Abstract
Factor D (FD) is an essential component of the complement alternative pathway (AP). It is an attractive pharmaceutical target because it is an AP-specific protease circulating in blood. Most components of the complement activation pathways are produced by the liver, but FD is highly expressed by adipose tissue. Two critical questions are: First, to what degree does adipose tissue contribute to circulating FD levels? Second, what quantity of FD is sufficient to maintain a functional AP? To address these issues, we studied a novel mouse strain with complete lipodystrophy (LD), the fld mouse with partial lipodystrophy, a FD-deficient mouse and samples from lipodystrophic patients. FD was undetectable in the serum of LD mice which also showed minimal AP function. Reconstitution with purified FD, serum mixing experiments and studies of partial LD mice all demonstrated that a low level of serum FD is sufficient for normal AP activity in the mouse system. This conclusion was further supported by experiments in which wildtype adipose precursors were transplanted into LD mice. Our results indicate that almost all FD in mouse serum is derived from adipose tissue. In contrast, FD levels were reduced ~50% in the sera of patients with congenital generalized LD. Our studies further demonstrate that a relatively small amount of serum FD is sufficient to facilitate significant time-dependent AP activity in humans and in mice. Further, this observation highlights the potential importance of obtaining near complete inhibition of FD in treating alternative complement activation in various autoimmune and inflammatory human diseases.
Keywords: adipokine, adipose tissue, adipose tissue metabolism, complement system, Factor D, innate immunity, lipodystrophy
Introduction
Complement is an important arm of innate immunity against bacteria and viruses and its activated products also serve as a bridge between innate and adaptive immunity. Three distinct but related pathways are responsible for complement activation: classical (CP), alternative (AP) and lectin (LP). All three enzymatic pathways converge on the cleavage of C3. This results in the generation of proinflammatory complement fragment C3a and a major opsonin C3b. The latter also serves as gateway to the membrane attack complex (MAC) and a substrate to engage the AP’s amplification loop. In the CP, C1, via its C1q subcomponent, binds antibody-antigen complexes, thus attacking foreign agents. In the lectin pathway, a plasma lectin, like Abs ficolins, collectins, or mannose binding lectin (MBL), binds to the microbial surface. In the AP, upon C3b’s covalent binding to a microbial surface, it engages the protease precursor (zymogen) factor B (FB) to form C3bB. This bimolecular complex is cleaved by FD, a serine protease unique to AP. The cleavage results in C3bBb, known as the AP C3 convertase, which is stabilized by properdin (P), an AP specific positive regulator. Factor D itself is cleaved from a pro-FD zymogen to a mature FD. This cleavage is mediated by members of the mannose-binding protein-associated serine protease (MASP) family. Mice deficient in both MASP1 and MASP3 only have Pro-FD in the serum and no AP activity, suggesting that MASP1/3 serve as proteases to cleave Pro-FD into FD (1). MASP3 is thought to be the major pro-FD convertase where MASP1 is the minor convertase (2–4).
Due to its low concentration in human serum [2–4 µg/ml (5, 6) and our data herein] and highly selective function, FD is a strong candidate for pharmaceutical approaches to block the powerful AP and its feedback loop for the treatment of numerous inflammatory diseases such as atypical hemolytic uremic syndrome (7, 8), paroxysmal nocturnal hemoglobinuria (8) and age-related macular degeneration (AMD) (9–13). Antibodies to FD can be used to neutralize AP activity in mice (14) and more recently have been developed for the treatment of patients with AMD (15, 16). A monoclonal antibody to FD was shown to block AP in primates without affecting the classical pathway (CP). Alternative pathway components FB and C3 in blood are largely produced by the liver but also locally by bone marrow derived and many other cell types (17). FD is also known as adipsin because it was independently identified as an abundant transcript in 3T3-L1 adipocytes (18), but it was soon identified as identical to Factor D (19, 20). In addition to the high level of mRNA expression of adipsin/FD in adipose tissue, adipsin/FD expression has been described in other tissues including muscle and lung in humans (19) and the sciatic nerve (21) as well as by the adrenal gland and ovary in mice (22). The Spiegelman group went on to demonstrate that paradoxically adipsin mRNA is decreased in genetic and chemical models of obesity (23–26). More recently, they demonstrated that adipsin plays a role in pancreatic beta cell function (27).
We have assessed FD levels and AP activity in a variety of lipodystrophic mice. These data, along with that derived from a series of dilution and serum mixing experiments, have enabled us to determine that little FD is required for AP activity. In other words, the “safety factor” for FD appears to be high. These results have clinical implications for the treatment of AP mediated disease with FD blocking Abs or small FD inhibitory molecules (28).
Materials and Methods
Mice
Lipodystrophic mice were created by crossing adiponectin-Cre mice (29) with homozygous LSL-ROSA-DTA mice (30). Of note, we observed these lipodystrophic mice die if born at room temperature (RT) and to survive must be maintained at thermoneutrality (30°C) until weaning age. Following weaning, animals were maintained in a temperature-controlled room (22°C) on a 12-h light-dark cycle. FD−/−, FB−/−, P−/− and C3−/− mice have been described (31–34). Animal work was performed according to the policies of Animal Studies Committee (ASC) at Washington University School of Medicine in St. Louis. Mice were analyzed under approved protocols and were provided appropriate care while undergoing research which complies with the standards in the Guide for the Use and Care of Laboratory Animals and the Animal Welfare Act.
Mouse Embryonic Fibroblast Differentiation
Primary mouse embryonic fibroblasts (MEFs) were prepared from WT C57/BL6 E14 embryos as described (35). Cells were allowed to become confluent and then differentiated 3 d later. White adipocytes were generated by using a cocktail of 1 µM dexamethasone, 5 µg/ml insulin, and 500 µM IBMX (DIX cocktail) for 3 d followed by insulin alone (I) for 3 d and then base media (High-glucose DMEM+10% FBS) for 3 d such that adipocytes are harvested 9 d after differentiation. Brown adipocytes were generated by adding the thiazolidinedione Troglitazone (15 µM) to the white adipocyte cocktail (DIXγ cocktail for 3 d and Iγ for 3 d) (35). All adipocytes were harvested on day 9 of differentiation with radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors. We have previously demonstrated that cells differentiated with DIX express white adipose tissue (WAT) markers and not brown adipose tissue (BAT) markers whereas cells differentiated with DIXγ express BAT markers without suppressing WAT markers (unpublished data).
Fat Transplant Experiments
Primary MEFs were prepared from WT histocompatible C57/BL6 E14 embryos as described (35). MEFs were injected subcutaneously at the sternum as reported (36). Mice were sacrificed 2 months after transplantation and sera were used to measure FD levels and AP activity.
PNGase Assays
Recombinant mouse FD from Sino Biological Inc. (Beijing, China) and freshly isolated mouse serum were used in assays to remove N-linked glycosylated sugars by PNGase F (QA-Bio, Inc, Palm Desert, CA). Following the manufacturer’s protocol, 1 µl of serum and various amounts of recombinant mouse FD were mixed with 30 µl of H2O, 10 µl of 5× buffer and 2.5 µl denaturant prior to being heated at 100°C for 5 min. After cooling for 10 min and then adding 2.5 µl Triton X-100 and 2.0 µl of PNGase F to the reaction, the sample was incubated for 3 h at 37°C. Reaction solution (50 µl) was then mixed with 50 µl SDS Sample Buffer containing reducing reagent (Sigma, St. Louis, Missouri). After heating for 60°C for 5 min, 30 µl of solution, equivalent to 0.3 µl of serum, was added and analyzed by SDS-PAGE.
LPS Assay for AP Activity in Plasma (37)
Plasma samples were harvested from mice using sterile technique. ELISA plates (cat# 3855, ThermoFisher Scientific, Waltham, MA) were coated with LPS (2 µg/well, catalog# L2762 Sigma-Aldrich, St. Louis, MO) at 4°C overnight. After washing 3× in buffer (PBS/0.05% Tween 20) and blocking with BSA (250 µl/well, 1% in PBS buffer), diluted mouse plasma (1:5 or 1:10 in Mg2+-EGTA buffer) was incubated on an ELISA plate at 37°C for 1 h (50 µl/well). The plates were washed 3× in washing buffer and then goat anti-mouse C3 Ab (100 µl/well, 1:4000 dilution in 1% BSA in PBS buffer; MP Cappel, CA) was added for 1 h. Wells were washed 3× as above and incubated with HRP-conjugated anti-goat IgG (100 µl/well, 1:2000 dilution in 1% BSA in PBS buffer; Jackson ImmunoResearch, PA) for 1 h. After 3 washes, Substrate Reagent (R&D Systems, MN) was added for 10 min (100 µl/well). The reaction was stopped with 50 µl 1 M H2SO4, and the OD of samples was measured at 450 nm. Similar experiments were performed with normal human serum and FD-depleted human serum supplemented with purified human FD (CompTech, Tyler, Texas). The primary and second antibodies used in the LPS assay with human serum are: Mouse anti-human C3d (ThermoScientific, #HYB 005-01-02) and HRP Donkey anti-Mouse IgG (Jackson Immunoresearch) respectively.
Rabbit RBC Assay for AP Activity
A standard rabbit RBC hemolysis assay was employed to measure AP activity of mouse serum (34). Rabbit RBCs (1 µl) (Colorado Serum Company, Denver, Colorado) were placed in AP buffer (GVB with 20 mM MgCl2 and 8 mM EGTA). Following centrifugation, the pellet was resuspended in the AP buffer and aliquots of 20% serum from LD and various complement deficient mice as well as human serum samples were added as described. After a 2 h incubation at 37°C, released hemoglobin was measured at an OD of 405 nm. Lysis of rabbit RBCs in water served as the positive control whereas rabbit RBCs in AP buffer served as the negative control. Hemolysis was determined by an OD ratio (20% serum with RBC in AP buffer - 20% serum without RBC in AP buffer)/(RBC in water − RBC in AP buffer). To account for minor differences in hemolysis between experiments, in each experiment the values were normalized to WT sera being 100% hemolysis.
Serum Mixing Experiments
As sera makes up 20% of the rabbit RBC hemolysis assay (above), we kept the total serum concentration the same, but varied the contribution of WT serum and serum from knockout mice (C3, FB, FD or P). For human serum mixing experiments, normal human serum was mixed with serum depleted of C3 or FD (CompTech).
Western Blot
Fresh serum from mice was employed to measure C3, FB, P or FD by Western blotting. Goat anti-M C3 (MP Biomedicals), goat anti-Hu FB (CompTech), rabbit anti-M P (37), sheep anti-M FD (R&D) and rabbit anti-Hu FD (Abcam, Cambridge, MA) were incubated with the membranes for 2 h or overnight at 4° at RT. Secondary HRP-conjugated rabbit anti-goat IgG (Sigma-Aldrich), goat anti-rabbit IgG (GE Healthcare UK Limited), donkey anti-sheep IgG (R&D) or rabbit anti-human IgG (Jackson Immuno Research) was added for 1 h at 37°C. Membranes were developed with a SuperSignal West Kit (Pierce).
CVF Treatments
For in vitro experiments, 10 µl of serum, 10 µl of 30 µg/ml of CVF (Quidel, San Diego, CA) and 10 µl of AP buffer (GVB with 20 mM MgCl2 and 8 mM EGTA) were incubated at 37°C for 30 minutes. For in vivo experiments, 30 µg of CVF was injected I.P. into the mice and serum was harvested 2 hours after the CVF injection. All samples were subjected for Western blots under reducing condition.
Human Samples
Patients were enrolled in a study to evaluate the effects of recombinant human methionyl leptin (metreleptin) therapy on lipodystrophy (https://clinicaltrials.gov/ct2/show/NCT00025883). We only used baseline sera samples (before leptin treatment) for this study which was conducted at the Clinical Center of the National Institutes of Health (CCNIH) and was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Written informed consent was obtained from all participants. Samples were also collected from healthy volunteers with informed consent under a protocol approved by the Washington University Human Studies Committee. Human FD was determined by an ELISA kit from R&D according to the manufacturer’s protocol as well as by Western blots with anti-human FD Ab from Abcam.
Results
Mouse FD is synthesized as a glycosylated protein by white and brown adipocytes and adipose tissue
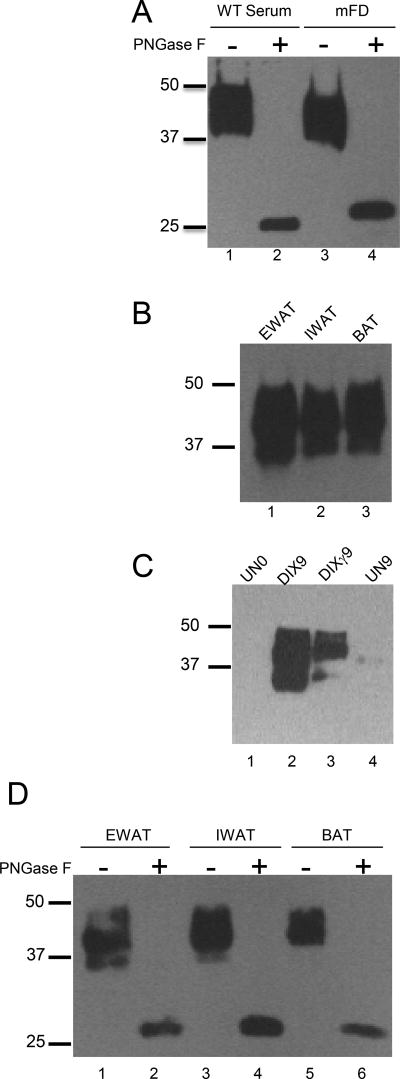
Both endogenous FD of mouse serum and recombinant mouse FD were a mixture of glycosylated isoforms with two prominent bands at an Mr between 37 and 50 k (Figure 1A). A similar sized full-length isoform was observed in whole cell lysates from inguinal and epididymal white adipose tissue as well as brown adipose tissue (Figure 1B). We also saw similar sized full-length isoforms in whole cell lysates of MEFs that had been differentiated into white as well as brown adipocytes, whereas no FD was observed in undifferentiated MEFs (Figure 1C). Treatment of sera or recombinant mouse FD (rmFD) with PNGaseF resulted in a single, 25 k deglycosylated isoform (Figure 1A). This also occurred when whole tissue lysates from visceral epididymal white adipose tissue, superficial inguinal white adipose tissue, and brown adipose tissue (which have different physiological roles) were treated with PNGaseF (Figure 1D).
FIGURE 1.
Western blot analysis of mouse FD in serum and adipose tissue. (A) Untreated serum sample from WT mouse (0.3 µl) and also of recombinant mouse FD (30 ng) were treated with PNGase F. (B) Western blot of mouse FD in various adipose tissues: EWAT (Epididymal White Adipose Tissue), IWAT (Inguinal White Adipose Tissue), BAT (Brown Adipose Tissue). (C) Western blot of 1) undifferentiated MEFS prior to differentiation (UN0, lane 1), 2) MEFs differentiated into white adipocytes with the Dexamethasone/Insulin/IBMX protocol (DIX) for 9 d (DIX9, lane 2), 3) MEFs differentiated into brown adipocytes with DIX plus the PPAR-γ agonist troglitazone (DIXγ) protocol for 9 d (DIXγ9, lane 3) and 4) undifferentiated MEFs at the conclusion of the differentiation period (UN9, lane 4). (D) Western blot of mouse FD in various fat tissues (EWAT, IWAT, and BAT) before and after treatment with PNGaseF. These results demonstrate that mouse FD both in serum and adipose tissues is highly and variably N-linked glycosylated.
Lipodystrophic mice
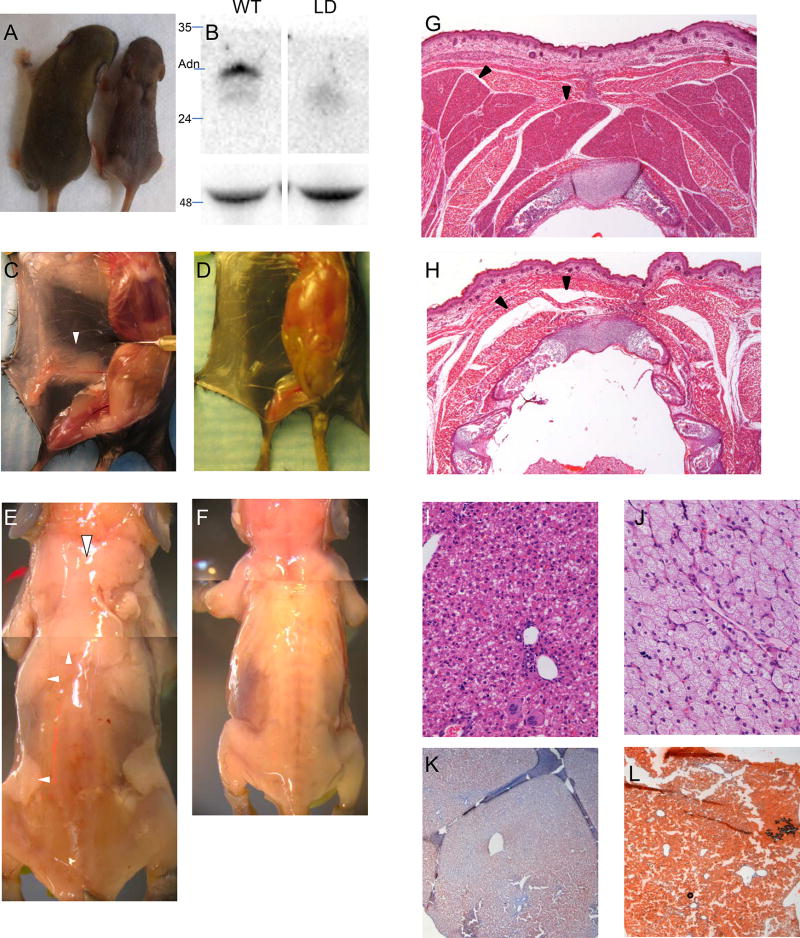
We set out to create lipodystrophic mice by crossing two publicly available lines (29, 30). When adiponectin-cre mice were crossed to homozygous LSL-DTA mice and pups were delivered at RT, adiponectin-cre+ mice were not found at Mendelian frequency (3/46 mice were Cre+ and lipodystrophic, P<0.05 by chi-square test). To assess whether this was due to non-specific leakage of adiponectin-cre in another tissue or whether it was a specific functional consequence due to the loss of adipose tissue, we postulated that the latter might be rescued by allowing mice to be born in a thermoneutral setting; i.e., the reduced viability was due to the loss of BAT-mediated thermoregulation and resultant hypothermia. The cross was repeated with the dam moved from RT to thermoneutrality once they appeared pregnant (~E14). Mutant double transgenic mice were now born at the expected Mendelian frequency. Subsequently, we found that pregnant mice carrying mutant progeny could be moved from RT to thermoneutrality up until the night before parturition without loss of viability in lipodystrophic pups (27/62 progeny Cre+, not statistically different from the Mendelian expected 50%). Double-transgenic lipodystrophic mice can be recognized soon after birth due to an interscapular defect normally containing brown adipose tissue (Figure 2A). LD mice had undetectable adiponectin (Figure 2B) grossly absent white adipose tissue (Figure 2C–F) and brown adipose tissue (Figure 2G–H), and massive hepatic steatosis (Figure 2I–L)
FIGURE 2.
Lipodystrophic mice. (A) WT (left) and LD (right) littermates. Note interscapular defect in LD mouse. (B) Upper, Western blot for adiponectin; lower, loading control (heavy chain). Pinned view of WT (C) and LD (D) mouse showing absence of inguinal fat in LD. (E, F) skinned WT and LD P8 mice showing complete lack of adipose tissue in LD mice. Hematoxylin and Eosin (H&E) staining of newborn postnatal day 0 (P0) WT (G) and LD (H) demonstrating absence of BAT in LD. H&E of liver in WT (I) and LD (J) mice. Oil Red O staining of WT (K) and LD (L) mice. Note the hepatic steatosis in LD mice. White arrowheads, adipose depots in WT mice (missing in LD mice). Black arrowheads, brown adipose in WT mice. In LD mice, black arrowheads point to where BAT should be, but there is only a tissue plane between skeletal muscles.
AP activity in LD mice
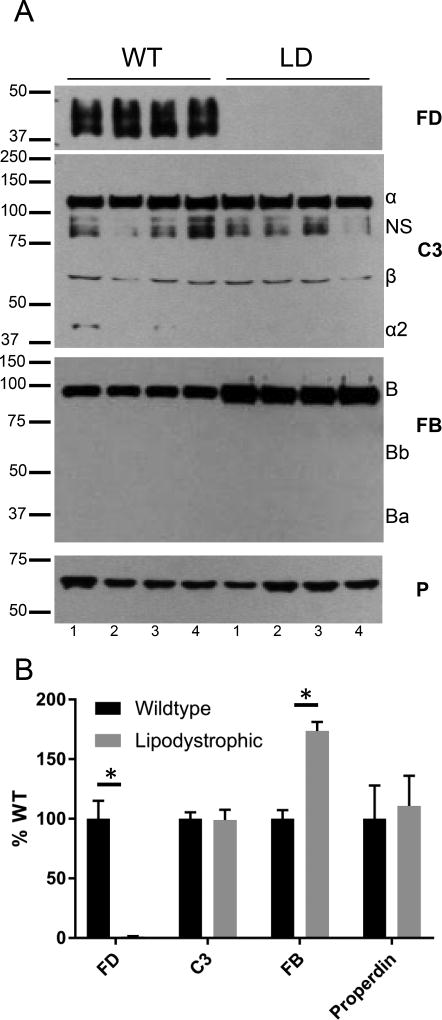
We next asked if FD could be detected in the plasma of LD mice. Western blotting revealed FD in the serum of WT mice, but FD was undetectable in the plasma of LD mice (Figure 3A–B). We also examined the relative abundance of the other AP complement proteins in serum. C3 and P levels were similar in WT and LD mice, but, interestingly, the FB concentration was approximately twice the level in LD compared to WT mice (Figure 3A–B).
FIGURE 3.
Absence of FD in LD mice. (A)Western blot analysis of complement components (FD, C3, FB and P) in sera of WT and LD mice. FD was undetectable while FB was increased approximately two-fold in the serum of LD mice. Serum C3 and P levels were similar between WT and LD mice. NS, non-specific band. A small amount of C3 alpha 2, a degradation fragment of complement activation produced during sample collection, was observed in some samples. (B) Quantification of (A) by densitometry. *, p<0.05.
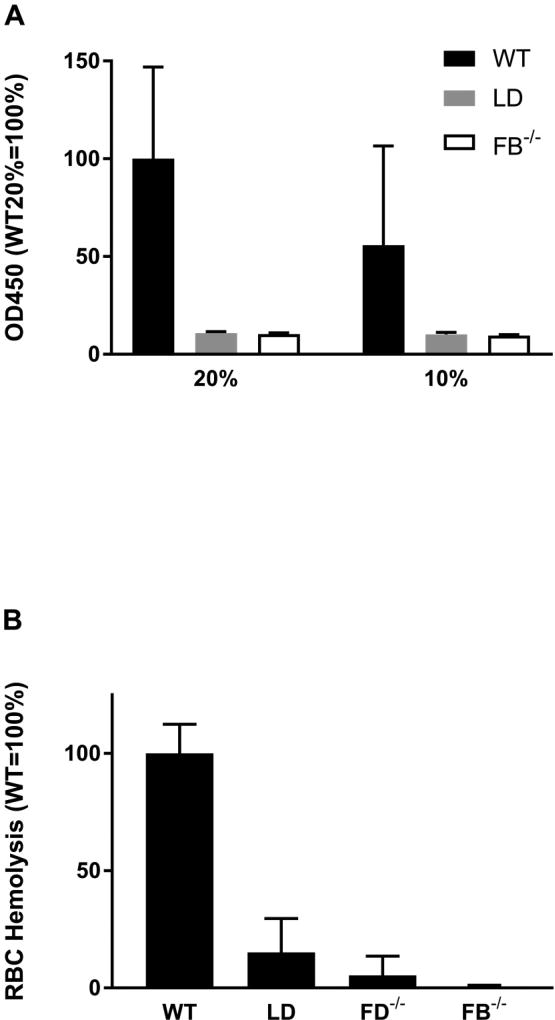
We subsequently used two functional assays to determine the activity of the AP activity in the LD mouse. There was no detectable AP in LD plasma in a LPS-coated microtiter-plate assay whereas WT plasma demonstrated robust activity (Figure 4A). In this assay, plasma is added to LPS coated wells and then the quantity of deposited C3 fragments is determined. We performed a second AP assay testing the ability of serum to lyse rabbit RBCs. In this experiment LD sera AP activity was 15% of the WT activity (Figure 4B).
FIGURE 4.
Deficiency of AP activity in sera of LD mice. (A) LPS-based in vitro binding assay in sera of WT (Black, n=8), LD (Grey, n=7), and FB−/− (White, n=4) mice. Left, assay performed with 20% serum; Right, assay performed with 10% serum. Samples normalized to WT 20% sera result equals 100% activity. (B) Rabbit RBC based-hemolysis assay on sera from WT (n=14), LD (n=9), FD−/− (n=8) or FB−/− (n=3) mice. Data normalized to WT equals 100% activity. These functional assays demonstrate a severe defect in AP activity of LD mice.
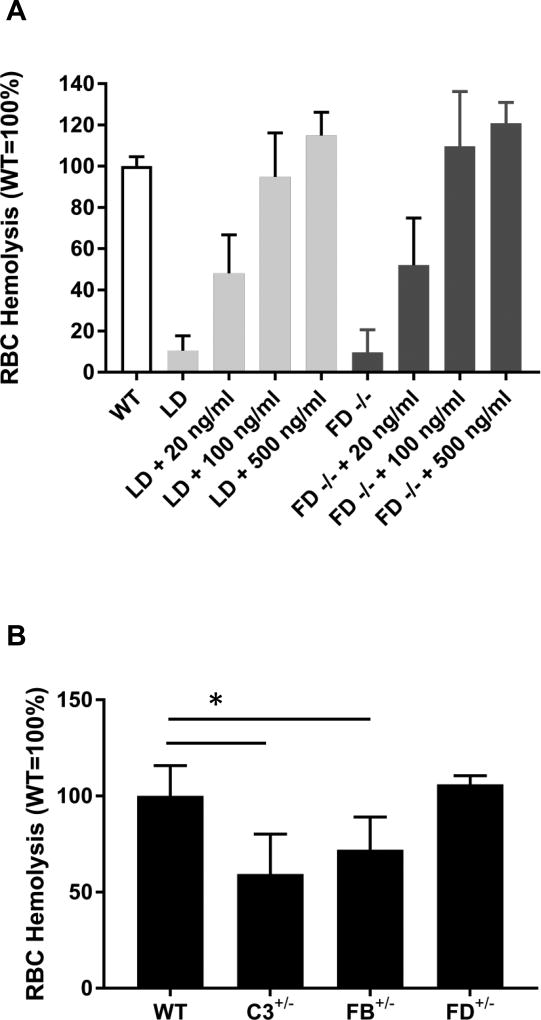
We followed these experiments by investigating whether purified human FD could rescue the AP defect in sera from LD mice and FD−/− mice. FD (20 ng/ml) rescued ~50% of the AP defect in both LD and FD-null mice and 100 ng/ml FD completely rescued the AP defect in sera from both LD and FD−/− mice (Figure 5A). These data further indicate that LD mice have virtually no FD and that relatively small amounts of FD are sufficient to rescue a FD deficit.
FIGURE 5.
Rescue of AP defect in LD and FD−/− mice by purified human FD. (A) Rabbit RBC-based hemolysis assay was performed to assess AP activity in WT (White), LD (Grey), and FD−/− (Black) sera (n=3 for all groups). Various quantities of FD were added to the reaction mixture. Human FD (100 ng/ml) rescued AP defect in the LD and FD−/− mice. All values were normalized to WT equals 100%. (B) RBC hemolytic assays were with sera from WT, C3+/−, FB+/− and FD+/− mice (n=3). All values were normalized to WT equals 100%.*, p<0.05.
We next examined sera from mice heterozygous for C3, FB or FD. There was a modest decrease in AP activity of C3 (63.3 ± 22.1%, p<0.05 vs WT) and FB heterozygous mice (76.7 ± 18.8%, p<0.05 vs WT), but no difference between FD heterozygous mice (109.3 ± 4.3%) and WT mice (100 ± 18.9%, Figure 5B).
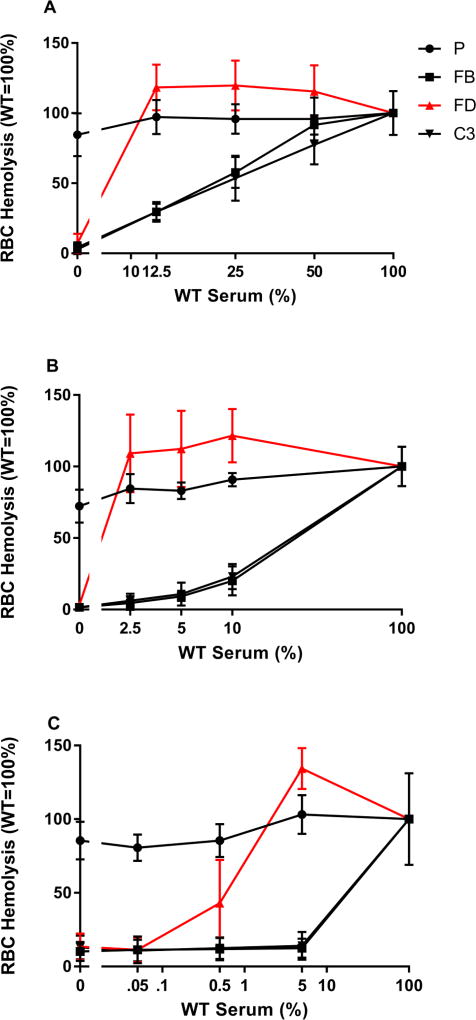
To more directly investigate the relationship between mouse FD levels and AP activity, we conducted mouse serum mixing experiments. In hemolysis assays, the total amount of serum was held constant at 20% (i.e. 20 µl of sera in a 100 µl RBC lysis reaction) but the contribution of WT serum and mutant serum was altered. This was accomplished by mixing WT sera with that from FD−/− mice, P−/−, FB−/− or C3−/− mice. The absence of P only had a small effect on the AP activity as measured by RBC hemolysis assay (Figure 6). Reduction of FB or C3 in the reaction by mixing WT serum with either FB−/− or C3−/− sera resulted in a linear relationship between the amount of FB or C3 and AP activity. However, FD showed a strikingly different relationship. When the FD concentration was lowered to 50, 25 or 12.5% of the WT levels (by mixing with FD−/− sera), the AP activity remained normal (Figure 6A). Experiments in which mixing serum at a lower range of WT sera showed that 2.5% of normal FD levels maintained full AP activity (Figure 6B). A further mixing experiment showed that a FD level of 0.5% (10 ng/ml using our measured value of FD of 10 µg/ml in mouse serum) supported 43% of AP activity (Figure 6C).
FIGURE 6.
Serum mixing experiments to assess the relative requirements of FD (Red triangle), C3 (Black inverted triangle), FB (Black square) or P (Black circle) in AP activation. Hemolytic assays were performed at a final concentration of 20% serum with different percentages of mixtures between WT and KO sera. For sake of clarity, 20% WT sera in the assay then correlates to 100% WT. (A) High-range WT sera (WT sera is 12.5–50% of total sera), (B) Mid-range (2.5–10%), (C) Low-range, (0.05–5%). Properdin levels affect AP modestly in the 20% serum assay system. Whereas FD, FB and C3 are all required for AP activity, the quantity of FD required is much lower than FB and C3. Note that 100% WT and 0% WT (100% KO) were performed with each experiment. Data are plotted with logarithmic x-axis to show spread of values with break to include 0% WT data.
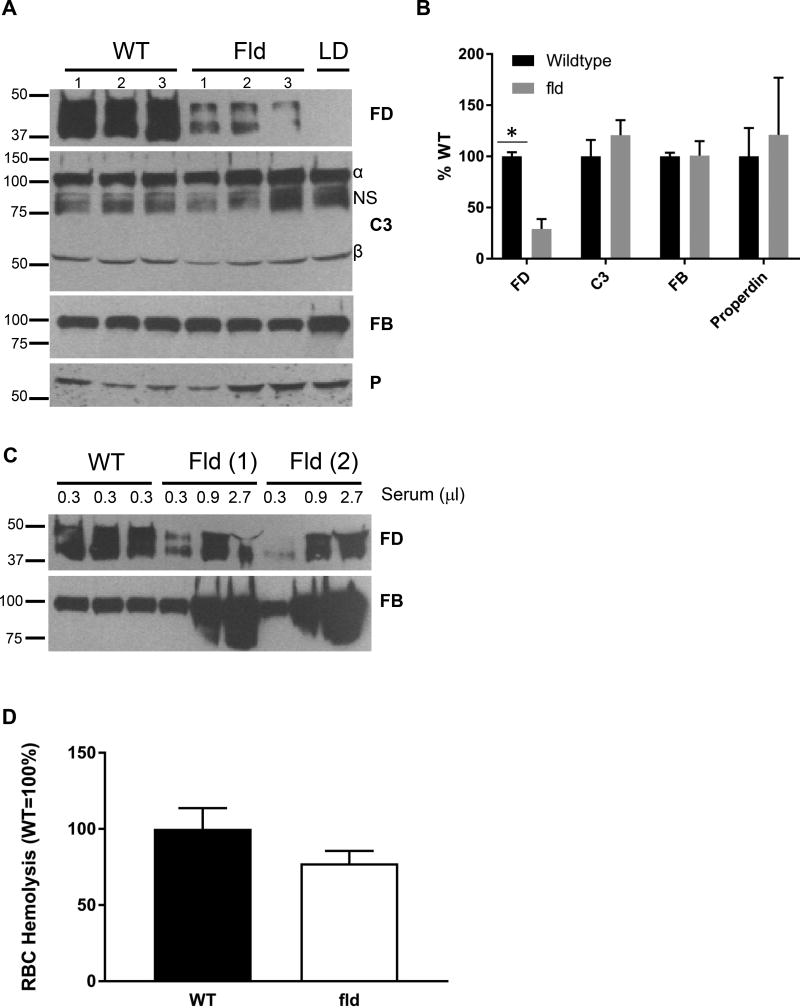
To gain additional insight into the relationship among adipose mass, FD levels and AP activity, we examined an additional mouse strain with partial lipodystrophy, fld. Fld mice have ~80% loss of adipose tissue (38). FD levels were reduced by ~ 70% in sera of fld mice (compared to WT mice, Figure 7A, B), whereas levels of other complement components, C3, FB, and P, were similar to WT mice (Figure 7A, B). Titration of plasma from fld mice also showed FD levels were approximately 30% of WT (Figure 7C). Despite only having ~30% of normal FD levels, serum from fld mice reconstituted 77.5 ± 8.1% of WT AP activity (non-significant compared to WT) in the rabbit RBC hemolysis assay (Figure 7D).
FIGURE 7.
FD level and AP activity in fld mice. (A) Western blot analysis for complement components in fld mice.(B) Quantification of (A). (C) Titration of FD level in fld mice. (D) Rabbit RBC-based hemolytic assay to measure AP activity in fld mice. Although there was ~30% of FD remaining in fld mice, fld sera (white) had ~80% of AP activity compared to WT mice (black), non-significant, n=3 per group. NS, non-specific band. *, p<.0.05.
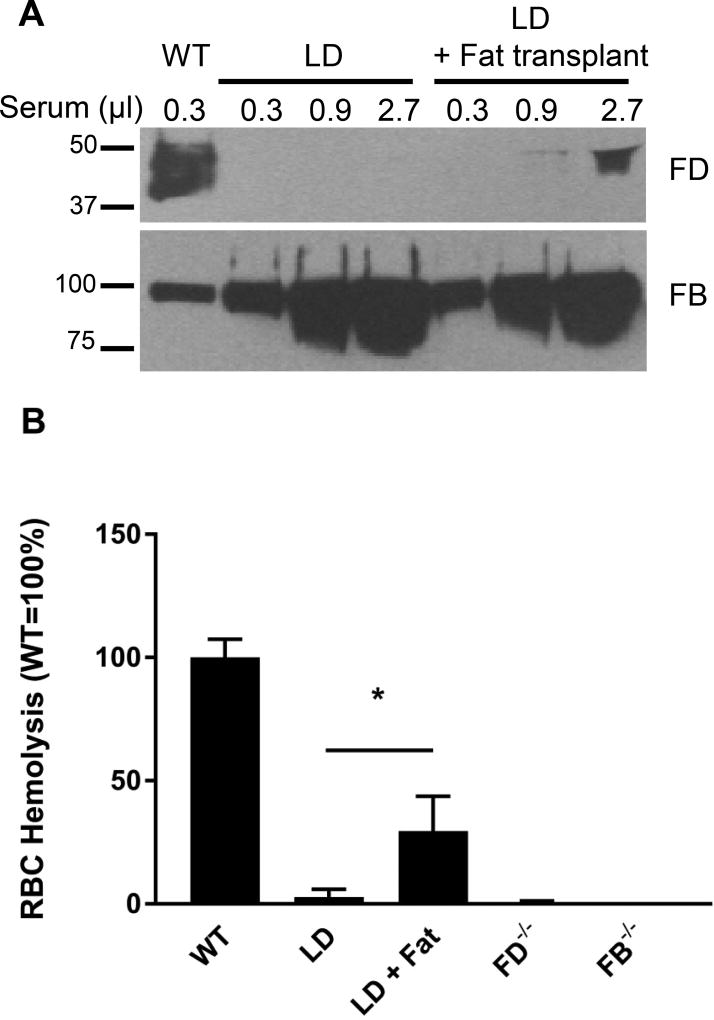
The other approach we used to probe the relationship among adipose mass, FD levels and AP activity was to perform rescue experiments with LD mice. We harvested MEFs from histocompatible C57/BL6 E14 embryos and injected the cells subcutaneously under the sternum of LD mice (36). A large fat pad developed at the injection site within 4 weeks of injection. Mice were sacrificed 8 weeks after injection. The fat pads weighed 500–1000 mg, and therefore constituted 10–20% of the normal fat mass of age-matched WT mice. Remarkably, many of the metabolic derangements (e.g. fatty liver) were rescued by the formation of the ectopic fat pad. We were now also able to detect circulating FD in LD mice that had been rescued by MEF injection. However, the FD level in transplanted mice only represented about 1–5% of WT circulating FD levels (Figure 8A). Despite these reduced FD levels, sera from the rescued LD mice were able to support AP activity at a level of ~30% of WT levels (Fig. 8B).
FIGURE 8.
FD concentrations and AP activity profile in LD mice following an adipose tissue transplant. (A) Western blot analysis of FB and FD in mice. In the titration experiments, FD levels in LD mice receiving a fat transplant were estimated to be about 1–5% of the WT mice; FB served as a loading control. (B) Rabbit RBC-based hemolytic assay to measure AP activity in WT, LD, and LD mice (n=3–5) that had received pre-adipocyte transplant (LD+Fat), *, p<0.05. FD−/− and FB−/− served as controls.
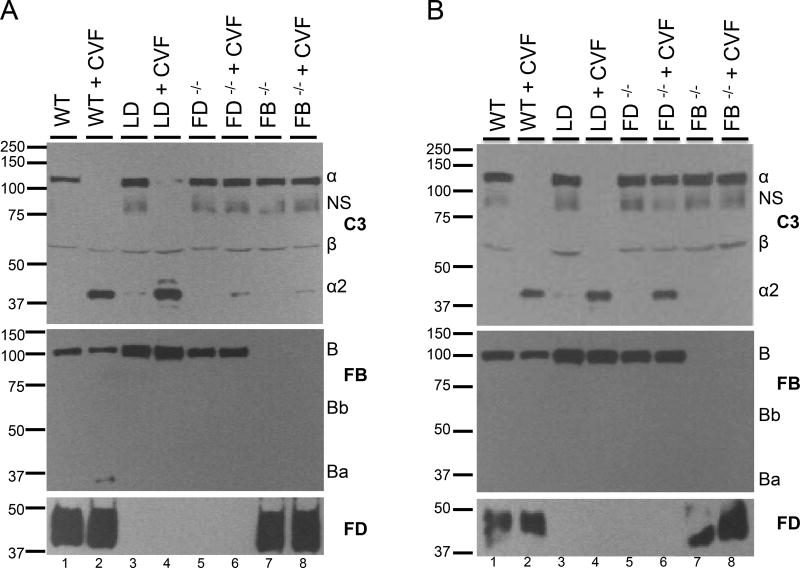
Cobra Venom Factor (CVF) is similar to host derived C3b in that it can work together with FB to trigger the AP. Consequently, we examined the ability of CVF to induce C3 cleavage (to C3b) in sera of WT, LD, FD−/− or FB−/− mice in vitro. CVF robustly induced cleavage of C3 in WT and LD sera, whereas very little cleavage occurred in sera from either FD−/− or FB−/− mice (Figure 9A). The FB and FD blots confirmed the absence of FD in LD and FD−/− sera and the increased FB in LD sera.
FIGURE 9.
Cobra venom factor (CVF) leads to C3 cleavage in sera of LD mice. (A) CVF induces cleavage in vitro of C3 in sera of WT and LD mice, but not FD−/− or FB−/− mice. (B) CVF injection results in C3 cleavage in WT, LD and FD−/− mice, but not in FB−/− mice. All gels were run under reducing conditions. All experiments were performed at least twice with representative results shown. NS, non-specific band.
We utilized the ability of CVF to induce C3 cleavage in vivo when injected into WT, LD, FD−/− or FB−/− mice. CVF injection into WT mice resulted in C3 cleavage. CVF injection into FB−/− mice resulted in no cleavage of C3, indicating Factor B is absolutely required for C3 cleavage. Surprisingly, injection of CVF into LD or FD−/− mice resulted in cleavage of C3, suggesting that FD may be dispensable for CVF induced cleavage of C3 in vivo (Figure 9B). This observation is similar to that in experiments performed when FD−/− mice were initially described (31). For these experiments, Western blotting again demonstrated an absence of FD in the sera of FD−/− and LD mice and increased FB in the sera of LD mice.
FD/Adipsin in Human Lipodystrophy
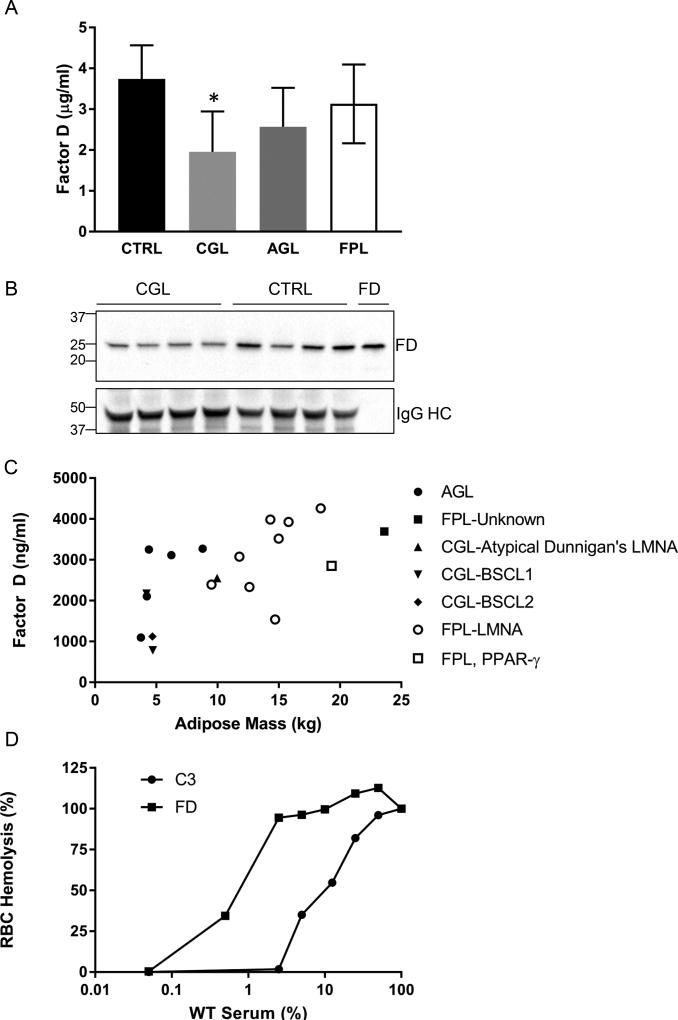
We next pursued whether the absolute requirement of adipose tissue for circulating FD levels observed in the mouse system applied to humans. We tested circulating FD levels in 1) normal subjects, 2) patients with congenital generalized lipodystrophy (CGL) due to mutations in acyl-glycerol acyltransferase 2 (AGPAT2, N=3) or Berardinelli-Seip Congenital Lipodystrophy 2 (BSCL2, N=1), 3) patients with familial partial lipodystrophy due to mutations in Lamin A (LMNA, N=7), and 4) patients with acquired generalized LD (N=5) using an ELISA. We found that FD was detectable in patients with these forms of LD, but that FD levels were significantly lower in CGL than in controls. Specifically, CGL patients had circulating FD levels ~50% of that in controls (Figure 10A). Patients with familial partial lipodystrophy (FPL) or acquired generalized LD had levels of FD between those of controls and CGL. We performed a Western blot on the sera from patients with CGL and controls and observed that FD levels were reduced in CGL patients compared to controls (see Legend, Figure 10B). Our data on human FD with Westerns showed that there was a single unglycosylated band with molecular weight of approximately 25 k. Because of the variability in fat mass in these patients, we created a plot of adipose mass vs FD levels and saw a linear relationship between adipose mass and FD levels (Figure 10C, R2=0.388). We performed the RBC hemolysis assay for AP on patient sera and observed equivalent levels of RBC hemolysis with CGL and control sera.
FIGURE 10.
Reduction in circulating FD levels in patients with CGL. (A) FD levels were measured by ELISA for the following groups: 1) control (Black, n=4), 2) CGL due to AGPAT2 or BSCL2 mutations (Light Gray, n=5), 3) AGL (Dark Gray, n=5) and 4) FPL due to mutations in LMNA or PPARG (White, n=8). *, p<0.05. (B) Reduced FD in CGL patients observed in Western blot. P<0.05 CGL vs. controls. FD lane is 10 ng of purified human FD. HC, heavy chain of IgG. Gel was run under reducing condition. (C) Scatter plot of adipose mass (kg) vs FD levels (ng/ml). R2=0.388. AGL, Black circle; FPL, unknown type, Black square; CGL-atypical Dunnigan’s, Black triangle; CGL-BSCL1, inverted Black triangle; CGL-BSCL2, Black diamond; FPL-LMNA, White circle; FPL, PPAR-γ, White square. (D) Mixing of normal human sera control with either FD or C3 depleted sera. Experiment was repeated three times.
To determine if the low concentrations of FD were also sufficient for activation of AP activity in the human system, we performed mixing experiments between normal human sera and human sera that had been immunodepleted of either C3 or FD. These experiments mirrored what was seen in mice; namely, a low concentration of human FD was needed to activate AP (approximately 1% for half-maximal activity) whereas there was more of a linear relationship between human C3 for AP activation (Figure 10D).
Requirement for Factor D in two quantitative assays
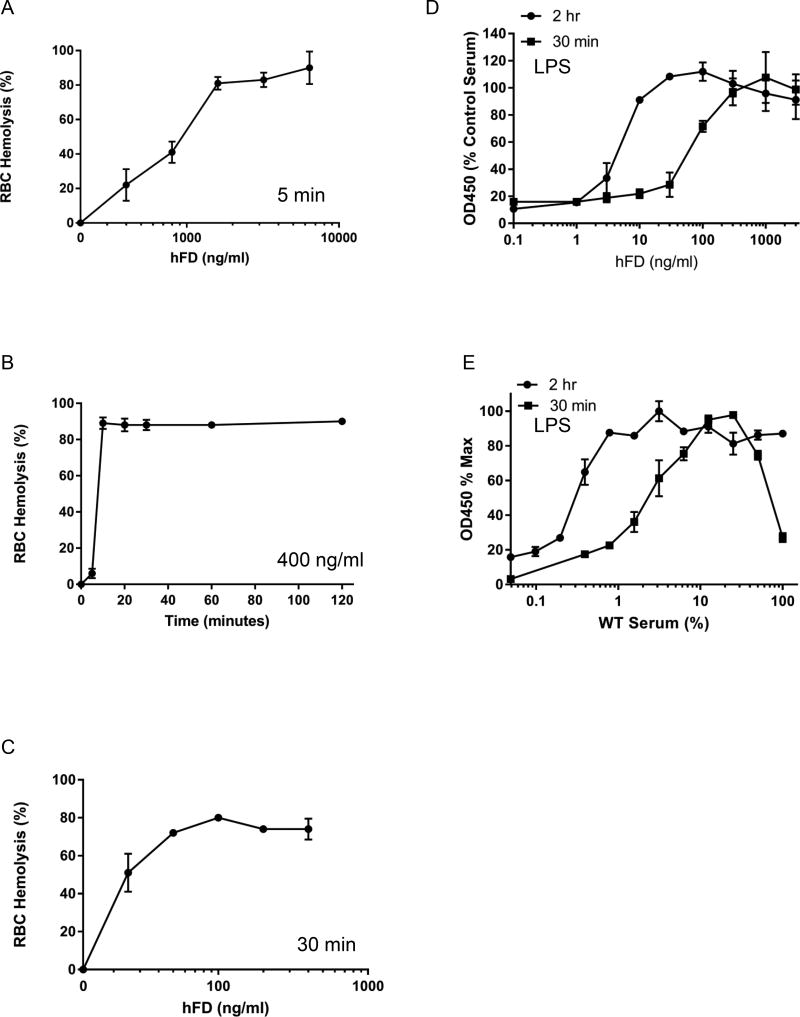
The literature from the 1970’s suggests that FD is the limiting factor in AP activation (39). That view was based on rabbit hemolysis assays performed using the addition of purified human FD or human serum to substitute for diisopropylfluorophosphate-inactivated FD in human plasma (39). Those experiments showed that adding more than physiological amounts of FD (up to 9-fold) resulted in increased AP activity. Those experiments, however, were carried out with incubation times of only 5 min. We have performed equivalent assays using purified human FD and FD-depleted human serum with a similar result at 5 min incubation (Figure 11A, B). In contrast, at longer incubation times much less added FD was required to attain WT-level AP activity (Figure 11C). Note that in this lytic system employing RBCs, by 10 min there was over 90% lysis when using 400 ng/ml hFD (Figure 11B). Moreover, substantial lysis was obtained at 25 ng/ml of FD in the standard 30 min incubation commonly employed in this assay system (Figure 11C). We also performed a dose response curve for FD in the LPS microplate assay using different incubation times both in human (adding hFD to FD depleted serum) and mouse (mixing WT and FD-null sera) systems (Figures 11D and E). When we added various amounts of hFD back to FD-depleted human serum we observed an EC50 of 76ng/ml for the activation of AP with a 30 minute incubation and an EC50 of 5ng/ml with a 2 hour incubation (Figure 11D). Addition of hFD to WT serum did not increase AP activity (not shown). We also conducted similar experiments by mixing WT mouse sera with FD-null mouse sera. For this experiment the total percentage of serum in the assay was held constant (20%) but the proportion of WT and FD-null serum varied. Using our measured values of 10 µg/ml for WT serum, the amount of FD required for 50% activation in this assay was 1.8% (36 ng/ml) and 0.3% (6 ng/ml) for the 30 minute and 2 hour incubation times respectively (Figure 11E). These numbers are quite similar to what we saw with the RBC hemolysis assay.
FIGURE 11.
Kinetics and dose response curve for human Factor D in the rabbit RBC hemolysis and LPS microplate assays for AP. A-D were performed in FD-depleted human serum reconstituted with hFD. A) Dose response curve for hFD with a 5 min incubation. B) Kinetics of hemolysis when using 400 ng/ml hFD. C) Dose response curve of hFD when used with a 30 min incubation. RBC hemolysis data are expressed as percent hemolysis. D) Dose response curve for hFD added to FD-depleted human serum in the LPS microplate assay. E) Dose response curve for FD mixing experiment using WT and FD−/− mouse serum in the LPS microplate assay.
Discussion
Our studies using lipodystrophic and FD−/− mice establish that FD/adipsin is almost entirely produced from adipose tissue. FD was not detected in LD mice. Although FD was previously described to be highly expressed in adipose, others had reported significant expression in non-adipose tissues such as by the sciatic nerve in mice (21) as well as by muscle and lung in humans (19). Our experiments indicate though that while other tissues might have the capacity to produce FD, it is the adipose tissue that accounts for almost all of the circulating FD in mice. It should be noted when comparing the mouse and human systems that circulating mouse FD is found at higher concentrations [10–30 µg/ml, our data and (40)] than human FD (2–4 µg/ml).
In contrast to the mouse, there was reduced (~50%, p<0.05) FD levels in the sera of patients with CGL. This is most likely because the patients with lipodystrophy still have a small amount of adipose tissue as compared to the lipodystrophic mice that were genetically engineered to be completely devoid of adipose tissue. Alternatively, either FD is normally secreted from non-adipose tissues in humans, or non-adipose tissues [e.g. the liver which highly expresses PPAR-γ and some adipocyte markers in the lipodystrophic state (41)], compensate to secrete FD in the lipodystrophic state. FD levels were reduced to a lesser extent in the sera of patients with acquired generalized lipodystrophy (AGL) or FPL, a state of partial loss of adipose mass. It is unclear why patients with AGL did not have as severe a reduction in FD levels compared to CGL patients. One potential explanation is the fact that AGL patients retain variable amounts of visceral adipose whereas it is completely absent in CGL patients (42–44). In addition, marrow adipose tissue is retained in AGL and absent in both CGL1 and CGL2 (42–44), the patients studied here. Also, it is perhaps not too surprising that patients with CGL have detectable FD and AP activity as an increased incidence of Neisseria infections is not observed in these patients as has been described in patients with loss of function FD mutations (45–47).
Another interesting observation was that lipodystrophic mice had increased levels of circulating FB. This is unlikely to be due solely to a compensatory effect in the absence of FD as it was not seen in FD−/− mice (Figure 8). Factor B is an acute phase response protein (17). We favor a hypothesis that the hepatic steatosis in LD mice induces hepatic inflammation and this in turn stimulates hepatic FB expression.
A finding of note is that CVF was able to facilitate in vitro C3 cleavage to a greater extent in LD than FD−/− sera in vitro (Figure 9A). This may be due to a small amount of FD in sera of LD mice that is not detected by our assay systems (LPS microplate assay, RBC hemolysis or Western blotting). Another possibility is that the increased FB in sera of LD mice accounts for augmented CVF-mediated C3 cleavage. However, when CVF was injected into WT, FD−/−, LD or FB−/− mice, C3 was cleaved in WT, FD−/− and LD mice, but not in FB−/− mice (Figure 9B). These data point to a FD independent mechanism of CVF-induced C3 cleavage. One possibility is that a second “compensatory” protease is responsible (31). Kallikrein is a possible candidate given its similar protease activity to FD (48).
Using both mixing experiments with sera from FD−/− mice and using LD mice rescued with preadipocyte transplant, we found that only a small percentage of the normal FD level is required to support significant in vitro AP activity in a time-dependent manner (Figure 11 and see below). This was observed with two different assays: rabbit RBC hemolysis and the LPS microplate C3 deposition assay. Utilizing the LPS assay for FD, there was scant, if any, AP activity detected at 5 min (even with “normal” FD levels). At 30 min 3 to 10% of normal FD levels were required for maximal activity. Remarkably, at 120 min nearly maximal deposition occurred and required with less than 1% of the serum FD levels. These data strongly support the concept that the FD dosage for maximum AP activation is not assay specific (like for properdin) but is especially dependent on kinetics (duration of the incubation). Thus, time of incubation and FD concentration are key features in AP assays and will play out in a distinctive fashion at various tissue sites based in part as well on the type and magnitude of cellular/tissue damage or degeneration. We are in the process of comparing other assays for the AP.
This finding likely has clinical implications. Near complete inhibition of FD may be necessary to fully suppress AP complement activation in autoimmune and innate immune mediated inflammatory human diseases, a conclusion in contrast to that of prior studies that FD is the rate limiting component of AP activation (39). As we have shown, this dissimilarity is based on differences of in vitro reaction times. FD blocking Abs have been and are being developed for the treatment of diseases featuring AP activation such as AMD and have entered clinical trials (15, 16). This approach was prompted by the observation of complement system proteins being deposited in the retina of patients with AMD (49) and then followed by the discovery that common (9–12) and rare variants (50, 51) in complement FH are associated with risk for AMD. It awaits future studies to determine whether FD inhibition will result in immunosuppression and opportunistic infections [which could be addressed prophylactically with meningococcal vaccination (52, 53)] and whether low levels of functional FD that may survive FD inhibition are sufficient to sustain pathogenesis in AP-mediated disease models, especially in chronic inflammatory conditions such as AMD.
We further speculate that these data are likely to impact on how we envision complement activation in extravascular tissue sites. Rather amazingly, the results indicate that low levels of FD are sufficient to mediate AP activation. We propose that this process is a fundamental mechanism by which the host responds to altered self; that is, cellular and tissue debris that is continuously generated (both for intracellular and extracellular disposal).
Following this line of reasoning, we hypothesize that this disposal activity was part of the original complement system. It was then adapted by the host to facilitate recognition of a foreign agent. With the subsequent evolutionary development of circulatory systems, reaction speed becomes more critical. However, the AP, in particular, has maintained its ability to recognize and opsonize debris.
Acknowledgments
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. We would like to acknowledge Abhimanyu Garg for assistance in genotyping patients with lipodystrophy, Brian Finck for providing the fld mice and Madonna Bogacki and Lorraine Schwartz for manuscript editing.
Abbreviations
- AGL
acquired generalized lipodystrophy
- AGPAT2
acyl-glycerol acyltransferase 2
- AMD
age-related macular degeneration
- AP
alternative pathway
- APL
acquired partial lipodystrophy
- ASC
animal studies committee
- BAT
brown adipose tissue
- BLAST
basic local alignment search tool
- BSA
bovine serum albumin
- BSCL2
Berardinelli-Seip Congenital Lipodystrophy 2
- CGL
congenital generalized lipodystrophy
- CP
classical pathway
- CVF
cobra venom factor
- DIX
dexamethasone, insulin, IBMX
- DMEM
Dulbecco’s Modified Eagle Medium
- DTA
diphtheria toxin
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FB
Factor B
- FBS
fetal bovine serum
- FD
Factor D
- FH
Factor H
- Fld
fatty liver dystrophy
- FPL
familial partial lipodystrophy
- I
insulin
- IBMX
3-isobutyl-1-methylxanthine
- LD
lipodystrophy
- LPS
lipopolysaccharide
- LSL
lox-stop-lox
- MAC
membrane attack complex
- MASP
mannose-binding protein-associated serine protease
- MBL
mannose binding lectin
- MEF
mouse embryonic fibroblast
- P
properdin
- PBS
phosphate buffered saline
- PNGase
Peptide-N-Glycosidase
- PPAR
peroxisome proliferator-activated receptor
- RBC
red blood cell
- RIPA
radioimmunoprecipitation assay
- RT
room temperature
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- WAT
white adipose tissue
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Author Contributions: XW and CH performed experiments, analyzed and interpreted data, wrote and revised the manuscript. IH, AA and SM performed experiments, analyzed and interpreted data. CP, DEH, RB, and JPA provided resources, interpreted data and revised the manuscript.
References
- 1.Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. The Journal of experimental medicine. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pihl R, Jensen L, Hansen AG, Thogersen IB, Andres S, Dagnaes-Hansen F, Oexle K, Enghild JJ, Thiel S. Analysis of Factor D Isoforms in Malpuech-Michels-Mingarelli-Carnevale Patients Highlights the Role of MASP-3 as a Maturase in the Alternative Pathway of Complement. J Immunol. 2017 doi: 10.4049/jimmunol.1700518. [DOI] [PubMed] [Google Scholar]
- 3.Dobo J, Szakacs D, Oroszlan G, Kortvely E, Kiss B, Boros E, Szasz R, Zavodszky P, Gal P, Pal G. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep. 2016;6:31877. doi: 10.1038/srep31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oroszlan G, Kortvely E, Szakacs D, Kocsis A, Dammeier S, Zeck A, Ueffing M, Zavodszky P, Pal G, Gal P, Dobo J. MASP-1 and MASP-2 Do Not Activate Pro-Factor D in Resting Human Blood, whereas MASP-3 Is a Potential Activator: Kinetic Analysis Involving Specific MASP-1 and MASP-2 Inhibitors. J Immunol. 2016;196:857–865. doi: 10.4049/jimmunol.1501717. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The complement factsbook. Elsevier; Boston, MA: 2017. [Google Scholar]
- 7.Berger BE. The Alternative Pathway of Complement and the Evolving Clinical-Pathophysiological Spectrum of Atypical Hemolytic Uremic Syndrome. Am J Med Sci. 2016;352:177–190. doi: 10.1016/j.amjms.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Gavriilaki E, Thanassi JA, Yang G, Baines AC, Podos SD, Huang Y, Huang M, Brodsky RA. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica. 2017;102:466–475. doi: 10.3324/haematol.2016.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 11.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 12.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hageman GS, Hancox LS, Taiber AJ, Gehrs KM, Anderson DH, Johnson LV, Radeke MJ, Kavanagh D, Richards A, Atkinson J, Meri S, Bergeron J, Zernant J, Merriam J, Gold B, Allikmets R, Dean M, A. M. D. C. S. Group Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual M, Catana E, White T, Spiegelman BM, Schifferli JA. Inhibition of complement alternative pathway in mice with Fab antibody to recombinant adipsin/factor D. Eur J Immunol. 1993;23:1389–1392. doi: 10.1002/eji.1830230632. [DOI] [PubMed] [Google Scholar]
- 15.Le KN, Gibiansky L, van Lookeren Campagne M, Good J, Davancaze T, Loyet KM, Morimoto A, Strauss EC, Jin JY. Population Pharmacokinetics and Pharmacodynamics of Lampalizumab Administered Intravitreally to Patients With Geographic Atrophy. CPT Pharmacometrics Syst Pharmacol. 2015;4:595–604. doi: 10.1002/psp4.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do DV, Pieramici DJ, van Lookeren Campagne M, Beres T, Friesenhahn M, Zhang Y, Strauss EC, Phase Ia I. A phase ia dose-escalation study of the anti-factor D monoclonal antibody fragment FCFD4514S in patients with geographic atrophy. Retina. 2014;34:313–320. doi: 10.1097/IAE.0b013e3182979ddd. [DOI] [PubMed] [Google Scholar]
- 17.Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188:183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986;83:3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. The Journal of biological chemistry. 1992;267:9210–9213. [PubMed] [Google Scholar]
- 20.Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- 21.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 24.Flier JS, Lowell B, Napolitano A, Usher P, Rosen B, Cook KS, Spiegelman B. Adipsin: regulation and dysregulation in obesity and other metabolic states. Recent Prog Horm Res. 1989;45:567–580. doi: 10.1016/b978-0-12-571145-6.50017-0. discussion 580-561. [DOI] [PubMed] [Google Scholar]
- 25.Platt KA, Min HY, Ross SR, Spiegelman BM. Obesity-linked regulation of the adipsin gene promoter in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:7490–7494. doi: 10.1073/pnas.86.19.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowell BB, Napolitano A, Usher P, Dulloo AG, Rosen BS, Spiegelman BM, Flier JS. Reduced adipsin expression in murine obesity: effect of age and treatment with the sympathomimetic-thermogenic drug mixture ephedrine and caffeine. Endocrinology. 1990;126:1514–1520. doi: 10.1210/endo-126-3-1514. [DOI] [PubMed] [Google Scholar]
- 27.Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, Banks AS, Khandekar MJ, Dietrich A, Flier JS, Cinti S, Bluher M, Danial NN, Berggren PO, Spiegelman BM. Adipsin is an adipokine that improves beta cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loyet KM, Good J, Davancaze T, Sturgeon L, Wang X, Yang J, Le KN, Wong M, Hass PE, van Lookeren Campagne M, Haughney PC, Morimoto A, Damico-Beyer LA, DeForge LE. Complement inhibition in cynomolgus monkeys by anti-factor d antigen-binding fragment for the treatment of an advanced form of dry age-related macular degeneration. J Pharmacol Exp Ther. 2014;351:527–537. doi: 10.1124/jpet.114.215921. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Ma M, Ippolito GC, Schroeder HW, Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci U S A. 2001;98:14577–14582. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci U S A. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai AJ, Briles DE, Volanakis JE, Wetsel RA, Colten HR. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 34.Stover CM, Luckett JC, Echtenacher B, Dupont A, Figgitt SE, Brown J, Mannel DN, Schwaeble WJ. Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol. 2008;180:3313–3318. doi: 10.4049/jimmunol.180.5.3313. [DOI] [PubMed] [Google Scholar]
- 35.Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV., Jr DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52:657–667. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkey CJ, Spiegelman BM. A functional peroxisome proliferator-activated receptor-gamma ligand-binding domain is not required for adipogenesis. The Journal of biological chemistry. 2008;283:24290–24294. doi: 10.1074/jbc.C800139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertram P, Akk AM, Zhou HF, Mitchell LM, Pham CT, Hourcade DE. Anti-mouse properdin TSR 5/6 monoclonal antibodies block complement alternative pathway-dependent pathogenesis. Monoclon Antib Immunodiagn Immunother. 2015;34:1–6. doi: 10.1089/mab.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reue K, Xu P, Wang XP, Slavin BG. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J Lipid Res. 2000;41:1067–1076. [PubMed] [Google Scholar]
- 39.Lesavre PH, Muller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med. 1978;148:1498–1509. doi: 10.1084/jem.148.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. The Journal of clinical investigation. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab. 2003;88:5433–5437. doi: 10.1210/jc.2003-030835. [DOI] [PubMed] [Google Scholar]
- 43.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82:129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Scheller EL, Rosen CJ. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprong T, Roos D, Weemaes C, Neeleman C, Geesing CL, Mollnes TE, van Deuren M. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood. 2006;107:4865–4870. doi: 10.1182/blood-2005-07-2820. [DOI] [PubMed] [Google Scholar]
- 46.Hiemstra PS, Langeler E, Compier B, Keepers Y, Leijh PC, van den Barselaar MT, Overbosch D, Daha MR. Complete and partial deficiencies of complement factor D in a Dutch family. The Journal of clinical investigation. 1989;84:1957–1961. doi: 10.1172/JCI114384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biesma DH, Hannema AJ, van Velzen-Blad H, Mulder L, van Zwieten R, Kluijt I, Roos D. A family with complement factor D deficiency. The Journal of clinical investigation. 2001;108:233–240. doi: 10.1172/JCI12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiemstra PS, Daha MR, Bouma BN. Activation of factor B of the complement system by kallikrein and its light chain. Thromb Res. 1985;38:491–503. doi: 10.1016/0049-3848(85)90182-3. [DOI] [PubMed] [Google Scholar]
- 49.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 50.Kavanagh D, Yu Y, Schramm EC, Triebwasser M, Wagner EK, Raychaudhuri S, Daly MJ, Atkinson JP, Seddon JM. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015;24:3861–3870. doi: 10.1093/hmg/ddv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon J, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61:118–125. doi: 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horiuchi T, Tsukamoto H. Complement-targeted therapy: development of C5- and C5a-targeted inhibition. Inflamm Regen. 2016;36:11. doi: 10.1186/s41232-016-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]