Abstract
Background
Selenium and coenzyme Q10 are both necessary for optimal cell function in the body. The intake of selenium is low in Europe, and the endogenous production of coenzyme Q10 decreases as age increases. Therefore, an intervention trial using selenium and coenzyme Q10 for four years as a dietary supplement was performed. The main publication reported reduced cardiovascular mortality as a result of the intervention. In the present sub-study the objective was to determine whether reduced cardiovascular (CV) mortality persisted after 12 years, in the supplemented population or in subgroups with diabetes, hypertension, ischemic heart disease or reduced functional capacity due to impaired cardiac function.
Methods
From a rural municipality in Sweden, four hundred forty-three healthy elderly individuals were included. All cardiovascular mortality was registered, and no participant was lost to the follow-up. Based on death certificates and autopsy results, mortality was registered.
Findings
After 12 years a significantly reduced CV mortality could be seen in those supplemented with selenium and coenzyme Q10, with a CV mortality of 28.1% in the active treatment group, and 38.7% in the placebo group. A multivariate Cox regression analysis demonstrated a reduced CV mortality risk in the active treatment group (HR: 0.59; 95%CI 0.42–0.81; P = 0.001). In those with ischemic heart disease, diabetes, hypertension and impaired functional capacity we demonstrated a significantly reduced CV mortality risk.
Conclusions
This is a 12-year follow-up of a group of healthy elderly participants that were supplemented with selenium and coenzyme Q10 for four years. Even after twelve years we observed a significantly reduced risk for CV mortality in this group, as well as in subgroups of patients with diabetes, hypertension, ischemic heart disease or impaired functional capacity. The results thus validate the results obtained in the 10-year evaluation.
The protective action was not confined to the intervention period, but persisted during the follow-up period. The mechanisms behind this effect remain to be fully elucidated, although various effects on cardiac function, oxidative stress, fibrosis and inflammation have previously been identified. Since this was a small study, the observations should be regarded as hypothesis-generating.
Trial registration
Clinicaltrials.gov NCT01443780.
Introduction
Selenium is a trace element that can be found in all living cells[1, 2]. Important selenoproteins in the body are selenoprotein P, glutathione peroxidases, and thioredoxin reductase, all protecting against oxidative stress. Increased vascular oxidative stress and endothelial dysfunction in patients with coronary heart disease have been reported, although the results are conflicting[3, 4]. In European populations with low dietary selenium intakes as a result of the low selenium content in the soil, biofortification has been regarded as logical[5, 6], as opposed to the status in the United States where the selenium soil content is generally high. The estimated serum selenium concentrations in US citizens are generally above 120 μg/L,[7, 8] whereas concentrations below 90 μg/L are invariably reported from European countries.[9–13].
Xia et al. demonstrated an interrelationship between selenium and coenzyme Q10 (ubiquinone) in the metabolic pathway to the active form of coenzyme Q10 (ubiquinol) [14]. Moreover, an adequate presence of coenzyme Q10 is needed for optimal functioning of selenoproteins. Similarly, a deficiency of selenium could influence the ability to obtain adequate concentrations of active coenzyme Q10 in cellular compartments. Coenzyme Q10 is a powerful anti-oxidant protecting against lipid peroxidation[15]. It has been shown that ubiquinone reduces the inflammatory response [16]. The endogenous production of coenzyme Q10 decreases continually after the age of 20, and the endomyocardial production is reduced to half at the age of 80 [17]. Thus, elderly people living in geographical areas with low selenium content in the soil and food may be at increased risk of heart disease and premature death due to a possible deficiency of these antioxidants. Our research group have recently reported higher CV mortality in a community population with low plasma selenium concentration [18].
We have previously reported on a dietary supplementation trial with both selenium and coenzyme Q10 to 443 elderly Swedish community members performed during 2003 until 2010 [19]. The intervention time was four years, and the follow-up after 5.2 years showed significantly reduced CV mortality, improved cardiac function as evaluated by echocardiography, and a reduced increase of the N-terminal fragment of proBNP (NT-proBNP), a cardiac peptide biomarker. The long-term effects as seen after ten years have also been reported by our group, where we still observed a significant reduction of CV mortality, even if the intervention lasted for only four years[20]. Positive effects could also be seen in some subgroups of the study population.
The primary aim of the present study was to evaluate possible CV effects of the intervention in the same population 12 years after the introduction of a four-year period of supplementation as a way to validate the surprising results from the 10-year evaluation. A secondary aim was to determine whether positive effects on CV risk could also be seen after 12 years in the two genders, in those with diabetes, ischemic heart disease (IHD), hypertension and impaired functional capacity as measured by the New York Heart Association functional Class (NYHA class).
Materials and methods
The design of the main study has been published elsewhere [19]. In brief, 443 elderly healthy participants were given dietary supplementation of 200 mg/day of coenzyme Q10 capsules (Bio-Quinon 100 mg B.I.D, Pharma Nord, Vejle, Denmark) and 200 μg/day of organic selenium yeast tablets (SelenoPrecise 200 μg, Pharma Nord, Vejle, Denmark), or a similar placebo during 48 months, and then the interventions was finished. The study supplementation was taken in addition to regular medication if used. All study medications (active drug and placebo) not consumed were returned and counted. All participants were examined by one of three experienced cardiologists. A new clinical history was recorded, and a clinical examination was performed, including blood pressure, assessment of New York Heart Association functional class (NYHA class) as well as ECG and echocardiography. Doppler echocardiographical examinations were performed with the participant in the left lateral position. The ejection fraction (EF) readings were categorized into four classes with interclass limits placed at 30%, 40% and 50% [21, 22]. Normal systolic function was defined as EF≥ 50%, while severely impaired systolic function was defined as EF< 30%. The first participant was included in January 2003, and the last participant concluded the study in February 2010.
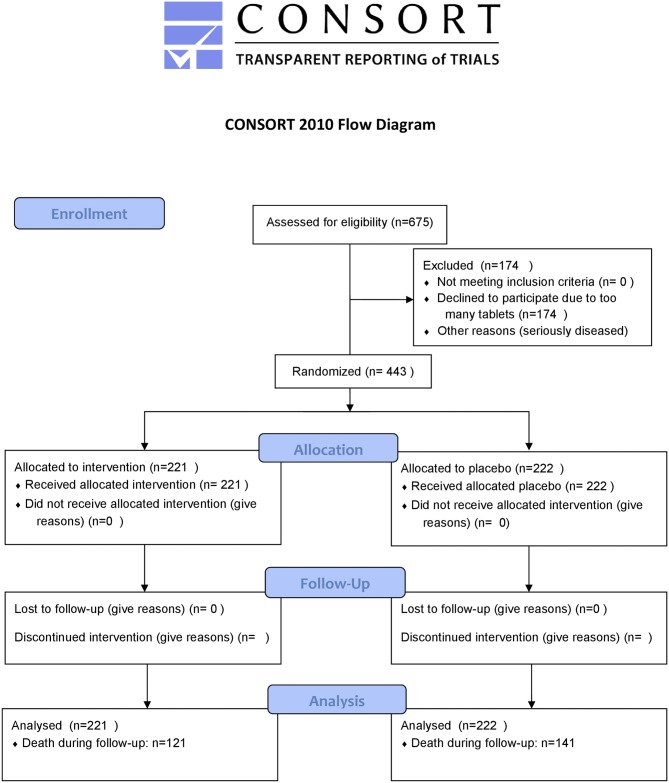
As the intervention time was unusually long for the main intervention study (48 months) only 228 participants completed the study; 86 died during the intervention time, and 129 (29.1%) decided not to complete the study. The reasons for the latter have been presented in detail in the main publication, but the main reason was there were too many tablets to take [19]. A flowchart of the total follow-up period is presented as Fig 1.
Fig 1. CONSORT flow chart of the study.
Written, informed consent was obtained from all patients. The study was approved by the Regional Ethical Committee in Linköping, Sweden and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. This study was registered at Clinicaltrials.gov, and has the identifier NCT01443780. As the trial started when registration in Clinicaltrials.gov, or elsewhere was optional, the trial was not registered at the start. Registration took place at the time of publication, when this became obligatory. There is presently no ongoing study from the research group with the described preparations.
Biochemical analyses
All blood samples were obtained while the patients were at rest in a supine position. The blood samples were collected in plastic vials containing EDTA (ethylenediamine tetracetic acid). The vials were placed on ice before chilled centrifugation at 3000g, and then frozen at -70 °C. No sample was thawed more than twice.
Mortality
All instances of cardiovascular mortality (CV mortality) were registered. The mortality information was obtained from the National Board of Health and Welfare in Sweden, which registers all deaths of Swedish citizens based on death certificates or autopsy reports. Written, informed consent was obtained from all patients.
Statistical methods
Descriptive data are presented as percentages. Chi-square tests were used for discrete variables. All evaluations are performed according to the intention-to-treat principle. Kaplan-Meier analysis was used to demonstrate CV mortality during the follow-up period. Cox proportional hazard regression analysis was used to evaluate risk of CV mortality. The independent variables included in the multivariate model were variables known to be associated with CV mortality: age, male gender, smoking, hypertension, diabetes, IHD, NYHA class III, treatment with beta blocker, ACE-inhibitor, Hb<120g/L, EF<40% and active treatment with selenium and coenzyme Q10.
A P-value of <0.05 was considered statistically significant. All data were analyzed using standard software (Statistica v. 13.2, Dell Inc, Tulsa, OK).
Results
The study population were followed regarding CV mortality during a median follow-up time of 4233 days (range 348–5275), i.e. about 12 years. In the survival group the median follow-up time was 4484 days (range 404–5275), and in the CV mortality group a median follow-up time of 2818 days (range 348–4869) was recorded.
From the basal characteristics it could be seen that the two populations were balanced at the start of the intervention in all but one variable, use of ACE-inhibitors (15.8% vs 24.3%; P = 0.03). After 12 years there was no significant difference between the basal characteristics variables (Table 1).
Table 1. Population characteristics at inclusion, and after 12 years.
| At study start | After 12 years | |||||
|---|---|---|---|---|---|---|
| Active | p-value | Placebo | Active | p-value | Placebo | |
| n | 221 | 222 | 100 | 81 | ||
| Age, mean | 78 | 78 | 75 | 76 | ||
| Males/Females, n | 115/106 | 110/112 | 42/58 | 33/48 | ||
| Smokers, n (%) | 21 (9.5) | 0.86 | 20 (9.0) | 2 (2.0) | 0.27 | 4 (4.9) |
| Diabetes, n (%) | 47 (21.3) | 0.95 | 48 (21.6) | 19 (19.0) | 0.60 | 13 (16.1) |
| Hypertension, n (%) | 158 (71.5) | 0.28 | 168 (75.7) | 69 (69.0) | 0.84 | 57 (70.4) |
| IHD, n (%) | 47 (21.3) | 0.51 | 53 (23.9) | 13 (13.0) | 0.35 | 7 (8.6) |
| NYHA class III, n (%) | 41 (18.6) | 0.49 | 47 (21.2) | 12 (12) | 0.46 | 7 (8.6) |
| Medical Treatment | ||||||
| ACEI, n (%) | 35 (15.8) | 0.03 | 54 (24.3) | 10 (10.0) | 0.32 | 12 (14.8) |
| Beta blockers, n (%) | 81 (36.7) | 0.40 | 73 (32.9) | 37 (37.0) | 0.11 | 21 (25.9) |
| Statins, n (%) | 45 (20.7) | 0.50 | 51 (23.0) | 20 (20.0) | 0.97 | 16 (19.8) |
| Examinations | ||||||
| Hb<120g/L, n (%) | 23 (10.4) | 0.39 | 29 (13.1) | 10 (10.0) | 0.98 | 8 (9.9) |
| EF<40%, n (%) | 16 (7.2) | 0.87 | 17 (7.7) | 5 (5.0) | 0.98 | 4 (4.9) |
Note: ACEI: ACE- inhibitors; EF: Ejection fraction according to echocardiography; IHD: Ischemic heart disease; NYHA: New York Heart Association functional class
Upon analyses of the basal characteristics, it could be seen that at the start of the intervention the active treatment group, and the placebo group had the same age (78 years), whereas in survivors after 12 years the mean age had decreased to 75 years in the active treatment group, and to 76 years in the placebo group. At the start of the intervention equal proportions of the two groups had diagnosed diabetes (21%), whereas after 12 years a reduction of the diabetic proportion could be seen in both groups, though it was more prominent in the placebo group (Table 1). The numbers with diagnosed hypertension were equal both in the groups at the start of the intervention, and after 12 years. Regarding IHD, the intervention and placebo groups were balanced both at the start and after 12 years; however, both groups exhibited a decrease in the number with IHD after 12 years.
Cardiovascular mortality within 12 years
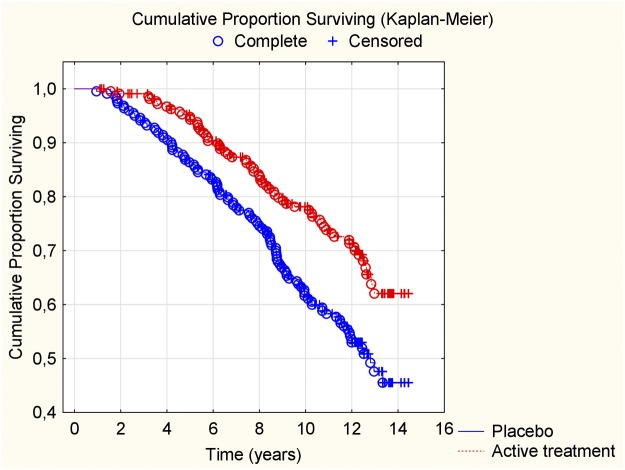
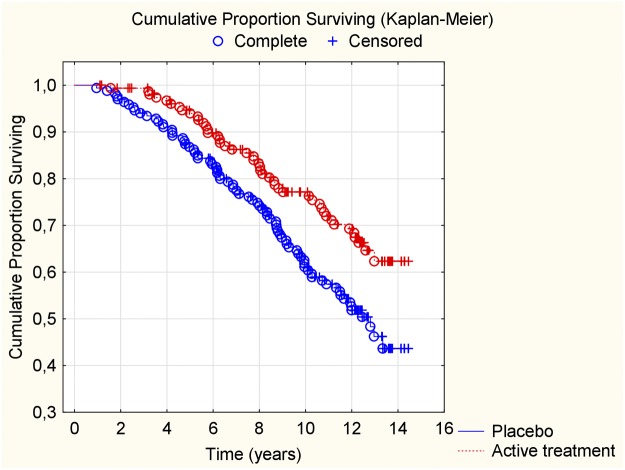
When evaluating the CV mortality within the 12-year period, we found that those on active treatment during the intervention had a significantly lower mortality also after 12 years (active treatment group: 62/221; 28.1%, vs placebo: 86/222; 38.7%; χ2:13.8; P<0.0001). The differences in CV mortality during the follow-up were also assessed and displayed in a Kaplan-Meier graph, showing a clear separation of the two groups (Fig 2).
Fig 2. Kaplan-Meier graph illustrating cardiovascular mortality during a follow-up period of 12 years of those supplemented with selenium and coenzyme Q10 versus placebo for four years on top of regular pharmaceutical treatment.
When applying the CV mortality results in univariate Cox regressions, we observed a highly significant risk reduction resulting from supplementing the participants with selenium and coenzyme Q10 for four years, also after 12 years (HR:0.58; 95%CI: 0.42–0.79; P<0.0007). Applying the data into a multivariate Cox regression model where well-known variables influencing the cardiovascular risk were included, a cardiovascular risk reduction appeared to remain also after 12 years (HR:0.59; 95%CI: 0.42–0.81: P = 0.001) (Table 2).
Table 2. Cox proportional hazard regression analysis evaluating risk of cardiovascular mortality by supplementation of selenium and coenzyme Q10 combined in a multivariate model after 12 years of follow-up after 4 years of intervention to an elderly community population.
| Variables | Hazard ratio | 95%CI | P-value |
|---|---|---|---|
| Age | 1.16 | 1.11–1.22 | <0.0001 |
| Male gender | 1.80 | 1.30–2.51 | <0.0001 |
| Smoking | 1.71 | 1.08–2.71 | 0.02 |
| Hypertension | 1.23 | 0.85–1.78 | 0.27 |
| Diabetes | 1.30 | 0.92–1.86 | 0.14 |
| IHD | 1.50 | 1.02–2.21 | 0.04 |
| NYHA class 3 | 2.01 | 1.45–3.03 | <0.0001 |
| Beta blocker | 0.85 | 0.59–1.21 | 0.37 |
| ACE-inhibitor | 1.11 | 0.76–1.61 | 0.59 |
| Hb<120g/L | 1.04 | 0.66–1.65 | 0.85 |
| EF<40% | 0.88 | 0.50–1.55 | 0.66 |
| Active treatment | 0.59 | 0.42–0.81 | 0.001 |
Notes: EF: Ejection fraction; IHD: Ischemic heart disease; NYHA: New York Heart Association functional class; Q4: 4th quartile
Subgroup analyses
When analyzing the two genders separately, we found a highly significant difference in CV mortality between the female intervention and the placebo groups, 20/106, and 46/112, respectively (χ2:12.7; P = 0.0004) after up to 12 years of follow-up. Similarly, in the male group a trend for difference between the groups was found (active group: 42/115, compared with the placebo group; 54/110; χ2:3.63; P = 0.057).
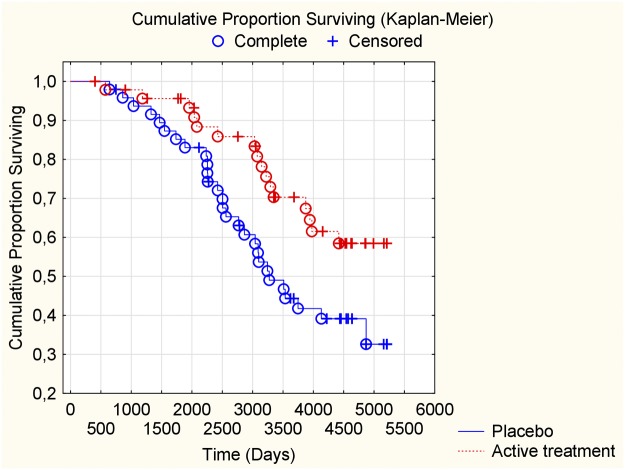
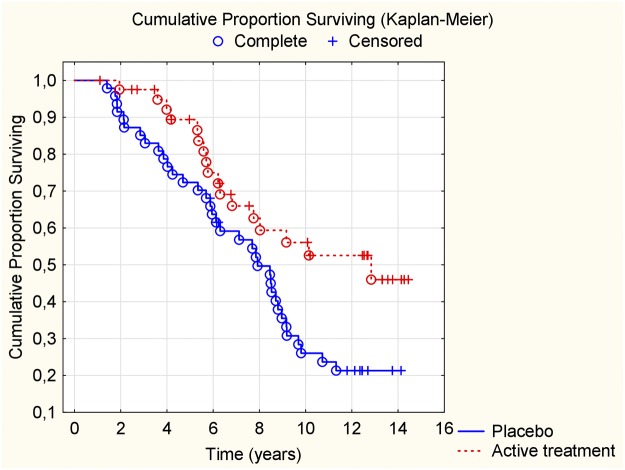
In the group with IHD we found a reduced CV mortality risk after supplementation with selenium and coenzyme Q10, also after 12 years of follow-up, in comparison with those on placebo (HR: 0.52; 95%CI: 0.30–0.90; P = 0.02) (Fig 3).
Fig 3. Kaplan-Meier graph illustrating cardiovascular mortality in participants with ischemic heart disease during a follow-up period of 12 years of those supplemented with selenium and coenzyme Q10 versus placebo for four years on top of regular pharmaceutical treatment.
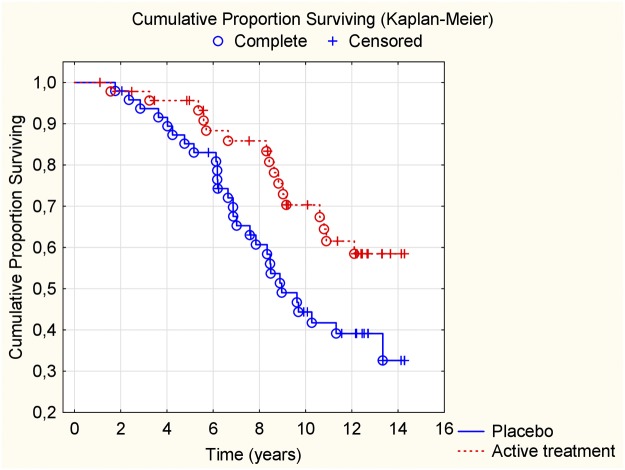
For the group with diabetes we also observed a reduced CV mortality risk after 12 years in the active supplementation group (HR: 0.50; 95%CI: 0.27–0.93; P = 0.03) (Fig 4).
Fig 4. Kaplan-Meier graph illustrating cardiovascular mortality in participants with diabetes during a follow-up period of 12 years of those supplemented with selenium and coenzyme Q10 versus placebo for four years on top of regular pharmaceutical treatment.
In the group with hypertension, a reduced CV mortality risk reduction could be seen as well (HR: 0.60; 95%CI: 0.41–0.85; P = 0.005) (Fig 5).
Fig 5. Kaplan-Meier graph illustrating cardiovascular mortality in participants with hypertension during a follow-up period of 12 years of those supplemented with selenium and coenzyme Q10 versus placebo for four years on top of regular pharmaceutical treatment.
For those with a severe functional impairment due to reduced cardiac function (NYHA functional class III) a significant reduction of CV mortality risk could be observed (HR: 0.49; 95%CI: 0.27–0.88; P = 0.002) (Fig 6).
Fig 6. Kaplan-Meier graph illustrating cardiovascular mortality in participants with NYHA functional class III during a follow-up period of 12 years of those supplemented with selenium and coenzyme Q10 versus placebo for four years on top of regular pharmaceutical treatment.
If combining those with IHD and those with impaired systolic cardiac function (EF<40%), a reduced risk for CV death within the 12-year period could be found with an HR of 0.56 (95%CI: 0.33–0.05; P = 0.03) (Table 3).
Table 3. Model testing the effect of intervention after 12 years on risk for cardiovascular mortality in different groups.
| Variable | HR | p-Value | 95%CV |
|---|---|---|---|
| Total study population | 0.58 | 0.0007 | 0.42–0.70 |
| HT | 0.59 | 0.005 | 0.41–0.85 |
| IHD | 0.52 | 0.02 | 0.30–0.90 |
| DM | 0.50 | 0.03 | 0.27–0.93 |
| NYHA III | 0.49 | 0.02 | 0.27–0.88 |
| IHD+EF<40% | 0.56 | 0.03 | 0.33–0.95 |
| IHD+EF<40%+HT | 0.60 | 0.004 | 0.42–0.84 |
| IHD+EF<40%+HT+DM | 0.59 | 0.003 | 0.42–0.83 |
Note: CV: Coefficient of variation; DM: Diabetes; HT: Hypertension; HR: Hazard ratio; IHD: Ischemic heart disease; NYHA III: New York Heart Association functional class III
In order to further increase the group at CV risk, we combined the groups with hypertension, IHD and impaired systolic function. In this group, we found a significant risk reduction with an HR of 0.60 (95%CI: 0.42–0.84; P = 0.004) as a result of the intervention (Table 3).
By adding those with diabetes to the above group, a significant risk reduction was still observed, with an HR of 0.59 (95%CI: 0.42–0.83; P = 0.003)(Table 3).
Discussion
In an intervention study in 443 elderly healthy persons, selenium and coenzyme Q10 were given as a combined dietary supplement for four years. After this period no intervention was given, and thus some of the participants have been without the selenium and coenzyme Q10 intervention for ten years. Our results show a continual and significant reduction in CV mortality during the whole follow-up period of 12 years, which also included the eight-year period after termination of the intervention (Table 4).
Table 4. Difference in cardiovascular mortality within 5, 10 and 12 years after intervention of selenium and coenzyme Q10 combined or placebo for four years.
| Follow-up time | Mortality in active treatment group (%) | Mortality in placebo group (%) | χ2-value | P-value |
|---|---|---|---|---|
| 5 years | 5.9 | 12.6 | 5.97 | 0.015 |
| 10 years | 20.8 | 38.7 | 17.01 | <0.0001 |
| 12 years | 28.1 | 45.0 | 13.78 | 0.0002 |
This appears clearly from the calculated mortality rates of the active treatment group, in comparison with those of the placebo group (Table 5), as we also did in the 10 years evaluation [20].
Table 5. Mortality rate after 5 years, 10 years and 12 years in the active treatment group compared to the placebo group, and to official mortality statistics.
| 5.2 years of follow-up | 10 years of follow-up | 12 years of follow-up | ||||
|---|---|---|---|---|---|---|
| All-cause mort rate | Cardiovasc mort rate | All-cause mort rate | Cardiovasc mort rate | All-cause mort rate | Cardiovasc mort rate | |
| Active group | 2433 | 1130 | 4427 | 2079 | 4542 | 2327 |
| Placebo group | 3115 | 2423 | 5400 | 3870 | 5993 | 3754 |
| Reference pop | 5794 | 2144 | 15,241* | 6998* | 15,420** | 6326** |
Note: Mortality rate expressed as mortality /100,000/year
Note: Reference group: Official Swedish mortality statistics based on the age group 80–84 in the 5.2 year follow-up, and on the age group 85 years and above in the 10 years follow-up;
* Figures based on statistics from 2014;
** Figures based on statistics from 2016
In Table 5, the figures from official Swedish mortality statistics are also added for comparison. The sample size of the present study is relatively small; therefore, the figures should be interpreted with caution. However, it is striking that the reduction in CV mortality also remains after 12 years. Even though the reduction in CV risk measured as reduced CV mortality is still significant, the effect seems somewhat less after 12 years than after ten years [21], especially in the male group.
This can probably be explained by the fact that ever-existing pathogenic factors catch up with the positive effects obtained by our intervention. Also, as reported previously, females have lower levels of coenzyme Q10 than males, presumably explaining why the females benefited more than males from the supplementation [23].
The CV mortality risk reduction is significant and stable in all the well-known risk populations, including those with IHD, hypertension or diabetes. Even after combining groups in order to increase stepwise the size to the subpopulations, the risk reduction is stable and comparable to the risk reduction obtained in the separate subgroups, indicating a robust effect caused by the intervention. It is tempting to speculate that permanent or progressive structural changes took place in the subjects of the placebo group during the interventional four-year period, explaining the apparent slowing down of CV pathogenesis in the supplemented group.
Selenium-containing enzymes, as well as the coenzyme ubiquinone are strong antioxidants, both being required in adequate amounts for normal cell function. Positive effects of supplementation of coenzyme Q10 on endothelial function have also been reported [24]. It has been shown that increased inflammatory activity[25–27], and oxidative stress occur in the elderly [28], and as one of the effects of the present intervention is to reduce signs of inflammatory activity [29], and oxidative stress [30], it is tempting to speculate that this effect is partially responsible for the long-lasting protection obtained in the present study. Moreover, endothelial cells and platelets may be particularly vulnerable to oxidative stress as they are surrounded by continuous oxygen transport in the circulation, and also exposed to increasing inflammatory burden with age [31, 32].
Furthermore, the persisting effects even eight years after termination of the supplementation could be related to the unusually long intervention time of four years, which as discussed above, presumably was paralleled by structural changes in the placebos. The mean plasma concentration in this population was 67 μg/L[18]. For full expression of the extracellular selenoprotein P, a concentration of >90–140 μg/L is needed [33–35], implying that a selenium deficiency in fact existed before the intervention and during the whole period in the placebo group. Apparently, the size of the intervention, 200 μg Se/day was enough to give the needed blood concentration, which has been reported previously [36]. On average, the supplemented group had an estimated total daily selenium intake of about 235 μg, which is well above the requirement to optimize selenoprotein P. It was also below a tolerable upper intake level of 300 μg as recommended by the European Food Safety Authority [37] and Nordic Nutrition Recommendations [38]. In contrast, the population studied in the American SELECT trial had a basal Se intake of at least 120 μg/d before supplementation; thus, their supplementation of 200 μg Se/d brought them into a marginal or hazardous zone of above 300 μg [39, 40].
The long-lasting effect of the supplementation might also be related to the fact that a major component of yeast selenium is selenomethionine, which is, at the expense of methionine, incorporated into non-seleno proteins, which constitute the unspecific selenium pool having a long elimination half-life [41]. Another partial explanation of the long-lasting effects might be that the municipality under study developed an increased interest in supplementation with selenium and coenzyme Q10 after the intervention period, and therefore some of the participants of the project may have continued to take supplementation. However, if this was the case, even though it was not investigated in this study, we would expect that the amount randomized to the placebo group that might have started post-project supplementation by random be approximately as those that previously belonged to the active treatment group, and as a result of the post-project supplementation, the difference between the groups should disappear.
The present 12-year evaluation of cardiovascular mortality after four years of intervention is unique, and should be regarded as a validation of the surprising results from the 10-year evaluation, and shows that the positive effects of the intervention persist.
Therefore, the hypothesis arising from our results remains that the intervention with selenium and coenzyme Q10 inhibits the pathogenesis of irreversible, presumably structural, changes preceding cardiovascular events.
Limitations
The presented study has a limited sample size, making the interpretation of the results difficult. However, the statistical evaluations are extensive due to the many evaluations performed in the study population, including those previously published.
The evaluations of the subgroups are even more uncertain as the sample sizes are smaller compared to the main study group. However, also in this respect we would like to argue that the different types of statistical methods used all point in the same direction; a reduction of mortality was obtained by the intervention, confirming the previous suggestions that the optimal selenium intake lies in the range 100–300 μg/d.
In this report the CV mortality within 12 years has been analyzed, a variable that could be uncertain as it was based mainly on death certificates, and not on autopsy in a majority of the participants. However, it is likely that the uncertainties in this study are of the same extent as previous mortality studies that are not based on autopsies only. Hence, in spite of the limitation we think our results provide interesting information.
The limited age span of the analyzed study population also represents a limitation, making extrapolation of the results to larger populations difficult. However, the fact that the incidences of CV as well as of other types of disease are higher in an elderly population compared to younger persons makes the obtained results of this 12-year follow-up even more intriguing.
Conclusion
We present a 12-year analysis of CV mortality in an elderly Swedish population that had been given supplementation with selenium and coenzyme Q10 as a contribution to their diet for four years. The present follow-up revealed a reduced CV mortality risk of more than 40%, and a significant risk reduction in those with hypertension, IHD, impaired cardiac function, and diabetes. We consider that the presented data, based on small sample sizes, should be regarded as hypothesis-generating, as the data are both intriguing and surprising.
Supporting information
(DOCX)
(DOC)
Data Availability
Under Swedish Law, the authors cannot share the data underlying this study and cannot do any further research than what is specified in the ethical permissions application. For inquires on the data, researchers should first reach out to the owner of the database, the University of Linköping. Please reach out to the corresponding author with requests and for assistance with data requests. If the university approves the request, researchers can submit an application to the Regional Ethical Review Board for the specific research question that the researcher wants to examine.
Funding Statement
Part of the analysis costs was supported by grants from Pharma Nord Aps, Denmark, the County Council of Östergötland, Linköping University. The funding organizations had no role in the design, management, analysis, or interpretation of the data, nor in the preparation, review or approval of the manuscript. No economic compensation was distributed.
References
- 1.Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–83. Epub 2010/09/04. doi: 10.1089/ars.2010.3275 . [DOI] [PubMed] [Google Scholar]
- 2.Selenius M, Rundlof AK, Olm E, Fernandes AP, Bjornstedt M. Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid Redox Signal. 2010;12(7):867–80. Epub 2009/09/23. doi: 10.1089/ars.2009.2884 . [DOI] [PubMed] [Google Scholar]
- 3.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–8. Epub 2001/11/28. . [DOI] [PubMed] [Google Scholar]
- 4.Vassalle C, Bianchi S, Bianchi F, Landi P, Battaglia D, Carpeggiani C. Oxidative stress as a predictor of cardiovascular events in coronary artery disease patients. Clin Chem Lab Med. 2012;50(8):1463–8. Epub 2012/08/08. doi: 10.1515/cclm-2011-0919 . [DOI] [PubMed] [Google Scholar]
- 5.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–68. Epub 2012/03/03. doi: 10.1016/S0140-6736(11)61452-9 . [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Agriculture ARS. Nutrient Intakes from Food: Mean amounts conusmed per individual, one day, 2005–2006. www.ars.usda.gov/ba/bhnrc/fsrg Accessed March 2010. 2008.
- 7.Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third National Health and Nutrition Examination Survey, 1988–1994. J Trace Elem Med Biol. 2003;17(1):13–8. Epub 2003/05/21. doi: 10.1016/S0946-672X(03)80040-8 . [DOI] [PubMed] [Google Scholar]
- 8.Bleys J, Navas-Acien A, Laclaustra M, Pastor-Barriuso R, Menke A, Ordovas J, et al. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003–2004. Am J Epidemiol. 2009;169(8):996–1003. Epub 2009/02/18. doi: 10.1093/aje/kwn414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cauwenbergh R, Robberecht H, Van Vlaslaer V, Deelstra H. Comparison of the serum selenium content of healthy adults living in the Antwerp region (Belgium) with recent literature data. J Trace Elem Med Biol. 2004;18(1):99–112. Epub 2004/10/19. doi: 10.1016/j.jtemb.2004.04.004 . [DOI] [PubMed] [Google Scholar]
- 10.Burri J, Haldimann M, Dudler V. Selenium status of the Swiss population: assessment and change over a decade. J Trace Elem Med Biol. 2008;22(2):112–9. Epub 2008/06/21. doi: 10.1016/j.jtemb.2007.11.002 . [DOI] [PubMed] [Google Scholar]
- 11.Letsiou S, Nomikos T, Panagiotakos D, Pergantis SA, Fragopoulou E, Antonopoulou S, et al. Serum total selenium status in Greek adults and its relation to age. The ATTICA study cohort. Biol Trace Elem Res. 2009;128(1):8–17. Epub 2008/10/28. doi: 10.1007/s12011-008-8252-2 . [DOI] [PubMed] [Google Scholar]
- 12.Spina A, Guallar E, Rayman MP, Tigbe W, Kandala NB, Stranges S. Anthropometric indices and selenium status in British adults: the U.K. National Diet and Nutrition Survey. Free Radic Biol Med. 2013;65:1315–21. Epub 2013/10/08. doi: 10.1016/j.freeradbiomed.2013.09.025 . [DOI] [PubMed] [Google Scholar]
- 13.Galan-Chilet I, Tellez-Plaza M, Guallar E, De Marco G, Lopez-Izquierdo R, Gonzalez-Manzano I, et al. Plasma selenium levels and oxidative stress biomarkers: A gene-environment interaction population-based study. Free Radic Biol Med. 2014;74C:229–36. Epub 2014/07/16. doi: 10.1016/j.freeradbiomed.2014.07.005 . [DOI] [PubMed] [Google Scholar]
- 14.Xia L, Nordman T, Olsson JM, Damdimopoulos A, Bjorkhem-Bergman L, Nalvarte I, et al. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J Biol Chem. 2003;278(4):2141–6. doi: 10.1074/jbc.M210456200 . [DOI] [PubMed] [Google Scholar]
- 15.Bullon P, Roman-Malo L, Marin-Aguilar F, Alvarez-Suarez JM, Giampieri F, Battino M, et al. Lipophilic antioxidants prevent lipopolysaccharide-induced mitochondrial dysfunction through mitochondrial biogenesis improvement. Pharmacol Res. 2015;91:1–8. doi: 10.1016/j.phrs.2014.10.007 . [DOI] [PubMed] [Google Scholar]
- 16.Lee BJ, Tseng YF, Yen CH, Lin PT. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: a randomized, placebo-controlled trial. Nutr J. 2013;12(1):142 doi: 10.1186/1475-2891-12-142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579–84. Epub 1989/07/01. . [DOI] [PubMed] [Google Scholar]
- 18.Alehagen U, Johansson P, Bjornstedt M, Rosen A, Post C, Aaseth J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur J Clin Nutr. 2016;70(1):91–6. doi: 10.1038/ejcn.2015.92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alehagen U, Johansson P, Bjornstedt M, Rosen A, Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013;167(5):1860–6. doi: 10.1016/j.ijcard.2012.04.156 . [DOI] [PubMed] [Google Scholar]
- 20.Alehagen U, Aaseth J, Johansson P. Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. PLoS One. 2015;10(12):e0141641 doi: 10.1371/journal.pone.0141641 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen-Urstad K, Bouvier F, Hojer J, Ruiz H, Hulting J, Samad B, et al. Comparison of different echocardiographic methods with radionuclide imaging for measuring left ventricular ejection fraction during acute myocardial infarction treated by thrombolytic therapy. Am J Cardiol. 1998;81(5):538–44. . [DOI] [PubMed] [Google Scholar]
- 22.van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77(10):843–50. . [DOI] [PubMed] [Google Scholar]
- 23.Onur S, Niklowitz P, Jacobs G, Lieb W, Menke T, Doring F. Association between serum level of ubiquinol and NT-proBNP, a marker for chronic heart failure, in healthy elderly subjects. Biofactors. 2015;41(1):35–43. doi: 10.1002/biof.1198 . [DOI] [PubMed] [Google Scholar]
- 24.Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors. 2011;37(5):366–73. Epub 2011/06/16. doi: 10.1002/biof.154 . [DOI] [PubMed] [Google Scholar]
- 25.Hubbard RE, Woodhouse KW. Frailty, inflammation and the elderly. Biogerontology. 2010;11(5):635–41. doi: 10.1007/s10522-010-9292-5 . [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008;84(4):900–14. doi: 10.1189/jlb.0108023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumley A, Emberson JR, Wannamethee SG, Lennon L, Whincup PH, Lowe GD. Effects of older age on fibrin D-dimer, C-reactive protein, and other hemostatic and inflammatory variables in men aged 60–79 years. J Thromb Haemost. 2006;4(5):982–7. doi: 10.1111/j.1538-7836.2006.01889.x . [DOI] [PubMed] [Google Scholar]
- 28.Violi F, Loffredo L, Carnevale R, Pignatelli P, Pastori D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxid Redox Signal. 2017. doi: 10.1089/ars.2016.6963 . [DOI] [PubMed] [Google Scholar]
- 29.Alehagen U, Lindahl TL, Aaseth J, Svensson E, Johansson P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS One. 2015;10(9):e0137680 doi: 10.1371/journal.pone.0137680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alehagen U, Aaseth J, Johansson P. Less increase of copeptin and MR-proADM due to intervention with selenium and coenzyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors. 2015;41(6):443–52. doi: 10.1002/biof.1245 . [DOI] [PubMed] [Google Scholar]
- 31.Conti V, Corbi G, Simeon V, Russomanno G, Manzo V, Ferrara N, et al. Aging-related changes in oxidative stress response of human endothelial cells. Aging Clin Exp Res. 2015. doi: 10.1007/s40520-015-0357-9 . [DOI] [PubMed] [Google Scholar]
- 32.Gradinaru D, Borsa C, Ionescu C, Prada GI. Oxidized LDL and NO synthesis-Biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev. 2015. doi: 10.1016/j.mad.2015.03.003 . [DOI] [PubMed] [Google Scholar]
- 33.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15(4):804–10. doi: 10.1158/1055-9965.EPI-05-0950 . [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92(3):525–31. Epub 2010/06/25. doi: 10.3945/ajcn.2010.29642 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst R, Collings R, Harvey LJ, King M, Hooper L, Bouwman J, et al. EURRECA-Estimating selenium requirements for deriving dietary reference values. Crit Rev Food Sci Nutr. 2013;53(10):1077–96. doi: 10.1080/10408398.2012.742861 . [DOI] [PubMed] [Google Scholar]
- 36.Alehagen U, Alexander J, Aaseth J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS One. 2016;11(7):e0157541 doi: 10.1371/journal.pone.0157541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EFSA Scientific Committe on Dietetic Products NaA. Tolerable upper intake levels for vitamins and minerals. 2004.
- 38.Ministers NCo. Nordic Nutrition Recommendations 2012. 2014.
- 39.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. Epub 2008/12/11. doi: 10.1001/jama.2008.864 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD009671 Epub 2013/02/27. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander J. Selenium. Nordberg GF, Fowler BA, Nordberg M, editors: Academic Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
Under Swedish Law, the authors cannot share the data underlying this study and cannot do any further research than what is specified in the ethical permissions application. For inquires on the data, researchers should first reach out to the owner of the database, the University of Linköping. Please reach out to the corresponding author with requests and for assistance with data requests. If the university approves the request, researchers can submit an application to the Regional Ethical Review Board for the specific research question that the researcher wants to examine.