Abstract
The composition of the diet (what we eat) has been widely related to the microbiota profile. However, whether the timing of food consumption (when we eat) influences microbiota in humans is unknown. A randomized, crossover study was performed in 10 healthy normal-weight young women to test the effect of the timing of food intake on the human microbiota in the saliva and fecal samples. More specifically, to determine whether eating late alters daily rhythms of human salivary microbiota, we interrogated salivary microbiota in samples obtained at 4 specific time points over 24 h, to achieve a better understanding of the relationship between food timing and metabolic alterations in humans. Results revealed significant diurnal rhythms in salivary diversity and bacterial relative abundance (i.e., TM7 and Fusobacteria) across both early and late eating conditions. More importantly, meal timing affected diurnal rhythms in diversity of salivary microbiota toward an inverted rhythm between the eating conditions, and eating late increased the number of putative proinflammatory taxa, showing a diurnal rhythm in the saliva. In a randomized, crossover study, we showed for the first time the impact of the timing of food intake on human salivary microbiota. Eating the main meal late inverts the daily rhythm of salivary microbiota diversity which may have a deleterious effect on the metabolism of the host.—Collado, M. C., Engen, P. A., Bandín, C., Cabrera-Rubio, R., Voigt, R. M., Green, S. J., Naqib, A., Keshavarzian, A., Scheer, F. A. J. L., Garaulet, M. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study.
Keywords: feces, eating time, diurnal rhythm, alpha diversity, beta diversity
Recent studies suggest that not only what we eat, but also when we eat may have a significant role in obesity treatment and metabolic alterations (1–6). Unusual feeding times can induce a disruption of the circadian system, which may lead to metabolic dysfunction (7–11). For example, in a longitudinal study of an overweight and obese Mediterranean population, our group demonstrated that those who ate lunch later in the day (main meal for this population) lost significantly less weight than those who ate lunch early, although early eaters (EEs) and late eaters (LEs) showed similar food intake, physical activity, sleep duration, and appetite-related hormone levels (2). Moreover, LEs had significantly higher insulin resistance. In the same line, a randomized, crossover study demonstrated that meal timing affects glucose tolerance, suggesting that eating late increases metabolic risk, even in healthy women (7).
The importance of caloric distribution across the day in weight loss effectiveness was supported by a 12-wk experimental study showing that participants assigned to high caloric intake during breakfast lost significantly more weight than those assigned to high caloric intake during dinner (12). Moreover, fasting glucose, insulin, and the insulin resistance index (homeostatic model assessment-insulin resistance) decreased significantly more in the large-breakfast group. These results suggest that eating late may negatively impact metabolic regulation and thus affect obesity and impair the success of weight loss therapies. The mechanism underlying this LE–induced metabolic syndrome and dysregulated metabolism is not fully understood. Several factors could be involved in the effect of food intake timing on metabolic alterations, such as substrate oxidation and circadian-related variables (7), as well as differences in diet-induced thermogenesis (13). However, other physiologic changes that contribute to obesity and metabolic syndrome may be implicated in the changes induced by the timing of food intake.
Recent evidence from animal and human studies indicates that the composition and function of the gut microbiota may be involved in obesity, weight loss, and metabolic alterations (14–17). Moreover, studies performed in extremely obese subjects have demonstrated that weight loss improves the obesity-associated gut microbiota composition toward a lean microbiome phenotype (18).
The salivary microbiota composition and diversity have also been linked to obesity, and metabolic alterations (19, 20), and a positive association between obesity and bacterial cellular abundance in oral subgingival biofilms has been found in adolescents (21). More important, recent results suggest that the oral cavity serves as a reservoir for potential intestinal pathobionts that can exacerbate intestinal disease or other inflammatory diseases (22). Indeed, ectopic colonization of oral bacteria in the intestine drives T helper 1 cell induction and inflammation (22).
The impact of timing of food intake on microbiota in humans has not been investigated. However, studies in rodents on feeding patterns (23), in equines on feeding frequency (24) and in humans on daily energy intake distribution and gut microbial abundances (25) have provided compelling evidence to support the hypothesis that several aspects related to the time of eating can affect microbiota and could be one of the mechanisms for late-eating–induced metabolic dysfunction.
It has been shown that high-fat-diet–induced obesity dampens the rhythmic changes in the gut microbiota of mice. This blunted rhythm is partially restored by time-restricted feeding, in which feeding was restricted to the night (their active phase) (23, 26). Furthermore, it has been proposed that the changes in gut microbiota related to time-restricted feeding influence host metabolism and protect against obesity and metabolic diseases (23, 26–28). Thus, feeding pattern and time of food intake, in addition to dietary composition, are important parameters when assessing the microbiota’s contribution to the metabolism of the mouse. However, to our knowledge no interventional studies have been published in humans showing the impact of changes in the timing of food intake on digestive tract microbiota.
The purpose of our study was to fill this gap in knowledge by determining the effect of the timing of food intake on human digestive tract microbiota (saliva and feces). More specifically, to test whether eating late alters daily rhythms of the human salivary microbiota, we conducted a randomized, crossover interventional study in healthy nonobese participants (National Library of Medicine, Bethesda, MD, USA; NCT03147703; https://clinicaltrials.gov/).
MATERIAL AND METHODS
Study participants
The study was performed in 10 healthy young women (25.6 ± 4.1 yr) of normal weight [body mass index (BMI) 22.5 ± 2.7 kg/m2]. Eighteen volunteers were initially contacted during September 2015, and those who fulfilled the established criteria (n = 10) (7), such as healthy women with no endocrine, renal, hepatic, or psychiatric disorders, and who were not using prescribed drugs or pharmacologic treatment other than oral contraceptives, were included in the study. Every participant completed both food timing interventions. General characteristics of the study sample are presented in Table 1. All research methods and procedures were performed in accordance with the Declaration of Helsinki for human studies and were approved by the Ethics Committee of the University of Murcia. All participants provided written informed consent.
TABLE 1.
General characteristics of the population
| Characteristic | Means ± sd |
|---|---|
| Age (yr) | 25.6 ± 4.1 |
| Weight (kg) | 60.9 ± 8.9 |
| Height (m) | 1.65 ± 0.62 |
| BMI (kg/m2) | 22.5 ± 2.7 |
| Body fat (%) | 24.8 ± 6.6 |
| Hip circumference (cm) | 96.9 ± 6.5 |
| Waist circumference (cm) | 77.8 ± 9.5 |
| Habitual dietary intake | |
| Total energy intake (kcal/d) | 1952 ± 308 |
| Carbohydrates (g) | 205 ± 48 |
| Fats (g) | 94 ± 21 |
| Proteins (g) | 81 ± 14 |
| Carbohydrates (%) | 41 ± 5 |
| Fats (%) | 43 ± 7 |
| Proteins (%) | 16 ± 2 |
| Habitual meal timing | |
| Breakfast | 0859 h ± 0038 h |
| Lunch | 1433 h ± 0030 h |
| Dinner | 2131 h ± 0019 h |
| Sleep characteristics | |
| Bed time | 0034 h ± 0044 h |
| Wake time | 0822 h ± 0037 h |
| Sleep duration | 0748 h ± 0031 h |
Women (n = 10). WHR, waist–hip ratio.
Experimental protocol
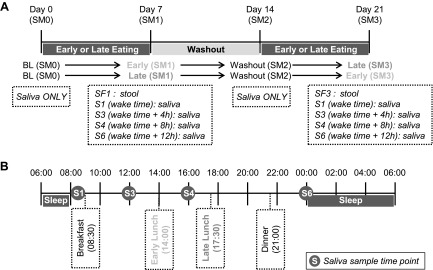
The study had a randomized, crossover design (Fig. 1). Each participant was studied under 2 food timing conditions: in the early eating condition (EE) lunch was eaten at 2:00 pm, and in the late eating condition (LE), lunch was eaten at 5:30 pm. Therefore, the 1600 h sample was taken ∼2 h after the main meal in the EE condition and ∼7 h after the last meal (breakfast), in randomized order, with a 1 wk washout period between visits, during which they consumed lunch at their habitual time (means ± sd; 1433 ± 0030 h; Table 1). Randomization was performed by the University of Murcia staff with block size of 2 in a balanced design by computer-executed software (http://www.randomization.com).
Figure 1.
The study protocol. A) An overall longitudinal view of the randomized, crossover trial in which each participant (n = 10) was studied under 2 food timing conditions: EE (meal at 2:00 pm) and LE (meal at 5:30 pm) during 1 wk for each condition. B) A summary of what occurred in the last day of intervention in each condition (meal times, saliva samples, sleep hours). Sampling was based on each participant’s usual awakening time. SM = week (SM0: baseline week; SM1: intervention wk 1; SM2: washout wk 2, and SM3: intervention wk 3).
All participants were given the same controlled diet with 3 standardized meals (breakfast, lunch, and dinner) during these two 7-d experimental periods, to provide sufficient energy for body-weight maintenance, which was calculated based on their habitual dietary intake derived from a 7-d record before enrollment in the study. We verified that the habitual dietary intake for each participant was within the European recommendations for women, at their age and BMI (29). The mean daily energy intake during the 2 intervention weeks was 1868 ± 234 kcal/d (50% of energy was supplied by carbohydrate, 35% by fat, and 15% by protein), fiber intake was of 30 ± 4 g without significant differences between the 2 experimental weeks. During these 2 wk, the distribution of energy during the day was 26% for breakfast; 47% for lunch, and 27% for dinner. The day of the saliva sampling, energy intake was 1858 ± 162 kcal/d (50% of energy was supplied by carbohydrate, 35% by fat, and 15% by protein), fiber intake was 20 ± 2 g. Total energy intake and macronutrient composition were determined with the Grunumur (v.2.0; University of Murcia, Murcia, Spain) (30), a nutrition evaluation program, in conjunction with Spanish food composition tables (31) (Supplemental Table 1). The timing of food intake was fixed during the whole intervention and was at 8:30 am for breakfast, and 9:00 pm for dinner, participants were not allowed to eat between meals and were instructed to follow their habitual sleep patterns, which were required to be the same during both meal timing intervention weeks.
Anthropometric measurements
Body weight was determined in barefooted participants wearing light clothes, with a digital scale accurate to the nearest 0.1 kg. Height was measured with a digital stadiometer (rank, 0.7–2.05) (Harpenden; Holtain, Crymych, Wales, United Kingdom). The subject was positioned upright, relaxed, and with the head in the Frankfurt plane. Height and weight measurements were obtained at the same time of day in the morning.
Total body fat was measured by bioelectrical impedance, using Tanita TBF-300 equipment (Tanita Corp. of America, Arlington Heights, IL, USA). Body fat distribution was assessed by the measurement of several circumferences and skinfolds in the upright position: waist circumference, at the level of the umbilicus, and hip circumference, and the widest circumference over the greater trochanters.
Sleep measurements
Participants were instructed to keep a sleep diary designed by the Murcia University Chronobiology Laboratory (32). The following data were obtained for every subject on a daily basis: time to bed, time of lights off, nocturnal awakenings lasting more than 10 min, sleep offset, and the time the subject got up. Sleep duration was calculated as the difference between lights off and sleep offset.
Saliva and fecal specimen collections
Saliva collection was performed using the Salivette system (Sarstedt, Barcelona, Spain). Salivary samples were collected on the last day of each intervention week in both food timing conditions at different times of day, depending on the habitual wake time (at awakening, 4 and 8 h after awakening, and at bedtime; i.e., for habitual waking time at 8:00 am, samples were taken at 8:00 am, 12:00 pm, 4:00 pm, and 12:00 am) as described in Fig. 1, to study the microbial daily rhythm. Participants were not allowed to eat or drink any food or beverage 1 h before the saliva sampling.
Salivary samples were collected and extracted from d 0 baseline, before starting the food timing intervention; d 7, one wk after exposure to either the EE or LE intervention; d 14 washout, 1 wk after following their habitual food timing; and d 21, one wk after exposure to the other food timing intervention. To avoid the impact of oral hygiene habits on the oral microbiome, volunteers were not allowed to use toothpaste during the night before and during the day of the saliva collection (24 h). Fecal samples were collected at the end of each EE or LE intervention week. Samples were maintained in the refrigerator until frozen at −80°C within 24 h of collection.
Microbiota profiling and bioinformatics analyses
Total genomic DNA was extracted, via bead beating, from isolated pelleted saliva and fecal samples with a commercially available kit (MasterPure Complete DNA and RNA Purification Kit; Epicentre Biotechnologies, Madison, WI, USA) that included an incubation of 30 min at 37°C with lysozyme (1 mg/ml final concentration) and mutanolysin (5 U/ml) according to the manufacturer’s recommend protocol. DNA concentrations were measured with a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and diluted to 5 ng/μl. Microbial small subunit (SSU or 16S) rRNA gene amplicons were generated by following the 16S rRNA Metagenomic Sequencing Library Preparation Illumina protocol (33). Primers (S-D-Bact-0341-b-S-17/S-D-Bact-0785-a-A-21) (34), targeting the V3–V4 variable region of the 16S rRNA gene, were amplified by PCR with the Illumina adapter overhang nucleotide sequences, according to Illumina protocols. After 16S rRNA gene amplification, the multiplexing step was performed, using a Nextera XT Index Kit (Illumina). A PCR product of 1 μl volume was checked with a Bioanalyzer DNA 1000 chip, and libraries were sequenced with a 2 × 300 bp paired-end run (MiSeq Reagent Kit v.3; Illumina) on a MiSeq Sequencer, according to the Illumina protocol.
Raw FASTQ files for each sample were merged with the software package PEAR (paired-end-read merger) (v.0.9.8) (35). Merged reads were quality trimmed and sequences shorter than 250 bases were discarded (CLC Genomics Workbench, v.10.0; CLC Bio, Aarhus, Denmark). Sequences were screened for chimeras (usearch61 algorithm) (36), and putative chimeric sequences were removed from the dataset [Quantitative Insights Into Microbial Ecology (QIIME, v.1.8)] (37). Each sample was rarefied to 11,000 sequences (38), and data were pooled, renamed, and clustered into operational taxonomic units (OTU) at 97% similarity (usearch6.1 algorithm). Representative sequences from each OTU were extracted and classified using the uclust consensus taxonomy assigner (Greengenes 13_8 reference database); no annotation is provided at taxonomic levels for which robust classification was not possible. Most representative sequences were annotated to the taxonomic level of genus. A biologic observation matrix (39) was generated at each taxonomic level from phylum to genus (using the make-OTU-table algorithm) and analyzed and visualized using the software packages Primer 7 (40) and the R programming environment (41).
The α-diversity indices (within-sample) and β diversity (between-sample) were used to examine changes in microbial community structure associated with timing of food intake. The α-diversity indices were generated using the package “vegan” implemented in the R programing language (42). It included the Shannon index (H′ = −∑ sum [Pi/log (Pi)] where Pi is the relative abundance of each taxon); Pielou’s evenness index [J′ = H′/log (S)] where S is the number of taxa present in each sample), the richness index (number of taxa present in each sample), and the Simpson index [D = ∑ (Pi2)] where Pi is the relative abundance of each taxon. To examine differences in community composition between samples, the pairwise Bray-Curtis dissimilarity (nonphylogenetic) metric was generated with the Primer 7 software package and used to perform analysis of similarity (ANOSIM) calculations. ANOSIM was performed at the taxonomic level of genus, using log (x + 1)-transformed data.
Significant differences in the relative abundance of individual taxa between the saliva and fecal samples were detected with a Kruskal-Wallis test generating a Benjamini-Hochberg false-discovery rate (FDR) corrected P value (FDR P < 0.05), implemented within QIIME software package (37). Taxa with an mean abundance of <1% across the sample set were removed from the analysis. The relative abundance of individual taxa reported failed to meet significance, when corrected for FDR (P < 0.05). Heat maps were generated on the rarefied phylum and genus dataset using R package pheatmaps (43). Further analysis revealed that data could not be significantly fit to a cosine function, which could be related to the limited sample size and number of time points in this study. Likewise, the limited sample size and number of time points reduces the utility of using the JTK/MetaCycle (44) for analysis.
In the SPSS software package (v.22; IBM, Armonk, NY, USA), paired Student’s t tests were used to analyze differences for parametric data satisfying normality test assumptions, via histograms, skewness/kurtosis, and the Schapiro-Wilk test. The Wilcoxon signed ranked paired t test was used to analyze nonparametric data. Microbial relative abundances, diversity values and the Firmicutes/Bacteroidetes (F/B) and Prevotellaceae/Bacteroidaceae (P/B) ratios between conditions were studied. The most common bacteria in human microbiota are phyla gram-positive Firmicutes and gram-negative Bacteroidetes (45). Numerous human studies have used the ratio of relative abundance of Firmicutes-to-Bacteroidetes as an important parameter associated with factors such as host age (46) or disease (47). Within studies, the F/B ratio is an easily calculated parameter that is responsive to changes in the structure of the microbial community, as these 2 phyla are dominant in most mammalian gastrointestinal tract microbiota. However, the F/B ratio of sequences derived from members of these phyla is sensitive to nucleic extraction (48) and PCR conditions, including annealing temperature (49) and primer set used, and comparison of F/B ratios between studies may not be robust. Likewise, F/B ratios generated from amplicon data should not be directly compared with ratios generated from shotgun metagenomics sequence data. Furthermore, a recent meta-analysis showed no association between differences in the F/B ratio and obesity status (50).
Prism (v.5.00) software (GraphPad, La Jolla, CA, USA) was used to for repeated measures (RM) 1- and 2-factor ANOVA; Friedman test; and Tukey, Dunn, and Bonferroni multiple comparison post hoc tests.
Intervention analysis
The study design was such that the order of EE or LE intervention was randomized; thus, to determine whether the order of experimental conditions affected outcomes, the data were examined for taxon-specific differences using an overall microbial community analysis (ANOSIM) (40). If the order of the intervention did not influence outcomes, then data collected from the different sampling times could be pooled. No significant differences were observed in the overall microbiota compositions of saliva and feces for EE condition, whether it happened in the first or second intervention week nor for the LE condition, whether it happened in the first or second intervention week (Supplemental Table 2). Therefore, because there was no significant order effect, we compared the meal timing condition regardless of order. Moreover, saliva samples collected at baseline and after the washout period were statistically indistinguishable, suggesting that the washout between eating conditions was sufficient.
RESULTS
Salivary microbiota
Salivary microbiota communities were significantly affected by the food timing condition. For example, food timing influenced salivary α diversity at 1200, 1600, and 2400 h (but not at 0800 h) (Table 2 and Supplemental Tables 3–6). The LE condition had higher α diversity at 1200 and 1600 h and lower diversity at 2400 h. Despite these differences in α diversity, there were no significant differences in the relative abundance of individual bacteria at any taxonomic level between the eating conditions at any time of the day, as depicted in the heat maps indicating bacteria belonging to each phylum and genus (Supplemental Fig. 2). The most abundant genera were Streptococcus, within the Firmicutes phylum, in all samples. No differences due to food timing were observed for the F/B ratio (Wilcoxon: 0800 h, P = 0.695; 1200 h, P = 0.432; 1600 h, P = 0.160; and 2400 h, P = 0.99) or the P/B ratio (Wilcoxon; 0800 h, P = 0.905; 1200 h, P = 0.612; 1600 h, P = 0.770; and 2400 h, P = 0.161). Finally, no significant overall microbial community structure differences between both food timing conditions were observed at the taxonomic level of genus (ANOSIM; Supplemental Table 7). These findings indicate that, although food timing affected the α diversity of the salivary microbiota, it did not change the overall microbial community structure or any individual taxa.
TABLE 2.
Alpha diversity differences in salivary microbiota between EE and LE conditions at each time point
| Time/α diversity | EE | LE | P |
|---|---|---|---|
| 12:00 pm | |||
| Class Shannon | 1.9900 ± 0.038 | 2.1180 ± 0.035 | 0.025 |
| Class Simpson | 0.8130 ± 0.012 | 0.8450 ± 0.007 | 0.035 |
| Order Shannon | 2.1800 ± 0.040 | 2.3370 ± 0.050 | 0.023 |
| Order Simpson | 0.8320 ± 0.012 | 0.8650 ± 0.007 | 0.023 |
| Order evenness | 0.6190 ± 0.015 | 0.6600 ± 0.011 | 0.049 |
| 4:00 pm | |||
| Phylum Shannon | 1.519 ± 0.062 | 1.685 ± 0.035 | 0.025 |
| Phylum Simpson | 0.704 ± 0.022 | 0.757 ± 0.011 | 0.034 |
| Phylum evenness | 0.579 ± 0.028 | 0.643 ± 0.012 | 0.025 |
| Class evenness | 0.635 ± 0.024 | 0.672 ± 0.015 | 0.045 |
| 12:00 am | |||
| Family Shannon | 2.891 ± 0.053 | 2.734 ± 0.057 | 0.021 |
| Genus Shannon | 3.062 ± 0.058 | 2.868 ± 0.064 | 0.018 |
No significant differences were found at 0800 h. Number of paired saliva samples = 10. Data assessed across groups for normality (histograms, skewness/kurtosis, and Shapiro-Wilk test). Refer to Supplemental Tables S2–S5 for nonsignificant results. P < 0.05 (paired Student’s t test or nonparametric Wilcoxon signed paired rank t test).
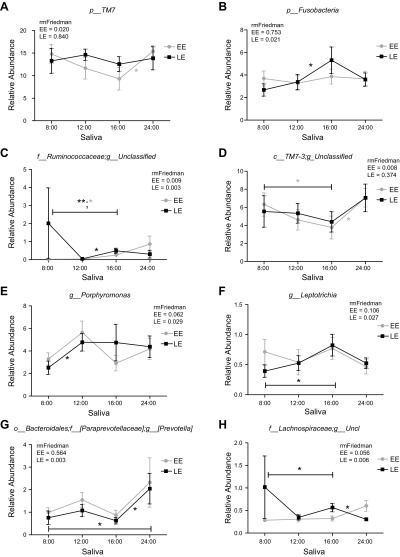
Next, we assessed diurnal rhythms in salivary microbiota. The α diversity exhibited a significant time of day variation in both EE and LE conditions (Table 3); these differences were observed for order, family, and genus levels in the EE condition and for phylum and class in LE. Time of day was a significant factor for some individual bacteria (relative abundances) at the taxonomic levels of phylum and genus (Fig. 2 and Table 4). Specifically, the following taxa exhibited a significant diurnal rhythm in the EE condition: phylum, TM7; and genus, Bacteroides, Ruminococcaceae_unclassified and TM7-3_unclassified; and in the LE condition: phylum, Fusobacteria; genus: Bacteroides, Porphyromonas, f_[Paraprevotellaceae]; g_[Prevotella], Lachnospiraceae_unclassified, Ruminococcaceae_unclassified, and Leptotrichia. Finally, ANOSIM test analyses revealed no significant differences in community structure across time in either eating condition at the taxonomic level of genus (Supplemental Table 7). These results show that diurnal rhythm is observed for α diversity and some bacterial taxa in salivary microbiota; however, time of day had no effect on overall microbial community structure.
TABLE 3.
Effect of time of day across each food timing condition on salivary α diversity
| α Diversity | F(3,39) | Pa | Time point comparisonb |
|---|---|---|---|
| EE saliva | |||
| Phylum Shannon | 2.535 | 0.078 | NS |
| Phylum Simpson | 1.392 | 0.267 | NS |
| Phylum richness | 2.309 | 0.099 | NS |
| Phylum evenness | 1.869 | 0.159 | NS |
| Class Shannon | 1.935 | 0.148 | NS |
| Class Simpson | 1.245 | 0.313 | NS |
| Class richness | 1.557 | 0.223 | NS |
| Class evenness | 1.384 | 0.269 | NS |
| Order Shannon | 3.044 | 0.046* | NS |
| Order Simpson | 1.445 | 0.251 | NS |
| Order richness | 1.572 | 0.219 | NS |
| Order evenness | 0.677 | 0.574 | NS |
| Family Shannon | 7.978 | 0.001* | 12:00 pm vs. 12:00 am**; 4:00 pm vs. 12:00 am*** |
| Family Simpson | 3.453 | 0.030* | 4:00 pm vs. 12:00 am* |
| Family richness | 1.616 | 0.209 | NS |
| Family evenness | 4.716 | 0.009* | 4:00 pm vs. 12:00 am** |
| Genus Shannon | 7.305 | 0.001* | 12:00 pm vs. 12:00 am**; 4:00 pm vs. 12:00 am*** |
| Genus Simpson | 2.691 | 0.066 | NS |
| Genus richness | 2.503 | 0.081 | NS |
| Genus evenness | 5.007 | 0.007* | 4:00 pm vs. 12:00 am** |
| LE saliva | |||
| Phylum Shannon | 2.706 | 0.065 | NS |
| Phylum Simpson | 2.182 | 0.113 | NS |
| Phylum richness | 2.725 | 0.064 | NS |
| Phylum evenness | 4.618 | 0.010* | 8:00 am vs. 12:00 pm*; 8:00 am vs. 4:00 pm* |
| Class Shannon | 1.274 | 0.303 | NS |
| Class Simpson | 1.324 | 0.287 | NS |
| Class richness | 3.236 | 0.038* | 8:00 am vs. 12:00 pm* |
| Class evenness | 3.622 | 0.026* | 8:00 am vs. 12:00 pm* |
| Order Shannon | 0.652 | 0.416 | NS |
| Order Simpson | 0.981 | 0.589 | NS |
| Order evenness | 2.615 | 0.126 | NS |
| Order richness | 2.080 | 0.072 | NS |
| Family Shannon | 0.378 | 0.769 | NS |
| Family Simpson | 0.726 | 0.545 | NS |
| Family richness | 2.528 | 0.078 | NS |
| Family evenness | 1.736 | 0.183 | NS |
| Genus Shannon | 0.324 | 0.808 | NS |
| Genus Simpson | 0.540 | 0.659 | NS |
| Genus richness | 2.235 | 0.107 | NS |
| Genus evenness | 1.478 | 0.243 | NS |
Forty total saliva samples/analysis: number of saliva samples/time point/group = 10. NS, no significance. *P < 0.05, **P < 0.01, ***P < 0.001 (aRMs 1-way ANOVA or bTukey’s multiple comparison test).
Figure 2.
Relative abundance of significant taxa in EE or LE conditions across time points: phylum TM7 (A); phylum Fusobacteria (B); genus Ruminococcaceae unclassified (C); genus TM7-3 unclassified (D); genus Porphyromonas (E); genus Leptotrichia (F); genus Prevotella (G); genus Lachnospiraceae unclassified (H). RM Friedman test indicated various individual taxa were significantly different across time in EE (A, C, D), LE (B, E, F, G, H) or both conditions (C). *Dunn’s multiple comparison test indicated significant differences between time points in either EE or LE. Error bars = SEM.
TABLE 4.
Effect of time of day for each food timing condition on saliva sample’s relative abundance of sequences derived from individual taxa
| Taxa | Pa | Time point comparisonb |
|---|---|---|
| EE saliva | ||
| p_TM7 | 0.020 | 4:00 pm vs. 12:00 am* |
| g_Bacteroides | 0.011 | ns |
| f_Ruminococcaceae; g_unclassified | 0.009 | 8:00 am vs. 4:00 pm* |
| c_TM7-3;g_unclassified | 0.008 | 8:00 am vs. 4:00 pm*; 4:00 pm vs. 12:00 am* |
| LE saliva | ||
| p_Fusobacteria | 0.021 | 12:00 pm vs. 4:00 pm* |
| g_Bacteroides | 0.014 | 12:00 pm vs. 4:00 pm* |
| g_Porphyromonas | 0.029 | 8:00 am vs. 12:00 pm* |
| f_[Paraprevotellaceae]; g_[Prevotella] | 0.003 | 8:00 am vs. 12:00 am*; 4:00 pm vs. 12:00 am* |
| f_Lachnospiraceae; g_unclassified | 0.006 | 8:00 pm vs. 4:00 pm*; 4:00 pm vs. 12:00 am* |
| f_Ruminococcaceae; g_unclassified | 0.003* | 8:00 am vs. 4:00 pm**; 12:00 pm vs. 4:00 pm* |
| g_Leptotrichia | 0.027* | 8:00 am vs. 4:00 pm* |
Forty total saliva samples/analysis: number of saliva samples/time point/group = 10. aRMs Friedman test: all significant at P < 0.05. bDunn’s multiple comparison test. *P < 0.05, **P < 0.01.
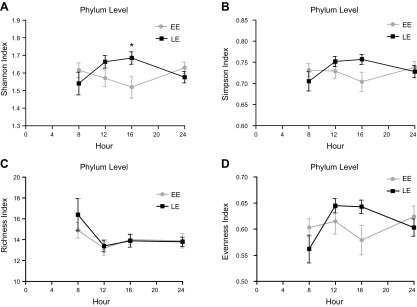
Finally, we assessed interactions between food timing condition (i.e., EE or LE) and time of day (2-way RM ANOVA) in salivary microbiota. Although food timing condition was not a significant factor, both time of day, and its interaction with food timing condition were significant factors for α diversity at many taxonomic levels (Fig. 3 and Tables 5 and 6). These data suggest that the food timing condition influences the daily rhythm in α-diversity salivary microbiota (Fig. 3 and Table 7).
Figure 3.
The α-diversity diurnal oscillations in EE vs. LE between time points. Analysis of phylum level α-diversity data revealed a significant main effect of time and a significant experimental condition (EE vs. LE) × time interaction. Bonferroni post hoc tests indicated a significant difference between index groups at 4:00 pm. Two-way RM ANOVA. *P < 0.05 (Table 7). Significant comparisons according to the Shannon index (interaction; P = 0.0044) (A); the Simpson index (P = 0.0395) (B); the richness index (time; P = 0.0061) (C); and the evenness index (interaction, P = 0.0090; and time. P = 0.0434) (D). Error bars = sem.
TABLE 5.
Effect of time of day between food timing conditions on diurnal rhythms of saliva sample’s α diversity
| α Diversity | Time of day | Food timing condition | P, interaction |
|---|---|---|---|
| Phylum Shannon | 0.763 | 0.485 | 0.004* |
| Phylum Simpson | 0.506 | 0.543 | 0.039* |
| Phylum richness | 0.006* | 0.626 | 0.586 |
| Phylum evenness | 0.043* | 0.712 | 0.009* |
| Class Shannon | 0.949 | 0.618 | 0.049* |
| Class Simpson | 0.812 | 0.739 | 0.089 |
| Class richness | 0.005* | 0.380 | 0.369 |
| Class evenness | 0.094 | 1.000 | 0.017* |
| Order Shannon | 0.997 | 0.696 | 0.127 |
| Order Simpson | 0.836 | 0.880 | 0.155 |
| Order richness | 0.028* | 0.547 | 0.231 |
| Order evenness | 0.338 | 0.929 | 0.068 |
| Family Shannon | 0.387 | 0.867 | 0.015* |
| Family Simpson | 0.571 | 0.773 | 0.041* |
| Family richness | 0.055 | 0.519 | 0.134 |
| Family evenness | 0.082 | 0.813 | 0.027* |
| Genus Shannon | 0.366 | 0.881 | 0.015* |
| Genus Simpson | 0.496 | 0.828 | 0.109 |
| Genus richness | 0.076 | 0.509 | 0.102 |
| Genus evenness | 0.023* | 0.882 | 0.063 |
Eighty saliva samples/analysis: number of saliva samples/time point/group = 10. *P < 0.05 (RMs, 2-way ANOVA).
TABLE 6.
RMs, 2-way ANOVA, matching by rows
| Source of variation | P, total variation (%) | |||
|---|---|---|---|---|
| A | B | C | D | |
| Interaction | 0.0044 (11.80) | 0.0395 (8.24) | 0.5866 (1.67) | 0.0090 (9.31) |
| Condition | 0.4852 (1.21) | 0.5437 (0.83) | 0.6266 (0.54) | 0.7125 (0.35) |
| Time | 0.7630 (0.93) | 0.5066 (2.18) | 0.0061 (11.86) | 0.0434 (6.34) |
Columns match panels A–D in Fig. 3.
TABLE 7.
Comparison of variation by index
| Source of variation | Total variation (%) | P* |
|---|---|---|
| Shannon index | ||
| Interaction | 11.80 | 0.0044* |
| Condition | 1.21 | 0.4852 |
| Time | 0.93 | 0.7630 |
| Simpson index | ||
| Interaction | 8.24 | 0.0395* |
| Condition | 0.83 | 0.5437 |
| Time | 2.18 | 0.5066 |
| Richness index | ||
| Interaction | 1.67 | 0.5866 |
| Condition | 0.54 | 0.6266 |
| Time | 11.86 | 0.0061* |
| Evenness index | ||
| Interaction | 9.31 | 0.0090* |
| Condition | 0.35 | 0.7125 |
| Time | 6.34 | 0.0434* |
P < 0.05 (RMs, 2-way ANOVA).
Fecal microbiota
There were no significant differences between EE and LE conditions for overall fecal microbiota composition structure. Specifically, no significant effects of food timing were observed for α diversity (Shannon, Simpson, richness, and evenness) (Supplemental Table 8); relative abundance of individual bacteria (data not shown); F/B ratio (Wilcoxon; P = 0.438); P/B ratio (Wilcoxon; P = 0.625); relative abundance of sequences from putative short-chain fatty acid–producing bacteria (Wilcoxon; P = 0.844); butyrate-producing anti-inflammatory genera (Wilcoxon; P = 1.00); or overall microbial community analyses at the taxonomic level of genus (ANOSIM: global R = −0.135; P = 0.965) (Supplemental Table 7). These findings indicate that EE vs. LE did not affect the fecal microbiota of healthy, normal-weight women.
DISCUSSION
The composition of the diet (what we eat) has been widely related to microbiota profile (51, 52). However, whether the timing of our meals (when we eat) influences microbiota in humans is unknown. We now show for the first time that food timing can influence the daily rhythms of the salivary microbiota diversity and abundance, even in a group of normal-weight and healthy women with no metabolic alterations. Indeed, only 1 wk of LE, while keeping the total caloric intake and dietary composition unchanged, caused changes in salivary microbiota.
Our randomized, crossover study was designed to maintain constant conditions (energy intake, dietary composition, and sleep duration) between both food timing intervention weeks during the whole experiment. Therefore, our data suggest that food timing per se affects daily rhythms in the salivary microbiota. The use of repeated saliva sampling enabled us to capture diurnal rhythms of salivary microbiota in free-living conditions in humans. Saliva samples are noninvasive and relatively unobtrusive, and allow serial sampling across 24 h.
We showed for the first time in human saliva microbiota the presence of daily rhythms in α diversity at several taxonomic levels. These diurnal fluctuations were present already at phylum and class level in the LE condition, whereas in the EE, daily rhythms were shown at lower levels, including order, family, and genus. These salivary microbiota results suggest that LE has a larger impact on daily rhythms in α diversity than EE. There was a significant effect of time of day as well as an interaction between time of day and food timing condition on α diversity at several taxonomic levels in salivary microbiota.
In saliva microbiota, it has been recently shown that good oral health is associated with lower phylogenetic diversity, whereas inflammatory problems such as periodontal diseases and high BMI are associated with higher diversity (20). Moreover, in infants, α-diversity scores in saliva tend to be higher in obese vs. nonobese children across all α-diversity metrics (53). In our late food timing condition, we found higher microbial diversity during the middle of the day, as compared with the early food timing condition in salivary microbiota. This finding suggests that eating late would drive changes in salivary microbial diversity in a similar direction to that observed in obesity and inflammation (54).
Furthermore, in the fecal samples, although no significant differences were found in diversity and relative abundances between EE and LE conditions, we observed a trend to a higher abundance of the Fusobacteriaceae family (P = 0.09; FDR P = 0.93) in the LE vs. the EE condition. Higher abundance of Fusobacterium spp. in the human gut has been associated with an increased risk of intestinal inflammatory diseases and colorectal cancer (55, 56). In addition, we observed that the fecal sample’s abundance of the Peptostreptococcacee family tended to be higher (P = 0.07; FDR P = 0.51) in EE vs. LE. This bacterial family has been described to increase after weight loss interventions in obese and diabetic patients (57) and also, an inverse relationship has been shown with an inflammation marker C-reactive protein (58). Therefore, this observation in the gut microbiota under LE conditions may suggest a potential proinflammatory profile and a higher risk of intestinal inflammatory-related diseases with LE in fecal microbiota. Along those lines, a recent observational study has reported significant associations between the relative abundance of several gut bacterial families and the distribution of energy intake before or after 1400 h (25). Nevertheless, further food timing interventional studies in obese and diabetic subjects are needed to confirm these results.
In addition, food timing affected salivary microbiota diurnal rhythms in individual bacterial taxa. Recently, salivary microbiota in the human oral cavity have been shown to exhibit global daily rhythms in both the Firmicutes and Bacteroidetes phyla (59). Consistently, our salivary microbiota results showed similar trends in daily rhythms in the relative abundance of these microbial groups. At the saliva’s phylum level, TM7 displayed a significant daily rhythm specifically during the EE condition; whereas, in the LE condition, significant daily rhythms were observed in phylum level Fusobacteria.
The differential oral microbial patterns noted in our study may result in a differential effect on metabolic alterations. Indeed, oral TM7 has been associated with a higher inflammatory score (60), a high risk of periodontitis and with human inflammatory mucosal diseases (61). Furthermore, Fusobacterium species have an important function in the subgingival biofilm which allows periodontal bacteria aggregation (62), increasing the risk of periodontitis, and has also been also related to other inflammatory problems, such as obesity (63, 64). Moreover, the presence of oral Fusobacteriaceae in the intestinal mucosal microbiota has been strongly correlated with disease status in Crohn’s disease (65), and this is an example of increased levels of microbes of oral origin that have been reported in the gut microbiota of patients with several diseases, including inflammatory bowel disease.
In a prior animal study, wrong time of eating induced diurnal rhythms in pathobiont sulfate-reducing bacteria, whereas it caused a loss of diurnal rhythms in health-promoting fermenter bacteria (66). Within our salivary microbiota study, putative periodontal pathogenic Fusobacteria genera Leptotrichia, a known sulfate-reducing bacteria to produce hydrogen sulfide (67), and Bacteroidetes genus Porphyromonas and Prevotella (belonging to the family Paraprevotellaceae) displayed daily rhythms only in LE conditions and not at EE (68–70). These results indicate that similar to what happens in animal studies, eating at an unusual time (during the day in nocturnal animal studies and late in humans) induces diurnal rhythms in those oral sulfate-reducing bacteria and pathobiont microbiota.
These results go along with those in other reports obtained in the gut microbiota in animal models (mice) which have shown that intestinal microbiota undergo diurnal oscillations (26, 71) and that, not only the type of diet, but also the timing of food intake can affect these daily rhythms (23, 66, 72). Indeed, the current randomized, crossover trial performed in humans revealed the impact of food timing on the diurnal rhythm in salivary microbiota, even though the participants followed the same diet during the two intervention weeks.
There are some limitations in our study. We had only four saliva sampling time points, (8:00 am, 12:00 pm, 4:00 pm, and 12:00 am) and for feces, just a single time point after each intervention. For salivary samples, further studies should include more time points during consecutive days and during the night. Moreover, the number of participants included in this study was relatively low (n = 10), and all of them were female and of normal weight. Future studies including additional time points would benefit from the use of circadian analysis, such as fitting data to a cosine function or the JTK/MetaCycle. The purpose of our study design was to identify the daily changes of bacterial abundance relative to each participant’s habitual wake time. Wake time is directly related to several synchronizers of the internal clock, such as morning light exposure, start of physical activity, and start of food intake. Nevertheless, a limitation of the current design is that the sample collected 8 h after habitual awakening (at 4:00 pm in the example displayed in Fig. 1) is going to be affected by eating 2 h before the sample in the EE condition, as opposed to eating nearly 7 h before the sample when the participant is in the LE condition, as indicated in Fig. 1. Timing after a meal might impact the microbiota because of the time the subjects have had to metabolize dietary substrates and reproduce or be exposed to lysozymes in saliva that destroy bacteria. It is likely that the changes observed in oral microbiota throughout the day are caused by an interaction of LE vs. EE, as well as the time of last food consumption. These considerations must be taken into account when interpreting the results. Nevertheless, our results reflect the real-life consequences of LE as compared to EE, in which the impact of meal timing on oral microbiota would also be a result of this interaction. Despite these limitations, our results indicate that food timing affects salivary microbial diversity and to a lesser extent the structure of the salivary microbial community composition.
The impact of these food timing–derived changes in the daily rhythm of salivary microbiota on the host’s metabolism should be further explored in future studies. In experimental animals, the time of food intake, in addition to dietary composition, is an important parameter when assessing the microbiota’s contribution to host metabolism (23). Indeed, changes in gut microbiota because of the timing of feeding have shown to have consequences for the circadian system because they can affect circadian clock activity in different tissues (66, 73–75). This has shown to be particularly critical for metabolic homeostasis of the host, as failure to rhythmically control the microbiota results in dysbiosis that promotes obesity and other manifestations of the metabolic syndrome (72, 76, 77).
To summarize, food timing has an impact on the daily rhythms of human salivary microbiota. Eating late causes changes in the daily rhythm of α diversity in salivary microbiota toward an opposite pattern to EE and in a direction similar to that previously observed in obesity, inflammation, and insulin resistance. LE is associated with the presence of diurnal rhythms in the saliva relative abundance of oral sulfate-reducing bacteria and pathobiont microbiota. Although our study provides information on the effect of timing of food intake on the microbiota, further research is needed to explore the complex and dynamic interrelation between food timing, daily rhythms in microbiota composition and health. Our study provides a scientific rationale for studying microbiota rhythms as a potential biomarker for risk of metabolic disorders and also as a therapeutic target for metabolic disorders such as obesity and metabolic syndrome.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by Spanish Government of Economy and Competitiveness (MINECO) Grants AGL2015-707487-P (to M.C.C.) and SAF2014-52480R; the European Regional Development Fund (ERDF); U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK102696 and DK1-R01DK_105072-01A1 (to M.G.), and R01 DK099512 and R01DK105072 (to F.A.J.L.S.); and NIH National, Heart, Lung, and Blood Institute Grants R01 HL094806, R01 HL140574 and R01 HL118601 (to F.A.J.L.S.). F.A.J.L.S. has received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, and Kellogg Company. The remaining authors declare no conflicts of interest.
Glossary
- ANOSIM
analysis of similarity
- BMI
body mass index
- EE
early eater
- F/B
Firmicutes/Bacteroidetes
- FDR
false discovery rate
- LE
late eater
- P/B
Prevotellaceae/Bacteroidaceae
- RM
repeated measure
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. C. Collado, F. A. J. L. Scheer, and M. Garaulet designed the research; C. Bandin and M. C. Collado performed the research; P. A. Engen, R. Cabrera-Rubio, R. M. Voigt, S. J. Green, A. Naqib, and A. Keshavarzian analyzed the data and performed statistical analyses; M. C. Collado, P. A. Engen, F. A. J. L. Scheer, A. Keshavarzian, and M. Garaulet wrote the paper; and M. C. Collado, P. A. Engen, F. A. J. L. Scheer, A. Keshavarzian, and M. Garaulet are primarily responsibility for the final content.
REFERENCES
- 1.Garaulet M., Gómez-Abellán P. (2014) Timing of food intake and obesity: a novel association. Physiol. Behav. 134, 44–50 10.1016/j.physbeh.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Garaulet M., Gómez-Abellán P., Alburquerque-Béjar J. J., Lee Y. C., Ordovás J. M., Scheer F. A. (2013) Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 37, 604–611 10.1038/ijo.2012.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garaulet M., Vera B., Bonnet-Rubio G., Gómez-Abellán P., Lee Y. C., Ordovás J. M. (2016) Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: the ONTIME (obesity, nutrigenetics, timing, mediterranean) study. Am. J. Clin. Nutr. 104, 1160–1166 10.3945/ajcn.116.134528 [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Lozano T., Vidal J., de Hollanda A., Scheer F. A. J. L., Garaulet M., Izquierdo-Pulido M. (2016) Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin. Nutr. 35, 1308–1314 10.1016/j.clnu.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Johnston J. D., Ordovás J. M., Scheer F. A., Turek F. W. (2016) Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv. Nutr. 7, 399–406 10.3945/an.115.010777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHill A. W, Phillips A. J., Czeisler C. A., Keating L., Yee K., Barger L. K., Garaulet M., Scheer F. A., Klerman E. B. (2017) Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 106, 1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandín C., Scheer F. A., Luque A. J., Ávila-Gandía V., Zamora S., Madrid J. A., Gómez-Abellán P., Garaulet M. (2015) Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int. J. Obes. 39, 828–833 10.1038/ijo.2014.182 [DOI] [PubMed] [Google Scholar]
- 8.Arble D. M., Bass J., Laposky A. D., Vitaterna M. H., Turek F. W. (2009) Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102 10.1038/oby.2009.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson M. P., Allison D. B., Fontana L., Harvie M., Longo V. D., Malaisse W. J., Mosley M., Notterpek L., Ravussin E., Scheer F. A., Seyfried T. N., Varady K. A., Panda S. (2014) Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 111, 16647–16653 10.1073/pnas.1413965111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado-Delgado R., Angeles-Castellanos M., Saderi N., Buijs R. M., Escobar C. (2010) Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 151, 1019–1029 10.1210/en.2009-0864 [DOI] [PubMed] [Google Scholar]
- 11.Stokkan K. A., Yamazaki S., Tei H., Sakaki Y., Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 10.1126/science.291.5503.490 [DOI] [PubMed] [Google Scholar]
- 12.Jakubowicz D., Barnea M., Wainstein J., Froy O. (2013) High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 21, 2504–2512 10.1002/oby.20460 [DOI] [PubMed] [Google Scholar]
- 13.Morris C. J., Garcia J. I., Myers S., Yang J. N., Trienekens N., Scheer F. A. (2015) The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring) 23, 2053–2058 10.1002/oby.21189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collado M. C., Isolauri E., Laitinen K., Salminen S. (2008) Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 [DOI] [PubMed] [Google Scholar]
- 15.Kalliomäki M., Collado M. C., Salminen S., Isolauri E. (2008) Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 87, 534–538 [DOI] [PubMed] [Google Scholar]
- 16.Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. (2005) Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102, 11070–11075 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 18.Damms-Machado A., Mitra S., Schollenberger A. E., Kramer K. M., Meile T., Königsrainer A., Huson D. H., Bischoff S. C. (2015) Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. BioMed Res. Int. 2015, 806248. 10.1155/2015/806248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodson J. M., Groppo D., Halem S., Carpino E. (2009) Is obesity an oral bacterial disease? J. Dent. Res. 88, 519–523 10.1177/0022034509338353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita T., Kageyama S., Furuta M., Tsuboi H., Takeuchi K., Shibata Y., Shimazaki Y., Akifusa S., Ninomiya T., Kiyohara Y., Yamashita Y. (2016) Bacterial diversity in saliva and oral health-related conditions: the Hisayama study. Sci. Rep. 6, 22164. 10.1038/srep22164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeigler C. C., Persson G. R., Wondimu B., Marcus C., Sobko T., Modéer T. (2012) Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity (Silver Spring) 20, 157–164 10.1038/oby.2011.305 [DOI] [PubMed] [Google Scholar]
- 22.Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., Kiguchi Y., Yasuma K., Watanabe E., Tanoue T., Thaiss C. A., Sato M., Toyooka K., Said H. S., Yamagami H., Rice S. A., Gevers D., Johnson R. C., Segre J. A., Chen K., Kolls J. K., Elinav E., Morita H., Xavier R. J., Hattori M., Honda K. (2017) Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 10.1126/science.aan4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarrinpar A., Chaix A., Yooseph S., Panda S. (2014) Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 10.1016/j.cmet.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venable E. B., Fenton K. A., Braner V. M., Reddington C. E., Halpin M. J., Heitz S. A., Francis J. M., Gulson N. A., Goyer C. L., Bland S. D., Cross T. L., Holscher H. D., Swanson K. S. (2017) Effects of feeding management on the equine cecal microbiota. J. Equine Vet. Sci. 49, 113–121 10.1016/j.jevs.2016.09.010 [DOI] [Google Scholar]
- 25.Kaczmarek J. L., Musaad S. M., Holscher H. D. (2017) Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am. J. Clin. Nutr. 106, 1220–1231 [DOI] [PubMed] [Google Scholar]
- 26.Thaiss C. A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A. C., Abramson L., Katz M. N., Korem T., Zmora N., Kuperman Y., Biton I., Gilad S., Harmelin A., Shapiro H., Halpern Z., Segal E., Elinav E. (2014) Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 10.1016/j.cell.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 27.Chaix A., Zarrinpar A., Miu P., Panda S. (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longo V. D., Panda S. (2016) Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 10.1016/j.cmet.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia Gabarra A., Castella Soley M., Calleja Fernandez A. (2017) Ingestas de energía y nutrientes recomendadas en la Unión Europea: 2008–2016. Nutr. Hosp. 34, 490–498 [DOI] [PubMed] [Google Scholar]
- 30.Perez-Llamas F., Garaulet M., Torralba C., Zamora S. (2012) Development of a current version of a software application for research and practice in human nutrition (GRUNUMUR 2.0). Nutr. Hosp. 27, 1576–1582 [DOI] [PubMed] [Google Scholar]
- 31.Mataix Verdú J., Mañas Almendros M., Llopis González J., Martínez de Victoria Muñoz E. (1995) Tabla De Composicion De Alimentos Españoles, Instituto de Nutricion y Tecnologia, Universidad de Granada, Granada, Spain [Google Scholar]
- 32.Azevedo C. V., Sousa I., Paul K., MacLeish M. Y., Mondejar M. T., Sarabia J. A., Ángeles Rol M., Madrid J. A. (2008) Teaching chronobiology and sleep habits in school and university. Mind Brain Educ. 2, 34–47 10.1111/j.1751-228X.2008.00027.x [DOI] [Google Scholar]
- 33.Sinclair L., Osman O. A., Bertilsson S., Eiler A. (2015) Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the illumina platform. PLoS One 10, e0116955. 10.1371/journal.pone.0116955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F. O. (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, e1. 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmieder R., Edwards R. (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864 10.1093/bioinformatics/btr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar R. C. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 37.Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gihring T. M., Green S. J., Schadt C. W. (2012) Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ. Microbiol. 14, 285–290 10.1111/j.1462-2920.2011.02550.x [DOI] [PubMed] [Google Scholar]
- 39.McDonald D., Clemente J. C., Kuczynski J., Rideout J. R., Stombaugh J., Wendel D., Wilke A., Huse S., Hufnagle J., Meyer F., Knight R., Caporaso J. G. (2012) The biological observation matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience 1, 7. 10.1186/2047-217X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke K. R. (1993) Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143 10.1111/j.1442-9993.1993.tb00438.x [DOI] [Google Scholar]
- 41.Dean C. B., Nielsen J. D. (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal. 13, 497–512 10.1007/s10985-007-9065-x [DOI] [PubMed] [Google Scholar]
- 42.Oksanen J., Blanchet F., Kindt R., Legendre P., Minchin P., O’Hara R., Simpson G., Solymos P., Stevens M., Wagner H. (2016) Package ‘vegan’: community ecology package, version 2.0-4. Retrieved from https://cran.r-project.org/web/packages/vegan/index.html
- 43.Kolde R. (2012) “Pheatmap: pretty heatmaps.” R package version 61. Retrieved from https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (Version 1.0.8)
- 44.Wu G., Anafi R. C., Hughes M. E., Kornacker K., Hogenesch J. B. (2016). MetaCycle: an integrated R package to evaluate periodicity in large scale data (v.1.1.0). Bioinformatics 32, 3351–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M., Gill S. R., Nelson K. E., Relman D. A. (2005) Diversity of the human intestinal microbial flora. Science 308, 1635–1638 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., Corthier G., Furet J. P. (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9, 123. 10.1186/1471-2180-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokol H., Seksik P., Furet J. P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Doré J. (2009) Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 15, 1183–1189 10.1002/ibd.20903 [DOI] [PubMed] [Google Scholar]
- 48.Bahl M. I., Bergström A., Licht T. R. (2012) Freezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysis. FEMS Microbiol. Lett. 329, 193–197 10.1111/j.1574-6968.2012.02523.x [DOI] [PubMed] [Google Scholar]
- 49.Green S. J., Venkatramanan R., Naqib A. (2015) Deconstructing the polymerase chain reaction: understanding and correcting bias associated with primer degeneracies and primer-template mismatches. PLoS One 10, e0128122. 10.1371/journal.pone.0128122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sze M. A., Schloss P. D. (2016) Looking for a signal in the noise: revisiting obesity and the microbiome. MBio 7,e01018-16 10.1128/mBio.01018-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ANR MicroObes Consortium (2013) Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 52.Fontana L., Partridge L. (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118 10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janem W. F., Scannapieco F. A., Sabharwal A., Tsompana M., Berman H. A., Haase E. M., Miecznikowski J. C., Mastrandrea L. D. (2017) Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes. PLoS One 12, e0172647. 10.1371/journal.pone.0172647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodson J. M., Hartman M. L., Shi P., Hasturk H., Yaskell T., Vargas J., Song X., Cugini M., Barake R., Alsmadi O., Al-Mutawa S., Ariga J., Soparkar P., Behbehani J., Behbehani K. (2017) The salivary microbiome is altered in the presence of a high salivary glucose concentration. PLoS One 12, e0170437. 10.1371/journal.pone.0170437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kostic A. D., Gevers D., Pedamallu C. S., Michaud M., Duke F., Earl A. M., Ojesina A. I., Jung J., Bass A. J., Tabernero J., Baselga J., Liu C., Shivdasani R. A., Ogino S., Birren B. W., Huttenhower C., Garrett W. S., Meyerson M. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 10.1101/gr.126573.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han Y. W. (2015) Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147 10.1016/j.mib.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Remely M., Hippe B., Zanner J., Aumueller E., Brath H., Haslberger A. G. (2016) Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. [E-pub ahead of print] Endocr. Metab. Immune Disord. Drug Targets. 10.2174/1871530316666160831093813 [DOI] [PubMed] [Google Scholar]
- 58.Hansen T. H., Gøbel R. J., Hansen T., Pedersen O. (2015) The gut microbiome in cardio-metabolic health. Genome Med. 7, 33. 10.1186/s13073-015-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takayasu L., Suda W., Takanashi K., Iioka E., Kurokawa R., Shindo C., Hattori Y., Yamashita N., Nishijima S., Oshima K., Hattori M. (2017) Circadian oscillations of microbial and functional composition in the human salivary microbiome. DNA Res. 24, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demmer R. T., Breskin A., Rosenbaum M., Zuk A., LeDuc C., Leibel R., Paster B., Desvarieux M., Jacobs D. R., Jr., Papapanou P. N. (2017) The subgingival microbiome, systemic inflammation and insulin resistance: the oral infections, glucose intolerance and insulin resistance study. J. Clin. Periodontol. 44, 255–265 10.1111/jcpe.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abusleme L., Dupuy A. K., Dutzan N., Silva N., Burleson J. A., Strausbaugh L. D., Gamonal J., Diaz P. I. (2013) The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 7, 1016–1025 10.1038/ismej.2012.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Signat B., Roques C., Poulet P., Duffaut D. (2011) Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 13, 25–36 [PubMed] [Google Scholar]
- 63.Bullon P., Newman H. N., Battino M. (2014) Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2000 64, 139–153 10.1111/j.1600-0757.2012.00455.x [DOI] [PubMed] [Google Scholar]
- 64.Genco R. J., Grossi S. G., Ho A., Nishimura F., Murayama Y. (2005) A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 76(11 Suppl), 2075–2084 10.1902/jop.2005.76.11-S.2075 [DOI] [PubMed] [Google Scholar]
- 65.Gevers D., Kugathasan S., Denson L. A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S. J., Yassour M., Morgan X. C., Kostic A. D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier R. J. (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leone V., Gibbons S. M., Martinez K., Hutchison A. L., Huang E. Y., Cham C. M., Pierre J. F., Heneghan A. F., Nadimpalli A., Hubert N., Zale E., Wang Y., Huang Y., Theriault B., Dinner A. R., Musch M. W., Kudsk K. A., Prendergast B. J., Gilbert J. A., Chang E. B. (2015) Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 10.1016/j.chom.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carbonero F., Benefiel A. C., Alizadeh-Ghamsari A. H., Gaskins H. R. (2012) Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 3, 448. 10.3389/fphys.2012.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langendijk-Genevaux P. S., Grimm W. D., van der Hoeven J. S. (2001) Sulfate-reducing bacteria in relation with other potential periodontal pathogens. J. Clin. Periodontol. 28, 1151–1157 10.1034/j.1600-051X.2001.281210.x [DOI] [PubMed] [Google Scholar]
- 69.Nakajima M., Arimatsu K., Kato T., Matsuda Y., Minagawa T., Takahashi N., Ohno H., Yamazaki K. (2015) Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 10, e0134234. 10.1371/journal.pone.0134234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y., Zou C. G., Fu Y., Li Y., Zhou Q., Liu B., Zhang Z., Liu J. (2016) Oral microbial community typing of caries and pigment in primary dentition. BMC Genomics 17, 558. 10.1186/s12864-016-2891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thaiss C. A., Levy M., Korem T., Dohnalova L., Shapiro H., Jaitin D. A., David E., Winter D. R., Gury-BenAri M., Tatirovsky E., Tuganbaev T., Federici S., Zmora N., Zeevi D., Dori-Bachash M., Pevsner-Fischer M., Kartvelishvily E., Brandis A., Harmelin A., Shibolet O., Halpern Z., Honda K., Amit I., Segal E., Elinav E. (2016) Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510.e1412 [DOI] [PubMed] [Google Scholar]
- 72.Thaiss C. A., Zeevi D., Levy M., Segal E., Elinav E. (2015) A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 6, 137–142 10.1080/19490976.2015.1016690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asher G., Sassone-Corsi P. (2015) Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 10.1016/j.cell.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 74.Mukherji A., Kobiita A., Ye T., Chambon P. (2013) Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153, 812–827 10.1016/j.cell.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 75.Murakami M., Tognini P., Liu Y., Eckel-Mahan K. L., Baldi P., Sassone-Corsi P. (2016) Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 17, 1292–1303 https://doi.org/10.15252/embr.201642463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voigt R. M., Forsyth C. B., Green S. J., Engen P. A., Keshavarzian A. (2016) Circadian rhythm and the gut microbiome. Int. Rev. Neurobiol. 131, 193–205 10.1016/bs.irn.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 77.Voigt R. M., Forsyth C. B., Green S. J., Mutlu E., Engen P., Vitaterna M. H., Turek F. W., Keshavarzian A. (2014) Circadian disorganization alters intestinal microbiota. PLoS One 9, e97500. 10.1371/journal.pone.0097500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.