Abstract
We have previously shown that decidualization of human endometrial stromal cells (ESCs) causes a genome-wide increase in the levels of acetylation of histone-H3 Lys-27 (H3K27ac). We also reported that the distal gene regions, more than 3 kb up- or downstream of gene transcription start sites have increased H3K27ac levels. Insulin-like growth factor-binding protein-1 (IGFBP-1) is a specific decidualization marker and has increased H3K27ac levels in its distal upstream region (−4701 to −7501 bp). Here, using a luciferase reporter gene construct containing this IGFBP-1 upstream region, we tested the hypothesis that it is an IGFBP-1 enhancer. To induce decidualization, we incubated ESCs with cAMP and found that cAMP increased luciferase expression, indicating that decidualization increased the transcriptional activity from the IGFBP-1 upstream region. Furthermore, CRISPR/Cas9-mediated deletion of this region in HepG2 cells significantly reduced IGFBP-1 expression, confirming its role as an IGFBP-1 enhancer. A ChIP assay revealed that cAMP increased the recruitment of the transcriptional regulators CCAAT enhancer-binding protein β (C/EBPβ), forkhead box O1 (FOXO1), and p300 to the IGFBP-1 enhancer in ESCs. Of note, C/EBPβ knockdown inhibited the stimulatory effects of cAMP on the levels of H3K27ac, chromatin opening, and p300 recruitment at the IGFBP-1 enhancer. These results indicate that the region −4701 to −7501 bp upstream of IGFBP-1 functions as an enhancer for IGFBP-1 expression in ESCs undergoing decidualization, that C/EBPβ and FOXO1 bind to the enhancer region to up-regulate IGFBP-1 expression, and that C/EBPβ induces H3K27ac by recruiting p300 to the IGFBP-1 enhancer.
Keywords: endocrinology, epigenetics, histone acetylation, CCAAT-enhancer-binding protein (C/EBP), cyclic AMP (cAMP)
Introduction
Human endometrial stromal cells (ESCs)2 undergo cyclic changes during the menstrual cycle, including proliferation and differentiation that are controlled by estrogen and progesterone. One of these changes, called decidualization, is the progesterone-induced differentiation of fibroblastoid ESCs of the endometrium into decidual cells. Impairment of decidualization can lead to miscarriage, implantation failure, and unexplained infertility (1–4).
Genome-wide analyses revealed a number of genes that are up-regulated or down-regulated by decidualization in human ESCs (5–8). Gene expression including transcription involves a change of chromatin structure, which can be regulated by epigenetic mechanisms such as histone modifications (9, 10). Acetylation of histone-H3 lysine-27 (H3K27ac) is one of the active histone modifications and is highly enriched at the active promoter or enhancer regions (11). By using a genome-wide ChIP-sequence approach, we identified a number of genes in which the H3K27ac level was increased during decidualization in human ESCs (8). Interestingly, 80% of the H3K27ac-increased regions were located in the distal regions, which are more than 3 kb upstream or downstream of the transcription start site (TSS). We also found that the increase of H3K27ac in the distal regions as well as in the proximal regions was associated with the up-regulation of gene expression during decidualization (8). These facts led us to speculate that the H3K27ac-increased regions in the distal regions play key roles as enhancer regions in the regulation of gene expressions during decidualization. To identify the transcriptional mechanisms that regulate the expression of decidualization-related genes, so far, studies have focused on only the proximal promoter regions. The function of the enhancer regions in this regulation remains poorly understood.
IGF-binding protein-1 (IGFBP-1) is preferentially induced during decidualization in ESCs and is therefore recognized as a marker of decidualization (2, 12). Only the roles of the proximal promoter region and transcription factors in the regulation of IGFBP-1 expression have been reported so far (13–16). Our genome-wide analysis using ChIP sequencing revealed a remarkable decidualization-induced increase in the H3K27ac level in the 4.7-kb upstream region of TSS, and that the H3K27ac level was much higher in this region than in the proximal promoter region. This upstream region has not previously been examined in the regulation of IGFBP-1 expression in any type of cells, including ESCs. Therefore, there is a possibility that this region is a novel enhancer region involved in IGFBP-1 expression.
In this study, we identified a novel enhancer region for IGFBP-1 expression by a genome-wide epigenome analysis of ESCs and genome editing. Furthermore, we show how transcription factors and coactivators regulate IGFBP-1 expression through the activation of the enhancer region during decidualization.
Results
Increase in H3K27ac levels in the distal region of IGFBP-1 gene by decidualization
According to our previous ChIP-sequence data of human ESCs, the H3K27ac level was increased by decidualization in many distal regions, which are more than 3 kb upstream or downstream of TSS (8). As shown in Fig. 1A, in two individuals (cases 1 and 2), the remarkable increase in H3K27ac levels was observed in the 5′ upstream distal region (−4701 to −7501 bp) of the IGFBP-1 gene by decidualization. H3K27ac levels in decidualized ESCs were remarkably higher in this region than in the promoter region. Because H3K27ac is one of the marks of an enhancer region, we designated this H3K27ac-increased region as an IGFBP-1 enhancer region.
Figure 1.
Increase in H3K27ac levels in the distal region of IGFBP-1 gene by decidualization. A, genome-browser snapshot showing the H3K27ac ChIP-sequence data around the IGFBP-1 gene in non-decidualized ESCs (non-dESC, treated with control medium) and decidualized ESC (dESCs, treated with estradiol (E2) and MPA). Data from two individuals (case 1 and case 2) are shown. The position of the TSS is designated as +1. The region outlined by a dashed line (4701 to 7501 bp upstream of the transcription start site of IGFBP-1 gene) shows the common region with increased signal of H3K27ac between the two individuals. This region is designated as the IGFBP-1 enhancer region. The horizontal black line at the bottom indicates the IGFBP-1 promoter region. B, validation of the results of ChIP-sequence experiment. ESCs were treated with E2 + MPA for 14 days (left panel) or cAMP for 4 days (right panel) to induce decidualization and then the H3K27ac level in the IGFBP-1 enhancer region was analyzed by ChIP-qPCR. Normal mouse IgG was used as a negative control. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control; b, p < 0.01 versus control. C, H3K4me1 levels in the IGFBP-1 enhancer region. ESCs were treated with or without cAMP for 4 days and then H3K4me1 levels in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. The intergenic region, 35 kb upstream of IGFBP-1, was used as a negative control region that does not have H3K4me1. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus intergenic region. D, effect of cAMP on the changes of chromatin structure in the IGFBP-1 enhancer region. ESCs were treated with or without cAMP for 4 days and then chromatin accessibility in the IGFBP-1 enhancer region was analyzed by FAIRE–qPCR. The relative ratio of FAIRE enrichment was calculated and values were expressed as a ratio of control. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus control. E, effect of cAMP on the enhancer activities of the IGFBP-1 enhancer region in ESCs. The IGFBP-1 enhancer region was subcloned into pGL4.23 (enhancer/pGL4.23). ESCs were transfected with reporter vector (pGL4.23-basic or enhancer/pGL4.23) and pRL-TK vector as a normalization control. After 5 h of transfection, cells were treated in the presence or absence of cAMP for 4 days. The firefly luciferase activity was normalized according to Renilla luciferase activities. Values of the luciferase activities were expressed as a ratio of control with pGL4.23-basic. Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control of enhancer/pGL4.23.
To validate the results of the ChIP-sequence data, ChIP–qPCR was performed with primers designed for the IGFBP-1 enhancer region. The H3K27ac level of this region was significantly increased by decidualization induced by estradiol (E2) + medroxyprogesterone acetate (MPA) stimulation (Fig. 1B, left panel). Dibutyryl-cAMP (cAMP), a decidualization stimulus (17), also increased the H3K27ac level in this region (Fig. 1B, right panel). In this study, to focus on the molecular mechanism on IGFBP-1 expression such as an interaction between the enhancer region and transcription factors, we used cAMP alone as a simple decidualization stimulus in the following experiments.
The levels of H3K4me1, which deposits in a poised enhancer region (18), were examined in the IGFBP-1 enhancer region. As shown in Fig. 1C, in non-decidualized ESCs (control), the H3K4me1 levels were higher in the IGFBP-1 enhancer region than those in the intergenic region, and remained high after cAMP stimulation, which indicates that H3K4me1 modification exists in the enhancer region even before cAMP stimulation and remains high after cAMP stimulation. Therefore, the IGFBP-1 enhancer region is in a poised state in non-decidualized ESCs. Combined with the result that H3K27ac levels were increased by cAMP stimulation, the IGFBP-1 enhancer region changes from a poised state to an activated state during decidualization.
The effect of cAMP on the changes of chromatin structure in the IGFBP-1 enhancer region were examined by FAIRE (formaldehyde-assisted isolation of regulatory elements)–qPCR. cAMP significantly increased the relative ratio of FAIRE enrichment in the IGFBP-1 enhancer region (Fig. 1D), indicating that the chromatin structure of the IGFBP-1 enhancer region becomes looser during decidualization.
To determine whether the IGFBP-1 enhancer region has an enhancer activity, a luciferase construct containing the IGFBP-1 enhancer region (enhancer/pGL4.23) was transfected into ESCs. cAMP significantly increased the luciferase activities of enhancer/pGL4.23, whereas no increase of luciferase activity was observed by cAMP when cells were transfected with pGL4.23-basic (Fig. 1E). These results showed that the IGFBP-1 enhancer region has transcriptional activity following a decidualization stimulus.
Effects of the deletion of endogenous IGFBP-1 enhancer region on IGFBP-1 mRNA expression
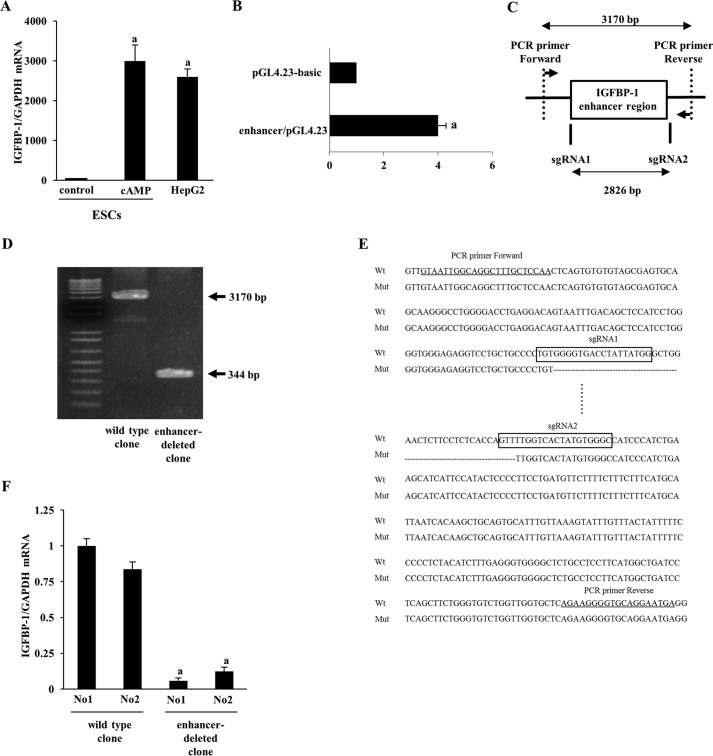
Although we showed that the IGFBP-1 enhancer region has a transcriptional activity in ESCs, it is still unclear whether the enhancer region is actually responsible for IGFBP-1 expression. To test the endogenous function of the enhancer region in regulating IGFBP-1 expression, we established cell lines in which the IGFBP-1 enhancer region was deleted from its endogenous locus by CRISPR/Cas9 editing. We first tried using ESC cell lines, but were unable to establish ESC clones due to the low efficiency of transfection and the difficulty of culturing single cells for cloning (data not shown). As a result, we then tried using human hepatocellular carcinoma (HepG2) cells. HepG2 cells expressed IGFBP-1 mRNA as highly as decidualized ESCs (Fig. 2A), and also showed transcriptional activity of the IGFBP-1 enhancer region (Fig. 2B), suggesting that the enhancer region is responsible for high expression of IGFBP-1 mRNA in HepG2 cells. Therefore, we used HepG2 cells to examine the endogenous function of the IGFBP-1 enhancer region by a genome editing approach. Two small guide RNAs (sgRNAs) were designed to surround the IGFBP-1 enhancer region (Fig. 2C). HepG2 cells were co-transfected with CRISPR/Cas9 and the two sgRNA constructs. After cell cloning, the genomic DNAs of each clone were analyzed by PCR amplification with primers surrounding the two sgRNAs (Fig. 2C). Deleting the 2826-bp region between the two sgRNAs and repairing the DNA by non-homologous end joining would be expected to produce a 344-bp product, whereas a 2826 + 344 = 3170-bp product would be expected from genomic DNAs in wildtype clones. Fig. 2D shows the result of PCR amplification from genomic DNAs of representative wildtype and enhancer-deleted clones. Sequencing the DNA confirmed the deletion of the desired region in the enhancer-deleted clones (Fig. 2E). We picked up two enhancer-deleted clones that had the same deletion on both alleles. IGFBP-1 mRNA expression was analyzed in two wildtype clones and two enhancer-deleted clones. The enhancer-deleted clones showed significantly lower expression of IGFBP-1 mRNA than the wildtype clones (Fig. 2F). These results demonstrate that the enhancer region is responsible for IGFBP-1 mRNA expression.
Figure 2.
Effects of the deletion of endogenous IGFBP-1 enhancer region on IGFBP-1 mRNA expression. A, comparison of IGFBP-1 mRNA expression between non-decidualized ESCs (control), decidualized ESCs (cAMP), and HepG2 cells. Relative mRNA expression levels were quantified by real-time RT-PCR. Values of IGFBP-1 mRNA were normalized to those of GAPDH and expressed as a ratio of the control of ESCs. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus control in ESCs. B, enhancer activity of the IGFBP-1 enhancer region in HepG2 cells. HepG2 cells were transfected with reporter vector (pGL4.23-basic or enhancer/pGL4.23) and pRL-TK vector as a normalization control. The firefly luciferase activity was normalized according to Renilla luciferase activities. Values of the luciferase activities were expressed as a ratio of pGL4.23-basic. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus pGL4.23-basic. C, the vertical lines represent the locations of sgRNA1 and sgRNA2 surrounding the IGFBP-1 enhancer region. Two PCR primers surrounding two sgRNAs were used to amplify the genomic DNAs of each clone. D, PCR amplification products from genomic DNA of the representative clones. 3170-bp PCR product was observed in wildtype clone. Successful deletion of the 2826-bp region between the two sgRNAs generated a smaller PCR product of 344 bp in enhancer-deleted clones. E, representative DNA sequencing result around both deletion junctions amplified from genomic DNA of enhancer-deleted clone (Mut). Part of the deleted 2826-bp region is shown in dashed lines. The sequences of each sgRNAs are boxed (sgRNA1 and sgRNA2). Primer sequences for genomic PCR are underlined. F, IGFBP-1 mRNA expression was analyzed in two wildtype clones and two enhancer-deleted clones by quantitative real-time RT-PCR. Values of IGFBP-1 were normalized to those of GAPDH and expressed as a ratio of wildtype clone, No1. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus wildtype clones.
Recruitment of transcription factors to the IGFBP-1 enhancer region by decidualization
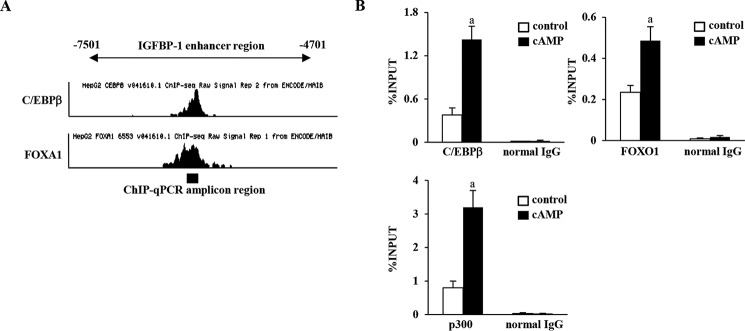
CCAAT enhancer-binding protein β (C/EBPβ) and Forkhead box O1 (FOXO1) are critical transcription factors for decidualization (5, 19). They are known to be involved in the regulation of IGFBP-1 expression during decidualization (5, 19–21). We therefore investigated whether these transcription factors are recruited to the enhancer region by cAMP stimulation. First, we examined whether the potential binding sites of C/EBPβ and FOXO1 exist within the IGFBP-1 enhancer region, using ChIP-sequence data on genome-wide binding of transcription factors in HepG2 cells, which are available in the Encyclopedia of DNA Elements (ENCODE) project (22). HepG2 cells have a C/EBPβ-binding peak in the center of the IGFBP-1 enhancer region (Fig. 3A). Because ENCODE does not have ChIP-sequence data on FOXO1, we checked the binding peaks of Forkhead box protein A1 (FOXA1) because it is a Forkhead box protein and shares the same DNA consensus binding sequence with FOXO1 (23). A FOXA1-binding peak was also observed in the center of the IGFBP-1 enhancer region (Fig. 3A). The DNA sequences around these binding peaks were submitted to the JASPAR database (http://jaspar.binf.ku.dk/),3 which predicted the consensus binding sequences of C/EBPβ and FOXA1 within the IGFBP-1 enhancer region. Therefore, ChIP–qPCR primers were designed to cover these regions to examine the recruitment of C/EBPβ and FOXO1 (Fig. 3A). ChIP–qPCR revealed that cAMP significantly increased the recruitment of C/EBPβ and FOXO1 to the IGFBP-1 enhancer region (Fig. 3B).
Figure 3.
Recruitment of transcription factors to the IGFBP-1 enhancer region by decidualization. A, ChIP-sequence data on C/EBPβ and FOXA1 binding in HepG2 cells from ENCODE. It showed the binding peak of C/EBPβ and FOXA1 in the center of the IGFBP-1 enhancer region. ChIP–qPCR was performed in ESCs with the primers surrounding these binding sites. Black bar at the bottom indicates the ChIP–qPCR amplicon region. B, ESCs were treated with or without cAMP for 4 days. The recruitment of transcription factors (C/EBPβ, FOXO1, and p300) to the IGFBP-1 enhancer region was analyzed by ChIP assay. Normal rabbit IgG was used as a negative control. The relative recruitment levels were analyzed by real-time PCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control.
p300 is a transcription coactivator that has histone acetyltransferase (HAT) activities and induces H3K27ac (24). p300 binds to the enhancer regions (25). Therefore, to elucidate the mechanism by which H3K27ac levels were increased in the IGFBP-1 enhancer region by decidualization, we examined p300 recruitment by ChIP–qPCR with the same primers. cAMP significantly increased p300 recruitment to the IGFBP-1 enhancer region (Fig. 3B). These results suggest that C/EBPβ and FOXO1 bind to the enhancer region to up-regulate IGFBP-1 expression during decidualization and that p300 is one of coactivators that increase H3K27ac levels in the enhancer region.
Involvement of C/EBPβ in the changes of H3K27ac status and the chromatin structure of the IGFBP-1 enhancer region
C/EBPβ interacts with cofactors with HAT activities and increases the H3K27ac levels in the IGFBP-1 promoter region by cAMP stimulation in ESCs (20, 26, 27). Therefore, we hypothesized that C/EBPβ recruitment is responsible for the increase in H3K27ac levels in the IGFBP-1 enhancer region. To test this hypothesis, the effect of C/EBPβ knockdown on the changes of H3K27ac status and chromatin structure of the IGFBP-1 enhancer region was examined. C/EBPβ protein expression was knock downed by C/EBPβ siRNA (Fig. 4A). The cAMP-induced increase in the IGFBP-1 mRNA level was significantly inhibited by C/EBPβ siRNA (Fig. 4B) and the cAMP-induced increase in the H3K27ac level and FAIRE enrichment of the IGFBP-1 enhancer region was significantly inhibited by C/EBPβ knockdown (Fig. 4, C and D). The H3K4me1 levels were not decreased by C/EBPβ knockdown in both ESCs and decidualized ESCs (Fig. 4E).
Figure 4.
Effects of C/EBPβ knockdown on the changes of H3K27ac status and the chromatin structure of the IGFBP-1 enhancer region. A, ESCs were transfected with an siRNA targeted against C/EBPβ or with a nontargeting siRNA as a control. 48 h after siRNA transfection, ESCs were treated with or without cAMP for 4 days. Whole-cell lysates were prepared and subjected to Western blotting to confirm the knockdown of C/EBPβ protein expression. β-Tubulin was used as an internal control. The immunoblot is representative of three different incubations. B, IGFBP-1 mRNA expression was analyzed by quantitative real-time RT-PCR. Values of IGFBP-1 were normalized to those of GAPDH and expressed as a ratio of the cAMP treatment sample, which was transfected with control siRNA. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus control treatment; b, p < 0.01 versus cAMP treatment in the control siRNA. C, H3K27ac level in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control; b, p < 0.05 versus cAMP treatment in the control siRNA. D, chromatin accessibility in the IGFBP-1 enhancer region was analyzed by FAIRE–qPCR. The relative ratio of FAIRE enrichment in the IGFBP-1 enhancer region was calculated and values were expressed as a ratio of control sample, which was transfected with control siRNA. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus control in the control siRNA; b, p < 0.05 versus cAMP treatment in the control siRNA. E, H3K4mel levels in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations.
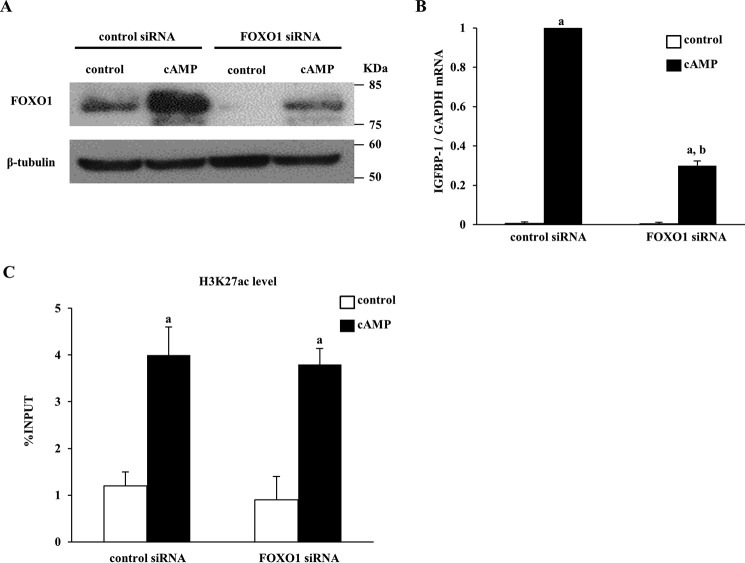
Because FOXO1 also interacts with cofactors with HAT activities and affects the status of sH3K27ac (28, 29), we also examined the effect of FOXO1 knockdown on the status of H3K27ac. FOXO1 protein expression was knock downed by FOXO1 siRNA (Fig. 5A). The IGFBP-1 mRNA level increased by cAMP was significantly inhibited by FOXO1 siRNA (Fig. 5B). However, knockdown of FOXO1 did not affect the cAMP-increased H3K27ac level in the IGFBP-1 enhancer region (Fig. 5C).
Figure 5.
Effects of FOXO1 knockdown on the H3K27ac status in the IGFBP-1 enhancer region. A, ESCs were transfected with an siRNA targeted against FOXO1 or with a nontargeting siRNA as a control. 48 h after siRNA transfection, ESCs were treated with or without cAMP for 4 days. Whole-cell lysates were prepared and subjected to Western blotting to confirm the knockdown of FOXO1 protein expression. β-Tubulin was used as an internal control. The immunoblot is representative of three different incubations. B, IGFBP-1 mRNA expression was analyzed by quantitative real-time RT-PCR. Values of IGFBP-1 were normalized to those of GAPDH and expressed as a ratio of the cAMP treatment sample, which was transfected with control siRNA. Values are mean ± S.E. of three different incubations. a, p < 0.01 versus control treatment; b, p < 0.01 versus cAMP treatment in the control siRNA. C, H3K27ac level in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control.
Involvement of C/EBPβ in the p300 recruitment to the IGFBP-1 enhancer region
Because p300 is reported to interact with both C/EBPβ and FOXO1 (27, 29), we examined whether knockdown of C/EBPβ or FOXO1 results in the reduction of the p300 recruitment to the IGFBP-1 enhancer region. Knockdown of C/EBPβ decreased the cAMP-increased p300 recruitment to the IGFBP-1 enhancer region (Fig. 6A), whereas knockdown of FOXO1 did not (Fig. 6B).
Figure 6.
Effects of knockdown of C/EBPβ or FOXO1 on the p300 recruitment to the IGFBP-1 enhancer region. A, ESCs were transfected with an siRNA targeted against C/EBPβ or with a nontargeting siRNA as a control. 48 h after siRNA transfection, ESCs were treated with or without cAMP for 4 days. The p300 recruitment level in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control; b, p < 0.05 versus cAMP treatment in the control siRNA. B, ESCs were transfected with an siRNA targeted against FOXO1 or with a nontargeting siRNA as a control. 48 h after siRNA transfection, ESCs were treated with or without cAMP for 4 days. The p300 recruitment level in the IGFBP-1 enhancer region was analyzed by ChIP–qPCR. Data were plotted as the ratio of IP DNA to the total INPUT DNA sample (%INPUT). Values are mean ± S.E. of three different incubations. a, p < 0.05 versus control.
Discussion
The transcriptional regulation of IGFBP-1 during decidualization is well studied, but so far mainly only in the proximal promoter region (13–16). By performing genome-wide epigenome analysis (ChIP-sequence), we found that the increase of H3K27ac, an active enhancer mark, by decidualization was more significant in the distal region than in the proximal promoter region. These facts led us to focus on the distal region of IGFBP-1 as a potential enhancer region because not only the regions near TSS, but also distal regions are considered as important cis-elements for transcription (30, 31). In the present study, we showed that this region has H3K4me1, a poised enhancer mark, before decidualization and is activated by decidualization with the acquisition of H3K27ac. We also showed that the chromatin structure of this region becomes looser by decidualization. In addition, our luciferase assay revealed that this region actually has transcriptional activities in ESCs. These results show that the distal region we focused on is a novel enhancer region that is in a poised state in non-decidualized ESCs and is activated during decidualization. In addition to the luciferase assay, we used a CRISPR/Cas9 technique to test the endogenous function of the enhancer region in IGFBP-1 expression. So far, it has been difficult to validate the endogenous function of a putative enhancer. A reporter assay confirms that the DNA sequence of the enhancer region transfected exogenously has a transcriptional activity, but it does not demonstrate whether an adjacent gene can be activated by this enhancer in vivo. By establishing cell lines that lack the IGFBP-1 enhancer region by genome editing, we clearly showed that the IGFBP-1 enhancer region is involved in IGFBP-1 expression. Because we used HepG2 cells, we cannot neglect the possibility that regulation of IGFBP-1 in decidualized ESCs is different from that in HepG2 cells. Taken together, our work shows that genome-wide epigenome analysis can be used to find novel enhancer regions and that genome editing can demonstrate the endogenous function of the enhancer region. Combining these two methods will contribute to the analysis of enhancers on the regulation of decidualization-related gene expressions.
Our study also showed a potential molecular mechanism by which transcription factors and coactivators regulate IGFBP-1 expression in the enhancer region. C/EBPβ interacts with p300, which is a coactivator that has HAT activities and induces H3K27 acetylation (24, 27). We found that p300 was recruited to the IGFBP-1 enhancer region by cAMP, suggesting that p300 is one of coactivators that increase the H3K27ac levels in the IGFBP-1 enhancer region. In addition, because knockdown of C/EBPβ inhibited the p300 recruitment to the IGFBP-1 enhancer region, C/EBPβ appears to play a key role in the increase of H3K27ac levels by recruiting p300 to the IGFBP-1 enhancer region. Furthermore, our results showed that C/EBPβ was involved in opening the chromatin structure of this region. Taken together, C/EBPβ acts as a pioneering factor initiating the process of chromatin remodeling in the enhancer region. This is supported by the finding that C/EBPβ acts as a pioneering transcription factor to trigger the opening of the chromatin status of the enhancer region of myb-inducible myelomonocytic-1 gene (32). C/EBPβ was not involved in the modification of H3K4me1 in the IGFBP-1 enhancer region. Therefore, C/EBPβ is not involved in de novo formation of this enhancer, and it contributes to activation of the enhancer region.
FOXO1 has also been reported to interact with p300 and to be involved in the induction of H3K27ac as a pioneering factor (33, 34). In contrast to C/EBPβ, FOXO1 did not interact with p300 and was not involved in the induction of H3K27ac in this region. FOXO proteins have dual roles as both pioneer and non-pioneering transcription factors (23). FOXO3, another forkhead box protein, binds to the regulatory elements with open chromatin structures that already display high H3K27ac in colon cancer cells (35). According to these reports, FOXO1 does not always work as a pioneering factor to initiate chromatin opening. Therefore, our results indicate that chromatin remodeling induced by C/EBPβ and p300 allows the following FOXO1 recruitment and activates the IGFBP-1 enhancer region.
We previously reported that the H3K27ac level was increased by cAMP in the promoter region of IGFBP-1 and that knockdown of C/EBPβ decreased it (20). Interestingly, the recruitment of FOXO1 and p300 by cAMP was not observed in the promoter region,4 suggesting that there are different mechanisms to increase H3K27ac levels between the enhancer and promoter regions. Further studies are needed to clarify what cofactors interact with C/EBPβ to increase H3K27ac levels in the promoter region.
The present study identified a novel enhancer region for IGFBP-1 expression by a genome-wide epigenome analysis and genome editing in ESCs. This enhancer region is activated by the recruitment of C/EBPβ and FOXO1 to up-regulate IGFBP-1 expression. Especially, C/EBPβ, cooperating with p300, increases H3K27ac levels and opens the chromatin structure of the enhancer region. Our work shows a novel molecular mechanism by which IGFBP-1 expressions is induced during decidualization in human ESCs.
Experimental procedures
Reagents
DMEM, l-glutamine, 1× trypsin-EDTA, streptomycin, and penicillin were purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from Biological Industries Ltd. (Beit Haemek, Israel). Collagenases, estradiol (E), MPA and dibutyryl-cAMP (Bt2cAMP) were obtained from Sigma. Tissue flasks were from BD Biosciences.
ESC isolation
Human endometrial tissues were obtained at hysterectomy from patients with a normal menstrual cycle, aged 40–45 years, who underwent surgery for myoma uteri or early stage cervical cancer. The patients were not on hormonal therapy at the time of surgery. Informed consent was obtained from all participating patients, and ethical approval was obtained from Institutional Review Board of Yamaguchi University Hospital. All experiments were performed in accordance with Tenets of the Declaration of Helsinki. Endometrial samples utilized for ESC isolation were histologically diagnosed as being in the late proliferative phase according to published criteria (36). Tissue samples were washed with phenol red-free DMEM containing 4 mm glutamine, 50 μg/ml of streptomycin, and 50 IU/ml of penicillin, and minced into pieces of <1 mm3. ESCs were isolated as reported previously (10). In brief, tissues were minced, enzymatically digested with 0.2% collagenase in a shaking water bath for 2 h at 37 °C and filtered through a 70-μm nylon mesh. Stromal cells in the filtrates were washed three times with the medium, and the number of viable cells was counted by Trypan blue dye exclusion. Under the microscope, all of the cells reacted with the stromal-reacting antibody vimentin (data not shown), indicating that they were homogeneous. The cells were also verified to be negative for the epithelial cell-reacting antibody (cytokeratin) (data not shown). Cells were seeded at 105 cells/cm2 in 75-cm2 tissue culture flasks and incubated in phenol red-free DMEM containing glutamine, antibiotics, and 10% dextran-coated charcoal-stripped FBS at 37 °C, 95% air and 5% CO2. At confluence, cells were treated with 1× trypsin-EDTA and subcultured into 25-cm2 tissue culture flasks. At 80% confluence after the first passage, the cell culture medium was changed to the treatment medium.
Cell culture
HepG2 cells were seeded in 75-cm2 tissue culture flasks and incubated in DMEM containing glutamine, antibiotics, and 10% FBS at 37 °C, 95% air and 5% CO2. At confluence, cells were treated with 1× trypsin-EDTA and subcultured into 6-well plates and used for the experiment of luciferase assay and genome editing as described below. To induce decidualization, ESCs were incubated with treatment medium (phenol red-free DMEM supplemented with glutamine, antibiotics, and 2% dextran-coated charcoal-stripped FBS) containing E2 (10−8 mol/liter) and MPA (10−6 mol/liter) for 14 days or Bt2cAMP (0.5 mm) for 4 days at 37 °C in an atmosphere of 95% air and 5% CO2. The cells were then used for the following experiments described below. The concentrations of ovarian steroids and cAMP and the period of incubation used in this study were based on our previous report (17). The medium was changed every other day. Cells isolated from one patient were incubated one time in triplicate. Cells from three individuals were incubated in a single experiment.
Real-time RT-PCR
Total RNA was isolated from the cultured cells with an RNeasy® Mini Kit. (Qiagen, Valencia, CA). The RNA was reverse transcribed as reported previously (37). For PCR amplification, first strand cDNA was synthesized from 1 μg of total RNA with reverse transcriptase (Invitrogen) in 20 μl of reaction mixture. Amplicons of IGFBP-1 and GAPDH were amplified by real-time RT-PCR as reported previously (38) with sequence-specific primer sets (Table S1).
Luciferase assay
Luciferase assay was performed to examine the enhancer activity of the IGFBP-1 enhancer region. The IGFBP-1 enhancer region was amplified by PCR from the human genomic DNA. The PCR products were subcloned upstream of the luciferase gene into firefly luciferase vector pGL4.23 (Promega, Madison, WI), which contains minimal promoter. The constructs were termed enhancer/pGL4.23. ESCs or HepG2 cells were cultured on a 24-well plate (5 × 104 cells/well) for 24 h and then transfected with reporter vector (pGL4.23-basic or enhancer/pGL4.23) and pRL-TK vector (Promega) as a normalization control using Lipofectamine LTX (Invitrogen). The firefly and Renilla luciferase activities were measured using a Dual-luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Western blotting
Whole cell lysates were prepared using loading buffer reagents (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) without trypsin treatment. Equal amounts of total protein were electrophoresed on a 10% SDS-polyacrylamide gel. The proteins were transferred to polyvinylidene difluoride membranes (ATTO, Tokyo, Japan). The membranes were blocked with blocking solution (5% skimmed milk with 0.1% Tween 20 dissolved in Tris-buffered saline (pH 7.5)), incubated with the first antibody for C/EBPβ, FOXO1 (Santa Cruz Biotechnology), and β-tubulin (Sigma), which were diluted in blocking solution, incubated with the peroxidase-conjugated second antibody diluted in blocking solution, visualized with the ECL-Western blotting detection system (Amersham Biosciences, Aylesburg, UK) according to the manufacturer's protocol, and used to expose hyperfilm-ECL (Amersham Biosciences). To reuse the blot, the membranes were stripped in Restore Western stripping buffer (Pierce).
Lipid-mediated transfection of siRNA duplexes
C/EBPβ ON-TARGET plus SMART pool, FOXO1 ON-TARGET plus SMART pool, and ON-TARGET plus Non-Targeting pool siRNA were purchased from Dharmacon (Lafayette, CO). siRNA was transfected to ESCs as reported previously (39). In brief, ESCs were plated in medium lacking antibiotics at ∼5 × 104 cells in 6-well plates. At 50% confluence, siRNA duplexes (20 nm) and RNAi MAX (2.5 μl/well; Invitrogen) diluted in Opti-MEM (Invitrogen) were transfected to ESCs. The medium was changed 4 h later. After 48 h of transfection, cells were incubated in the presence or absence of cAMP for 4 days.
ChIP assay
The levels of transcription factor recruitment and histone modification status were examined by ChIP assay according to the protocol for the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) as reported previously (20, 40) with some modifications. Cells were cross-linked by addition of formaldehyde into the medium at a final concentration of 1% and incubated for 10 min at 37 °C. Cross-linking was terminated by addition of glycine (0.125 m, final concentration). Cells were washed with ice-cold PBS containing protease inhibitors (Sigma) and resuspended in ChIP lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris-HCl, pH 8.0, with protease inhibitors). The lysates were sonicated using a Bioruptor ultrasonicator (Cosmo-bio, Tokyo, Japan), precleared with salmon sperm DNA-protein A at 4 °C for 4 h, and diluted with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl, pH 8.0, 167 mm NaCl, with protease inhibitors). Five percent of the supernatants were kept as input controls (INPUT). Dynabeads Protein A (Invitrogen) were incubated with antibodies for H3K27ac, H3K4me1 (generous gifts from Dr. Kimura) (41), C/EBPβ, p300 (Santa Cruz Biotechnology), FOXO1 (Active Motif, Carlsbad, CA), and normal mouse or rabbit IgG (Invitrogen) at 4 °C overnight. The precleared chromatin was incubated with antibody-bound Dynabeads for 6 h at 4 °C. Immune complexes were collected and washed once for 5 min on a rotating platform with 1 ml each of the following buffers in sequence: low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.0, 150 mm NaCl), high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.0, 1500 mm NaCl), LiCl wash buffer (250 mm LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mm EDTA, 10 mm Tris-HCl, pH 8.0), and twice with TE (10 mm Tris-HCl, pH 8.0, 1 mm EDTA). Immune complexes were eluted with 200 μl of elution buffer (1% SDS, 0.1 m NaHCO3, 10 mm DTT). The immunoprecipitated (IP) chromatin complexes and INPUT were incubated at 65 °C overnight to reverse the cross-linking and subjected to proteinase K treatment. Purified DNA using a QIAquick PCR purification kit (Qiagen) served as the template for PCR using primer sets (Table S1) to amplify specific regions. Real-time PCR was used to determine the relative levels of transcription factor recruitments and statuses of H3K27ac and H3K4me1 in the IGFBP-1 enhancer region. The intergenic region, 35 kb upstream of IGFBP-1, was used as a negative control region, which does not have H3K4me1. The ratio of IP DNA to the INPUT DNA sample (%INPUT) was calculated as reported previously (42).
FAIRE–qPCR
Chromatin accessibility in the IGFBP-1 enhancer region was investigated with FAIRE–qPCR as reported previously (43). The precleared chromatin was prepared as described in the ChIP assay. 10% of the chromatin solution was kept as input controls (INPUT). The remaining chromatin solutions were purified by 3 cycles of phenol/chloroform/isoamyl (FAIRE sample). They were incubated at 65 °C overnight to reverse the cross-linking and subjected to proteinase K treatment. Purified DNA using a QIAquick PCR purification kit (Qiagen) served as the template for PCR using primer sets to amplify the IGFBP-1 enhancer region (Table S1). Real-time PCR was used to determine the relative levels of FAIRE enrichment. The ratio of the FAIRE sample DNA relative to INPUT DNA was calculated.
Deletion of the IGFBP-1 enhancer region by CRISPR/Cas9 system
In the CRISPR/Cas9 system, a sgRNA targets the Cas9 protein to a specific genomic location and generates double-strand break. Random mutations can be generated when the DNA break is repaired via nonhomologous end joining (44, 45). By introducing two sgRNAs surrounding the IGFBP-1 enhancer region into a cell, two DNA breaks may be generated simultaneously on the same chromosome, leading to the deletion of the IGFBP-1 enhancer region. To construct sgRNA expression vectors, each 20-bp target sequence was subcloned into pCAGmCherry-gRNA Vector (Addgene plasmid number 87110, a gift from Dr. Juan Carlos Izpisua Belmonte). The CRISPR/Cas9 target sequences used in this study are: sgRNA1, GCATAATAGGTCACCCCACAGGG and sgRNA2, GCCCACATAGTGACCAAAACTGG, in which the 3-bp PAM sequences are underlined (Fig. 2, C and E). HepG2 cells were plated in medium lacking antibiotics at ∼3 × 105 cells in a 6-well plate 1 day before transfection. At 50% confluence, Cas9 plasmid (1 μg) (Addgene plasmid number 44719, a gift from Kiran Musunuru) and two gRNA plasmids (0.5 μg each) were transfected to HepG2 cells with Lipofectamine 3000 (Invitrogen). After 48 h of transfection, cells were trypsinized, diluted, and plated in 6-well plates. After 10 days, individual colonies were isolated, harvested, seeded in 24-well plates, and grown until confluence when they were transferred to 6-well plates. To screen the enhancer deletion, the genomic DNAs of each clone were analyzed by PCR amplification with primers surrounding the two sgRNAs (Fig. 2, C and E). Twenty ng of genomic DNA was used and amplified with PrimeSTAR GXL DNA Polymerase (TaKaRa, Ohtsu, Japan). The resulting products were subjected to agarose gel electrophoresis and purified using a QIAquick gel extraction kit (Qiagen). The PCR products were cloned into pGEM-T easy vector (Promega, Tokyo, Japan) and sequencing was performed using an ABI automated sequencer with BigDye terminators (Applied Biosystems, Foster City, CA). Two wildtype clones and two enhancer-deleted clones were used as representative clones. IGFBP-1 mRNA expression was analyzed by real-time RT-PCR.
Statistical analysis
Statistical significance was determined by one-way analysis of variance. After analysis of variance, the Tukey-Kramer test was applied to analyze differences between groups. An unpaired t test was applied to analyze the difference between two groups. All statistical analyses were performed using SPSS for Windows version 11 (SPSS Inc., Chicago, IL). Differences were considered significant at p < 0.05.
Author contributions
I. T. conceptualization; I. T. data curation; I. T. formal analysis; I. T. funding acquisition; I. T., K. J., Y. S., and M. S. validation; I. T. and S. S. investigation; I. T. visualization; I. T., S. S., and R. M. methodology; I. T. writing-original draft; I. T. and N. S. writing-review and editing; T. T., H. A., H. T., and N. S. supervision.
Supplementary Material
Acknowledgment
We thank Dr. Hiroshi Kimura (Tokyo Kogyo University, Tokyo, Japan) for the gift of anti-H3K27ac and H3K4me1 antibodies.
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grants 15K10720, 15K20146, 16K11091, 16K11142, 16K20191, 16K20194, 17K11240, and 17K11239. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
I. Tamura, K. Jozaki, S. Sato, Y. Shirafuta, M. Shinagawa, R. Maekawa, T. Taketani, H. Asada, H. Tamura, and N. Sugino, unpublished data.
- ESC
- endometrial stromal cell
- H3K27ac
- acetylation of histone-H3 lysine-27
- TSS
- transcription start site
- IGFBP-1
- insulin-like growth factor-binding protein-1
- MPA
- medroxyprogesterone acetate
- FAIRE
- formaldehyde-assisted isolation of regulatory elements
- sgRNA
- small guide RNA
- C/EBPβ
- CCAAT enhancer-binding protein β
- FOXO1
- forkhead box O1
- HAT
- histone acetyltransferase
- DMEM
- Dulbecco's mofified Eagle's medium
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IP
- immunoprecipitated.
References
- 1. Zhang Q., and Yan J. (2016) Update of Wnt signaling in implantation and decidualization. Reprod. Med. Biol. 15, 95–105 10.1007/s12522-015-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gellersen B., and Brosens J. (2003) Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J. Endocrinol. 178, 357–372 10.1677/joe.0.1780357 [DOI] [PubMed] [Google Scholar]
- 3. Salker M., Teklenburg G., Molokhia M., Lavery S., Trew G., Aojanepong T., Mardon H. J., Lokugamage A. U., Rai R., Landles C., Roelen B. A., Quenby S., Kuijk E. W., Kavelaars A., Heijnen C. J., Regan L., Macklon N. S., and Brosens J. J. (2010) Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS ONE 5, e10287 10.1371/journal.pone.0010287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laird S. M., Tuckerman E. M., and Li T. C. (2006) Cytokine expression in the endometrium of women with implantation failure and recurrent miscarriage. Reprod. Biomed. Online 13, 13–23 [DOI] [PubMed] [Google Scholar]
- 5. Wang W., Taylor R. N., Bagchi I. C., and Bagchi M. K. (2012) Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Mol. Endocrinol. 26, 2016–2030 10.1210/me.2012-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aghajanova L., Horcajadas J. A., Weeks J. L., Esteban F. J., Nezhat C. N., Conti M., and Giudice L. C. (2010) The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 151, 1341–1355 10.1210/en.2009-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popovici R. M., Kao L. C., and Giudice L. C. (2000) Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 141, 3510–3513 10.1210/endo.141.9.7789 [DOI] [PubMed] [Google Scholar]
- 8. Tamura I., Ohkawa Y., Sato T., Suyama M., Jozaki K., Okada M., Lee L., Maekawa R., Asada H., Sato S., Yamagata Y., Tamura H., and Sugino N. (2014) Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol. Endocrinol. 28, 1656–1669 10.1210/me.2014-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li B., Carey M., and Workman J. L. (2007) The role of chromatin during transcription. Cell 128, 707–719 10.1016/j.cell.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 10. Tamura I., Taketani T., Lee L., Kizuka F., Taniguchi K., Maekawa R., Asada H., Tamura H., and Sugino N. (2011) Differential effects of progesterone on COX-2 and Mn-SOD expressions are associated with histone acetylation status of the promoter region in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 96, E1073–E1082 10.1210/jc.2010-2489 [DOI] [PubMed] [Google Scholar]
- 11. Wang Z., Zang C., Rosenfeld J. A., Schones D. E., Barski A., Cuddapah S., Cui K., Roh T. Y., Peng W., Zhang M. Q., and Zhao K. (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40, 897–903 10.1038/ng.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sultana S., Kajihara T., Mizuno Y., Sato T., Oguro T., Kimura M., Akita M., and Ishihara O. (2017) Overexpression of microRNA-542–3p attenuates the differentiating capacity of endometriotic stromal cells. Reprod. Med. Biol. 16, 170–178 10.1002/rmb2.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim J. J., Taylor H. S., Akbas G. E., Foucher I., Trembleau A., Jaffe R. C., Fazleabas A. T., and Unterman T. G. (2003) Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol. Reprod. 68, 24–30 10.1095/biolreprod.102.009316 [DOI] [PubMed] [Google Scholar]
- 14. Gao J., Mazella J., Suwanichkul A., Powell D. R., and Tseng L. (1999) Activation of the insulin-like growth factor binding protein-1 promoter by progesterone receptor in decidualized human endometrial stromal cells. Mol. Cell. Endocrinol. 153, 11–17 10.1016/S0303-7207(99)00096-9 [DOI] [PubMed] [Google Scholar]
- 15. Tang M., Mazella J., Zhu H. H., and Tseng L. (2005) Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol. Hum. Reprod. 11, 237–243 10.1093/molehr/gah149 [DOI] [PubMed] [Google Scholar]
- 16. Tamura I., Asada H., Maekawa R., Tanabe M., Lee L., Taketani T., Yamagata Y., Tamura H., and Sugino N. (2012) Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology 153, 5612–5621 10.1210/en.2012-1420 [DOI] [PubMed] [Google Scholar]
- 17. Matsuoka A., Kizuka F., Lee L., Tamura I., Taniguchi K., Asada H., Taketani T., Tamura H., and Sugino N. (2010) Progesterone increases manganese superoxide dismutase expression via a cAMP-dependent signaling mediated by noncanonical Wnt5a pathway in human endometrial stromal cells. J. Clin. Endocrinol Metab. 95, E291–E299 10.1210/jc.2010-0619 [DOI] [PubMed] [Google Scholar]
- 18. Creyghton M. P., Cheng A. W., Welstead G. G., Kooistra T., Carey B. W., Steine E. J., Hanna J., Lodato M. A., Frampton G. M., Sharp P. A., Boyer L. A., Young R. A., and Jaenisch R. (2010) Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U.S.A. 107, 21931–21936 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takano M., Lu Z., Goto T., Fusi L., Higham J., Francis J., Withey A., Hardt J., Cloke B., Stavropoulou A. V., Ishihara O., Lam E. W., Unterman T. G., Brosens J. J., and Kim J. J. (2007) Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol. Endocrinol. 21, 2334–2349 10.1210/me.2007-0058 [DOI] [PubMed] [Google Scholar]
- 20. Tamura I., Sato S., Okada M., Tanabe M., Lee L., Maekawa R., Asada H., Yamagata Y., Tamura H., and Sugino N. (2014) Importance of C/EBPβ binding and histone acetylation status in the promoter regions for induction of IGFBP-1, PRL, and Mn-SOD by cAMP in human endometrial stromal cells. Endocrinology 155, 275–286 10.1210/en.2013-1569 [DOI] [PubMed] [Google Scholar]
- 21. Kim J. J., Buzzio O. L., Li S., and Lu Z. (2005) Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol. Reprod. 73, 833–839 10.1095/biolreprod.105.043182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ENCODE Project Consortium. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lalmansingh A. S., Karmakar S., Jin Y., and Nagaich A. K. (2012) Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim. Biophys. Acta 1819, 707–715 10.1016/j.bbagrm.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 24. Jin Q., Yu L. R., Wang L., Zhang Z., Kasper L. H., Lee J. E., Wang C., Brindle P. K., Dent S. Y., and Ge K. (2011) Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 10.1038/emboj.2010.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z., Zang C., Cui K., Schones D. E., Barski A., Peng W., and Zhao K. (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019–1031 10.1016/j.cell.2009.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiper-Bergeron N., Salem H. A., Tomlinson J. J., Wu D., and Haché R. J. (2007) Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPβ by GCN5. Proc. Natl. Acad. Sci. U.S.A. 104, 2703–2708 10.1073/pnas.0607378104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mink S., Haenig B., and Klempnauer K. H. (1997) Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol. 17, 6609–6617 10.1128/MCB.17.11.6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yalley A., Schill D., Hatta M., Johnson N., and Cirillo L. A. (2016) Loss of interdependent binding by the FoxO1 and FoxA1/A2 forkhead transcription factors culminates in perturbation of active chromatin marks and binding of transcriptional regulators at insulin-sensitive genes. J. Biol. Chem. 291, 8848–8861 10.1074/jbc.M115.677583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perrot V., and Rechler M. M. (2005) The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol. Endocrinol. 19, 2283–2298 10.1210/me.2004-0292 [DOI] [PubMed] [Google Scholar]
- 30. Li G., Ruan X., Auerbach R. K., Sandhu K. S., Zheng M., Wang P., Poh H. M., Goh Y., Lim J., Zhang J., Sim H. S., Peh S. Q., Mulawadi F. H., Ong C. T., Orlov Y. L., et al. (2012) Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 10.1016/j.cell.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y., Wong C. H., Birnbaum R. Y., Li G., Favaro R., Ngan C. Y., Lim J., Tai E., Poh H. M., Wong E., Mulawadi F. H., Sung W. K., Nicolis S., Ahituv N., Ruan Y., and Wei C. L. (2013) Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature 504, 306–310 10.1038/nature12716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plachetka A., Chayka O., Wilczek C., Melnik S., Bonifer C., and Klempnauer K. H. (2008) C/EBPβ induces chromatin opening at a cell-type-specific enhancer. Mol. Cell. Biol. 28, 2102–2112 10.1128/MCB.01943-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hatta M., Liu F., and Cirillo L. A. (2009) Acetylation curtails nucleosome binding, not stable nucleosome remodeling, by FoxO1. Biochem. Biophys. Res. Commun. 379, 1005–1008 10.1016/j.bbrc.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 34. Hatta M., and Cirillo L. A. (2007) Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J. Biol. Chem. 282, 35583–35593 10.1074/jbc.M704735200 [DOI] [PubMed] [Google Scholar]
- 35. Eijkelenboom A., Mokry M., Smits L. M., Nieuwenhuis E. E., and Burgering B. M. (2013) FOXO3 selectively amplifies enhancer activity to establish target gene regulation. Cell Rep. 5, 1664–1678 10.1016/j.celrep.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 36. Noyes R. W., Hertig A. T., and Rock J. (1975) Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 122, 262–263 10.1016/S0002-9378(16)33500-1 [DOI] [PubMed] [Google Scholar]
- 37. Maekawa R., Sato S., Okada M., Lee L., Tamura I., Jozaki K., Kajimura T., Asada H., Yamagata Y., Tamura H., Yamamoto S., and Sugino N. (2016) Tissue-specific expression of estrogen receptor 1 is regulated by DNA methylation in a T-DMR. Mol. Endocrinol. 30, 335–347 10.1210/me.2015-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okada M., Lee L., Maekawa R., Sato S., Kajimura T., Shinagawa M., Tamura I., Taketani T., Asada H., Tamura H., and Sugino N. (2016) Epigenetic changes of the Cyp11a1 promoter region in granulosa cells undergoing luteinization during ovulation in female rats. Endocrinology 157, 3344–3354 10.1210/en.2016-1264 [DOI] [PubMed] [Google Scholar]
- 39. Tamura I., Shirafuta Y., Jozaki K., Kajimura T., Shinagawa M., Maekawa R., Taketani T., Asada H., Sato S., Tamura H., and Sugino N. (2017) Novel function of a transcription factor WT1 in regulating decidualization in human endometrial stromal cells and its molecular mechanism. Endocrinology 158, 3696–3707 10.1210/en.2017-00478 [DOI] [PubMed] [Google Scholar]
- 40. Lee L., Asada H., Kizuka F., Tamura I., Maekawa R., Taketani T., Sato S., Yamagata Y., Tamura H., and Sugino N. (2013) Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 154, 458–470 10.1210/en.2012-1610 [DOI] [PubMed] [Google Scholar]
- 41. Kimura H., Hayashi-Takanaka Y., Goto Y., Takizawa N., and Nozaki N. (2008) The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct. Funct. 33, 61–73 10.1247/csf.07035 [DOI] [PubMed] [Google Scholar]
- 42. Qiu X., Aiken K. J., Chokas A. L., Beachy D. E., and Nick H. S. (2008) Distinct functions of CCAAT enhancer-binding protein isoforms in the regulation of manganese superoxide dismutase during interleukin-1β stimulation. J. Biol. Chem. 283, 25774–25785 10.1074/jbc.M801178200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simon J. M., Giresi P. G., Davis I. J., and Lieb J. D. (2012) Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat. Protoc. 7, 256–267 10.1038/nprot.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A., and Zhang F. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., Norville J. E., and Church G. M. (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.