Abstract
The effects of osmotic stress due to sorbitol on the photosynthetic machinery were investigated in the cyanobacterium Synechococcus R-2. Incubation of cells in 1.0 m sorbitol inactivated photosystems I and II and decreased the intracellular solute space by 50%. These effects of sorbitol were reversible: Photosynthetic activity and cytoplasmic volume returned to the original values after removal of the osmotic stress. A blocker of water channels prevented the osmotic-stress-induced inactivation and shrinkage of the intracellular space. It also prevented the recovery of photosynthetic activity and cytoplasmic volume when applied just before release from osmotic stress. Inhibition of protein synthesis by lincomycin had no significant effects on the inactivation and recovery processes, an observation that suggests that protein synthesis was not involved in these processes. Our results suggest that osmotic stress decreased the amount of water in the cytoplasm via the efflux of water through water channels (aquaporins), with resultant increases in intracellular concentrations of ions and a decrease in photosynthetic activity.
Cyanobacteria are prokaryotes that perform oxygenic photosynthesis (Pfenning, 1978). Cyanobacterial cells resemble the chloroplasts of higher plants in terms of membrane structure (Golden et al., 1987; Houmard and Tandeau de Marsac, 1988; Gantt, 1994), composition of membrane lipids (Murata and Wada, 1995; Nishida and Murata, 1996), and structure of the photosynthetic machinery (Anderson and Andersson, 1988; Bryant, 1991; Öquist et al., 1995). Moreover, cyanobacteria are able to acclimate to a wide range of environmental conditions (Tandeau de Marsac and Houmard, 1993; Nishida and Murata, 1996; Hagemann and Erdmann, 1997). These characteristics suggest that cyanobacteria should be a good model for studies of molecular mechanisms of stress responses in higher plants.
The responses of cyanobacteria to salt stress have been investigated in some detail. Under high-salt conditions, the Na+/H+ antiporter is activated in Synechococcus sp. PCC 6311 (Blumwald et al., 1984). Stimulation of respiration has been observed in Synechococcus sp. PCC 6311, Anacystis nidulans, and Synechocystis sp. PCC 6803 (Fry et al., 1986; Molitor et al., 1986; Jeanjean et al., 1990). Accumulation under high-salt conditions of compatible solutes such as Suc, trehalose, Gly betaine, and glucosylglycerol has often been found in association with tolerance to salt stress in cyanobacteria (Reed and Stewart, 1988; Joset et al., 1996; Hagemann and Erdmann, 1997; Hayashi and Murata, 1998; Papageorgiou et al., 1998). The synthesis de novo of a number of proteins under salt stress has been observed in several species (Bhagwat and Apte, 1989; Hagemann et al., 1990, 1991). Our recent work indicates that unsaturation of the fatty acids in membrane lipids plays an important role in the tolerance of the photosynthetic machinery to salt stress in Synechocystis sp. PCC 6803 (Allakhverdiev et al., 1999). However, salt stress usually has both ionic and osmotic effects and, in most earlier studies, these effects have not been examined separately.
In the present study, we investigated the effects of osmotic stress on the photosynthetic machinery, focusing on the role of water channels, in the cyanobacterium Synechococcus R-2. We found that osmotic stress due to sorbitol decreased the cytoplasmic volume via the efflux of water through water channels (aquaporins). The subsequent increase in the intracellular concentration of ions might have been the cause of the concomitant inactivation of the photosynthetic machinery.
MATERIALS AND METHODS
Growth Conditions and Exposure of Cells to Osmotic Stress
A strain of Synechococcus R-2 (that corresponds to Synechococcus sp. PCC 7942) was obtained from Dr. W.E. Borrias (University of Utrecht, The Netherlands). Cells were grown photoautotrophically in glass tubes (80 mL) at 32°C under constant illumination at 70 μE m−2 s−1 from incandescent lamps in BG-11 medium (Stanier et al., 1971) supplemented with 20 mm 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-NaOH (pH 7.5). Cultures were aerated with sterile air that contained 1% CO2 (Ono and Murata, 1981). After 4 d, cells were harvested by centrifugation at 9,000g for 10 min and resuspended in fresh BG-11 medium (pH 7.5) at a density of 10 μg chlorophyll (Chl) mL−1. Cells were incubated with shaking every 15 min at 32°C in light at 70 μE m−2 s−1 in the presence of 1.0 m sorbitol or in its absence. After designated periods of time, a portion of each suspension of cells was withdrawn. The cells were washed twice with BG-11 medium by centrifugation at 9,000g for 10 min and resuspension, and then finally suspended in BG-11 medium.
Preparation of Thylakoid Membranes
Thylakoid membranes were isolated from cells as described previously (Mamedov et al., 1991) with minor modifications. A 100-mL suspension of cells with a cell density corresponding to 30 μg Chl mL−1 was centrifuged at 9,000g for 10 min. The pelleted cells were resuspended in 50 mL of a solution that contained 50 mm HEPES-NaOH (pH 7.5) and 20 mm CaCl2 and the suspension was centrifuged at 9,000g for 10 min. Finally, cells were suspended in 40 mL of 50 mm HEPES-NaOH (pH 7.5) that contained 1.0 m Gly betaine, 800 mm sorbitol, 10 mm CaCl2, and 1 mm 6-amino-n-caproic acid. The above procedures were performed at 25°C, but all subsequent steps were performed at 4°C. The suspension of cells was passed through a French pressure cell (SLM Instruments, Urbana, IL) at 160 MPa, and the resultant homogenate was centrifuged at 6,000g for 10 min to remove unbroken cells and cell debris. The supernatant was centrifuged at 20,000g for 30 min and the sedimented thylakoid membranes were suspended in 4 mL of a solution containing 50 mm HEPES-NaOH (pH 7.5), 400 mm Suc, 10 mm CaCl2, and 1 mm 6-amino-n-caproic acid.
Measurement of Electron Transport Activities
The electron transport activities of PSII and PSI in intact cells were measured at 32°C by monitoring the light-induced evolution and uptake of oxygen, respectively, with a Clark-type oxygen electrode (Hansatech Instruments, Kings Lynn, UK). Actinic light, at 2 mE m−2 s−1 at the surface of the cuvette, was obtained by passage of light from an incandescent lamp through a red optical filter (R-60; Toshiba, Tokyo) and an infrared-absorbing filter (HA-50, Hoya Glass, Tokyo). The oxygen-evolving activity of PSII was measured in the presence of 1.0 mm 1,4-benzoquinone (BQ) or 1.0 mm 2,6-dichloro-1,4-benzoquinone (DCBQ) as electron acceptors. The electron transport activity of PSI was determined in the presence of 15 μm DCMU, 5 mm ascorbate, 0.1 mm 2,6-dichloroindophenol (DCIP), and 0.1 mm methyl viologen (MV).
The light-induced reduction of DCIP by thylakoid membranes was determined at 25°C in a reaction mixture that contained 50 mm HEPES-NaOH (pH 7.5), 400 mm Suc, and 5 mm CaCl2 by monitoring changes in A580 with a reference beam of light at 500 nm in a dual-wavelength spectrophotometer (model UV-300, Shimadzu, Kyoto) as described previously (Allakhverdiev et al., 1999).
The transport of electrons from the reduced form of DCIP to MV by thylakoid membranes was measured at 25°C by monitoring the uptake of oxygen with the Clark-type oxygen electrode in the same reaction mixture as described above after the addition of 15 μm DCMU, 5 mm ascorbate, 0.1 mm DCIP, and 0.1 mm MV. Red actinic light at 1.2 mE m−2 s−1 at the surface of the cuvette was obtained by passage of light from an incandescent lamp through the two optical filters mentioned above.
The yield of Chl fluorescence from intact cells was measured with a pulse amplitude modulation fluorometer (PAM-101, Walz, Effeltrich, Germany). The initial fluorescence of Chl (Fo) was determined after excitation with dim light at 650 nm and 10 μE m−2 s−1, which was modulated at 600 Hz. The maximum yield of fluorescence (Fmax) was determined after the addition of continuous actinic light at 2 mE m−2 s−1 (Schreiber et al., 1993). Concentrations of Chl were determined as described by Arnon et al. (1974).
Measurement of Cell Volume
Cell volume (cytoplasmic volume) was determined by electron paramanetic resonance (EPR) spectrometry as described previously (Blumwald et al., 1983). For measurements, cells were harvested and resuspended at 400 μg Chl mL−1 in a solution of 1.0 mm 2,2,6,6-tetramethyl-4-oxopiperidinooxy free radical (TEMPO, a spin probe), 20 mm K3[Fe(CN)6]3, and 75 mm Na2Mn-EDTA. TEMPO that was oxidized by K3[Fe(CN)6]3 penetrated the plasma membrane rapidly and reached an equilibrium in all phases of the suspension of cells. The addition of the paramagnetic quencher Na2Mn-EDTA, which cannot permeate the plasma membrane, broadened the EPR signals from everywhere except from within the plasma membrane-bound space. The internal volume of cells could be calculated from the difference between the EPR spectra obtained with the cells and the control. The cells were enclosed in a sealed-glass capillary (i.d., 0.02 cm) in a final volume of 40 μL and EPR spectra were recorded at room temperature in an EPR spectrometer (model ER 200-D, Bruker, Karlsruhe, Germany). The EPR signal from the 40-μL capillary was measured with 1.0 mm TEMPO alone as controls. The measurements were made in darkness under the following conditions: 100 kHz field modulation at a microwave frequency of 11.72 GHz; a modulation amplitude of 0.4 mT; microwave power of 10 mW; a time constant of 80 ms; and a scan rate of 0.4 G s−1.
Uptake of Sorbitol by Cells
To examine the possible uptake of sorbitol by cells, cells at a concentration that corresponded to 100 μg Chl mL−1 were incubated with 1.0 m sorbitol plus 18.5 kBq mL−1 [U-14C]sorbitol (equivalent to 1.7 nm; Amersham-Pharmacia Biotech, Tokyo). At designated times, a portion of the cell suspension (equivalent to 20 μg of Chl) was withdrawn and vacuum filtered on a glass fiber filter (i.d., 3 cm). Cells on the filter were then rinsed with 6 mL of 1.0 m sorbitol in BG-11, and the remaining radioactivity on the filter was determined with a scintillation counter.
RESULTS
Sorbitol-Induced Inactivation of Photosynthetic Activity in Vivo
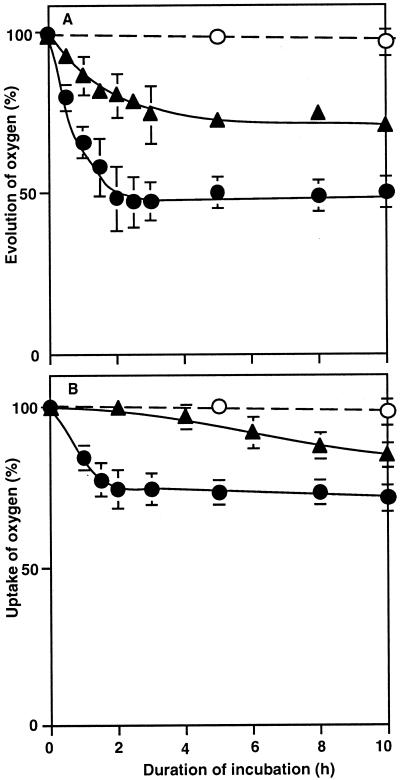
We examined the effects of osmotic stress on the activities of PSII and PSI in intact cells by monitoring the evolution and uptake of oxygen, respectively. During incubation with 1.0 m sorbitol for 2 h, the oxygen-evolving activity of PSII in the presence of BQ declined to 50% of the original level and stayed at the same low level for 10 h (Fig. 1A). When we used DCBQ as an artificial acceptor of electrons, we obtained essentially the same results (data not shown).
Figure 1.
Effects of sorbitol and a water-channel blocker on the photosynthetic electron-transport activities of PSII and PSI in intact cells. Cells were incubated in the presence of 1.0 m sorbitol and 100 μm p-chloromercuriphenyl-sulfonic acid (the water-channel blocker) or in their absence. At designated times, a portion of each cell suspension was withdrawn and the activities of PSII and PSI were determined. A, The oxygen-evolving activity of PSII was examined after the addition of 1.0 mm BQ to the suspension. The activity that corresponded to 100% was 533 ± 38 μmol O2 mg−1 Chl h−1. B, The electron-transport activity of PSI was determined by monitoring the uptake of oxygen at 32°C after the addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm ascorbate, and 0.1 mm MV to the suspension. The rate of oxygen uptake that corresponded to 100% was 353 ± 52 μmol O2 mg−1 Chl h−1 in the absence of sorbitol and p-chloromercuriphenyl-sulfonic acid (○); in the presence of 1.0 m sorbitol (●); and in the presence of 1.0 m sorbitol and 100 μm p-chloromercuriphenyl-sulfonic acid (▴). Each point represents the average with se of results from four independent experiments.
The transport of electrons by PSI from the reduced form of DCIP to MV declined to 70% of the original rate during incubation with 1.0 m sorbitol for 2 h, and the activity was barely affected during a further 8-h incubation (Fig. 1B). These results indicated that osmotic stress decreased the activities of both PSII and PSI in the cyanobacterial cells.
Effects of a Water-Channel Blocker
We next examined the effects of a water-channel blocker on the sorbitol-induced inactivation of PSII and PSI in cells. The water-channel blocker p-chloromercuriphenyl-sulfonic acid at 100 μm (Pfeuffer et al., 1998; Tyerman et al., 1999, and refs. therein) effectively protected the oxygen-evolving activity of PSII against the sorbitol-induced inactivation. After incubation with 1.0 m sorbitol for 10 h, the oxygen-evolving activity remained at 75% of the original level in the presence of the water-channel blocker (Fig. 1A). Suc and mannitol at 1.0 m also decreased the oxygen-evolving activity of PSII as sorbitol did, and the water-channel blocker protected PSII against the inactivation (data not shown). The water-channel blocker also markedly suppressed the sorbitol-induced inactivation of PSI (Fig. 1B). These findings suggested that the water-channel blocker had protected PSII and PSI against osmotic-stress-induced inactivation.
Reversibility of Sorbitol-Induced Inactivation
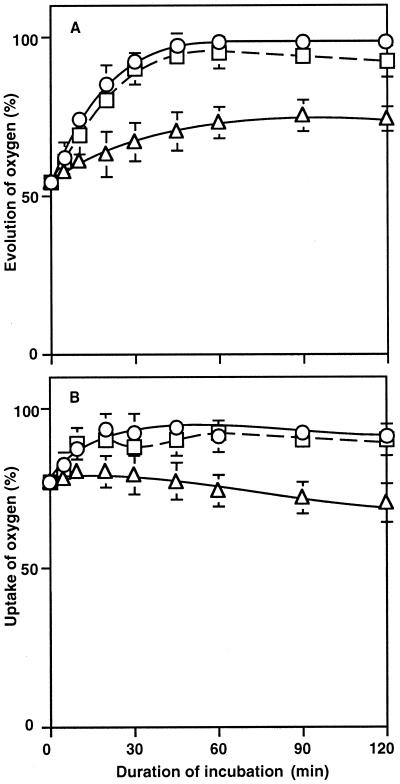
To examine the recovery of the activities of PSII and PSI after removal of sorbitol from the medium, cells were incubated with 1.0 m sorbitol for 2 h and then washed with BG-11 medium as described in “Materials and Methods.” When these cells were incubated in the absence of sorbitol, the activities of PSII and PSI returned to the original levels within 1 h (Fig. 2).
Figure 2.
Recovery of PSII and PSI in intact cells after removal of sorbitol. After cells had been incubated for 2 h with 1.0 m sorbitol, they were washed twice with fresh BG-11 medium and incubated in the presence of either lincomycin or p-chloromercuriphenyl-sulfonic acid and in the absence of these chemicals. At designated times, a portion of each cell suspension was withdrawn and the activities of PSII and PSI were determined. A, The oxygen-evolving activity of PSII was examined after the addition of 1.0 mm BQ to the suspension. The activity that corresponded to 100% was 544 ± 38 μmol O2 mg−1 Chl h−1. B, The activity of PSI was determined by monitoring the uptake of oxygen after the addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm ascorbate, and 0.1 mm MV to the suspension. The rate of oxygen uptake that corresponded to 100% was 364 ± 52 μmol O2 mg−1 Chl h−1 in the absence of both chemicals (○); in the presence of 100 μm p-chloromercuriphenyl-sulfonic acid (▵); in the presence of 100 μg lincomycin mL−1 (□). Each point represents the average with se of results from four independent experiments.
Lincomycin, an inhibitor of protein synthesis, had no effect on the recovery of the activities of PSII and PSI (Fig. 2). Therefore, the recovery process appeared not to involve the synthesis of proteins de novo. By contrast, the water-channel blocker significantly suppressed the recovery of both PSII and PSI. It seems likely that water channels were involved not only in the sorbitol-induced inactivation but also in the recovery from inactivation.
Sorbitol-Induced Changes in Chl Fluorescence
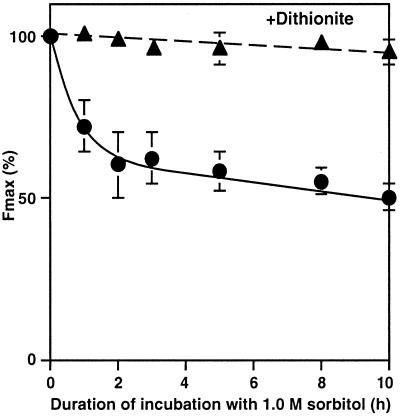
To identify the site of inactivation in PSII by osmotic stress, we examined changes in the maximum fluorescence of Chl (Fmax) during incubation of cells in the presence of sorbitol. Fmax declined during incubation with 1.0 m sorbitol (Fig. 3) and the pattern of the decline was similar to that in the oxygen-evolving activity (see Fig. 1A). When dithionite was added to reduce QA, the primary electron acceptor of the PSII complex, Fmax returned to the original level. This result suggested that the site of sorbitol-induced inactivation was the electron-donating side of PSII and not the photochemical reaction center. This suggestion was supported by the observation that addition of DCMU increased Fmax to a level similar to that observed after the addition of dithionite (data not shown).
Figure 3.
Changes in the yield of Chl fluorescence in intact cells during incubation with 1.0 m sorbitol. At designated times, a portion of each cell suspension was withdrawn and kept in darkness at 32°C for 15 min; the maximum fluorescence of Chl (Fmax) was then determined at 25°C before and after the addition of 1 mg mL−1 dithionite. ●, No dithionite; ▴, after addition of dithionite. Each point represents the average with se of results from three independent experiments.
Effects of Sorbitol and the Water-Channel Blocker on Cell Volume
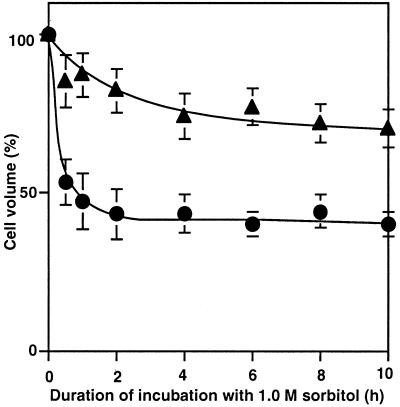
We analyzed changes in cell volume (cytoplasmic volume) during incubation with sorbitol by monitoring EPR (see “Materials and Methods”). The cell volume prior to exposure of cells to osmotic stress was 0.75 ± 0.05 fL per cell. When cells were incubated in 1.0 m sorbitol, cell volume fell to 50% of the original level within 30 min and remained at this low level for 10 h (Fig. 4). In the presence of 100 μm p-chloromercuriphenyl-sulfonic acid, the decrease in cell volume was limited to about 20%.
Figure 4.
Changes in cell volume during incubation in the presence of sorbitol. Cells were incubated with 1.0 m sorbitol in the presence of 100 μm p-chloromercuriphenyl-sulfonic acid and in its absence. At designated times, a portion of each cell suspension was withdrawn and the cell volume was determined from measurements of EPR. The cell volume that corresponded to 100% was 0.75 ± 0.05 fL per cell in the absence of p-chloromercuriphenyl-sulfonic acid (●); in the presence of 100 μm p-chloromercuriphenyl-sulfonic acid (▴). Each point represents the average with se of results from four independent experiments.
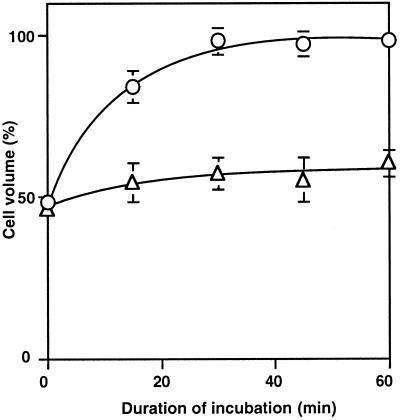
When cells were released from osmotic stress, cell volume returned to its original value within 30 min (Fig. 5), but the water-channel blocker markedly inhibited such recovery. These results indicated that the osmotic stress due to sorbitol had shrunk cells reversibly and that the water-channel blocker prevented both shrinkage and the recovery from shrinkage.
Figure 5.
Recovery of cell volume after removal of sorbitol. After cells had been incubated for 2 h with 1.0 m sorbitol, they were washed twice with fresh BG-11 medium and then incubated in the presence and absence of 100 μm p-chloromercuriphenyl-sulfonic acid. At designated times, a portion of each cell suspension was withdrawn, and the cell volume was determined as described in the text in the absence of p-chloromercuri-phenyl-sulfonic acid (○); in the presence of 100 μm p-chloromercuriphenyl-sulfonic acid (▵). Each point represents the average with se of results from four independent experiments.
Effects of Sorbitol on PSII- and PSI-Mediated Transport of Electrons in Vitro
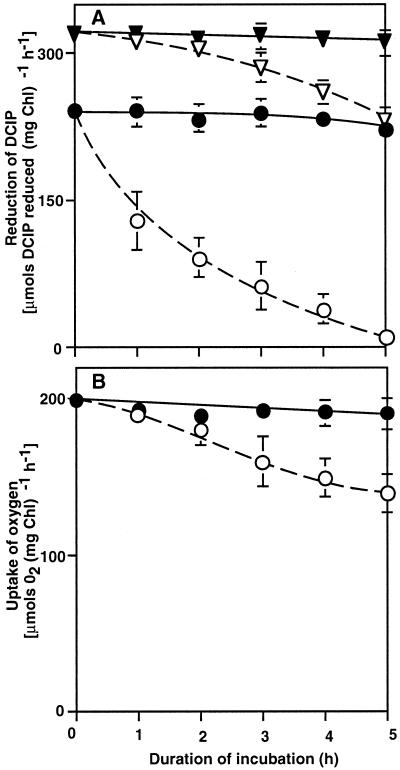
We examined the direct effects of sorbitol on the PSII- and PSI-mediated transport of electrons in isolated thylakoid membranes. When thylakoid membranes were incubated at 32°C in the absence of sorbitol, the PSII-mediated transport of electrons from water to DCIP was completely lost within 5 h (Fig. 6A). The presence of 1.0 m sorbitol in the reaction mixture completely protected this electron transport system from inactivation. The transport of electrons from diphenylcarbazide (DPC) to DCIP was inhibited in the absence of sorbitol but to a much lesser extent than electron transport from water to DCIP. The presence of 1.0 m sorbitol also fully protected the system for transport of electrons from DPC to DCIP. These results indicated that the presence of 1.0 m sorbitol stabilized the oxygen-evolving machinery on the donor side of PSII in isolated thylakoid membranes during prolonged incubation at 32°C.
Figure 6.
Changes in electron-transport activities in isolated thylakoid membranes during incubation in the presence and absence of sorbitol. Thylakoid membranes at a Chl concentration of 10 μg mL−1 were incubated at 32°C in darkness in the presence of 1.0 m sorbitol and in its absence. At designated times, a portion of each suspension of membranes was withdrawn and the activities of PSII and PSI were determined. A, The transport of electrons by PSII from water to DCIP (○, ●) and from DPC to DCIP (▵, ▴) was monitored by following the light-induced reduction of DCIP after the addition of 0.1 mm DCIP and of 0.1 mm DCIP plus 0.5 mm DPC, respectively. B, The transport of electrons by PSI from the reduced form of DCIP to MV was monitored by following the light-induced uptake of oxygen after addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm ascorbate, and 0.1 mm MV to the suspension. Black symbols, In the presence of 1.0 m sorbitol; white symbols, in the absence of sorbitol. Each point represents the average with se of results from three independent experiments.
During incubation of thylakoid membranes for 5 h in the absence of sorbitol, PSI activity decreased by 30% (Fig. 6B). However, the presence of 1.0 m sorbitol completely protected this activity. These results revealed the totally opposite effects of sorbitol on photosynthetic electron transport activities in vivo and in vitro: in intact cells, sorbitol inhibited the activities of both PSI and PSII; in isolated thylakoid membranes, sorbitol protected both PSI and PSII from decay over time.
Effects of the Water-Channel Blocker on the Uptake of Sorbitol
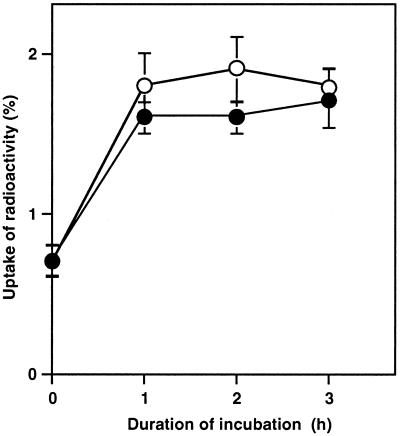
The above-mentioned observations strongly suggested that in intact cells, the water-channel blocker protected PSI and PSII against inactivation due to osmotic stress by decreasing the water-permeating activity of water channels. However, it also seemed possible that the water-channel blocker would have modified water channels so that they were permeable to sorbitol, and that the sorbitol that was influxed into the cytoplasm protected PSI and PSII against inactivation. Thus, we examined the effect of the water-channel blocker on the uptake of 14C-labeled sorbitol by cells. The result in Figure 7 revealed that cells incorporated sorbitol to some extent, but this incorporation was not affected by the presence of the water-channel blocker. These results suggested that sorbitol might be incorporated in the periplasmic space but not in the cytoplasm, and that water channels were impermeable to sorbitol in the presence of the water-channel blocker.
Figure 7.
Effects of a water-channel blocker on the uptake of sorbitol by Synechococcus R-2 cells. Cells at 100 μg Chl mL−1 were incubated with 1.0 m sorbitol plus [U-14C]sorbitol (18.5 kBq mL−1, 1.7 nm) in the presence of 100 μm p-chloromercuriphenyl-sulfonic acid (●) or in its absence (○). At designated times a portion of cell suspension was withdrawn and the uptake of radioactivity in cells was determined as described in “Materials and Methods.” Uptake of radioactivity is expressed as the radioactivity remaining on the glass-fiber filter divided by the radioactivity supplied as a percentage. Each value represents the average with se of results from three independent experiments.
The latter statement was confirmed by another set of experiments using 2-mercaptoethanol that inhibits the effect of p-chloromercuriphenyl-sulfonic acid on water channels (Maggio and Joly, 1995; Tyerman et al., 1999, and refs. therein). When cells were incubated with 1.0 m sorbitol for 2.5 h in the absence of the water-channel blocker, the oxygen-evolving activity decreased to one-half the original level (Table I). The presence of the water-channel blocker diminished the inactivation. A further addition of 2-mercaptoethanol removed this effect of p-chloromercuriphenyl-sulfonic acid (Table I). These results suggested that sorbitol did not penetrate the cytoplasm membrane even in the presence of the water-channel blocker.
Table I.
Effects of 2-mercaptoethanol (ME) and a water-channel blocker on the sorbitol-induced inactivation of PSII
| Addition | Oxygen-Evolving Activity
|
|
|---|---|---|
| −ME | +ME | |
| μmol mg−1 Chl h−1 | ||
| Control (no addition) | 557 ± 42 (100) | 551 ± 39 (99) |
| 1.0 m Sorbitol | 251 ± 26 (45) | 240 ± 18 (43) |
| 1.0 m Sorbitol + 100 μm pCMPSA | 457 ± 33 (82) | 256 ± 21 (46) |
Cells were incubated for 2.5 h in the presence of 1.0 m sorbitol and 100 μm p-chloromercuriphenyl-sulfonic acid (pCMPSA, a water-channel blocker) or in their absence, and then incubated in the presence of 500 μm 2-mercaptoethanol or in its absence for 10 min, and the oxygen-evolving activity of PSII was measured as described in the legend to Figure 1. Each value represents the average ± se of results from four independent experiments. Values in parentheses are relative activities.
DISCUSSION
We investigated the effects of osmotic stress on photosynthetic activity in Synechococcus R-2 using sorbitol as the external osmoticum. This polyol did not penetrate the plasma membrane (Fig. 7; Table I). Osmotic stress due to 1.0 m sorbitol inactivated the oxygen-evolving machinery of PSII and disrupted the electron-transport activity of PSI in intact cells (Fig. 1). The effect on PSII was greater than that on PSI (Fig. 1). These effects of sorbitol were observed in intact cells but not in isolated thylakoid membranes. In the latter, sorbitol effectively protected both PSI and PSII from inactivation during prolonged incubation at 32°C (Fig. 6).
We examined the oxygen-evolving activity of intact cells in the presence of BQ as an artificial acceptor of electrons (Figs. 1 and 2). In this system, electrons are transported from water to BQ through the Mn cluster, P680 (a form of Chl at the photochemical reaction center), pheophytin a, the primary electron acceptor of plastoquinone (QA) and the secondary electron acceptor of plastoquinone (QB). Analysis of Chl fluorescence provided a clue to the site that was affected by osmotic stress in intact cells (Fig. 3). During incubation with sorbitol, the extent of the reduction of QA decreased. However, QA was fully reduced when dithionite was added to sorbitol-treated cells. These findings suggest that the photochemical reaction center complex that includes QA, pheophytin, and P680 was undamaged by osmotic stress due to sorbitol. Therefore, it is likely that the transport of electrons from water to P680 was inhibited as a consequence of the osmotic stress.
Osmotic stress due to 1.0 m sorbitol decreased the cytoplasmic space by about 50% (Fig. 4). This shrinkage was completed during incubation of cells for 30 min, indicating that the shrinkage occurred more rapidly than the inactivation of PSI and PSII, which required about 2 h (Fig. 1). Thus, the first event after the exposure of cells to 1.0 m sorbitol might be the efflux of water through water channels in the plasma membrane. We verified this possibility by showing that shrinkage could be prevented, not fully but to a considerable extent, by the water-channel blocker p-chloromercuriphenyl-sulfonic acid (Fig. 4). Shrinkage might have increased the concentrations of major intracellular ions such as K+ and Cl−, leading to the gradual inactivation of PSI and PSII. We demonstrated previously that an increase in the ambient ionic concentration inactivates the oxygen-evolving PSII complex in vitro (Miyao and Murata, 1983; Murata and Miyao, 1985).
When cells were released from osmotic stress, photosynthetic activity and intracellular volume returned to normal (Figs. 2 and 5). The recovery of intracellular volume was faster than that of the PSII activity: 30 versus 60 min. The water-channel blocker markedly suppressed the sorbitol-induced inactivation of the photosynthetic machinery, shrinkage of the cytoplasm, and recovery after release from osmotic stress (Figs. 1, 2, 4, and 5). The correspondence between intracellular volume and photosynthetic activity suggests that the efflux of water through water channels during osmotic stress might have increased the concentration of ions in the cytoplasm and this increase might have been responsible for the reversible inactivation. Each cell of Synechococcus sp. PCC 6301, which is very similar to Synechococcus R-2, contains approximately 200 mm intracellular K+ ions (Ono and Murata, 1981). Moreover, it has been demonstrated that the intracellular concentration of K+ ions in Synechocystis sp. PCC 6714 increases upon addition of sorbitol to the medium (Reed and Stewart, 1985).
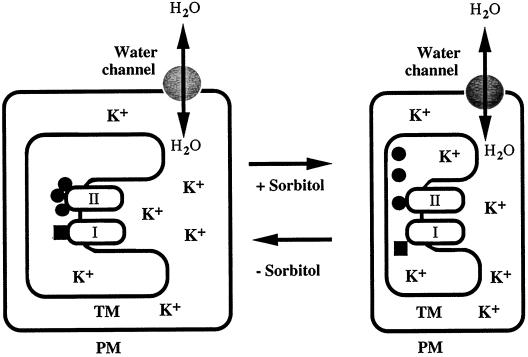
Figure 8 shows a hypothetical model that explains the osmotic stress-induced inactivation of the photosynthetic machinery and its recovery from inactivation. Water channels are assumed to be present in the plasma membrane, even though no gene for a water channel has been identified in Synechococcus R-2. However, a gene for a putative water channel has been found in the genome of Synechocystis sp. PCC 6803 (Kaneko et al., 1996).
Figure 8.
A hypothetical schematic explanation of the osmotic stress-induced inactivation of PSI and PSII in cyanobacterial cells. ●, Three extrinsic proteins, namely, the 33-kD protein, Cyt c550, and PsbU, of the oxygen-evolving machinery of PSII; ▪, plastocyanin or Cyt c553 associated with PSI. PM, Plasma membrane; TM, thylakoid membrane; I, PSI complex; II, PSII complex.
The oxygen-evolving machinery of the PSII complex is located on the luminal side of thylakoid membranes. In cyanobacteria, this machinery is stabilized by three extrinsic proteins, a 33-kD protein, Cyt c550, and PsbU (Enami et al., 1998; Shen et al., 1998; Nishiyama et al., 1999). Among these proteins, Cyt c550 and PsbU are loosely bound to the donor side of the core complex of PSII (Nishiyama et al., 1997, 1999).
In our model, when the extracellular osmotic pressure increases, the water in the intracellular space leaves the cell through the water channels and the cytoplasmic concentration of K+ ions increases. This increase leads, in turn, to an increase in the concentration of K+ ions in the intrathylakoid space and, as a result, the oxygen-evolving machinery is partially inactivated by dissociation of the extrinsic proteins. A similar mechanism can be postulated for the osmotic-stress-induced inactivation of PSI. An increase in the intrathylakoid concentration of K+ ions results in the dissociation of plastocyanin or Cyt c553 from the PSI complex, which causes partial inactivation of the PSI-mediated transport of electrons. When the cell is released from the osmotic stress, water enters through the water channels and the cytoplasmic concentration of K+ ions decreases. The intrathylakoid concentration of K+ ions then also decreases and, as a result, the integrity of PSII and PSI is restored by the renewed binding of the extrinsic proteins to these complexes.
Footnotes
This work was financially supported in part by a Grant-in-Aid for Specially Promoted Research (no. 08102011 to N.M.) from the Ministry of Education, Science and Culture, Japan, and by the National Institute for Basic Biology Cooperative Research Program on the Stress Tolerance of Plants.
LITERATURE CITED
- Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N. Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc Natl Acad Sci USA. 1999;96:5862–5867. doi: 10.1073/pnas.96.10.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Andersson B. The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci. 1988;13:352–355. doi: 10.1016/0968-0004(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae: I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bhagwat AA, Apte SK. Comparative analysis of proteins induced by heat shock, salinity, and osmotic stress in the nitrogen-fixing cyanobacterium Anabaena sp. strain L-31. J Bacteriol. 1989;171:5187–5189. doi: 10.1128/jb.171.9.5187-5189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Mehlhorn RJ, Packer L. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc Natl Acad Sci USA. 1983;80:2599–2602. doi: 10.1073/pnas.80.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Wolosin JM, Packer L. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem Biophys Res Commun. 1984;122:452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- Bryant DA. Cyanobacterial phycobilisomes: progress towards complete structural and functional analysis via molecular genetics. In: Bogorad L, Vasil IK, editors. Cell Culture and Somatic Cell Genetics of Plants. 7B. San Diego: Academic Press; 1991. pp. 257–300. [Google Scholar]
- Enami I, Kikuchi S, Fukuda T, Ohta H, Shen JR. Binding and functional properties of four extrinsic proteins of photosystem II from a red alga Cyanidium caldarium, as studied by release-reconstitution experiments. Biochemistry. 1998;37:2787–2793. doi: 10.1021/bi9724624. [DOI] [PubMed] [Google Scholar]
- Fry JV, Huflejt M, Erber WWA, Peschek GA, Packer L. The role of respiration during adaptation of the freshwater cyanobacterium Synechococcus 6311 to salinity. Arch Biochem Biophys. 1986;244:686–691. doi: 10.1016/0003-9861(86)90637-5. [DOI] [PubMed] [Google Scholar]
- Gantt E. Supramolecular membrane organization. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 119–138. [Google Scholar]
- Golden SS, Brusslan J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Hagemann M, Erdmann N. Environmental stresses. In: Rai AK, editor. Cyanobacterial Nitrogen Metabolism and Environmental Biotechnology. New Delhi, India: Narosa Publishing House; 1997. pp. 156–221. [Google Scholar]
- Hagemann M, Techel D, Rensing L. Comparison of salt- and heat-induced alterations of protein synthesis in the cyanobacterium Synechocystis sp. PCC 6803. Arch Microbiol. 1991;155:587–592. [Google Scholar]
- Hagemann M, Wolfel L, Kruger B. Alterations of protein synthesis in the cyanobacterium Synechocystis sp. PCC 6803 after a salt shock. J Gen Microbiol. 1990;136:1393–1399. [Google Scholar]
- Hayashi H, Murata N. Genetically engineered enhancement of salt tolerance in higher plants. In: Sato K, Murata N, editors. Stress Responses of Photosynthetic Organisms. Molecular Mechanisms and Molecular Regulation. Amsterdam: Elsevier; 1998. pp. 133–148. [Google Scholar]
- Houmard J, Tandeau de Marsac N. Cyanobacterial genetic tools: current status. Methods Enzymol. 1988;167:808–847. doi: 10.1016/0076-6879(88)67092-3. [DOI] [PubMed] [Google Scholar]
- Jeanjean R, Onana B, Peschek GA, Joset F. Mutants of the cyanobacterium Synechocystis sp. PCC 6803 impaired in respiration and unable to tolerate high salt concentrations. FEMS Microbiol Lett. 1990;68:125–130. [Google Scholar]
- Joset F, Jeanjean R, Hagemann M. Dynamics of the response of cyanobacteria to salt stress: deciphering the molecular events. Physiol Plant. 1996;96:738–744. [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly RJ. Effects of mercuric chloride on the hydraulic conductivity of tomato root systems: evidence for a channel-mediated water pathway. Plant Physiol. 1995;109:331–335. doi: 10.1104/pp.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedov MD, Hayashi H, Wada H, Mohanty PS, Papageorgiou GC, Murata N. Glycinebetaine enhances and stabilizes the evolution of oxygen and the synthesis of ATP by cyanobacterial thylakoid membranes. FEBS Lett. 1991;294:271–274. doi: 10.1016/0014-5793(91)81446-f. [DOI] [PubMed] [Google Scholar]
- Miyao M, Murata N. Partial disintegration and reconstitution of the photosynthetic oxygen-evolution system: binding of 24 kD and 18 kD polypeptides. Biochim Biophys Acta. 1983;725:87–93. [Google Scholar]
- Molitor V, Erber WWA, Peschek GA. Increased levels of cytochrome-c-oxidase and sodium antiporter in the plasma membrane of Anacystis nidulans after growth in sodium-enriched media. FEBS Lett. 1986;204:251–255. [Google Scholar]
- Murata N, Miyao M. Extrinsic membrane proteins in the photosynthetic oxygen-evolving complex. Trends Biochem Sci. 1985;10:122–124. [Google Scholar]
- Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Los DA, Hayashi H, Murata N. Thermal protection of the oxygen-evolving machinery by PsbU, an extrinsic protein of photosystem II in Synechococcus sp. PCC 7002. Plant Physiol. 1997;115:1473–1480. doi: 10.1104/pp.115.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Los DA, Murata N. PsbU, a protein associated with photosystem II, is required for the acquisition of cellular thermotolerance in Synechococcus sp. PCC 7002. Plant Physiol. 1999;120:301–308. doi: 10.1104/pp.120.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Murata N. Chilling susceptibility of the blue-green alga Anacystis nidulans: effect of growth temperature. Plant Physiol. 1981;67:176–182. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öquist G, Campbell D, Clarke A, Gustafsson P. The cyanobacterium Synechococcus modulates photosystem II function in response to excitation stress through D1 exchange. Photosynth Res. 1995;46:151–158. doi: 10.1007/BF00020425. [DOI] [PubMed] [Google Scholar]
- Papageorgiou GC, Alygizaki-Zorba A, Ladas N, Murata N. A method to probe the cytoplasmic osmolality and osmotic water and solute fluxes across the cell membrane of cyanobacteria with chlorophyll a fluorescence: experiments with Synechococcus sp. PCC 7942. Physiol Plant. 1998;103:215–224. [Google Scholar]
- Pfenning N. General physiology and ecology of photosynthetic bacteria. In: Clayton RK, Sistrom WR, editors. The Photosynthetic Bacteria. New York: Plenum Press; 1978. pp. 3–18. [Google Scholar]
- Pfeuffer J, Flogel U, Leibfritz D. Monitoring of cell volume and water-exchange time in perfused cells by diffusion-weighted 1H NMR spectroscopy. NMR Biomed. 1998;11:11–18. doi: 10.1002/(sici)1099-1492(199802)11:1<11::aid-nbm498>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Reed RH, Stewart WDP. Evidence for turgor-sensitive K+ influx in the cyanobacteria Anabaena variabilis ATCC 29413 and Synechocystis PCC 6714. Biochim Biophys Acta. 1985;812:155–162. [Google Scholar]
- Reed RH, Stewart WDP. The responses of cyanobacteria to salt stress. In: Rogers LJ, Gallan JR, editors. Biochemistry of the Algae and Cyanobacteria. Vol. 12. Oxford: Clarendon Press; 1988. pp. 217–231. [Google Scholar]
- Schreiber U, Neubauer C, Schliwa U. PAM fluorometer based on medium frequency pulsed Xe-flash measuring light: a highly sensitive new tool in basic and applied photosynthesis research. Photosynth Res. 1993;36:65–72. doi: 10.1007/BF00018076. [DOI] [PubMed] [Google Scholar]
- Shen J-R, Qian M, Inoue Y, Burnap RL. Functional characterization of Synechocystis sp. PCC 6803 ΔpsbU and ΔpsbV mutants reveals important roles of cytochrome c-550 in cyanobacterial oxygen evolution. Biochemistry. 1998;37:1551–1558. doi: 10.1021/bi971676i. [DOI] [PubMed] [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N, Houmard J. Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiol Rev. 1993;104:119–190. [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot. 1999;50:1055–1071. [Google Scholar]