We provided a developmental explanation for the patterns of molar crown configuration present during hominoid evolution.
Abstract
The detailed anatomical features that characterize fossil hominin molars figure prominently in the reconstruction of their taxonomy, phylogeny, and paleobiology. Despite the prominence of molar form in human origins research, the underlying developmental mechanisms generating the diversity of tooth crown features remain poorly understood. A model of tooth morphogenesis—the patterning cascade model (PCM)—provides a developmental framework to explore how and why the varying molar morphologies arose throughout human evolution. We generated virtual maps of the inner enamel epithelium—an indelibly preserved record of enamel knot arrangement—in 17 living and fossil hominoid species to investigate whether the PCM explains the expression of all major accessory cusps. We found that most of the variation and evolutionary changes in hominoid molar morphology followed the general developmental rule shared by all mammals, outlined by the PCM. Our results have implications for the accurate interpretation of molar crown configuration in hominoid systematics.
INTRODUCTION
Teeth are the most durable part of the skeletal system and form the dominant part of the hominin fossil record. Hence, analyses of hominin dental anatomy represent a cornerstone of paleoanthropological inquiry. Variation in one key aspect of tooth anatomy—molar crown configuration, or the expression in number, size, shape, and spatial disposition of molar cusps—has long been used for species diagnoses, phylogenetic and functional inferences, and population history reconstructions of Homo sapiens and our fossil hominin relatives (1–6). The developmental mechanisms that generate this variation, however, are poorly understood, obscuring interpretations of well-known evolutionary trends in molar cusp expression. Here, we test the applicability of a single morphogenetic rule to determine the number, size, and spatial distribution of molar cusps. In doing so, we contribute to a better understanding of the genotype-to-phenotype relationship driving variation in molar occlusal design over the past 15 million years (Ma) of hominoid evolution.
Over the past two decades, developmental studies of mammalian tooth germs suggest that growth of multicuspid teeth (premolars and molars) resembles a Turing-like mechanism governed by the iterative activation and silencing of embryonic signaling centers known as enamel knots (EKs) (7–10). EKs direct the growth and folding of the inner enamel epithelium, which acts as a “blueprint” for the final size and shape of the tooth crown (11, 12). EKs produce diffusible molecules that inhibit the formation of new knots nearby such that these new knots can only form outside the zones of inhibition of their previously initiated counterparts (10). The primary EK appears at the tip of the first cusp to initiate formation and controls the induction of secondary EKs arising along the inner enamel epithelium at the sites of the future cusps. The patterning cascade model (PCM) of tooth morphogenesis therefore postulates that molar cusp expression is determined by the interplay between the timing and spacing of EK initiation and the duration of crown growth before mineralization (7, 8, 13, 14). In a simplified example, a short duration of growth of the inner enamel epithelium, together with the late initiation of EK formation and increased inhibition, either reduces the likelihood of new cusp development or limits a cusp’s potential to attain a large size, assuming a constant rate of growth in time and space. The PCM has successfully reconstructed molar cusp variation of seal teeth and has been hypothesized to work across mammals (7–9, 13–15). Seal molars, however, are comparatively simple structures with a single row of mesiodistally oriented cusps and are unlike the quadrate molars of humans and most other primates. Thus, although predictions of the PCM in seals can be addressed using a single dimension (z axis), those in primates require mapping EK spatiotemporal relationships in three dimensions (3D), the approach taken in this paper. To date, supporting evidence of the PCM’s ability to explain hominoid molar morphological diversity has been equivocal and limited to one species, cusp, or molar type (16–20).
Here, we investigate the extent to which the PCM explains variation in molar cusp expression within Hominidae. Our sample comprises 763 molar teeth (M1 to M3) from six hominoid genera [Australopithecus (AUS), Paranthropus (PAR), Homo (HOM), Pan (PAN), Gorilla (GOR), and Pongo (PON)] and includes representatives of most hominin species currently recognized (see Materials and Methods). All major accessory cusps that are not primitively shared by all therian mammals were evaluated, including the hypocone, cusps 5 and 6, and Carabelli’s cusp (protoconal cingulum) of the upper molars, as well as cusps 5, 6 (“single” and “double”), and 7 of the lower molars (Fig. 1). Because the model was tested on fully formed teeth, we used the 3D form of the enamel-dentine junction (EDJ), which exactly reflects the endpoint of growth of the inner enamel epithelium, the embryonic anlage from which tooth crowns develop (11, 12, 18). Hence, dentine horns visible at the EDJ mark the precise location of EK initiation.
Fig. 1. Anatomical and developmental configuration of molar cusps.
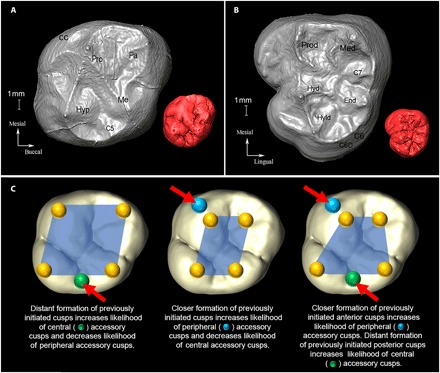
Occlusal view of the EDJ of an (A) upper and (B) lower molar with cusp nomenclature. (A) Paranthropus robustus (SK831a, ULM3) and (B) Australopithecus africanus (STW560b, LLM3). An occlusal view of outer enamel surface (OES) is shown in red as an inset (not to scale). pro, protocone; pa, paracone; me, metacone; hyp, hypocone; C5, cusp 5; CC, Carabelli’s cusp; prod, protoconid; med, metaconid; end, entoconid; hyd, hypoconid; hyld, hypoconulid/cusp 5; C6, cusp 6 (tuberculum sextum); C6D, double cusp 6; C7, cusp 7. The OES in (A) exhibits both cusps 5 and 6 (cusp 6 not present at EDJ). (C) Schematic of potential developmental pathways during tooth crown morphogenesis. Location of EKs (yellow spheres) superimposed onto an OES and exaggerated for visualization purposes. Note that in developing teeth, cusp spacing is regulated by EKs, affecting the growth of the intercuspal regions and distances (denoted by blue polygons) at which new EKs and cusps form. Growth of tooth after the cusp patterning can further modify the cusp distances.
The 3D surface area of the EDJ and 3D intercusp distances were used as proxies for the size of the inner enamel epithelium and the spatiotemporal arrangement of EK formation, respectively. Following the logic of the PCM, the disposition of EKs across the epithelial landscape influences other aspects of molar crown configuration, such as cusp number and size (8, 13, 14). We classified all cusps according to their position on the crown: The hypocone and Carabelli’s cusp occur on the periphery, whereas all other accessory cusps were classified as central given that they develop between two previously initiated cusps. For peripheral cusps, we quantified the mean intercusp distance (as a proportion of 3D surface area) between all earlier-initiating EKs. Consistent with the PCM, this approach assumes a close relationship between the size of the inhibitory fields and the proximity of EKs and predicts that a relatively small mean intercusp distance between previously initiated EKs makes an additional cusp more likely to initiate on the crown periphery (17, 19). Central cusps, on the other hand, face an additional spatial limitation such that they are more likely to be present and/or more strongly expressed when their two adjacent and earlier-initiated cusps are more widely spaced (Fig. 1) (18).
RESULTS AND DISCUSSION
Our analyses of samples of Pleistocene to recent H. sapiens and 10 species of fossil hominins using rank correlation coefficients and odds logistic regression revealed that most of the diversity in molar cusp features can be explained by the PCM. A similar pattern was found for all great apes, suggesting that this developmental mechanism of cusp expression was present in the last common ancestor of extant hominoids. For peripheral cusps, we found that hypocone presence was significantly and negatively correlated with mean relative intercusp distance of previously formed cusps. Our results for Carabelli’s cusp, however, show a negative relationship between cusp expression and mean relative intercusp distance only in Australopithecus and Gorilla (Fig. 2, Table 1, figs. S1 and S2, and table S1). For central cusps of the upper molars, cusp 5 expression is positively correlated with relative metacone-hypocone spacing in Australopithecus, Paranthropus (P ≤ 0.05), and Gorilla (P ≤ 0.05), whereas the occasional presence of cusp 6 is also associated with the greatest metacone-hypocone distances. Similarly, lower molar cusps 5 and 6 are more likely to develop and to be larger in teeth with expanded hypoconid-entoconid and entoconid-hypoconulid distances, respectively (P ≤ 0.05 in all six hominoid genera examined). Our results for double cusp 6—observed in our Australopithecus, Paranthropus, and Homo samples—are also suggestive of the validity of the PCM in that we found a positive relationship between cusp expression and hypoconulid–tuberculum sextum spacing, although results were significant only in Paranthropus. Results for cusp 7 were less clear, with a positive but nonsignificant relationship between cusp 7 and metaconid-entoconid distance in Australopithecus and Homo (Figs. 2 and 3, Table 1, and table S1).
Fig. 2. Accessory cusp expression of hominoid upper molars.
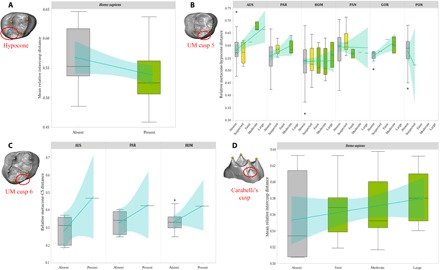
Box plots of relative intercusp distance by degree of expression for the hypocone (A) in H. sapiens and for cusp 5 (B), cusp 6 (C), and Carabelli’s cusp (D) in hominoids (generic analysis for Carabelli’s cusp in fig. S1). Line of fit and 95% confidence intervals are shown in light blue for visualization of trends only. The PCM predicts a negative relationship between trait expression and mean intercusp distance for the hypocone and Carabelli’s cusp and a positive relationship between trait expression and expanded intercusp distance for cusps 5 and 6. For box plots, cusp absence is in gray, cusp “suspected” is in yellow, and cusp presence is in green. Cusp analyzed in each box plot is circled in red, and landmarks used to calculate intercusp distance are in yellow. Genera are not depicted if the accessory cusp was invariably absent/present in our sample. The asterisk indicates outliers. UM, upper molar.
Table 1. Kendall’s rank correlation coefficient between accessory cusp expression and relative intercusp distance.
Sample size for each pairwise comparison in parentheses. Significant values (at P < 0.05) bolded. Blank cells indicate that the feature was invariably absent/present or that samples did not have enough grades of expression for statistical analysis. AUS, Australopithecus; PAR, Paranthropus; HOM, Homo; HSr, recent H. Sapiens; PAN, Pan; GOR, Gorilla; PON, Pongo.
| Taxon | HYP | UMC5 | CC | LMC5 | LMC6 | LMC6D | LMC7 |
| AUS | — | 0.21 (35) | −0.31* (24) | — | 0.39* (48) | 0.29 (13) | 0.26 (19) |
| PAR | — | 0.22* (39) | −0.09 (24) | — | 0.37* (46) | 0.38* (38) | — |
| HOM | −0.24* (94) | −0.03 (113) | −0.01 (79) | 0.55* (144) | 0.36* (127) | 0.17 (29) | 0.14 (39) |
| HSr | −0.23* (48) | 0.07 (49) | 0.14 (35) | 0.56* (104) | 0.39* (67) | — | — |
| PAN | — | −0.02 (49) | 0.09 (35) | — | 0.28* (69) | — | −0.04 (46) |
| GOR | — | 0.51* (16) | −0.16 (11) | — | — | — | — |
| PON | — | −0.26 (16) | 0.13 (14) | — | 0.33* (29) | — | — |
*Adherence to PCM predictions (only significant results considered).
Fig. 3. Accessory cusp expression of hominoid lower molars.
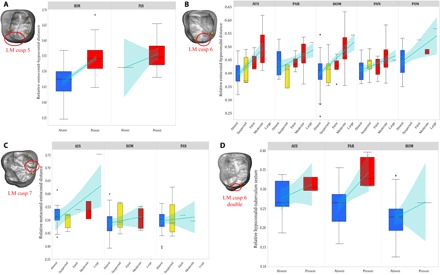
Box plots of relative intercusp distance by degree of expression for cusp 5 (A), cusp 6 (B), cusp 7 (C), and cusp 6 double (D) in hominoids. Line of fit and 95% confidence intervals are shown in light blue for visualization of trends only. The PCM predicts a positive relationship between trait expression and intercusp distance for cusps 5, 6 (single and double), and 7. For box plots, cusp absence is in blue, cusp suspected is in yellow, and cusp presence is in red. Cusp analyzed in each box plot is circled in red, and landmarks used to calculate intercusp distance are in yellow. Genera are not depicted if the accessory cusp was invariably absent/present in our sample. The asterisk indicates outliers. LM, lower molar.
Although direct experimental testing is impossible in fossils and unfeasible in most extant mammals, the application of a developmental-based model evaluating the complex form of the EDJ strongly suggests that the wide range of variation in hominoid cuspal patterns is primarily regulated by EKs such that the size of their inhibitory fields constrains the presence and size of subsequently developing cusps. The strongest support for the PCM comes from the hypocone and lower molar cusps 5 and 6 (single and double). Reported frequencies of cusp expression for hominoids provide an additional way to validate the PCM as tested in seals. When cusp variation is analyzed along the z axis and all other parameters are held constant, in high-cusped molars, EKs initiate farther apart than in bunodont molars. Hence, bunodont molars should exhibit both lower cusp height differentials and higher frequencies of accessory cusps (8, 9, 13, 14). Although the bunodont molars of Paranthropus and Australopithecus have higher frequencies of accessory cusps relative to later and higher-cusped hominin molars, high-cusped Gorilla molars do not have a distinctly high frequency of extra cusps (1, 21). Given the limited differential variability for selection to act upon, it is believed that going from high-cusped to a bunodont configuration is easier than the reverse (15). Thus, considering the highly variable and heritable nature of enamel thickness (22), it has been suggested that the evolution of hyperthick enameled teeth in Paranthropus was the “least resistant” adaptive response for fracturing tough food in otherwise suboptimal flat molars (23).
Although we highlight the power of the PCM to explain variation in cusp expression within the hominin lineage, our results also point to some deviations from this highly conserved developmental program. The PCM does not fully explain variation of Carabelli’s cusp and cusp 7, and, to a lesser extent, upper molar cusp 5. We hypothesize that one reason for these disparities in Carabelli’s cusp and cusp 7 is that mild expressions of these two features do not involve a dentine horn. In contrast, even the weakest degrees of expression of all other cusps analyzed are invariably associated with a dentine horn, and thus with the unequivocal presence of an EK (24, 25). This suggests that a slightly different developmental pathway may account for Carabelli’s cusp and cusp 7 morphogenesis. A recent study has identified variants in the transcription factor FOXI3 that lead to incomplete development of lingually oriented cusps (26). The PCM derives from a wide range of studies on mice and seals, mammals with highly derived dentitions. Considering that humans and mice share an ancestry going back ~100 Ma and a shared ancestry with seals that goes even deeper in time, we hypothesize that minor genetic alterations in humans and other primates have led to small deviations from the PCM. The relatively prolonged life span of hominoids, and its associated protraction of time devoted to developing teeth, makes humans and their closest relatives more prone to accumulate mutations that may lead to bifurcations to the original developmental program (27). Experimental evidence also indicates that environmental stressors can alter gene expression (28, 29), and recently, it was found that malnutrition and systemic diseases in humans lead to developmental disruptions that increased variability in molar cusp expression, including the presence of extra cusps (30).
Broad themes in molar cuspal patterns exist and have shaped various phylogenetic or taxonomic schemes of human origins and diversity, including the differential expansion of the talonid in P. robustus and Paranthropus boisei (2), the higher frequency of lower molar cusps 6 and 7 in Paranthropus and Australopithecus, respectively (1, 31), the large and protruding hypocones of Neandertals (4), or the trend toward the reduction or loss of the hypocone and lower molar cusp 5 in recent Europeans (3). By testing the PCM, we have provided a developmental explanation for these and other long-standing patterns of molar crown configuration observed throughout human evolution. Our results are consistent with the emerging picture that a single basic developmental program (with the differential composition of its parameters) is responsible for the generation of morphological novelties and the myriad dental phenotypes found in mammalian teeth. These results also point to the highly homoplastic nature of accessory cusps and have implications for the accurate interpretation of dental phenotypic variation in hominoid systematics.
MATERIALS AND METHODS
Materials
The study sample consisted of 276 and 487 upper and lower molars, respectively (Table 2). The extinct hominin sample included 12 Australopithecus anamensis, 13 Australopithecus afarensis, 80 A. africanus, 2 Paranthropus aethiopicus, 9 P. boisei, 83 P. robustus, 15 Homo habilis sensu lato, 9 Homo erectus sensu lato, 5 Middle Pleistocene hominins, 91 Homo neanderthalensis, and 37 Pleistocene H. sapiens. The complete list of the fossil specimens used is provided in tables S2 and S3. Reference samples of 177 recent H. sapiens, 115 Pan troglodytes, 27 Pan paniscus, 38 Gorilla spp., and 40 Pongo spp. molars were also examined. No antimeres were included except for rare cases where cusp number differed between the left and right sides (tables S2 and S3).
Table 2. Sample composition for hominoid upper and lower molars used in this study.
| Taxon | Upper molars | Lower molars | ||||||||
| UM1 | UM2 | UM3 | UM | Total | LM1 | LM2 | LM3 | LM | Total | |
| A. anamensis | 2 | 3 | — | — | 5 | 3 | 4 | — | — | 7 |
| A. afarensis | 2 | 1 | 1 | 1 | 5 | 3 | 4 | 2 | — | 9 |
| A. africanus | 10 | 11 | 7 | 1 | 29 | 14 | 21 | 16 | — | 51 |
| P. aethiopicus | — | — | — | — | — | — | 2 | — | — | 2 |
| P. boisei | 1 | 1 | 1 | — | 3 | — | 4 | 1 | 1 | 6 |
| P. robustus | 16 | 12 | 8 | — | 36 | 20 | 14 | 13 | — | 47 |
| Homo sp./habilis s.l. | 4 | 2 | — | — | 6 | 5 | 3 | 1 | — | 9 |
| H. erectus s.l. | 2 | 3 | — | — | 5 | 1 | 2 | 1 | — | 4 |
| Middle Pleistocene hominins | 1 | 1 | — | — | 2 | 1 | 1 | 1 | — | 3 |
| H. neanderthalensis | 19 | 19 | 3 | 1 | 42 | 18 | 24 | 7 | — | 49 |
| H. sapiens (Pleistocene) | 4 | 5 | 1 | 2 | 12 | 3 | 10 | 9 | 3 | 25 |
| H. sapiens (recent) | 14 | 20 | 2 | 13 | 49 | 37 | 64 | 24 | 3 | 128 |
| P. troglodytes | 16 | 21 | 6 | — | 43 | 28 | 34 | 10 | — | 72 |
| P. paniscus | 5 | 1 | — | — | 6 | 10 | 11 | — | — | 21 |
| Gorilla | 1 | 7 | 8 | — | 16 | 1 | 10 | 11 | — | 22 |
| Pongo | 7 | 7 | 3 | — | 17 | 12 | 15 | 6 | — | 33 |
Data collection procedures and statistical analyses
Each molar was subjected to microcomputed tomography to produce 3D surface models of the internal tooth structure at the EDJ. These models were subsequently manipulated on Avizo/Amira (FEI Visualization Sciences Group) for landmark digitizing and trait scoring. Accessory cusp expression was collected following standardized scoring protocols summarized in table S4. Intercusp distances (Euclidean distances) were derived from homologous landmarks placed on the dentine horns. Tooth size was calculated using the 3D surface area of the EDJ crown (32) following the parameters recommended by Pampush et al. (33). Following the study of Hunter et al. (17), both nonparametric Kendall’s rank correlation tests and ordered logistic regression models were used to examine the relationship between relative intercusp spacing (that is, intercusp distance divided by tooth size) and the presence and degree of expression of accessory cusps. Most analyses were performed at the genus level given the small samples of most fossil species available for study. A detailed explanation of the methods used can be found in the Supplementary Materials.
Supplementary Material
Acknowledgments
Access to specimens was provided by the following institutions: Croatian Museum of Natural History, Ditsong National Museum of Natural History, Francisc Rainer Anthropology Institute, National Museums of Kenya, Musée d’Art et d’Archéologie du Périgord, Musée d’Archéologie Nationale de Saint-Germain-en-Laye, Musée d’Angoulême, Musée National de Préhistoire des Eyzies, Musée de l’Homme, Museo Nacional de Ciencias Naturales, Max Planck Institute for Evolutionary Anthropology, Royal Museum for Central Africa, Museum für Vor- und Frühgeschichte, National Museum of Ethiopia, Museum National d’Histoire Naturelle, American Museum of Natural History, Rockefeller Museum, Royal Belgian Institute of Natural Sciences, Senckenberg Research Institute, Sackler School of Medicine, Tel Aviv University, Leipzig University, University of Witwatersrand, Direction du Patrimoine Culturel, and Musée Archéologique de Rabat. We also thank C. Menter for access to material from Drimolen, B. Kimbel for access to material from Hadar, and F. Schrenck and O. Kullmer for access to material from Sangiran. We thank H. Temming, D. Plotzki, L. Westphal, S. Antón, T. Harrison, B. Kimbel, J. Lawrence, and S. Daly for help and comments. Finally, we thank two anonymous reviewers and the editor for their comments and constructive criticism that helped improve the manuscript. Funding: This research was supported by the NSF, the Wenner-Gren Foundation, the Leakey Foundation, the New York University’s Graduate School of Arts and Science James Arthur Fellowship, the Institute of Human Origins at Arizona State University, and the Max Planck Society. A.O. was a John Templeton Foundation–funded postdoctoral fellow during the completion of this research. Author contributions: A.O., S.E.B., and M.M.S. conceived and designed the study; J.-J.H. and M.M.S. acquired the raw data; A.O. and M.M.S. processed the data; G.T.S. and J.-J.H. provided materials and tools; A.O. analyzed the data; A.O. and G.T.S. prepared the figures. A.O. wrote the paper with input from all coauthors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The data of the landmarks, dental measurements, and trait scores can be accessed on the Edmond online repository (https://edmond.mpdl.mpg.de/imeji/collection/pNs4X4pEY1D8GjO7?q=).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaar2334/DC1
Supplementary Materials and Methods
fig. S1. Relationship between mean relative intercusp distance and Carabelli’s cusp per genus.
fig. S2. Relationship between mean relative intercusp distance and Carabelli’s cusp per molar type in Homo.
fig. S3. Right lower molar with example of homologous landmarks (yellow dots) placed at the cusp tips from which Euclidean distances were calculated.
fig. S4. Ordinary least squares regression of upper (top row) and lower (bottom row) molar size comparisons estimated from crown outline, centroid size, and 3D surface area.
table S1. Ordered logistic regression of cusp expression and relative intercusp distance.
table S2. Fossil hominin upper molars used in this study including accession number, locality/site, and source.
table S3. Fossil hominin lower molars used in this study including accession number, locality/site, and source.
table S4. System used in this study for scoring accessory cusps.
table S5. Tooth size comparisons estimated from crown outline, centroid size, and 3D surface area.
REFERENCES AND NOTES
- 1.Wood B. A., Abbott S. A., Analysis of the dental morphology of Plio-Pleistocene hominids. I. Mandibular molars: Crown area measurements and morphological traits. J. Anat. 136, 197–219 (1983). [PMC free article] [PubMed] [Google Scholar]
- 2.Suwa G., Wood B. A., White T. W., Further analysis of mandibular molar crown and cusp areas in Pliocene and early Pleistocene hominids. Am. J. Phys. Anthropol. 93, 407–426 (1994). [DOI] [PubMed] [Google Scholar]
- 3.G. R. Scott, C. G. Turner, The Anthropology of Modern Human Teeth: Dental Morphology and Its Variation in Recent Human Populations (Cambridge Univ. Press, 1997). [Google Scholar]
- 4.S. E. Bailey, “Neandertal dental morphology: Implications for modern human origins,” thesis, Arizona State University (2002). [Google Scholar]
- 5.Ungar P. S., Dental topography and diets of Australopithecus afarensis and early Homo. J. Hum. Evol. 46, 605–622 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Martinón-Torres M., Bermúdez de Castro J. M., Gómez-Robles A., Arsuaga J. L., Carbonell E., Lordkipanidze D., Manzi G., Margvelashvili A., Dental evidence on the hominin dispersals during the Pleistocene. Proc. Natl. Acad. Sci. U.S.A. 104, 13279–13282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar-Ciudad I., Jernvall J., A gene network model accounting for development and evolution of mammalian teeth. Proc. Natl. Acad. Sci. U.S.A. 99, 8116–8120 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar-Ciudad I., Jernvall J., A computational model of teeth and the developmental origins of morphological variation. Nature 464, 583–586 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Salazar-Ciudad I., Tooth patterning and evolution. Curr. Opin. Genet. Dev. 22, 585–592 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Jernvall J., Thesleff I., Reiterative signalling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, 19–29 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Schour I., Massler M., Studies in tooth development. The growth pattern of the human teeth. J. Am. Dent. Assoc. 27, 1778–1793 (1940). [Google Scholar]
- 12.Butler P. M., The ontogeny of molar pattern. Biol. Rev. Camb. Philos. Soc. 31, 30–69 (1956). [Google Scholar]
- 13.Jernvall J., Mammalian molar cusp patterns: Developmental mechanisms of diversity. Acta Zool. Fenn. 198, 1–61 (1995). [Google Scholar]
- 14.Jernvall J., Linking development with generation of novelty in mammalian teeth. Proc. Natl. Acad. Sci. U.S.A. 97, 2641–2645 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polly P. D., Variability, selection, and constraints: Development and evolution in viverravid (Carnivora, Mammalia) molar morphology. Paleobiology 24, 409–429 (1998). [Google Scholar]
- 16.Hlusko L. J., Do N., Mahaney M. C., Genetic correlations between mandibular molar cusp areas in baboons. Am. J. Phys. Anthropol. 132, 445–454 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Hunter J. P., Guatelli-Steinberg D., Weston T. C., Durner R., Betsinger T. K., Model of tooth morphogenesis predicts Carabelli cusp expression, size, and symmetry in humans. PLOS ONE 5, e11844 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner M. M., Gunz P., The presence of accessory cusps in chimpanzee lower molars is consistent with a patterning cascade model of development. J. Anat. 217, 245–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormann S., Guatelli-Steinberg D., Hunter J., Metamerism, morphogenesis, and the expression of Carabelli and other dental traits in humans. Am. J. Phys. Anthropol. 150, 400–408 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Paul K. S., Astorino C. M., Bailey S. E., The Patterning Cascade Model and Carabelli’s trait expression in metameres of the mixed human dentition: Exploring a morphogenetic model. Am. J. Phys. Anthropol. 162, 3–18 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Ortiz A., Bailey S. E., Hublin J.-J., Skinner M. M., Homology, homoplasy and cusp variability at the enamel-dentine junction of hominoid molars. J. Anat. 231, 585–599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlusko L. J., Suwa G., Kono R. T., Mahaney M. C., Genetics and the evolution of primate enamel thickness: A baboon model. Am. J. Phys. Anthropol. 124, 223–233 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Ungar P. S., Hlusko L. J., The evolutionary path of least resistance. Science 353, 29–30 (2016). [DOI] [PubMed] [Google Scholar]
- 24.M. M. Skinner, “Enamel-dentine junction morphology of extant hominoid and fossil hominin lower molars,” thesis, George Washington University (2008). [Google Scholar]
- 25.Ortiz A., Skinner M. M., Bailey S. E., Hublin J.-J., Carabelli’s trait revisited: An examination of mesiolingual features at the enamel–dentine junction and enamel surface of Pan and Homo sapiens upper molars. J. Hum. Evol. 63, 586–596 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Kupczik K., Cagan A., Brauer S., Fischer M. S., The dental phenotype of hairless dogs with FOXI3 haploinsufficiency. Sci. Rep. 7, 5459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster G., Alberch P., Evolution and bifurcation of developmental programs. Evolution 36, 444–459 (1982). [DOI] [PubMed] [Google Scholar]
- 28.de Nadal E., Ammerer G., Posas F., Controlling gene expression in response to stress. Nat. Rev. Genet. 12, 833–845 (2011). [DOI] [PubMed] [Google Scholar]
- 29.F. J. de Brujin, Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria (Wiley-Blackwell, 2016). [Google Scholar]
- 30.Riga A., Belcastro M. G., Moggi-Cecchi J., Environmental stress increases variability in the expression of dental cusps. Am. J. Phys. Anthropol. 153, 397–407 (2014). [DOI] [PubMed] [Google Scholar]
- 31.S. E. Bailey, B. A. Wood, Trends in postcanine occlusal morphology within the hominin clade: The case of Paranthropus, in Dental Perspectives on Human Evolution, S. E. Bailey, J.-J. Hublin, Eds. (Springer-Verlag, 2007), chap. 3.
- 32.Ungar P. S., M’Kiera F., A solution to the worn tooth conundrum in primate functional anatomy. Proc. Natl. Acad. Sci. U.S.A. 100, 3874–3877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pampush J. D., Winchester J. M., Morse P. E., Vining A. Q., Boyer D. M., Kay R. F., Introducing molaR: A new R package for quantitative topographic analyses of teeth (and other topographic surfaces). J. Mamm. Evol. 23, 397–412 (2016). [Google Scholar]
- 34.Wollny G., Kellman P., Ledesma-Carbayo M-J., Skinner M. M., Hublin J.-J., Hierl T., MIA—A free and open source software for gray scale medical image analysis. Source Code Biol. Med. 8, 20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molnar S., Human tooth wear, tooth function and cultural variability. Am. J. Phys. Anthropol. 34, 175–189 (1971). [DOI] [PubMed] [Google Scholar]
- 36.C. G. Turner, C. R. Nichol, G. R. Scott, Scoring procedures for key morphological traits of the permanent dentition: The Arizona State University Dental Anthropology System, in Advances in Dental Anthropology, M. A. Kelley, C. S. Larsen, Eds. (Wiley-Liss, 1991), chap. 3.
- 37.J. T. Robinson, The Dentition of the Australopithecinae (Transvaal Museum, 1956). [Google Scholar]
- 38.C. A. W. Korenhof, Morphogenetical Aspects of the Human Upper Molar (Uitgeversmaatschappij Neerlandia, 1956). [Google Scholar]
- 39.J. E. Frisch, Trends in the evolution of the hominoid dentition, in Bibliotheca Primatologica No. 3 (Karger, 1964), pp. 1–130. [Google Scholar]
- 40.J. F. Van Reenen, C. Reid, The Carabelli trait in early South African hominids: A morphological study, in Aspects of Dental Biology: Paleontology, Anthropology and Evolution, J. Moggi-Cecchi, Ed. (International Institute for the Study of Man, 1995), pp. 291–298.
- 41.Guatelli-Steinberg D., Irish J.D., Brief communication: Early hominin variability in first molar dental trait frequencies. Am. J. Phys. Anthropol. 128, 477–484 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Nichol C. R., Turner C. G. II, Intra- and interobserver concordance in classifying dental morphology. Am. J. Phys. Anthropol. 69, 299–315 (1986). [DOI] [PubMed] [Google Scholar]
- 43.Ortiz A., Dental morphological variation among six pre-Hispanic South American populations with implications for the peopling of the New World. Dent. Anthropol. 26, 20–32 (2013). [Google Scholar]
- 44.B. S. Kraus, J. Jordan, The Human Dentition Before Birth (Lea and Febiger, 1965). [Google Scholar]
- 45.Swindler D. R., McCoy H. A., Primate odontogenesis. J. Dent. Res. 44, 283–295 (1965). [DOI] [PubMed] [Google Scholar]
- 46.Oka S. W., Kraus B. S., The circumnatal status of molar crown maturation among the Hominoidea. Arch. Oral Biol. 14, 639–659 (1969). [DOI] [PubMed] [Google Scholar]
- 47.Corruccini R. S., Molar cusp size variability in relation to odontogenesis in hominoid primates. Arch. Oral Biol. 24, 633–634 (1979). [DOI] [PubMed] [Google Scholar]
- 48.Hammer Ø., Harper D. A. T., Ryan P. D., PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9 (2001). [Google Scholar]
- 49.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012); www.R-project.org/.
- 50.A. Agresti, Analysis of Ordinal Categorical Data (Wiley, 2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaar2334/DC1
Supplementary Materials and Methods
fig. S1. Relationship between mean relative intercusp distance and Carabelli’s cusp per genus.
fig. S2. Relationship between mean relative intercusp distance and Carabelli’s cusp per molar type in Homo.
fig. S3. Right lower molar with example of homologous landmarks (yellow dots) placed at the cusp tips from which Euclidean distances were calculated.
fig. S4. Ordinary least squares regression of upper (top row) and lower (bottom row) molar size comparisons estimated from crown outline, centroid size, and 3D surface area.
table S1. Ordered logistic regression of cusp expression and relative intercusp distance.
table S2. Fossil hominin upper molars used in this study including accession number, locality/site, and source.
table S3. Fossil hominin lower molars used in this study including accession number, locality/site, and source.
table S4. System used in this study for scoring accessory cusps.
table S5. Tooth size comparisons estimated from crown outline, centroid size, and 3D surface area.


