Supplemental Digital Content is available in the text
Keywords: anterior cervical artificial disc replacement, anterior cervical decompression and fusion, bi-level cervical spondylosis, follow-up, meta-analysis
Abstract
Background:
Nowadays, anterior cervical artificial disc replacement (ACDR) has achieved favorable outcomes in treatment for patients with single-level cervical spondylosis. However, It is still controversial that whether or not it will become a potent therapeutic alternation in treating 2 contiguous levels cervical spondylosis compared with anterior cervical decompression and fusion (ACDF). Therefore, we conducted a systematic review and meta-analysis to compare the efficacy and safety of ACDR and ACDF in patients with 2 contiguous levels cervical spondylosis.
Methods:
According to the computer-based online search, PubMed, Embase, Web of Science, and Cochrane Library for articles published before July 1, 2017 were searched. The following outcome measures were extracted: neck disability index (NDI), visual analog scale (VAS) neck, VAS arm, Short Form (SF)-12 mental component summary (MCS), SF-12 physical component summary (PCS), overall clinical success (OCS), patient satisfaction (PS), device-related adverse event (DRAE), subsequent surgical intervention (SSI), neurological deterioration (ND), and adjacent segment degeneration (ASD). Methodological quality was evaluated independently by 2 reviewers using the Furlan for randomized controlled trial (RCT) and MINORS scale for clinical controlled trials (CCT). The chi-squared test and Higgin I2 test were used to evaluate the heterogeneity. A P < .10 for the chi-squared test or I2 values exceeding 50% indicated substantial heterogeneity and a random-effect model was applied; otherwise, a fixed-effect model was used. All quantitative data were analyzed by the Review Manager 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results:
Nine RCTs and 2 CCT studies containing 2715 patients were included for this meta-analysis. The pooled analysis indicated that the ACDR group is superior to ACDF in NDI, VAS neck, PCS score, OCS, PS, DRAE, ASD, and SSI. However, the pooled results indicate that there was no significant difference in the ND, VAS arm and in MCS score.
Conclusions:
The present meta-analysis suggests that for bi-level cervical spondylosis, ACDR appears to provide superior clinical effectiveness and safety effects than ACDF. In the future, more high-quality RCTs are warranted to enhance this conclusion.
1. Introduction
According to a recent public health report, the Global Burden of Disease Study, neck pain is the main cause of movement disorders, with current estimates of 349 million people affected worldwide.[1] This large number of patients will continue to increase further. A previous review of the literature, described neck pain as a chronic condition associated with intervertebral disc degeneration.[2,3] Current conservative treatment includes use of nonsteroidal anti-inflammatory drugs at earlier stages, but invasive interventions are standard treatments at later stages.[4]
Since the 1950s, anterior cervical decompression and fusion (ACDF) has been regarded as the “gold standard” of surgical therapy for symptomatic cervical myelopathy or radiculopathy, achieving neural decompression, segmental stabilization, and favorable results in clinical follow-up.[5–7] However, ACDF is associated with pseudarthrosis formation, limitation of index level, and accelerated adjacent segment degeneration (ASD).[8,9] Thus, anterior cervical artificial disc replacement (ACDR) represents a new, relative segmental motion-preserving procedure for cervical spondylosis. Compared with ACDF, ACDR can restore the interspace height of cervical vertebra, preserve the index/adjacent level, and also theoretically prevent ASD.[10,11] Each procedure has its own characteristic features, and most studies have compared single-level ACDR with ACDF, but the safety and efficacy of bi-level procedure remains controversial. To provide a high level of evidence for decision making by clinicians and patients, we performed a meta-analysis to compare outcomes after bi-level ACDR with those of bi-level ACDF, to evaluate which procedure yields more favorable patients.
2. Materials and methods
2.1. Search strategy
To search all of the relevant literature, we systematically searched literature published in the database (PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials). Search terms were subjected to the following: “anterior cervical artificial disc replacement,” “cervical total disc replacement,” “cervical artificial disc,” “disc arthroplasty,” ACDR, CTDA, CDA, “anterior cervical decompression and fusion,” “anterior interbody fusion,” ACDF, “2 level,” “two level,” “bi-level,” “double level” with various combinations of the operators “AND,” “NOT,” and “OR.” There were restriction of study design was controlled trial published between January 1, 2000 and July 1, 2017. Restriction of languages was English. References cited in the relevant articles were also reviewed (see in Supplement 1).
2.2. Inclusion criteria
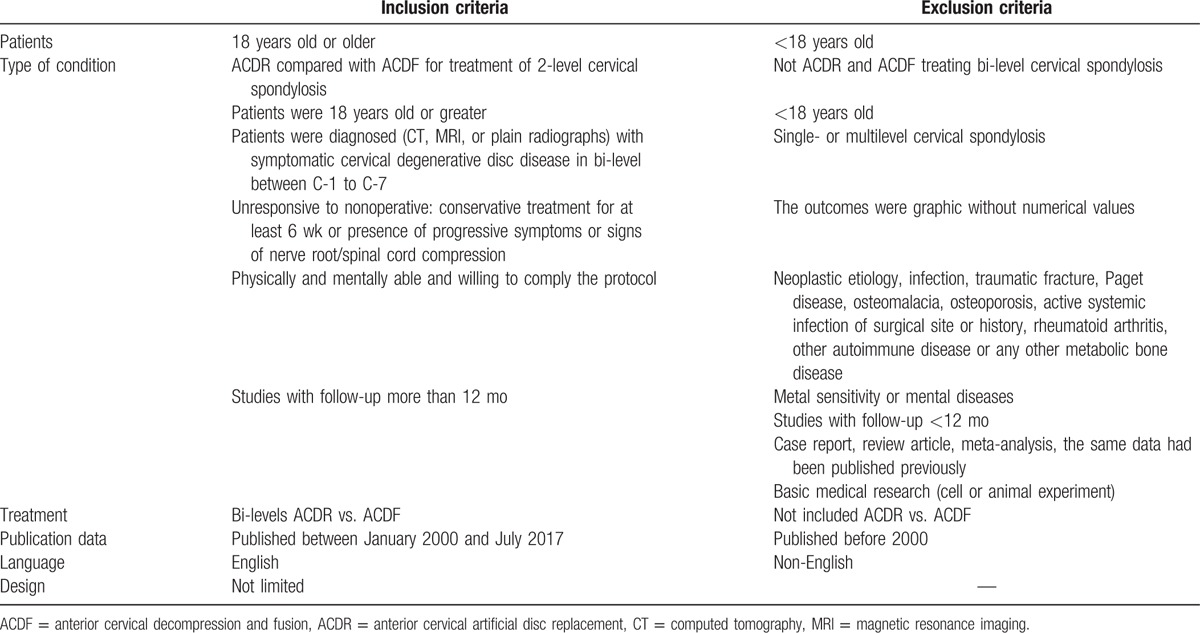
All studies on treatment of bi-level cervical spondylosis were reviewed. The criteria for inclusion of an article were ACDR compared with ACDF for treatment of 2-level cervical spondylosis; patients were 18 years old or greater; patients were diagnosed (computed tomography, magnetic resonance imaging, or plain radiographs) with symptomatic cervical degenerative disc disease in bi-level between C-1 to C-7; unresponsive to nonoperative: conservative treatment for at least 6 weeks or presence of progressive symptoms or signs of nerve root/spinal cord compression; physically and mentally able and willing to comply the protocol; and studies with follow-up more than 12 months (Table 1).
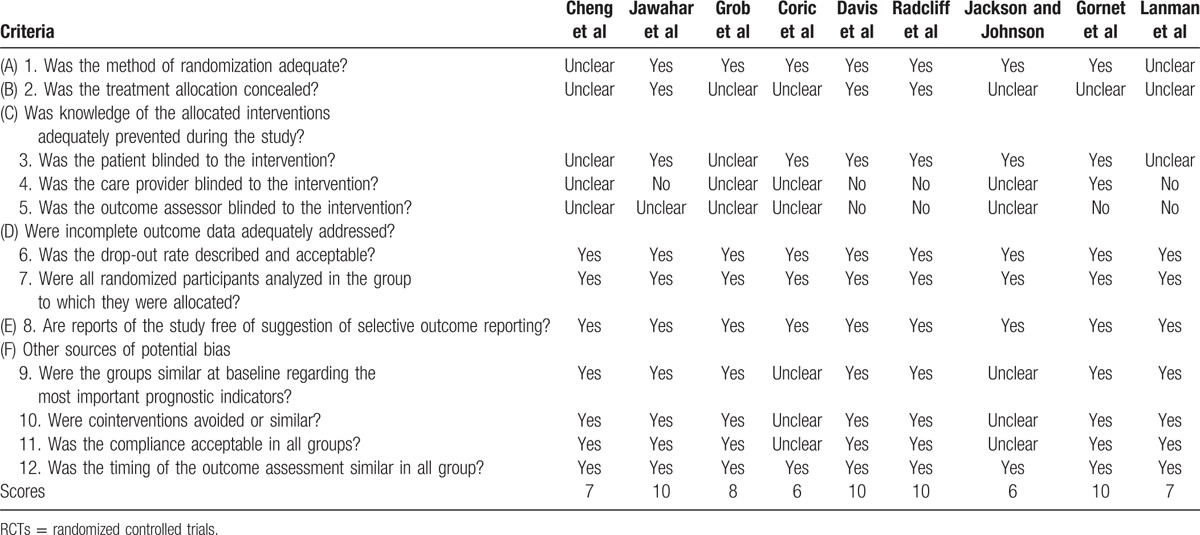
Table 1.
Quality assessment of included RCT studies by using the Furlan scores.
2.3. Exclusion criteria
Patients were excluded if they were associated with: not ACDR and ACDF treating bi-level cervical spondylosis; <18 years old; single- or multilevel cervical spondylosis; the outcomes were graphic without numerical values; neoplastic etiology, infection, traumatic fracture, Paget disease, osteomalacia, osteoporosis, active systemic infection of surgical site or history, rheumatoid arthritis, other autoimmune disease or any other metabolic bone disease; metal sensitivity or mental diseases; studies with follow-up <12 months; case report, review article, meta-analysis, the same data had been published previously; and basic medical research (cell or animal experiment) (Table 2).
Table 2.
Inclusion and exclusion criteria for article selection.
2.4. Data extraction
The following data were extracted by 2 authors independently using a purpose-designed form: first author and year, study design, region, details, intervention, follow-up (months), and outcomes. Disagreement between the 2 reviewers was arbitrated by the third reviewer. If any disagreements existed, a third author was consulted to discussion until consensus was reached. The outcome including at least one of the following outcomes (Table 2):
-
1.
NDI (neck disability index)
-
2.
VAS (visual analog scale) neck
-
3.
VAS arm
-
4.
SF-12 (Short Form 12) MCS (mental component summary)
-
5.
SF-12 (Short Form 12) PCS (physical component summary)
-
6.
OCS (overall clinical success)
-
7.
PS (patient satisfaction)
-
8.
DRAE (device-related adverse event)
-
9.
SSI (subsequent surgical intervention)
-
10.
ND (neurological deterioration)
-
11.
ASD (adjacent segment degeneration)
2.5. Quality assessment
The quality of the studies was independently assessed by the 2 authors according to The checklist by Furlan et al[12,13] was used to evaluate the methodological quality of randomized controlled trials (RCTs). Evaluation of clinical controlled studies was performed with the MINORS scale. Every study was assessed by 2 independent researchers and judgment of every item. Any disagreement with respect to eligibility during the extraction was discussed and resolved.
2.6. Statistical analysis
The risk ratio (RR) and the corresponding 95% confidence interval (CI) were assessed for the dichotomous outcomes, and the standardized mean difference (SMD) and 95% CI were assessed for the continuous outcomes. The chi-squared test and Higgin I2 test were used to evaluate the heterogeneity. A P value <.10 for the chi-squared test or I2 values exceeding 50% indicated substantial heterogeneity. A fixed-effect model was used if significantly statistical heterogeneity was absent; otherwise, a random-effect model was applied. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting 1 study in turn when necessary. Owing to the limited number (11) of included studies, publication bias was not assessed. P < .05 in 2-tailed tests was considered statistically significant. A meta-analysis was performed on the extracted data with RevMan 5.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Search results
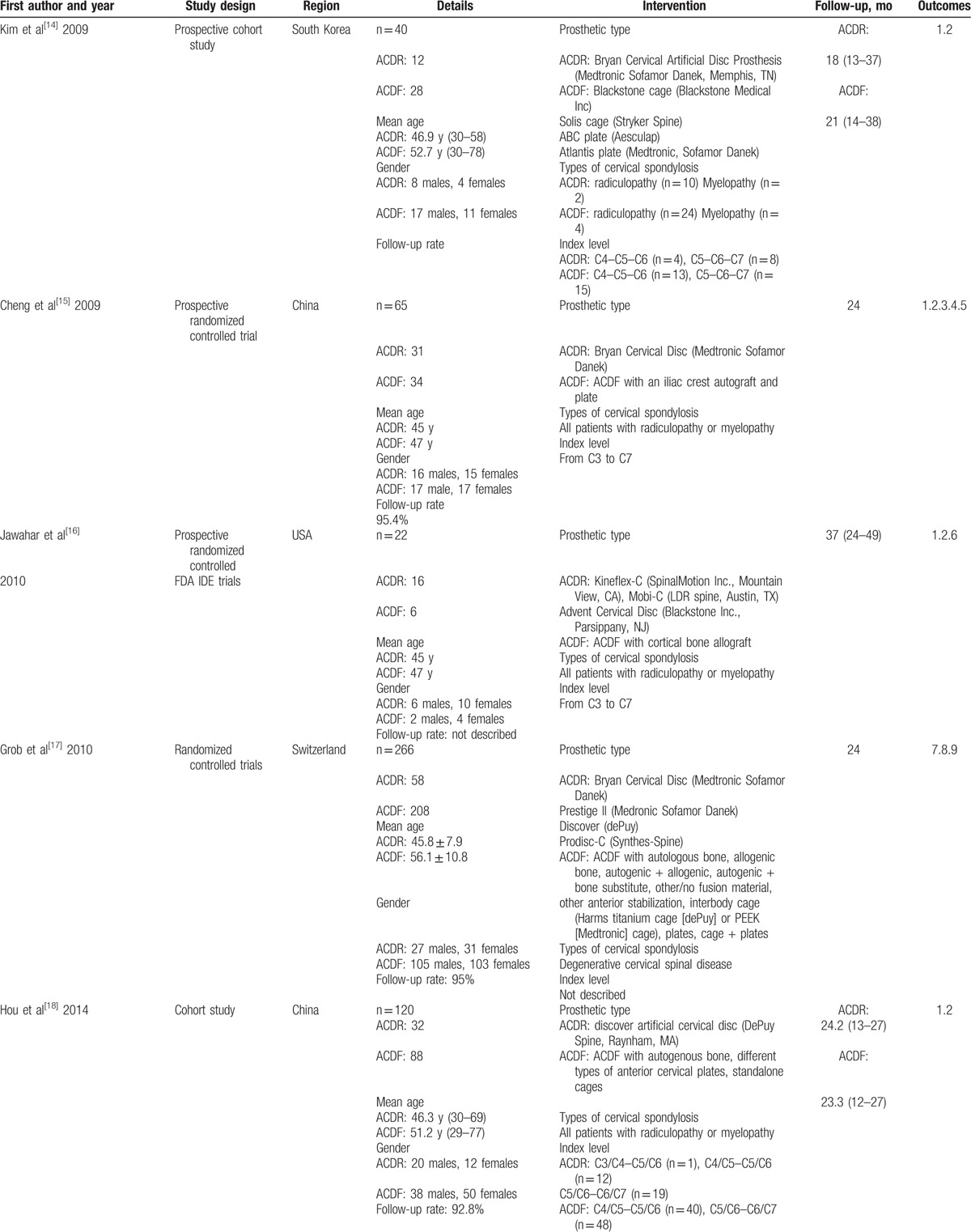
Flow chart for inclusion of studies is shown in Fig. 1. The literature search initially yielded 325 relevant trials from PubMed (N = 47), Embase (N = 113), Web of Science (N = 3), and The Cochrane Library (N = 162). After we reviewed the titles and abstracts of all trials, 128 trials were excluded. We continued to refine and exclude the 197 studies, 21 potentially studies were obtained, then 10 studies were excluded due to fail to meet criteria. Finally, 9 RCTs and 2 clinical controlled trials (CCTs) containing 2715 patients were included for this meta-analysis. We recorded the characteristics of the 11 included trials, as well as the details of the clinical outcome measurement (Table 3 ).
Figure 1.
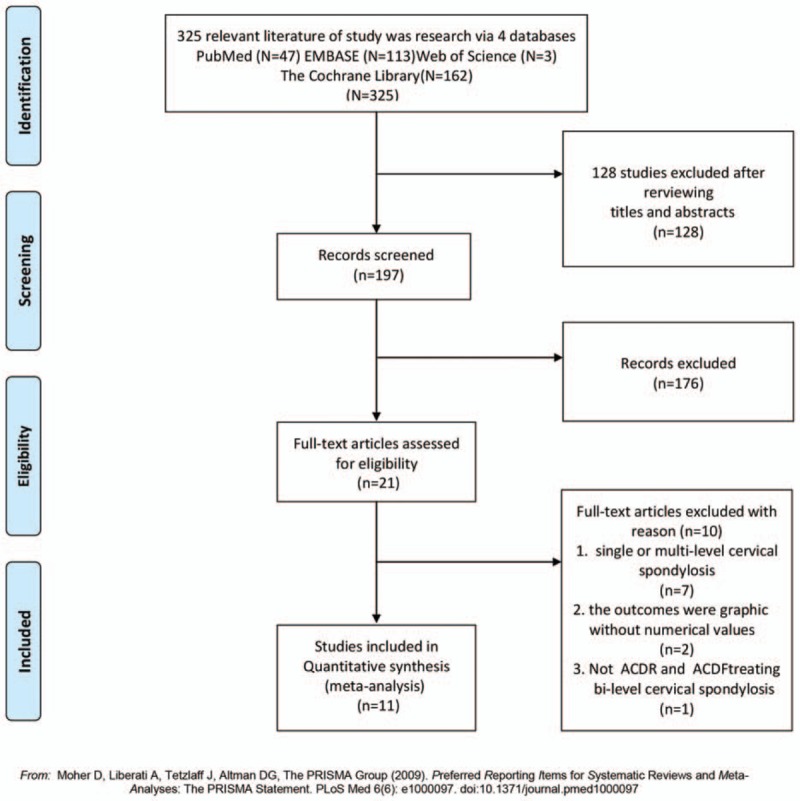
Flow chart for inclusion of studies.
Table 3.
Characteristics of included studies.
3.2. Quality assessment
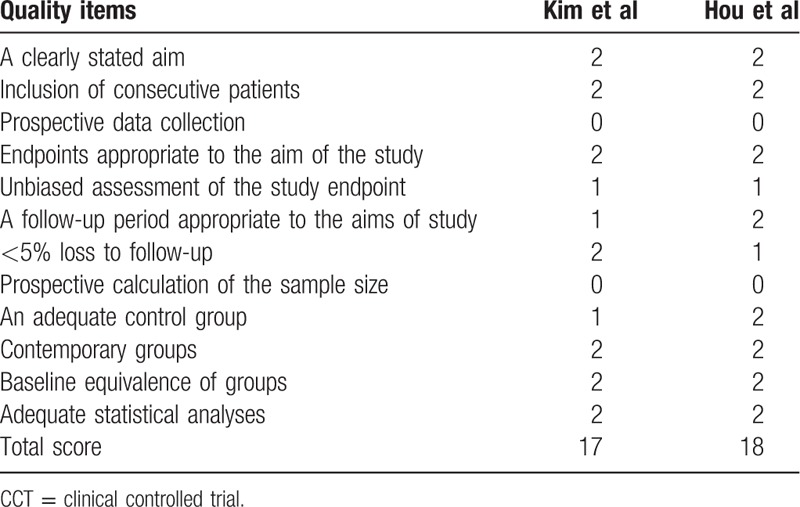
The Furlan scores in 9 RCTs were in the range from 6 to 10 of 12 (Table 1). Seven RCTs were scored 7 or higher, and 2 RCTs were scored lower than 7, suggesting overall high quality of studies. The MINORS scale of studies in both studies was 17 and 18 and judged as good quality (Table 4). They were considered high methodological quality.
Table 3 (Continued).
Characteristics of included studies.

Table 4.
Methodological quality of the CCT studies by using MINORS scale.
3.3. Clinical effectiveness
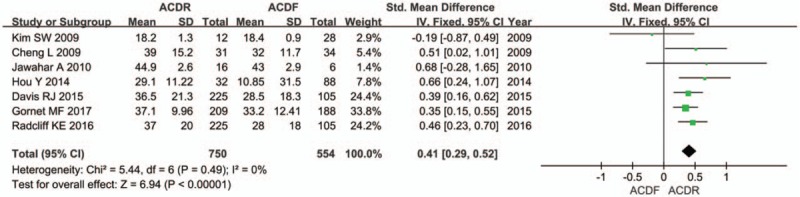
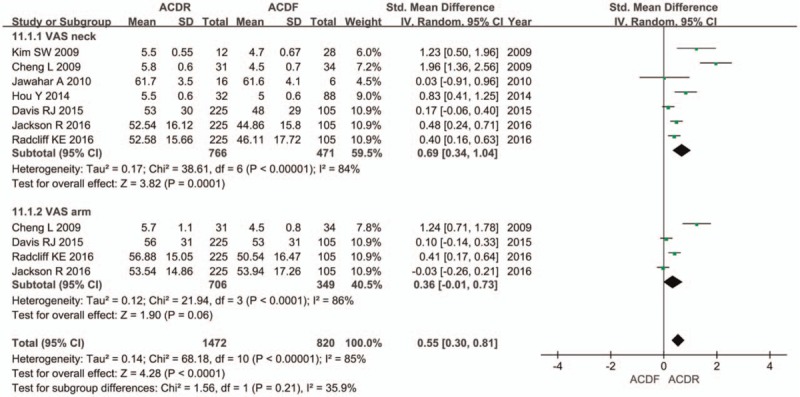
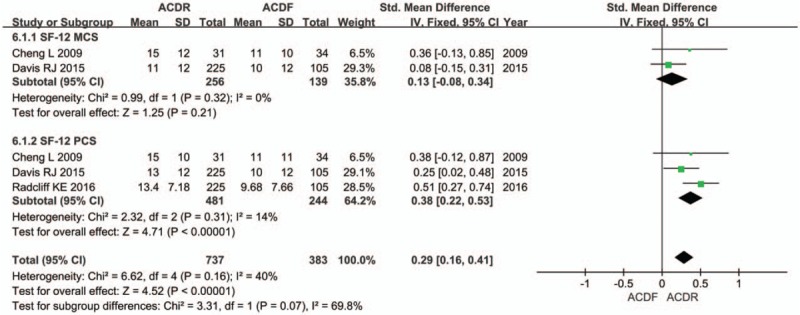
Seven studies provided NDI score. There was significant difference in the NDI score between 2 groups. The overall effect showed that the ACDR group had statistically higher NDI scores improvement than the ACDF group (SMD = 0.41 [0.29, 0.52], P < .00001, Fig. 2). Similarly, 7 studies with pooled results indicated that the ACDR group had statistically higher VAS scores in neck pain improvement than the ACDF group. VAS neck pain (SMD = 0.69 [0.34, 1.04], P = .0001, Fig. 3). However, there is no significant difference between 2 groups in VAS arm pain (SMD = 0.36 [−0.01, 0.73], P = .06, Fig. 3). Two studies and 3 studies provided SF-12 MCS and PCS score, respectively. The pooled results indicate that there was no significant difference in the MCS score between 2 groups (SMD = 0.13 [−0.08, 0.34], P = .21, Fig. 4). The pooled PCS score showed significant difference between the ACDR and ACDF group (SMD = 0.38 [0.22, 0.53], P < .00001, Fig. 4). There were 6 studies provided OCS, the pooled result showed that ACDR group is superior to ACDF group (SMD = 1.49 [1.20, 1.85], P = .0003, Fig. 5). PS was also reported in 5 studies, the overall effect showed that ACDR group had statistically higher rate improvement than the ACDF group (SMD = 1.06 [1.03, 1.09], P = .0003, Fig. 6), and 2 studies reported that one who experienced operation would also recommend their treatment to friends (SMD = 1.12 [1.05, 1.19], P = .0002, Fig. 6).
Figure 2.
The standardized mean difference estimate for the neck disability index score.
Figure 3.
The standardized mean difference estimate for the visual analog scale neck and arm pain.
Figure 4.
The standardized mean difference estimate for the Short Form 12 mental component summary and physical component summary.
Figure 5.
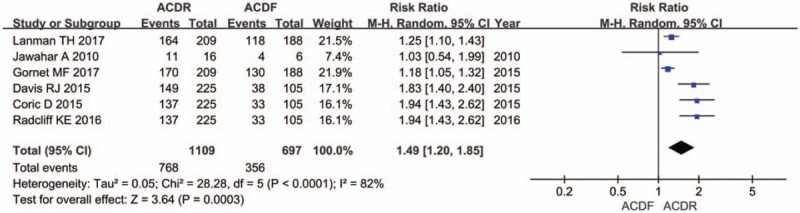
The risk ratio estimate for the clinical overall success rate.
Figure 6.
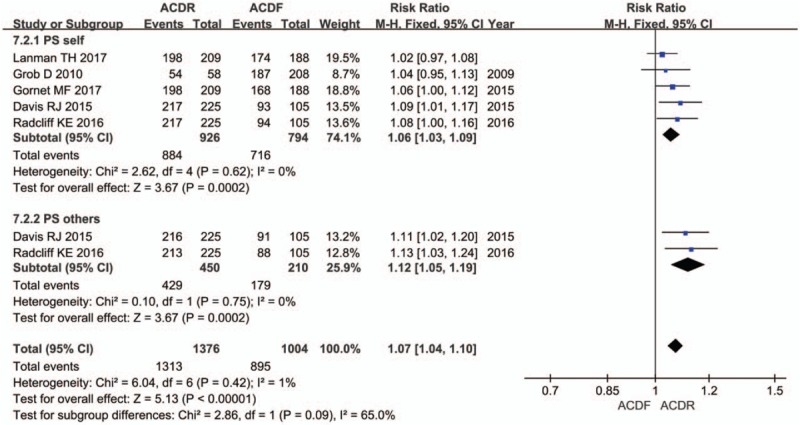
The risk ratio estimate for the patient satisfaction rate.
3.4. Clinical safety
Six studies reported DRAE. Patients in the ACDR group had statistically significant lower incidence of DRAE (RR = 0.59 [0.48, 0.73], P < .0001, Fig. 7). There was significantly more SSI rate extracted in 7 studies in the ACDF group than in the ACDR group (RR = 0.29 [0.21, 0.39], P < .00001, Fig. 8). However, ND rate also indicates that there was no statistically significant between 2 groups (RR = 0.61 [0.36, 1.01], P = .06, Fig. 9). In addition, 2 studies showed that there were also significantly more superior and inferior ASD rate in the ACDF group compared with ACDR (RR = 0.40 [0.35, 0.46], P < .00001, Fig. 10).
Figure 7.
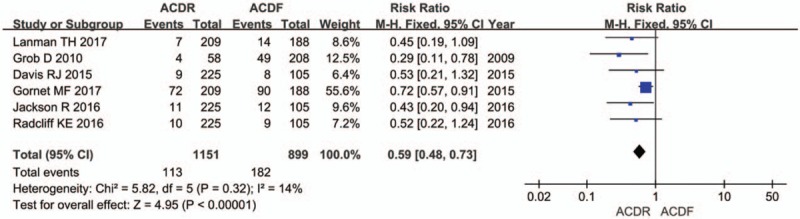
The risk ratio estimate for the device-related adverse events rate.
Figure 8.
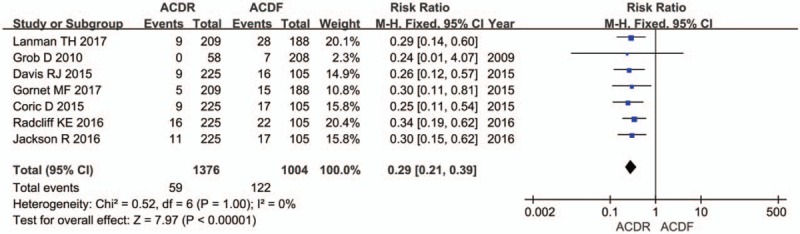
The risk ratio estimate for the subsequent surgical intervention.
Figure 9.
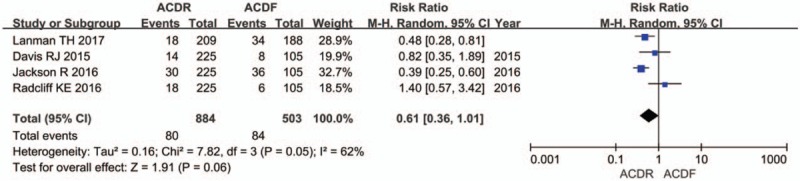
The risk ratio estimate for the neurological deterioration.
Figure 10.
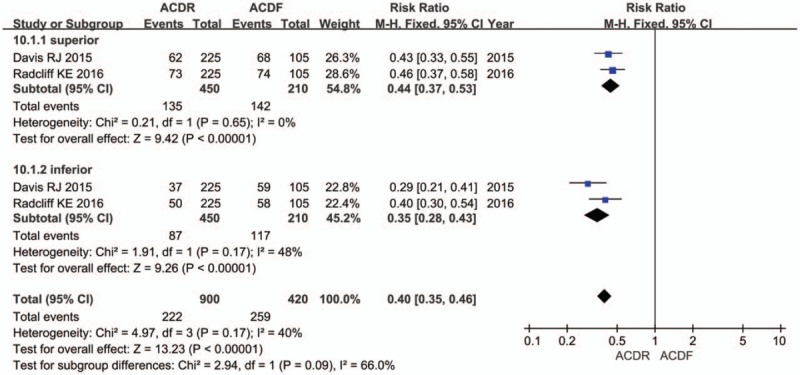
The risk ratio estimate for the adjacent-segment disc degeneration rate.
3.5. Sensitivity analysis
The sensitivity analysis was conducted to discover whether the lack of each study will change the pooled OR and SMD completely. After removing each, no original pooled results were significantly changed. It proves that the overall meta-analysis results were reliable.
4. Discussion
Through analysis of level 1 evidence from 9 prospective randomized well-controlled clinical trials and 2 high-quality cohort studies, it was demonstrated that ACDR is superior to ACDF. Both effectiveness and safety parameters were examined by using RevMan 5.3 software. Previously used indicators, including the NDI, VAS neck and arm pain, SF-12 MCS and PCS, OCS, and PS revealed that improvement with ACDR shown an advantage over ACDF. However, ND rate was not statistically different between the 2 groups. More recently used indicators, including device-related AE, subsequent surgical intervention (SSI), and ASD rate demonstrated a lower incidence rate for ACDR than for ACDF. Although a meta-analysis and an RCT offer level 1 evidence, a meta-analysis allows for pooling of results to obtain a quantitative and statistically significant estimate of treatment effects and the ability to draw more convincing conclusions.
Patient treated with ACDR showed greater NDI improvement than those treated with ACDF in follow-up. This result is consistent with single- or multilevel cervical treatment.[24–26] We surmise that neck vertebrae can sustain a more favorable physical structure after an ACDR procedure compared with ACDF. The VAS score data for neck and arm pain, and the SF-12, for clinical effectiveness assessment, were also analyzed. The overall effect on neck pain showed that ACDR has favorable outcomes, possibly as a result of preservation of mobility and restoration of the neck muscles. However, some previous studies reported that there was no difference in clinical outcome between 2 types of operation in midterm follow-up.[27] We speculate that the discrepancy was due to different standards for inclusion criteria and statistical methods. Similarly, although VAS neck pain improvement with ACDR was superior to that with ACDF, we performed subgroup analysis and found that there was no difference between the 2 groups in VAS arm pain. Unlike neck pain, we know that improvement in arm pain after surgical treatment depends on the degree of nerve root decompression that is vital guarantee for surgical effectiveness, which may else interpret why there is no statistical difference in neurological success. To determine whether different prostheses result in different outcomes between the 2 surgeries strategy, we conducted subgroup analysis. The result showed that ACDR is as effective as ACDF. Different from previous reports,[24,26] on the SF-12 PCS, ACDR is showed more positive results than ACDF. Interestingly, SF-12 MCS showed no statistical difference.
Whether ACDR or ACDF could reduce the incidence rate of ASD has remained controversial.[28] Based on current understanding, ACDR cannot completely prevent the occurrence of ASD, but it can alleviate ASD by maintaining the mobility of the index level and relieving the intradiscal pressure in adjacent segment discs. On the one hand, Goffin et al[29] reported that the intradiscal pressure in the adjacent segment in 2-level ACDR was clearly lower than that for ACDF, causing lower degeneration rate in adjacent levels. However, other spine surgeon found that multifusion induces ASD more extensively. Hilibrand[30] reported that ASD outcomes in multilevel ACDF were inferior to those with single-level ACDF, the possible explanation is the fusion of degenerated or potentially degenerated segments during the operation. Conversely, in contrast to the doctrine of biomechanics, other authors believe that natural degeneration is the main cause of ASD.[31] Although this is somewhat reasonable, some still believe that the physiological environment of the neck will inevitably be changed after surgery, apart from genetic predisposition,[32] compared with ACDR, which preserves mobility, and flexibility, and is closer to a normal anatomic state, a fusion procedure changes the mechanical environment and adds to compensatory movement of the adjacent level, with both factors exacerbating ASD. Therefore, we speculate that there are 2 reasons for the high ASD rate in ACDF. First, the 2 pathological changes mentioned above lead to higher intradiscal pressure compared with that in ACDR, thus stimulating abundant inflammatory mediators in the adjacent disc. Second, the dominant inflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α,[33,34] contribute significantly to ASD. Together, the main causes of ASD are based on natural degeneration and surgical intervention. Although ACDR has advantages over ACDF with regard to ASD development, the greater significance maintaining the range of motion and restoring neck function. Another focus of attention is the apparent correlation between reoperation rate and ASD.[35] In our study, we observed that SSI rate occurs more often with ACDF than ACDR. However, not all ASD requires reoperation, and vice versa. In addition, the author encounters a notable case in clinical, in which vertebrae between 2 prostheses developed a compression fracture resulting from excessive physiological load. Interestingly, no database search found a similar case report or study.
There are several limitations and merits of this study. First, only 11 studies were included in this study, the full text was available for 9, and the others were articles from conference proceeding. This may lead to bias due to missing data. Second, although our electronic and manual search encompassed a range of databases, we only included articles published in English, it may lead language bias. At last, some of RCTs with incomplete data may decrease the quality of evidence and strength of analysis. Although, limitations in this research, there were some merits existed. First, our up-to-date article retrieval yielded 11 eligible studies including 9 RCTs (evidence of level 1) and 2 CCTs (evidence of level 2), it provided more high-level literature from origin and generated more credible results by evidence-based medicine analysis. Moreover, 5 multicenter RCTs under the guidance of FDA out of 9 RCTs may further reinforce the quality of the evidence. Finally, more high-quality RCTs with large sample size are required to investigate the efficiency of ACDR compared with ACDF.
5. Conclusion
Although there was no significant difference between ACDR and ACDF in ND, VAS arm MCS score, most effective indices such as NDI, VAS neck, PCS score, PS, OCS, is superior to ACDR than ACDF. In addition, safety indices of ACDR including DRAE, ASD, and SSI were better than ACDF. In all, ACDR appears to be more effective and safety than ACDF; however, more well-designed studies with large samples are needed to provide further evidence for the effect and reliability of ACDR compared with ACDF in the treatment of cervical spondylosis.
Author contributions
Conceptualization: Y. Xiong.
Data curation: Y-D. Yang, Z.-G. Hu.
Investigation: D-Y. Zhao, L-J. Duan.
Methodology: X-S. Tang, Y-S. Gao.
Resources: C-H. Li.
Supervision: X. Yu.
Writing – original draft: H. Zhao.
Supplementary Material
Footnotes
Abbreviations: ACDF = anterior cervical decompression and fusion, ACDR = anterior cervical artificial disc replacement, ASD = adjacent segment degeneration, CCT = clinical controlled trial, CI = confidence interval, DRAE = device-related adverse event, MCS = mental component summary, ND = neurological deterioration, NDI = neck disability index, OCS = overall clinical success, PCS = physical component summary, PS = patient satisfaction, RCT = randomized controlled trial, RR = risk ratio, SF-12 = Short Form 12, SMD = standard mean difference, SSI = subsequent surgical intervention, VAS = visual analog scale.
Xing Yu contributed to the design of the study. He Zhao and Li-Jun Duan were responsible for data collection, data analysis, and drafting the manuscript. Yu-Shan Gao, Yong-Dong Yang, Ding-Yan Zhao, and Xiang-Sheng Tang contributed to analyzing the data with regard to its potential clinical significance. Yang Xiong, Zhen-Guo Hu, and Chuan-Hong Li conceived the meta-analysis and participated in its design and coordination. Xing Yu, He Zhao, and Li-Jun Duan screened titles and abstracts of eligible citations and determined if they met the inclusion criteria to this meta-analysis. All authors read and approved the final manuscript. All those who contributed to this meta-analysis meet the criteria for authorship and have been listed as authors.
The authors declare that National “Twelfth Five-Year” Plan for Science and Technology Support (2012BAI18B05) was received for this meta-analysis. The Manuscript submitted does not contain information about medical device(s)/drug(s). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Vos T, Ryan MB, Brad B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J 2006;6(suppl):190–7. [DOI] [PubMed] [Google Scholar]
- [3].Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 2007;60(supp 11):S7–13. [DOI] [PubMed] [Google Scholar]
- [4].May M. Regenerative medicine: rebuilding the backbone. Nature 2013;503:S7–9. [DOI] [PubMed] [Google Scholar]
- [5].Clements DH, O’Leary PF. Anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 1990;15:1023–5. [DOI] [PubMed] [Google Scholar]
- [6].Gore DR, Sepic SB. Anterior discectomy and fusion for painful cervical disc disease: a report of 50 patients with an average follow-up of 21 years. Spine (Phila Pa 1976) 1998;23:2047–51. [DOI] [PubMed] [Google Scholar]
- [7].Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5-to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 2005;30:2138–44. [DOI] [PubMed] [Google Scholar]
- [8].Matsumoto M, Okada E, Ichihara D, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine (Phila Pa 1976) 2010;35:36–43. [DOI] [PubMed] [Google Scholar]
- [9].Nanda A, Sharma M, Sonig A, et al. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: a single surgeon's experience of 1,576 patients. World Neurosurg 2014;82:1380–7. [DOI] [PubMed] [Google Scholar]
- [10].Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine 2005;3:417–23. [DOI] [PubMed] [Google Scholar]
- [11].Dmitriev AE, Cunningham BW, Hu N, et al. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976) 2005;30:1165–72. [DOI] [PubMed] [Google Scholar]
- [12].Furlan AD, Pennick V, Bombardier C, et al. 2009 Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929–41. [DOI] [PubMed] [Google Scholar]
- [13].Van Tulder M, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976) 2003;28:1290–9. [DOI] [PubMed] [Google Scholar]
- [14].Kim SW, Limson MA, Kim SB, et al. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur Spine J 2009;18:218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheng L, Nie L, Zhang L, et al. Fusion versus Bryan cervical disc in two-level cervical disc disease: a prospective, randomised study. Int Orthop 2009;33:1347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jawahar A, Cavanaugh DA, Kerr EJ, et al. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J 2010;10:1043–8. [DOI] [PubMed] [Google Scholar]
- [17].Grob D, Porchet F, Kleinstück FS, et al. A comparison of outcomes of cervical disc arthroplasty and fusion in everyday clinical practice: surgical and methodological aspects. Eur Spine J 2010;19:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hou Y, Liu Y, Yuan W, et al. Cervical kinematics and radiological changes after discover artificial disc replacement versus fusion. Spine J 2014;14:867–77. [DOI] [PubMed] [Google Scholar]
- [19].Coric D, Albert T, Radcliff K. Five-year results of 2-level cervical total disc replacement compared with anterior discectomy and fusion: an independent review of a prospective, randomized, controlled multicenter investigational device exemption clinical trial. Clin Neurosurg 2015;62:221–2. [DOI] [PubMed] [Google Scholar]
- [20].Davis RJ, Nunley PD, Kim KD, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine 2015;22:15–25. [DOI] [PubMed] [Google Scholar]
- [21].Radcliff K, Coric D, Albert T. Five-year clinical results of cervical total disc replacement compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled, multicenter investigational device exemption clinical trial. J Neurosurg Spine 2016;25:213–324. [DOI] [PubMed] [Google Scholar]
- [22].Jackson R, Johnson DE. Neurological outcomes of two-level total disk replacement versus anterior discectomy and fusion: 7-year results from a prospective, randomized, multicenter trial. Neurosurgery 2016;63(suppl 1):164. [Google Scholar]
- [23].Gornet MF, Lanman TH, Burkus JK, et al. Cervical disc arthroplasty with the Prestige LP disc versus anterior cervical discectomy and fusion, at 2 levels: results of a prospective, multicenter randomized controlled clinical trial at 24 months. J Neurosurg Spine 2017;26:653–67. [DOI] [PubMed] [Google Scholar]
- [24].Lanman TH, Burkus JK, Dryer RG, et al. Long-term clinical and radiographic outcomes of the Prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial. J Neurosurg Spine 2017;27:7–19. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y, Liang C, Tao Y, et al. Cervical total disc replacement is superior to anterior cervical decompression and fusion: a meta-analysis of prospective randomized controlled trials. PLoS ONE 2015;10:e0117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nunley PD, Jawahar A, Kerr EJ, et al. Choice of plate may affect outcomes for single versus multilevel ACDF: results of a prospective randomized single-blind trial. Spine J 2009;9:121–7. [DOI] [PubMed] [Google Scholar]
- [27].Mummaneni PV, Amin BY, Wu JC, et al. Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review comparing long-term follow-up results from two FDA trials. Evid Based Spine Care J 2012;3:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kepler CK, Brodt ED, Dettori JR, et al. Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review comparing multilevel versus single-level surgery. Evid Based Spine Care J 2012;3:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goffin J, Van Calenbergh F, van Loon J, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine (Phila Pa 1976) 2003;28:2673–8. [DOI] [PubMed] [Google Scholar]
- [30].Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519–28. [DOI] [PubMed] [Google Scholar]
- [31].Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976) 2007;32:2797–804. [DOI] [PubMed] [Google Scholar]
- [32].Nouri A, Tetreault L, Singh A. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 2015;40:E675–93. [DOI] [PubMed] [Google Scholar]
- [33].Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater 2015;30:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kepler CK, Hilibrand AS. Management of adjacent segment disease after cervical spinal fusion. Orthop Clin North Am 2012;43:53–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


