Abstract
Immunotherapies have shown promise in treatment of cancer, but more potent and targeted therapies are needed. Natural killer (NK) cells are lymphocytes with innate ability to recognize and lyse tumor cells. When activated, they also produce type II interferon (IFNγ) to orchestrate the activity of other immune cells. Strategies to elicit NK cell activation in vivo have potential usefulness in anti-tumor immunotherapies. Here, we report on a strategy to stimulate NK cell activation and anti-tumor activity in mice with established B16.F10 murine melanomas. We and others previously observed that NK cells are rapidly activated during infection by pathogens such as the bacterium Listeria monocytogenes (Lm). A secreted Lm virulence protein, p60, and a fragment of p60 termed L1S were previously shown to stimulate innate immune responses and promote NK cell activation. We purified recombinant L1S and characterized its activity in cell culture studies. Recombinant L1S protein was also observed to promote accumulation and robust NK cell activation in the lungs when given via intratracheal instillation to control and tumor-bearing mice. Importantly, therapeutic administration of a single L1S dose was found to significantly reduce the number and area of “metastatic” tumor nodules on the lungs of mice with established B16.F10 murine melanomas. Depletion studies showed that these antitumor effects were dependent on NK cells and IFNγ. These data provide proof of concept that administration of a single immune-modulating microbial polypeptide can be used to therapeutically boost NK cell in vivo activation and promote anti-tumor responses.
INTRODUCTION
Tumor cells that metastasize and establish in distal tissues are difficult or impossible to locate and resect. Hence metastatic tumors are the primary cause of cancer-related deaths (1). Immune cells can hunt and kill individual tumor cells. Strategies to boost the anti-tumor activity of immune cells thus have potential use in treatment of metastatic and hematologic cancers. Melanoma is a type of skin cancer that frequently metastasizes to the lungs, liver, bones and brain (2–3). Metastatic melanomas cause over 10,000 deaths annually in the United States (4). A subset of human patients with metastatic melanomas have been found to respond well to treatment with T cell activating checkpoint inhibitor immunotherapies and such therapies have also been effective in the commonly-used murine B16.F10 melanoma model (5–6). However, these current T cell-based therapies are not completely effective. Hence, there remains need for additional or improved immunotherapeutic approaches to treat metastatic melanoma and other cancers.
Boosting of innate anti-tumor immune responses could in theory be used synergistically to supplement or improve anti-cancer immunotherapies based on stimulation of adaptive immunity. Natural killer (NK) cells are an innate immune cell type that is a promising target for development of such immunotherapies. The presence of IFNγ-producing NK cells in tumor tissue corresponds with improved prognosis in both murine models and clinical studies of human patients (7–10). When appropriately primed and activated, NK cells recognize tumor cells and can induce cytolysis to directly kill the tumor cells in the absence of specific tumor antigens. Activated NK cells also produce cytokines such as IFNγ that can regulate other innate and adaptive immune cells. The ability of NK cells to mediate killing and cytokine production is regulated by the presence of activating and inhibitory cell surface receptors on the tumor cell, as well as by cytokines and other priming signals provided by dendritic cells (DC) or other accessory cells. Priming of NK cells increases their cytolytic activity and ability to produce immune activating cytokines such as IFNγ (11). Cytokines that are important for NK cell priming and activation include IL-18, IL-1β, IL-15, and IL-12 (7, 12–16). Previous work has shown that administration of specific NK cell-activating cytokines such as IL-2, IL-2 and IL-15, or a combination of IL-2, IFNα, and GM-CSF can promote NK cell responses in cancer patients (17). However, alternative or additional approaches may more specifically boost NK cell activity. The availability of multiple methods to prime or boost the activity of NK cells in vivo could also facilitate development of more effective combination immunotherapies or therapies involving sequential activation of patient NK cells.
Infections by a variety of bacterial and viral pathogens potently elicit NK cell activation and IFNγ secretion (18). Triggering of NK cell activation by pathogens has also been associated with anti-tumor activity (23). Listeria monocytogenes (Lm) is a Gram-positive bacterial pathogen known to potently stimulate NK cell activity in infected mice (20–22). A secreted Lm virulence protein, p60, contributes to this effect, promoting NK cell activation and IFNγ secretion both in the context of Lm infection and, importantly, as a recombinant protein in absence of intact Lm (20,23). The ability of recombinant p60 to promote NK cell activity maps to a fragment termed L1S (23). In cell culture models, intact p60 protein or the L1S region indirectly stimulate activation of murine and human NK cells to secrete IFNγ due to their effects on dendritic cells (DCs) (21, 23).
Given its stimulatory effects in vitro, we reasoned that recombinant L1S might also be useful to stimulate beneficial NK cell activity in disease settings. Here, we provide proof of concept that the recombinant L1S protein can be used to promote activation of lung NK cells in mice in a therapeutic setting. Administration of L1S protein to lungs of mice with established B16.F10 murine melanoma tumors triggered significant NK cell and IFNγ dependent reductions in tumor burden. Hence, L1S has potential use in promoting NK cell activity in the context of anti-cancer immunotherapies.
MATERIALS AND METHODS
Preparation of L1S Protein
L1S protein was expressed in E. coli with a pTrcHis (Invitrogen) construct and purified as previously described (23). Briefly, log phase bacteria were incubated 8 h with IPTG to induce protein expression. Following cell lysis and DNAse treatment, lysates with centrifuges to pellet cellular debris. Supernatants were filtered in a 0.22 μm syringe filter and loaded onto and ATKA FPLC for nickel (Ni) column affinity purification of the 6 x His tagged protein. Aliquots of fractions eluted from the column were evaluated using SDS-PAGE and Coomassie staining. In some experiments, polymixin B columns were used to further purify L1S protein. Fractions containing L1S protein were concentrated using Millipore 10k KD columns and protein concentrations were determined using BCA.
Mice
C57BL/6J (B6) and B6.tlr4−/− mice were obtained from Jackson Laboratories and maintained at National Jewish Health and the University of Colorado Anschutz Medical Campus. Mice were utilized at 8–12 weeks of age. Mice were anesthetized for i.t. instillations by i.p. inoculation with 150 μl containing 80–100 mg/kg ketamine and 5–10 mg/kg of Xylazine. A heat lamp was utilized to maintain body temperature until this point. To sacrifice mice, 500 μl Fatal Plus (pentobarbital) was given i.p. and mice were exsanguinated via cardiac stick. For depletion experiments, purified endotoxin-free antibodies were purchased from BioXcell. Clones used were: anti-NK1.1 clone PK136, anti-Ly6G clone 1A8, and anti-IFNγ clone XMG1.1. All depletion antibodies were given at 500 μg per mouse in a 200 μl i.p. injection.
Lung Digestion and Flow Cytometry
Lungs were perfused with 10 ml of ice cold PBS through the heart. One lobe was taken and frozen for lysate ELISA. Remaining lobes were separated and chopped utilizing surgical scissors. Lungs were digested in 1 mg/ml of Collagenase IV (Worthington) or 1 mg/ml of Collagenase VIII (Sigma) for 30 minutes at 37°C. To stop the reaction 60 μl of 5mM EDTA was added to each organ. Digested organs were brought into single cell suspension by processing through an 18G needle 3–5 times and then passed through at 100 μm cell strainer twice. 2 × 106 cells were obtained and plated in RPMI complete medium with Golgi Plug, Brefeldin A, protein transport inhibitor (Ebioscience) at 1:1000 for 3 h. Following treatment with protein transport inhibitor the cells were treated with FC block for 30 minutes. Surface staining used the following antibodies and was performed for 30 minutes in FACS buffer at 4C: Ly6G-A18 (PerCP-Cy5.5, Biolegend), NK1.1-PK136 (PerCP-Cy5.5, eBioscience), CD3-17A2 (BV510, Biolegend), NKp46-29A1.4 (Pe-Cy7, eBioscience). For intracellular staining, cells were treated with a PFA-saponin mixture for 15 minutes at RT to fix the cells and prepare them for intracellular staining. Cells were then stained for Intracellular cytokines in PBS-Saponin for 45 minutes at RT using the following antibodies: IFNγ-XMG1.2 (APC, eBioscience). Samples were run on LSRII and LSR Fortessa (BD Biosciences) and FACS analysis was done through FlowJo Software.
Isolation and growth of bone marrow DCs
Bone marrow was obtained from the hind-leg bones of mice. Bone marrow was extracted from 1–2 legs and plated in 3–6, T-75 flasks containing 50 ml of RPMI complete medium containing GM-CSF. Cells were grown over 7 days and new media was added on days 2 and 4. On day 7 non-adherent cells were collected and plated in 300 μl of RPMI complete media in 24-well plates at 3 × 105 per well overnight. On day 8 media was changed and experiments were conducted.
Cell culture and co-culture experiments
BMDCs were treated with 10 μg/well (33.3 ug/ml) of protein following priming with PBS (none), IL-12 (2 ng/ml), PIC (20 μg/ml), or LPS 10 ng/ml. Supernatants were collected 24 h after treatment and tested for IL-1β or IL-18 via ELISA. Nylon-wool non-adherent cells (NWNA) or purified NK cells were isolated for co-culture experiments and added at 3 × 104 cells per well. 24 h later supernatants were collected and tested for IFNγ via ELISA. BD-OptEIA kits were used to quantify IL-1β and IFNγ and eBioscience pre-coated plate kits were utilized to quantify IL-18.
Isolation of nylon wool non-adherent cells
Sterile nylon wool (0.5 g) was inserted into 20 ml syringe columns. A three-way stopcock was attached to the column and a 20 ml syringe containing RPMI complete medium. The nylon wool was primed with 20 ml of RP10 medium and placed at 37°C. Whole spleen was isolated and digested into single cell suspension using a 70 μm nylon sheets. Red blood cells were lysed using ACK RBC lysis buffer and re-suspended in RP10 medium. Splenic cells were placed onto nylon wool and incubated for 1 h at 37°C to remove adherent myeloid and B cells.
B16.F10 culture
B16.F10 murine melanoma cells were obtained from ATCC (CRL-6475). B16.F10/RFP murine melanoma cells were a gift from Divij Matthew and Raul Torres (University of Colorado). Cells were maintained in culture with DMEM as described (24–25). Cells were sequentially passaged at 80–90% confluency. Cells thawed from frozen aliquots were passaged 5–8 times prior to harvest at < 50% confluence for use in vivo. Cells were reconstituted into cold PBS prior to i.v. injection.
Lung immune activation model
Mice were given intratracheal inoculations of 125μg of protein, 125ng of LPS, or PBS in 50μl volume. The mice were sacrificed 24, 48, or 96 h to analyze immune parameters. Lungs were prepared for single cell suspension and flow cytometric analysis as above.
Assay for tumor cell lysis
To isolate effector NK cells, mice were given intratracheal inoculations of L1S protein or PBS as above and sacrificed 24 hours later. Lungs were perfused with 10 ml of ice cold PBS through the heart. The lobes were separated and chopped utilizing surgical scissors. Lungs were digested in 1ml of Collagenase/Hyluronidase mixture (STEMCELL Catalog# 07912), DNAse at 10μg/ml, and 1ml of RPMI complet media for 45 min at 37°C. 1 mg/ml of Collagenase IV (Worthington) or 1 mg/ml of Collagenase VIII (Sigma) for 30 minutes at 37°C. To stop the reaction 60 μl of 5mM EDTA was added. Digested lungs were brought into single cell suspension by processing through an 18G needle 3–5 times and then passed through at 100 μm cell strainer twice. NK cells were then isolated utilizing STEMCELL EasySep Mouse NK cell isolation kit (Catalog# 19855). Purified lung NK cells were added at the indicated effector:target (E:T) ratios to B16.F10/RFP target cells that had been plated the day before. After a 4 h co-culture, cells were lifted, stained for NK1.1, and analyzed by flow cytometry. A known quantity of fluorescent beads was spiked into samples to facilitate accurate counting of total cell numbers (CountBright absolute counting beads #C36950). Live single cells were gated and the number of RFP+NK1.1− cells per 1000 beads was determined. Specific lysis was quantified by calculating the reduction in number of tumor cells following the culture with NK cells: % specific lysis = 100* [(number of target cells in control) – (number of target cells with NK cells) / (number of target cells in control)]
Lung ‘metastasis’ model
Mice were injected with 2–6 × 105 B16.F10 cells via tail vein. Eight days later, tumor-inoculated mice were randomized into groups given intratracheal inoculations with 50 μl of PBS with or without 125 μg L1S protein or 125 ng LPS. The mice were sacrificed 24, 48, or 96 h to analyze tumor burden and immune parameters. Lungs were inflated with PBS and removed from the animal. Lobes were separated and imaged on both sides. Each black lung nodule was counted by clicker-counter with edges being counted on only one side. To estimate total tumor area, images were taken of the lung surface and analyzed using the FIJI package of ImageJ. To accommodate differences in the size of various lung images, tumor area measurements for each lung were normalized to total lung surface area within each image.
Peritoneal tumor model
Mice were injected i.p. with 1 × 105 B16.F10 cells on day 0. Five days later, mice were given a single i.p. injection of 200 μl PBS ± 0.5 mg L1S protein. Mice were sacrificed for analysis day 10 (5 d after treatment). Visible tumor masses from each mouse were dissected from the peritoneum and weighed to determine overall tumor burden.
Data analysis and statistics
Data was processed using GraphPad Prism Version 5.0b. One-Way ANOVA analysis was conducted utilizing Newman-Kuels post-test to calculate p-value. Significant differences are indicated with asterisks (*) when p < 0.05.
Ethics statement
The Animal Care and Use Committees for National Jewish Health (Protocol# AS2682-08-16) and the University of Colorado School of Medicine (Protocol# 105614(05)1E) approved all studies. These protocols adhere to standards of the United States Public Health Service and Department of Agriculture.
RESULTS
Purified recombinant L1S protein can be used to promote NK cell activation
Our previous results using cell co-culture assays support the model that p60 or its L1S fragment indirectly promote NK cell activation (Fig. 1A). L1S and p60 stimulate inflammasome-dependent production of IL-18 that is required for the NK cell activation in these cultures, as is cell-contact between bone marrow dendritic cells (BMDCs) and NK cells (23, 26). As for other NLRP3-inflammasome activators, the ability of L1S to induce IL-1β and IL-18 processing and secretion requires priming of cultured BMDCs using TLR agonists or cytokines.
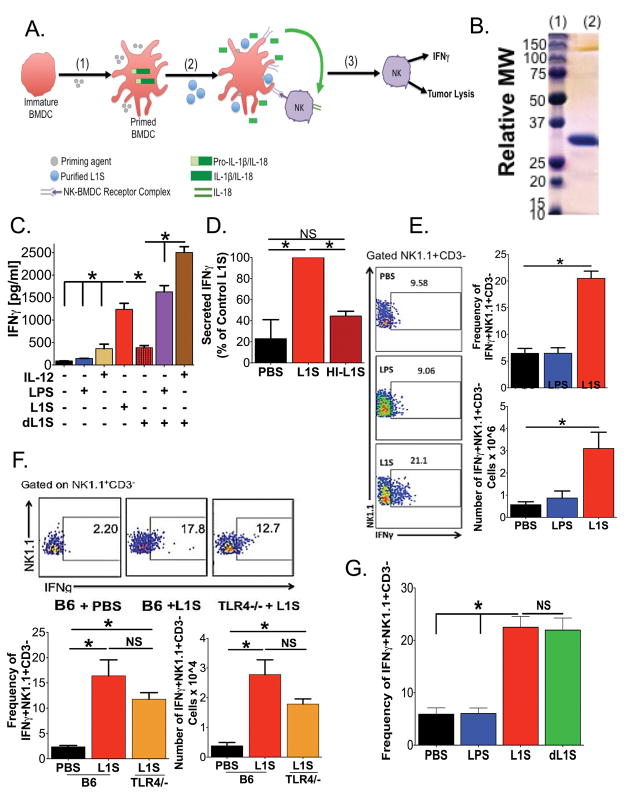
Figure 1. Treatment with L1S Protein Stimulates BMDCs to Induce Natural Killer Cell Activation.
(A) Model depicting the presumed mechanism for NK cell activation, based on Schmidt et al23: (1) Priming of immature BMDC induces production of pro-cytokines. (2) L1S treatment elicits inflammasome-dependent cytokine processing and promotes cell-contact-dependent NK cell activation. (3) Activated NK cells produce IFNγ and show increased cytolytic function. (B) Representative coomassie-stained SDS-PAGE with (lane 1) mass marker and (lane 2) 10 ug of purified L1S protein. (C) Supernatant IFNγ was quantified 24 h after establishing co-cultures of BMDC and NWNA cells (both from B6 mice). BMDC were treated with the indicated priming agent ± 30 ug/ml recombinant L1S. L1S was purified using nickel (Ni) affinity chromatography alone. Detoxified (d)L1S was further purified using a polymixin B column to remove LPS. (N=3) (D) Relative abundance of supernatant IFNγ 24 h after treatment of co-cultured LPS-primed BMDC and NWNA cells with PBS ± purified control or heat-inactivated (HI) L1S protein. (N=3) (E) Intracellular staining for IFNγ+ in gated NK1.1+CD3− cell populations isolated from lungs of B6 mice 24 h after instillation of PBS ± 125 ng LPS or 125 ug L1S (N=7). (F) Intracellular staining for IFNγ+ in gated NK1.1+CD3− cell populations from lungs of B6 and B6.tlr4−/− mice 24 h after instillation of PBS or 125 ug L1S (N=3). (G) Intracellular staining for IFNγ+ in gated NK1.1+CD3− cell populations isolated from lungs of B6 mice at 24 h after instillation of PBS ± 125 ng LPS, 125 μg L1S or 125 μg of dL1S (N=4). For E, F and G, representative plots are shown gated on Live → singlets →→ NK1.1+CD3− → IFNγ+. For all graphs data were pooled from the indicated number of experiments using at least 3 cultures or mice per group per experiment. Errors are SEM. (*) indicates p < 0.05 using ANOVA with Newman-Keuls comparison.
To study the potential for therapeutic use of L1S, we first expressed His-tagged recombinant L1S protein in Escherichia coli and purified the protein using Ni-affinity chromatography. The protein was free of contaminants as judged by SDS-PAGE (Fig. 1B). However, in some preparations limulus assays detected trace amounts of LPS (<10 ng/ug). Consistent with the presence of this priming agent, L1S purified using single step Ni-affinity chromatography stimulated secretion of IL-1β and IL-18 by unprimed BMDCs (Supplementary Figure 1). L1S purified in this way also promoted secretion of IFNγ in co-cultures comprised of nylon wool-non-adherent (NWNA) splenic lymphocytes and unprimed BMDCs (Fig. 1C). Activated NK cells are responsible for the IFNγ production in these co-cultures (23, 37). When “detoxified” L1S (dL1S) was prepared by a second purification step on polymixin B columns, the protein failed to stimulate NK cell IFNγ secretion in the cell co-cultures unless the BMDCs were first primed with IL-12 or LPS (Fig. 1C). These priming agents did not induce IFNγ production in the absence of L1S protein (Fig. 1C), nor was heat-inactivated L1S (HI-L1S) capable of inducing IFNγ secretion in these co-cultures (Fig. 1D). These data are consistent with our previous results and confirm that preparations of purified L1S exert protein-dependent immune stimulatory activity.
We next sought to evaluate the ability of purified L1S to stimulate NK cell activity in vivo. To this end, Ni-affinity purified protein was instilled directly into the lungs of naïve B6 mice using an established intratracheal (i.t.) inoculation method (28). Lungs were harvested 24 hrs later and digested to prepare single cell populations for flow cytometric analysis. Compared to mice given an equal volume of PBS ± 125 ng LPS, the proportion and number of lung NK1.1+CD3− NK cells staining positive for IFNγ+ was significantly increased in mice treated with 125 ug of purified L1S protein (Fig. 1E). The frequency of lung NK cells activated to produce IFNγ was proportional to the dose of L1S given (Supplementary Figure 1), and we primarily observed responding NK cells in the site inoculated with L1S (lung or peritoneum) at the doses used. Interestingly, lung NK cell activation was also seen to occur in B6.tlr4−/− mice (Fig. 1F), which lack the LPS receptor TLR4. Furthermore, detoxified L1S (dL1S) protein induced NK cell activation and IFNγ production when instilled into the lung (Fig. 1G). These data demonstrate that L1S protein itself is responsible for the NK cell activation. The absence of a priming agent requirement to induce NK cell activation in the lungs may be due to in situ priming of DCs by commensal lung bacteria. Taken together, these results demonstrate that purified L1S protein can be administered to naïve mice to elicit activation of lung NK cells in vivo.
L1S treatment induces a limited and transient inflammatory response in the lung
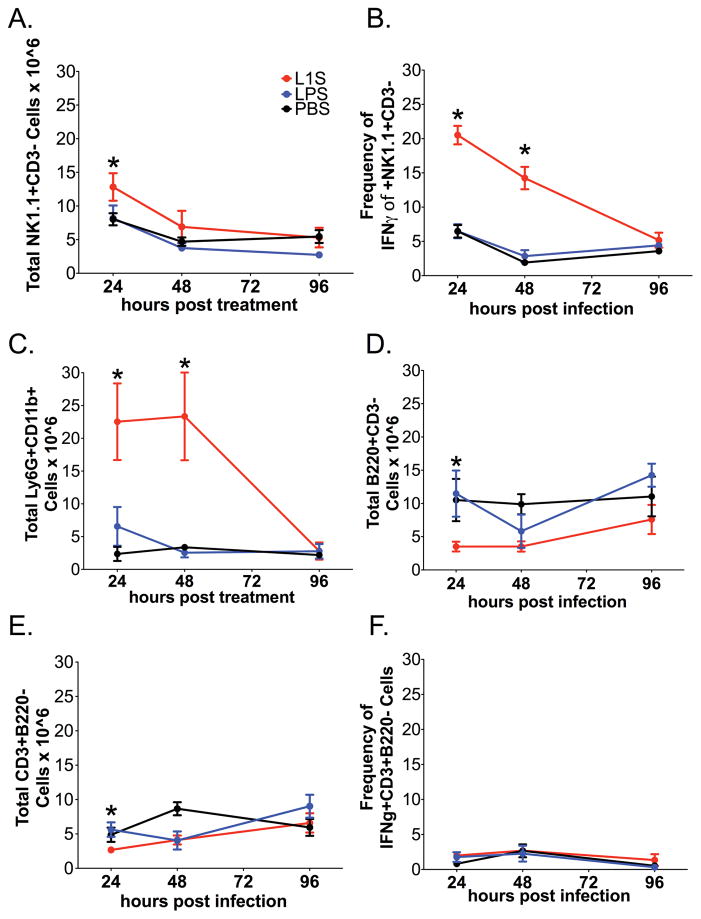
The kinetics and extent for the inflammatory response elicited by L1S treatment was further evaluated. A modest and transient, but significant, increase in the total number of NK recovered from the lungs was observed at 24 h after L1S-treatment (Fig. 2A) It was not determined if this increase resulted from recruitment of new NK cells or proliferation of lung-resident NK cells. The proportion of lung NK cells producing IFNγ increased substantially after L1S treatment, peaking at 24 h after treatment and returning to basal levels by 96 h (Fig. 2B). L1S instillation was also associated with a significant and transient increase in the number of neutrophils in the lungs (Fig. 2C). Kinetics of neutrophil accumulation correlated with those of NK cell IFNγ production, suggesting a generalized inflammatory response following the L1S treatment. Consistent with this interpretation, B and T cell numbers were also modestly increased at 24 after L1S installation (Fig. 2D–E). These data demonstrated the ability of L1S treatment to stimulate a rapid and transient accumulation and activation of NK cells in the lung, a response that was further associated with accumulation of other immune cells.
Figure 2. Kinetics of inflammatory cell accumulation and activation in lungs of naïve mice treated with L1S.
As above, mice received a single i.t. instillation with PBS ± L1S protein or LPS. At the indicated times, lungs were harvested and single cell suspensions prepared for flow cytometry analysis. (A) NK cell (NK1.1+CD3−) number per lung. (B) Proportion of activated NK cells (NK1.1+CD3−) as measured by intracellular staining for IFNγ+NK1.1+CD3−. (C) Neutrophil (Ly6G+CD11b+) number per lung. (D) B cell number per lung (B220+CD3−). (E) T cell (CD3+B220−) number per lung. (F) Proportion of T cells (CD3+B220−) staining positive for intracellular IFNγ+ at indicated time. Each data point represents data pooled from at least 3 experiments with at least 3 mice per experiment. Cells are gated on Live → singlets → Respective group(s) of interest. Error bars indicate SEM. (*) indicates p < 0.05 using ANOVA with Newman-Keuls comparison.
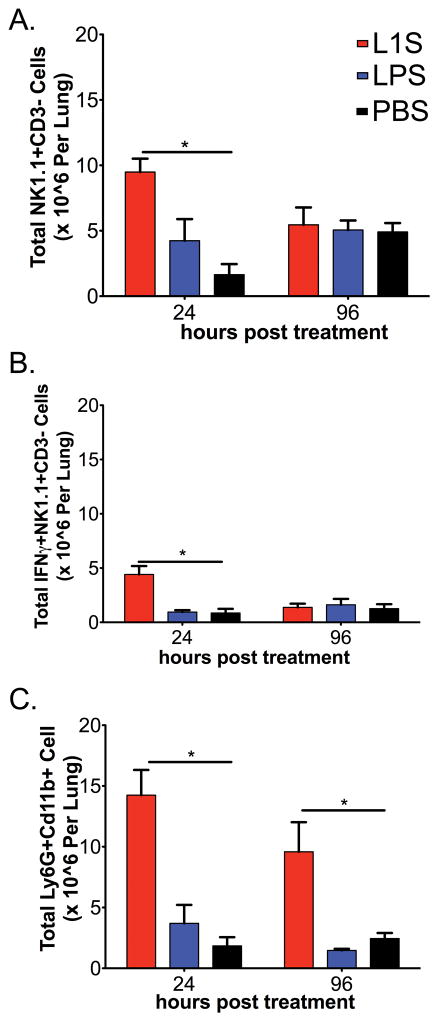
To evaluate the efficacy of L1S in stimulating NK cell activation in tumor-bearing mice, we instilled the protein i.t. into animals with established metastatic melanomas. Mice received a single dosing of L1S eight days after they received an i.v. inoculation with B16.F10 melanoma cells. As in the naïve mice above, the total number of NK cells was significantly increased in the lungs of tumor-bearing mice 24 h after L1S treatment (Fig. 3A). The number of total lung NK cells returned to baseline by 96 h after L1S treatment (Fig. 3A). Intracellular staining for IFNγ showed that the number of NK cell actively secreting IFNγ was also transiently increased after L1S treatment in the tumor-bearing mice (Fig. 3B). Neutrophils also transiently accumulated in lungs of tumor-bearing mice following the L1S stimulation (Fig. 3C). These data showed that the presence of established melanoma tumor nodules did not interfere with the ability of i.t. L1S treatment to trigger transient inflammation and NK cell activation in the lungs.
Figure 3. Kinetics of inflammatory cell accumulation and activation in lungs of tumor-bearing mice treated with L1S.
Mice were inoculated i.v. with B16.F10 melanoma cells. Eight days later, they received a single i.t. instillation with PBS ± L1S protein or LPS as above. Mice were sacrificed and lungs digested for flow cytometry analysis of total NK cells (NK1.1+CD3−) at (A) 24 and 96 h after L1S treatment. Intracellular staining for IFNγ+ was also used to determine proportion of activated NK cells (NK1.1+CD3−) (B) 24 and 96 h after the L1S treatment. (C) Total neutrophils (Ly6G+CD11b+) in the lung were analyzed at 24 and 96 h after L1S treatment. Each graphed bar represents data pooled from at least 3 experiments with at least 3 mice per experiment. Cells are gated on Live → singlets → Respective group(s) of interest. Error bars indicate SEM. (*) indicates p < 0.05 using ANOVA with Newman-Keuls comparison.
L1S instillation is therapeutic against metastatic disease
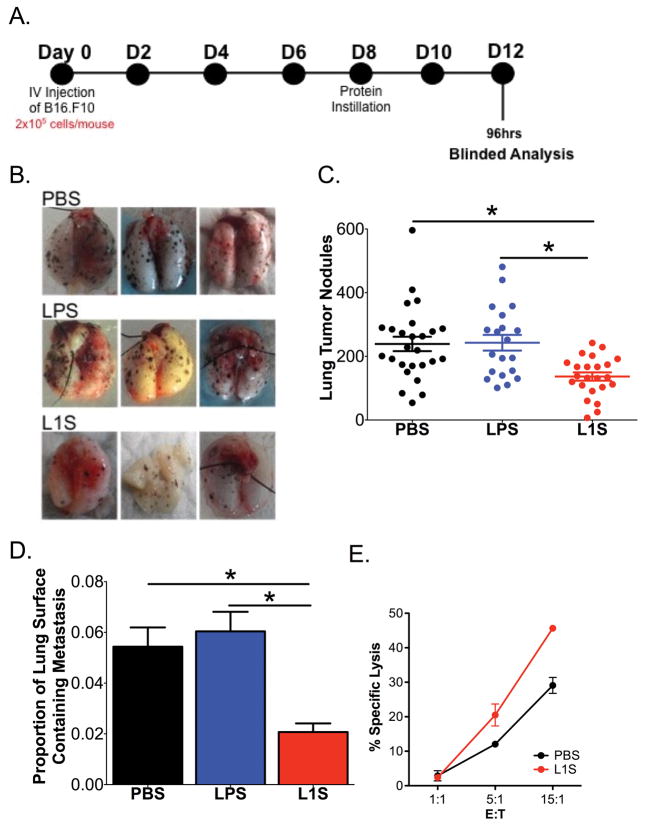
To evaluate if tumor burdens were reduced by the elicited inflammatory and NK cell response, mice were treated with L1S on d8 and harvested for analysis 4d later (Fig. 4A). Upon harvest, the lungs from mice treated with L1S were clearly distinguished by a reduced abundance of dark melanoma nodules (Fig. 4B). Blinded quantification of these visible nodules confirmed there was a significant reduction in their numbers in the L1S-treated mice (Fig. 4C). The average size of the tumor nodules also appeared to be reduced in the L1S treated group (see Fig. 4B). To quantify this parameter, we utilized image analysis methods to estimate the proportion of the lung surface area covered by tumors (Supplemental Figure 2). Results confirmed the ability of L1S treatment to significantly reduce lung tumor burden (Fig. 4D). NK cells isolated from lungs 24 h after L1S treatment were also found to have an increased ability to lyse B16.F10 tumor cells using a clone expressing RFP (Fig. 4E). A separate series of experiments showed that i.p. treatment with L1S could also reduce the overall mass of melanoma recovered from mice with established intraperitoneal B16.F10 tumors (Supplemental Fig. 3). These results together argue that the inflammatory response and/or NK cell activation triggered by a single L1S treatment was sufficient to significantly reduce masses of metastatic and local B16.F10 melanomas in the lung and peritoneum of mice with previously-established tumors.
Figure 4. L1S instillation stimulates a therapeutic anti-tumor response in mice with established “metastatic” B16.F10 melanomas.
Mice were given tumor cells on d0 and treated on d8 by instillation with 125 μg of PBS ± L1S or LPS. Mice were sacrificed on d12 (96 h after treatment). (A) Experimental timeline. (B) Representative images of mouse lungs at time of harvest. (C) Plot depicts quantified tumor nodules counted on lungs of the treated mice at harvest. Date are pooled from seven independent experiments. (D) The proportion of lung surface area covered with tumor nodules was quantified as a measure of total tumor size. Data are from the same experiments as in (C). (E) Lysis of RFP-expressing B16.F10 tumor cell targets by NK cells purified from lungs of mice at 24 h after instillation of PBS ± L1S protein. Data are from one of three experiments with similar results. All graphs depict means ± SEM. (*) indicates p < 0.05 using ANOVA with Newman-Keuls comparison.
Therapeutic effects of L1S treatment require NK cells and IFNγ
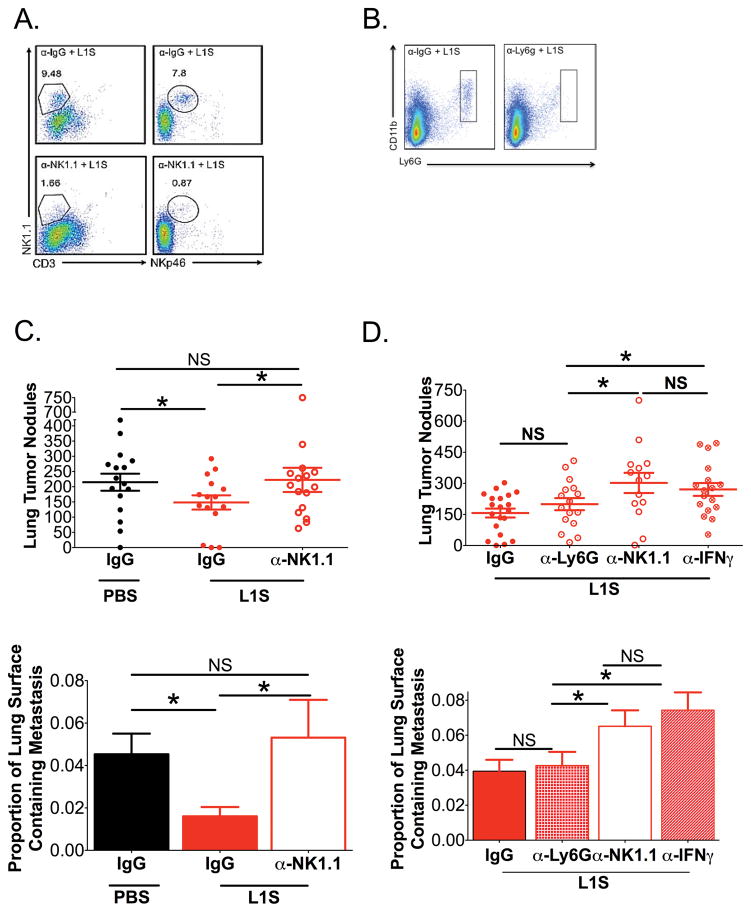
To further investigate the mechanism responsible for the therapeutic effects of L1S treatment, mice with established tumors were depleted of various immune effectors. Tumors were established by i.v. inoculation as above. On d7 after the tumor inoculation, mice were given single i.p. inoculations of PBS ± control IgG, α-NK1.1 (Clone PK136), or α-Ly6G (clone 1A8) to deplete NK cells or neutrophils, respectively. Flow cytometric analysis confirmed that lung NK cells and neutrophils were depleted effectively in the respective mice (Fig. 5A, B). The day following depletion (d8), mice received a single L1S treatment, then tumor burdens in the lungs were quantified on d12. Results showed that L1S significantly reduced tumor burdens in mice treated with L1S + IgG control antibody, relative to control mice given IgG but not L1S (Fig. 5C). However, L1S failed to reduce tumor nodules when given to mice depleted of NK cells prior to treatment (Fig. 5C, α-NK1.1). Subsequent experiments further showed that L1S treatment was ineffective at reducing tumor burdens in mice pre-treated with α-IFNγ (Fig. 5D). By contrast, tumor burdens were similar in mice treated with L1S and IgG or α-Ly6G (to deplete neutrophils) (Fig. 5D). These results together indicate that NK cells and IFNγ, are required for the anti-tumor effects of L1S, while neutrophils are not (Fig. 5D). Thus, the inflammatory response elicited by L1S was not sufficient to mediate anti-tumor effects. These therapeutic anti-tumor effects instead appear to be due to the accumulation of NK cells and their production of IFNγ.
Figure 5. Therapeutic effects of L1S treatment are dependent on NK cells and IFNγ.
Mice were inoculated with B16.F10 cells as above. Groups of these mice received i.p. inoculation with depleting antibodies on d7 and i.t. treatment with PBS± L1S on d8 as indicated. Harvest were performed on d12. (A) Dot plots depict NK cells (NK1.1+CD3−) from lung homogenates of mice treated with a control IgG (a-IgG) or α-NK1.1 by analysis of both NK1.1+CD3− and NKp46+CD3− after instillation of L1S purified by Ni-column chromatography treated mice. (B) Representative FACs dot plots of Neutrophils by analysis of Ly6G+CD11b+ in L1S purified by Ni-column chromatography treated mice given α-IgG control or α-Ly6G depletion. (C) Tumor nodules and tumor area are quantified on the lungs of mice with established tumors and are depleted of NK cells and treated with L1S purified by Ni-column chromatography or PBS (N=4). (D) Tumor nodules and tumor area are quantified on the lungs of mice with established tumors and are depleted of NK cells, IFNγ, or Neutrophils and treated with L1S purified by Ni-column chromatography (N=5). Cells are gated on Live → singlets → Respective group(s) of interest. For all graphs, * indicates p<.05 using ANOVA with Newman-Keuls comparison. Shown are mean ± SEM
DISCUSSION
As basic understanding of the immune system has increased, so has the ability of researchers and clinicians to identify and apply therapeutic approaches that harness the body’s immune response. Such efforts have potential to improve treatment of infectious, neoplastic, and other diseases. In this study, we investigated a novel approach to promote anti-tumor immune responses. This approach exploits a pathogen-derived immune modulating factor to boost NK cell responses in animals with established metastatic tumors. As shown here, highly-purified preparations of this factor (a recombinant polypeptide containing the L1S region from the L. monocytogenes p60 virulence protein) stimulate secretion of IL-18 by primed BMDC and IFNγ production by NK cell/BMDC co-cultures. We further showed that local instillation of the L1S protein into the lungs promoted activation of lung NK cells to secrete IFNγ in both naïve mice and mice with established metastatic tumors. The model tumor used in these experiments was the NK cell-sensitive B16.F10 melanoma (29–30). Consistent with the interpretation that NK cell activation exerted anti-tumor effects in treated mice, we observed significant reductions in the number and size of tumors in L1S-treated mice. NK cells isolated from lungs of L1S-treated mice also showed an increased ability to lyse RFP-expressing B16.F10 tumor cells. Therapeutic effects were absent in mice given L1S protein after depletion of NK cells or IFNγ, but not Ly6G+ neutrophils. Hence, our data suggest L1S can be used in vivo to boost the activation of tumor-reactive NK cells.
Our studies here were founded on prior work showing expression of the p60 virulence protein by Lm is associated with increased NK cell activation (20). Expression of p60 by Lm was also shown to boost activation and IFNγ secretion by NK cells co-cultured with infected BMDC (21,23). The ability to promote NK cell activation appears to benefit Lm because IFNγ secreting NK cells responding to p60/L1S rapidly switch to secretion of IL-10 (21). IL-10 has been argued both to promote anti-tumor resistance in the B16.F10 model (32), and to promote growth of B16.F10 melanoma cells (34). However, given our finding that IFNγ is important for the observed anti-tumor effects, we did not further explore NK cell IL-10 production in the present work.
Our depletion experiments indicated that NK1.1+ cells and IFNγ are important for the anti-tumor effects of L1S therapy. Accumulation of neutrophils (Ly6G+CD11b+) was also seen after L1S instillation, and neutrophils have previously been observed to associate with tumors (35–37). However, depletion of Ly6G+ cells did not impact L1S therapeutic efficacy. Like NK cells, NKT cells express the NK1.1 marker. Since we did not observe increased IFNγ production or numbers of NKT cells in L1S-treated lungs (not shown). The failure of mice treated with αNK1.1 to reduce tumor burdens in the presence of L1S is likely due to the depletion of NK, not NKT, cells. NK cells were first described as cytolytic innate effector cells in 1975 (38). The ability of NK cells to recognize and lyse THP-1 and YAC-1 tumor cells is also known to be induced or enhanced by exposure to cytokines such as TNF and IFNγ, which NK cells themselves produce (39–41). These cytokines increase target cell expression of NK cell activating or adhesion ligands, such as ICAM1 (39). IFNγ can also act on NK cells to increase their cytolytic anti-tumor activity by promoting expression of granule proteins and death receptor ligands such as TRAIL (42). Increased expression of the IFN-inducible activation marker CD69 has also been associated with increased NK cell cytolysis (43–44), and we observed increased CD69 expression on NK cells from L1S-treated lungs (not shown). IFNγ can also directly promote apoptosis of tumor cells (45), and can enhance M1 differentiation and thus tumoricidal activity of tumor associated monocytes and macrophages. Future studies will be necessary to better define which of these mechanisms is most important for the L1S induced anti-tumor effects.
In addition to mouse DC and NK cells, recombinant L1S protein stimulates human DC and NK cells in cell culture models (21). Activation in these cell cultures requires the presence of contaminating or exogenous priming agents in addition to purified recombinant L1S protein itself. The requirement for a priming agent could in theory limit the efficacy or safety of L1S in human anti-cancer therapies. However, we previously found that FDA-approved agents such as monophosphoryl lipid A suffice for priming in cell culture (23). We also show here that i.t. treatment with dL1S stimulated NK cell activation and secretion of IFNγ. This likely reflects the presence of endogenous priming agents within the lung microenvironment. Priming could be due to microbes or microbial products present in the air or commensal microbes in the lung or other mucosal tissues (46–47). Evidence that lung DC are constitutively primed comes from a study by Ichinohe et al, who found that depletion of microbiota via the use of an antibiotic cocktail lead to reductions in lung responses to inflammatory stimuli (48). Providing exogenous LPS to the antibiotic treated mice was found to restore their responsiveness to inflammatory stimuli. Further studies will be needed to directly test if the responses to dL1S observed are dependent on constitutive priming of lung DCs and suffice to induce anti-tumor responses.
Efforts to better understand immune regulation have led to the development and application of several novel immune-based therapies that show great potential in treatment of cancers (5–6). These existing immunotherapies focus on activation of T-cell responses in cancer patients. Unlike T cells, which recognize specific tumor antigens, NK cells recognize and lyse tumor cell targets independent of specific antigens – including tumor cells that lack expression of the MHC molecules. Therapies that promote NK cell activation might thus be useful in combination with T cell-stimulating immunotherapies. Our results here demonstrate use of a bacteria-derived polypeptide to stimulate NK cell activation and anti-tumor responses in vivo. Future efforts to further improve our understanding of how L1S promotes NK cell activation and anti-tumor responses could thus reveal new approaches to boost immune responses for therapy of cancer.
Supplementary Material
Acknowledgments
This work was supported by grants NIH grant R01AI065638 and a Research Grant from the Cancer League of Colorado.
We are thankful to Drs. Carrie Cole and Sarah Clark for reading and providing constructive feedback on this manuscript. We also acknowledge Dr. Daniel McDermott for helpful suggestions and Drs. Rebecca Schmidt and Elizabeth Redente for assistance and advice on methods for isolation of L1S protein and instillation procedures. Several Anschutz Medical Campus core facilities were used throughout this work.
References
- 1.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22(6):1136–51. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures 2014 [Internet] American Cancer Society; Atlanta: ACS; [cited 2017 Mar7] Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2014.html. [Google Scholar]
- 3.Metastatic Cancer [Internet] National Cancer Institute; [cited 2017Mar7]. Available from: https://www.cancer.gov/types/metastatic-cancer. [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results Program [Internet] National Cancer Institutes; [cited 2017Mar7]. Available from: https://seer.cancer.gov/statfacts/html/melan.html. [Google Scholar]
- 5.Pico de Coana Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Molr Med. 2015;21(8):482–491. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Page DB, Postwo MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 9.Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26(2):161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348(6230):136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev of Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 13.Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92(3):256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 14.Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012;18:270–285. doi: 10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol. 2012;2(88):1–13. doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeberlein S, Sebald H, Bogdan C, Schleicher U. IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo. Eur J Immunol. 2010;40(6):1708–1717. doi: 10.1002/eji.200939988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266(2):154–181. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol. 2011;2:88. doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudreau JE, Bonehill A, Thielemans K, Wan Y. Engineering dendritic cells to enhance cancer immunotherapy. Mol Ther. 2011;19(5):841–853. doi: 10.1038/mt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humann J, Bjordahl R, Andreasen K, Lenz LL. Expression of the p60 autolysin enhances NK cell activation and is required for listeria monocytogenes expansion in IFN-gamma-responsive mice. J Immunol. 2007;178(4):2407–2414. doi: 10.4049/jimmunol.178.4.2407. [DOI] [PubMed] [Google Scholar]
- 21.Clark SE, Filak HC, Guthrie BS, Schmidt RL, Jamieson A, Merkel P, Knight V, Cole CM, Raulet DH, Lenz LL. Bacterial manipulation of NK cell regulatory activity increases susceptibility to Listeria monocytogenes infection. PLoS Pathog. 2016;12(6):e1005708. doi: 10.1371/journal.ppat.1005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29(5):819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt RL, Filak HC, Lemon JD, Potter TA, Lenz LL. A LysM and SH3-domain containing region of the Listeria monocytogenes p60 protein stimulates accessory cells to promote activation of host NK cells. PLoS Pathog. 2011;7(11):e1002368. doi: 10.1371/journal.ppat.1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ya Z, Hailemichael Y, Overwijk W, Restifo NP. Mouse model for pre-clinical study of human cancer immunotherapy. Curr Protoc Immunol. 2015;108:20.1.1–43. doi: 10.1002/0471142735.im2001s108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol. 2001;Chapter 20(Unit 20.1) doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt RL, Lenz LL. Distinct Licensing of IL-18 and IL-1β secretion in response to NLRP3 inflammasome activation. PLoS One. 2012;7(9):e45186. doi: 10.1371/journal.pone.0045186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humann J, Lenz LL. Activation of naïve NK cells in response to Listeria monocytogenes requires IL-18 and contact with infected dendritic cells. J Immunol. 2010;184(9):5172–5178. doi: 10.4049/jimmunol.0903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL. Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J Vis Exp. 2011;51 doi: 10.3791/2702. pii:2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy MA, Zhang T, Sentman CL. NK cells rapidly remove B16F10 tumor cells in a perforin and interferon-gamma independent manner in vivo. Cancer Immunol Immunother. 2007;56(8):1153–1161. doi: 10.1007/s00262-006-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980;65(5):929–935. [PubMed] [Google Scholar]
- 31.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang XY, Catino JJ, King I. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184(2):579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human tumor-infiltrating myeloid cells: Phenotypic and functional diversity. Front Immunol. 2017;8:86. doi: 10.3389/fimmu.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassetta L, Pollard JW. Cancer immunosurveillance: role of patrolling monocytes. Cell Res. 2016;26(1):3–4. doi: 10.1038/cr.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Hernandez ML, Hernandez-Pando R, Gariglio P, Berumen J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumor and vascular cell proliferation. Immunology. 2002;105(2):231–243. doi: 10.1046/j.1365-2567.2002.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012;33(5):949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 36.Uribe-Querol E, Rosales C. Neutrophils in cancer: Two sides of the same coin. J Immunol Res. 2015;2015:983698. doi: 10.1155/2015/983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosales C, Demaurex N, Lowelland CA, Uribe-Querol E. Neutrophils: Their role in innate and adaptive immunity. J Immunol Res. 2016;2016:1469780. doi: 10.1155/2016/1469780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cell in the mouse. II. Cytotoxic cells with specificity for mouse Maloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Bioly. 2012;91(2):299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 41.Welsh RM., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978;121(5):1631–1635. [PubMed] [Google Scholar]
- 42.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita NH, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligant (Trail) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193(6):661–70. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borrego F, Robertson MJ, Ritz J, Pena J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97(1):159–165. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichlin A, Yokoyam WM. Natural killer cell proliferation induced by anti-NK1.1 and IL-2. Immunol Cell Biol. 1998;76(2):143–152. doi: 10.1046/j.1440-1711.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 45.Kubota A, Lian RH, Lohwasser S, Salcedo M, Takei F. IFN-gamma production and cytotoxicity of IL-2-activated murine NK cells are differentially regulated by MHC class I molecules. J Immunol. 1999;163(12):6488–6493. [PubMed] [Google Scholar]
- 46.Lighthart B, Prier K, Loper GM, Bromenshenk J. Bees scavenge airborne bacteria. Microb Ecol. 2000;39(4):314–321. [PubMed] [Google Scholar]
- 47.Dickson RP, Erb-Downward JR, Martinez FJ, Hufnagle GB. The microbiome and the respiratory tract. Ann Rev Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichinohe T, I, Pang K, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against repiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.