SUMMARY
Background.
Platelet (PLT) gel has been successfully used in tissue regeneration of diabetic and surgical wounds through the releasing of growth factors such as basic fibroblast and PLT-derived growth factors. Based on this background, our previous clinical trial have assessed the feasibility and efficacy of PLT gel for the treatment of muco-cutaneous lesions related to graft versus host disease (GvHD) after allogeneic haematopoietic stem cell transplantion (HSCT). The promising results reported in a small series of 6 patients, of whom 1 with oral ulcers, represent the rationale of the present study.
Materials and methods.
The aim of this study was to verify the efficacy and safety of PLT gel for treating oral ulcers due to chronic GvHD. Allogeneic hemocomponents were used to obtain PLT gel with an automated system for the on-site preparation and application of patient (autologous) or healthy blood donor (allogeneic)-derived fibrin sealant or PLT-rich fibrin (Vivostat system, Vivostat A/S). Ten patients with multiple oral lesions related to chronic GvHD underwent allogeneic PLT gel as local therapy alone or in combination with systemic therapy in half of the cases.
Results.
After the second PLT gel application, all patients resumed the feeding and a significant improvement of the oral pain was observed. After a median of five PLT gel applications (range, 2–15), 7 out of 10 patients showed a complete response. No side effects were documented.
Conclusion.
These data confirm that the PLT gel may be used as a safe and effective tool, alone or in combination with systemic therapy, for the treatment of mucosal lesions of mouth related to cGvHD.
Keywords: allogeneic HSCT, oral ulcers, cGvHD, PLT gel
Introduction
Allogeneic haematopoetic stem cell transplantation (HSCT) represents a potentially curative treatment for several haematological disorders, regardless the stem cell source and the donor’s type (1–6), but its success is still hampered by the onset of the graft versus host disease. Graft-versus-host disease (GvHD) is a major complication of allogeneic HSCT, associated with significant morbidity and mortality. Balancing the benefits and disadvantages, GvHD enhances survival by decreasing the risk of disease relapses counterbalanced by an increase of mortality due to life-threatening infections or organ failure events (7). Age and donor/recipient HLA matching, intensity of the conditioning regimen, source of stem cells and quality of the graft are the main risk factors influencing the incidence and seriousness of the side effects related to the transplant procedure, including the GvHD (8–12).
GvHD reflects a complex pathological interaction between the innate and adaptive immune systems of the host and donor. Over the past decades there has been a tremendous advancement in understanding of the cellular and molecular underpinnings of this disease, including novel extracellular mediators of inflammation, immune subsets, intra-cellular signal transduction, post-translation modifications and epigenetic regulation (13). Moreover, many recent changes in transplant practice have led to changing the clinical patterns of GvHD. These include the introduction of alternative donor sources, reduced intensity conditioning and novel prophylaxis for GvHD. However, skin and oral mucosa manifestations represent the most frequent localization of acute and chronic GvHD, respectively, occurring with an incidence ranging between 20 and 70%. In particular, the most of patients who develop cGvHD with oral cavity involvement presents dysgeusia, salivary glands hypofunction, xerostomia, lichenoid lesions and atrophy of buccal mucosa, tongue and lips whereas scleroderma, ulcers and mucocele occur in a minority of cases. Painful oral wounds may heavily impair the feeding function and quality of life of this setting of patients (7). To improve the management of these symptoms, in addition to the systemic therapy, several “ancillary therapy and supportive care therapies,” including immunosuppressive or anti-inflammatory topical efforts, have been administered (14). The oral cavity supportive care consists of dexamethasone and other corticosteroid rinse formulations that are held and swished in the mouth for 4 to 6 minutes and then expelled without swallowing, four to six times per day. In addition, topical analgesia with viscous lidocaine may be helpful when symptomatic mucosal GvHD impairs nutrition or communication. Some oral mucosa manifestations are refractory to the combination of the systemic therapy with the above-mentioned ancillary cares so that the investigation of novel therapeutic agents is required. The role of platelet (PLT) gel is well known in tissue regeneration of difficult diabetic/surgical wounds through the releasing of several growth factors (GFs) (15). In fact, wound healing is a complex process mediated by interacting molecular signals and regulated by a pattern of events including coagulation, inflammation, formation of granulation tissue, epithelization and tissue remodeling which allow regeneration. These events are mediated and modulated by interacting molecular signals, primarily cytokines, and GFs. The main factors of tissue repair are represented by a series of GFs: PLT-derived growth factor (PDGF), insulin-like growth factor (IGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF). Several experimental clinical trials have shown that chronic wounds may be related to the deficiency of the abovementioned GFs involved in the healing process by way of decreased production, impaired release, trapping, excess of degradation or a combination of these mechanisms. During the epithelial ulcers’ repair, the pivot role of the release of PLT alfa-granules, has been demonstrated by several studies. These granules containing many GFs like PDGF, EGF, IGF-1 and -2, transform growth factor-beta, and VEGF (16, 17). As a matter of fact, re-epithelization, angiogenesis by mesenchymal cell recruitment and extracellular matrix synthesis depends on the content of these granules. All this information led to the development of a PLT gel to facilitate the regeneration of tissues damaged by ulcers. The main clinical application of this gel has been indicated to treat chronic injuries of vascular, surgical and diabetic patients. Moreover, our previous experiences have shown the efficiency of fibrin glue and PLT gel in the treatment of two severe complications of allogeneic HSCT such as the hemorrhagic cystitis and muco-cutaneous lesions related to the GvHD, respectively (18–20).
Based on biological rationale and previous clinical data, the aim of this prospective cooperative study was to confirm the efficacy and safety of topical use of PLT gel in clinical management of cGvHD oral ulcers in patients who underwent allogeneic HSCT for hematological malignant diseases.
Materials and methods
From January 2006 to date, 10 patients with a median age of 48.5 years (39–61), affected by hematological malignancies, received an allogeneic HSCT at Rome Transplant Network of “Tor Vergata” Hematology and developed cGvHD. The Rome Transplant Network is a JACIE (Joint Accreditation Committee of ISCT and EBMT) accredited Metropolitan Transplant Program that performs about 70 allogeneic HSCT for year, according to an established policy for the donor identification and standard transplant procedures (21, 22). All patients developed extensive (N=5) or limited (N=5) cGvHD with oral involvement characterized by painful ulcers and impairment of feeding function. They received the same myeloablative (n=7) or reduced intensity (n=3) conditioning regimen, according to patient’s age or comorbidity index (23), consisting of thiotepa, single daily dose of i.v.busulphan and fludarabine (5), including ATG in matched unrelated transplant. As GvHD prophylaxis, cyclosporine and methotrexate combination was given. As stem cell source, all patients received peripheral blood stem cells from HLA identical sibling or unrelated donor in 6 and 4 cases, respectively. For grading cGvHD, National Institutes of Health Consensus was used (24). Platelet gel application alone or in combination with immunosuppressive agents was used according to the severity of cGvHD. Limited oral cGvHD ulcers were treated with PLT gel alone, whereas the most part of extensive cGvHD received PLT gel in association with steroids. Ulcers affected buccal mucosa, tongue, lips or mouth’s floor. Pre-treatment parameters were considered oral mucosa injuries, spontaneous widespread pain worsened by swallowing or feeding and microbiological contamination of lesions. Response to the treatment has been evaluated in relation to the pain’s involution, feeding function’s recovery and the ulcers’ disappearance. Complete response was defined as the resolution of oral ulcers and symptoms whereas partial response was classified as pain improvement associated with persistence of ulcers after 8 gel applications.
The Vivostat system (Vivolution A/S, Alleroed, Denmark) is an automated system for the on-site preparation and application of patient (autologous) or healthy blood donor (allogeneic) derived fibrin sealant or PLT-rich fibrin (PRF) (25). Platelet gel used in this study, is produced as final product of an aliquot of 8 ml with platelets concentration of 2×10^6/μl, from hemo components with Vivostat system. Vivostat is a device used for preparation and delivery of Platelet gel and fibrin sealant (also called fibrin glue). It takes 23 minutes to produce an homologous or autologous concentrate form 120 ml of whole blood. This system has 3 components: a processor for platelet gel preparation, one unit for quality control and one unit for delivery. Thanks to Vivostat system, platelets concentrations is 7–10 time higher when compared to blood donor. The first step of Vivostat procedure consists of the reaction amongst 60 mL of plasma and batroxobin for 10 minutes at 37°C. Batroxobin acts on plasmatic fibrinogen to catalyze the release of fibrin peptide A. The result is fibrin I polymer soluble in acid-ambient without the activation of Factor XIII. The next step consists of fibrin I polymer isolation by centrifugation and its addition to sodium acetate buffer at the concentration of 0.2 mol/L at pH 4. The purified fibrin I solution may be transferred to the applicator including a syringe of 1 ml of carbonate/bicarbonate buffer at the concentration of 0.75 mol/L (pH 10). In presence of Ca2+ at neutral pH, the final product of PLT gel is obtained: in these conditions, endogenous prothrombin is converted to thrombin cleaving the fibrinopep-tide B from fibrin I to fibrin II. The resulting final product cannot be frozen, while the fibrin I solution could be cryopreserved at −40°C for next using or may be stored at ambience temperature to be used within 6 hours. The Vivostat application system has an electromechanical innovative design incorporating a reusable applicator that houses the mechanical and electronic workings of the system and a disposable, single-use spray pen. On finger activation of the pen button, fibrin I and neutralizing solution are delivered at a controlled rate through separate tubes of a multi-lumen tube to the tip of the spray pen; on emission from the tip they mix in a stream of a compressed air to form a fine low-pressure spray of fibrin sealant. The distance between the spray pen and the target site was 2 to 2.5 cm and the application occurred by centripetal rotation at a delivery rate of 1.4 mL/min and at a pressure of 30 bar.
In a multidisciplinary context, first oral ulcers evaluation was performed by transplant physicians. dentists performed oral biopsy, if necessary, while the PLT gel preparation and application was carried out at Blood Bank. The PLT gel was applied on oral GvHD ulcers twice a week, away from meal in order to enhance activated platelets actions.
Statistical analysis
Patient characteristics and data are summarized using descriptive techniques, including absolute and relative frequencies for categorical variables, while continuous variables are expressed as median and range.
Results
The patients enrolled in this trial showed cGvHD characterized by either oral lesions alone or associated with macular-papular rash, sclerodermia or other organ involvement such as eyes, liver or lung. Table 1 describes patients’ and ulcers’ characteristics while Table 2 shows the timing of oral lesions’ occurrence after the allogeneic HSCT, the duration of ulcers prior to the PLT gel procedure, the number of gel applications and the response for each patient. The median time between the onset of cGvHD ulcers and allogeneic HSCT or gel application was 9 months (4–19) and 9 days (3–14), respectively. The microbiological assessment before the PLT gel application resulted negative in all cases except for one. After the second PLT gel application, all patients showed a significant improvement of the oral pain and resumed the feeding. After a median of five PLT gel applications (range, 2–15), 7 out of 10 patients showed a complete response, using only the PLT gel in 4 of them affected by a limited form of cGvHD characterized by oral injuries alone. Only one patient with limited oral cGvHD required a combination of local gel application and steroids to achieve complete remission. The other 5 patients who experienced extensive cGvHD received steroids and PLT gel for oral ulcers in 4 cases, showing a complete oral response in 3 of them. Finally, the Figure 1 documented the outcome of oral ulcers in a case of extensive chronic GvHD refractory to 2 previous lines of therapy. No side effects were observed during the procedure except for a burning sensation and/or itching on the involved sites, meanly referred during the first application. On the follow-up, no in situ recurrence of ulcers was observed. Moreover, the multidisciplinary approach including transplant, transfusion physicians and dentists allowed a quick diagnosis and a rapid planning/start up of this therapeutic procedure.
Table 1.
Patients’ characteristics.
| Patient Number | Sex/Age | Haematological disease | Status disease at HSCT | Ulcer occurrence (months) | Localization of oral ulcers | Number of oral ulcers |
|---|---|---|---|---|---|---|
| 1 | M/47 | ALL | 1st PR | 4 | Tongue, Buccal mucosae | 3 |
| 2 | F/39 | CML | CR | 10 | Gum, Mouth’s floor | 2 |
| 3 | F/44 | AML | PR | 6 | Buccal mucosae | 2 |
| 4 | M/49 | NHL | 3rd CR | 18 | Tongue | 1 |
| 5 | F/56 | NHL | 3rd PR | 19 | Buccal mucosae | 2 |
| 6 | F/41 | ALL | Resistant | 6 | Tongue | 1 |
| 7 | M/43 | AML | 1st CR | 10 | Lip, Tongue Buccal mucosae |
3 |
| 8 | F/59 | AML | 1st CR | 9 | Buccal mucosae Mouth’s floor |
3 |
| 9 | M/58 | AML | Resistant | 11 | TongueLip | 3 |
| 10 | M/61 | AML | 3rd CR | 9 | Tongue, Buccal mucosae | 3 |
HSCT = Haematopoietic Stem Cell Transplantation; M = Male; F = Female; ALL = Acute Lymphoblastic Leukemia; CML = Chronic Myelogenous Leukemia; AML = Acute Myeloid Leukemia; NHL = Non Hodgkin Lymphoma; CR = Complete Remission; PR = Partial Remission.
Table 2.
cGvHD grading, therapy and response.
| Patient number | Type of cGvHD | cGvHD treatments | Ulcer duration before gel (days) | Number of gel application | Response |
|---|---|---|---|---|---|
| 1 | Extensive | PDN*, Rituximab* PLT Gel, Photoferesis |
14 | 5 | Complete |
| 2 | Extensive | PDN | 10 | 2 | Partial |
| 3 | Limited | PLT Gel | 7 | 9 | Partial |
| 4 | Extensive | PDN, PLT Gel | 3 | 2 | Complete |
| 5 | Limited | PLT Gel | 5 | 2 | Complete |
| 6 | Extensive | PDN, PLT Gel | 9 | 15 | Complete |
| 7 | Limited | PLT Gel | 9 | 5 | Complete |
| 8 | Extensive | PDN, PLT Gel | 9 | 2 | Complete |
| 9 | Limited | PDN, PLT Gel | 11 | 7 | Partial |
| 10 | Limited | PLT Gel | 7 | 5 | Complete |
cGvHD = Chronic Graft versus Host Disease; PDN = Prednisone; PLT = Platelets;
= treatment before PLT gel starting.
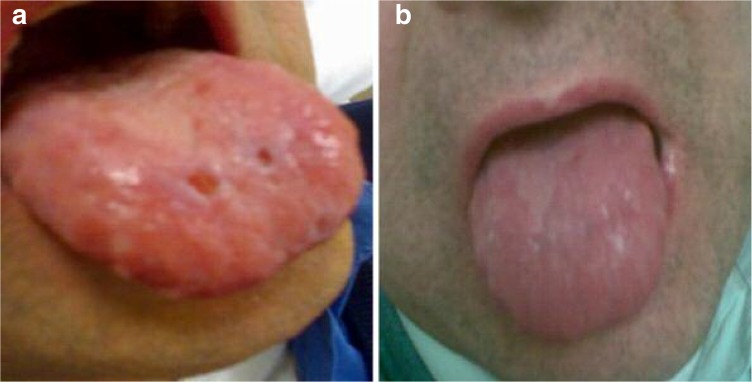
Figure 1.
a: Oral mucosal lesions pre-PLT gel applications; b: Oral mucosal lesions after 5 PLT gel applications.
Discussion
Platelets have a key role not only in hemostasis processes but also in tissue regeneration and wound healing (15). It’s known that fibroblasts and mesenchimal cells promote neo-angiogenesis and are stimulated by PDGF, VEGF, bFGF, IGF, PF and EGF released by the α granules of platelets. For this reason, in the last 2 decades an increasing interest for the non transfusional use of hemocomponents has been reported. Nowadays, platelet gel is used in maxillofacial, reconstructive-plastic, orthopedic, cardiovascular and dermatologic surgery with the aims to improve and accelerate healing processes (16, 17). Moreover, PLT gel is used as local therapy for the treatment of chronic cutaneous ulcers, typical manifestations of diabetic vasculopathy or arterio-venous insufficiency. Some reports have also shown the ability of platelets to accumulate in the liver and promote tissues regeneration, after hepatectomy. This proliferative effect seems due to the direct contact between platelets and hepatocytes, that starts transduction signaling with subsequent release of several growth factors such as the Hepatocyte growth factor (HGF) and IGF. More recently, three pilot studies have demonstrated the reparative effect of fibrin glue and PLT gel on the bladder mucosa of patients who developed refractory hemorrhagic cystitis and muco-cutaneous lesions related to the GvHD, respectively, after allogeneic HSCT (18–20).
Nowadays, several devices able to produce fibrin glue or PLT gel with different viscosity, elasticity or plasticity are available (Vivostat, PCCS, Harvest, Fibrinet, Autogel Process Cytomedix, Magellan Medtronic, Plateltex). Methods of PLT gel preparation highly influence the physical and biological characteristics of the final product with impact on the therapeutic efficiency. First of all, the platelet concentration. It has been observed that platelets count is related with the PDGF release and several studies have shown that platelets concentration is lower with the test tube technique comparing with the other methods (25). Great importance has GFs concentration in platelet derivates. Several studies analyzed differences between existing technique for gel preparation, related to GFs concentration (25–27). However, all GFs (PDGF-AB, PDGF-BB e TGF-beta in particular) seems to be in the same proportion despite the preparation’s method whereas there is a different white blood cells contamination. Contaminating cells are mostly mononucleated cells, like monocytes or lymphocytes and their role is unclear. Some studies suppose an antiseptic action in the site of wounds, due to the release of pro-inflammatory cytokine and myeloperoxidase (MPO), reducing risks of infection (27). Using Vivostat or Fibrinet, the contamination of final product is three Log less than with other techniques. On these biological and clinical evidences, the aims of our study was to confirm the feasibility and efficiency application of PLT gel (Vivostat system) for healing the oral ulcers related to cGvHD in patients undergoing an allogeneic HSCT. The major advantages of PLT gel are represented by its safety, prompt availability and limited cost. According to the Italian policy, the preparation of PLT gel is on behalf of the blood banks, so that it is accessible to nearly all transplant Centers. The machine of Vivostat System is small and easily transferable so that also critical patients may be treated inside the sterile room. The prompt availability is due to the short time of its preparation (30 minutes) and to the possibility of activation immediately before the clinical application that requires just one hour. Further, the PLT gel would be an important tool to reduce the infections from saprophyte germs of the oral cavity linked to the presence of ulcers. In fact, the oral wounds represent an entry site of bacterial infections on which the PLT gel application may act through two different mechanisms: 1) closing the door to entry for microbiological agents by fibrin clots and 2) exploiting the local anti-infective effect of substances, as myeloperoxidasis, released by leukocytes included in the gel preparation. The clinical confirmation of this feature of PLT gel is represented by the absence of septic events during the treatment period. Finally, the lack of systemic pharmacologic properties of PLT gel represents an additional advantage of this procedure in the management of ulcers related to GvHD. In fact, neither early nor long term side effects were observed and the ulcers resolution could lead to a faster withdrawal of the immuno-suppressive therapy, reducing the infective risk.
Conclusions
Our data show that all patients treated with PLT gel for cGvHD oral ulcers reached a quick improvement of pain in the oral cavity and food in-take, after 2 gel applications. Moreover, the achievement of complete response in 70% of cases, using PLT gel alone in 4 out of 5 patients affected by limited cGvHD, supports its use in the limited form of this allogeneic HSCT’s complication. The absence of ulcers relapse in the site of previous PLT gel application confirms that the content rich in growth factors makes it is a useful tool to maintain a long term tissue repair.
In conclusion, the results of our study are particularly promising and induce to recommend the PLT gel as a safe and effective therapeutic instruments for the management of oral ulcers related to chronic GvHD.
Authorship
Alessandra Picardi, MD, PhD, designated the study, drafted the manuscript and performed the allogeneic HSCT.
Angelo Ferraro, Alessandro Lanti, MD, made the PLT gel and its clinical application.
Gaspare Adorno, Director of Blood Bank, approved the clinical trial and revised the manuscript.
Michele Miranda, Dentist, performed the oral biopsy of the transplanted patients.
Federico Meconi, MD, collected data and performed the haematological follow-up of the transplanted patients.
William Arcese, Director of Transplant Unit, approved the clinical trial, revised the manuscript and performed the allogeneic HSCT.
Patrizio Bollero, DDS, PhD designated the study, drafted the manuscript and performed the oral biopsy.
Footnotes
Conflict of interest
The Authors declare that they have no conflict of interest relevant to the manuscript submitted to ORAL & Implantology.
References
- 1.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–289. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piñana JL, Sanz J, Picardi A, et al. Umbilical Cord Blood Transplantation From Unrelated Donors In Patients With Ph-Positive Acute Lymphoblastic Leukemia. Haematologica. 2014;99:378–392. doi: 10.3324/haematol.2013.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulatedhaploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1090. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 4.Arcese W, Picardi A, Santarone S, et al. On behalf of Rome Transplant Network Haploidentical, G-CSF primed, unmanipulated bone marrow transplantation for patients with high-risk hematological malignancies: an update. Bone Marrow Transplant. 2015;50:24–S30. doi: 10.1038/bmt.2015.91. [DOI] [PubMed] [Google Scholar]
- 5.Sanz J, Picardi A, HernándezBoluda JC, et al. Impact of Graft-versus-Host Disease Prophylaxis on Outcomes after Myeloablative Single-Unit Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2013;19:1387–1392. doi: 10.1016/j.bbmt.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Robin M, Sanz GF, Ionescu I, et al. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia. 2011;25:75–81. doi: 10.1038/leu.2010.219. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 8.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 9.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuna R, Loiseau P, Ruggeri A, et al. Impact of HLA mismatch direction on outcome after umbilical cord blood transplantation for hematological malignant disorders: a retrospective Eurocord-EBMT analysis. Bone Marrow Transplant. 2014;49:24–29. doi: 10.1038/bmt.2013.120. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto C, Ogawa H, Fukuda T, et al. Impact of a Low CD34 Dose on Allogeneic Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;28:S1083–91. doi: 10.1016/j.bbmt.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Picardi A, Arcese W. Quality assessment of cord blood units selected for unrelated transplantation: a transplant center perspective. Transfusion and Apheresis Science. 2010;42:289–297. doi: 10.1016/j.transci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Magenau J, Runaas L, Reddy P. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol. 2016;173:190–205. doi: 10.1111/bjh.13959. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–1018. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar VS, Shiwen X, Bostrom M, et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccin A, Di Pierro AM, Tagnin M, et al. Healing of a soft tissue wound of the neck and jaw osteoradionecrosis using platelet gel. Regen Med. 2016;11:459–463. doi: 10.2217/rme-2016-0031. [DOI] [PubMed] [Google Scholar]
- 18.Tirindelli MC, Flammia G, Sergi F, et al. Fibrin glue for refractory hemorrhagic cystitis after unrelated marrow, cord blood, and haploidentical hematopoietic stem cell transplantation. Transfusion. 2009;49:170–175. doi: 10.1111/j.1537-2995.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 19.Picardi A, Lanti A, Cudillo L, et al. Platelet gel for treatment of mucocutaneous lesions related to graft-versus-host disease after allogeneic hematopoietic stem cell transplant. Transfusion. 2010;50:501–506. doi: 10.1111/j.1537-2995.2009.02439.x. [DOI] [PubMed] [Google Scholar]
- 20.Tirindelli M, Flammia G, Bove P, et al. Fibrin glue therapy for severe hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1612–1617. doi: 10.1016/j.bbmt.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Picardi A, Arcese W, Pollichieni S, et al. The Rome Transplant Network model compared to the Italian Bone Marrow Donor Registry activity for unrelated donor search process and transplant efficiency for hematological malignancy. Transfusion. 2017;57:1734–1743. doi: 10.1111/trf.14131. [DOI] [PubMed] [Google Scholar]
- 22.Arcese W, Mangione I, Picardi A. Algorithm for donor selection in 2011. Cur Opin Hematol. 2011;18:401–407. doi: 10.1097/MOH.0b013e32834ba838. [DOI] [PubMed] [Google Scholar]
- 23.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;10:3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 25.Velada JL, Holingsbee DA. Physical Characteristics of Vivostat® Patient-Derived Sealant. EurSurg Res. 2001;33:399–404. doi: 10.1159/000049737. [DOI] [PubMed] [Google Scholar]
- 26.Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. 2013;53:138–146. doi: 10.1111/j.1537-2995.2012.03668.x. [DOI] [PubMed] [Google Scholar]
- 27.Leitner GC, Gruber R, Neumüller J, et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. VoxSanguinis. 2006;91:135–139. doi: 10.1111/j.1423-0410.2006.00815.x. [DOI] [PubMed] [Google Scholar]