. Immunol . 155 : 126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Doherty T. A., Scott D., Walford H. H., et al. 2014. . Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84 . J. Allergy Clin. Immunol . 133 : 1203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kearley J., Silver J. S., Sanden C., et al. 2015. . Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection . Immunity 42 : 566 . [DOI] [PubMed] [Google Scholar]
- 98. Hams E., Armstrong M. E., Barlow J. L., et al. 2014. . IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis . Proc. Natl Acad. Sci. USA 111 : 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li D., Guabiraba R., Besnard A. G., et al. 2014. . IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice . J. Allergy Clin. Immunol . 134 : 1422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gollwitzer E. S., Saglani S., Trompette A., et al. 2014. . Lung microbiota promotes tolerance to allergens in neonates via PD-L1 . Nat. Med . 20 : 642 . [DOI] [PubMed] [Google Scholar]
- 101. Kim B. S., Siracusa M. C., Saenz S. A., et al. 2013. . TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation . Sci. Transl. Med . 5 : 170ra16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roediger B., Kyle R., Yip K. H., et al. 2013. . Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells . Nat. Immunol . 14 : 564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Imai Y., Yasuda K., Sakaguchi Y., et al. 2013. . Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice . Proc. Natl Acad. Sci. USA 110 : 13921 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McHedlidze T., Waldner M., Zopf S., et al. 2013. . Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis . Immunity 39 : 357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li J., Razumilava N., Gores G. J., et al. 2014. . Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation . J. Clin. Invest . 124 : 3241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liang Y. Jie Z. Hou L. Aguilar-Valenzuela R. Vu D. Soong L. and Sun J . 2013. . IL-33 induces nuocytes and modulates liver injury in viral hepatitis . J. Immunol . 190 : 5666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Besnard A. G., Guabiraba R., Niedbala W., et al. 2015. . IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells . PLoS Pathog . 11 : e1004607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Russi A. E. Walker-Caulfield M. E. Ebel M. E. and Brown M. A . 2015. . Cutting edge: c-Kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice . J. Immunol . 194 : 5609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jiang H. R., Milovanovic M., Allan D., et al. 2012. . IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages . Eur. J. Immunol . 42 : 1804 . [DOI] [PubMed] [Google Scholar]
- 110. Li M. Li Y. Liu X. Gao X. and Wang Y . 2012. . IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice . J. Neuroimmunol . 247 : 25 . [DOI] [PubMed] [Google Scholar]
- 111. Milovanovic M. Volarevic V. Ljujic B. Radosavljevic G. Jovanovic I. Arsenijevic N. and Lukic M. L . 2012. . Deletion of IL-33R (ST2) abrogates resistance to EAE in BALB/C mice by enhancing polarization of APC to inflammatory phenotype . PLoS One 7 : e45225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Perry H. M., Oldham S. N., Fahl S. P., et al. 2013. . Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and B-1a B cell proliferation . Arterioscler. Thromb. Vasc. Biol . 33 : 2771 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mantani P. T., Duner P., Bengtsson E., et al. 2015. . IL-25 inhibits atherosclerosis development in apolipoprotein E deficient mice . PLoS One 10 : e0117255 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Munneke J. M., Bjorklund A. T., Mjosberg J. M., et al. 2014. . Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease . Blood 124 : 812 . [DOI] [PubMed] [Google Scholar]
- 115. Van Gool F., Molofsky A. B., Morar M. M., et al. 2014. . Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy . Blood 124 : 3572 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fahy J. V . 2014. . Type 2 inflammation in asthma - present in most, absent in many . Nat. Rev. Immunol . 15 : 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]