Abstract
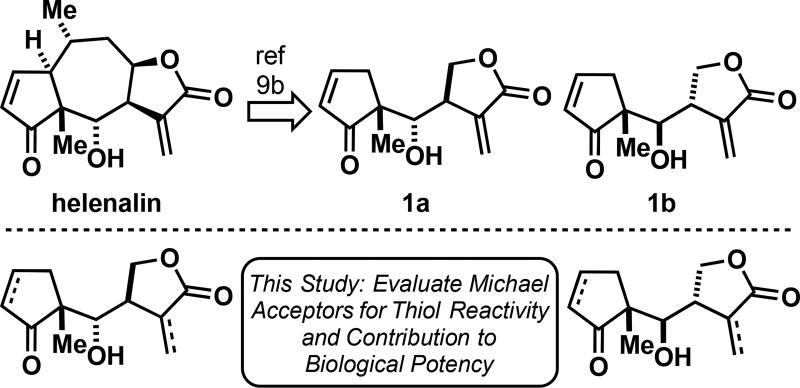
Helenalin is a pseudoguaianolide natural product that targets Cys38 within the DNA-binding domain of NF-κB transcription factor p65 (RelA). Helenalin contains two Michael acceptors that covalently modify cysteines, a α-methylene-γ-butyrolactone and a cyclopentenone. We recently reported two simplified helenalin analogues that mimic the biological activity of helenalin and contain both electrophilic moieties. To determine the individual contributions of the Michael acceptors toward NF-κB inhibition, we synthesized a small library of helenalin-based analogues containing various combinations of α-methylene-γ-butyrolactones and cyclopentenones. The kinetics of thiol addition to a subset of the analogues was measured to determine the relative thiol reactivities of the embedded electrophiles. Additionally, the cellular NF-κB inhibitory activities of the analogues were determined to elucidate the contributions of each Michael acceptor to biological potency. Our studies suggest the α-methylene-γ-butyrolactone contributes most significantly to the NF-κB inhibition of our simplified helenalin analogues.
Keywords: cysteine reactive, Michael acceptor, p65/RelA transcription factor, helenalin, Bis-electrophile
Graphical abstract
A library of small molecules containing α-methylene-γ-butyrolactones and/or cyclopentenones representative of the natural product helenalin was synthesized. The kinetics of hetero-Michael addition of thiols to each electrophile was characterized, as well as the abilities of each compound to regulate NF-κB signaling. We find the α-methylene-γ-butyrolactone reacts irreversibly with biological thiols and contributes most to biological activity amongst the two electrophiles.
Introduction
NF-κB transcription factors regulate the cellular inflammatory response, and deregulated activity can lead to tumorigenesis and cancer progression, coronary disease, and autoimmunity.[1] Although hundreds of small molecules have been reported that inhibit the NF-κB pathway, most target up-stream kinases or regulatory proteins that overlap with other signaling pathways.[2] Consequently, the probability of off-target effects is significant, which limits the utilities of most NF-κB inhibitors as chemical probes. Targeting the most downstream proteins in the pathway, the NF-κB (p50/p65) transcription factor heterodimer, may yield more specific NF-κB inhibitors.
Developing high affinity small molecule modulators of protein-protein or protein-DNA binding interfaces essential to transcription factor signaling is challenging.[3] One strategy in this regard is to utilize covalent modifiers, which have demonstrated utility for increasing potency and/or specificity to inhibit kinases, ATPases, and bromodomains.[4] Specifically, chemical probes and therapeutic molecules have been designed to have thiol reactive Michael acceptors to gain potency, selectivity, and overcome biological resistance.[5] Natural products have provided a rich source for cysteine reactive covalent modifiers that inhibit specific classes of proteins, many of which contain a α-methylene-γ-butyrolactone.[6] Helenalin is a pseudoguaianolide natural product isolated from plants in the Arnica and Helenium genera that is a covalent protein modifier (Figure 1).[7] This natural product features two Michael acceptors, a α-methylene-γ-butyrolactone and a cyclopentenone, which was discovered to target p65 of the NF-κB transcription factor heterodimer.[8]
Figure 1.
(top) Helenalin is a pseudoguaianolide natural product with two Michael acceptors. 1a and 1b mimic the biological activity of helenalin.[9b] (bottom) We report a synthesis of helenalin analogues with varied Michael acceptors, the kinetic reactivities of helenalin analogues to thiols, and the NF-κB inhibitory activities of a number of helenalin analogues.
Recently, our group synthesized structurally simplified helenalin molecules containing the same Michael acceptors as the natural product (1a and 1b, Figure 1).[9] Our helenalin-based probes were shown to inhibit the NF-κB pathway in a cellular reporter assay comparable to helenalin and were able to alkylate Cys38 within the DNA binding pocket of p65. However, the individual contributions of the cyclopentenone and α-methylene-γ-butyrolactone with respect to inhibition of NF-κB signaling were not determined. Previous studies suggest that helenalin derivatives without the α-methylene-γ-butyrolactone lose much, but not all, of their activity in biological assays compared to helenalin.[10] However, a comparable thiol reactivity and biological potency study of simplified helenalin analogues representative of 1a and 1b has not been previously reported, although other fully synthetic helenalin-inspired analogues have demonstrated NF-κB inhibitory activities.[11]
The current study reports the synthesis of simplified helenalin analogues containing various combinations of cyclopentenones and α-methylene-γ-butyrolactones, characterization of the kinetic reactivities of a subset of compounds to model biological thiols, and determination of the cellular NF-κB inhibitory activities of the library of simplified helenalin analogues.
Results and Discussion
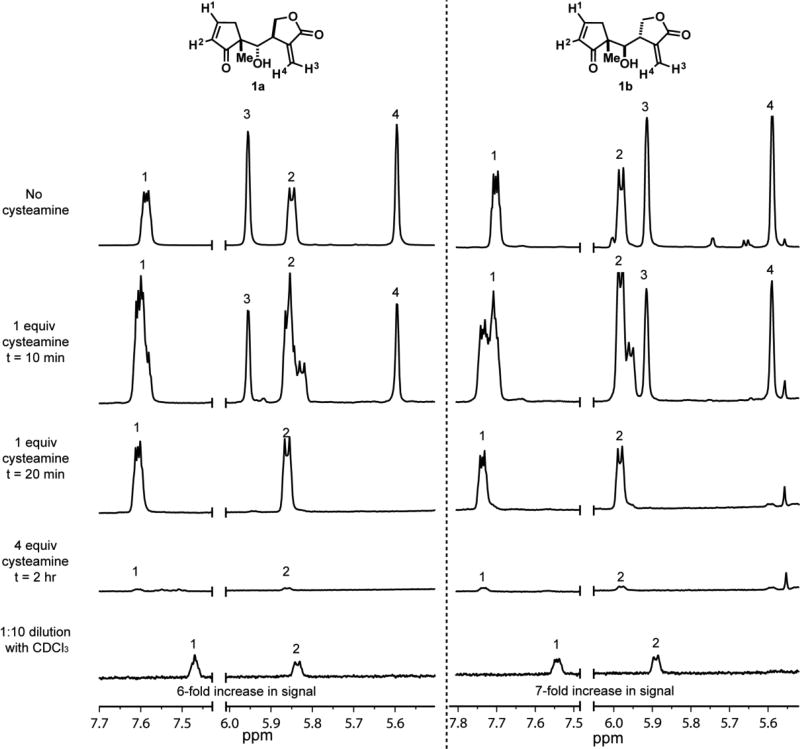
A qualitative evaluation of the reactivities of both Michael acceptors present on 1a and 1b was conducted using a previously reported NMR thiol reactivity assay.[12] Simplified helenalin derivatives 1a and 1b were incubated separately with cysteamine in DMSO-d6 at pH 9 (Figure 2). Both compounds behaved equivalently in this experiment. Addition of one equivalent of cysteamine yielded a mixture of hetero-Michael adducts that equilibrated exclusively to the α-methylene-γ-butyrolactone addition product. The observation of non-overlapping H1 and H2 resonances at 10 minutes that are distinct from starting material and product likely corresponds to the formation of a new stereocenter at the alpha position of the α-methylene-γ-butyrolactone upon hetero-Michael addition of cysteamine. Protonation of the resulting enolate from either the top or bottom face of the butyrolactone ring yields diastereomers at the α-carbon. However, at 20 minutes these peaks coalesce indicating equilibration to the more thermodynamically favored diastereomer. Nearly complete hetero-Michael addition to the cyclopentenone was achieved by adding more cysteamine, which required a total of four equivalents. Addition of cysteamine past four equivalents yielded no change in adduct resonances by NMR. To further query the reversibility of the hetero-Michael addition of cysteamine to both Michael acceptors, the DMSO-d6 solution containing 1a or 1b reacted with 4 equivalents of cysteamine was diluted 1:10 with CDCl3, which induces retro-hetero-Michael addition because of the decrease in solvent polarity and increased solubility of cysteamine in CDCl3.[13] Upon dilution of the reaction mixture, the endocyclic enone signals increased approximately 6-fold for 1a and 7-fold for 1b, but no signal returned corresponding to the exocyclic methylene for either compound. These data further support that hetero-Michael addition to the cyclopentenone is reversible and addition to the α-methylene-γ-butyrolactone is irreversible, which is consistent with previous reports.[8b,12,14] Although no Michael addition to the cyclopentenone was observed until full reaction with the α-methylene-γ-butyrolactone, we cannot rule out the possibility of cysteamine reacting at the cyclopentenone in a reversible fashion with comparable or faster kinetics than the α-methylene-γ-butyrolactone. This would require the reversible reaction to occur faster than the coalescence frequency of the observed NMR signals between the starting material and addition intermediate (enolate) or product.
Figure 2.
Simplified helenalin derivatives 1a (left spectra) and 1b (right spectra) were reacted with cysteamine in DMSO-d6.[12] The reactivities of the cyclopentenone and α-methylene-γ-butyrolactone of 1a and 1b were qualitatively assessed by 1H NMR analyses of protons H1–H4 as a function of cysteamine concentration and time. Reversibility of cysteamine adducts were determined by dilution studies with CDCl3. Proton signals were normalized to a TMS internal standard. These data are representative of three independent experiments.
NMR kinetic reactivity experiments were also performed in D2O to more closely mimic biological systems. Reaction of one equivalent of cysteamine with 1a and 1b in D2O (pH = 9) resulted in the same reactivity pattern as shown in Figure 2 (performed in DMSO-d6); however, hetero-Michael addition to the α-methylene-γ-butyrolactone with cysteamine occurred in less than 5 minutes. Addition of another equivalent of cysteamine (total of two equivalents) resulted in complete reaction with the cyclopentenone in less than 5 additional minutes. When two equivalents of cysteamine were added to 1a and 1b at once in D2O both Michael acceptors reacted to completion in less than five minutes.
Another biologically relevant thiol, N-acetyl-cysteine (N-Ac-Cys), yielded similar results when studied in D2O at neutral and basic pH (pH 7 and 9, respectively). These data suggest that the electrophilic cyclopentenones and α-methylene-γ-butyrolactones of 1a and 1b are competent to rapidly form hetero-Michael adducts with thiols.
Helenalin analogues 1a and 1b have been shown to covalently target the DNA-binding surface of p65, which contains solvent exposed cysteines.[9b] However, the individual contributions of both Michael acceptors of 1a and 1b to biological potency have not been established. Therefore, we sought to synthesize analogues of 1a and 1b with various combinations of Michael acceptors.
Attempts to selectively reduce the exocyclic methylene over the cyclopentenone starting from 1a and 1b resulted in non-selective reduction of both Michael acceptors or decomposition. Specifically, the utilization of boron (NaBH4, NaBH3CN), aluminum (LiAlH4, DIBAL-H), or copper hydride (Stryker’s reagent) sources for selective reduction of the exocyclic methylene over the cyclopentenone was unsuccessful due to decomposition of the starting material. Attempts to selectively hydrogenate the individual Michael acceptors using standard catalytic hydrogenation (Pd/C, 1 atm H2) with short reaction times (1–10 min) or catalytic transfer hydrogenation (Pd/C, NH4HCO2) were also unsuccessful. Therefore, a different strategy was developed to differentiate the Michael acceptors.
The approach involved the synthesis of 2 and 3 where the cyclopentenone was replaced with a cyclopentanone bearing a TBS-protected enol ether (Supplementary Scheme S1).[9b] These two building blocks permitted assembly of our small library (9–20) of simplified helenalin analogues utilizing metal-mediated hydrogenations followed by acid-catalyzed deprotections or Saegusa-Ito oxidations.[15]
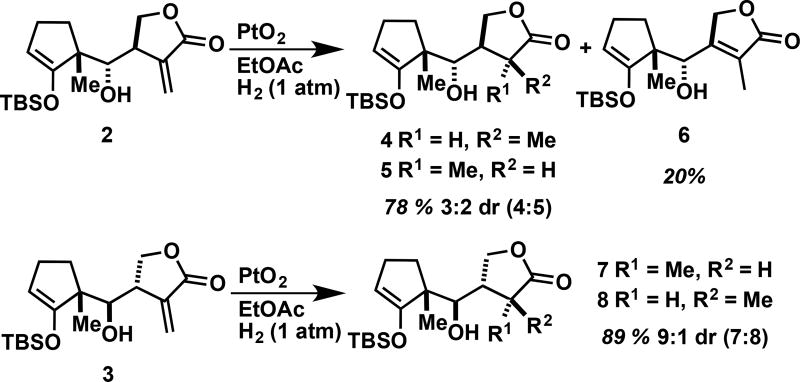
The exocyclic methylene of diastereomers 2 and 3 were hydrogenated with catalytic PtO2 (Scheme 1).[10b] Hydrogenation of 2 resulted in an inseparable mixture of 4 and 5 in 78% overall yield in a 3:2 dr favoring diastereomer 4. Interestingly, an isomerized product 6 was also isolated in 20% yield. This compound is likely the result of the platinum forming a bond to the α-carbon of the lactone ring after delivering one hydride to the exocyclic methylene, which would then leave the possibility for β-hydride elimination, giving rise to the stable quaternary olefin.[16] Hydrogenation of 3 using the same conditions as used for 2 resulted in two separable diastereomers 7 and 8 in a 9:1 dr (89% overall yield). The isomerized product analogous to 6 was surprisingly not observed in this reaction.
Scheme 1.
Reduction of the exocyclic methylene on derivatives 2 and 3.
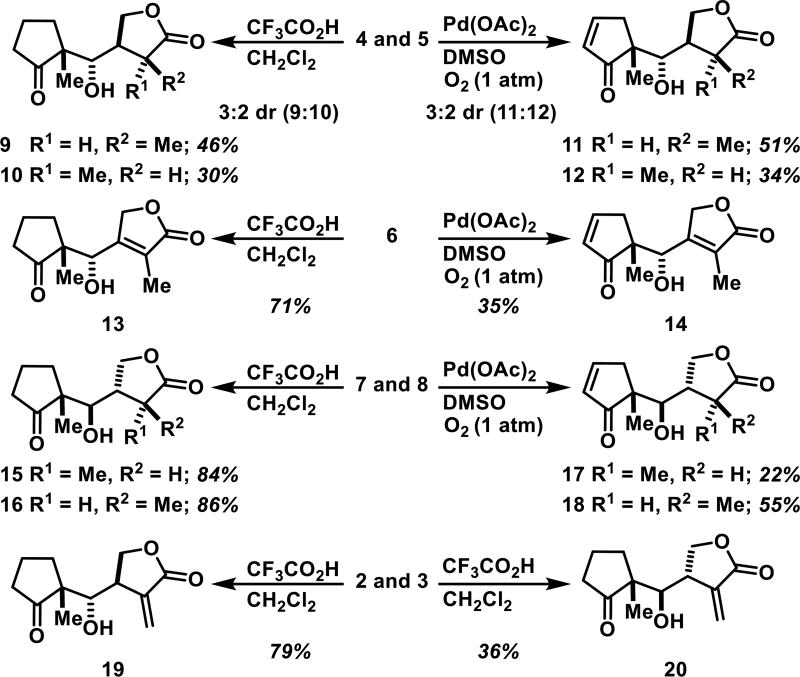
Inseparable diastereomers 4 and 5, the isomerized 6, and separated diastereomers 7 and 8 were either deprotected in the presence of CF3CO2H resulting in compounds without a cyclopentenone or subjected to Saegusa-Ito[15] oxidation conditions to install the endocyclic enone, yielding analogues bearing a cyclopentenone (Scheme 2). Acid-mediated deprotection of diastereomers 4 and 5 yielded separable diastereomers 9 and 10 in 76% yield (3:2 dr). Intermediates 4 and 5 were reacted as a mixture with Pd(OAc)2 in DMSO under O2 (1 atm) to afford separable diastereomers 11 and 12 in 85% yield (3:2 dr). Isomerized product 6 was similarly subjected to deprotection and Saegusa-Ito oxidation to give 13 and 14 in 71% and 35% yields, respectively. Separated 7 and 8 were individually deprotected with CF3CO2H to give 15 and 16 in 84% and 86% yields, respectively. Subjecting 7 and 8 to the Saegusa-Ito oxidation conditions yielded enones 17 and 18 (22% and 55% yields, respectively). Finally, intermediates 2 and 3 were deprotected to obtain 19 and 20 in 79% and 36% yields, respectively, which lack the endocyclic enone, but maintain the α-methylene-γ-butyrolactone electrophile.
Scheme 2.
Synthesis of simplified helenalin analogues 9 – 20.
The stereochemistry of starting materials 2 and 3 were previously reported;[9b] however, the stereochemistry of the methyl group resulting from PtO2-mediated hydrogenation required determination for each derivative. Attempts to annotate the stereochemistries of 4, 5, 7, and 8 utilizing various NMR experiments were unsuccessful because of their instabilities over the course of lengthy NMR experiments and interference of diagnostic resonances due to the tert-butyldimethylsilyl and methyl groups. Therefore, 9–11, 15, and 16 (all lacking the TBS group) were used to define the stereochemistry of the reduced exo-methylene using NOESY experiments. These data can be found in the Supporting Information (SI).
With analogues containing single Michael acceptors available, we then turned our attention to characterizing the individual thiol reactivities of the cyclopentenone and α-methylene-γ-butyrolactone. Because the hetero-Michael addition of thiols to the electrophiles occurred too quickly to be accurately determined using 1H NMR spectroscopy, we next utilized HPLC analysis of rapidly quenched reaction aliquots to calculate the kinetics of thiol addition to the embedded electrophiles. Selected analogues were incubated with one, two, or ten equivalents of N-Ac-Cys as the model biological thiol. The reactions were carried out in PBS (pH = 7) in the presence of tris(2-carboxyethyl)phosphine (TCEP) and benzophenone (an internal standard for integration of HPLC signals). The TCEP was added to prevent oxidation and dimerization of N-Ac-Cys. The reaction was initiated by mixing compounds with a solution of N-Ac-Cys, and aliquots were removed from the reaction mixture and quenched in acidic H2O (pH = 1; acidified with CF3CO2H). The quenched samples were then analyzed by HPLC using an eluent system of distilled and deionized (dd)H2O and MeCN (both containing 0.1% CF3CO2H). Previous studies have shown that thiol addition to α,β-unsaturated ketones do not occur, or occurs at a negligible rate, at or below pH 3.[17] Notably, compounds and products used in this assay were stable to the quenching conditions for up to 16 hours.
Analogues 19 and 20 were used to determine the rate of thiol addition to the α-methylene-γ-butyrolactone in the absence of the cyclopentenone. To measure the rate of thiol addition to the cyclopentenone, 11 was used because of its stability to the assay conditions and lack of apparent reversibility under acidic conditions. Simplified helenalin derivatives 1a and 1b were not stable under the acidic conditions (required for quenching the hetero-Michael addition reaction) to permit accurate annotations of the kinetics of thiol addition to the electrophiles by HPLC. When N-Ac-Cys was reacted with 11, 19, and 20 at equimolar concentrations, first-order reaction kinetics were observed (Table 1; for kinetic plots see the SI). As the concentration of N-Ac-Cys was increased to two and ten equivalents the rate increased approximately 2-fold and 10-fold, respectively, indicating that the reaction is first-order in the concentration of N-Ac-Cys. The rate of reaction of 19 (3.7×10−3 s−1) and 20 (4.4×10−3 s−1), containing only the α-methylene-γ-butyrolactone, was approximately 10-fold faster compared to 11 (4.5×10−4 s−1), containing only the cyclopentenone.
Table 1.
First-order reaction rates (s−1) of N-acetyl cysteine (N-Ac-Cys) addition to compounds 11, 19, and 20. Values shown are an average of two runs. Differences between replicates were ≤ 9.5 × 10−5 s−1 for all kinetic experiments. See Supporting Information for kinetic plots and analysis.
| first-order reaction rate (s−1) | |||
|---|---|---|---|
|
|
|||
| compound |
N-Ac-Cys (1 equiv.) |
N-Ac-Cys (2 equiv.) |
N-Ac-Cys (10 equiv.) |
| 11 | 4.5 × 10−4 | 8.8 × 10−4 | 7.3 × 10−3 |
| 19 | 3.7 × 10−3 | 6.2 × 10−3 | 3.9 × 10−2 |
| 20 | 4.4 × 10−3 | 1.0 × 10−2 | 6.4 × 10−2 |
Kinetics studies have not been performed between helenalin and N-Ac-Cys; however, it has been reported that cysteine reacts with helenalin in D2O within five minutes, which agrees with data in this study.[14] Conversely, thiol reactivity experiments (by 1H NMR) of helenalin and glutathione (GSH) found preferential reactivity to the cyclopentenone over the α-methylene-γ-butyrolactone at an approximate 14-fold faster rate (1.7×10−3 vs. 1.2×10−4 M−1 s−1) with second-order reaction kinetics.[14] To the contrary, the data presented here shows first-order reaction kinetics between N-Ac-Cys and the simplified helenalin analogues containing a single Michael acceptor. First-order reaction kinetics is atypical for thiol-Michael addition reactions.[5,14] Differences in compound structures and/or assay conditions (e.g., pH and the type of thiol evaluated) may likely explain the discrepancy. For example, in previous studies with helenalin, cysteine had the reverse reactivity of GSH in which addition to the α-methylene-γ-butyrolactone occurred first and was complete in less than five minutes.[14] Our reactivity studies of simplified helenalin analogues with cysteamine and N-Ac-Cys have revealed preferential thermodynamic reactivity at the α-methylene-γ-butyrolactone over the cyclopentenone, which is consistent with earlier work on the parent natural product. Additional studies are required to fully characterize the discrete mechanistic steps for thiol-Michael addition in our system.
Simplified helenalin analogues 9–20 were next screened to test the contribution of each Michael acceptor to NF-κB modulatory activity. In this assay, A549 cells with a stably transfected NF-κB luciferase reporter were dosed with compounds at the respective concentration 30 minutes prior to induction of NF-κB signalling with TNF-α for 7 h and determination of relative NF-κB activity (Table 2).[18]
Table 2.
Inhibition of canonical NF-κB signalling by simplified helenalin analogues. Values are the mean ± S.D. from n ≥ 3 biological replicates and were normalized to a no compound, induced control.
| no Michael acceptors |
NF-κB activity (%; Mean ± S.D.) | ||
|---|---|---|---|
|
| |||
| 250 µM | 100 µM | ||
| 9 | 86 ± 1 | 91 ± 3 | |
| 10 | 86 ± 5 | 97 ± 8 | |
| 13 | 78 ± 5 | 84 ± 1 | |
| 15 | 99 ± 8 | 99 ± 7 | |
| 16 | 79 ± 6 | 82 ± 2 | |
|
| |||
| enone only | 250 µM | 100 µM | 50 µM |
|
| |||
| 11 | 50 ± 5 | 85 ± 3 | 92 ± 6 |
| 12 | 34 ± 4 | 69 ± 4 | 93 ± 11 |
| 14 | 43 ± 4 | 73 ± 3 | - |
| 17 | 78 ± 9 | 86 ± 12 | 95 ± 5 |
| 18 | 63 ± 4 | 83 ± 8 | 88 ± 6 |
| S4 | 23 ± 4 | 62 ± 2 | - |
|
| |||
| butyrolactone only | 50 µM | 20 µM | 10 µM |
| 19 | 11 ± 2 | 43 ± 1 | 71 ± 2 |
| 20 | 12 ± 1 | 42 ± 2 | 69 ± 2 |
| 1b[a] | 7 ± 1 | 16 ± 8 | 52 ± 17 |
As expected, all fully reduced compounds containing no Michael acceptors (9, 10, 15, and 16) failed to substantially inhibit induced NF-κB activity at 250 µM, the highest concentration tested. Isomerized 13 displayed the most activity of all analogues in this cohort (remaining NF-κB activity: 78% [250 µM] and 84% [100 µM]).[19]
Compounds containing the cyclopentenone but not the α-methylene-γ-butyrolactone had only slightly improved activity compared to the fully reduced analogues. Cells dosed at 100 µM with 11 and 12 still had 85% and 69% residual NF-κB activity, respectively. Both compounds were slightly more potent at 250 µM. Enones 17 and 18 had little inhibitory activity against the NF-κB pathway at 100 µM (residual NF-κB activity: 86% and 83%, respectively), and increasing the concentration to 250 µM did not substantially improve potency. Cells dosed with isomerized 14 had similar potency (NF-κB activity: 43% [250 µM] and 73% [100 µM]). The stereochemistry of the reduced methyl group seemed to have little effect on the inhibitory activity of 11–12 and 17–18, which only contain the cyclopentenone.
On the other hand, analogues bearing α-methylene-γ-butyrolactones were substantially more potent. At 50 µM treatment both 19 and 20 showed inhibition of induced NF-κB activity with only 12% and 11% residual luciferase activity remaining, respectively. A slightly lower 20 µM treatment yielded significant activity (remaining NF-κB activity: 43% [19] and 42% [20]). In comparison, helenalin reduces induced NF-κB signaling to 54% at 2.5 µM and 14% at 5 µM in this same assay.[9b]
Our data show that simplified helenalin analogues without either Michael acceptor are inactive, whereas analogues containing only the cyclopentenone (11–12 and 17–18) have very modest activity. These data suggest some contribution of the cyclopentenone to the biological activity of helenalin and simplified analogues 1a and 1b. Conversely, analogues containing only the α-methylene-γ-butyrolactone (19–20) still maintain low micromolar inhibitory activity, akin to 1a and 1b.[9b]
Interestingly, S4 (Scheme S2), which is 17 but esterified with 5-pentynoic acid at the secondary hydroxyl group, has increased activity compared to the non-esterified derivatives (residual NF-κB activity: 23% [250 µM] and 62% [100 µM] for S4 versus 78% [250 µM] for 17). These results may be explained by the decreased polarity of S4 in comparison to non-esterified derivatives (e.g., 17), resulting in better cell uptake. However, more studies are needed to corroborate this initial result.
Conclusions
We report the synthesis of structurally simplified helenalin analogues bearing various combinations of cyclopentenone and α-methylene-γ-butyrolactone electrophiles. Evaluation of these molecules in reactivity assays with biological thiols revealed both electrophiles react with cysteamine to yield Michael adducts, with reactivity at the cyclopentenone occurring more slowly and reversibly. Conversely, α-methylene-γ-butyrolactones react more quickly with N-Ac-Cys to afford Michael adducts that are irreversible. Screening of the library of simplified helenalin analogues for inhibition of NF-κB signaling in a cellular luciferase reporter assay revealed that analogues containing α-methylene-γ-butyrolactones were significantly more potent than molecules only bearing a cyclopentenone, although the latter electrophile did seem to contribute to activity. Taken together, our study provides new insights into the rational design of covalent inhibitors targeting NF-κB p65 and provides a head-to-head comparison of the reactivity profiles of two common electrophiles, cyclopentenones and α-methylene-γ-butyrolactones, which are widely utilized in chemical probes and therapeutics.
Experimental Section
Thiol Reactivity Assay
Simplified helenalin analogues were stored as 100 mM solutions in DMSO, benzophenone was stored as a 40 mM solution in DMSO, and N-Ac-Cys was stored as a solid in a glovebox (VAC Omni-lab) under positive argon pressure. DMSO stock solutions of test compounds were stored in a −20 °C freezer. Aliquots of N-Ac-Cys were weighed and transferred from the glovebox just prior to use. A 2× thiol solution containing N-Ac-Cys, TCEP, and benzophenone was prepared in 1× PBS and adjusted to pH = 7 with aqueous KOH (6 M). To initiate the reaction, 500 µL of the above solution was added to 500 µL of a solution of the simplified helenalin analogue in PBS. The final reaction conditions consisted of 1, 2, or 10 equiv of N-Ac-Cys, 1, 2, or 10 equiv of TCEP, 200 µM benzophenone, and 1 mM (1 equiv) of simplified helenalin analogue in PBS at pH = 7 (containing 0.5% v/v DMSO). Aliquots (100 µL) of the reaction were taken at various time points (including t = 0; immediately following setup) and quenched by addition to aqueous CF3CO2H (1% v/v CF3CO2H solution; 100 µL; pH = 1). Each aliquot was then analyzed via HPLC using the same method described for purity testing of the synthesized compounds (see Supporting Information), except 0.1% CF3CO2H modifier was added to the eluents. Sample concentration was monitored by integrating the area under the curve of the remaining starting material for each time point, which was normalized to the internal standard benzophenone peak area and initial concentration measurement (t = 0 s). Experiments were run in duplicate and data are shown as an average of the two runs. Differences between replicates were ≤ 9.5×10−5 s−1 for all kinetic experiments.
NF-κB Luciferase Reporter Assay
Compounds were tested for inhibition of the NF-κB pathway in stably transfected A549 cells containing an NF-κB driven luciferase reporter as previously described.[18] Cell viability information can be found in the SI (Table S2).
General Methods and Materials
Unless otherwise noted, reactions were performed in flame-dried glassware under a nitrogen or argon atmosphere and stirred with a Teflon-coated magnetic stir bar. Liquid reagents and solvents were transferred via syringe and cannula using standard techniques. Reaction solvents dichloromethane (DCM), tetrahydrofuran (THF) and diethyl ether (Et2O) were dried by passage over a column of activated alumina using a solvent purification system (MBraun). All other chemicals were used as received unless otherwise noted. Reaction temperatures above 23 °C refer to oil bath temperature, which was controlled by a temperature modulator. Reaction progress was monitored by thin layer chromatography using EMD Chemicals Silica Gel 60 F254 glass plates (250 µm thickness) and visualized by UV irradiation (at 254 nm) and/or KMnO4 stain. All preparative TLC was done using a Silicycle glass backed extra hard layer preparative TLC plate (particle size: 60 Å, plate size: 20×20 cm, thickness: 1000 µm, indicator F-254). Silica gel chromatography was performed on a Teledyne-Isco Combiflash Rf-200 instrument utilizing Redisep Rf High Performance silica gel columns (Teledyne-Isco) or flash column chromatography was performed using SiliCycle silica gel (32–63 µm particle size, 60 Å pore size). 1H NMR (400, 500, or 600 MHz) and 13C NMR (100, 125, 150 MHz) spectra were recorded at room temperature on a Bruker NMR spectrometer. 1H and 13C chemical shifts (δ) are reported relative to the solvent signal, CHCl3 (δ = 7.26 for 1H NMR and δ = 77.00 for 13C NMR). Proton signals from hydroxyl groups were included in annotations for spectral shifts if visible. Some spectra contain TMS (0.05% v/v). High resolution mass spectral data were obtained at the Analytical Biochemistry Core Facility of the Masonic Cancer Center on an LTQ OrbiTrap Velos Mass Spectrometer (Thermo Fisher). All compounds tested in biological assays were >95% pure by HPLC (See Table S1 in Supporting Information for purities).
General Procedures
General Procedure A: Reduction of exocyclic methylene butyrolactone
The substrate (0.03–0.06 M) was dissolved in EtOAc, PtO2 was added (0.1 equiv), the reaction was degassed (3×) and backfilled with H2 (1 atm), and then allowed to react for 30 minutes. The reaction flask was then degassed and backfilled with N2 (2×), then opened to the air. The suspension was then filtered through Celite and rinsed with EtOAc (3×, 10 mL). The resulting solution was concentrated in vacuo.
General Procedure B: Deprotection of silyl enol ether
The substrate (0.5–0.1 M) was dissolved in DCM in an open atmosphere. TFA (0.1 mL) was added to the reaction and stirred for 15 minutes at RT. The reaction was quenched with aqueous NaHCO3 (10 mL) and extracted with DCM (10 mL, 3×). The resulting organic layer was dried over Na2SO4 and concentrated in vacuo.
General Procedure C: Saegusa-Ito oxidation.[15]
The substrate (0.01 M) was dissolved in DMSO and Pd(OAc)2 was added (0.5 equiv). The reaction was degassed and backfilled with O2 (1×, 1 atm). The reaction was stirred for 48 hrs at RT. Water (10 mL) was then added to the reaction and extracted with EtOAc (3×, 10 mL). The resulting organic layer was washed with H2O (1×, 15 mL) and brine (1×, 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo.
(3S,4R)-4-((S)-((R)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methyldihydrofuran-2(3H)-one (4), (3R,4R)-4-((S)-((R)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methyldihydrofuran-2(3H)-one (5), and 4-((S)-((R)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methylfuran-2(5H)-one (6)
Followed General Procedure A: Compound 2 (100 mg, 0.295 mmol); EtOAc (5 mL); PtO2 (7 mg, 0.030 mmol). The crude product was SiO2 (deactivated with 1% NEt3) purified with EtOAc (0–25%) in hexanes to give an inseparable mixture of diastereomers 4 & 5 (78 mg, 78% yield) and the isomerized product 6 (20 mg, 20% yield).
Data for 4 (major diastereomer, 3:2 dr): 1H NMR (500 MHz, CDCl3): δ 4.64 (t, J = 2.4 Hz, 1H)*, 4.29 (dd, J = 8.8, 7.6 Hz, 1H), 4.07 (dd, J = 11.3, 8.8 Hz, 1H), 3.62 (dd, J = 9.9, 3.9 Hz, 1H), 2.82 – 2.74 (m, 1H), 2.73 – 2.66 (m, 1H)*, 2.30 – 2.21 (m, 1H), 2.21 – 2.13 (m, 1H)*, 1.97 (d, J = 4.0 Hz, 1H), 1.95 – 1.87 (m, 1H)*, 1.60 – 1.48 (m, 1H)*, 1.34 (d, J = 7.6 Hz, 3H)*, 1.01 (s, 3H), 0.93 (s, 9H)*, 0.21 (s, 3H)*, 0.18 (s, 3H)*. 13C NMR (125 MHz, CDCl3): δ 180.2, 156.9, 101.6, 73.2, 68.6, 51.7, 42.8, 37.9, 29.4, 25.56*(3C, CH3), 25.53*, 22.1, 17.9, 10.4, −4.65*, −5.32*.
Data for 5 (minor diastereomer): 1H NMR (500 MHz, CDCl3): δ 4.66 (t, J = 2.4 Hz, 1H)*, 4.36 (t, J = 8.7 Hz, 1H), 4.01 (app t, J = 8.8 Hz, 1H), 3.57 (dd, J = 6.1, 4.2 Hz, 1H), 2.73 – 2.66 (m, 1H)*, 2.41 – 2.32 (m, 1H), 2.30 – 2.21 (m, 1H)*, 2.21 – 2.13 (m, 1H)*, 2.03 (d, J = 4.3 Hz, 1H), 2.01 – 1.94 (m, 1H)*, 1.60 – 1.48 (m, 1H)*, 1.36 (d, J = 7.4 Hz, 3H)*, 1.06 (s, 3H), 0.93 (s, 9H)*, 0.21 (s, 3H)*, 0.18 (s, 3H)*. 13C NMR (125 MHz, CDCl3): δ 180.3, 156.7, 101.9, 76.9, 70.3, 51.8, 45.0, 36.7, 30.0, 25.62*(3C, CH3), 25.54*, 22.3, 18.0, 16.9, −4.69*, −5.33*. *Overlapping signals for diastereomers. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H32O4Si 341.2143, found 341.2133.
Data for 6: 1H NMR (500 MHz, CDCl3): δ 4.91 (dq, J = 17.2, 1.9 Hz, 1H), 4.82 – 4.75 (m, 1H), 4.72 (d, J = 4.3 Hz, 1H), 4.70 (t, J = 2.4 Hz, 1H), 2.39 (d, J = 4.4 Hz, 1H), 2.29 – 2.20 (m, 1H), 2.18 – 2.11 (m, 1H), 2.00 – 1.92 (m, 1H), 1.87 (t, J = 2.0 Hz, 3H), 1.56 – 1.48 (m, 1H), 1.08 (s, 3H), 0.96 (s, 9H), 0.23 (s, 3H), 0.20 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 175.0, 159.4, 155.9, 125.3, 102.6, 71.8, 70.2, 52.4, 30.1, 25.6 (3C, CH3), 25.4, 21.8, 18.0, 9.7, −4.6, −5.4. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H30O4Si 339.1986, found 339.1980.
(3R,4S)-4-((R)-((R)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methyldihydrofuran-2(3H)-one (7) and (3S,4S)-4-((R)-((R)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methyldihydrofuran-2(3H)-one (8)
Followed General Procedure A: Compound 3 (50 mg, 0.148 mmol); EtOAc (5 mL); PtO2 (4 mg, 0.015 mmol). The crude product was SiO2 purified with EtOAc (0–25%) in hexanes to give separable diastereomers 7 (40 mg) and 8 (4 mg) with an 89% overall yield (9:1 dr).
Data for 7 (major diastereomer, contains <5% of minor diastereomer): 1H NMR (500 MHz, CDCl3): δ 4.58 (t, J = 2.4 Hz, 1H), 4.30 – 4.23 (m, 1H), 4.03 (dd, J = 11.2, 9.0 Hz, 1H), 3.73 (dd, J = 9.7, 2.6 Hz, 1H), 3.09 (d, J = 3.1 Hz, 1H), 2.81 – 2.70 (m, 2H), 2.30 – 2.21 (m, 1H), 2.21 – 2.12 (m, 1H), 1.73 – 1.63 (m, 1H), 1.54 – 1.46 (m, 1H), 1.32 (d, J = 6.9 Hz, 3H), 1.14 (s, 3H), 0.93 (s, 9H), 0.22 (d, J = 1.9 Hz, 3H), 0.17 (s, 3H), Impurities: 4.79 – 4.40, 1.9. 13C NMR (125 MHz, CDCl3): δ 180.4, 158.6, 101.2, 75.6, 68.1, 49.8, 43.1, 37.5, 31.6, 25.6 (3C, CH3), 25.5, 19.7, 18.0, 10.2, −4.4, −5.6. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H32O4Si 341.2143, found 341.2139.
Data for 8 (minor diastereomer): 1H NMR (500 MHz, CDCl3): δ 4.59 (t, J = 2.4 Hz, 1H), 4.32 (dd, J = 9.2, 8.3 Hz, 1H), 3.95 (app t, J = 9.1 Hz, 1H), 3.65 (dd, J = 7.4, 3.5 Hz, 1H), 2.74 (d, J = 3.6 Hz, 1H), 2.73 – 2.65 (m, 1H), 2.41 – 2.31 (m, 1H), 2.30 – 2.21 (m, 1H), 2.21 – 2.12 (m, 1H), 1.83 – 1.74 (m, 1H), 1.59 – 1.51 (m, 1H), 1.38 (d, J = 7.2 Hz, 3H), 1.13 (s, 3H), 0.93 (s, 9H), 0.21 (s, 3H), 0.18 (s, 3H). Impurities: 1.56 (H2O). 13C NMR (125 MHz, CDCl3): δ 180.2, 158.3, 101.1, 80.0, 69.9, 50.7, 45.9, 38.0, 31.7, 25.7 (3C, CH3), 25.5, 20.5, 18.0, 17.0, −4.4, −5.5. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H32O4Si 341.2143, found 341.2137.
(3S,4R)-4-((S)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methyldihydrofuran-2(3H)-one (9) and (3R,4R)-4-((S)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methyldihydrofuran-2(3H)-one (10)
Followed General Procedure B: Mixture of diastereomers 4 & 5 (78 mg, 0.288 mmol); DCM (5 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–50%) in hexanes to give separable diastereomers 9 (25 mg) and 10 (14 mg) with a 76% overall yield (3:2 dr).
Data for 9 (major diastereomer): 1H NMR (500 MHz, CDCl3): δ 4.30 (dd, J = 8.9, 7.6 Hz, 1H), 4.06 (dd, J = 11.1, 8.8 Hz, 1H), 3.97 (dd, J = 9.7, 3.8 Hz, 1H), 2.82 – 2.69 (m, 2H), 2.48 (ddt, J = 12.5, 6.7, 1.8 Hz, 1H), 2.29 – 2.21 (m, 1H), 2.20 – 2.12 (m, 1H), 2.12 – 2.07 (m, 1H), 2.07 – 2.01 (m, 1H), 1.95 – 1.83 (m, 1H), 1.75 (ddt, J = 12.5, 6.7, 1.8, Hz, 1H), 1.32 (d, J = 7.2 Hz, 3H), 0.91 (s, 3H). Impurities: 1.56 (H2O). 13C NMR (125 MHz, CDCl3): δ 222.7, 179.5, 72.2, 68.0, 54.0, 42.8, 37.6, 37.4, 30.2, 18.8, 18.3, 10.5. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H18O4 227.1278, found 227.1270.
Data for 10 (minor diastereomer): 1H NMR (500 MHz, CDCl3): δ 4.39 (app t, J = 8.8 Hz, 1H), 4.00 (dd, J = 9.1, 8.1 Hz, 1H), 3.90 (t, J = 4.7 Hz, 1H), 2.77 – 2.65 (m, 1H), 2.45 – 2.34 (m, 2H), 2.32 – 2.23 (m, 1H), 2.20 – 2.09 (m, 1H), 2.07 – 1.99 (m, 1H), 1.93 – 1.81 (m, 1H), 1.74 – 1.67 (m, 1H), 1.35 (d, J = 7.3 Hz, 3H), 0.95 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 222.9, 179.8, 75.5, 69.8, 54.0, 44.9, 37.7, 36.5, 30.5, 19.3, 18.4, 16.8. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H18O4 227.1278, found 227.1271.
(3S,4R)-4-((S)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methyldihydrofuran-2(3H)-one (11) and (3R,4R)-4-((S)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methyldihydrofuran-2(3H)-one (12)
Followed General Procedure C: Mixture of diastereomers 4 & 5 (8 mg, 0.022 mmol); Pd(OAc)2 (3 mg, 0.011 mmol); DMSO (2 mL). The crude product was purified using preparative TLC with an EtOAc (70%) in hexanes eluent system to give the separated diastereomers 11 (3 mg) and 12 (2 mg) in a 3:2 dr and 85% overall yield.
Data for 11 (major diastereomer): 1H NMR (500 MHz, CDCl3): δ 7.80 – 7.76 (m, 1H), 6.19 (dt, J = 5.9, 2.2 Hz, 1H), 4.28 (dd, J = 8.8, 7.5 Hz, 1H), 4.10 (dd, J = 11.1, 8.8 Hz, 1H), 4.01 – 3.95 (m, 1H), 2.90 (dt, J = 18.9, 2.5 Hz, 1H), 2.81 – 2.70 (m, 2H), 2.41 (ddd, J = 18.9, 2.2, 2.0 Hz, 1H), 2.14 (d, J = 6.1 Hz, 1H), 1.34 (d, J = 7.3 Hz, 3H), 1.07 (s, 3H). Impurities: 1.56 (H2O). 13C NMR (125 MHz, CDCl3): δ 213.7, 179.3, 164.3, 132.2, 72.7, 68.0, 51.5, 43.6, 38.8, 37.6, 21.2, 10.6. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 225.1113.
Data for 12 (minor diastereomer): 1H NMR (500 MHz, CDCl3): δ 7.80 – 7.77 (m, 1H), 6.19 (dt, J = 5.8, 2.2 Hz, 1H), 4.37 (app t, J = 8.8 Hz, 1H), 4.03 (app t, J = 8.9 Hz, 1H), 3.94 (t, J = 5.6 Hz, 1H), 2.99 (dt, J = 18.7, 2.5 Hz, 1H), 2.77 – 2.68 (m, 1H), 2.48 – 2.36 (m, 2H), 2.18 (d, J = 6.1 Hz, 1H), 1.35 (d, J = 7.2 Hz, 3H), 1.09 (s, 3H). Impurities: 5.33 (CH2Cl2), 5.1 (s), 1.56 (H2O), 1.26 and 0.86 (grease). 13C NMR (125 MHz, CDCl3): δ 213.8, 179.7, 164.6, 132.4, 74.7, 69.6, 51.6, 45.3, 39.1, 36.0, 21.5, 16.8. Impurities: 29.9 (grease). HRMS-ESI+ (m/z) calc’d [M+H]+ for C12H16O4 225.1121, found 225.1113.
4-((S)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methylfuran-2(5H)-one (13)
Followed General Procedure B: Compound 6 (11 mg, 0.033 mmol); DCM (3 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–80%) in hexanes to give 13 (5 mg, 71% yield) as a white solid. 1H NMR (500 MHz, CDCl3): δ 5.00 (bs, 1H), 4.90 (dq, J = 17.5, 2.0 Hz, 1H), 4.73 – 4.66 (m, 1H), 2.50 – 2.45 (m, 1H), 2.43 (dt, J = 8.3, 1.8 Hz, 1H), 2.32 – 2.23 (m, 1H), 2.23 – 2.13 (m, 1H), 2.10 – 2.01 (m, 1H), 1.94 – 1.81 (m, 1H), 1.89 (t, J = 2.0 Hz, 3H), 1.64 (ddt, J = 12.2, 6.9, 1.7 Hz, 1H), 0.95 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 222.0, 174.6, 157.9, 125.5, 70.5, 70.1, 53.9, 38.0, 30.3, 19.4, 18.4, 9.7. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 225.1115.
4-((S)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methylfuran-2(5H)-one (14)
Followed General Procedure C: Compound 6 (31 mg, 0.092 mmol); Pd(OAc)2 (10 mg, 0.046 mmol); DMSO (2 mL). The crude product was SiO2 purified with EtOAc (0–70%) in hexanes to give 14 (7 mg, 35% yield) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.80 – 7.75 (m, 1H), 6.24 (dt, J = 5.8, 2.2 Hz, 1H), 5.04 (d, J = 5.6 Hz, 1H), 4.92 (dq, J = 17.2, 2.0 Hz, 1H),,4.69 – 4.62 (m, 1H), 2.93 (dt, J = 18.5, 2.5 Hz, 1H), 2.60 (d, J = 5.7 Hz, 1H), 2.41 – 2.34 (m, 1H), 1.93 (t, J = 2.0 Hz, 3H), 1.08 (s, 3H). Impurities: 1.26 and 0.86 (grease) 13C NMR (125 MHz, CDCl3): δ 212.6, 174.4, 164.3, 157.4, 132.3, 126.2, 70.4, 69.8, 51.1, 38.7, 21.6, 9.6. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H14O4 223.0965, found 223.0958.
(3R,4S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methyldihydrofuran-2(3H)-one (15)
Followed General Procedure B: Compound 7 (21 mg, 0.062 mmol); DCM (3 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–50%) in hexanes to give 15 (12 mg, 84% yield) as a white solid. 1H NMR (500 MHz, CDCl3): δ 4.47 (s, 1H), 4.19 (t, J = 8.0 Hz, 1H), 3.95 (t, J = 9.9 Hz, 1H), 3.75 (d, J = 9.1 Hz, 1H), 2.87 – 2.75 (m, 2H), 2.49 (dd, J = 19.6, 8.7 Hz, 1H), 2.30 – 2.18 (m, 1H), 2.08 – 1.99 (m, 1H), 1.99 – 1.87 (m, 1H), 1.72 – 1.59 (m, 2H), 1.34 (d, J = 6.7 Hz, 3H), 1.14 (s, 3H). Impurities: 1.56 (H2O), 1.26 and 0.86 (grease) 13C NMR (125 MHz, CDCl3): δ 225.8, 179.8, 73.0, 67.4, 50.4, 41.9, 37.1, 37.0, 34.0, 18.7, 15.0, 10.2. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H18O4 227.1278, found 227.1270.
(3S,4S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methyldihydrofuran-2(3H)-one (16)
Followed General Procedure B: Compound 8 (6 mg, 0.002 mmol); DCM (3 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–40%) in hexanes to give 16 (4 mg, 86% yield) as a white solid. 1H NMR (500 MHz, CDCl3): δ 4.25 (s, 1H), 4.20 (t, J = 8.7 Hz, 1H), 3.84 (t, J = 9.1 Hz, 1H), 3.61 (d, J = 7.2 Hz, 1H), 2.70 – 2.59 (m, 1H), 2.41 (dd, J = 19.5, 8.6 Hz, 1H), 2.33 – 2.22 (m, 1H), 2.22 – 2.09 (m, 1H), 2.02 – 1.77 (m, 2H), 1.69 – 1.55 (m, 2H), 1.34 (d, J = 7.1 Hz, 3H), 1.06 (s, 3H). Impurities: 4.8 – 4.6 (m), 1.56 (H2O), 1.26 and 0.86 (grease). 13C NMR (125 MHz, CDCl3): δ 225.4, 179.8, 72.1, 68.7, 51.1, 45.3, 37.9, 37.0, 34.1, 18.6, 16.8, 15.2. Impurities: 29.8 (grease). HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H18O4 227.1278, found 227.1270.
(3R,4S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methyldihydrofuran-2(3H)-one (17)
Followed General Procedure C: Compound 7 (27 mg, 0.080 mmol); Pd(OAc)2 (9 mg, 0.040 mmol); DMSO (4 mL). The crude product was SiO2 purified with EtOAc (0–100%) in hexanes to give 17 (4 mg, 22% yield). 1H NMR (500 MHz, CDCl3): δ 7.78 – 7.70 (m, 1H), 6.23 – 6.17 (m, 1H), 4.14 – 4.06 (m, 1H), 4.02 – 3.95 (m, 2H), 3.78 (d, J = 9.3 Hz, 1H), 2.87 – 2.74 (m, 2H), 2.54 (d, J = 19 Hz, 1H), 2.38 (d, J = 19 Hz, 1H), 1.33 (d, J = 6.0 Hz, 3H), 1.24 (s, 3H). Impurities: 1.56 (H2O). 13C NMR (125 MHz, CDCl3): δ 214.8, 179.6, 163.6, 132.1, 72.0, 67.5, 48.7, 42.8, 40.4, 37.2, 19.1, 10.1. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 225.1115.
(3S,4S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methyldihydrofuran-2(3H)-one (18)
Follows General Procedure C: Compound 8 (21 mg, 0.063 mmol); Pd(OAc)2 (7 mg, 0.031 mmol); DMSO (4 mL). The crude product was SiO2 purified with EtOAc (0–100%) in hexanes to give 18 (8 mg, 55% yield). 1H NMR (500 MHz, CDCl3): δ 7.75 (dt, J = 5.9, 2.7 Hz, 1H), 6.19 (dt, J = 5.8, 2.1 Hz, 1H), 4.20 (t, J = 8.7 Hz, 1H), 3.91 (t, J = 10.2 Hz, 1H), 3.75 (d, J = 2.5 Hz, 1H), 3.71 (dd, J = 7.0, 2.4 Hz, 1H), 2.76 – 2.68 (m, 1H), 2.64 (dt, J = 19.2, 2.5 Hz, 1H), 2.42 (ddd, J = 19.2, 2.9, 1.9 Hz, 1H), 2.36 – 2.28 (m, 1H), 1.40 (d, J = 6.9 Hz, 3H), 1.23 (s, 3H). Impurities: 1.56 (H2O). 13C NMR (125 MHz, CDCl3): δ 214.4, 179.5, 163.6, 132.3, 76.2, 69.1, 49.7, 45.9, 40.5, 37.8, 19.5, 16.8. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 227.1114.
(R)-4-((S)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methylenedihydrofuran-2(3H)-one (19)
Followed General Procedure B: 2 (20 mg, 0.059 mmol); DCM (1 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–70%) in hexanes to give 20 (5 mg) in 36% yield. 1H NMR (500 MHz, CDCl3): δ 6.44 – 6.42 (m, 1H), 5.87 – 5.86 (m, 1H), 4.41 (dd, J = 9.3, 7.8 Hz, 1H), 4.19 (dd, J = 9.3, 3.9 Hz, 1H), 3.94 – 3.89 (m, 1H), 3.28 – 3.21 (m, 1H), 2.45 – 2.29 (m, 3H), 2.23 – 2.13 (m, 1H), 2.07 – 1.98 (m, 1H), 1.92 – 1.80 (m, 1H), 1.76 – 1.69 (m, 1H), 1.01 (s, 3H). Impurities: 1.56 (H2O), 1.26 and 0.86 (grease). 13C NMR (125 MHz, CDCl3): δ 222.3, 170.4, 134.6, 126.5, 75.5, 69.6, 53.8, 41.6, 38.0, 30.9, 19.5, 18.6. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 225.1114.
(S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopentyl)methyl)-3-methylenedihydrofuran-2(3H)-one (20)
Followed General Procedure B: 3 (20 mg, 0.059 mmol); DCM (1 mL); TFA (0.1 mL). The crude product was SiO2 purified with EtOAc (0–70%) in hexanes to give 19 (10 mg) in 79% yield. 1H NMR (500 MHz, CDCl3): δ 6.39 (dd, J = 2.8, 1.1 Hz, 1H), 6.15 (dd, J = 2.5, 1.1 Hz, 1H), 4.37 (d, J = 1.5 Hz, 1H), 4.33 (app t, J = 8.5 Hz, 1H), 4.09 (dd, J = 9.1, 6.6 Hz, 1H), 3.78 (dd, J = 8.3, 1.5 Hz, 1H), 3.40 – 3.34 (m, 1H), 2.47 (ddt, J = 19.4, 8.4, 2.1 Hz, 1H), 2.30 – 2.19 (m, 1H), 2.08 – 2.00 (m, 1H), 1.98 – 1.88 (m, 1H), 1.88 – 1.80 (m, 1H), 1.78 – 1.71 (m, 1H), 1.15 (s, 3H). Impurities: 1.56 (H2O), 1.26 and 0.86 (grease). 13C NMR (125 MHz, CDCl3): δ 224.9, 170.5, 135.0, 126.4, 76.1, 67.6, 51.2, 41.8, 37.2, 34.8, 18.7, 15.8. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H16O4 225.1121, found 225.1115.
Supplementary Material
Acknowledgments
We gratefully acknowledge research funding from the NIH (R21-CA194661), The V Foundation for Cancer Research (V Scholar Award to DAH), Hyundai Hope on Wheels (Hope Grant), University of Minnesota (UMN) Academic Health Center (Seed Grant #2010.01), UMN College of Pharmacy (Bighley Graduate Fellowship to JCW; Melendy/Peters Summer Research Scholarship to HMS), UMN Department of Chemistry (Heisig/Gleysteen Summer Undergraduate Research Fellowship awards to JWB and JTE), and the UMN Undergraduate Research Opportunities Program (awards to JWB, JTE, and TJB). Mass spectrometry was performed at the Analytical Biochemistry Core Facility of the UMN Masonic Cancer Center, which is supported by the NIH (P30-CA77598 and S10RR-024618).
References
- 1.a) Xiao G, Fu J. Am. J. Cancer Res. 2011;1:192–221. [PMC free article] [PubMed] [Google Scholar]; b) Aggarwal BB. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]; c) Herrington FD, Carmody RJ, Goodyear CS. J. Biomol. Screen. 2016;21:223–242. doi: 10.1177/1087057115617456. [DOI] [PubMed] [Google Scholar]; d) Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Clin. Sci. 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]; e) Basseres DS, Baldwin AS. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]; f) Hayden MS, Ghosh S. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]; g) Huxford T, Ghosh G. Cold Spring Harbor Perspect. Biol. 2009;1:a000075. doi: 10.1101/cshperspect.a000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilmore TD, Herscovitch M. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 3.a) Darnell JE., Jr Nat. Rev. Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]; b) Berg T. Curr. Opin. Chem. Biol. 2008;12:464–471. doi: 10.1016/j.cbpa.2008.07.023. [DOI] [PubMed] [Google Scholar]; c) Koehler AN. Curr. Opin. Chem. Biol. 2010;14:331–340. doi: 10.1016/j.cbpa.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Lagoutte R, Patouret R, Winssinger N. Curr. Opin. Chem. Biol. 2017;39:54–63. doi: 10.1016/j.cbpa.2017.05.008. [DOI] [PubMed] [Google Scholar]; b) Huhn AJ, Guerra RM, Harvey EP, Bird GH, Walensky LD. Cell Chem. Biol. 2016;23:1123–1134. doi: 10.1016/j.chembiol.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lagoutte R, Serba C, Abegg D, Hoch DG, Adibekian A, Winssinger N. Nat. Comm. 2016;7:12470. doi: 10.1038/ncomms12470. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Potashman MH, Duggan ME. J. Med. Chem. 2009;52:1231–1246. doi: 10.1021/jm8008597. [DOI] [PubMed] [Google Scholar]; e) Singh J, Petter RC, Baillie TA, Whitty A. Nat. Rev. Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 5.Jackson PA, Widen JC, Harki DA, Brummond KM. J. Med. Chem. 2017;60:839–885. doi: 10.1021/acs.jmedchem.6b00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Kitson RR, Millemaggi A, Taylor RJ. Angew. Chem. Int. Ed. 2009;48:9426–9451. doi: 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]; b) Merfort I. Curr. Drug Targets. 2011;12:1560–1573. doi: 10.2174/138945011798109437. [DOI] [PubMed] [Google Scholar]; c) Pande V, Sousa SF, Ramos MJ. Curr. Med. Chem. 2009;16:4261–4273. doi: 10.2174/092986709789578222. [DOI] [PubMed] [Google Scholar]
- 7.a) Adams, W RH. J. Am. Chem. Soc. 1949;71:2546–2551. [Google Scholar]; b) Kos O, Lindenmeyer MT, Tubaro A, Sosa S, Merfort I. Planta Med. 2005;71:1044–1052. doi: 10.1055/s-2005-871284. [DOI] [PubMed] [Google Scholar]; c) Pettit GR, Budzinsk JC, Cragg GM, Brown P, Johnston LD. J. Med. Chem. 1974;17:1013–1016. doi: 10.1021/jm00255a024. [DOI] [PubMed] [Google Scholar]
- 8.a) Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. J. Biol. Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]; b) Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]; c) Buchele B, Zugmaier W, Lunov O, Syrovets T, Merfort I, Simmet T. Anal. Biochem. 2010;401:30–37. doi: 10.1016/j.ab.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.1a and 1b in this work correspond to compounds 6a and 6b, in our earlier publication [9b]; Widen JC, Kempema AM, Villalta PW, Harki DA. ACS Chem. Biol. 2017;12:102–113. doi: 10.1021/acschembio.6b00751.
- 10.a) Lee KH, Ibuka T, Mar EC, Hall IH. J. Med. Chem. 1978;21:698–701. doi: 10.1021/jm00205a022. [DOI] [PubMed] [Google Scholar]; b) Lee KH, Kim SH, Furukawa H, Piantadosi C. J. Med. Chem. 1975;18:59–63. doi: 10.1021/jm00235a013. [DOI] [PubMed] [Google Scholar]
- 11.Duplan V, Serba C, Garcia J, Valot G, Barluenga S, Hoerle M, Cuendet M, Winssinger N. Org. Biomol. Chem. 2014;12:370–375. doi: 10.1039/c3ob42049c. [DOI] [PubMed] [Google Scholar]
- 12.Avonto C, Taglialatela-Scafati O, Pollastro F, Minassi A, Di Marzo V, De Petrocellis L, Appendino G. Angew. Chem. Int. Ed. 2011;50:467–471. doi: 10.1002/anie.201005959. [DOI] [PubMed] [Google Scholar]
- 13.Appendino G, Minassi A, Collado JA, Pollastro F, Chianese G, Taglialatela-Scafati O, Ayyari M, Garcia V, Munoz E. Eur. J. Org. Chem. 2015:3721–3726. [Google Scholar]
- 14.Schmidt TJ. Bioorg. Med. Chem. 1997;5:645–653. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 15.a) Ito Y, Hirao T, Saegusa T. J. Org. Chem. 1978;43:1011–1013. [Google Scholar]; b) Larock RC, Hightower TR, Kraus GA, Hahn P, Zheng D. Tetrahedron Lett. 1995;36:2423–2426. [Google Scholar]
- 16.Siegel S, Smith GV. J. Am. Chem. Soc. 1960;82:6082–6087. [Google Scholar]
- 17.Schmidt TJ, Lyss G, Pahl HL, Merfort I. Bioorg. Med. Chem. 1999;7:2849–2855. doi: 10.1016/s0968-0896(99)00234-5. [DOI] [PubMed] [Google Scholar]
- 18.Hexum JK, Tello-Aburto R, Struntz NB, Harned AM, Harki DA. ACS. Med. Chem. Lett. 2012;3:459–464. doi: 10.1021/ml300034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Previous work has found that sesquiterpenes can inhibit the luciferase enzyme directly.[19b] However, our previous[9b,18] and unpublished studies evaluating the natural product parthenolide have yielded comparable, low micromolar NF-κB inhibitory activities by the luciferase reporter assay used in this study and a related NF-κB secreted alkaline phosphatase (SEAP) reporter assay in HEK293 cells. Nonetheless, those compounds containing thiol reactive electrophiles reported in Table 2 with NF-κB inhibitory activities requiring high concentrations (>100 µM) should be evaluated in companion assays to corroborate the NF-κB inhibitory activities reported here. Lindenmeyer MT, Garcia-Pineres AJ, Castro V, Merfort I. Anal. Biochem. 2004;328:147–154. doi: 10.1016/j.ab.2004.01.021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.