Abstract
BACKGROUND
To investigate uniformly successful results from a statewide program of patient navigation (PN) for colonoscopy, this comparison study evaluated the effectiveness of the PN intervention by comparing outcomes for navigated versus non-navigated patients in one of the community health clinics included in the statewide program. Outcomes measured included screening completion, adequacy of bowel preparation, missed appointments and cancellations, communication of test results, and consistency of follow-up recommendations with clinical guidelines.
METHODS
The authors compared a subset of 131 patients who were navigated to a screening or surveillance colonoscopy with a similar subset of 75 non-navigated patients at one endoscopy clinic. The prevalence and prevalence odds ratios were computed to measure the association between PN and each study outcome measure.
RESULTS
Patients in the PN intervention group were 11.2 times more likely to complete colonoscopy than control patients (96.2% vs 69.3%; P<.001), and were 5.9 times more likely to have adequate bowel preparation (P =.010). In addition, intervention patients had no missed appointments compared with 15.6% of control patients, and were 24.8 times more likely to not have a cancellation <24 hours before their appointment (P<.001). All navigated patients and their primary care providers received test results, and all follow-up recommendations were consistent with clinical guidelines compared with 82.4% of patients in the control group (P<.001).
CONCLUSIONS
PN appears to be effective for improving colonoscopy screening completion and quality in the disparate populations most in need of intervention. To the best of our knowledge, the results of the current study demonstrate some of the strongest evidence for the effectiveness of PN to date, and highlight its value for public health.
Keywords: colonoscopy, disparate populations, outcome assessment, patient adherence, patient navigation, public health
INTRODUCTION
Colorectal cancer (CRC) is largely preventable through effective screening; in addition, screening can detect cancer early, thereby increasing the likelihood of survival. Nonetheless, CRC remains the second leading cause of cancer death in the United States among cancers affecting both men and women.1 Despite compelling evidence2,3 and strong recommendations,4 only 65.7% of average-risk adults aged 50 to 75 years were reported to be up to date with CRC screening in 2014.5 This is below the Healthy People 2020 target of 70.5%6 and further below the goal of 80% by 2018 suggested by the National Colorectal Cancer Roundtable (http://nccrt.org/). Of national significance, large disparities in CRC screening exist based on race, ethnicity, income, education, and health insurance status.5 Increasing high-quality screening is essential for the prevention and early detection of CRC, and finding interventions that can effectively address disparities is critical to reaching that goal.
Colonoscopy is the most widely used CRC screening test in the United States7; through the detection and removal of potentially precancerous polyps, it can prevent CRC.2,8 However, several specific barriers have been shown to reduce the use of colonoscopy and thereby undermine the prevention and early detection of CRC. Patient navigation (PN) is individualized assistance to help patients overcome personal and healthcare system barriers, and to facilitate understanding and timely access, thereby enabling those patients to complete CRC screening.9
Several types of barriers contribute to the perpetuation of disparities in CRC screening.10–18 Health system barriers include inadequate insurance or access to payment resources, lack of a medical home, or lack of a primary care provider (PCP) recommendation to undergo screening.10–15,17,18 Personal and cultural barriers include lack of knowledge and misconceptions regarding screening, distrust of the medical system, poor understanding of bowel preparation instructions, absence of language interpretation services, no transportation home after colonoscopy with sedation, lack of understanding concerning scheduling appointments and completing paperwork, challenges to arranging time off work, embarrassment, fear of the procedure, and fatalistic attitudes regarding cancer.10–14,16–18 These barriers all contribute to the inability to undergo screening and exacerbate disparities in CRC.
Although several prior studies have suggested that PN might be a powerful tool with which to address patient barriers and thereby support the success of CRC screening,19–24 others have not demonstrated such effects.25–28 In the current study, we present an evaluation of the New Hampshire Colorectal Cancer Screening Program (NHCRCSP) PN model (Dartmouth-Hitchcock Medical Center) that used structured, telephonic navigation delivered by registered nurse navigators with physician (endoscopist) oversight and aimed at increasing quality colonoscopy screening. Quality screening includes ensuring an informed patient who is due for screening or surveillance, keeps his or her appointment, has good preparation for the colonoscopy, and also receives test results and guideline-adherent recommendations for subsequent screening. The PN model evaluated herein was designed to address these quality aspects of screening colonoscopy, in addition to increasing completion of colonoscopy.
The PN model was designed and implemented by the NHCRCSP and funded as part of the Centers for Disease Control and Prevention’s (CDC) Colorectal Cancer Control Program (CRCCP).29 The model was implemented to serve low-income, uninsured, and underinsured patients by providing colonoscopies at 12 endoscopy clinics across the state of New Hampshire. To evaluate consistently successful colonoscopy completion and quality outcomes achieved for approximately 2000 patients statewide, we conducted a comparison group study at one of the endoscopy clinics. In this comparison study, we investigate the hypothesis that navigated patients will achieve better screening outcomes than non-navigated patients. It is important to note that we are advancing previously reported PN outcomes by not only assessing colonoscopy screening completion and bowel preparation quality,19–24 but also the frequency of missed appointments and cancellations, communication of screening results to patients and PCPs, and the consistency of patients’ recommended rescreening intervals with clinical guidelines.
MATERIALS AND METHODS
Study Design and Participants
Using a retrospective, nonequivalent comparison group research design, we compared clinical outcomes for a subset of NHCRCSP patients at 1 of the 12 endoscopy clinics served by the NHCRCSP (intervention group, all of whom received PN as part of the NHCRCSP) with those of a similar subset of patients at the same clinic (control group, non-NHCRCSP and who did not receive PN) who received usual care. Patients in the intervention and control study groups received medical care at the same federally qualified health center, and all were referred for colonoscopies at the same endoscopy center, thereby allowing for a comparison of a very similar population of navigated versus usual-care patients. Because the current study was a retrospective study of clinical outcomes, there was no physical recruitment of patients, nor was there a selection process that predetermined a subgroup of patients to refer to navigation versus to usual care. There were no patients in the usual-care group who had been offered NHCRCSP navigation and refused. We abstracted demographic and clinical outcomes data from existing clinic records for navigated and non-navigated patients. To maximize comparability among the 2 patient groups, all patient records met 5 inclusion criteria: 1) patients were aged 50 to 64 years; 2) patients had an income level <250% of the federal poverty level, with all patients uninsured and having an alternate source of payment for colonoscopy; 3) patients were scheduled and notified of the colonoscopy test date between July 1, 2012 and September 30, 2013; 4) patients were scheduled for a screening or surveillance colonoscopy; and 5) patients had no diagnosis of CRC from the completed test. The small number of patient records with a diagnosis of cancer were excluded to ensure confidentiality. CDC funds supported the costs for NHCRCSP-navigated patients and existing uncompensated care programs from the clinic supported the costs for patients in the comparison group. The Institutional Review Board of the CDC and appropriate Dartmouth-Hitchcock committees approved the study protocol and methods.
NHCRCSP PN Intervention
As per CDC grant guidelines, CRCCPs including the NHCRCSP provided free screening (to address disparities) using a defined portion of their grant funding; the grant also was intended to increase CRC screening rates overall in grantee states. Accordingly, NHCRCSP staff divided their time between those 2 goals. For the colonoscopy PN program, 2 registered nurse navigators (totaling 1.2 full-time equivalents) were trained by the NHCRCSP staff and navigated all NHCRCSP patients. This training included detailed education regarding colonoscopy screening and patient care processes developed by the NHCRCSP. The nurses delivered PN with support from other NHCRCSP staff members including the medical director (endoscopist providing oversight of the PNs), program director (a registered nurse providing nurse management of the PNs), data manager, and secretary. A central navigation model was used in which the navigators were part of the NHCRCSP staff, rather than working for a specific clinic; therefore, they operated externally to the clinic setting. Due to the statewide presence of the program, navigation was delivered telephonically. A language translation telephone service was used for non-English-speaking patients, and the NHCRCSP provided translated written materials for bowel preparation instruction in the 26 different languages needed for the specific population being served.
Patients were referred to the NHCRCSP by their PCPs or by self-referral after hearing about the program and contacting the NHCRCSP directly. The secretary collected all the necessary enrollment information, ensured that patients met non-clinical eligibility criteria (ie, were low income, uninsured, and a resident of New Hampshire) and sent patients an information packet and the necessary forms for the patient to complete and return. Using the patient’s medical history form and prior screening information, the program director approved all enrollments; high-risk and questionably symptomatic patients were reviewed by the medical director. All NHCRCSP patients were confirmed (based on prior test results and pathology if applicable) to be due and appropriate for colonoscopy; however, this outcome was not one of the outcomes assessed within the comparison study described herein because this information often could not be obtained for usual-care patients for whom the NHCRCSP could not request prior records. Once the patient was determined to be eligible, medically appropriate, and due for colonoscopy, he or she was referred to a PN and scheduled for colonoscopy at a geographically convenient endoscopy center.
Navigators followed a standardized protocol that was developed and implemented by the NHCRCSP. The protocol required a minimum of 6 topic-specific contacts with each patient, designed to gain the trust of, educate, and support the patient throughout the entire screening process. Because the population was comprised of under-served patients, many with language barriers (which were especially prevalent in the study clinic population), an essential first step was gaining agreement to undergo colonoscopy. The full, detailed protocol is available at www.cdc.gov/cancer/crccp/pn-replication-manual.htm. Briefly, the 6 required topic-specific contacts with each patient included: 1) initially contacting the patient to obtain agreement to undergo colonoscopy, confirm appointment scheduling, establish rapport, and assess barriers; 2) reviewing bowel preparation instructions and directions for how and where to obtain the bowel preparation, addressing barriers, and confirming transportation and patient escort plans (5–7 days before colonoscopy); 3) reviewing bowel preparation instructions in specific detail and addressing any challenges and remaining barriers (1–2 days before colonoscopy); 4) confirming the appointment details (including location and transportation), discussing bowel preparation progress, and answering any remaining questions (the day before colonoscopy); 5) evaluating the colonoscopy experience and providing any necessary support shortly after the procedure; and 6) confirming patient receipt and understanding of the results and recommended rescreening interval from the endoscopist (within 2–4 weeks after colonoscopy, if possible).
All 6 topic-specific communications were conducted over the telephone, with e-mail contact limited to messages regarding non-medical information such as confirming a date and time to contact the patient. No text messages were used.
After the colonoscopy results were available, as part of collecting comprehensive data for reporting to the CDC, navigators reviewed individual patient risk and procedure findings including pathology results with the medical director, and compared the endoscopist’s recommended rescreening interval with clinical CRC screening and surveillance guidelines.4,30 If inconsistent, navigators or the medical director contacted the endoscopist’s office to resolve the discrepancy. Navigators also contacted the endoscopist’s office if patients did not receive follow-up information, and confirmed that the results had been copied to the PCP. Navigators recorded detailed service delivery data in a real-time database system known as Catalyst (Spectrum Health Policy Research [SHPR], Atlanta, Ga) used by the NHCRCSP. All patients signed a release of medical information form at the time of enrollment into the NHCRCSP, allowing navigators access to patient medical information, including prior screening results. Payment for the colonoscopies and the bowel preparation was provided by the NHCRCSP through the CDC grant.
Usual Care
Usual care (status quo) was provided by the endoscopy clinic for all patients and included: 1) scheduling the procedure and determining medical eligibility to undergo colonoscopy (by telephone through a series of defined questions); 2) mailing the patient written bowel preparation and pharmacy instructions and forwarding bowel preparation prescriptions to the patient’s preferred pharmacy; 3) educating the patient about bowel preparation and clinic policies and reviewing medical details by telephone (5–7 days before the procedure); 4) reminding the patient and providing the appointment time and clinic arrival instructions by telephone (2–4 days before colonoscopy); 5) mailing a letter to the patient with pathology results (after colonoscopy, within <2–3 weeks after colonoscopy, if possible); and 6) documenting the recommended rescreening or surveillance interval in the patient’s health maintenance records. A language translation service for non–English-speaking patients was provided.
Sampling Strategy and Method
The NHCRCSP program collaborated with 12 endoscopy sites located in geographic proximity to target populations across the state. For this comparison study, we chose a clinic with a sufficient number of low-income patients and a sample size sufficient to achieve an estimated 7% detectable difference between the intervention and control groups, based on 1-directional (1-tailed) tests with a 5% type I error rate and 80% power.
Using purposive sampling, we selected all intervention group and control group patient records that met the established inclusion criteria. Purposive sampling was appropriate given the need to sample patient records on the basis of prespecified inclusion criteria so that outcomes could be measured between comparable populations.31,32 We applied a 100% sampling rate to all intervention and control patient records (ie, total population sampling) meeting the established inclusion criteria given that the total size of the population was relatively small. The final sample included 131 NHCRCSP (intervention group) and 75 non-NHCRCSP (control group) patient records.
Data Sources and Collection Procedures
Data sources
Data for the patients in the intervention group were extracted from the NHCRCSP database system, Catalyst, which is a cloud-based software system used to record, track, and manage detailed patient data. The NHCRCSP worked with SHPR to design enhancements and manage the Catalyst system to allow for optimal functionality for real-time recording, tracking, and management of patient administrative and medical data. Data variables included patient demographics and socioeconomic indicators, comorbidities and CRC personal and family history, patient barriers to colonoscopy, appointment dates, missed appointments and cancellations, bowel preparation quality, colonoscopy procedure details and results including pathology, communication of results to patients and PCPs, and recommended rescreening intervals.
Three members of the NHCRCSP team, including a gastroenterologist with extensive endoscopic experience, a registered nurse with experience in data abstraction, and a data manager, abstracted data from the medical records for the 75 control patients at the selected clinic. Data variables abstracted comprised the same data variables included for the patients in the intervention group. Data sources included the electronic medical record to obtain the colonoscopy report, patient risk factors, and office visit notes and to view copies of results letters or telephone communication with the patient and/or the PCP, and the appointment record system to confirm no-shows and cancellations. A structured abstraction form, created by SHPR and directly linked to an Excel spreadsheet (Microsoft Corporation, Redmond, Wash), was used to collect all the variables as outlined in Tables 1 to 3. Direct linking of the data abstraction form to the Excel spreadsheet avoided potential errors in data entry into the spreadsheet. All collected data points were reviewed by a minimum of 2 individuals, always including the endoscopist and either the data manager or registered nurse. A final deidentified spreadsheet was provided to the CDC team.
TABLE 1.
Study Outcome Measures
| Variable | Definition | Numerator | Denominator |
|---|---|---|---|
| Colonoscopy completed | A colonoscopy is completed within 12 mo of the patient receiving confirmation of the scheduled test date | No. of patients with completed colonoscopy within 12 mo | No. of patients scheduled for a colonoscopy during the study period |
| Adequate bowel preparation qualitya | Bowel preparation is considered adequate (excellent, good, or fair) by the endoscopist performing the colonoscopy | No. of patients with adequate bowel preparation | No. of patients with a performedb colonoscopy during the study period |
| Missed appointment/no show | Patient does not appear for his or her scheduled appointment and did not cancel in advance | No. of missed appointments/no shows without prior cancellation | No. of scheduled colonoscopies during the study period |
| Cancellation <24 h prior to appointment | Patient cancels his or her appointment <24 h before the scheduled appointment | No. of cancellations <24 h before the scheduled appointment | No. of scheduled colonoscopies during the study period |
| Results communicated to the patient | Records indicate that communication was received by the patient regarding results of the colonoscopy examination | No. of patients who received communication about their results | No. of patients with a completed colonoscopy during the study period |
| Results communicated to the PCPc | Records show that communication was received by the PCP regarding results of the colonoscopy examination | No. of patients whose PCP received communication about their results | No. of patients with a completed colonoscopy during the study period |
| Final recommended rescreening interval consistent with clinical guidelines | The no. of mo/y recommended by the endoscopist until the next colonoscopy is consistent with US Preventive Services Task Force and US Multi-Society Task Force on Colorectal Cancer clinical guidelines (for PN group this is after navigator intervention if it was needed to ensure consistency with guidelines) | No. of patients who were recommended a screening interval that was consistent with clinical guidelines | No. of patients with a completed colonoscopy during the study period |
Abbreviations: PCP, primary care provider; PN, patient navigation.
Nearly all endoscopy centers in New Hampshire participate in a research-funded colonoscopy registry called the New Hampshire Colonoscopy Registry. The New Hampshire Colonoscopy Registry Colonoscopy Procedure Form instructs endoscopists to score the bowel preparation based on the worst prepared segment after clearing all colon segments, using the following categories: excellent (essentially 100% visualization), good (very unlikely to impair visualization), fair (possibly impairing visualization), and poor (definitely impairing visualization). Poor preparation is considered inadequate. Therefore, bowel preparation assessment in the current study was likely to be more consistent (and consistently noted) by endoscopists than in centers without similar research.
A performed colonoscopy is one that was initiated, regardless of whether it was complete or incomplete. A colonoscopy may be incomplete for several reasons, including inadequate bowel preparation.
Results communicated to the patient and PCP were defined differently for navigated versus non-navigated patients. For navigated patients, we were able to assess whether the results were received by the patient because this was documented by the navigator in the Catalyst records after discussion with the patient. Navigators also confirmed that results had been sent to the PCPs. For the non-navigated patients, we were able to assess whether the results had been sent to the patients and to their PCP through documentation in the clinic records (ie, via letter or telephone message).
TABLE 3.
Outcome Results for the Intervention Group Versus the Control Group
| Intervention Group N = 131 |
Control Group N = 75 |
Intervention Group Versus Control Group |
||
|---|---|---|---|---|
|
| ||||
| Outcome | % | % | ORa | Pb |
| Colonoscopy completedc | 96.2 | 69.3 | 11.2 | <.001 |
| Adequate bowel preparation quality | 97.6 | 87.5 | 5.9 | .010 |
| Missed appointment/no show | 0.0 | 15.6 | 48.4d | <.001 |
| Cancellation <24 h before appointment | 0.8 | 16.0 | 24.8 | <.001 |
| Results communicated to patient | 100.0 | 96.2 | 10.1d | .084 |
| Results communicated to PCP | 100.0 | 48.1 | 272.2d | <.001 |
| Final recommended rescreening interval consistent with clinical guidelines | 100.0 | 82.4 | 54.0d | <.001 |
Abbreviations: OR, odds ratio; PCP, primary care provider.
Unadjusted ORs.
P values were derived using the Fisher exact test.
Colonoscopy was completed within 12 months of the patient receiving confirmation of the scheduled test date.
Intervention group status was found to predict perfect success. ORs were computed after adjusting zero cells to 0.5.
Study Outcomes
The CDC and the NHCRCSP collaborated to define study variables and outcomes. Study outcomes included: 1) colonoscopy completion (including completion of colonoscopies for which the patient did not show up or that were incomplete due to inadequate preparation on the first attempt); 2) adequate bowel preparation quality; 3) missed appointments/no-shows; 4) cancellations <24 hours before the appointment; 5) results communicated to the patient; 6) results communicated to the PCP; and 7) consistency between the endoscopist’s recommended rescreening or surveillance interval and clinical CRC guidelines. All study outcome measures were defined from data variables abstracted from the Catalyst system (patients in the intervention group) and clinic medical records (patients in the control group). Each is defined in Table 1.
Data Analysis
To describe demographic characteristics, we compiled descriptive statistics and used the chi-square test to detect any differences between the 2 groups. We used the Fisher exact test of independence to evaluate the association between PN and study outcome measures. We computed the prevalence odds ratio to measure the association between PN and the prevalence of each study outcome. All analyses were conducted using Stata statistical software (version 14; StataCorp LLC, College Station, Tex).
RESULTS
Patient Characteristics
The characteristics of the patient sample are presented in Table 2. The groups were similar with regard to age, family history of CRC, and diabetic status. Greater than 80% of patients were aged 50 to 59 years in both groups. The intervention population included fewer white patients (61.1% vs 77.3%), more female patients (62.6% vs 46.7%), and more individuals who required a language interpreter (37.4% vs 10.7%). In addition, smaller percentages of patients in the intervention group had been screened previously (24.4% vs 32.0%), had a prior personal history of CRC or polyps (9.9% vs 18.7%), or were smokers (18.3% vs 36.0%).
TABLE 2.
Patient Characteristics
| Intervention Group N = 131 |
Control Group N = 75 |
||
|---|---|---|---|
| Patient Characteristics | No. (%) | No. (%) | Pa |
| Age, y | |||
| 50–59 | 108 (82.4) | 65 (86.7) | .705 |
| 60–64 | 23 (17.6) | 10 (13.3) | |
| Sex | |||
| Female | 82 (62.6) | 35 (46.7) | .026 |
| Male | 49 (37.4) | 40 (53.3) | |
| Race | |||
| White | 80 (61.1) | 58 (77.3) | .005 |
| Black/African American | 7 (5.3) | 5 (6.7) | |
| Asian | 28 (21.4) | 3 (4.0) | |
| Other race | 1 (0.8) | 2 (2.6) | |
| Do not know/refused to answer | 15 (11.5) | 7 (9.4) | |
| Ethnicity | |||
| Hispanic | 32 (24.4) | 2 (2.7) | <.001 |
| Non-Hispanic | 97 (74.1) | 65 (86.7) | |
| Do not know/refused to answer | 2 (1.5) | 8 (10.6) | |
| Primary language | |||
| English | 72 (55.0) | 65 (86.7) | .006 |
| Spanish | 30 (22.9) | 4 (5.3) | |
| Otherb,c | 29 (22.1) | 6 (7.9) | |
| Interpreter needed | 49 (37.4) | 8 (10.7) | <0.001 |
| Previously been screened for CRC | 32 (24.4) | 24 (32.0) | .049 |
| Family history of CRC | 13 (9.9) | 8 (10.7) | .637 |
| Personal history of CRC or polyps | 13 (9.9) | 14 (18.7) | .056 |
| Diabetic | 19 (14.5) | 11 (14.7) | .944 |
| Smoker | 24 (18.3) | 27 (36.0) | <0.001 |
Abbreviation: CRC, colorectal cancer.
P values were derived from Pearson chi-square tests.
Other language for the intervention group included Vietnamese, Arabic, Nepali, Mandarin, Portuguese, Cantonese, Kurdish, Tagalog, Bosnian, Gujarati, Bengali, Krahn, and American Sign Language.
Other language for the control group included Vietnamese, Arabic, Korean, Romanian, and Dinka.
Outcomes
Patients in the intervention group completed colonoscopy screening at a prevalence rate of 96.2% compared with 69.3% for patients in the control group, and were 11.2 times more likely to complete colonoscopy screening than control patients (P<.001) (Table 3). Patients in the intervention group also were found to be 5.9 times more likely to have adequate bowel preparation quality than control patients (P =.010). In addition, patients in the intervention group had no missed appointments or no-show episodes compared with 15.6% for control group patients (P<.001), and were 24.8 times more likely to not have a cancellation <24 hours before their appointment compared with control patients. No significant difference was detected between the 2 groups with regard to results being communicated to patients; however, patients in the intervention group were significantly more likely to have their results communicated to their PCP (100% vs 48.1%; P<.001) and to have a final recommended rescreening interval that was consistent with clinical guidelines (100% vs 82.4%; P<.001).
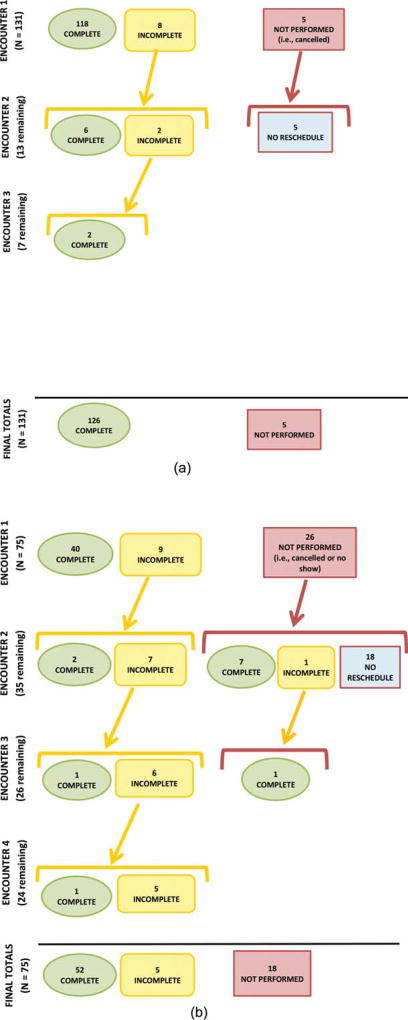
We evaluated the number of complete and incomplete colonoscopies at each test encounter level, and the number of colonoscopies not performed due to missed appointments and cancellations during the study time period. As shown in Figure 1 Top and Bottom, a notation of “incomplete” denotes that the colonoscopy was performed but not completed by the endoscopist. This could be due to inadequate bowel preparation, an inability to reach the cecum (anatomical endpoint), incomplete polyp removal, or medical complications during the procedure. “Not performed” denotes that the colonoscopy never occurred due to either a missed appointment (“no-show”) or cancellation by the patient. We evaluated these outcomes for all patients to illustrate the number of test encounters needed to move patients to completion of their colonoscopy screening process. The same group of endoscopists performed the colonoscopies for all patients, regardless of whether the patients were in the intervention or control groups. All the patients in the intervention group with an initial incomplete colonoscopy completed the process to the final test outcome (8 of 8 patients), whereas fewer than one-half of patients in the control group completed the process to the final test outcome (4 of 9 patients). It is important to note that patients in the intervention group required fewer test encounters to complete screening compared with control patients.
Figure 1.
(Top) Flowchart of colonoscopy completions in the intervention group by test encounter level. (Bottom) Flowchart of colonoscopy completions in the control group by test encounter level. Both panels illustrate the number of complete and incomplete colonoscopies at each test encounter level and the number of colonoscopies not performed due to missed appointments and cancellations during the study time period. “Incomplete” denotes that the colonoscopy was performed but not completed by the endoscopist. This could be due to inadequate bowel preparation, inability to reach the cecum (anatomical endpoint), incomplete polyp removal, or medical complications during the procedure. “Not performed” denotes that the colonoscopy never occurred due to either a missed appointment (“no show”) or cancellation by the patient.
DISCUSSION
The findings of the current study highlight the public health benefit of PN for increasing colonoscopy completion among a diverse, low-income population. Based on a model using nurse navigators with physician (endoscopist) oversight and experienced nurse management and following a rigorous 6 topic-specific communication protocol, >96% of navigated patients completed colonoscopy. Ultimately, navigated patients were found to be 11 times more likely to complete colonoscopy and nearly 6 times more likely to have an adequate bowel preparation than patients receiving usual care. In addition, none of the navigated patients missed an appointment (no shows) in comparison with 15.6% of the control patients, and <1% of navigated patients cancelled within 24 hours of the scheduled appointment compared with 16.0% of patients in the control group. Outcomes similar to those demonstrated within the NHCRCSP cohort used for this comparison study were found for the entire statewide NHCRCSP navigated group consisting of nearly 2000 colonoscopies at 12 unrelated endoscopy centers over the course of 6 years (ie, > 96% colonoscopy completion rate, <1% inadequate preparation rate, and 0.1% no-show rate).33
Although prior studies have reported the success of PN in helping patients complete the colonoscopy procedure,19–24 the results of the current study not only demonstrated extremely high completion effectiveness but also extended beyond completion outcomes in demonstrating that PN can improve the communication of colonoscopy results by the endoscopist to the patient’s referring PCP, and the frequency with which endoscopist follow-up recommendations are consistent with screening and surveillance guidelines. This latter outcome is essential to screening quality because repeat screening and surveillance intervals that are shorter than recommended intervals expose patients to unnecessary risks, and intervals that are longer than recommended decrease the effectiveness of screening for the prevention and early detection of CRC.30,34,35 Screening is not a 1-time event, and ensuring consistently appropriate and high-quality screening is essential to screening effectiveness. This PN model, using registered nurses and including physician oversight, provided the opportunity to investigate how these outcomes could be addressed. To the best of our knowledge, no previous study has investigated PN effectiveness across such a broad set of outcomes. We believe these results demonstrate some of the strongest evidence to date for the effectiveness of PN in improving colonoscopy completion and quality. As a result, a replication manual containing specific information and tools for other entities to replicate the NHCRCSP PN model has been created by the NHCRCSP and the CDC and is available online,36 and a study has been funded to evaluate this PN model in new, diverse settings, which will allow for the future comparison and evaluation of the effectiveness and examine the potential scalability of the intervention.
The cost implications of PN also must be recognized. Reducing the number of incomplete colonoscopies (often due to inadequate preparation) and avoiding inappropriately short rescreening intervals could result in reductions in overall health care spending. The increase in high-quality, completed colonoscopy though use of PNs could contribute to long-term outcomes of decreased CRC rates and associated treatment costs, estimated in the United States at $14 billion in 2010 and projected to reach $17 billion by 2020.37 Furthermore, the financial cost of lost productivity resulting from CRC was estimated to be $12 billion in 2010.38 Endoscopy and health care centers also might benefit by avoiding significant lost reimbursement caused by late cancellations and missed appointments/no-show patients, which are estimated at rates of 12% to 42%, with the latter number reported for vulnerable populations.39,40 Late cancellations and no-show patients cannot be replaced by other procedures at the last minute, thereby creating a loss of revenue that could be avoided through the PN results demonstrated herein. A NHCRCSP study currently is underway to assess the cost-effectiveness of this PN intervention by comparing costs and screening outcomes between navigated and usual-care patients.
Several factors might have contributed to the effectiveness of this particular PN model. Given the clinical complexity of colonoscopy,41 nurse navigators with endoscopist oversight might be especially adept at addressing patient concerns and questions. Furthermore, the establishment of a standardized 6-topic communication protocol to guide the content and timing of navigator-patient interaction ensured that the needed patient education and support were delivered consistently by the navigator. The frequency and timing of calls also might have contributed to this effectiveness given that increased intervention dose has been shown to improve health outcomes.42 It is interesting to note that the usual-care control group also received multiple calls, suggesting that it might be call content and the relationship with the navigator that achieved the demonstrated effect, beyond simple dose. The navigated patients received telephone calls from a single navigator who actively tried to establish a relationship with the patient as part of the call content. The patients in the control group also received multiple telephone calls, but those calls were made by a variety of individuals whose primary goal was to communicate information. Other factors that might have contributed to effectiveness were ongoing mentoring as well as characteristics and training of the navigators; management by an experienced public health nurse; and continuing collection and review by project staff of extensive program data, which were used consistently to monitor program implementation, quality, and outcomes.
Finally, in addition to PN, increasing population CRC screening rates from 64% overall (much lower for underserved populations) to the National Colorectal Cancer Roundtable target of 80% demands a comprehensive approach, including provider-oriented and patient-oriented evidence-based strategies implemented in health systems.43–46 Individuals of lower income, education, and health literacy are disproportionately more likely to have never undergone screening or not be up to date with screening.7 For these individuals, PN can help to reduce this disparity, as can consideration of alternative test options such as the fecal occult blood test/fecal immunochemical test for patients who are at average risk of CRC.47 An important area for future research would be the adaptation of this PN model for programs that include fecal occult blood test/fecal immunochemical test testing options.
The limitations of the current study should be acknowledged. Although attempts were made to identify a comparable group, we found some significant differences between navigated and usual-care patients. However, several of these differences (eg, the percentage of the intervention vs control groups, respectively, that were of nonwhite race, required an interpreter, previously were screened for CRC, had a history of polyps or CRC) might have predicted a greater rate of colonoscopy completion and other related outcomes for the control group rather than the intervention group. Second, although the possibility of cross-contamination represents a potential limitation given that patients came from the same clinic, the navigators operated externally to the clinic, thereby reducing the likelihood of contamination. Third, all patients had a payment source for colonoscopy; given the significant barrier that cost presents for this procedure, screening rates for both groups might not have been as high otherwise. In addition, selection bias is possible because some patients in the intervention group might have been more motivated to complete screening than controls, given their desire to enroll in the PN program. However, patients in both groups in this retrospective study were sufficiently motivated to agree to undergo a colonoscopy. Many patients referred to the NHCRCSP were unaware that their providers had referred them for colonoscopy (most likely due to office time constraints and language barriers) or did not have an understanding of the procedure; for those patients, the first step in PN was to explain what a colonoscopy is, and to gain patient acceptance of testing, for which navigators can be particularly skilled. In terms of study design, although a randomized, prospective methodology often is desirable for investigations of effectiveness, CDC funding for the NHCRCSP was restricted for program purposes and did not allow for a research protocol. Therefore, once the highly effective program outcomes were noted, the retrospective design of the current study was chosen to allow for detailed evaluation and comparison with an appropriate group. Finally, data from 2010 through 2014 demonstrated that New Hampshire consistently has had CRC screening rates >70%, which is higher than the national average of 64%5; therefore, existing social and peer norms might have contributed toward higher screening rates for both groups.
PN is a pivotal intervention for increasing colonoscopy screening. The results of the current study highlight the significant effectiveness of navigation in achieving this goal. In particular, the results demonstrated that the NHCRCSP model of PN (involving registered nurse navigators, physician oversight, a minimum of 6 topic-specific telephone contacts, strong program monitoring, and experienced nurse management) were highly effective in increasing colonoscopy completion among an under-served population in New Hampshire. In addition, these results extend the evidence of potential outcomes affected by navigation, including decreasing no-show patients and cancellations <24 hours before colonoscopy, improving bowel preparation quality, improving the communication of results and follow-up recommendations, and increasing the frequency of guideline-appropriate rescreening and surveillance intervals. To establish generalizability, an important next step involves further evaluation of the model in different settings and with different populations. Of ultimate significance, the increase in screening completion and quality that can be delivered through effective PN can make a critical difference in decreasing morbidity and mortality from CRC.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING SUPPORT
No specific funding was disclosed.
CONFLICT OF INTEREST DISCLOSURES
Joanne Gersten and Lynn Butterly have received a grant from the Centers for Disease Control and Prevention for work performed as part of the current study. They also report that a copyright has been issued to the New Hampshire Colorectal Cancer Screening Program for the patient navigation model described in the current study.
AUTHOR CONTRIBUTIONS
Ketra Rice: Conceptualization and design, acquisition of the data, methodology, formal analysis and interpretation of the data, investigation, validation, data curation, writing–original draft, writing–review and editing, and critical revision of the article for important intellectual content. Lindsay Gressard: Conceptualization and design, acquisition of the data, methodology, formal analysis and interpretation of the data, investigation, validation, data curation, writing–review and editing, and critical revision of the article for important intellectual content. Amy DeGroff: Conceptualization and design, writing–original draft, writing–review and editing, critical revision of the article for important intellectual content, supervision, and project administration. Joanne Gersten: Conceptualization and design, acquisition of the data, writing– review and editing, resources, and critical revision of the article for important intellectual content. Janene Robie: Conceptualization and design, acquisition of the data, writing–review and editing, resources, and critical revision of the article for important intellectual content. Steven Leadbetter: Conceptualization and design, formal analysis and interpretation of the data, writing–review and editing, and critical revision of the article for important intellectual content. Rebecca Glover-Kudon: Conceptualization and design, formal analysis and interpretation of the data, writing–review and editing, and critical revision of the article for important intellectual content. Lynn Butterly: Conceptualization and design, acquisition of the data, writing–original draft, writing–review and editing, resources, critical revision of the article for important intellectual content, and supervision.
References
- 1.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-Based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015. [Accessed July 6, 2016]. www.cdc.gov/uscs. [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypec-tomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;336:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force. [Accessed July 7, 2016];Final Recommendation Statement: Colorectal Cancer: Screening. 2008 Oct; http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ colorectal-cancer-screening.
- 5.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [Accessed January 27, 2016]. http://www.cdc.gov/brfss/index.html. [Google Scholar]
- 6.Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. [Accessed March 22, 2016];Healthy People 2020. https://www.healthypeople.gov.
- 7.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 9.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21:1614–1617. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 10.Griffith KA, Passmore SR, Smith D, Wenzel J. African Americans with a family history of colorectal cancer: barriers and facilitators to screening. Oncol Nurs Forum. 2012;39:299–306. doi: 10.1188/12.ONF.299-306. [DOI] [PubMed] [Google Scholar]
- 11.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20:989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasser KE, Ayanian JZ, Fletcher RH, Good MJ. Barriers to colorectal cancer screening in community health centers: a qualitative study. BMC Fam Pract. 2008;9:15. doi: 10.1186/1471-2296-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendren S, Chin N, Fisher S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011;103:701–710. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson CM, Cassells AN, Greene MA, Beach ML, Tobin JN, Dietrich AJ. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011;103:746–753. doi: 10.1016/s0027-9684(15)30414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN National Colorectal Cancer Roundtable Screening Among the 65 Plus Task Group. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Jones R, Woolf S, Cunningham T, et al. Patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38:508–516. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sly JR, Edwards T, Shelton RC, Jandorf L. Identifying barriers to colonoscopy screening for nonadherent African American participants in a patient navigation intervention. Health Educ Behav. 2013;40:449–457. doi: 10.1177/1090198112459514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 19.Honeycutt S, Green R, Ballard D, et al. Evaluation of a patient navigation program to promote colorectal cancer screening in rural Georgia, USA. Cancer. 2013;119:3059–3066. doi: 10.1002/cncr.28033. [DOI] [PubMed] [Google Scholar]
- 20.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 21.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandorf L, Guitierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Green BB, Anderson ML, Wang CY, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. J Am Board Fam Med. 2014;27:789–795. doi: 10.3122/jabfm.2014.06.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leone LA, Reuland DS, Lewis CL, et al. Reach, usage, and effectiveness of a Medicaid patient navigator intervention to increase colorectal cancer screening, Cape Fear, North Carolina, 2011. Prev Chronic Dis. 2013;10:E82. doi: 10.5888/pcd10.120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells KJ, Lee JH, Calcano ER, et al. A cluster randomized trial evaluating the efficacy of patient navigation in improving quality of diagnostic care for patients with breast or colorectal cancer abnormalities. Cancer Epidemiol Biomarkers Prev. 2012;21:1664–1672. doi: 10.1158/1055-9965.EPI-12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freund KM, Battaglia TA, Calhoun E, et al. Writing Group of the Patient Navigation Research Program. Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst. 2014;106:dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph DA, DeGroff AS, Hayes NS, Wong FL, Plescia M. The Colorectal Cancer Control Program: partnering to increase population level screening. Gastrointest Endosc. 2011;73:429–434. doi: 10.1016/j.gie.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR United States Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Teddlie C, Yu F. Mixed methods sampling: a typology with examples. J Mixed Methods Res. 2007;1:77–100. [Google Scholar]
- 32.Laerd Dissertation. [Accessed December 21, 2016];Total population sampling. http://dissertation.laerd.com/total-population-sampling.php.
- 33.Gressard L, DeGroff A, Glover-Kudon R, et al. A closer look at a highly effective patient navigation intervention for colorectal cancer screening. Poster session presented at: American Public Health Association 144th Annual Meeting and Exposition; October 29–November 2, 2016; Denver, CO. [Google Scholar]
- 34.Schoenfeld PS, Cohen J. Quality indicators for colorectal cancer screening for colonoscopy. Tech Gastrointest Endosc. 2013;15:59–68. doi: 10.1016/j.tgie.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson JC, Butterly LF. Colonoscopy: quality indicators. Clin Transl Gastroenterol. 2015;6:e77. doi: 10.1038/ctg.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. [Accessed January 30, 2017];New Hampshire Colorectal Cancer Screening Program patient navigation model. https://www.cdc.gov/cancer/crccp/pdf/nhcrcsp_pn_manual.pdf.
- 37.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkin EB, Shapiro E, Snow JG, Zauber AG, Krauskopf MS. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer. 2012;118:5982–5988. doi: 10.1002/cncr.27595. [DOI] [PubMed] [Google Scholar]
- 39.Berg B, Murr M, Chermak D, et al. Estimating the cost of no-shows and evaluating the effects of mitigation strategies. Med Decis Making. 2013;33:976–985. doi: 10.1177/0272989X13478194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazarian ES, Carreira FS, Toribara NW, Denberg TD. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol. 2008;6:438–442. doi: 10.1016/j.cgh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Rohan E, Boehm J, DeGroff A, Glover-Kudon R, Preissle J. Implementing the CDC’s Colorectal Cancer Screening Demonstration Program: wisdom from the field. Cancer. 2013;119(suppl 15):2870–2883. doi: 10.1002/cncr.28162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol. 2008;41:327–350. doi: 10.1007/s10464-008-9165-0. [DOI] [PubMed] [Google Scholar]
- 43.National Colorectal Cancer Roundtable. [Accessed February 9, 2017];Paying for colorectal cancer screening navigation toolkit strategies for payment and sustainability. http://nccrt.org/wp-content/uploads/v.22016_paying_pntoolkit_full_final.pdf.
- 44.Baron RC, Rimer BK, Breslow RA, et al. Task Force on Community Preventive Services. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med. 2008;35(suppl 1):S34–S55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Baron RC, Melillo S, Rimer BK, et al. Task Force on Community Preventive Services. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: a systematic review of provider reminders. Am J Prev Med. 2010;38:110–117. doi: 10.1016/j.amepre.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 46.Sabatino SA, Habarta N, Baron RC, et al. Task Force on Community Preventive Services. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35(suppl 1):S67–S74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC) Vital Signs: colorectal cancer screening test use-United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]