Abstract
Charcot-Marie-Tooth (CMT) disease is the most commonly inherited neurologic disorder, but its molecular mechanisms remain unclear. One variant of CMT, 2F, is characterized by mutations in heat shock protein 27 (Hsp27). As bioactive sphingolipids have been implicated in neurodegenerative diseases, we sought to determine if their dysregulation is involved in CMT. Here, we show that Hsp27 knockout mice demonstrated decreases in ceramide in peripheral nerve tissue and that the disease-associated Hsp27 S135F mutant demonstrated decreases in mitochondrial ceramide. Given that Hsp27 is a chaperone protein, we examined its role in regulating ceramide synthases (CerSs), an enzyme family responsible for catalyzing generation of the sphingolipid ceramide. We determined that CerSs colocalized with Hsp27, and upon the presence of S135F mutants, CerS1 lost its colocalization with mitochondria suggesting that decreased mitochondrial ceramides result from reduced mitochondrial CerS localization rather than decreased CerS activity. Mitochondria in mutant cells appeared larger with increased interconnectivity. Furthermore, mutant cell lines demonstrated decreased mitochondrial respiratory function and increased autophagic flux. Mitochondrial structural and functional changes were recapitulated by blocking ceramide generation pharmacologically. These results suggest that mutant Hsp27 decreases mitochondrial ceramide levels, producing structural and functional changes in mitochondria leading to neuronal degeneration.—Schwartz, N. U., Linzer, R. W., Truman, J.-P., Gurevich, M., Hannun, Y. A., Senkal, C. E., Obeid, L. M. Decreased ceramide underlies mitochondrial dysfunction in Charcot-Marie-Tooth 2F.
Keywords: sphingolipid, CerS, mitochondria, neuropathy, CMT2F
Charcot-Marie-Tooth (CMT) disease is the most commonly inherited peripheral neuropathy, with a prevalence of ∼1 in 2500 (1). CMT was initially defined clinically by its alternate name, hereditary motor and sensory neuropathy, describing the typical patient presentation of symmetric distal polyneuropathy, gait abnormalities, pes cavus (hammer toes), and diminished sensation and deep tendon reflexes (2, 3). Over 70 genes have been implicated in CMT, highlighting its genetic heterogeneity (4). CMT type 1 (CMT1) comprises 80% of cases and presents with reduced motor nerve conduction velocity, whereas CMT2 presents with normal motor nerve conduction velocity but decreased compound muscle action potential (4). Unsurprisingly, most genes implicated in CMT1 are involved in homeostasis of myelin. Interestingly, despite causing symptoms only in peripheral neurons, genes involved in CMT2 are implicated in basic cellular processes. Current treatments for CMT are still quite limited.
CMT2F is specifically caused by mutations in heat shock protein 27 (Hsp27) (5). Hsp27 is a member of the class of small Hsps that are vital in cellular homeostatic mechanisms, such as inflammation, longevity, cell division, cell survival, and apoptosis (6). Although lacking intrinsic folding capacity, small Hsps act as ATP-independent molecular chaperones, maintaining client proteins in a folding-competent state before passing them on to ATP-dependent chaperones for refolding or denaturation (6, 7). There is an extensive literature on the protective effects of Hsp27 in the nervous system. Overexpression of Hsp27 reduces neuronal cell death and the severity of drug-induced seizures (8), and Hsp27 injection reduces severity of sequelae of cerebral ischemia in mice (9). Hsp27 expression is upregulated in neuronal injury and thought to have a role protecting injury-induced motor neuron death (10). Hsps are vital in promoting proteostasis—the homeostatic and quality-control mechanisms assuring protein integrity—in neurons by preventing the accumulation and aggregation of misfolded proteins that can cause neural diseases (11). Evidence suggests that Hsp27 restricts cell death by reducing protein aggregation or other pathologic mechanisms seen in Alzheimer’s, Huntington’s, and Parkinson’s diseases and amylotrophic lateral sclerosis (ALS) (12, 13). However, the pathogenesis of Hsp27 in CMT2F remains unknown but is thought to be a result of defects in axonal transport of mitochondria (14).
Sphingolipid metabolism involves myriad enzymes responsible for generating sphingoid bases and converting them into an array of structurally distinct sphingolipid molecules with a complex diversity of functions (15). Ceramides are a key sphingolipid intermediate, predominantly produced by ceramide synthases (CerSs), and they function as bioactive molecules regulating growth arrest, senescence, apoptosis, and autophagy (15–18). CerSs generate ceramides predominantly through the de novo generation of dihydroceramide from acylation of dihydrosphingosine or through the salvage pathway by acylating sphingosine generated from breakdown of complex sphingolipids (17). Six CerS isoforms (CerS1–6) have been identified, each of which uses different length fatty acyl-coenzyme A chains to produce distinct ceramide species (15, 19). CerSs are active at the endoplasmic reticulum (ER), with enzymatic activity thought to occur on the cytosolic face of the ER (20, 21). CerSs have also been detected in additional compartments, including mitochondria and nuclear membranes. CerS1, which catalyzes the formation of C18 and C18:1 ceramides (22, 23), is highly expressed in the nervous system, skeletal muscles, and testes (24). Mice with nonsense or certain point mutations in CerS1 show a neurodegenerative phenotype with reduced C18 ceramide biosynthesis, accumulation of ubiquitylated proteins and lipofuscin in the nervous system, neuronal cell death, and an ataxic phenotype (25). A mutation in the initial enzyme of the de novo pathway (serine palmitoyltransferase) causes hereditary sensory neuropathy type 1, which has a similar phenotype to CMT2F (26), suggesting that alterations in the de novo pathway of ceramide genesis may produce CMT-like phenotypes.
In this study, we performed liquid chromatography/mass spectrometry (LC/MS) on Hsp27 knockout (KO) mouse sciatic nerve tissue, yielding the novel observation that the Hsp27 KO sciatic nerve demonstrates decreased ceramides. We determine that this decrease in ceramide occurs acutely in the mitochondria in CMT2F mutant cells, which present with less mitochondrial CerS1. We propose that a novel CerS:Hsp27 interaction draws CerS away from the mitochondria, reducing mitochondrial ceramide species. This results in mitochondrial morphologic and functional/metabolic changes that parallel decreases in ceramide. Our work provides a novel mechanism for CMT degeneration through dysregulation of the CerS pathway, implicating sphingolipids in CMT pathobiology.
MATERIALS AND METHODS
Cell culture and viruses
HT-22 cells were used for biologic studies and human embryonic kidney 293T cells were cultured for viral production. HT-22 and HCT-293T were cultured with DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum. Cells were monitored with MycoAlert to detect potential Mycoplasma contaminations. Human Hsp27 was cloned into pcDNA3.1 with an N-terminal hemagglutinin (HA) tag. A QuikChange site-directed mutagenesis kit (Agilent, Santa Clara, CA, USA) was used to generate S135F, P136W, and P182L mutations. HT-22 cells were transiently transfected with pcDNA3.1 constructs for 48 h with X-tremeGene 9, with a ratio of 1 μg DNA:4 μl X-tremeGene:100 μl Opti-MEM. Cells were exposed to fumonisin B1 (FB1; Enzo Life Sciences, Farmingdale, NY, USA) or myriocin (Sigma-Aldrich, St. Louis, MO, USA) for the time period indicated. Dorsal root ganglia (DRG) cells were collected and cultured from mice with modifications, as described previously (27). DRGs were collected from 7 mo old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) for cellular imaging experiments. Siliconized circular coverslides (22 mm; Hampton Research, Laguna Niguel, CA, USA), placed in a 6-well plate, were pretreated 16 h before dissection with 0.1 mg/ml poly-l-lysine (Sigma-Aldrich) in PBS. Four hours before plating DRGs, the poly-l-lysine solution was removed, washed for 5 min with PBS, and replaced with 20 μg/ml laminin (Thermo Fisher Scientific) in PBS. DRGs were collected in ice-cold Neurobasal medium (Thermo Fisher Scientific) and digested in 20 U/ml papain (Worthington Biochemical, Lakewood, NJ, USA) and papain activation solution (0.4 mg/ml L-cysteine, 50 nM EDTA, 1.5 μM CaCl in HBSS, pH 7.4) for 20 min at 37°C and subsequently digested in a mixture of 5 mg/ml displase (Sigma-Aldrich) and collagenase type IV (Worthington Biochemical) for 20 min at 37°C. Cells were then washed with PBS and triturated with 3 subsequently smaller fire-polished Pasteur pipettes in F:12 Ham’s DRG culture medium, consisting of F12 nutrient Ham’s medium (11330-032; Thermo Fisher Scientific), 10% fetal bovine serum (GE Healthcare HyClone, Logan, UT, USA), 1× minimal essential medium vitamins (Thermo Fisher Scientific), and 1:100 penicillin/streptomycin (Thermo Fisher Scientific). The cells were subsequently centrifuged at 2000 rpm and resuspended in F:12 Ham’s DRG culture medium before straining (Thermo Fisher Scientific). Laminin was removed from the plates and washed with PBS, and the cells were resuspended, plated, and allowed to adhere for 3 h before another 3 ml medium was gently added. Cells were cultured for 4 d before imaging. A Neon transfection machine was used for electroporation. Neuronal identify was confirmed by costaining for transient receptor potential cation channel subfamily V member 1 (TrpV1) or neuron-specific class III β-tubulin (Tuj1).
Immunoprecipitation
Anti-FLAG immunoprecipitation (IP) was performed using anti-FLAG M2 affinity gel (Sigma-Aldrich), according to the manufacturer’s guidelines.
Immunoblotting and antibodies
Cells were collected from 60-mm dishes, 72 h after plating, washed in PBS, and lysed in a Triton X-100 lysis buffer, as used in IPs. Lysates were sonicated and centrifuged at 14,000 rpm for 10 min to pellet debris. Protein was measured using SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA), and samples were normalized and mixed with loading buffer (NuPage LDL sample buffer; 4×) and 0.72 M 2-ME (Sigma-Aldrich) and boiled for 5 min. Protein samples were resolved on 4–20% gels (Thermo Fisher Scientific) at 150 V using the Criterion system (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to PVDF membrane in Tris buffer (100 V, 60 min, 4°C). Membranes were blocked with 5% milk/PBS containing 0.1% Tween for 1 h at room temperature, probed with primary antibody in 5% bovine serum albumin (Sigma-Aldrich) overnight at 4°C. Then they were washed quickly and then twice for 15 min, probed with horseradish peroxidase-conjugated species-specific secondary antibody (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h at room temperature, and then washed transiently and twice for 15 min. Immunoblots were quantified using ImageJ. The antibodies used in this study were as follows: anti-FLAG (F3165; Sigma-Aldrich), anti-actin (A5441; Sigma-Aldrich), anti-HA (rabbit polyclonal, 3724; Cell Signaling Technology, Danvers, MA, USA); goat polyclonal, Y-11; Santa Cruz Biotechnology), anti-α-tubulin (TU-02; Santa Cruz Biotechnology), anti-acetyl-α-tubulin (6-11B-1; Santa Cruz Biotechnology), anti-Hsp27 (C-20; Santa Cruz Biotechnology), anti-LASS1/CerS1 (H-170; Santa Cruz Biotechnology), anti-calnexin (H-70; Santa Cruz Biotechnology), anti-LC3B (2775S; Cell Signaling Technology), anti-TOM20 (FL-145; Santa Cruz Biotechnology), TrpV1 (rabbit; EMD Millipore, Billerica, MA, USA), Tuj1 (mouse; BioLegend, San Diego, CA, USA), and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology).
Immunofluorescence
HT-22 cells were plated on poly-d-lysine-coated, 35 mm confocal dishes (MatTek, Ashland, MA, USA) for 72 h. Cells were washed and fixed with 4% freshly prepared paraformaldehyde (Sigma-Aldrich) in PBS for 10 min, washed, permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 5 min, washed, blocked in 2% human serum for 1 h, incubated with primary antibody in 2% serum for 1 h at 37°C, washed, incubated with secondary antibodies conjugated with Alexa Fluor dyes (Thermo Fisher Scientific) for 1 h, and then washed once quickly and twice for 15 min each. Vectashield DAPI-mounting medium (H-1200; Vector Laboratories, Burlingame, CA, USA) was applied for 15 min before imaging. All washes were performed with sterile, 0.22 μm-filtered PBS, and all steps were done at room temperature unless indicated otherwise. Images were obtained using a Leica TSC SP8 laser-scanning confocal microscope. Mitochondria were visualized using 500 nM MitoTracker Deep Red FM (Thermo Fisher Scientific) for 30 min, according to the manufacturer’s instructions. Mitochondrial morphology was quantified using the macro Mitochondrial Morphology, developed by Dagda et al. (28). Images were imported to ImageJ v2.0.0. Cells of interest were selected by confirming that they were transfected with Hsp27 by visualizing a fluorophore-conjugated antibody attached to an Hsp27 antibody. Cells were then encircled using the polygon selection tool and processed by removing both the nuclear DAPI channel and Hsp27 channel, thereby only displaying the mitochondria channel. The Process function within the Mitochondria Morphology macro was run on the image by applying the Autothreshold tool. Additional manual threshold adjustments were then made to ensure appropriate signal was present without any impeding background signal. The image was then evaluated using the Mitochondrial Morphology Analyze function. This macro takes measurements of all present mitochondria in the region of interest and provides quantification of mitochondria size, area, perimeter, count, circularity, and solidity. The tool also provides an area/perimeter value as an index for mitochondrial interconnectivity. Data were transferred to Microsoft Excel for analysis.
Mitochondrial function
A Seahorse XF96 machine (Agilent) was used to measure oxygen consumption rates, according to the manufacturer’s instructions. HT-22 cells (10,000) were used to seed XF96 plates, and experiments were performed according to guidelines established by the manufacturer. Three temporal measurements were taken and averaged to compute each basal condition. Following the experiment, cell cartridge was frozen, and data were normalized to total protein.
Lipid analysis
Cells were washed with ice-cold PBS before collection. Protein levels of tissue samples and subcellular fractions were measured, and masses submitted were normalized to total. HPLC and MS of ceramide species were performed by the Lipidomics Core at Stony Brook University, as previously described (29). Lipids from cell culture experiments were normalized to total phosphate levels following LC/MS readings.
Cell assays
Cell viability was measured using the oxidation of the aqueous solution 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to the insoluble formazan (Sigma-Aldrich), as previously described (30).
Subcellular fractionation
Protein lysate was collected with a Mitochondrial Isolation Kit for Cultured Cells (Thermo Fisher Scientific), according to instructions as provided by the manufacturer. Spin speeds of 1000 g and subsequently 3000 g were, respectively, used to obtain an enhanced purity mitochondrial fraction. Sample pelleting at 1000 g was deemed nuclear; the supernatant, subsequently pelleting at 3000 g and also following a subsequent wash and 3000 g spin, was deemed mitochondrial. Following the first 3000 g spin, supernatants were spun at 13,000 g, and the resulting fraction was deemed cytosolic/microsomal.
Mouse tissue
All animal procedures were approved by the Stony Brook University Institutional Animal Care and Use Committee and followed the guidelines of the American Veterinary Medical Association. Sciatic nerve and brain tissue were dissected from C57BL/6 and Hsp27 KO mice (31) and flash frozen in liquid nitrogen and later homogenized using a TissueRuptor (Qiagen, Germantown, MD, USA). Results were normalized to protein.
Statistical analysis
Unpaired Student’s t test for single comparisons with a P < 0.05 was considered statistically significant for comparison of 2 groups, unless noted otherwise. Data are represented as means with se, unless noted otherwise; n = number of independent experiments, unless noted otherwise.
RESULTS
Hsp27 KO results in alterations in sphingolipid metabolism
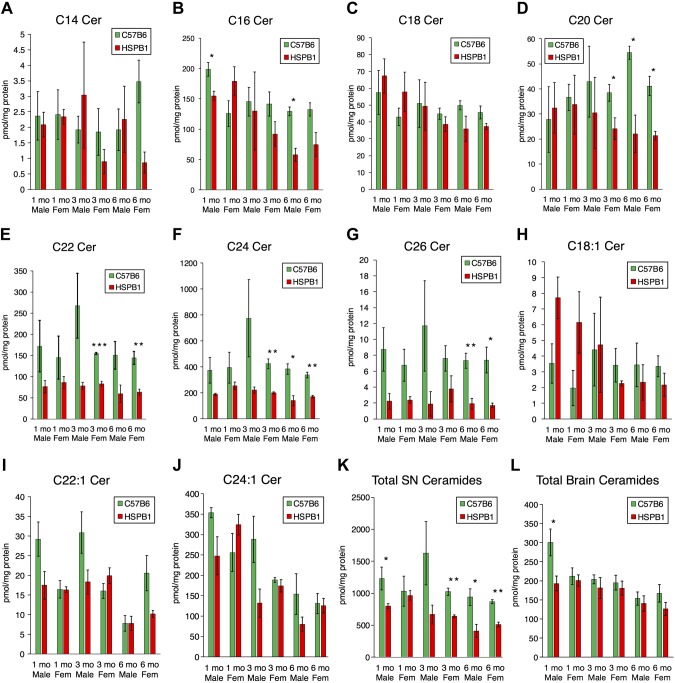
To investigate the effects of the chaperone protein Hsp27 on ceramide regulation, we measured ceramide species in brain and sciatic nerve samples from Hsp27 KO mice. Interestingly, sciatic nerve tissue showed decreased ceramide, particularly at 6 mo of age. These changes were most notable in longer chain-length ceramides with saturated acyl chains (Fig. 1A–J). Although large decreases in total ceramides occurred in sciatic nerve from older mice, they were not unanimously seen in brain tissue (Fig. 1K, L and Supplemental Fig. 1). These data, demonstrating selective sphingolipid decreases in sciatic nerve tissue from adult animals, suggested that sphingolipid metabolism may be altered when peripheral neuropathy presents in CMT2F.
Figure 1.
Decreased ceramides in Hsp27 KO sciatic nerve tissue. A–J) Sciatic nerve tissue from mice was homogenized and used for lipid measurements with LC/MS. C14, C16, C18, C20, C22, C24, C26, C18:1, C22:1, and C24:1 ceramides, are shown, respectively (n = 3). K, L) Total ceramide levels from sciatic nerve (SN) and brain tissue (n = 3). HSPB1, Hsp-β1. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine if there were acutely altered sphingolipid levels in CMT2F in a cellular model, we established conditions for transfection of wild-type (WT) or S135F mutant Hsp27 into HT-22 cells (Supplemental Fig. 2), an immortalized line derived from mouse HT-4 hippocampal cells (32, 33), as a biologic model. In addition to replicating the neuronal intracellular environment, decreased endogenous Hsp27 make HT-22 cells a strong model for the study of biologic functions of Hsp27 in CMT2F pathology by introducing WT or mutant genes. With the use of LC/MS, ceramides were measured in HT-22 cells transfected with vector (V), WT Hsp27, and S135F Hsp27. Surprisingly, the S135F mutation did not cause altered cellular ceramide levels compared with the WT condition (Supplemental Fig. 3). Nevertheless, a lack of change did not rule out compensatory mechanisms in the sphingolipid network that could mask the primary effects. To determine if the S135F mutant specifically inhibited CerSs, cells were labeled with the nonendogenous precursor C17 sphingosine for 20 min to evaluate the synthesis of ceramide species. However, labeling revealed no differences between HT-22 cells either transiently or stably transfected with WT and S135F (Supplemental Fig. 4A, B). These results raised the possibility that changes in ceramides may be compartment or tissue dependent.
Hsp27 mutant decreases mitochondrial ceramides
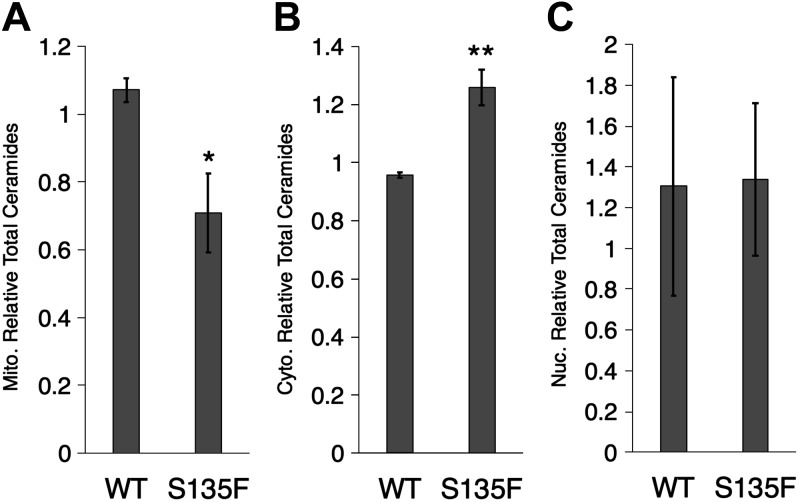
Mitochondrial trafficking defects have been shown in CMT2F, and mitochondrial proteins are known to be mutated in CMT2A, suggesting that acute alterations in sphingolipid metabolism may be restricted to mitochondria (34–36). To test this hypothesis, we established conditions for fractionating cells to isolate crude mitochondrial, nuclear, and cytoplasmic (including microsomes) fractions from V-, WT-, or S135F Hsp27-expressing cells (Supplemental Fig. 5) and then measured ceramide species in these fractions. The S135F Hsp27 cells showed a significant decrease in total mitochondrial ceramide levels, whereas cytoplasmic ceramides were slightly increased, and nuclear ceramides were unchanged compared with WT Hsp27 cells (Fig. 2). Ceramides from V cells were used for normalization, and levels were similar to WT. These data suggest that ceramide metabolism is altered in CMT2F.
Figure 2.
Decreased mitochondrial ceramides in S135F mutant HT-22 cells. Mitochondrial (Mito.; A), cytoplasmic (Cyto.; B), and nuclear (Nuc.; C) fractions of HT-22 cells were isolated and used for lipid measurements with LC/MS. Mitochondrial fractions demonstrate reduced total ceramide (*P < 0.05, n = 4) and cytoplasmic fractions with increased total ceramide (**P < 0.01, n = 4), and there is no change in nuclear ceramides (P = 0.95, n = 4).
Hsp27 mutant displays interactions with CerSs
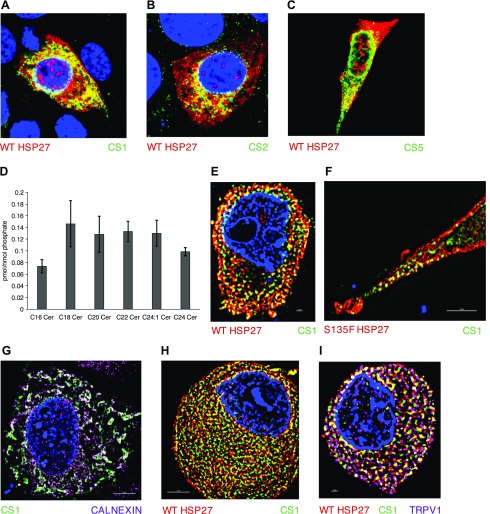
Next, we sought to evaluate how Hsp27 affected ceramide levels. As CerSs are the predominant regulators of de novo CerS, we hypothesized that they may be dysregulated in CMT2F, producing deficits in ceramide production. To address this hypothesis, CerS regulation by Hsp27 was examined in HT-22 cells. First, to determine if Hsp27 interacted with CerS, FLAG tags were subcloned onto CerS1, CerS2, and CerS5, and each was coexpressed with Hsp27 in HT-22 cells. Having established conditions for transfection and immunocytochemistry in HT-22 cells (Supplemental Fig. 6A), we determined that each of these CerSs displayed colocalization with Hsp27 (Fig. 3A–C).
Figure 3.
Hsp27:CerS1 interaction localizes to the ER. A–C) WT Hsp27 (red) colocalizes with FLAG-tagged CerS1, CerS2, and CerS5, respectively, (green) in HT-22 cells by confocal microscopy. D) Ceramide profile from DRGs cultured for 4 d from 7 mo female mice (n = 3). E, F) WT and S135F Hsp27 (red), respectively, colocalize with FLAG-tagged CerS1 (green) in HT-22 cells by SIM microscopy. G) FLAG-tagged CerS1 (green) and calnexin (pink) strongly colocalize. H) Endogenous WT-Hsp27 (red) and anti-CerS1 (green) colocalize in mouse DRG. I) Electroporated S135F Hsp27 (red) and FLAG-tagged CerS1 (green) colocalize in mouse DRG cells. Confirmation of neuronal identity with TrpV1 (pink).
To evaluate the relative importance of different CerS in mouse peripheral neurons, sphingolipid levels were measured in DRG from 7 mo old female mice. C18 ceramide was one of the highest species, suggesting that CerS1, which predominantly generates C18 ceramide, is highly expressed in these cells (Fig. 3D). This falls in line with other evidence linking high CerS1 expression in the nervous system. This prompted us to use CerS1 as a target to investigate the effects of sphingolipid biology on CMT2F.
CerS1 function has been shown to be highly dependent on cellular localization, and CerS1 mutations can induce dysfunction by altering its cellular location (19, 37). Furthermore, overexpressed ectopic expression of CerS1 does not alter its cellular localization (19). We next conducted studies to evaluate the colocalization of Hsp27 and CerS1 microscopically and determine the cellular localization of this interaction. Super-resolution structured illumination microscopy (SIM) was used to determine that overexpressed WT and S135F Hsp27 and CerS1 further colocalize, as indicated by the yellow overlap of the green anti-FLAG staining-transfected CerS1 and red anti-Hsp27 staining (Fig. 3E, F). As expected, transfected CerS1 showed strong colocalization to the ER, as evidenced by the white overlap of green anti-FLAG and purple anti-calnexin (Fig. 3G). These data demonstrate the proximity of Hsp27 and CerS1, especially in the ER.
We further sought to demonstrate this interaction in DRGs that were similarly isolated and cultured from mice, allowing introduction of genes through electroporation (Supplemental Fig. 6B). Endogenous Hsp27 (red) and CerS1 (green) in DRGs were confirmed to colocalize by the presence of yellow (Fig. 3H). Furthermore, electroporated WT and S135F Hsp27 also colocalized with CerS1, as shown by a representative image of S135F and CerS1 cotransfection (Fig. 3I). Hence, these results suggest that the colocalization occurs with endogenous proteins in primary neurons, as it would in the disease.
To evaluate further the interaction of CerSs and Hsp27, an HA tag was subcloned onto Hsp27 to aide in its discrimination and facilitate co-IP experiments. HT-22 cells were transiently transfected with CerS1, along with empty V, WT Hsp27, or S135F mutant Hsp27s. Pulldown of CerS1 with anti-FLAG beads and immunoblotting for Hsp27 in HT-22 cells confirmed the interaction of Hsp27 and CerS1 and showed the increased interaction of the S135F mutant compared with WT Hsp27 (Supplemental Fig. 7A–C). Furthermore, co-IP supernatants were immunoblotted to determine if the increased S135F interaction to CerS1 resulted in a substantially decreased proportion of free S135F in the cell; however, there was no significant change between WT and S135F. These results demonstrate a very close interaction between CerS1 and Hsp27, resulting in co-IP of the 2 proteins, and furthermore, show altered interaction between CerS and mutant Hsp27, which may contribute to the dysregulated ceramide levels in CMT2F.
Hsp27 modulates localization of CerS1
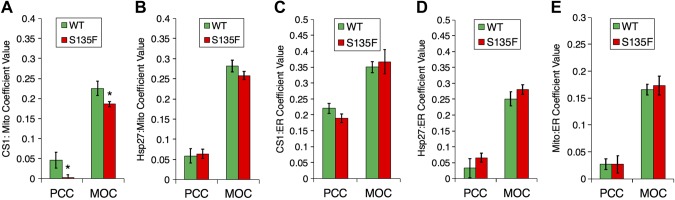
Mitochondria, as well as mitochondrial-associated membranes, have been shown to possess distinct ceramide pools through endogenous CerSs (38). Decreased mitochondrial ceramides suggested that the alteration of ceramide homeostasis may be a result of change in the localization of CerSs. To determine if WT or S135F altered the cellular localization of CerS1, we simultaneously stained HT-22 cells for CerS1 and either mitochondria or ER markers. Analysis of images from SIM microscopy using Pearson’s correlation coefficient (PCC) and Manders’ overlap coefficient (MOC) as quantitative measures of colocalization showed that expression of the S135F Hsp27 mutant caused a decrease in the localization of CerS1 to mitochondria when compared with cells expressing WT Hsp27 (Fig. 4A). However, WT and S135F Hsp27 themselves both displayed similar localization to mitochondria, irrespective of the mutation (Fig. 4B). With the use of a similar approach by staining with an ER instead of mitochondrial marker, we determined that neither CerS1 nor Hsp27 had any differential localization to ER with expression of the S135F mutant compared with WT Hsp27 (Fig. 4C, D). As a proxy for mitochondrial-associated membranes, we also analyzed ER and mitochondrial overlap in the presence of WT or S135F Hsp27; however, the mutant did not alter this parameter (Fig. 4E). We did not observe a notable difference in cells untransfected with Hsp27. Taken all together, these data suggest that mitochondrial association of CerS1 is altered by mutant Hsp27, causing a diversion of ceramide production away from the mitochondria.
Figure 4.
S135F mutant decreases CerS1 localization to mitochondria. A) CerS1 localizes to mitochondria in the presence of WT Hsp27 more than S135F in HT-22 cells. Quantification of super-resolution SIM colocalization of FLAG (CerS1) and MitoTracker Deep Red (mitochondrial marker) used PCC and MOC (*P < 0.05 for PCC, *P < 0.05 for MOC, n = 31 and n = 35). B) SIM quantification of FLAG and calnexin (ER marker) colocalization (P = 0.797 for PCC, P = 0.188 for MOC, n = 24 and n = 27). C) CerS1 localizes to the ER in the presence of WT and S135F Hsp27 in HT-22 cells. SIM quantification of FLAG and calnexin colocalization using PCC and MOC (P = 0.131 for PCC, P = 0.70 for MOC, n = 15 and n = 18). D) SIM quantification of FLAG and calnexin colocalization was quantified using PCC and MOC (P = 0.324 for PCC, P = 0.287 for MOC, n = 9 and n = 10). E) SIM quantification of calnexin and MitoTracker Deep Red (P = 0.324 for PCC, P = 0.287 for MOC, n = 9 and n = 10).
Hsp27 mutant produces alterations in mitochondrial morphology and in a sphingolipid-dependent manner
Next, it became imperative to determine whether the observed decreased mitochondrial ceramides and decreased mitochondrial localization of CerS1 by S135F mutants reflected substantial alterations in mitochondrial structure and function induced by mutant Hsp27 and whether sphingolipids play a role in these effects. It should be noted that Hsp27 mutants have been associated with decreased mitochondrial trafficking (33) but not mitochondrial morphologic effects.
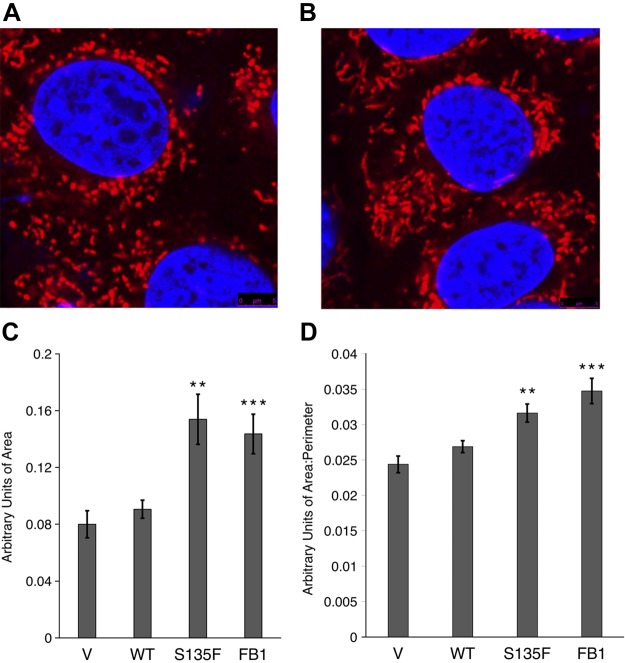
Therefore, mitochondrial morphology in cells transfected with either WT or S135F Hsp27 was evaluated. Compared with mitochondria in WT cells (Fig. 5A), mitochondria in S135F cells appeared larger (Fig. 5B), with increased area and greater interconnectivity, as quantitated by an increased ratio of area/perimeter (Fig. 5C, D). Thus, mutant Hsp27 exerts significant effects on mitochondrial morphology.
Figure 5.
S135F mutant produces enlarged mitochondria consistent with decreased CerS function. A, B) Representative images of mitochondria in HT-22 cells transfected with WT and S135F Hsp27, respectively. C) Increased area is observed in S135F mutant-transfected HT-22 cells (n = 41, 44) and cells treated with FB1 (n = 15, 13). D) Increased area/perimeter ratio is observed in S135F mutant-transfected HT-22 cells (n = 41, 44) and cells treated with FB1 (n = 15, 13). **P < 0.01, ***P < 0.001.
To determine if the reduced ceramide generation is involved in these changes, CerS was inhibited with FB1 to mimic the decrease in ceramides observed with mutant Hsp27. FB1 application at 50 μM for 24 h reproduced the increases in mitochondrial size and interconnectivity, as seen in the presence of the S135F mutant (Fig. 5C, D). These results show that attenuation of ceramide specifically (with FB1) recapitulates the morphologic effects of the decrease in ceramides induced by mutant Hsp27.
Hsp27 mutants decrease mitochondrial respiration involving sphingolipids
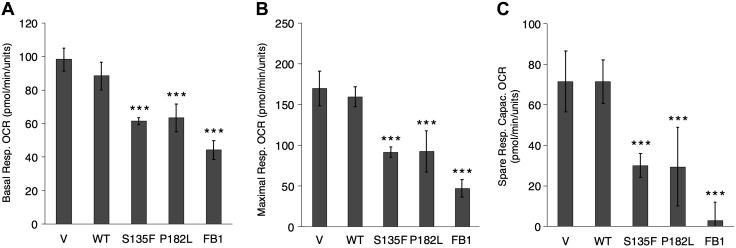
Next, we sought to determine if the observed swollen mitochondria in mutant cells were associated with decreased respiratory functioning. Indeed, mutant HT-22 cells (S135F, P182L) displayed decreased basal and maximal oxygen consumption rates and substantial decreases in spare respiratory capacity (Fig. 6). These studies reveal important functional effects of mutant Hsp27 on mitochondrial function.
Figure 6.
S135F and P182L mutants display decreased mitochondrial function consistent with decreased CerS function. A) Mutants (S135F and P182L) and FB1 display decreased basal respiration (Resp.) Oxygen Consumption Rate (OCR) compared with WT and V, respectively. B) Mutants and FB1 display decreased maximal respiration compared with WT and V, respectively. C) Mutants and FB1 display decreased spare respiratory capacity (Capac.) compared with WT and V, respectively. All experiments were repeated at least twice with similar results; error bars represent sd of a single experiment. ***P < 0.001.
To determine if this decrease in mitochondrial functioning is also a result of the decreased ceramides, FB1 was applied. The results showed that FB1 caused a significant decrease in the same respiratory parameters (Fig. 6), suggesting that CerS inhibition underlies the defective respiration in CMT2F mutants.
Hsp27 mutant increases autophagic flux but not cellular proliferation
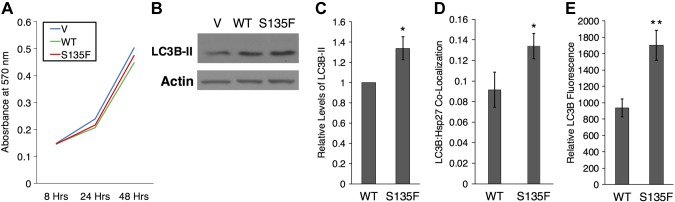
A decreased respiratory ability of cells may affect their ability to replicate and survive. The S135F mutant transiently transfected into N2a cells was previously shown to decrease cellular survival by ∼30% at 48 h post-transfection (5). As increased ceramide is linked to apoptosis, we set out to determine if observed, altered ceramides could modulate cell viability in the HT-22 model. Analysis of V-, WT-, and S135F-transfected cells showed no difference in proliferation within 48 h (Fig. 7A). To evaluate if the S135F mutant modulated growth responses to metabolic stress, we applied 2-deoxyglucose at concentrations of 2.5 and 25 μM, as determined empirically (and glucose as osmotic controls) to decrease HT-22 cell viability (39). Cell counts were not altered, even in the presence of 2-deoxyglucose (Supplemental Fig. 8A). This suggests that CMT2F pathology is not directly related to apoptosis or necrosis, which supports clinical and mouse model findings of substantial atrophy, aberrant myelination, and axon loss that is mild and often accompanied by regeneration (40–42).
Figure 7.
Increased autophagy but no change in growth of S135F mutant cells. A) The S135F mutation does not affect cell proliferation. B) Representative Western blot showing a mildly increased LC3B signal in S135F compared with WT-transfected HT-22 cells. C) There is an ∼1.3-fold increase in autophagy in S135F Hsp27, as indicated by the LC3B-II signal by immunoblotting (n = 6). D) Quantification of confocal images reveals an increase in autophagy, as indicated by LC3B:Hsp27 colocalization (n = 22, 23). E) Quantification of intensity of LC3B staining as a measure of autophagy reveals increased autophagy in S135F (n = 12, 19). *P < 0.05, **P < 0.01.
An important biologic response involved in clearing compromised mitochondria is autophagy, a process in which autophagosomes sequester damaged cellular components, including mitochondria, and target them for degradation (43). Although autophagy has been shown to be involved in some forms of CMT and suggested as a common pathologic mechanism (44), it has not been shown to be altered in CMT2F. However, activated Hsp27 has been shown to be a potent stimulator of autophagy in lipid clearance (45) and induces autophagy in response to cisplatin (46) and acute kidney injury to prevent cell death (47). Furthermore, ceramide has been shown to be an important regulator of cellular autophagy (23). Therefore, given these associations, we set out to determine if the S135F mutant regulates cellular autophagy in CMT2F. HT-22 cells were transfected with WT and S135F Hsp27 and immunoblotted for LC3B-II, a common marker of autophagy. S135F mutants display greater LC3B-II than WT-transfected Hsp27 HT-22 cells (Fig. 7B, C), suggesting that autophagy is upregulated in CMT2F mutants. To validate this result, HT-22 cells, transfected with WT and S135F Hsp27, were stained for LC3B and Hsp27. Increased autophagic flux in mutant cells was also demonstrated by increased LC3B colocalization with S135F rather than WT Hsp27 and an increased relative LC3B signal (Fig. 7D, E). Through 3 different methods, these results suggest that autophagic flux and autophagy are increased in CMT2F. This suggests an entirely novel cellular view of CMT2F, with decreased ceramide production leading to impaired mitochondrial function, resulting in enhanced autophagy that leads to neuronal degeneration.
DISCUSSION
Alterations in sphingolipids have been associated with a wide variety of neurodegenerative diseases. Several sphingolipids serve as bioactive signaling molecules in a plethora of pathways and have been implicated in Alzheimer’s (48, 49), Parkinson’s (50), and ALS (51). We sought to determine if changes in sphingolipid metabolism may mediate CMT phenotypes. This study presents the first evidence that directly and mechanistically links CMT to sphingolipid dysregulation. We identified a novel interaction of Hsp27 and CerSs and determine that decreased mitochondrial localization of CerS1 occurs in CMT2F mutation, reducing mitochondrial ceramide levels. The CMT2F mutation produces alterations in mitochondrial morphology that are accompanied by decreases in respiratory function. Importantly, these alterations are reproduced by decreasing CerS pharmacologically.
The results specifically begin to connect mutant Hsp27 to mitochondrial ceramide levels and function. WT and S135F mutant Hsp27 interact with CerS in neuronal cells, with evidence of increased interaction of the mutant. Whereas most CerS localize to the ER, there is a population of CerS at the mitochondria in neuronal cells. The S135F mutant decreases this mitochondrial CerS population, whereas increasing cytoplasmic CerS. Although this shift does not alter total cellular ceramide production, it results in decreased mitochondrial ceramides, without affecting the intrinsic activity of total cellular populations of CerS. Decreased ceramide production by pharmacological inhibition of CerS recapitulates all of the major effects of mutant Hsp27 noted in this study; thus, FB1 causes increases in size and connectivity of neuronal mitochondria that are also observed in the S135F mutant. Moreover, decreased ceramide production also reduced mitochondrial respiratory function, which again, was observed in mutant mitochondria. These mitochondrial dysfunctions in the mutant underlie increased autophagic flux, suggesting that decreased mitochondrial ceramides are responsible for cellular pathology in CMT2F.
The function of endogenous ceramides generated in the mitochondria is still poorly understood, especially in the context of neuronal dysfunction. Ceramide has been innately connected to mitochondrial apoptosis (52) and has been implicated as an inhibitor of the mitochondrial permeability transition pore (53). In other cellular systems related to cancer pathology, ceramide has been shown to modulate the targeting of autophagosomes to mitochondria in promoting mitophagy (23). Our results demonstrate an important role for steady-state levels of mitochondrial ceramide in maintenance of mitochondrial functions. Such a role does not negate a role for increased mitochondrial ceramide in inducing pathophysiologic responses.
Further understanding of how these mitochondrial pools of ceramide species are dysregulated in nervous system diseases and their specific effects on mitochondria will further enhance our understanding of CMT pathology. It also remains unknown why particularly long-chain ceramides, such as C24 and C26 ceramide, demonstrate the largest decreases in Hsp27 KO sciatic nerve tissue. In this context, CerSs have been shown to form heterodimers, with the implication that different CerSs have complex regulatory effects upon each of their activity (54). Such interactions may explain the complex changes in ceramides noted in this study. Additionally, CerS1 lacks a homeobox-like domain region, unlike the other human and mouse CerS, which may make it more suited for mitochondrial functions than other CerS, which may be more preferentially targeted to the nucleus (17, 55). Recent evidence suggests that homeobox-like domains found in CerS may not be necessary for CerS activity but serve to increase transcriptional activation independently from their direct effects catalyzing ceramide synthesis (56, 57).
The understanding of the regulatory environment of different types of neural tissue on the sphingolipid network will provide greater clarity on why sphingolipid changes in Hsp27 KO mice occurred predominantly in sciatic nerve tissue rather than the brain. As CMT symptomatology is thought to present exclusively in the peripheral nervous system, these results further suggest that sphingolipid alterations are involved in CMT2F.
Additional evidence is beginning to implicate sphingolipids in other forms of CMT. Deficiency of the gene responsible for clearing sphingolipids, sphingosine 1-phosphate (S1P) lyase, was recently discovered to be a novel cause of CMT, with a greatly increased sphingosine:sphinganine ratio in the disease state (58), yet a relatively much smaller increase in S1P levels. Such a drastic alteration of sphingosine:sphinganine ratio content without a similar increase in S1P from S1P lyase dysfunction is difficult to explain from simple, canonic, mechanistic models of sphingolipid metabolism and suggests that alterations to sphingolipid networks may produce dramatic and complex effects on sphingolipid content and metabolism in the cell. Although our study identifies depletion in mitochondrial ceramides, it is unknown whether these decreases are a result of a global change in CerS or compensations in the sphingolipid network. More complex alterations in ceramide production may involve modulation in the activity and formation of complexes of CerSs.
The impairment of mitochondria is in line with other research on CMT. Electron micrography from CMT2F mutant mice demonstrates increased total mitochondrial content, although number and size were not precisely quantified (59). Additionally, other types of CMT2 have shown mitochondrial dysfunctions, such as CMT2A, which is caused directly by mutations in the mitofusin (35, 36). The increased size of mitochondria in the S135F mutant suggests that there may be an increase in fusion in CMT2F. This underlies the possibility that a common mechanism may exist between CMT2A and CMT2F. It is currently unknown whether the genetic etiologies of CMT2 subtypes have distinct mechanisms or converge on a pathway that will offer common targets for treatment. Mitochondrial morphology also has been linked to other neurodegenerative diseases, including Parkinson’s disease and ALS (28, 60–62). Enlarged, swollen mitochondria similarly produced dysfunctional changes in Ullrich congenital muscular dystrophy (63). In addition to our data, demonstrating that mutant mitochondria have reduced respiratory functioning, S135F mutants have been found to hyperstabilize the microtubule network and thus, decrease anterograde mitochondrial trafficking (34). It is unclear if these processes are connected and how they contribute to overall mitochondrial function.
Although CMT1C has been associated with increased autophagy (64), studies on CMT2 and autophagy are lacking. Interestingly, curcumin, which stimulates protein translocation to the plasma membrane and as such, is thought to increase autophagy, has been shown to be effective in mouse models (4, 65). Curcumin has also been shown to stimulate de novo CerS (54), suggesting that its therapeutic effects may potentially be mediated by ceramide. With the use of both immunoblotting and multiple imaging methods, our results suggest that CMT2F also presents with increased autophagy in cellular models. Whether this is a compensatory response to increased cellular stress by clearing misfolded Hsp27 proteins or functions as a primary pathologic insult is unclear. Although observed increases in autophagy are likely downstream of mitochondrial affliction, it is unknown whether these changes relate to increased mitophagy in cells. Further work should focus on studying autophagy in other types of CMT and proteasomal inhibitors as potential pharmacologically therapies.
We also sought to determine if other reported cellular phenotypes were altered in our neuronal model. S135F mouse sciatic nerve has been reported to reduce tubulin acetylation, resulting in diminished microtubule-mediated transport of mitochondria (34). Some studies using cellular models have confirmed a decrease in tubulin acetylation in S135F cells (42), whereas others have failed to observe a difference (66). In our model, we were not able to observe a change in tubulin acetylation. To investigate a potential effect of sphingolipid metabolism on acetylation of tubulin in CMT2F, HT-22 cells were transfected both stably and transiently with V, WT, and S135F but showed no difference in the ratio of α-tubulin acetylation (Supplemental Fig. 8B, C). S135F has also been linked to tubulin function by increased colocalization of S135F Hsp27 with Tuj1 (or TUBB3) (66).
In summary, the data presented in this study strongly suggest that sphingolipid alterations underlie CMT2F and also provide evidence that mitochondrial dysfunction and autophagy play a role in the disease pathogenesis. We provide evidence of a novel Hsp27:CerS interaction and decreased CerS in the mitochondria along with decreased mitochondrial ceramides in the S135F mutant. Through these studies, we explore a topic—ceramide synthesis—that could lead to new targets for novel treatments to prevent or reduce the severity of CMT2F or other neuropathies with similar mechanisms. In particular, the validation of the implications presented here would create an imperative for developing small molecule inhibitors of the Hsp27:CerS interaction that could be therapeutic for this disease paradigm.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Chiara Luberto (Stony Brook University) for overall advice. The authors also thank the Stony Brook Lipidomics Core for measurement and analysis of sphingolipids, the Stony Brook Proteomics Core for proteomic measurement and analysis of samples, and Dr. Ashley Snider (Stony Brook University) for assistance in managing mice. Peter Dong (University of Pennsylvania, Philadelphia, PA, USA) and Dr. Tanvir Khan (Stony Brook University) assisted in training for DRG dissection. The research in this manuscript was supported by the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant GM062887 and Veterans Affairs Merit Award 5I01BX000156-08 (to L.M.O.), NIH National Cancer Institute Grant P01CA097132 (to Y.A.H. and L.M.O.). The authors declare no conflicts of interest.
Glossary
- ALS
amylotrophic lateral sclerosis
- CerS
ceramide synthase
- CMT
Charcot-Marie-Tooth
- CMT1/2/2F
Charcot-Marie-Tooth type 1/2/2F
- co-IP
coimmunoprecipitation
- DRG
dorsal root ganglia
- ER
endoplasmic reticulum
- FB1
fumonisin B1
- HA
hemagglutinin
- Hsp27
heat shock protein 27
- IP
immunoprecipitation
- KO
knockout
- LC/MS
liquid chromatography/mass spectrometry
- MOC
Manders’ overlap coefficient
- PCC
Pearson’s correlation coefficient
- S1P
sphingosine-1-phosphate
- SIM
structured illumination microscopy
- TrpV1
transient receptor potential cation channel subfamily V member 1
- Tuj1
neuron-specific class III β-tubulin
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. U. Schwartz, Y. A. Hannun, C. E. Senkal, and L. M. Obeid conceived of and designed experiments; N. U. Schwartz, R. W. Linzer, J.-P. Truman, M. Gurevich, and C. E. Senkal performed experiments; N. U. Schwartz and R. W. Linzer analyzed data; and N. U. Schwartz, C. E. Senkal, and L. M. Obeid wrote the manuscript.
REFERENCES
- 1.Timmerman V., Strickland A. V., Züchner S. (2014) Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes (Basel) 5, 13–32https://doi.org/10.3390/genes5010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck P. J., Lambert E. H. (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch. Neurol. 18, 603–618https://doi.org/10.1001/archneur.1968.00470360025002 [DOI] [PubMed] [Google Scholar]
- 3.MacMillan J. C., Harper P. S. (1994) The Charcot-Marie-Tooth syndrome: clinical aspects from a population study in South Wales, UK. Clin. Genet. 45, 128–134https://doi.org/10.1111/j.1399-0004.1994.tb04009.x [DOI] [PubMed] [Google Scholar]
- 4.Harel T., Lupski J. R. (2014) Charcot-Marie-Tooth disease and pathways to molecular based therapies. Clin. Genet. 86, 422–431https://doi.org/10.1111/cge.12393 [DOI] [PubMed] [Google Scholar]
- 5.Evgrafov O. V., Mersiyanova I., Irobi J., Van Den Bosch L., Dierick I., Leung C. L., Schagina O., Verpoorten N., Van Impe K., Fedotov V., Dadali E., Auer-Grumbach M., Windpassinger C., Wagner K., Mitrovic Z., Hilton-Jones D., Talbot K., Martin J.-J., Vasserman N., Tverskaya S., Polyakov A., Liem R. K. H., Gettemans J., Robberecht W., De Jonghe P., Timmerman V. (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 36, 602–606https://doi.org/10.1038/ng1354 [DOI] [PubMed] [Google Scholar]
- 6.Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 12, 842–846https://doi.org/10.1038/nsmb993 [DOI] [PubMed] [Google Scholar]
- 7.Bagnéris C., Bateman O. A., Naylor C. E., Cronin N., Boelens W. C., Keep N. H., Slingsby C. (2009) Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J. Mol. Biol. 392, 1242–1252https://doi.org/10.1016/j.jmb.2009.07.069 [DOI] [PubMed] [Google Scholar]
- 8.Akbar M. T., Lundberg A. M. C., Liu K., Vidyadaran S., Wells K. E., Dolatshad H., Wynn S., Wells D. J., Latchman D. S., de Belleroche J. (2003) The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J. Biol. Chem. 278, 19956–19965https://doi.org/10.1074/jbc.M207073200 [DOI] [PubMed] [Google Scholar]
- 9.Teramoto S., Shimura H., Tanaka R., Shimada Y., Miyamoto N., Arai H., Urabe T., Hattori N. (2013) Human-derived physiological heat shock protein 27 complex protects brain after focal cerebral ischemia in mice. PLoS One 8, e66001.https://doi.org/10.1371/journal.pone.0066001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn S. C., Perrelet D., Kato A. C., Scholz J., Decosterd I., Mannion R. J., Bakowska J. C., Woolf C. J. (2002) Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 36, 45–56 [DOI] [PubMed] [Google Scholar]
- 11.Smith H. L., Li W., Cheetham M. E. (2015) Molecular chaperones and neuronal proteostasis. Semin. Cell Dev. Biol. 40, 142–152https://doi.org/10.1016/j.semcdb.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tóth M. E., Szegedi V., Varga E., Juhász G., Horváth J., Borbély E., Csibrány B., Alföldi R., Lénárt N., Penke B., Sántha M. (2013) Overexpression of Hsp27 ameliorates symptoms of Alzheimer’s disease in APP/PS1 mice. Cell Stress Chaperones 18, 759–771https://doi.org/10.1007/s12192-013-0428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp P. S., Akbar M. T., Bouri S., Senda A., Joshi K., Chen H. J., Latchman D. S., Wells D. J., de Belleroche J. (2008) Protective effects of heat shock protein 27 in a model of ALS occur in the early stages of disease progression. Neurobiol. Dis. 30, 42–55https://doi.org/10.1016/j.nbd.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Kim J. Y., Woo S. Y., Hong Y. B., Choi H., Kim J., Choi H., Mook-Jung I., Ha N., Kyung J., Koo S. K., Jung S. C., Choi B. O. (2016) HDAC6 inhibitors rescued the defective axonal mitochondrial movement in motor neurons derived from the induced pluripotent stem cells of peripheral neuropathy patients with HSPB1 mutation. Stem Cells Int. 2016, 9475981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullen T. D., Hannun Y. A., Obeid L. M. (2012) Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441, 789–802https://doi.org/10.1042/BJ20111626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannun Y. A., Obeid L. M. (2011) Many ceramides. J. Biol. Chem. 286, 27855–27862https://doi.org/10.1074/jbc.R111.254359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: insights into the regulation of ceramide synthesis. J. Biol. Chem. 281, 25001–25005https://doi.org/10.1074/jbc.R600010200 [DOI] [PubMed] [Google Scholar]
- 18.Lee H., Rotolo J. A., Mesicek J., Penate-Medina T., Rimner A., Liao W. C., Yin X., Ragupathi G., Ehleiter D., Gulbins E., Zhai D., Reed J. C., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2011) Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One 6, e19783.https://doi.org/10.1371/journal.pone.0019783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridevi P., Alexander H., Laviad E. L., Min J., Mesika A., Hannink M., Futerman A. H., Alexander S. (2010) Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing. Exp. Cell Res. 316, 78–91https://doi.org/10.1016/j.yexcr.2009.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morell P., Radin N. S. (1970) Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A’s by brain microsomes. J. Biol. Chem. 245, 342–350 [PubMed] [Google Scholar]
- 21.Hirschberg K., Rodger J., Futerman A. H. (1993) The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem. J. 290, 751–757https://doi.org/10.1042/bj2900751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J. C., Sullards M. C., Merrill A. H., Jr., Futerman A. H. (2002) Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 277, 35642–35649https://doi.org/10.1074/jbc.M205211200 [DOI] [PubMed] [Google Scholar]
- 23.Sentelle R. D., Senkal C. E., Jiang W., Ponnusamy S., Gencer S., Selvam S. P., Ramshesh V. K., Peterson Y. K., Lemasters J. J., Szulc Z. M., Bielawski J., Ogretmen B. (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8, 831–838https://doi.org/10.1038/nchembio.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J. C., Kirchman P. A., Zagulski M., Hunt J., Jazwinski S. M. (1998) Homologs of the yeast longevity gene LAG1 in Caenorhabditis elegans and human. Genome Res. 8, 1259–1272https://doi.org/10.1101/gr.8.12.1259 [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., Spassieva S. D., Jucius T. J., Shultz L. D., Shick H. E., Macklin W. B., Hannun Y. A., Obeid L. M., Ackerman S. L. (2011) A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 7, e1002063.https://doi.org/10.1371/journal.pgen.1002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penno A., Reilly M. M., Houlden H., Laurá M., Rentsch K., Niederkofler V., Stoeckli E. T., Nicholson G., Eichler F., Brown R. H., Jr., von Eckardstein A., Hornemann T. (2010) Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187https://doi.org/10.1074/jbc.M109.092973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming M. S., Vysochan A., Paixão S., Niu J., Klein R., Savitt J. M., Luo W. (2015) Cis and trans RET signaling control the survival and central projection growth of rapidly adapting mechanoreceptors. eLife 4, e06828.https://doi.org/10.7554/eLife.06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagda R. K., Cherra S. J., III, Kulich S. M., Tandon A., Park D., Chu C. T. (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284, 13843–13855https://doi.org/10.1074/jbc.M808515200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91https://doi.org/10.1016/j.ymeth.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Corbacho M. J., Canals D., Adada M. M., Liu M., Senkal C. E., Yi J. K., Mao C., Luberto C., Hannun Y. A., Obeid L. M. (2015) Tumor necrosis factor-α (TNFα)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J. Biol. Chem. 290, 25356–25373https://doi.org/10.1074/jbc.M115.658658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowe J., Aubareda A., McNamee K., Przybycien P. M., Lu X., Williams R. O., Bou-Gharios G., Saklatvala J., Dean J. L. E. (2013) Heat shock protein B1-deficient mice display impaired wound healing. PLoS One 8, e77383.https://doi.org/10.1371/journal.pone.0077383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanderson T. H., Raghunayakula S., Kumar R. (2015) Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol. Cell. Neurosci. 64, 116–122https://doi.org/10.1016/j.mcn.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Li L., Suo W. Z. (2009) HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci. 84, 267–271https://doi.org/10.1016/j.lfs.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 34.D’Ydewalle C., Krishnan J., Chiheb D. M., Van Damme P., Irobi J., Kozikowski A. P., Vanden Berghe P., Timmerman V., Robberecht W., Van Den Bosch L. (2011) HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 17, 968–974https://doi.org/10.1038/nm.2396 [DOI] [PubMed] [Google Scholar]
- 35.Neusch C., Senderek J., Eggermann T., Elolff E., Bähr M., Schneider-Gold C. (2007) Mitofusin 2 gene mutation (R94Q) causing severe early-onset axonal polyneuropathy (CMT2A). Eur. J. Neurol. 14, 575–577https://doi.org/10.1111/j.1468-1331.2006.01688.x [DOI] [PubMed] [Google Scholar]
- 36.Züchner S., Mersiyanova I. V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E. L., Zappia M., Nelis E., Patitucci A., Senderek J., Parman Y., Evgrafov O., Jonghe P. D., Takahashi Y., Tsuji S., Pericak-Vance M. A., Quattrone A., Battaloglu E., Polyakov A. V., Timmerman V., Schröder J. M., Vance J. M. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 36, 449–451https://doi.org/10.1038/ng1341 [DOI] [PubMed] [Google Scholar]
- 37.Min J., Mesika A., Sivaguru M., Van Veldhoven P. P., Alexander H., Futerman A. H., Alexander S. (2007) (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol. Cancer Res. 5, 801–812https://doi.org/10.1158/1541-7786.MCR-07-0100 [DOI] [PubMed] [Google Scholar]
- 38.Bionda C., Portoukalian J., Schmitt D., Rodriguez-Lafrasse C., Ardail D. (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 382, 527–533https://doi.org/10.1042/BJ20031819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundaram K., Mather A. R., Marimuthu S., Shah P. P., Snider A. J., Obeid L. M., Hannun Y. A., Beverly L. J., Siskind L. J. (2016) Loss of neutral ceramidase protects cells from nutrient- and energy -deprivation-induced cell death. Biochem. J. 473, 743–755https://doi.org/10.1042/BJ20150586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang B., Liu X., Zhao G., Luo W., Xia K., Pan Q., Cai F., Hu Z., Zhang C., Chen B., Zhang F., Shen L., Zhang R., Jiang H. (2005) Mutation analysis of the small heat shock protein 27 gene in chinese patients with Charcot-Marie-Tooth disease. Arch. Neurol. 62, 1201–1207https://doi.org/10.1001/archneur.62.8.1201 [DOI] [PubMed] [Google Scholar]
- 41.Luigetti M., Fabrizi G. M., Madia F., Ferrarini M., Conte A., Del Grande A., Tasca G., Tonali P. A., Sabatelli M. (2010) A novel HSPB1 mutation in an Italian patient with CMT2/dHMN phenotype. J. Neurol. Sci. 298, 114–117https://doi.org/10.1016/j.jns.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 42.Lee J., Jung S. C., Joo J., Choi Y. R., Moon H. W., Kwak G., Yeo H. K., Lee J. S., Ahn H. J., Jung N., Hwang S., Rheey J., Woo S. Y., Kim J. Y., Hong Y. B., Choi B. O. (2015) Overexpression of mutant HSP27 causes axonal neuropathy in mice. J. Biomed. Sci. 22, 43.https://doi.org/10.1186/s12929-015-0154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J., Giordano S., Zhang J. (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441, 523–540https://doi.org/10.1042/BJ20111451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haidar M., Timmerman V. (2017) Autophagy as an emerging common pathomechanism in inherited peripheral neuropathies. Front. Mol. Neurosci. 10, 143.https://doi.org/10.3389/fnmol.2017.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen L., Qi Z., Zhu Y., Song X., Xuan C., Ben P., Lan L., Luo L., Yin Z. (2016) Phosphorylated heat shock protein 27 promotes lipid clearance in hepatic cells through interacting with STAT3 and activating autophagy. Cell. Signal. 28, 1086–1098https://doi.org/10.1016/j.cellsig.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 46.Chen R., Dai R. Y., Duan C. Y., Liu Y. P., Chen S. K., Yan D. M., Chen C. N., Wei M., Li H. (2011) Unfolded protein response suppresses cisplatin-induced apoptosis via autophagy regulation in human hepatocellular carcinoma cells. Folia Biol. (Praha) 57, 87–95 [PubMed] [Google Scholar]
- 47.Matsumoto T., Urushido M., Ide H., Ishihara M., Hamada-Ode K., Shimamura Y., Ogata K., Inoue K., Taniguchi Y., Taguchi T., Horino T., Fujimoto S., Terada Y. (2015) Small heat shock protein beta-1 (HSPB1) is upregulated and regulates autophagy and apoptosis of renal tubular cells in acute kidney injury. PLoS One 10, e0126229.https://doi.org/10.1371/journal.pone.0126229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakthisaran R., Tangirala R., Rao ChM. (2015) Small heat shock proteins: role in cellular functions and pathology. Biochim. Biophys. Acta 1854, 291–319https://doi.org/10.1016/j.bbapap.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 49.Mielke M. M., Bandaru V. V. R., Haughey N. J., Xia J., Fried L. P., Yasar S., Albert M., Varma V., Harris G., Schneider E. B., Rabins P. V., Bandeen-Roche K., Lyketsos C. G., Carlson M. C. (2012) Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology 79, 633–641https://doi.org/10.1212/WNL.0b013e318264e380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccinini M., Scandroglio F., Prioni S., Buccinnà B., Loberto N., Aureli M., Chigorno V., Lupino E., DeMarco G., Lomartire A., Rinaudo M. T., Sonnino S., Prinetti A. (2010) Deregulated sphingolipid metabolism and membrane organization in neurodegenerative disorders. Mol. Neurobiol. 41, 314–340https://doi.org/10.1007/s12035-009-8096-6 [DOI] [PubMed] [Google Scholar]
- 51.Henriques A., Croixmarie V., Priestman D. A., Rosenbohm A., Dirrig-Grosch S., D’Ambra E., Huebecker M., Hussain G., Boursier-Neyret C., Echaniz-Laguna A., Ludolph A. C., Platt F. M., Walther B., Spedding M., Loeffler J. P., Gonzalez De Aguilar J. L. (2015) Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum. Mol. Genet. 24, 7390–7405https://doi.org/10.1093/hmg/ddv439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patwardhan G. A., Beverly L. J., Siskind L. J. (2016) Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 48, 153–168https://doi.org/10.1007/s10863-015-9602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novgorodov S. A., Gudz T. I., Obeid L. M. (2008) Long-chain ceramide is a potent inhibitor of the mitochondrial permeability transition pore. J. Biol. Chem. 283, 24707–24717https://doi.org/10.1074/jbc.M801810200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laviad E. L., Kelly S., Merrill A. H., Jr., Futerman A. H. (2012) Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 287, 21025–21033https://doi.org/10.1074/jbc.M112.363580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkataraman K., Futerman A. H. (2002) Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett. 528, 3–4https://doi.org/10.1016/S0014-5793(02)03248-9 [DOI] [PubMed] [Google Scholar]
- 56.Mesika A., Ben-Dor S., Laviad E. L., Futerman A. H. (2007) A new functional motif in Hox domain-containing ceramide synthases: identification of a novel region flanking the Hox and TLC domains essential for activity. J. Biol. Chem. 282, 27366–27373https://doi.org/10.1074/jbc.M703487200 [DOI] [PubMed] [Google Scholar]
- 57.Tirodkar T. S., Lu P., Bai A., Scheffel M. J., Gencer S., Garrett-Mayer E., Bielawska A., Ogretmen B., Voelkel-Johnson C. (2015) Expression of ceramide synthase 6 transcriptionally activates acid ceramidase in a c-Jun N-terminal Kinase (JNK)-dependent manner. J. Biol. Chem. 290, 13157–13167https://doi.org/10.1074/jbc.M114.631325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atkinson D., Nikodinovic Glumac J., Asselbergh B., Ermanoska B., Blocquel D., Steiner R., Estrada-Cuzcano A., Peeters K., Ooms T., De Vriendt E., Yang X. L., Hornemann T., Milic Rasic V., Jordanova A. (2017) Sphingosine 1-phosphate lyase deficiency causes Charcot-Marie-Tooth neuropathy. Neurology 88, 533–542https://doi.org/10.1212/WNL.0000000000003595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava A. K., Renusch S. R., Naiman N. E., Gu S., Sneh A., Arnold W. D., Sahenk Z., Kolb S. J. (2012) Mutant HSPB1 overexpression in neurons is sufficient to cause age-related motor neuronopathy in mice. Neurobiol. Dis. 47, 163–173https://doi.org/10.1016/j.nbd.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie W., Chung K. K. (2012) Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 122, 404–414https://doi.org/10.1111/j.1471-4159.2012.07769.x [DOI] [PubMed] [Google Scholar]
- 61.Xu H. B., Jiang R. H., Li L. (2014) Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 33, 347–358 [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez G. E., González D. M. C., Monachelli G. M. G., Costa J. J., Nicola A. F., Sica R. E. P. (2012) Morphological abnormalities in mitochondria of the skin of patients with sporadic amyotrophic lateral sclerosis. Arq. Neuropsiquiatr. 70, 40–44https://doi.org/10.1590/S0004-282X2012000100010 [DOI] [PubMed] [Google Scholar]
- 63.Angelin A., Tiepolo T., Sabatelli P., Grumati P., Bergamin N., Golfieri C., Mattioli E., Gualandi F., Ferlini A., Merlini L., Maraldi N. M., Bonaldo P., Bernardi P. (2007) Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc. Natl. Acad. Sci. USA 104, 991–996https://doi.org/10.1073/pnas.0610270104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S. M., Olzmann J. A., Chin L.-S., Li L. (2011) Mutations associated with Charcot-Marie-Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome-autophagy pathways. J. Cell Sci. 124, 3319–3331https://doi.org/10.1242/jcs.087114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouhy D., Timmerman V. (2013) Animal models and therapeutic prospects for Charcot-Marie-Tooth disease. Ann. Neurol. 74, 391–396https://doi.org/10.1002/ana.23987 [DOI] [PubMed] [Google Scholar]
- 66.Almeida-Souza L., Asselbergh B., d’Ydewalle C., Moonens K., Goethals S., de Winter V., Azmi A., Irobi J., Timmermans J.-P., Gevaert K., Remaut H., Van Den Bosch L., Timmerman V., Janssens S. (2011) Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J. Neurosci. 31, 15320–15328https://doi.org/10.1523/JNEUROSCI.3266-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.