Abstract
Socioeconomic disadvantage (SED) experienced in early life is linked to a range of risk behaviors and diseases. Neuroimaging research indicates that this association is mediated by functional changes in corticostriatal reward systems that modulate goal‐directed behavior, reward evaluation, and affective processing. Existing research has focused largely on adults and within‐household measures as an index of SED, despite evidence that broader community‐level SED (e.g., neighborhood poverty levels) has significant and sometimes distinct effects on development and health outcomes. Here, we test effects of both household‐ and community‐level SED on resting‐state functional connectivity (rsFC) of the ventral striatum (VS) in 100 racially and economically diverse children and adolescents (ages 6–17). We observed unique effects of household income and community SED on VS circuitry such that higher community SED was associated with reduced rsFC between the VS and an anterior region of the medial prefrontal cortex (mPFC), whereas lower household income was associated with increased rsFC between the VS and the cerebellum, inferior temporal lobe, and lateral prefrontal cortex. Lower VS‐mPFC rsFC was also associated with higher self‐reported anxiety symptomology, and rsFC mediated the link between community SED and anxiety. These results indicate unique effects of community‐level SED on corticostriatal reward circuitry that can be detected in early life, which carries implications for future interventions and targeted therapies. In addition, our findings raise intriguing questions about the distinct pathways through which specific sources of SED can affect brain and emotional development.
Keywords: adolescence, children, nucleus accumbens, socioeconomic status, ventral striatum
1. INTRODUCTION
Socioeconomic disadvantage (SED) is a global health concern. In the US alone, one in every five children is born into poverty (Jiang, Ekono, & Skinner, 2016), making SED one of the most pervasive types of childhood stress. SED in early life has been linked to a wide range of psychiatric disorders (e.g., major depression, conduct disorder, antisocial personality; Kessler, et al., 2014; Wadsworth, Evans, Grant, Carter, and Duffy, 2016; Yoshikawa, Aber, and Beardslee, 2012), risk‐related behaviors (e.g., substance use, risky sexual behavior), and impairments in affective and cognitive functioning (e.g,. delays in reading and language acquisition, poorer grades in school, impaired decision‐making; Farah, et al., 2008; Hackman and Farah, 2009; Noble, Farah, and McCandliss, 2006a; Noble, McCandliss, and Farah, 2007; Noble, Norman, and Farah, 2005; Noble, Wolmetz, Ochs, Farah, and McCandliss, 2006b; Raizada and Kishiyama, 2010; Raizada, Richards, Meltzoff, and Kuhl, 2008). Notably, these disparities persist throughout life, irrespective of socioeconomic factors later in life (Adler & Rehkopf, 2008; Birnie, et al., 2011; Cohen, Janicki, Deverts, Chen, & Matthews, 2010; Poulton, et al., 2002; Shonkoff, Boyce, & McEwen, 2009; Wadsworth, et al., 2016).
A landmark neuroimaging study in adults provided the first evidence that alterations in corticostriatal reward circuitry may reflect the neurological embedding of early SED. Gianaros and colleagues (2011) demonstrated that adults with lower parental education, a presumptive indicator of early SED, showed lower activation and task‐related functional connectivity among corticostriatal reward regions, including the ventral striatum (VS), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC), during reward processing. Interestingly, these effects were not explained by the participants’ current socioeconomic status. Other studies of early SED‐related exposures (e.g., cumulative stress, low environment enrichment and parental care) in humans and animal models concur with these findings, pointing to alterations in corticostriatal circuitry and reward‐driven behavior (e.g., Brake, Zhang, Diorio, Meaney, and Gratton, 2004; Casement, et al., 2014; Hanson, et al., 2016). It is not surprising, then, that dysfunction in VS and corticostriatal circuitry has long been implicated in a number of conditions linked to early SED, including addictive behaviors, impulsive decision‐making, and depressive disorders (Chein, Albert, O'brien, Uckert, & Steinberg, 2011; Stringaris, et al., 2015; Weissman, et al., 2015).
Given that childhood and adolescence are considered periods of high‐risk for the emergence of emotional and cognitive impairments linked to SED, research on the functional neural correlates of SED during development is critically important. Corticostriatal circuitry undergoes functional refinement over the course of childhood and adolescence, which may render it particularly vulnerable to certain rearing conditions (Fareri, et al., 2015; Mills, Lalonde, Clasen, Giedd, & Blakemore, 2014; Porter, et al., 2015; Raznahan, et al., 2014; Silverman, Jedd, & Luciana, 2015; Tamnes, et al., 2010). Given these developmental changes, effects of early SED in adults may differ from those observed in children/adolescents, which is important for understanding the etiopathology of SED‐related disorders and for identifying avenues for early intervention. In addition, relative to research in adults, research on SED in children and adolescents may be less susceptible to retrospective biases, influence of long‐term compensatory mechanisms, added contribution of exposures in adulthood (e.g., alcohol use), and social selection biases (i.e., children are less likely to choose where they live than adults). To our knowledge, only one study has examined the effects of SED on corticostriatal circuitry in children and/or adolescents. In that study, Romens and colleagues (2015) demonstrated that early SED (i.e., household receipt of public assistance) predicted heightened neural response in the mPFC during a monetary reward task at age 16. Moreover, the researchers observed that mPFC activity mediated the relationship between SED and depressive symptoms at age 16 (Romens, et al., 2015), an observation that links early alterations in this circuitry to psychological health.
SED is a multidimensional construct, with indicators ranging from household‐level (e.g., family income, parental education) to broader, more community‐based measures (e.g., neighborhood quality, community poverty levels). Household‐level measures reflect the availability of family resources whereas community measures reflect the availability of social and community resources (e.g., access to health care and/or education, physical threats present in the neighborhood). The few studies examining functional neural correlates of SED have relied almost exclusively on household‐level measures, despite compelling evidence that the broader community context also has strong effects on child development and health outcomes (see review by (Leventhal & Brooks‐Gunn, 2000). Community‐level SED may be a particularly salient determinant of well‐being in young people, given the emerging focus on social and other rewards outside of the household. Indeed, it is estimated that neighborhood‐ and community‐level SED accounts for up to 10% of variation in child development, well‐being, and health (Leventhal & Brooks‐Gunn, 2000), with children and adolescents from disadvantaged neighborhoods exhibiting more risk‐related behaviors (e.g., substance use, delinquent acts, sexual activity), poorer educational outcomes (achievement and attainment), disrupted social networks and connections, and poorer physical and mental health (i.e., internalizing and externalizing problems) relative to their more advantaged peers (Boardman & Saint Onge, 2005). There is also evidence for increased mortality rates among individuals living in more distressed communities (Meijer, Rohl, Bloomfield, & Grittner, 2012). Studies show that community SED has effects above and beyond individual‐ and family‐level SED indicators, and in some cases may have unique effects (for e.g., expression of internalizing symptoms in children; Vine, et al., 2012). Evaluation of both household‐ and community‐level SED should provide greater understanding of how environmental context can shape neurodevelopment, and provide new insight into the biological embedding of socioeconomic health disparities.
Here, we examine the unique contributing effects of both household‐ (i.e., annual income) and community‐level (i.e., community distress) indicators of SED on resting‐state functional connectivity (rsFC) of brain reward circuitry in children and adolescents. Importantly, this study was centered in urban Detroit, a region with geographic inequality in race and poverty (Darden, Rahbar, Jezierski, Li, & Velie, 2010) and entrenched socioeconomic health and education disparities (Schulz, Williams, Israel, & Lempert, 2002). Indeed, there is an appreciable 14‐year life expectancy difference between individuals residing in the most deprived areas of Detroit relative to other areas of the city (Chetty, et al., 2016), reflecting the substantial SED health disparities in this urban community. We recruited a relatively large number (N = 100) of children and adolescents who ranged broadly in racial and socioeconomic makeup, with dense sampling in minority, urban, low‐income communities, though participants were not selected on this basis. We focus on rsFC of the VS as a central reward region, and predict that youth from households with lower annual income and those from less economically advantaged communities will exhibit decreased connectivity between VS and corticostriatal regions (e.g., OFC and mPFC). This would support a model in which early SED contributes to later mental health and maladaptive behaviors by affecting integrity of key developing reward circuitries
2. MATERIALS AND METHODS
2.1. Participants
This study reports on 100 children and adolescents (63 female) between the ages of 6–17 years (see Table 1). Participants were recruited through advertisements posted on Craigslist (Detroit), Wayne State University's website, printed fliers, and through Metropolitan Detroit mental health clinics. Individuals who exhibited any of the following were excluded: contraindication for magnetic resonance imaging (MRI), neurological or movement disorders, history of brain injury, English as a second language, or severe learning disability. Written parental informed consent as well as child and/or adolescent assent were obtained prior to participation. Participants were not excluded based on prior exposure to interpersonal adversity or psychopathology to match the characteristics of our urban study population, but these factors were examined and/or included as covariates in analyses. The experimental design and all study procedures were approved by the Wayne State University Institutional Review Board.
Table 1.
Participant Demographics (N = 100)
| N | Percentage of total (%) | Range | Mean (SD) | |
|---|---|---|---|---|
| Demographic Information | ||||
| Age | 6.3–17.7 | 11.32 (2.74) | ||
| Pubertal stage (Tanner) | 1.0–5.0 | 2.86 (1.44) | ||
| Gender (female) | 63 | |||
| IQ | 58.0–139.0 | 100.97 (16.71) | ||
| Zipcode distress scores | 0.9–99.4 | 56.51 (35.84) | ||
| Annual income | ||||
| Less than $40,000 | 51 | 52.58 | ||
| $40–60,000 | 20 | 20.62 | ||
| $60–80,000 | 10 | 10.31 | ||
| $80–100,000 | 2 | 2.06 | ||
| More than $100,000 | 14 | 14.43 | ||
| Race/ethnicity | ||||
| African‐American | 44 | 47.31 | ||
| Caucasian | 38 | 40.86 | ||
| Latino | 3 | 3.23 | ||
| Biracial/other | 8 | 8.60 | ||
| Parental marital status | ||||
| Unpartnered | 48 | 48.48 | ||
| Partnered | 51 | 51.52 | ||
| Parental education (reporting parent) | ||||
| No GED/High‐school diploma | 7 | 7.07 | ||
| GED/High‐school diploma | 16 | 16.16 | ||
| Some college | 30 | 30.30 | ||
| 2‐year degree | 8 | 8.08 | ||
| 4‐year degree | 22 | 22.22 | ||
| Master's | 15 | 15.15 | ||
| Doctorate | 1 | 1.01 | ||
| Clinical Measures | ||||
| Anxiety (SCR) | 0.0–73.0 | 21.72 (16.12) | ||
| Depression (CDI) | 0.0–23.0 | 3.10 (3.97) | ||
| Reward sensitivity (BIS/BAS) | 9.0–17.3 | 13.41 (1.71) |
2.2. Measures
Our main factors of interest were household income and community SED. Annual household income information was obtained via parent‐report. Parents of participants were asked to indicate annual household income from a set of binned responses ranging from <$40k to >$120k (see Table 1). Participant zipcodes were used to obtain measures of community distress via the Distressed Communities Index (DCI; available at http://eig.org/dci), which utilizes population and US Census data. The DCI is a composite of seven socioeconomic indicators: (1) population without high school degree, (2) housing vacancy, (3) adults not working, (4) poverty, (5) median income relative to state, (6) change in employment, and (7) change in business establishments (see Figure 1). Community distress is scored on a 0–100 scale, with higher numbers representing greater distress.
Figure 1.
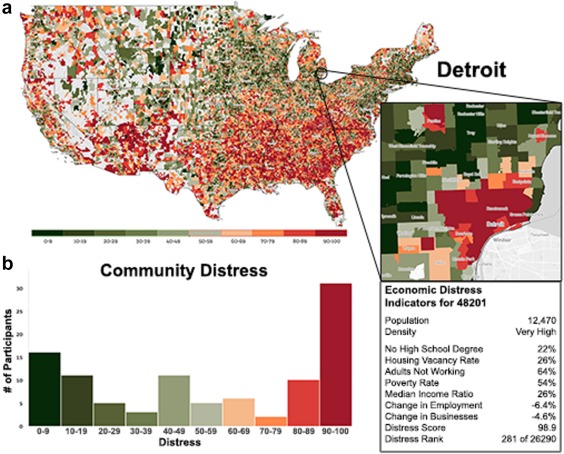
(a) Community distress map of the USA, and sub‐selection of the Detroit Metropolitan Area and community distress indicators from one example zip code. Based on US Census and population data, via the Economic Innovation Group (EIG; http://eig.org/dci). (b) Distribution of community distress scores across included participants [Color figure can be viewed at http://wileyonlinelibrary.com]
Three self‐report symptom dimensions were measured: anxiety (Screen for Child Anxiety‐Related Emotional Disorders; Birmaher, et al., 1999), depression (Children's Depression Inventory, short form; Sitarenios & Kovacs, 1999), and reward sensitivity (Behavioral Inhibition and Activation Scales, BIS/BAS; Carver & White, 1994). IQ was measured using the Kaufman Brief Intelligence Test, version 2 (Bain & Jaspers, 2010). Pubertal maturation was assessed with the self‐reported Tanner stages questionnaire (Tanner, 1962).
To characterize and control for other forms of childhood adversity in this sample, we measured exposure to adverse interpersonal experiences, using parent and/or child endorsements of trauma items indicated on the Children's Trauma Assessment Center Screen Checklist, as used in previous research (Marusak, Martin, Etkin, & Thomason, 2015). Three checklist items were excluded prior to administration, to narrow interpersonal adversity to deprivation (e.g., neglect) and threats to safety (e.g., abuse and violence exposure), distinct, but central types of adverse interpersonal experiences (McLaughlin, Sheridan, & Lambert, 2014). The three items removed from the trauma checklist were: (i) exposure to drug activity, (ii) parental/caregiver drug use/substance abuse, and (iii) frequent and multiple moves or homelessness. Of note, 43% of participants reported exposure to one or more form of interpersonal adversity, and no participants reported exposure to deprivation only (e.g., neglect).
2.3. Neuroimaging data acquisition
Neuroimaging data were collected at the Wayne State University School of Medicine MRI Research Facility, using a 3.0 T Siemens MAGNETOM Verio scanner. Participants underwent a 6 min resting‐state scan, during which they were instructed to remain awake with their eyes closed. We checked in with participants immediately following the resting‐state scan to verify that they were awake. All participants were awake following the resting‐state scan, and none endorsed falling asleep. To maximize sample size, data were combined across two functional magnetic resonance imaging (fMRI) sequences. 77% of the sample underwent the following fMRI sequence: 180 volumes, repetition time [TR] = 2000 ms; echo time [TE] = 25 ms; flip angle = 90°; voxel size = 3.44 × 3.44 × 4 mm; matrix = 220 × 220 and 29 slices. The remaining participants underwent the following fMRI sequence: 240 volumes, TR = 1500 ms; TE = 31 ms; flip angle = 83°; voxel size = 2.9 × 2.9 × 2.9 mm; matrix = 186 × 186 and 51 slices. Additionally, a high‐resolution T1‐weighted image was obtained for anatomical reference within the same imaging session, for both sequences. Of note, sequence parameters were accounted for when computing rsFC values. Importantly, community distress scores, t(98) = .178, p = .859, did not differ by sequence; however, household income did differ by fMRI sequence, Mann‐Whitney U = 799, p = .008. Follow‐up analyses examined effects of income on VS rsFC both by using sequence as a covariate, and by performing analyses within sequence groups, separately.
2.4. Neuroimaging data preprocessing
Image preprocessing was carried out using SPM8 (Statistical Parametric Mapping; http:/http://www.fil.ion.ucl.ac.uk/spm/). Images were slice‐time corrected, realigned, spatially normalized to the Montreal Neurological Institute (MNI) template, and smoothed using a 6‐mm Gaussian kernel.
2.5. Motion
To mitigate the potential influence of motion artifact in the rsFC analyses, we implemented several complementary approaches. First, functional image volumes were realigned to the mean and band‐pass filtered to 0.008–0.09 Hz. Then a despiking procedure was implemented in CONN (see https://www.nitrc.org/projects/conn/), to mitigate the impact of potential outlier scans (via a squashing function), and realignment parameters, and signal from white matter and cerebral spinal fluid (aCompCor; Chai, Castanon, Ongur, & Whitfield‐Gabrieli, 2012) were removed using covariate regression analysis. Measures of motion across the scan (i.e., maximum and mean framewise displacement [FD]) were within accepted standards (maximum FD: M = .46, SD = .06; mean FD: M = .2, SD = .07; e.g., Fair et al., 2012) and importantly, were not correlated with variables of interest (i.e., income and community distress), p's < .38.
2.6. Seed‐based functional connectivity analysis
Right and left VS seed regions were defined using a 4 mm radius sphere around peaks of interest (x = ±10, y = 14, z = 0), following resting‐state studies in adults and in youth (Di Martino, et al., 2008; Porter, et al., 2015). CONN toolbox (v. 15.h; https://www.nitrc.org/projects/conn/) was used to calculate rsFC of each seed, using Pearson bivariate correlation.
2.7. Statistical analyses
To establish the basic features of VS‐based network topology and compare this to prior research, we first performed one‐sample t tests to examine connectivity of left and right VS across the sample. Our two main analyses evaluated rsFC of left and right VS using separate regression analyses with (1) community SED and (2) household income as regressors of interest. Age was entered as a nuisance regressor, given the relatively wide age range of our sample, and the evidence of significant age‐related changes in corticostriatal circuitry across childhood and adolescence (Di Martino et al., 2008; Porter et al., 2015). In addition to our two main analyses, secondary analyses tested for potential interactions between community SED and household income, and for effects of age and age × SED interactions on observed connectivity measures. Given our a priori interest in effects of community SED and income on corticostriatal connectivity, and prior research documenting effects of SED on several anterior frontal regions, including anterior cingulate cortex (ACC), OFC, and mPFC (Forbes et al., 2010; Gianaros & Manuck, 2010; Gianaros et al., 2011; Romens et al., 2015), we evaluated two medial frontal anatomical regions of interest (ROIs) that encompassed the following areas: mPFC/OFC (Brodmann Area [BA] 10) and adjacent ACC (BA 32). BA 10 and BA 32 ROIs were defined using the Automated Anatomical Labeling atlas (dilated by 2 voxels, following Tong et al., 2016). A small‐volume familywise error (FWE) corrected threshold of pFWE < .05 was applied. A complementary whole brain threshold of p < .001, cluster extent >98 voxels was also applied, as determined by AFNI's 3dFWHMx and 3dClustSim (https://afni.nimh.nih.gov). The spatial autocorrelation of data and non‐Gaussian nature of the fMRI signal were accounted for when computing this threshold.
Given the range of experiences in this sample and the documented effects of intepersonal adverse experiences on corticostriatal circuitry (e.g., Goff et al., 2013), secondary analyses tested robustness of results when additionally controlling for interpersonal adversity exposure, as well as IQ. We also compared individual connectivity values to scores on self‐reported symptom measures. VS connectivity values were extracted for each participant using a 6 mm radius sphere around peak areas related to income or community distress. These values were then submitted to SPSS for Pearson bivariate correlation analysis, and an alpha of 0.05 (two‐tailed) was applied. For symptom measures that were correlated with VS connectivity values, we examined potential meditation effects using SPSS PROCESS software (Hayes, 2013).
3. RESULTS
3.1. Participant demographics
Household incomes ranged broadly across the sample, with 53% reporting annual incomes < $40,000 and 16% reporting incomes > $80,000 (Table 1). Community distress scores ranged from 0.9 (very minimal distress) to 99.4 (very high distress; M = 56.51, SD = 35.84), with the highest percentage of scores falling in the 90–100 distress range (Figure 1b). Not surprisingly, community distress was negatively correlated with household income (p < .001). In addition, child IQ was positively related to income (p < .005) and negatively related to community distress scores (p < .001). Income and community distress were not related to participant age (p > .21), nor self‐report clinical measures (anxiety, depression, and reward‐seeking; p's > .29). In addition, gender distribution did not differ based on income or community SED levels (p's > .12). Consistent with prior reports (e.g., Gillespie, et al., 2009), children exposed to interpersonal adversity (violence, abuse, neglect) were more likely to be from lower income families, Mann‐Whitney U = 799, p = .008, and resided in more distressed communities, t(97) = –3.50, p = .001.
3.2. VS rsFC across Sample
Bilateral positive VS connectivity was observed with several regions, including OFC, subgenual ACC, ventrolateral frontal regions (e.g., insula), and basal ganglia (see Figure 2). Positive connectivity was also observed in the both dorsal and ventral striatum, thalamus, cerebellum, and superior parietal lobule. Negative VS connectivity was observed in several regions, including parahippocampal gyrus, precuneus, and ventral and medial frontal regions. Overall, this pattern of connectivity is consistent with prior reports in adolescents and adults (Barnes et al., 2010; Choi, Yeo, & Buckner, 2012; Di Martino et al., 2008; Porter et al., 2015).
Figure 2.
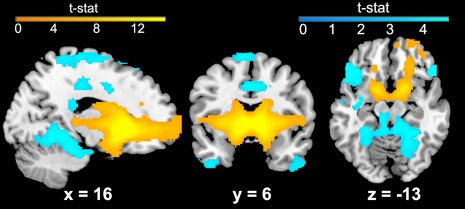
Resting‐state functional connectivity (rsFC) of the ventral striatum (VS) across the sample. Results are given at whole‐brain corrected threshold for visualization of the basic network topology, p < .001, >98 voxels. Results of the right VS seed are given; similar results were observed for the left VS. Areas of positive VS connectivity are shown in warm colors; areas of negative VS connectivity are shown in cool colors [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Impact of community socioeconomic distress on VS rsFC
Our main analyses tested for effects of community and household‐level SED on corticostriatal circuitry. Controlling for age, we found that youth living in more distressed communities displayed reduced positive connectivity between the right VS and an anterior region of the mPFC, localized to the frontal pole (see Figure 3 and Table 2). This result was significant using small‐volume correction in BA 10 (x = 10, y = 74, z = 6; pFWE = .037, Z = 4.19, 267 voxels), and also reached significance at the corrected whole‐brain threshold (x = 10, y = 74, z = 6, see Table 2). No significant results were found within the BA 32 ROI. The effect in right VS held when controlling for exposure to interpersonal adversity, scan sequence, IQ, and gender, as well as when controlling for household income, suggesting unique effects of community SED. In addition, the effect of community SED on right VS‐mPFC rsFC was significant when testing sequence groups separately (p's < .05). Right VS‐mPFC connectivity was not related to age or the age × community SED interaction (p's > .05). There was, however, an association between VS‐mPFC rsFC and anxiety, such that more positive VS‐mPFC connectivity was associated with lower anxiety symptoms, r(90) = –.236, p = .25. Given this association, we conducted mediation analyses to test whether VS‐mPFC connectivity statistically mediated the link between community SED and anxiety symptomology. A mediation relationship (indirect effects) was significant (β = .04, SE = .02, lower limit confidence interval [LLCI] = .004, upper limit confidence interval [ULCI] = .08). Direct effects of community SED on anxiety were not significant, suggesting that VS‐mPFC connectivity fully mediated the pathway between community SED and anxiety symptomology. Reversal of this model (community SED→anxiety→VS‐mPFC connectivity) yielded nonsignificant effects (LLCI = −.002, ULCI = .00001), suggesting that connectivity mediates anxiety but not the reverse. There were no significant effects of community SED on rsFC of the left VS in a priori regions of interest or at the whole brain corrected threshold.
Figure 3.
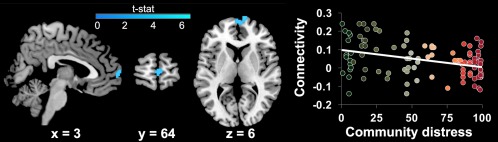
Reduced resting‐state functional connectivity (rsFC) between right ventral striatum (VS) and an anterior portion of the mPFC (frontal pole, Brodmann Area 10) in youth residing in more distressed communities. Results are displayed at the whole‐brain corrected threshold of p < .001, >98 voxels (see also Table 2) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Whole brain effects of community and household‐level socioeconomic disadvantage (SED) on ventral striatal (VS) resting‐state functional connectivity (rsFC)
|
Contrast Region |
BA | Voxels | Peak Z‐stat | X | Y | Z | |
|---|---|---|---|---|---|---|---|
| Lower rsFC of the right VS with higher community SED | |||||||
| Medial prefrontal cortex | 10 | 118 | 4.19 | 10 | 74 | 6 | |
| Lower rsFC of the right VS with lower household income | |||||||
| Inferior temporal lobe | 20, 21, 37 | 107 | 4.13 | −66 | −48 | −18 | |
| Lower rsFC of the left VS with lower household income | |||||||
| Cerebellum | 137 | 4.84 | 46 | −80 | −30 | ||
| Lateral prefrontal cortex | 9, 45 | 128 | 4.16 | 56 | 8 | 30 | |
Cluster coordinates in MNI‐152 2 mm standard space. Only clusters that are significant at the whole‐brain corrected threshold (p < .001, >100) voxels are given. No effects of community distress reached significance for the left VS.
3.4. Impact of household income on VS rsFC
ROI analyses showed no effect of household income on connectivity of left or right VS with BA 10 or BA 32. Whole brain analyses revealed several areas showing altered VS connectivity with household income (see Figure 4 and Table 2). Controlling for age, we found that lower income was associated with increased connectivity between the right VS and an area of the inferior temporal lobe (see Table 2). Lower income was also associated with increased connectivity between the left VS and cerebellum and right lateral prefrontal cortex (see Table 2). These effects held when controlling for interpersonal adversity and gender. However, when controlling for IQ and scan sequence type, only the effect in the cerebellum remained significant (x = 46, y = −80, z = −30; Z = 4.61, 126 voxels). Effects of household income on VS‐cerebellar rsFC remained significant within the larger (r[75] = −0.19, p = .033) but not smaller (r[22] = −0.241, p = .14) scan sequence group.
Figure 4.
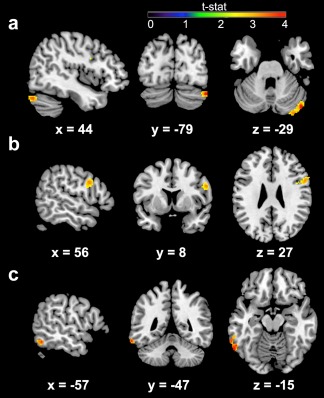
Increased resting‐state functional connectivity (rsFC) between the ventral striatum (VS) and the cerebellum (a), inferior temporal lobe (b), and lateral prefrontal cortex (c) in youth from lower income households. Results are displayed at the whole‐brain corrected threshold of p < .001, >98 voxels (see also Table 2) [Color figure can be viewed at http://wileyonlinelibrary.com]
In addition, there was a correlation between higher VS‐cerebellar connectivity and higher depressive symptoms, r(100) = .23, p = .21. Given this association, we conducted mediation analyses to test whether VS‐cerebellar connectivity statistically mediated the link between household income and depressive symptomology. The mediation relationship (indirect effects) was not significant (LLCI = −.001, ULCI = .016). There were no effects of age or age × income interactions for VS connectivity with the cerebellum, lateral prefrontal cortex, or inferior temporal lobe. There were also no areas showing lower VS connectivity for lower income youth. These results suggest that observed effects of SED on corticostriatal connectivity may differ from those related to household income.
3.5. Interactive effects of community socioeconomic distress and household income on VS rsFC
There was no significant interaction effect between community distress and household income in ROI analyses nor at the whole brain threshold for either the left or right VS.
4. DISCUSSION
This study examined the impact of household‐ (i.e., income) and community‐level (i.e., community distress) measures of SED on rsFC of VS‐based corticostriatal circuitry. We tested this in a diverse sample of children and adolescents from racially and economically distinct backgrounds, with a large number at high sociodemographic risk (i.e., lower income, more distressed communities). This approach is fitting with calls for larger, more representative samples in SED (Gianaros & Hackman, 2013; Hackman, Farah, & Meaney, 2010), and neuroimaging (Falk et al., 2013) research. This approach is also consistent with observations that the strongest effects of SED on neural development are often seen among the most disadvantaged youth (Noble et al., 2015).
As could be expected, children from lower income families tended to reside in more economically distressed communities. However, household income and community SED had distinct effects on VS connectivity: higher community SED was associated with reduced VS‐mPFC rsFC, whereas lower household income was associated with increased VS rsFC with more distributed brain circuitries. Specifically, VS rsFC to inferior temporal lobe, cerebellum, and lateral prefrontal cortex was increased in those with lower household income. The effects of community SED on corticostriatal circuitry were independent of exposure to interpersonal adversity (i.e., abuse, violence, neglect), household income, gender, IQ, and scan sequence type, however, only the result for income in the cerebellum remained significant when controlling for IQ and scan sequence type. There were no interactive effects of community SED and household income on VS connectivity. Taken together, these data add to the growing body of literature suggesting that household‐ and community‐level indicators of SED have differential effects on child health and behavioral outcomes (Kaplan, 1996; Macintyre & Ellaway, 2000; Robert, 1999), and demonstrate, for the first time, that community SED impacts corticostriatal reward circuitry during development.
Given that the mPFC is implicated in higher‐order decision‐making and control (Ernst & Fudge, 2009; Galvan et al., 2006; Miller & Cohen, 2001), lower VS‐mPFC rsFC in youth may reflect reduced frontal control over incentive salience or reward “wanting”—a core function of the VS (Berridge & Robinson, 1998). Disinhibition of VS reactivity may contribute to problem behaviors (e.g., more risky decision making, greater impulsivity) or sensitize disadvantaged youth to the reinforcing properties of drugs (Weiland et al., 2013). A recent meta‐analysis demonstrates that the mPFC is involved not only when individuals receive valuable outcomes themselves, but also when they observe others receiving such outcomes (Morelli, Sacchet, & Zaki, 2015). Thus, lower VS‐mPFC connectivity in children reared in more stressful communities may reflect lower ability to integrate observed reward associations in the environment (via VS) into personal drives and internal decision‐making (via mPFC). Of note, the peak in the mPFC appeared to be localized to the anterior portion of the mPFC (frontal pole, BA 10). Connectivity between the VS and the frontal pole has been reported in previous studies (Koehler et al., 2013), and research implicates the frontal pole in the evaluation of self‐generated decisions (Tsujimoto, Genovesio, & Wise, 2010). Future research should evaluate whether these neural patterns predict behavioral problems or problematic use of alcohol and other drugs (e.g., tobacco, marijuana).
Given that blunted connectivity between ventral striatum and mPFC is also reported in individuals with depression (Furman, Hamilton, & Gotlib, 2011), SED‐related alterations in corticostriatal circuitry may contribute to elevated risk of affective dysfunction. Indeed, blunted reward sensitivity and disrupted translation of reward motivation into goal‐directed behavior are centrally implicated in vulnerability to depressive disorders (Epstein et al., 2006; Nestler et al., 2002; Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008). It has been suggested that neighborhood‐level adversity contributes to risk for depression during adolescence (Silk et al., 2007). Although community‐level SED was not assessed in their study, Romens et al. (2015) linked household‐level SED (i.e., public assistance) with mPFC reward response and depressive symptoms at age 16. We observed that VS‐mPFC rsFC mediated the link between community SED and anxiety in our sample. Together with these findings, our data provide initial evidence that disruptions in development of corticostriatal circuitry may underlie the link between early SED and the development of affective disorders.
Of note, we found distinct effects of household income and community SED on VS rsFC. While community SED was related to VS‐mPFC circuitry, lower income was associated with higher rsFC between the VS and lateral prefrontal cortex and cerebellum, previously linked to addictive behaviors, including gambling and drug abuse (Cservenka, Casimo, Fair, & Nagel, 2014; Koehler et al., 2013; Moulton, Elman, Becerra, Goldstein, & Borsook, 2014). These problem behaviors are observed more frequently among low‐income populations (see review by Hanson & Chen, 2007). Recent research has highlighted the important role of the cerebellum in modulating attention, language, cognition, and affect (Allen, Buxton, Wong, & Courchesne, 1997; Riva & Giorgi, 2000; Schmahmann, 1991; Schmahmann, 1998; Schmahmann, 2000), as well as in regulating cell body regions for primary norepinephrine and dopamine projection in the brain (Reis & Golanov, 1997; Snider, Maiti, & Snider, 1976). Neuroimaging work shows that the cerebellum possesses a high density of glucocorticoid receptors (Lawson, Ahima, Krozowski, & Harlan, 1992; Pavlik & Buresova, 1984; Sanchez, Young, Plotsky, & Insel, 2000), and indicates that this region may play an important role in mediating the stress response (Del Bo, Ross, Pardal, Saavedra, & Reis,1983a; Del Bo, Sved, & Reis, 1983b; Dietrichs, Haines, Roste, & Roste, 1994). Animal studies have suggested that exposure to early stress may significantly affect the cerebellum and that this area may, in fact, mediate some of the neurobehavioral consequences of early stress or neglect (Harlow, Dodsworth, & Harlow, 1965; Harlow & Harlow, 1966; Heath, 1972; Mason & Berkson, 1975; Prescott, 1980). Given these data, in addition to those supporting associations between cerebellar abnormalities and psychiatric disorders such as substance abuse, schizophrenia, autism spectrum disorder, and depression (Andreasen, Paradiso, & O'leary, 1998; Courchesne, 1991; Cservenka et al., 2014; Liu et al., 2012; Sweeney, Strojwas, Mann, & Thase, 1998), income‐related alterations in rsFC between cerebellum and VS may carry implications for individuals’ ability to modulate attention, reward, response to stress, and their susceptibility to developing certain psychiatric conditions.
Our findings also indicated that lower income was associated with higher VS rsFC with the inferior temporal lobe, a region implicated in visual learning and memory (see review by Miyashita, 1993). In this context, increased VS connectivity with this region in children from lower income households may reflect altered coordination between regions involved in reward (i.e., VS) and memory‐related processes (e.g., inferior temporal lobe; Toni, Rowe, Stephan, & Passingham, 2002). Of note, prior studies also document rsFC between these regions, despite the apparent lack of direct anatomical connections (Haber & McFarland, 1999). This suggests that VS connectivity with inferior temporal lobe and cerebellum may be mediated by a third area, such as thalamus (Cauda et al., 2011).
Our data suggest that corticostriatal circuitry may be an important mechanistic target for understanding the link between SED and outcomes (e.g., mental health, academic achievement, cognitive functioning). However, it is unclear which aspects of SED are driving neurological variation. At the household‐level, potential mediators might include family stress, prenatal factors, parental care, toxins, nutrition, and cognitive stimulation in the home (e.g., linguistic exposure; Ursache & Noble, 2016). Household SED could also covary with parental depression and low parental nurturing, involvement, and supervision, all of which may modify child neurodevelopment (Dawson et al., 2003; Guo & Harris, 2000; McLaughlin et al., 2015). Community‐level effects may be driven by inequalities in resource distribution (e.g., access to healthcare and education), pollutants, physical threats in the neighborhood (e.g., violence, harmful substances, crime rates, run‐down buildings), and various factors related to the “social climate” (e.g., residential stability, neighborhood cohesion, collective efficacy, and social support, norms, and control; see review by Sellstrom & Bremberg, 2006). These factors are difficult to disentangle in human studies. Animal models may be leveraged to define a more causal relationship between SED‐related exposures and neural development.
While our dataset includes a relatively large, diverse sample of youth from a range of Metropolitan Detroit communities, it is important to consider specific aspects of this urban community that influence results. For example, a survey from Data Driven Detroit found that a third of city lots and homes are abandoned (Data Driven Detroit; http://datadrivendetroit.org/cdad-residential-typology-analysis/), which may affect how children perceive the safety or experience cohesion in their communities. There is evidence to suggest that neighborhood stability and the related ability to form lasting and meaningful social networks may be important coping sources in children, which can buffer negative effects of SED (Johns et al., 2012). Neighborhood SED and disorganization (e.g., building damage, vacant buildings, and boarded up windows) have been linked to problematic drug and alcohol use among adults (Boardman, Finch, Ellison, Williams, & Jackson, 2001; Keyes et al., 2012). Another potential influence is environment toxicants, such as lead exposure, which have known disruptive effects on dopamine (see review by Lidsky & Schneider, 2003), the primary neuromodulator within brain reward circuitry. Blood lead levels are elevated among children residing in more distressed communities (Moody, Darden, & Pigozzi, 2016), which may be linked to lead‐based paint exposure in older homes and/or high demolition rates. It is also possible that some of these effects are evident at or before the time of birth, as neighborhood‐level factors predicts greater risk for adverse perinatal outcomes (e.g., preterm birth, small‐for‐gestational age; see meta‐analysis by Vos, Posthumus, Bonsel, Steegers, & Denktas, 2014).
Although this study had several strengths, including a relatively large sample size, inclusion of multiple SED and psychosocial indicators, and evaluation of a racially and economically diverse sample, there were some limitations that suggest directions for future research. First, use of a cross‐sectional design precludes investigation of behavioral and health outcomes relating to aberrant connectivity. The present study was designed to evaluate how SED experienced at the household and community level impacts the organization of corticostriatal circuitry critical for reward processing and decision making. Future longitudinal research is needed, however, to clarify how variation in corticostriatal circuitry fits into the widely‐studied pathways linking SED to health disparities. Such longitudinal studies would benefit from mediation analyses that integrate multidimensional measurements of SED indicators across level of analysis and measures of intermediary factors (e.g., cognitive stimulation in the home, parenting practices, family stress) across development (cf. Gianaros & Hackman, 2013). A longitudinal design should also provide insight into sensitive periods in reward circuitry, and when behavioral correlates are likely to manifest. Second, we used resting‐state fMRI; thus, the functional and behavioral relevance of altered reward circuitry is unclear. However, recent work suggests that rsFC patterns can predict activity during an array of tasks (Tavor et al., 2016). Third, our measure of household income was based on parent‐report, which may be inaccurate. However, income is the most common measure of SED and is typically obtained via self‐report, given that access to administrative data (e.g., income tax returns) is often limited (Farah, 2017). Our measure of community SED was estimated based on US Census data geocoded to participants’ zip codes. This approach is consistent with the few published studies examining community SED and brain organization (Gianaros et al., 2017; Krishnadas et al., 2013). As others have noted, however, there is no best approach for measuring within‐household or community‐level SED (Farah, 2017; Ursache & Noble, 2016). Therefore, future studies should examine different SED indicators, such as perceived level of social standing, which has been shown to contribute to neural function in adults (Gianaros et al., 2008). Among children, there is some evidence that child's perceptions of neighborhood safety predict health‐related behaviors (e.g., physical activity) and outcomes (e.g., psychological distress; Nichol, Janssen, & Pickett, 2010).
5. CONCLUSIONS
We found that corticostriatal circuitry in childhood and adolescence is influenced by the households and communities that young people live in, and that these factors have unique effects. Our findings promote taking different approaches into consideration when crafting future targeted interventions based on whether SED is occurring within‐ or outside household, or both. These data suggest that corticostriatal circuitry may be an important mechanistic target for understanding the link between SED and outcomes (e.g., mental health, academic achievement, cognitive functioning). Given the observed effects of community SED on corticostriatal circuitry, these data add to a growing literature indicating the importance of neighborhood in early development and suggesting that interventions that target disadvantaged neighborhoods could reduce the prospect of poor health outcomes.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank Yashwanth Katkuri, Pavan Jella, and Zahid Latif of Wayne State University (WSU) for their assistance in neuroimaging data acquisition; Andrea Bedway, Maria Tocco, Rita Elias, Gregory Baldwin, Kayla Martin, Stephen Shen, Kelly Quednau, Farrah Elrahal, Lauren Grove, Shelley Paulisin, Craig Peters, Limi Sharif, Xhenis Brahimi, Sajah Fakhoury, Aneesh Hehr, Allesandra Iadipaolo, Farah Sheikh, Brian Silverstein, and Suzanne Brown of WSU for assistance in participant recruitment and data collection; and thank the children and their families who generously shared their time.
Research reported in this publication was supported, in part, by the Merrill Palmer Skillman Institute, Department of Pediatrics and Department of Pharmacy Practice of WSU, NIH National Institute of Environmental Health Sciences awards P30 ES020957 and R21 ES026022 (MET), NIH National Institute of Mental Health award R01 MH110793 (MET), and a NARSAD Young Investigator Award (MET). Dr. Marusak is supported by American Cancer Society award 129368‐PF‐16–057‐01‐PCSM. Dr. Rabinak is supported by National Institute of Mental Health awards K01 MH101123 and R61 MH111935.
Marshall NA, Marusak HA, Sala‐Hamrick KJ, Crespo LM, Rabinak CA, Thomason ME. Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Hum Brain Mapp. 2018;39:1982–1994. 10.1002/hbm.23978
Funding information Merrill Palmer Skillman Institute; Department of Pediatrics and Department of Pharmacy Practice of WSU; NIH National Institute of Environmental Health Sciences, Grant/Award Numbers: P30 ES020957 and R21 ES026022; NIH National Institute of Mental Health, Grant/Award Number: R01 MH110793; NARSAD Young Investigator Award; American Cancer Society, Grant/Award Number: 129368‐PF‐16‐057‐01‐PCSM; National Institute of Mental Health, Grant/Award Numbers: K01 MH101123 and R61 MH111935.
REFERENCES
- Adler, N. E. , & Rehkopf, D. H. (2008). US disparities in health: Descriptions, causes, and mechanisms. Annual Review of Public Health, 29, 235–252. [DOI] [PubMed] [Google Scholar]
- Allen, G. , Buxton, R. B. , Wong, E. C. , & Courchesne, E. (1997). Attentional activation of the cerebellum independent of motor involvement. Science, (New York, N.Y.), 275, 1940–1943. [DOI] [PubMed] [Google Scholar]
- Andreasen, N. C. , Paradiso, S. , & O'leary, D. S. (1998). Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical‐subcortical‐cerebellar circuitry? Schizophrenia Bulletin, 24, 203–218. [DOI] [PubMed] [Google Scholar]
- Bain, S. K. , & Jaspers, K. E. (2010). Test review: Review of Kaufman brief intelligence test. Second Edition. Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman Brief Intelligence Test, Second Edition. Bloomington, MN: Pearson, Inc . Journal of Psychoeducational Assessment, 28:167–174. [Google Scholar]
- Barnes, K. A. , Cohen, A. L. , Power, J. D. , Nelson, S. M. , Dosenbach, Y. B. , Miezin, F. M. , … Schlaggar, B. L. (2010). Identifying basal ganglia divisions in individuals using resting‐state functional connectivity MRI. Frontiers in Systems Neuroscience, 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K. C. , & Robinson, T. E. (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews, 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Birmaher, B. , Brent, D. A. , Chiappetta, L. , Bridge, J. , Monga, S. , & Baugher, M. (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. Journal of the American Academy of Child & Adolescent Psychiatry, 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Birnie, K. , Cooper, R. , Martin, R. M. , Kuh, D. , Sayer, A. A. , Alvarado, B. E. , … Cooper, C. (2011). Childhood socioeconomic position and objectively measured physical capability levels in adulthood: A systematic review and meta‐analysis. PloS One, 6, e15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman, J. D. , Finch, B. K. , Ellison, C. G. , Williams, D. R. , & Jackson, J. S. (2001). Neighborhood disadvantage, stress, and drug use among adults. Journal of Health and Social Behavior, 151–165. [PubMed] [Google Scholar]
- Boardman, J. D. , & Saint Onge, J. M. (2005). Neighborhoods and adolescent development. Children, Youth and Environments, 15, 138. [PMC free article] [PubMed] [Google Scholar]
- Brake, W. G. , Zhang, T. Y. , Diorio, J. , Meaney, M. J. , & Gratton, A. (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience, 19, 1863–1874. [DOI] [PubMed] [Google Scholar]
- Carver, C. S. , & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67, 319. [Google Scholar]
- Casement, M. D. , Guyer, A. E. , Hipwell, A. E. , McAloon, R. L. , Hoffmann, A. M. , Keenan, K. E. , & Forbes, E. E. (2014). Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience, 8, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda, F. , Cavanna, A. E. , D'agata, F. , Sacco, K. , Duca, S. , & Geminiani, G. C. (2011). Functional connectivity and coactivation of the nucleus accumbens: A combined functional connectivity and structure‐based meta‐analysis. Journal of Cognitive Neuroscience, 23, 2864–2877. [DOI] [PubMed] [Google Scholar]
- Chai, X. J. , Castanon, A. N. , Ongur, D. , & Whitfield‐Gabrieli, S. (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage, 59, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein, J. , Albert, D. , O'brien, L. , Uckert, K. , & Steinberg, L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science, 14, F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty, R. , Stepner, M. , Abraham, S. , Lin, S. , Scuderi, B. , Turner, N. , … Cutler, D. (2016). The association between income and life expectancy in the United States, 2001–2014. JAMA, 315, 1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, E. Y. , Yeo, B. T. , & Buckner, R. L. (2012). The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108, 2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. , Janicki‐Deverts, D. , Chen, E. , & Matthews, K. A. (2010). Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences, 1186, 37–55. [DOI] [PubMed] [Google Scholar]
- Courchesne, E. (1991). Neuroanatomic imaging in autism. Pediatrics, 87, 781–790. [PubMed] [Google Scholar]
- Cservenka, A. , Casimo, K. , Fair, D. A. , & Nagel, B. J. (2014). Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Research, 221, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden, J. , Rahbar, M. , Jezierski, L. , Li, M. , & Velie, E. (2010). The measurement of neighborhood socioeconomic characteristics and black and white residential segregation in Metropolitan Detroit: Implications for the study of social disparities in health. Annals of the Association of American Geographers, 100, 137–158. [Google Scholar]
- Dawson, G. , Ashman, S. B. , Panagiotides, H. , Hessl, D. , Self, J. , Yamada, E. , & Embry, L. (2003). Preschool outcomes of children of depressed mothers: Role of maternal behavior, contextual risk, and children's brain activity. Child Development, 74, 1158–1175. [DOI] [PubMed] [Google Scholar]
- Del Bo, A. , Ross, C. A. , Pardal, J. F. , Saavedra, J. M. , (1983a). Fastigial stimulation in rats releases adrenomedullary catecholamines. The American Journal of Physiology, 244, R801–R809. [DOI] [PubMed] [Google Scholar]
- Del Bo, A. , Sved, A. F. , (1983b). Fastigial stimulation releases vasopressin in amounts that elevate arterial pressure. The American Journal of Physiology, 244, H687–H694. [DOI] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. M. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18, 2735–2747. [DOI] [PubMed] [Google Scholar]
- Dietrichs, E. , Haines, D. E. , Roste, G. K. , & Roste, L. S. (1994). Hypothalamocerebellar and cerebellohypothalamic projections – Circuits for regulating nonsomatic cerebellar activity? Histology and Histopathology, 9, 603–614. [PubMed] [Google Scholar]
- Epstein, J. , Pan, H. , Kocsis, J. H. , Yang, Y. , Butler, T. , Chusid, J. , … Stern, E. (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry, 163, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Ernst, M. , & Fudge, J. L. (2009). A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews, 33, 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Nigg, J. T. , Iyer, S. , Bathula, D. , Mills, K. L. , Dosenbach, N. U. , … Milham, M. P. (2012). Distinct neural signatures detected for ADHD subtypes after controlling for micro‐movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience, 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, E. B. , Hyde, L. W. , Mitchell, C. , Faul, J. , Gonzalez, R. , Heitzeg, M. M. , … Schulenberg, J. (2013). What is a representative brain? Neuroscience meets population science. Proceedings of the National Academy of Sciences of the United States of America, 110, 17615–17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, M. J. (2017). The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron, 96, 56–71. [DOI] [PubMed] [Google Scholar]
- Farah, M. J. , Betancourt, L. , Shera, D. M. , Savage, J. H. , Giannetta, J. M. , Brodsky, N. L. , … Hurt, H. (2008). Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science, 11, 793–801. [DOI] [PubMed] [Google Scholar]
- Fareri, D. S. , Gabard‐Durnam, L. , Goff, B. , Flannery, J. , Gee, D. G. , Lumian, D. S. , … Tottenham, N. (2015). Normative development of ventral striatal resting state connectivity in humans. Neuroimage, 118, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. E. , Ryan, N. D. , Phillips, M. L. , Manuck, S. B. , Worthman, C. M. , Moyles, D. L. , … Dahl, R. E. (2010). Healthy adolescents' neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 162–172. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, D. J. , Hamilton, J. P. , & Gotlib, I. H. (2011). Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disorders, 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A. , Hare, T. A. , Parra, C. E. , Penn, J. , Voss, H. , Glover, G. , & Casey, B. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk‐taking behavior in adolescents. Journal of Neuroscience, 26, 6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , & Hackman, D. A. (2013). Contributions of neuroscience to the study of socioeconomic health disparities. Psychosomatic Medicine, 75, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , Horenstein, J. A. , Hariri, A. R. , Sheu, L. K. , Manuck, S. B. , Matthews, K. A. , & Cohen, S. (2008). Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience, 3, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , Kuan, D. C. , Marsland, A. L. , Sheu, L. K. , Hackman, D. A. , Miller, K. G. , & Manuck, S. B. (2017). Community socioeconomic disadvantage in midlife relates to cortical morphology via neuroendocrine and cardiometabolic pathways. Cerebral Cortex, 27, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , & Manuck, S. B. (2010). Neurobiological pathways linking socioeconomic position and health. Psychosomatic Medicine, 72, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , Manuck, S. B. , Sheu, L. K. , Kuan, D. C. , Votruba‐Drzal, E. , Craig, A. E. , & Hariri, A. R. (2011). Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex, 21, 896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, C. F. , Bradley, B. , Mercer, K. , Smith, A. K. , Conneely, K. , Gapen, M. , … Ressler, K. J. (2009). Trauma exposure and stress‐related disorders in inner city primary care patients. General Hospital Psychiatry, 31, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, B. , Gee, D. G. , Telzer, E. H. , Humphreys, K. L. , Gabard‐Durnam, L. , Flannery, J. , & Tottenham, N. (2013). Reduced nucleus accumbens reactivity and adolescent depression following early‐life stress. Neuroscience, 249, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, G. , & Harris, K. M. (2000). The mechanisms mediating the effects of poverty on children's intellectual development. Demography, 37, 431–447. [DOI] [PubMed] [Google Scholar]
- Haber, S. N. , & McFarland, N. R. (1999). The concept of the ventral striatum in nonhuman primates. Annals of the New York Academy of Sciences, 877, 33–48. [DOI] [PubMed] [Google Scholar]
- Hackman, D. A. , & Farah, M. J. (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. A. , Farah, M. J. , & Meaney, M. J. (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Albert, D. , Iselin, A.‐M. R. , Carré, J. M. , Dodge, K. A. , & Hariri, A. R. (2016). Cumulative stress in childhood is associated with blunted reward‐related brain activity in adulthood. Social Cognitive and Affective Neuroscience, 11, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, M. D. , & Chen, E. (2007). Socioeconomic status and health behaviors in adolescence: A review of the literature. Journal of Behavioral Medicine, 30, 263–285. [DOI] [PubMed] [Google Scholar]
- Harlow, H. F. , Dodsworth, R. O. , & Harlow, M. K. (1965). Total social isolation in monkeys. Proceedings of the National Academy of Sciences of the United States of America, 54, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, H. F. , & Harlow, M. (1966). Learning to love. American Scientist, 54, 244–272. [PubMed] [Google Scholar]
- Hayes, A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach. New York, NY: Guilford Press.
- Heath, R. G. (1972). Electroencephalographic studies in isolation‐raised monkeys with behavioral impairment. Diseases of the Nervous System, 33, 157–163. [PubMed] [Google Scholar]
- Jiang, Y. , Ekono, M. M. , & Skinner, C. (2016). Basic facts about low‐income children, children under 18 years, 2014. New York, NY: NCCP.
- Johns, L. E. , Aiello, A. E. , Cheng, C. , Galea, S. , Koenen, K. C. , & Uddin, M. (2012). Neighborhood social cohesion and posttraumatic stress disorder in a community‐based sample: Findings from the Detroit Neighborhood Health Study. Social Psychiatry and Psychiatric Epidemiology, 47, 1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, G. A. (1996). People and places: Contrasting perspectives on the association between social class and health. International Journal of Health Services, 26, 507–519. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Duncan, G. J. , Gennetian, L. A. , Katz, L. F. , Kling, J. R. , Sampson, N. A. , … Ludwig, J. (2014). Associations of housing mobility interventions for children in high‐poverty neighborhoods with subsequent mental disorders during adolescence. JAMA, 311, 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Keyes, K. M. , McLaughlin, K. A. , Koenen, K. C. , Goldmann, E. , Uddin, M. , & Galea, S. (2012). Child maltreatment increases sensitivity to adverse social contexts: Neighborhood physical disorder and incident binge drinking in Detroit. Drug Alcohol Depend, 122, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler, S. , Ovadia‐Caro, S. , van der Meer, E. , Villringer, A. , Heinz, A. , Romanczuk‐Seiferth, N. , & Margulies, D. S. (2013). Increased functional connectivity between prefrontal cortex and reward system in pathological gambling. PloS One, 8, e84565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas, R. , McLean, J. , Batty, G. D. , Burns, H. , Deans, K. A. , Ford, I. , … Cavanagh, J. (2013). Socioeconomic deprivation and cortical morphology: Psychological, social, and biological determinants of ill health study. Psychosomatic Medicine, 75, 616–623. [DOI] [PubMed] [Google Scholar]
- Lawson, A. , Ahima, R. S. , Krozowski, Z. , & Harlan, R. E. (1992). Postnatal development of corticosteroid receptor immunoreactivity in the rat cerebellum and brain stem. Neuroendocrinology, 55, 695–707. [DOI] [PubMed] [Google Scholar]
- Leventhal, T. , & Brooks‐Gunn, J. (2000). The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin, 126, 309. [DOI] [PubMed] [Google Scholar]
- Lidsky, T. I. , & Schneider, J. S. (2003). Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain, 126, 5–19. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Zeng, L. L. , Li, Y. , Ma, Q. , Li, B. , Shen, H. , & Hu, D. (2012). Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PloS One, 7, e39516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre, S. , & Ellaway, A. (2000). Ecological approaches: Rediscovering the role of the physical and social environment. Social Epidemiology, 9, 332–348. [Google Scholar]
- Marusak, H. A. , Martin, K. R. , Etkin, A. , & Thomason, M. E. (2015). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, W. A. , & Berkson, G. (1975). Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys: A study with artificial mothers. Developmental Psychobiology, 8, 197–211. [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Sheridan, M. A. , & Lambert, H. K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Sheridan, M. A. , Tibu, F. , Fox, N. A. , Zeanah, C. H. , & Nelson, C. A. (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences, 112, 5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, M. , Rohl, J. , Bloomfield, K. , & Grittner, U. (2012). Do neighborhoods affect individual mortality? A systematic review and meta‐analysis of multilevel studies. Social Science & Medicine (1982), 74, 1204–1212. [DOI] [PubMed] [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Mills, K. L. , Lalonde, F. , Clasen, L. S. , Giedd, J. N. , & Blakemore, S.‐J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita, Y. (1993). Inferior temporal cortex: Where visual perception meets memory. Annual Review of Neuroscience, 16, 245–263. [DOI] [PubMed] [Google Scholar]
- Moody, H. A. , Darden, J. T. , & Pigozzi, B. W. (2016). The relationship of neighborhood socioeconomic differences and racial residential segregation to childhood blood lead levels in Metropolitan Detroit. Journal of Urban Health, 93, 820–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli, S. A. , Sacchet, M. D. , & Zaki, J. (2015). Common and distinct neural correlates of personal and vicarious reward: A quantitative meta‐analysis. NeuroImage, 112, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton, E. A. , Elman, I. , Becerra, L. R. , Goldstein, R. Z. , & Borsook, D. (2014). The cerebellum and addiction: Insights gained from neuroimaging research. Addiction Biology, 19, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler, E. J. , Barrot, M. , DiLeone, R. J. , Eisch, A. J. , Gold, S. J. , & Monteggia, L. M. (2002). Neurobiology of depression. Neuron, 34, 13–25. [DOI] [PubMed] [Google Scholar]
- Nichol, M. , Janssen, I. , & Pickett, W. (2010). Associations between neighborhood safety, availability of recreational facilities, and adolescent physical activity among Canadian youth. Journal of Physical Activity and Health, 7, 442–450. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Farah, M. J. , & McCandliss, B. D. (2006a). Socioeconomic background modulates cognition‐achievement relationships in reading. Cognitive Development, 21, 349–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , … Sowell, E. R. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , McCandliss, B. D. , & Farah, M. J. (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10, 464–480. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Norman, M. F. , & Farah, M. J. (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8, 74–87. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Wolmetz, M. E. , Ochs, L. G. , Farah, M. J. , & McCandliss, B. D. (2006b). Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9, 642–654. [DOI] [PubMed] [Google Scholar]
- Pavlik, A. , & Buresova, M. (1984). The neonatal cerebellum: The highest level of glucocorticoid receptors in the brain. Brain Research, 314, 13–20. [DOI] [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Iosifescu, D. , Hallett, L. A. , Ratner, K. G. , & Fava, M. (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research, 43, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, J. N. , Roy, A. K. , Benson, B. , Carlisi, C. , Collins, P. F. , Leibenluft, E. , … Ernst, M. (2015). Age‐related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Developmental Cognitive Neuroscience, 11, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton, R. , Caspi, A. , Milne, B. J. , Thomson, W. M. , Taylor, A. , Sears, M. R. , & Moffitt, T. E. (2002). Association between children's experience of socioeconomic disadvantage and adult health: A life‐course study. The Lancet, 360, 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott, J. W. (1980). Somatosensory affectional deprivation (SAD) theory of drug and alcohol use. NIDA Research Monograph, 30, 286–296. [PubMed] [Google Scholar]
- Raizada, R. D. , & Kishiyama, M. M. (2010). Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada, R. D. , Richards, T. L. , Meltzoff, A. , & Kuhl, P. K. (2008). Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage, 40, 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan, A. , Shaw, P. W. , Lerch, J. P. , Clasen, L. S. , Greenstein, D. , Berman, R. , … Giedd, J. N. (2014). Longitudinal four‐dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences, 111, 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, D. J. , & Golanov, E. V. (1997). Autonomic and vasomotor regulation. International Review of Neurobiology, 41, 121–149. [DOI] [PubMed] [Google Scholar]
- Riva, D. , & Giorgi, C. (2000). The contribution of the cerebellum to mental and social functions in developmental age. Fiziologiia Cheloveka, 26, 27–31. [PubMed] [Google Scholar]
- Robert, S. A. (1999). Socioeconomic position and health: The independent contribution of community socioeconomic context 1. Annual Review of Sociology, 25, 489–516. [Google Scholar]
- Romens, S. E. , Casement, M. D. , McAloon, R. , Keenan, K. , Hipwell, A. E. , Guyer, A. E. , & Forbes, E. E. (2015). Adolescent girls' neural response to reward mediates the relation between childhood financial disadvantage and depression. Journal of Child Psychology and Psychiatry, 56, 1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, M. M. , Young, L. J. , Plotsky, P. M. , & Insel, T. R. (2000). Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20, 4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. (1991). An emerging concept. The cerebellar contribution to higher function. Archives of Neurology, 48, 1178–1187. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. (1998). Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences, 2, 362–371. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. (2000). The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics, 13, 189–214. [Google Scholar]
- Schulz, A. J. , Williams, D. R. , Israel, B. A. , & Lempert, L. B. (2002). Racial and spatial relations as fundamental determinants of health in Detroit. Milbank Quarterly, 80, 677–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellstrom, E. , & Bremberg, S. (2006). The significance of neighbourhood context to child and adolescent health and well‐being: A systematic review of multilevel studies. Scandinavian Journal of Public Health, 34, 544–554. [DOI] [PubMed] [Google Scholar]
- Shonkoff, J. P. , Boyce, W. T. , & McEwen, B. S. (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA, 301, 2252–2259. [DOI] [PubMed] [Google Scholar]
- Silk, J. S. , Vanderbilt‐Adriance, E. , Shaw, D. S. , Forbes, E. E. , Whalen, D. J. , Ryan, N. D. , & Dahl, R. E. (2007). Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Development and Psychopathology, 19, 841–865. [DOI] [PubMed] [Google Scholar]
- Silverman, M. H. , Jedd, K. , & Luciana, M. (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta‐analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarenios, G. , & Kovacs, M. (1999). Use of the Children's Depression Inventory. In M. Maruish, Ed. The use of psychological testing for treatment planning and outcomes assessment (2nd ed., pp. 267–298). Mahwah, NJ: Lawrence Erlbaum Associates, Publishers.
- Snider, R. S. , Maiti, A. , & Snider, S. R. (1976). Cerebellar pathways to ventral midbrain and nigra. Experimental Neurology, 53, 714–728. [DOI] [PubMed] [Google Scholar]
- Stringaris, A. , Vidal‐Ribas Belil, P. , Artiges, E. , Lemaitre, H. , Gollier‐Briant, F. , Wolke, S. , … Consortium, I. (2015). The brain's response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community‐based sample. American Journal of Psychiatry, 172, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney, J. A. , Strojwas, M. H. , Mann, J. J. , & Thase, M. E. (1998). Prefrontal and cerebellar abnormalities in major depression: Evidence from oculomotor studies. Biological Psychiatry, 43, 584–594. [DOI] [PubMed] [Google Scholar]
- Tamnes, C. K. , Østby, Y. , Fjell, A. M. , Westlye, L. T. , Due‐Tønnessen, P. , & Walhovd, K. B. (2010). Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex, 20, 534–548. [DOI] [PubMed] [Google Scholar]
- Tanner, J. M. (1962). Growth at adolescence, 2nd Ed. Oxford: Blackwell.
- Tavor, I. , Jones, O. P. , Mars, R. , Smith, S. , Behrens, T. , & Jbabdi, S. (2016). Task‐free MRI predicts individual differences in brain activity during task performance. Science, 352, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y. , Chen, Q. , Nichols, T. E. , Rasetti, R. , Callicott, J. H. , Berman, K. F. , … Mattay, V. S. (2016). Seeking optimal region‐of‐interest (ROI) single‐value summary measures for fMRI studies in imaging genetics. PloS One, 11, e0151391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni, I. , Rowe, J. , Stephan, K. E. , & Passingham, R. E. (2002). Changes of cortico‐striatal effective connectivity during visuomotor learning. Cerebral Cortex, 12, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Tsujimoto, S. , Genovesio, A. , & Wise, S. P. (2010). Evaluating self‐generated decisions in frontal pole cortex of monkeys. Nature Neuroscience, 13, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache, A. , & Noble, K. G. (2016). Neurocognitive development in socioeconomic context: Multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology, 53, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine, M. , Stoep, A. V. , Bell, J. , Rhew, I. C. , Gudmundsen, G. , & McCauley, E. (2012). Associations between household and neighborhood income and anxiety symptoms in young adolescents. Depression and Anxiety, 29, 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, A. A. , Posthumus, A. G. , Bonsel, G. J. , Steegers, E. A. , & Denktas, S. (2014). Deprived neighborhoods and adverse perinatal outcome: A systematic review and meta‐analysis. Acta Obstetricia Et Gynecologica Scandinavica, 93, 727–740. [DOI] [PubMed] [Google Scholar]
- Wadsworth, M. E. , Evans, G. W. , Grant, K. , Carter, J. S. , & Duffy, S. (2016). Poverty and the development of psychopathology. Developmental Psychopathology, 4, 1–44. [Google Scholar]
- Weiland, B. J. , Welsh, R. C. , Yau, W.‐Y. W. , Zucker, R. A. , Zubieta, J.‐K. , & Heitzeg, M. M. (2013). Accumbens functional connectivity during reward mediates sensation‐seeking and alcohol use in high‐risk youth. Drug and Alcohol Dependence, 128, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, D. G. , Schriber, R. A. , Fassbender, C. , Atherton, O. , Krafft, C. , Robins, R. W. , … Guyer, A. E. (2015). Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Developmental Cognitive Neuroscience, 16, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa, H. , Aber, J. L. , & Beardslee, W. R. (2012). The effects of poverty on the mental, emotional, and behavioral health of children and youth: Implications for prevention. American Psychologist, 67, 272. [DOI] [PubMed] [Google Scholar]


