Abstract
Emphysema is characterized by degradation of lung alveoli that leads to poor airflow in lungs. Irreversible elastic fiber degradation by matrix metalloproteinases (MMPs) and reactive oxygen species (ROS) activity leads to loss of elasticity and drives the progression of this disease. We investigated if a polyphenol, pentagalloyl glucose (PGG) can increase elastin production in pulmonary fibroblasts. We also studied the effect of PGG treatment in reducing MMP activity and ROS levels in cells. We exposed rat pulmonary fibroblasts to two different types of inflammatory environments i.e., tumor necrosis factor-α (TNF-α) and cigarette smoke extract (CSE) to mimic the disease. Parameters like lysyl oxidase (LOX) and elastin gene expression, MMP-9 activity in the medium, lysyl oxidase (LOX) activity and ROS levels were studied to assess the effect of PGG on pulmonary fibroblasts. CSE inhibited lysyl oxidase (LOX) enzyme activity that resulted in a decreased elastin formation. Similarly, TNF-α treated cells showed less elastin in the cell layers. Both these agents caused increase in MMP activity and ROS levels in cells. However, when supplemented with PGG treatment along with these two inflammatory agents, we saw a significant increase in elastin deposition, reduction in both MMP activity and ROS levels. Thus PGG, which has anti-inflammatory, anti-oxidant properties coupled with its ability to aid in elastic fiber formation, can be a multifunctional drug to potentially arrest the progression of emphysema.
Keywords: COPD, emphysema, ECM degradation
Background
Emphysema, a subset of chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation, oxidative stress, elastin damage, and progressive alveolar destruction [1]. Improvement in lung function is not possible even after smoking cessation [2]. Cigarette smoke insult triggers the inflammatory response in lungs and over a period of time recruitment of inflammatory cells causes excessive release of enzyme mediators leading to a disruption of ECM balance in the lungs. Cigarette smoke also activates cellular apoptosis and inhibits alveolar elastin repair, which makes the emphysema condition irreversible [3–5].
Elastic fibers provide elastic recoil to organs like the lungs. Loss of lung elasticity has been correlated to a loss of lung function in emphysema patients [6].. The inability of mature cells to regenerate elastin in large quantities has been attributed to the lack of reparative process [7]. Moreover, elastin degradation products (EDPs) act as chemo- attractants for monocytes and therefore EDPs further increase the inflammatory burden on lungs [8]. Inflammatory cells release matrix metalloproteinases (MMPs) that has been shown to cause protease-antiprotease imbalance in emphysematous lungs. Many varieties of MMPs have now been shown to overexpress in emphysema; however, MMP 12 and MMP 9 are more pronounced [9–12]. Stopping elastin degradation has received little attention as a potential therapy for treatment of emphysema
Pentagalloyl glucose (PGG) is a derivative of tannic acid found in green tea, red wine, and nuts. We have previously shown from in vitro and in vivo experiments that PGG can preserve elastin, inhibit MMP activity, and help restore lost elastin in aneurysmal aorta o rats [13–15]. We hypothesize that PGG treatment, due to its anti-oxidant and anti- inflammatory properties combined with its affinity to bind to elastin, would stop the emphysema progression by inhibiting MMP activity and restoring elastin deposition [16, 17]. As a preliminary step, we investigated the effects of PGG on rat pulmonary fibroblasts cell cultures for inhibition of matrix metalloproteinases (MMPs) and reactive oxygen species (ROS) activity, elastin gene and protein expression, and elastin deposition under inflammatory conditions mimicking emphysema.
Methods (expanded methods are provided as a supplement)
Preparation of cigarette smoke extract (CSE)
Cigarette smoke extract was prepared using a custom designed instrument with slight modifications to the procedure described in literature [18].
Pulmonary fibroblast cell culture
Primary rat pulmonary fibroblasts (Cell Biologics Inc©, IL, USA) were grown in Dulbecco’s Modified Eagle Medium (DMEM), (ScienCell™, CA, USA) supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich®, St. Louis, MO), 1% fibroblast growth substrate (FGS) (ScienCell™, CA, USA), and 1% Penicillin-Streptomycin. Cells from passages 2–6 only were used for all experiments. The cells were allowed to grow for one day in normal growth medium before the start of the treatments.
To mimic inflammatory conditions in vitro, cells were treated with either tumor necrosis factor (TNF-α) (Peprotech Inc. ®, Rocky Hill, NJ) or Cigarette smoke extract (CSE). Cells were divided into six groups depending on the combination of substances they were treated with. The groups were: DMEM only (DMEM group), PGG (10 μg/mL) (PGG group), tumor necrosis factor (TNF-α) (50 ng/ml) (TNF-α group), TNF-α (50 ng/mL) + PGG (10 μg/mL) (TNF-α +PGG group), CSE (5% final concentration) (CSE group), and CSE (5%) + PGG (10 μg/mL) (CSE+PGG group). Cells were grown under these conditions for up to 21 days, and the medium was replenished twice every week. The cell cultures were analyzed at days 7, 14 and 21 for cell viability, total protein, total elastin in matrix and medium, collagen in matrix and medium, MMP activity in the medium, and ROS activity in cells.
mRNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR)
Cells grown in the aforementioned conditions were lysed at weeks 1 and 2, and mRNA was extracted using RNeasy RNA extraction kit (Qiagen, Valencia, CA) as per manufacturer’s protocol.
Quantitative PCR
The quantitative PCR (qPCR) was performed and analyzed using a Rotorgene qPCR machine (Qiagen, Valencia, CA) to measure the relative expression levels of Lysyl Oxidase (LOX), Lysyl Oxidase 1(LOXL1) and Elastin (ELN) genes with respect to Beta-2 Microglobulin (B2MG). Description and primer sequences are included in the supplementary material.
Lysyl oxidase activity
Lysyl oxidase activity in the medium was measured using Amplite fluorimetric lysyl oxidase activity assay kit (AAT Bioquest, Sunnyvale, CA) according to manufacturer’s instructions.
Gelatin zymography and Reverse zymography
Active MMP activity was analyzed in the medium collected from cell cultures using gelatin zymography as described previously [19].
FASTIN assay
Total insoluble elastin deposited in the cell layers and soluble tropoelastin in the media were quantified using the Fastin™ Elastin assay kit (Biocolor, UK) according to the manufacturer’s protocol. Matrix elastin was normalized to total lysate protein released by the cells, which is assumed to be directly proportional to the total cell count, while the tropoelastin in medium was normalized to the total protein content in the medium.
Reactive oxygen species (ROS) analysis
ROS activity in pulmonary fibroblasts was analyzed under aforementioned growth conditions using CellRox® deep red reagent, as per the manufacturer’s protocol.
Results
Initially we checked the cell numbers in different conditions using Picogreen dsDNA assay. Addition of TNF-α did not affect cell growth or viability (Supplementary Figure 1 A). However, cell numbers in CSE group remained significantly lower than DMEM group (Supplementary Figure 1B). PGG at the used concentration (10 μg/mL) did not show any toxicity to the cells.
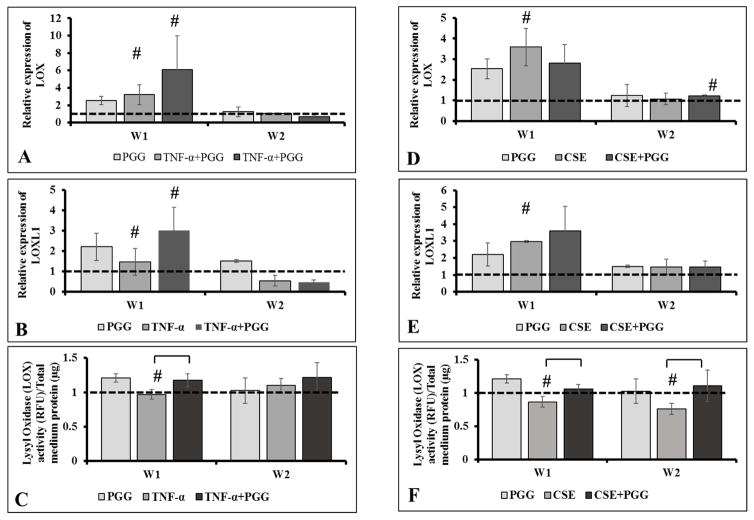
Gene expression and activity of Lysyl Oxidase (LOX)
Quantitative PCR analysis of mRNA showed that expression of LOX and LOXL1 genes was upregulated in all of the groups as compared to the DMEM control group at week 1. The TNF-α+PGG group showed 6-fold upregulation of LOX expression. By week 2, the LOX expression returned to control levels (Figure 1 A–B). Treating cells with TNF-α did not seem to affect LOX activity in the medium. PGG addition slightly increased LOX protein activity levels at week 1 and 2 under TNF-α inflammatory condition (Figure 1C). CSE and CSE+PGG groups showed an increase in LOX and LOXL1 gene expression(~3–4 fold) during week 1. However, they returned to control levels by week 2 (Figure 1 D–E). Addition of CSE impeded LOX protein levels in the media despite increases seen in the mRNA level; however, addition of PGG to CSE restored LOX protein levels (Figure 1F).
Figure 1.
Relative gene expression of lysyl oxidase (LOX and LOX1) gene (A–D). Lysyl Oxidase (LOX) protein activity in cell culture medium for all groups (E–F). Dashed lines represent levels in DMEM control group. # represents significantly different from DMEM group. (n=6)
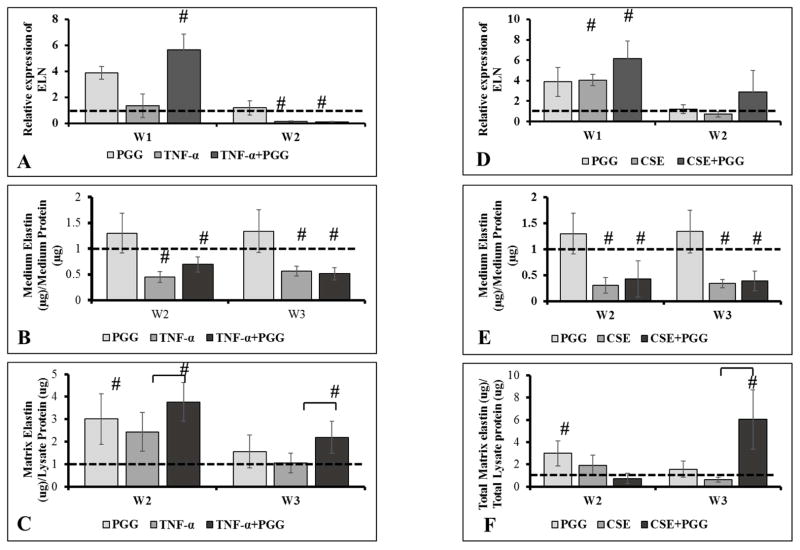
Gene expression and deposition of elastin protein
Addition of TNF-α did not affect ELN gene expression. Addition of PGG in presence of TNF-α enhanced ELN gene expression by ~6-fold at week 1. (Figure 2A). The ELN gene levels were down by week 2. Elastin levels in the media was not altered by addition of TNF-α by week 2 but it dropped by week 3. (Figure 2B). When matrix elastin was quantified, PGG addition showed increased deposition of insoluble matrix elastin at week 3; clearly suggesting that PGG binds to soluble elastin and coacervates it leading to the deposition of insoluble elastin. (Figure 2C).
Figure 2.
Relative gene expression of elastin (ELN) gene (A–B). Elastin quantified in cell culture medium for all groups (C–D). Matrix elastin quantified from cell layers for all groups (n=6) (E–F). Dashed lines represent levels in DMEM control group. # represents significantly different from DMEM group. (n=6)
Expression of elastin gene in CSE and CSE+PGG groups showed a significant increase (~ 4–6 fold) after one week. These levels came back to normal (same as DMEM group) by week 2 (Figure 2D). Elastin protein levels in the media; however, were significantly lower CSE and CSE+PGG groups as compared to DMEM group at both 2 and 3 weeks (Figure 2E). When matrix elastin was quantified, we observed a significant increase in cross-linked elastin deposition in the CSE+PGG group, suggesting that PGG mitigates the effects of CSE and helps elastin deposition similar to what was seen in TNF-α groups (Figure 2F).
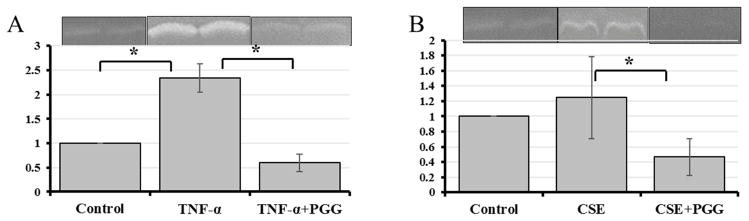
Gelatin zymography and Reverse zymography for MMP and TIMP activities
Gelatin zymography from TNF-α group at week 1 and 2 time points showed substantial increase in MMP-9 activity as compared to control DMEM group, which was diminished when PGG was added (TNF-α+PGG) (Figure 3A). On the other hand, CSE addition did not affect MMPs but PGG further suppressed MMP activity in that group as well (Figure 3B). To test if PGG prevents MMP activity at the gene or protein level, zymography gels from TNF-α and CSE groups were incubated in development buffer along with PGG (20 μg/ml). It showed reduction in MMP-9 (92kDa) bands in TNFα group (data not shown) suggesting that PGG decreases MMP activity at the protein level. TIMP activity analyzed by reverse zymography also showed that PGG treated cells have lower TIMP activity (Supplemental Figure 2) probably due to the reduction of MMPs seen in PGG groups.
Figure 3.
MMP-9 (92kDa) activity as visualized by gelatin gel zymography at 2 weeks for TNF-α groups (A) and CSE groups (B). PGG inhibits MMP activity, as shown by diminished band intensity. * represents significantly different (n=6).
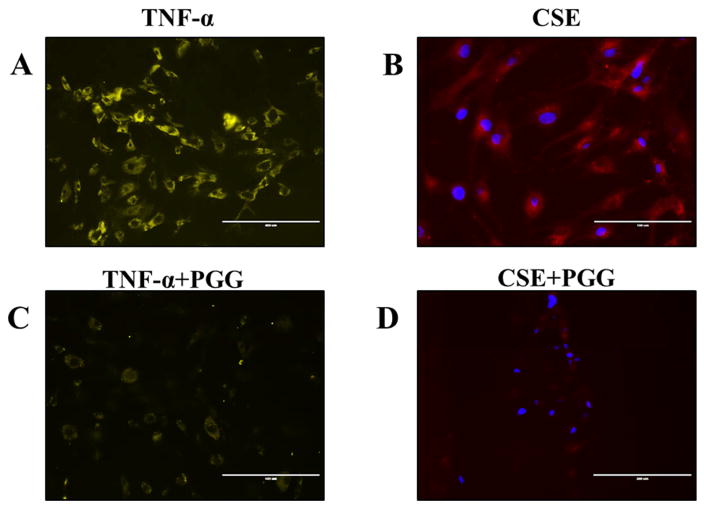
ROS analysis
We observed a significant reduction in the ROS found in rat pulmonary fibroblasts in the TNF-α+PGG and CSE+PGG groups compared to the TNF-α and CSE groups. (Figure 4).
Figure 4.
Reactive oxygen species (ROS) in rat lung fibroblasts was visualized using CellRox Deep red reagent. Yellow (A–C, for TNF-α and TNF-α+PGG groups) and red (B–D, for CSE and CSE+PGG groups) represents ROS and blue represents DAPI staining of nuclei. Significant inhibition of ROS could be seen in PGG treated groups. (n=3).
Discussion
Pulmonary fibroblasts are important in the pathology of COPD as they participate in ECM remodeling and are used as in vitro models to study the disease [20, 21]. Various agents have been used to mimic the inflammatory conditions that exist in COPD including TNF-α, but cigarette smoke extract use has emerged as a key model [22–24]. The main advantage of using CSE is that it correlates well to in vivo exposure. Components of cigarette smoke need to have solubility to pass through the mucus layer before reaching the epithelial cells of alveoli that remain distal to the airways. There are many different CSE preparations reported (type and number of cigarettes and different volumes), for both in vitro and in vivo purposes [3] [18, 25]. We used method from Baglole et al [18]. Lysyl oxidase family of enzymes are copper-dependent enzymes, which facilitate covalent crosslinking of elastin. Inhibition of LOX activity during postnatal lung growth has shown to result in irreversible structural changes [26]. LOXL1 has been shown to interact with fibulin-5, fibrillin, and with tropoelastin in ligand-binding assays [27]. Inactivation of LOX and LOXL1 individually leads to enlarged airspaces in the lung [28, 29]. CSE has been shown to inhibit cross-linking of tropoelastin molecules to form elastic fibers [30]. Li et al., suggest that LOX could be affected at many stages including mRNA expression [31]. We found an increase in LOX mRNA expression at week 1 when cells were treated with TNFα and CSE. Gao et al., have shown that LOX mRNA transcription can be perturbed using cigarette smoke condensate, but not extract, at a concentration of 80 μg/ml or more [32]. Interestingly we observed an increased LOX mRNA expression in cells treated with PGG, and its activity remained higher than respective non-PGG treated controls. However, the mechanisms of why PGG treatment increases LOX is unknown at this time. We have shown earlier that PGG binds to tropoelastin secreted by cells and coacervates it in the matrix [15]. This may have caused increased local concentration of elastin surrounding the cells and caused signaling to increase LOX production. However, this needs to be tested further.
We observed that CSE treatment caused an increase in elastin mRNA expression at week 1 while TNF-α treatment did not. Kahari et al., and DiCamillo et al., report that TNF-α suppresses elastin mRNA production in fibroblasts and aortic smooth muscle cells [33] [34]. The increase in elastin due to CSE correlates well to increased elastin expression seen in COPD patients due to smoking [35, 36]. However, elastin protein in the medium was significantly lower than the control group. Cells supplemented with PGG showed more matrix elastin production compared to their respective controls (TNF-α+PGG> TNF-α, CSE+PGG>CSE) suggesting that PGG allowed coacervation and deposition of elastin in the matrix. Precipitation of elastin with PGG has been shown in our previous studies by measuring the kinetics of tropoelastin coacervation and maturation confirming that PGG binds to elastin and coacervates it [37]. Our results can be compared to that observed by Jimenez et al. for the effect of tannic acid and ellagic acid in dermal fibroblast cultures [38]. They have also observed lesser elastin degradation and more deposition of insoluble cross-linked elastin when cells were treated with tannic acid. Elastin degradation by MMPs is an important step in the pathophysiological path of COPD. Elastin degradation results in elastin derived peptides (EDPs), which have been shown to be chemoattractants for monocytes. Sellami et al., have even shown that intra- tracheal injection of VGVAPG amino acid (one of the EDP sequences) causes emphysema in a murine model [39]. With gelatin zymography, we clearly show a decrease in MMP activity in the TNF-α +PGG and CSE+PGG groups compared to the TNF-α and CSE groups respectively. These results are in agreement with many other studies that reported decreased MMP activity after treatment with polyphenols [40, 41]. To further test if PGG mediated suppression of MMPs was at the protein level and not at the gene level, we incubated zymography gels in a solution containing 20 μg/mL PGG. Addition of PGG to the developing buffer caused suppression of MMP bands clearly suggesting that PGG inhibits MMP activity outside the cells. Lindner et al., have shown a spike in MMP-9 expression (90 fold) in human pulmonary fibroblasts after 24 hours of exposure to 10 ng/ml of TNF-α [42]. Ning et al., have observed that cigarette smoke stimulates MMP-2 activity in human fibroblast cultures [43]. However, we have only observed MMP-9 activity but not MMP-2 in zymography. We also saw decreased TIMP activity in the cultures which had PGG added to them. This TIMP activity is not affected by incubation of the gel in the development buffer containing PGG. We believe reduced TIMP levels seen in PGG groups is due to balance of MMPs and TIMPs. As MMPs were lower in PGG groups, it might have also reduced TIMP activity [44]. Seifart et al., also show that all-trans retinoic acid (ATRA) treatment reduces MMP and therefore TIMP activity [45]. Inflammatory conditions are also known to increase reactive oxygen species in the local environment [46]. Oxidative stress is an important factor present in COPD, which is caused by cigarette smoking in patients [47]. The anti-oxidant properties of polyphenols have already been documented [16, 48]. We saw a clear eradication of the signal from the fluorescent ROS substrate in cells supplemented with PGG along with TNF-α or CSE treatment. It is also interesting to note that this oxidative stress could have caused increased elastin expression in CSE and TNF-α groups of our experiment, as observed by Deslee et al., [49]. We believe the anti-oxidant property of PGG might help in controlling oxidative stress in COPD and thereby controlling damage in lungs.
We also tested for collagen in cell cultures to investigate if PGG increases collagen deposition in the matrix (data provided in supplementary material; Figure 3). Finlay et al., [50] showed that collagen remodeling is a consistent feature of emphysematous lungs which was supported by Mosquero et al [51]. Increased collagen was seen as a process of repair by the lungs. We found that PGG treatment does not cause any extra collagen deposition either in soluble or insoluble forms and hence we believe PGG will not cause increased fibrosis, which could be detrimental to the lung function.
Conclusion
In this paper, we show that PGG treatment leads to increased elastin production and deposition in rat pulmonary fibroblast cell cultures under simulated inflammatory conditions and at the same time, it suppresses MMP and ROS activity. Elastin damage has been shown to drive lung damage in COPD. Thus, stabilizing and regenerating elastin without altering collagen deposition can be a way to arrest the progression of this disease. Our data shows that PGG could be used as a multifunctional drug in the treatment of COPD.
Supplementary Material
Supplementary Figure 1: Cell numbers of all treatment groups grown for week 1 and 2 as determined by Picogreeen dsDNA quantification assay (n=6).
Supplementary Figure 2: Active TIMPs, visualized by reverse zymography technique, show reduction of TIMP levels in PGG groups. (n=6)
Supplementary Figure 3: Collagen quantification in cell culture medium and cell-matrix for PGG, TNF-α and TNF-α+PGG groups (A–B). Dashed line represents DMEM group value. (n=3). # represents significantly different from DMEM group.
Table 1.
Primer sequences for LOX, LOXL1, ELN and B2MG genes
| Gene | Primer sequence | Primer Length | Amplicon size |
|---|---|---|---|
| Lysyl Oxidase-1 (LOXL1) | |||
| FWD | 5′-CACTACACAGGTCGCTTC-3′ | 17 | 137 |
| REV | 5′-AGGCGGCTATGCTTCTT-3′ | 16 | |
| Lysyl Oxidase (LOX) | |||
| FWD | 5′-TACCTGGTGCCTGAATCA-3′ | 17 | 138 |
| REV | 5′-GTACTGCTTCATCCTTTGGG-3′ | 19 | |
| Beta (β2) Microglobulin (B2MG) | |||
| FWD | 5′-CGTGATCTTTCTGGTGCTTGTC-3′ | 21 | 122 |
| REV | 5′-ACGTAGCAGTTGAGGAAGTTGG-3′ | 21 | |
| Elastin (ELN) | |||
| FWD | 5′-AAAGCAGCGAAGTATGCAGG-3′ | 20 | 110 |
| REV | 5′-CCTGTAATGCCTCCAATCCC-3′ | 20 |
Highlights.
Emphysema, characterized by degradation of lung alveoli that leads to poor airflow in lungs, is a major cause of mortality and morbidity with no known treatments.
We found that lung alveolar fibroblasts when treated with a polyphenol namely pentagalloyl glucose (PGG) leads to inhibition of MMP and ROS activity that is responsible for ECM degradation
PGG also binds to tropoelastin molecules secreted by cells and increases matrix elastin production, thus may lead to increased alveolar compliance.
Acknowledgments
We gratefully acknowledge funding from the NIH grant P20GM103444 and the Hunter Endowment to NRV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5(4):475–7. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godtfredsen NS, Lam TH, Hansel TT, Leon ME, Gray N, Dresler C, et al. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008;32(4):844–53. doi: 10.1183/09031936.00160007. [DOI] [PubMed] [Google Scholar]
- 3.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35(6):681–8. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, et al. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2005;33(2):121–9. doi: 10.1165/rcmb.2003-0341OC. [DOI] [PubMed] [Google Scholar]
- 5.Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. Am Rev Respir Dis. 1983;127(2):189–92. doi: 10.1164/arrd.1983.127.2.189. [DOI] [PubMed] [Google Scholar]
- 6.Black PN, Ching PS, Beaumont B, Ranasinghe S, Taylor G, Merrilees MJ. Changes in elastic fibres in the small airways and alveoli in COPD. Eur Respir J. 2008;31(5):998–1004. doi: 10.1183/09031936.00017207. [DOI] [PubMed] [Google Scholar]
- 7.Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc. 2006;3(5):428–33. doi: 10.1513/pats.200601-009AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uemura Y, Okamoto K. Elastin-derived peptide induces monocyte chemotaxis by increasing intracellular cyclic GMP level and activating cyclic GMP dependent protein kinase. Biochem Mol Biol Int. 1997;41(1):57–64. doi: 10.1080/15216549700201061. [DOI] [PubMed] [Google Scholar]
- 9.Mecham RP, Broekelmann TJ, Fliszar CJ, Shapiro SD, Welgus HG, Senior RM. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997;272(29):18071–6. doi: 10.1074/jbc.272.29.18071. [DOI] [PubMed] [Google Scholar]
- 10.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J. 2012;39(1):197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 11.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–4. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 12.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62(8):706–13. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosoudi N, Chowdhury A, Siclari S, Karamched S, Parasaram V, Parrish J, et al. Reversal of Vascular Calcification and Aneurysms in a Rat Model Using Dual Targeted Therapy with EDTA- and PGG-Loaded Nanoparticles. Theranostics. 2016;6(11):1975–1987. doi: 10.7150/thno.16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115(13):1729–37. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 15.Sinha A, Nosoudi N, Vyavahare N. Elasto-regenerative properties of polyphenols. Biochem Biophys Res Commun. 2014;444(2):205–11. doi: 10.1016/j.bbrc.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Li L, Kim SH, Hagerman AE, Lu J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res. 2009;26(9):2066–80. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlton AJ, Baxter NJ, Lilley TH, Haslam E, McDonald CJ, Williamson MP. Tannin interactions with a full-length human salivary proline-rich protein display a stronger affinity than with single proline-rich repeats. FEBS Lett. 1996;382(3):289–92. doi: 10.1016/0014-5793(96)00186-x. [DOI] [PubMed] [Google Scholar]
- 18.Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, et al. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: implications for emphysema. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L19–29. doi: 10.1152/ajplung.00306.2005. [DOI] [PubMed] [Google Scholar]
- 19.Nosoudi N, Nahar-Gohad P, Sinha A, Chowdhury A, Gerard P, Carsten CG, et al. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles. Circ Res. 2015;117(11):e80–9. doi: 10.1161/CIRCRESAHA.115.307207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, et al. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J. 2004;24(4):575–9. doi: 10.1183/09031936.04.00143703. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wu L, Feng MX, Sexton P, Bai CX, Qu JM, et al. Pulmonary fibroblasts from COPD patients show an impaired response of elastin synthesis to TGF-beta1. Respir Physiol Neurobiol. 2011;177(3):236–40. doi: 10.1016/j.resp.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Krimmer DI, Oliver BGG. What can in vitro models of COPD tell us? Pulm Pharmacol Ther. 2011;24(5):471–477. doi: 10.1016/j.pupt.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNF alpha in pulmonary pathophysiology. Resp Res. 2006:7. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churg A, Wang RD, Tai H, Wang XS, Xie CS, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Resp Crit Care. 2004;170(5):492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 25.Yang GY, Zhang CL, Liu XC, Qian G, Deng DQ. Effects of cigarette smoke extracts on the growth and senescence of skin fibroblasts in vitro. Int J Biol Sci. 2013;9(6):613–23. doi: 10.7150/ijbs.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kida K, Thurlbeck WM. Lack of recovery of lung structure and function after the administration of beta-amino-propionitrile in the postnatal period. Am Rev Respir Dis. 1980;122(3):467–75. doi: 10.1164/arrd.1980.122.3.467. [DOI] [PubMed] [Google Scholar]
- 27.Thomassin L, Werneck CC, Broekelmann TJ, Gleyzal C, Hornstra IK, Mecham RP, et al. The pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. Journal of Biological Chemistry. 2005;280(52):42848–42855. doi: 10.1074/jbc.M506832200. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 29.Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167(4):927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent P, Janoff A, Kagan HM. Cigarette-Smoke Blocks Cross-Linking of Elastin Invitro. Chest. 1983;83(5):S63–S65. doi: 10.1378/chest.83.5_supplement.63s. [DOI] [PubMed] [Google Scholar]
- 31.Li WD, Zhou J, Chen LJ, Luo ZJ, Zhao YZ. Lysyl Oxidase, A Critical Intra- and Extra-Cellular Target in the Lung for Cigarette Smoke Pathogenesis. Int J Env Res Pub He. 2011;8(1):161–184. doi: 10.3390/ijerph8010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao S, Chen KY, Zhao YZ, Rich CB, Chen LJ, Li SJ, et al. Transcriptional and posttranscriptional inhibition of lysyl oxidase expression by cigarette smoke condensate in cultured rat fetal lung fibroblasts. Toxicol Sci. 2005;87(1):197–203. doi: 10.1093/toxsci/kfi212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahari VM, Chen YQ, Bashir MM, Rosenbloom J, Uitto J. Tumor- Necrosis-Factor-Alpha down-Regulates Human Elastin Gene-Expression - Evidence for the Role of Ap-1 in the Suppression of Promoter Activity. Journal of Biological Chemistry. 1992;267(36):26134–26141. [PubMed] [Google Scholar]
- 34.DiCamillo SJ, Carreras I, Panchenko MV, Stone PJ, Nugent MA, Foster JA, et al. Elastase-released epidermal growth factor recruits epidermal growth factor receptor and extracellular signal-regulated kinases to down- regulate tropoelastin mRNA in lung fibroblasts. Journal of Biological Chemistry. 2002;277(21):18938–18946. doi: 10.1074/jbc.M200243200. [DOI] [PubMed] [Google Scholar]
- 35.Deslee G, Woods JC, Moore CM, Liu L, Conradi SH, Milne M, et al. Elastin expression in very severe human COPD. European Respiratory Journal. 2009;34(2):324–331. doi: 10.1183/09031936.00123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Wu L, Qu JM, Bai CX, Merrilees MJ, Black PN. Pro- inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med. 2012;16(7):1522–1532. doi: 10.1111/j.1582-4934.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha A, Nosoudi N, Vyavahare N. Elasto-regenerative properties of polyphenols. Biochem Bioph Res Co. 2014;444(2):205–211. doi: 10.1016/j.bbrc.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez F, Mitts TF, Liu K, Wang YT, Hinek A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J Invest Dermatol. 2006;126(6):1272–1280. doi: 10.1038/sj.jid.5700285. [DOI] [PubMed] [Google Scholar]
- 39.Sellami M, Meghraoui-Kheddar A, Terryn C, Fichel C, Bouland N, Diebold MD, et al. Induction and regulation of murine emphysema by elastin peptides. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2016;310(1):L8–L23. doi: 10.1152/ajplung.00068.2015. [DOI] [PubMed] [Google Scholar]
- 40.Philips N, Samuel M, Arena R, Chen YJ, Conte J, Natarajan P, et al. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J Cosmet Sci. 2010;61(2):125–32. [PubMed] [Google Scholar]
- 41.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478(1):51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 42.Lindner D, Zietsch C, Becher PM, Schulze K, Schultheiss HP, Tschope C, et al. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem Res Int. 2012;2012:875742. doi: 10.1155/2012/875742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning W, Dong Y, Sun J, Li C, Matthay MA, Feghali-Bostwick CA, et al. Cigarette smoke stimulates matrix metalloproteinase-2 activity via EGR-1 in human lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36(4):480–90. doi: 10.1165/rcmb.2006-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parasaram V, Nosoudi N, LeClair RJ, Binks A, Vyavahare N. Targeted drug delivery to emphysematous lungs: Inhibition of MMPs by doxycycline loaded nanoparticles. Pulm Pharmacol Ther. 2016;39:64–73. doi: 10.1016/j.pupt.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seifart C, Muyal JP, Plagens A, Yildirim AO, Kohse K, Grau V, et al. All- trans retinoic acid results in irregular repair of septa and fails to inhibit proinflammatory macrophages. Eur Respir J. 2011;38(2):425–39. doi: 10.1183/09031936.00123809. [DOI] [PubMed] [Google Scholar]
- 46.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD- -implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–24. doi: 10.2147/COPD.S51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacNee W. Treatment of stable COPD: antioxidants. European Respiratory Review. 2005;14(94):12–22. [Google Scholar]
- 48.Husari A, Hashem Y, Bitar H, Dbaibo G, Zaatari G, El Sabban M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. Int J Chron Obstruct Pulmon Dis. 2016;11:227–37. doi: 10.2147/COPD.S97027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deslee G, Adair-Kirk TL, Woods JC, Moore CM, Pierce RA. Oxidative Stress Induces Elastin Expression in Human Lung Fibroblasts. Am J Resp Crit Care. 2009:179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Cell numbers of all treatment groups grown for week 1 and 2 as determined by Picogreeen dsDNA quantification assay (n=6).
Supplementary Figure 2: Active TIMPs, visualized by reverse zymography technique, show reduction of TIMP levels in PGG groups. (n=6)
Supplementary Figure 3: Collagen quantification in cell culture medium and cell-matrix for PGG, TNF-α and TNF-α+PGG groups (A–B). Dashed line represents DMEM group value. (n=3). # represents significantly different from DMEM group.