Summary
A decline in female reproduction is one of the earliest hallmarks of aging in many animals, including invertebrates and mammals [1–4]. The insulin/insulin-like growth factor-1 signaling (IIS) pathway has a conserved role in regulating longevity [5], and also controls reproductive aging [2,6]. Although IIS transcriptional targets that regulate somatic aging have been characterized [7,8], it was not known whether the same mechanisms influence reproductive aging. We previously showed that Caenorhabditis elegans daf-2 IIS receptor mutants extend reproductive span by maintaining oocyte quality with age [6], but IIS targets in oocytes had not been identified. Here, we compared the transcriptomes of aged daf-2(−) and wild-type oocytes, and distinguished IIS targets in oocytes from soma-specific targets. Remarkably, IIS appears to regulate reproductive and somatic aging through largely distinct mechanisms, although the binding motif for longevity factor PQM-1 [8] was also overrepresented in oocyte targets. Reduction of oocyte-specific IIS targets decreased reproductive span extension and oocyte viability of daf-2(−) worms, and pqm-1 is required for daf-2(−)’s long reproductive span. Cathepsin B-like gene expression and activity levels were reduced in aged daf-2(−) oocytes, and RNAi against cathepsin B-like W07B8.4 improved oocyte quality maintenance and extended reproductive span. Importantly, adult-only pharmacological inhibition of cathepsin B proteases reduced the age-dependent deterioration in oocyte quality, even when treatment was initiated in mid-reproduction. This suggests that it is possible to pharmacologically slow age-related reproductive decline through mid-life intervention. Oocyte-specific IIS target genes thereby revealed potential therapeutic targets for maintaining reproductive health with age.
Results and Discussion
Identification of oocyte-specific IIS transcriptional targets
The insulin/IGF-1-like signaling (IIS) pathway coordinates nutrient availability with energy homeostasis, in turn regulating lifespan and reproductive aging [2,5,6]. The extended longevity of C. elegans IIS receptor mutants (i.e., daf-2(−) worms) is dependent on transcriptional targets of the FoxO transcription factor DAF-16 [7,8], many of which are primarily expressed in the adult hypodermis and intestine [8–10]. However, developing oocytes are themselves transcriptionally active (before being silenced prior to fertilization), producing mRNAs for their own use and for embryonic development [11], and IIS targets in oocytes had not been identified. Reproductive decline in both humans and C. elegans is largely due to deterioration of oocyte quality [6,12]. Therefore, the identification of oocyte-specific IIS transcriptional targets may reveal mechanisms by which insulin/IGF-1-like signaling slows reproductive aging and maintains oocyte quality with age.
Like daf-2(−) mutation, daf-2 RNAi doubles the mated reproductive span (i.e., period of adulthood with reproductive competence) of wild-type [6] and fem-1(hc17) (self-spermless) worms (Figure 1A). To identify IIS targets that regulate oocyte quality maintenance with age, we isolated unfertilized oocytes from control- and daf-2(RNAi)-treated fem-1(hc17) worms on day 8 of adulthood, when mated daf-2(−) worms still produce viable progeny but mated wild-type worms produce predominantly low-quality oocytes [6]. 126 genes were significantly upregulated and 30 genes were significantly downregulated in aged daf-2(−) oocytes (FDR = 1%; Figures 1B,C, Data S1).
Figure 1. Characterization of daf-2(−) oocyte gene expression.
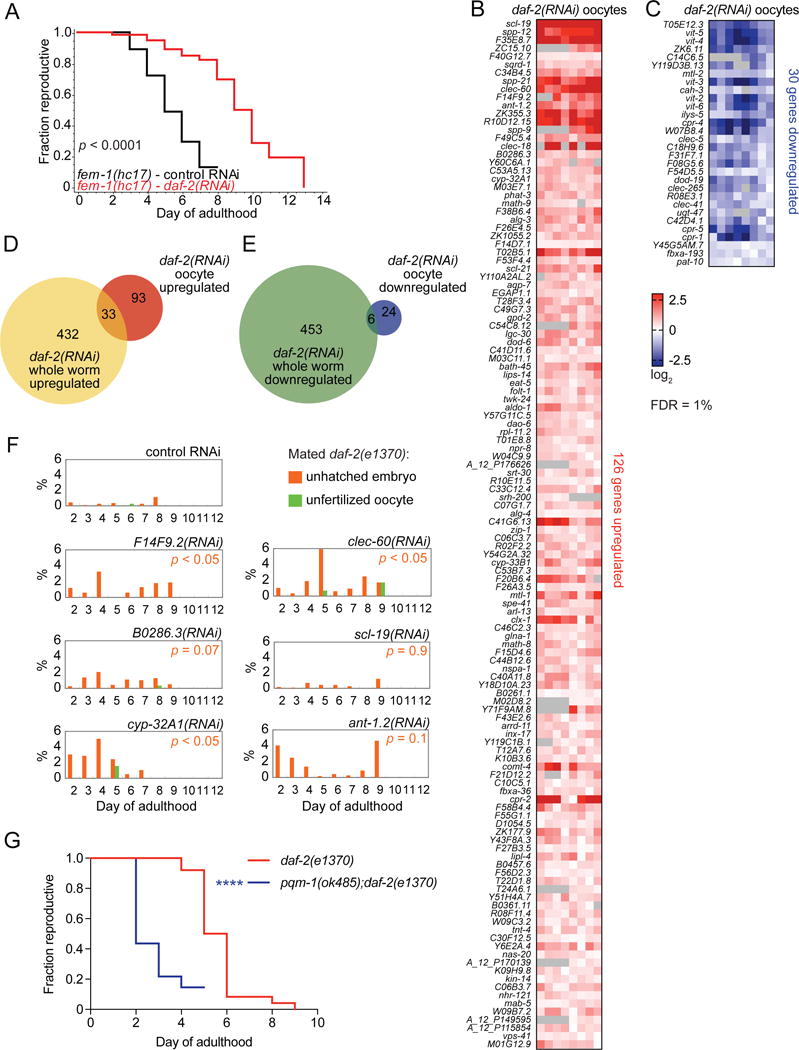
(A) daf-2 RNAi extends fem-1(hc17) mated reproductive span (n = 59–60, p < 0.0001). Eight independent expression comparisons of Day 8 daf-2(RNAi);fem-1(hc17) vs. fem-1(hc17) oocytes reveal 126 upregulated genes (B) and 30 downregulated genes (C); (FDR = 1%; ranked by significance (SAM)). See also Data S1. Overlap between oocyte-specific and whole worm gene sets that are (D) upregulated with daf-2 RNAi and (E) downregulated with daf-2 RNAi (FDR = 1%; see also Figures S1D,E, and Data S2). (F) Percentages unhatched embryos (orange) and unfertilized oocytes (green) produced daily from mated daf-2(e1370) mutant worms treated with RNAis against top-scoring genes upregulated in daf-2(RNAi) oocytes. Normal progeny are not displayed. P values: differences in unhatched embryo production with age between target gene RNAi-treated group vs. control RNAi-treated group. See also Figure S2. (G) Loss of pqm-1 decreases daf-2(e1370) self-fertilized reproductive span by 50% (n = 40, **** p < 0.0001).
Many daf-2(RNAi) oocyte-upregulated genes have known functions related to the germline and/or reproduction. For instance, scl-19/dct-4, the gene with the highest Significance Analysis of Microarrays (SAM) score, encodes an SCP-like extracellular protein that affects the formation of germline tumors [13]. DOD-6 (downstream of DAF-16) is predicted [14] to interact with IMA-3, an importin homolog essential for organizing the nuclear pore complex in the germline [15]. The metallothionein mtl-1 has a role in determining brood size [16], and the triacylglycerol lipase lipl-4 regulates lifespan extension of germline-less worms by activating autophagy [17,18]. These oocyte IIS targets are all canonical Class I IIS target genes (i.e., upregulated in daf-2(−), and downregulated in daf-16(−);daf-2(−) whole worms; [7,8]); additional Class I IIS targets detected in isolated oocytes included C-type lectin clec-18, adenine nucleotide translocase ant-1.2, and cytochrome P450 cyp-33B1. However, more than 70% of the genes upregulated in daf-2(RNAi) oocytes were not previously identified as IIS targets (Figure S1A). This class included two small RNA pathway Argonaute proteins (alg-3 and -4) involved in male gametogenesis [19], and folt-1 (folate transporter 1, orthologous to human SLC19A folate transporters), which impacts germline formation and oogenesis [20].
Many genes downregulated in daf-2(RNAi) oocytes encode proteins with functions in nutrient reservoir activity and lipid transport, as well as lysosomal and peptidase/proteolysis activity (Figure S1C). Vitellogenins (vit-2, vit-3, vit-4, vit-5, and vit-6) encode major yolk components and are strong Class II whole-worm IIS target genes (i.e., downregulated in daf-2(−), and upregulated in day 1 daf-16(−);daf-2(−) whole worms; [7]). Other canonical Class II IIS targets downregulated in daf-2(RNAi) oocytes include dod-19 and the metallothionein mtl-2. 30% of the genes significantly downregulated in daf-2(RNAi) oocytes were not previously identified as IIS transcriptional targets [8] (Figure S1A). Four of the 30 downregulated genes are known or predicted cathepsin B-like cysteine proteases (cpr-1, cpr-4, cpr-5 and W07B8.4), two of which (cpr-1, W07B8.4) were not previously identified as IIS targets [8].
Collectively, many genes that affect reproduction, germline formation and function, or oogenesis were regulated in the higher-quality aged oocytes of daf-2 RNAi-treated worms, including oocyte-specific targets that were not previously known to be IIS-regulated.
IIS has distinct target gene sets in oocytes versus somatic tissue
To determine the degree to which somatic IIS targets are similar to oocyte IIS targets, we compared the expression profiles of day 8 control- and daf-2(RNAi)-treated fem-1(hc17) whole worms (Figures S1D,E). We expected oocyte-level changes to account for some of the whole-worm differential expression (as whole worms contain oocytes), therefore we compared whole worm IIS target genes to isolated oocyte IIS targets. However, only 33 of the 465 genes that were upregulated (~7%) and 6 of the 459 genes that were downregulated (~1%) in daf-2(RNAi) whole worms were also significantly altered in isolated daf-2(RNAi) oocytes (Figures 1D,E, Data S2); that is, more than 95% of the IIS targets detected in day 8 whole worm assessments were soma-specific (Figures S1D,E). Pathways and functions associated with the oocyte-specific daf-2(RNAi)-regulated gene sets were largely distinct from those of the soma-specific sets (Figures S1B,C). The paucity of shared Gene Ontology (GO) terms suggests that daf-2(−)’s regulation of oocyte quality maintenance involves gene functions that are distinct from those that influence somatic aging.
IIS’s regulation of reproductive and somatic aging can be dissociated both temporally and spatially. daf-2(RNAi) treatment after early adulthood does not alter reproductive span, whereas IIS actively regulates lifespan into adulthood [6,21]. daf-2(−)-mediated reproductive span extension requires DAF-16 activity in the intestine and muscle but not in neurons [6], while lifespan extension of daf-2(−) worms requires DAF-16 in the intestine and hypodermis but not in muscle [9,22]. The soma-specific IIS target set is enriched for predicted hypodermal and/or intestinal genes [10], and depleted for neuronal and/or germline genes (Figure S1F), in agreement with previous work [8,9], suggesting that soma-specific IIS targets are more likely to impact somatic aging than reproductive aging.
Functional analyses of IIS oocyte transcriptional targets
To determine the contribution of IIS’s oocyte gene targets to oocyte quality maintenance, we performed functional analyses on a subset of top daf-2(RNAi) oocyte-upregulated targets, ranked by SAM score and fold-change. Reduction of daf-2(RNAi) oocyte-upregulated genes slc-19, cyp-32A1, ant-1.2, and B0286.3 reduced the mated reproductive span of daf-2(e1370) by approximately 10% each (Figure S2A), consistent with the 5–10% reduction of lifespan with loss of individual daf-2/daf-16 targets (Figure S2D; [7]). dod-6, a shared soma/oocyte IIS target gene, is required for both daf-2(e1370)’s longevity and extended reproductive span (p < 0.0001; Figures S2D,E). By contrast, life-long knockdown of soma-specific IIS targets gst-3 or sod-3, which are required for daf-2’s longevity (Figure S2D; [7]) but are not transcriptionally altered in daf-2(RNAi) oocytes, had no significant effect on daf-2(e1370)’s reproductive span (Figure S2E). IIS targets in the oocytes may therefore act cumulatively to impact reproductive aging.
Examining progeny viability further confirmed an effect of daf-2(RNAi) oocyte-upregulated targets on reproductive function. daf-2(−) worms normally produce healthy offspring until day 8 of adulthood [6], but RNAi-mediated knockdown of F14F9.2, clec-60, or cyp-32A1 significantly inhibited hatching of embryos produced by daf-2(e1370) worms (Figure 1F). These results suggest that at least a subset of the highly upregulated daf-2(RNAi) oocyte genes contribute to IIS’s regulation of oocyte quality maintenance with age.
The DAE-binding factor PQM-1 regulates reproductive aging
Previously, we found that IIS target gene promoters are enriched for two motifs: TGTTTAC, the DAF-16-binding element (DBE), and CTTATCA, the DAF-16-Associated Element (DAE) [7]. DNA motif analysis of the 1 kb upstream of soma-specific and oocyte-specific IIS targets revealed that the DBE and DAE were overrepresented in promoter regions of the soma-specific but not oocyte-specific daf-2(RNAi)-upregulated genes (Figure S1G). The DAE motif was overrepresented in the promoter regions of both the oocyte-specific and the soma-specific daf-2(RNAi) downregulated targets (Figure S1H), suggesting a regulatory role for this element in oocytes. The DAE motif is bound by the C2H2 Zn finger transcription factor PQM-1, a key component of daf-2 longevity [8]. We found that loss of pqm-1 also greatly reduced the reproductive span of daf-2 mutants (50%; p < 0.0001; Figure 1G), indicating that PQM-1 plays a major role in determining IIS’s effects on both somatic aging and reproductive aging.
Effects of cathepsin B-like cysteine proteases on reproductive aging
We were struck by the fact that four of the 30 IIS oocyte-specific downregulated genes (Figures 1C,2A; Data S1) encoded cathepsin B-like proteases, and lysosomal and proteolysis/peptidase activity were significantly enriched GO terms for oocyte-specific but not somatic daf-2(RNAi)-downregulated genes (Benjamini-Hochberg adjusted p values < 0.0016; Figure S1C). We noted that these genes also tended to be downregulated in day 8 high-quality TGF-β Sma/Mab(−) mutant oocytes, and in young day 3 fem-1(hc17) oocytes compared to day 8 fem-1(hc17) oocytes (Figure S3A); that is, cathepsin B proteases were downregulated in all high-quality oocyte conditions.
Figure 2. Cathepsin B-like cysteine proteases inhibit oocyte quality maintenance with age.
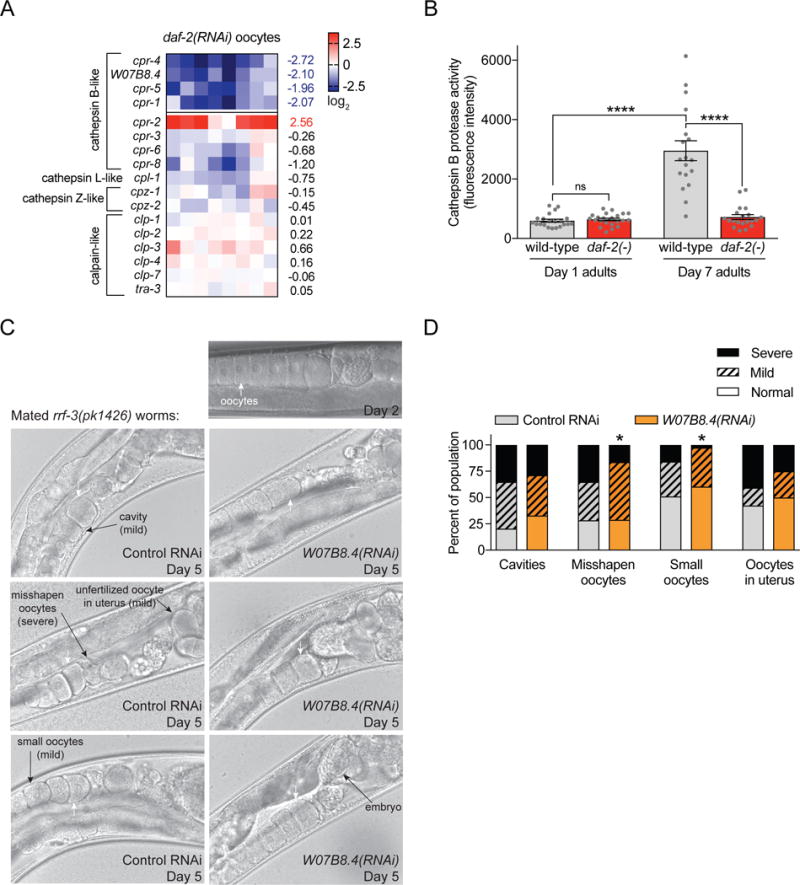
(A) Unlike other members of the clan CA of cysteine proteases, a group of cathepsin B-like genes are significantly downregulated in day 8 daf-2(RNAi);fem-1(hc17) oocytes (FDR = 1%; ranked by SAM significance score). (B) Cathepsin B protease activity (Magic Red Cathepsin B Kit) of the most mature unfertilized oocyte of day 1 and mated day 7 wild-type and daf-2(1370) (± SEM; n = 18–22 individuals; see Figure S3B for representative images). (C) Representative images and (D) scored oocyte morphology defects show significant improvements in oocyte quality for day 5 mated rrf-3(pk1426) worms with life-long feeding of W07B8.4(RNAi) vs. control (L4440 vector) RNAi (n = 73–88). For reference, a young (day 2) mated rrf-3(pk1426) worm on control RNAi is shown; no obvious differences were noted in the oocyte morphology of day 2 W07B8.4(RNAi) vs. control RNAi worms. * p ≤ 0.05.
Cathepsin B is a lysosomal cysteine protease involved in many mammalian physiological functions, including intra- and extra-cellular protein degradation and turnover, apoptosis, cleavage of extracellular matrix proteins, and key regulatory steps of cancer biology [23]. We found that while cathepsin B protease activity levels were low in the high-quality oocytes of young adult wild-type worms, by day 7 of adulthood, cathepsin B protease activity in the oocytes had more than tripled (Figures 2B, S3B). Importantly, day 7 daf-2(e1370) oocytes did not exhibit this age-dependent elevation in cathepsin B protease activity (Figures 2B, S3B), consistent with the lower expression of cathepsin B-like genes in high-quality aged daf-2(−) oocytes.
Deteriorating oocyte quality is a major cause of age-dependent reproductive decline [6,12]. An increase in cathepsin B gene expression and protease activity with age could either be due to 1) a protective response to age-related damage, or 2) the loss of suppression of a potentially deleterious activity. Directly reducing cathepsin B cysteine proteases should only improve oocyte quality with age in the latter case (i.e., if they are associated with a deleterious activity); loss of cathepsin B proteases in the former case should lead to increased oocyte damage. Therefore, we evaluated whether cathepsin B cysteine proteases affected oocyte quality with age by knocking down individual cathepsin B-like genes in adult rrf-3(pk1426) (RNAi-sensitive) worms. Adult-only RNAi-mediated knockdown of the predicted cathepsin B-like gene W07B8.4 increased reproductive span by ~10% (p < 0.05; Figures S3D). More strikingly, reduction of W07B8.4 significantly improved the morphology of aging oocytes, significantly reducing the frequency of irregularly shaped or abnormally small oocytes in day 5 mated rrf-3(pk1426) worms (Figures 2C,D). These results suggested a deleterious role for at least one cathepsin B-like gene in the regulation of reproductive aging, rather than an increase in cathepsin B protease activity due to an adaptive response to damage. Notably, any influences on reproductive aging appeared independent of somatic aging effects, as no change in lifespan was observed with adult-only knockdown of any of the individual cathepsin B-like genes (Figure S3F).
We also explored whether pharmacological treatment of aging animals could improve the quality of aged oocytes. MDL-28170, a potent cell-permeable cathepsin B inhibitor utilized previously in C. elegans [24,25], broadly suppresses all cathepsin B proteases. Treatment of adult wild-type worms with MDL-28170 significantly slowed the decline of oocyte quality with age, as demonstrated by reduced incidences of cavities between oocytes, misshapen oocytes, and abnormally small oocytes in day 5 mated worms exposed to MDL-28170 after day 1 of adulthood, compared to DMSO-exposed controls (Figures 3A,B).
Figure 3. Inhibition of cathepsin B-like cysteine proteases reduces age-dependent oocyte quality deterioration.
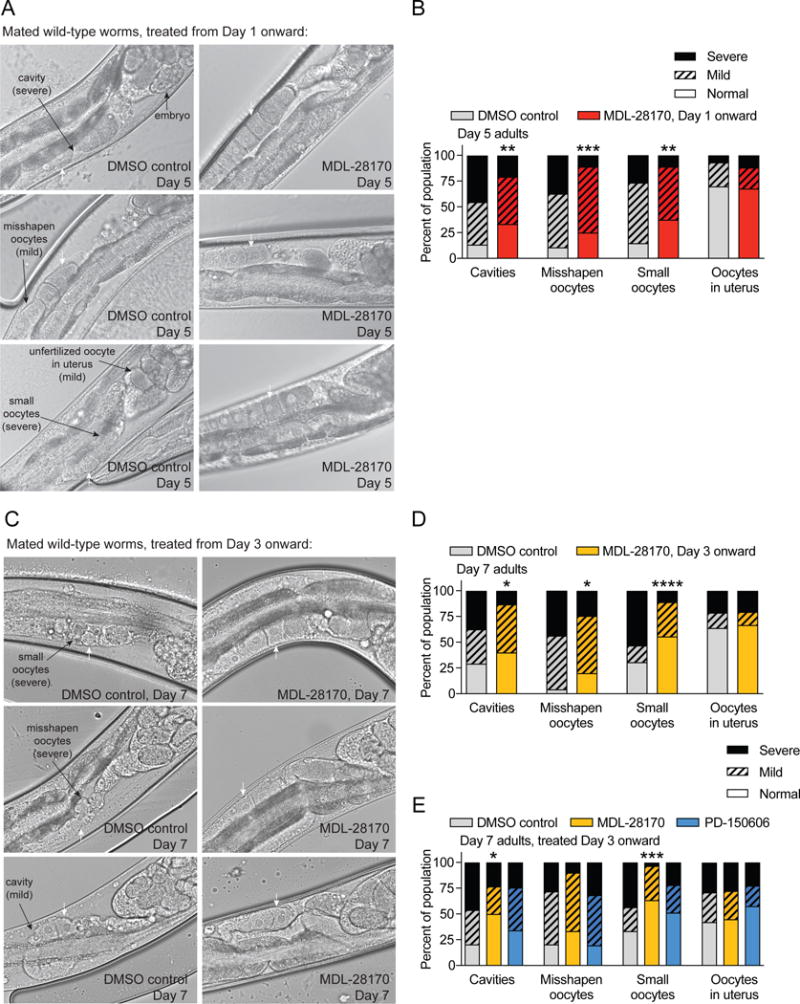
(A) Representative images and (B) oocyte morphology defect scoring show that MDL-28170 treatment after day 1 of adulthood significantly improves day 5 mated wild type oocyte quality, compared to DMSO-exposed controls (n = 72–75). (C) Representative images and (D) oocyte morphology defect scoring show that MDL-28170 after day 3 of adulthood significantly improves oocyte quality of day 7 mated wild-type worms, compared to DMSO-exposed controls (n = 45–49). White arrows indicate oocytes. (E) Oocyte morphology scoring of Day 7 mated wild-type worms treated from day 3 of adulthood onward with DMSO control (n = 39), the cathepsin B + calpain inhibitor MDL-28170 (n = 30), or the calpain inhibitor PD-150606 (n = 41) indicates that MDL-28170 treatment in mid-reproduction significantly improves oocyte quality, unlike PD-150606. * p ≤ 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 in comparison with DMSO control group.
Remarkably, initiating MDL-28170 treatment on day 3 of adulthood – an age at which oocyte quality and reproductive competence of wild-type worms has already begun to decline (e.g., Figure 1A) – still improved the quality of oocytes in aging worms (Figures 3C,D). This was particularly evident in the reduced incidence of severely small oocytes in day 7 mated worms treated with MDL-28170 from day 3 onward (Figures 3C,D).
MDL-28170 inhibits both cathepsin B and calpain cysteine proteases, although only the former were downregulated in high-quality daf-2(RNAi) oocytes (Figure 2A). To distinguish between these classes of proteases, we also evaluated the effects of a calpain-specific inhibitor, PD-150606 [26]; we found that this calpain inhibitor did not significantly affect oocyte morphology maintenance (Figure 3E), suggesting that cathepsin B inhibition is responsible for the improved oocyte quality we observed upon MDL-28170 treatment.
Together, our results show that inhibition of cathepsin B cysteine proteases significantly reduces age-dependent deterioration of oocyte quality. Moreover, significant improvements to oocyte quality were evident even when cathepsin B proteases were not inhibited until partway through the reproductive period, suggesting the potential for late-life interventions to slow reproductive aging.
Conclusions
In humans, the dramatic increase in rates of infertility, birth defects, and miscarriages occurs more than a decade before the exhaustion of oocyte supply (menopause), indicating that decreasing oocyte quality, rather than depletion, is the major contributing factor for reproductive aging [1,12]. Reproductive decline in C. elegans is also caused by decreasing oocyte quality [2–4,6], and there appears to be a high degree of evolutionary conservation – spanning worms, mice, and humans – of molecular processes that regulate oocyte quality with age [2,6,27]. Here, we have uncovered a set of oocyte-specific IIS transcriptional targets that are largely distinct from soma-specific IIS targets, indicating that IIS regulates reproductive and somatic aging through distinct molecular mechanisms. We confirmed that a number of oocyte-specific IIS target genes regulate the maintenance of oocyte viability with age. While the roles of IIS in reproductive and somatic maintenance are likely coordinated, reproductive status decisions can be dissociated from lifespan regulation [28], and a separate oocyte-specific IIS transcriptome could be one mechanism to fine tune control of reproduction under stressful or nutrient-poor conditions. Distinct oocyte- and soma-specific IIS targets may also illustrate differences in regulating replicative versus non-replicative cells, as the only cells in adult C. elegans with the capacity for proliferation are germ cells.
We identified a set of cysteine proteases whose inhibition during adulthood slows the decline of oocyte quality with age, indicating that cathepsin B proteases might affect the renewal and maintenance of oocyte competence with age. Intriguingly, the age-dependent increase in cathepsin B gene expression and protease activity is not likely to be in response to damage, but rather a deleterious cause of oocyte aging, since cathepsin B inhibition preserves oocyte morphology with age. Suppressing a rise in cathepsin B protease activity in oocytes could be a mechanism by which aging daf-2(−) worms maintain oocyte quality. Interestingly, elevated cathepsin B mRNA and activity levels in cumulus cells are also associated with compromised developmental competence of bovine oocytes [29,30], suggesting that increased cathepsin B levels are an evolutionarily conserved process associated with decreased oocyte quality.
Our results demonstrate that it is possible to use a pharmacological agent to slow reproductive aging. Importantly, the success of cathepsin B inhibitor treatment of “reproductively aged” mothers suggests that this could be a viable approach for late-life interventions to slow age-related reproductive decline in mammals. Our study provides insights into fundamental mechanisms of oocyte quality control, and suggests that a transcriptomic approach could uncover further mechanisms to extend reproductive span, facilitating the development of therapies to address the issue of maternal reproductive decline with age.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Coleen Murphy (ctmurphy@princeton.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
C. elegans genetics
All strains were maintained at 20°C on plates made from standard nematode growth medium (NGM: 3 g/L NaCl, 2.5 g/L Bacto-peptone, 17 g/L Bacto-agar in distilled water, with 1mL/L cholesterol (5 mg/mL in ethanol), 1 mL/L 1M CaCl2, 1 mL/L 1M MgSO4, and 25 mL/L 1M potassium phosphate buffer (pH 6.0) added to molten agar after autoclaving; [31]) or high growth medium (HGM: NGM recipe modified as follows: 20 g/L Bacto-peptone, 30 g/L Bacto-agar, and 4mL/L cholesterol (5 mg/mL in ethanol); all other components same as NGM), with OP50 E. coli as the food source. Experiments that did not involve RNAi treatments were performed using NGM plates seeded with OP50 E. coli for ad libitum feeding [31]; for RNAi experiments, the standard NGM molten agar was supplemented with 1 mL/L 1M IPTG (isopropyl β-d-1-thiogalactopyranoside) and 1mL/L 100mg/mL carbenicillin, and plates were seeded with HT115 E. coli or OP50(xu3363) E. coli for ad libitum feeding. Hypochlorite-synchronization to developmentally synchronize experimental animals was performed by collecting eggs from gravid hermaphrodites via exposure to an alkaline-bleach solution (e.g., 5.5 mL water, 1.5 mL 5N KOH, 3.0 mL sodium hypochlorite), followed by repeated washing of collected eggs in M9 buffer (6 g/L Na2HPO4, 3 g/L KH2PO4, 5 g/L NaCl and 1 mL/L 1M MgSO4 in distilled water; [31]).
The following strains were used in this study: the N2 Bristol strain as wild-type worms, rrf-3(pk1426), pqm-1(ok485);daf-2(e1370), daf-2(e1370), fem-1(hc17), fog-2(q71), and W07B8.4(ok2537). RNAi clones were obtained from the Ahringer RNAi library or were a gift from the Kenyon lab [21], and in the case of cpr-1, -4, -5, and W07B8.4 RNAis (and an L4440 vector control), RNAi plasmids were transformed into an OP50(xu3363) E. coli background before use [32].
METHOD DETAILS
Oocyte and whole-worm selection for microarrays
On day 8 of adulthood, collections were made of whole worms or isolated oocytes (extracted by cutting worms in egg salts buffer, followed by repeated nylon net filtration/centrifugation; [33]) from hypochlorite-synchronized fem-1;control(RNAi) and fem-1;daf-2(RNAi) animals. RNA was extracted and purified using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA), cRNA was linearly amplified and Cy3/Cy5 labeled (Agilent, Santa Clara, CA, USA), and hybridized to Agilent 44k C. elegans microarrays, as previously performed [34]. In all cases, RNA used for hybridizing onto individual arrays was derived from biological replicates.
Microarray analyses
Data were filtered for quality (PUMAdb; http://puma.princeton.edu), and Cluster 3.0 was used for hierarchical clustering [35]. One-class SAM analyses (FDR = 1%) were performed in RStudio to identify day 8 daf-2(RNAi) oocyte targets and day 8 daf-2(RNAi) whole-worm targets [36]. Genes were submitted to DAVID 6.8 (https://david.ncifcrf.gov/) for Gene Ontology analysis [37]. Visualization of overlap between gene lists was generated using BioVenn (http://www.biovenn.nl/) [38].
Tissue enrichment analyses
Gene sets were submitted to http://worm-tissue.princeton.edu/ to determine tissue enrichment scores [10].
Motif analyses
1 kb of 5′ upstream sequences were retrieved and submitted to oligo-analysis using Regulatory Sequence Analysis Tools (RSAT) [39] to identify overrepresented sequences.
Reproductive span and lifespan assays
As previously described [4], self-fertilized or mated reproductive spans were performed by moving individually plated hermaphrodites to fresh plates daily until progeny production had ceased for two subsequent days; the last day of viable progeny production preceding two days without progeny was designated as the day of reproductive cessation (assessed by evaluating plates for progeny 1–2 days after the hermaphrodites had been moved off plates). Synchronized hermaphrodites were individually plated as L4 larvae for self-fertilized reproductive span assays, or mated to young (day 1) wild-type or fog-2(q71) males at a 1:3 ratio for 24 h before being individually plated, with successful mating ascertained by evaluating the fraction of male progeny produced. Animals were censored on the day of matricide.
Lifespan assays were carried out in accordance with previously described protocols [4]. In brief, synchronized hermaphrodites (~10 per plate) were transferred away from progeny to fresh plates every 1–2 days, until the end of their reproductive period. Worms were designated as alive or dead based on their responsiveness to touch stimuli, with incidences of matricide, aberrant vulval structures, or lost/dehydrated worms leading to censoring on the day of the event. The first day of adulthood was defined as t = 0 for the standard Kaplan-Meier log-rank survival tests.
Progeny viability assay
As previously described [6], synchronized hermaphrodites were transferred to fresh plates every day, and each individual’s daily production of embryos that failed to hatch or oocytes that were unfertilized (versus healthy, hatched embryos) was counted.
Magic Red cathepsin B activity assay
The Magic Red Cathepsin B Kit (Immunochemistry Technologies, Bloomington, MN, USA) was used, based on manufacturers’ instructions and previous descriptions of use in C. elegans experiments [40]. In brief, Magic Red reagent dye dissolved in DMSO and further diluted with water was spread over 3 mL NGM plates previously seeded with OP50 E. coli, giving a final 1X concentration of the dye. Once the liquid had dried, hypochlorite-synchronized L4 larvae or mated day 6 adults (previously mated at a 1:2 ratio with fog-2(q71) males from the L4 stage to day 1 of adulthood) were placed on the plates and incubated at 20°C overnight in th e dark, then cleared of residual dye by being placed on fresh plates seeded with OP50 E. coli for 35 minutes prior to imaging. The Magic Red reagent contains a target sequence peptide that is cleaved by cathepsin B to produce a red fluorescent product; red fluorescence intensity was measured using Nikon Elements Advanced Research software.
Oocyte morphology experiments
For all oocyte morphology experiments, hypochlorite-synchronized hermaphrodites were placed on plates at a 1:2 ratio with fog-2(q71) males for at least 24 h (from the L4 stage to day 1 of adulthood) for mating, and then maintained on plates without males or accumulating progeny from day 1 onward. For cathepsin B and/or calpain inhibition experiments, wild-type hermaphrodites were exposed to MDL-28170, PD-150606, or the DMSO control as either mated day 1 adults or, in the delayed treatment experiments, as mated day 3 adults. Individually plated mated hermaphrodites were exposed to a final concentration of 20 μM MDL-28170 (Sigma-Aldrich, St. Louis, MO, USA) [25], 20 μM PD-150606 (Sigma-Aldrich) [26], or Dimethylsulfoxide (DMSO; ATCC: The Global Bioresearch Center, Manassas, Virginia, USA) as the control. MDL-28170 and PD-150606 were both dissolved in DMSO to a concentration of 65.4 mM, and immediately before use, each stock mixture (or the same volume of DMSO alone) was diluted in water to 0.04 mM; 100 μL was spread over each 4 mL NGM plate, and plates were seeded with OP50 E. coli after the liquid had dried. Worms were transferred to these drug-treated plates within 24 h, and were transferred to freshly treated plates every 24 or 48 h until oocyte images were taken.
For scoring oocyte images, the image order was randomized and the scorer was blinded to the treatment represented in each image while assigning a score for each of the signs of deterioration according to the severity of the phenotype. Images were scored based on criteria described in [6]; in brief, a score of either normal, mild, or severe was assigned for each category, based on the severity of the phenotype. With respect to the ‘cavities’ phenotype, normal indicated no cavities, mild indicated some loss of contact between oocytes, and severe indicated the presence of large splits between oocytes; with respect to the ‘misshapen oocytes’ category, normal indicated that all oocytes were similarly shaped (generally cuboidal), mild indicated some slight irregularities, and severe indicated one or more oocytes appearing damaged or very irregularly shaped; with respect to the ‘small oocytes’ category, normal indicated no small oocytes, mild indicated that the oocytes did not tend to fill the given space between the body wall and germline arm, and severe indicated incidences of very tiny oocytes; with respect to the ‘oocytes in uterus’ category, normal indicated no recognizable unfertilized oocytes in the uterus (only embryos), while mild indicated at least one recognizable unfertilized oocyte in the uterus, and severe indicated the presence of either multiple individual unfertilized oocytes or a clump of them in the uterus.
QUANTIFICATION AND STATISTICAL ANALYSIS
Lifespan and reproductive span assays were assessed using Kaplan-Meier log rank tests. The Friedman test (with Dunn’s test for multiple comparisons) was used to compare the target gene RNAi-treated group versus the control RNAi-treated group with respect to the production of either unhatched embryos or unfertilized oocytes over time. To compare red fluorescence levels in oocytes for cathepsin B protease activity experiments, 2-way ANOVAs were used for evaluating effects of age (day 1 versus day 7) and genotype (wild-type versus daf-2(e1370)), with a significant interaction between factors (p < 0.0001) leading to the performance of Bonferroni post-hoc comparisons to determine differences between individual groups. For oocyte quality experiments, chi square analyses were used to determine whether there were significant differences between populations for each category of scored oocyte phenotypes. Experiments were repeated on separate days with separate populations, to confirm that results were reproducible. Prism 7 software was used for all statistical analyses; software and further statistical details used for microarray analyses are described in the method details section of the STAR methods. Additional statistical details of experiments, including sample size (with n representing the number of worms), can be found in the figure legends.
DATA AND SOFTWARE AVAILABILITY
Raw microarray data set is publically available through PUMAdb (http://puma.princeton.edu).
Supplementary Material
Data S1: Significantly (A) upregulated and (B) downregulated genes in isolated oocytes of daf-2(RNAi);fem-1(hc17) vs. fem-1(hc17) on control RNAi (FDR = 1%).
Related to Figure 1.
Data S2: Shared genes that are significantly (A) upregulated or (B) downregulated in both oocytes and whole worms (FDR = 1% for both) in a comparison of daf-2(RNAi);fem-1(hc17) vs. fem-1(hc17) on control RNAi.
Related to Figure 1.
Acknowledgments
We thank the Caenorhabditis Genetics Center (CGC) for strains, members of the Murphy Lab for comments on the manuscript, and Jessica Landis for discussion on microarray data analyses. NMT was supported by a Banting Postdoctoral Fellowship. The study was supported by the Glenn Foundation for Medical Research, and NIH New Innovator (1DP2OD004402-01) and March of Dimes awards to CTM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
SL, NMT, RK, and CTM planned experiments; SL, NMT, RK, and CS performed experiments and analyzed data, with assistance from JA and WK; NMT, SL, and CTM wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Armstrong DT. Effects of maternal age on oocyte developmental competence. Theriogenology. 2001;55:1303–22. doi: 10.1016/s0093-691x(01)00484-8. [DOI] [PubMed] [Google Scholar]
- 2.Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4:e1000295. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo S, Shaw WM, Ashraf J, Murphy CT. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009;5:e1000789. doi: 10.1371/journal.pgen.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 6.Luo S, Kleemann GA, Ashraf JM, Shaw WM, Murphy CT. TGF-β and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell. 2010;143:299–312. doi: 10.1016/j.cell.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–83. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 8.Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell. 2013;154:676–690. doi: 10.1016/j.cell.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Judy M, Lee SJ, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikina MD, Huttenhower C, Murphy CT, Troyanskaya OG. Global prediction of tissue-specific gene expression and context-dependent gene networks in Caenorhabditis elegans. PLoS Comput Biol. 2009;5:e1000417. doi: 10.1371/journal.pcbi.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker AK, Boag PR, Blackwell TK. Transcription reactivation steps stimulated by oocyte maturation in C. elegans. Dev Biol. 2007;304:382–93. doi: 10.1016/j.ydbio.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–54. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 13.Pinkston-Gosse J, Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat Genet. 2007;39:1403–9. doi: 10.1038/ng.2007.1. [DOI] [PubMed] [Google Scholar]
- 14.Wong AK, Park CY, Greene CS, Bongo LA, Guan Y, Troyanskaya OG. IMP: a multi-species functional genomics portal for integration, visualization and prediction of protein functions and networks. Nucleic Acids Res. 2012;40:W484–90. doi: 10.1093/nar/gks458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geles KG, Adam SA. Germline and developmental roles of the nuclear transport factor importin alpha3 in C. elegans. Development. 2001;128:1817–30. doi: 10.1242/dev.128.10.1817. [DOI] [PubMed] [Google Scholar]
- 16.Hughes S, Stürzenbaum SR. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits. Environ Pollut. 2007;145:395–400. doi: 10.1016/j.envpol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Lapierre LR, Gelino S, Meléndez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–14. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–40. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2010;107:3588–93. doi: 10.1073/pnas.0911685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin MU, Liau WS, Balamurugan K, Ashokkumar B, Said HM, LaMunyon CW. Knockout of the folate transporter folt-1 causes germline and somatic defects in C. elegans. BMC Dev Biol. 2010;10:46. doi: 10.1186/1471-213X-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–4. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 22.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 23.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syntichaki P, Xu K, Driscoll M, Tavernarakis N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 2002;419:939–44. doi: 10.1038/nature01108. [DOI] [PubMed] [Google Scholar]
- 25.Aggad D, Vérièpe J, Tauffenberger A, Parker JA. TDP-43 toxicity proceeds via calcium dysregulation and necrosis in aging Caenorhabditis elegans motor neurons. J Neurosci. 2014;34:12093–103. doi: 10.1523/JNEUROSCI.2495-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng L, Zhang A, Jin Y, Yan D. Regulation of neuronal axon specification by glia-neuron gap junctions in C. elegans. Elife. 2016;5:e19510. doi: 10.7554/eLife.19510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–8. doi: 10.1016/s1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 28.Antebi A. Regulation of longevity by the reproductive system. Exp Gerontol. 2013;48:596–602. doi: 10.1016/j.exger.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettegowda A, Patel OV, Lee KB, Park KE, Salem M, Yao J, Ireland JJ, Smith GW. Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: functional and diagnostic implications. Biol Reprod. 2008;79:301–9. doi: 10.1095/biolreprod.107.067223. [DOI] [PubMed] [Google Scholar]
- 30.Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol Reprod Dev. 2010;77:439–48. doi: 10.1002/mrd.21164. [DOI] [PubMed] [Google Scholar]
- 31.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao R, Chun L, Ronan EA, Friedman DI, Liu J, Xu XZS. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 2015;11:1123–33. doi: 10.1016/j.celrep.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MA. Sperm and oocyte isolation methods for biochemical and proteomic analysis. Methods Mol Biol. 2006;351:193–201. doi: 10.1385/1-59745-151-7:193. [DOI] [PubMed] [Google Scholar]
- 34.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–45. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Hoon MJL, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–4. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 36.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina-Rivera A, Defrance M, Sand O, Herrmann C, Castro-Mondragon JA, Delerce J, Jaeger S, Blanchet C, Vincens P, Caron C, et al. RSAT 2015: Regulatory Sequence Analysis Tools. Nucleic Acids Res. 2015;43:W50–6. doi: 10.1093/nar/gkv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keith SA, Maddux SK, Zhong Y, Chinchankar MN, Ferguson AA, Ghazi A, Fisher AL. Graded proteasome dysfunction in Caenorhabditis elegans activates an adaptive response involving the conserved SKN-1 and ELT-2 transcription factors and the autophagy-lysosome pathway. PLOS Genet. 2016;12:e1005823. doi: 10.1371/journal.pgen.1005823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–12. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Significantly (A) upregulated and (B) downregulated genes in isolated oocytes of daf-2(RNAi);fem-1(hc17) vs. fem-1(hc17) on control RNAi (FDR = 1%).
Related to Figure 1.
Data S2: Shared genes that are significantly (A) upregulated or (B) downregulated in both oocytes and whole worms (FDR = 1% for both) in a comparison of daf-2(RNAi);fem-1(hc17) vs. fem-1(hc17) on control RNAi.
Related to Figure 1.


