SUMMARY
Vitamin B12 functions as a cofactor for methionine synthase to produce the anabolic methyl donor S-adenosylmethionine (SAM) and for methylmalonyl-CoA mutase to catabolize the short-chain fatty acid propionate. In the nematode Caenorhabditis elegans, maternally supplied vitamin B12 is required for the development of offspring. However, the mechanism for exporting vitamin B12 from the mother to the offspring is not yet known. Here, we use RNAi of more than 200 transporters with a vitamin B12-sensor transgene to identify the ABC transporter MRP-5 as a candidate vitamin B12 exporter. We show that the injection of vitamin B12 into the gonad of mrp-5 deficient mothers rescues embryonic lethality in the offspring. Altogether, our findings identify a maternal mechanism for the transit of an essential vitamin to support the development of the next generation.
In Brief
How dietary vitamin B12 is transported from the mother to developing offspring is unknown. Na et al. demonstrate that the ABC transporter MRP-5 (multidrug resistance protein 5) transports vitamin B12 from the intestine of C. elegans mothers to the offspring to promote embryonic viability and development
INTRODUCTION
Maternal micronutrient status during pregnancy greatly affects embryonic development and fetal health (Dror and Allen, 2008; Fall et al., 2003; Owens and Fall, 2008; Skjærven et al., 2016). For instance, the intake of folate supplements by pregnant women reduces the occurrence of neural tube defects in newborns (Czeizel and Dudás, 1992; Viswanathan et al., 2017). Folate functions together with vitamin B12, or cobalamin, in the one carbon cycle to recycle methionine from homocysteine (Moreno-Garcia et al., 2013) (Figure 1A). One of the main functions of the one-carbon cycle is to produce S-adenosylmethionine (SAM), a major methyl donor required to synthesize phosphatidylcholine, an important component of cellular membranes, and to modify histones and DNA to ensure proper genomic functions (Rosenblatt and Whitehead, 1999). The other function of vitamin B12 is as a cofactor in the breakdown of the short-chain fatty acid propionate (Shchelochkov et al., 2016) (Figure 1A). Humans obtain vitamin B12 through the ingestion of animal products in which this stable micronutrient is efficiently stored (Martens et al., 2002). Deficiency of vitamin B12 in newborns is mostly caused either by maternal depletion or malabsorption, although rare recessive inborn errors of vitamin B12 processing or utilization have also been identified (Rosenblatt and Cooper, 1990; Rosenblatt and Whitehead, 1999).
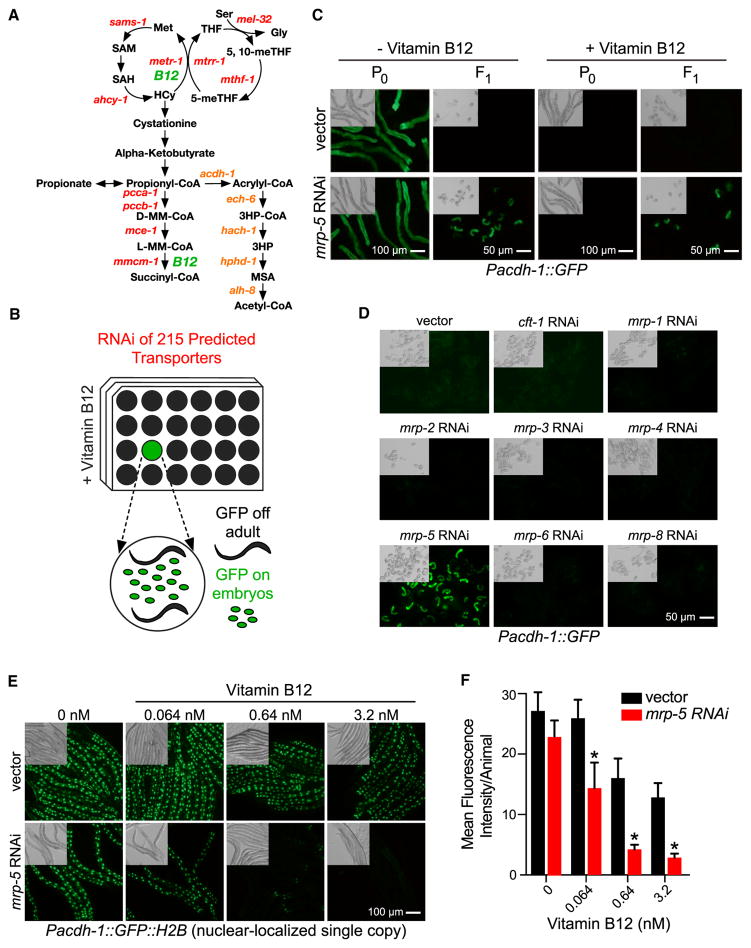
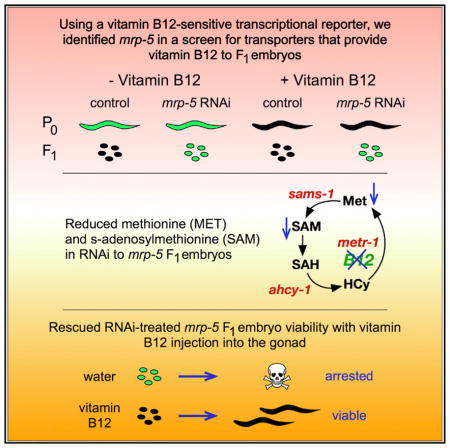
Figure 1. A C. elegans Transporter RNAi Screen Identifies mrp-5 as a Potential Vitamin B12 Exporter.
(A) Cartoon of the two vitamin-B12-dependent metabolic pathways. Gene names are from C. elegans. 3HP, 3-hydroxypropionate; 5-meTHF, 5-methyltetrahydrofolate; 5,10-meTHF, 5,10-methylenetetrahydrofolate; D-MM-CoA, D-methylmalonyl-CoA; Gly, glycine; HCy, homocysteine; L-MM-CoA, L-methylmalonyl-CoA; Met, methionine; MSA, malonic semialdehyde; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate. The acdh-1 branch in orange text indicates the vitamin-B12-independent propionate shunt.
(B) Diagram of C. elegans transporter RNAi library screen. RNAi-treated animals that displayed GFP fluorescence in the F1, but not the P0, generation in the presence of 64 nM vitamin B12 were considered hits.
(C) Fluorescence and differential interference contrast (DIC) microscopy images of Pacdh-1::GFP animals subjected to RNAi of mrp-5 in E. coli HT115 compared to vector control at P0 and F1 generations in the presence or absence of 64 nM vitamin B12. Scale bars, 50 μm for embryos and 100 μm for adults.
(D) Fluorescence and DIC microscopy images of Pacdh-1::GFP animals subjected to RNAi of mrp family members in E. coli HT115 compared to control RNAi in the F1 generation. An RNAi clone for mrp-7 was not available. Scale bar, 50 μm.
(E) Fluorescence microscopy images of single-copy insertion Pacdh-1::GFP::H2B animals subjected to mrp-5 RNAi in E. coli OP50 compared to control RNAi with low-dose titration of vitamin B12. Scale bar, 100 μm.
(F) Images from (E) were quantitated using ImageJ to determine the mean fluorescence intensity per animal. At least five animals were analyzed per condition. Error bars represent SD. The p value was determined by unpaired multiple t tests, with a false discovery rate of 1% (*p < 0.0001).
See also Figures S1 and S2.
Recently, the nematode Caenorhabditis elegans has been established as a powerful model for studying vitamin B12-dependent processes (Yilmaz and Walhout, 2014). C. elegans is a bacterivore that can be fed individual bacterial species and strains that can elicit different effects on the animal’s life history traits and gene expression. For instance, when fed Comamonas aquatica DA1877 bacteria, the animals develop faster, have reduced fecundity, and have a shorter lifespan relative to animals fed the standard laboratory diet of Escherichia coli OP50 (MacNeil et al., 2013; Watson et al., 2013). These dietary differences are due, at least in part, to the fact that Comamonas synthesizes vitamin B12, whereas E. coli does not (Watson et al., 2014). Dietary vitamin B12 is required to support C. elegans development, as the animal itself cannot synthesize this cofactor (Bito et al., 2013; Watson et al., 2014). Vitamin B12 is ingested by the mother and needs to be exported from the intestine into the gonad to support the development of her offspring. However, the mechanism by which this occurs is not yet known. Here, we report that the ABC transporter mrp-5 controls vitamin B12 export from the intestine to support C. elegans embryonic development.
RESULTS
An RNAi Screen Identifies the ABC Transporter MRP-5 as a Candidate to Export Vitamin B12 from the C. elegans Intestine
We have previously established the Pacdh-1::GFP transgenic C. elegans strain as a reporter of dietary vitamin B12 status: in this strain, GFP is highly expressed when vitamin B12 is low and repressed when levels of this micronutrient are high (Arda et al., 2010; MacNeil et al., 2013; Watson et al., 2014; Watson et al., 2016). GFP expression in this strain is under the control of the promoter of the acyl-coenzyme A (CoA) dehydrogenase acdh-1. This gene encodes the first enzyme of an alternate propionate breakdown pathway, or propionate shunt, which does not require vitamin B12 and is transcriptionally activated when this cofactor is in low supply (Watson et al., 2016) (Figure 1A). We reasoned that a defect in vitamin B12 transport from the mother to her offspring would result in parental generation (P)0 animals with low levels of GFP in the presence of vitamin B12 and F1 embryos with high levels of GFP expression. Further, these embryos would be expected to have developmental defects, because vitamin B12 is required to produce SAM, which is needed to generate biomass and, thus, support development (Watson et al., 2014). We performed RNAi knockdown of 215 predicted C. elegans transporters of the ABC transporter and solute carrier families by feeding animals E. coli HT115 bacteria expressing double-stranded RNA in the presence of 64 nM vitamin B12 (Figure 1B). We identified a single gene, mrp-5, that, when knocked down, resulted in mothers with low levels of GFP expression and dead embryos with high GFP expression (Figure 1C). mrp-5 encodes an ABC transporter belonging to the multi-drug resistance protein (MRP) family (Korolnek et al., 2014). MRP proteins are highly similar, and the C. elegans genome encodes nine family members (http://www.wormbase.org). To test for specificity, we retested eight of the nine members of this family for which RNAi clones were available and found that only mrp-5 RNAi caused activation of GFP expression in the F1 generation (Figure 1D). We then titrated vitamin B12 from 6.4 nM to 6.4 μM and found that GFP expression remains activated in mrp-5 RNAi embryos even under very high vitamin B12 conditions (Figure S1A).
MRP-5 is expressed in the intestine (Korolnek et al., 2014) (Figure S2). If mrp-5, indeed, encodes a transporter that exports vitamin B12 from the C. elegans intestine to support embryonic development, this would lead to the prediction that higher levels of vitamin B12 should be retained in the intestine when the transporter is perturbed. To test this prediction, we devised a highly sensitive experimental setup by using (1) a single-copy Pacdh-1::GFP::H2B strain that expresses GFP in the nucleus, enabling easier monitoring of differences in GFP levels, and (2) an RNAi-compatible strain of E. coli OP50 (Xiao et al., 2015), because animals fed this diet express higher levels of GFP than those fed E. coli HT115 (Figure S1B). Importantly, RNAi of mrp-5 is equally efficient in E. coli HT115 and E. coli OP50, as measured by qRT-PCR (Figure S1C). We found that much lower concentrations of vitamin B12 were sufficient to repress intestinal GFP in mrp-5 RNAi animals compared to vector control animals (Figures 1E and 1F). This finding suggests that vitamin B12 is retained in the intestine when mrp-5 is knocked down. Together, these findings indicate that mrp-5 encodes a transporter that exports vitamin B12 from the intestine of the mother to support embryonic development of her offspring.
Injection of Vitamin B12 into the Gonad Rescues Embryonic Lethality Caused by Loss of mrp-5
We predicted that direct supplementation of vitamin B12 into the gonad should bypass the requirement for vitamin B12 export from the intestine and rescue embryonic lethality caused by perturbation of mrp-5. To test this prediction, we injected either vitamin B12 or water (vehicle) as a control into the gonad of Pacdh-1::GFP animals that were exposed to mrp-5 or vector control RNAi. If the vitamin B12 injection into the gonad bypassed MRP-5-dependent export, we expected to observe live animals with low GFP expression, while injecting water would lead to dead embryos with high GFP expression (Figure 2A). For this experiment, we used the E. coli OP50 RNAi strains we generated (see also Figure 1E), because transgenic Pacdh-1::GFP embryos express high levels of GFP, while they express low GFP levels when fed E. coli HT115 bacteria (Figure S1). Using E. coli OP50 thus enabled us to observe a decrease in GFP expression, while using E. coli HT115 would not.
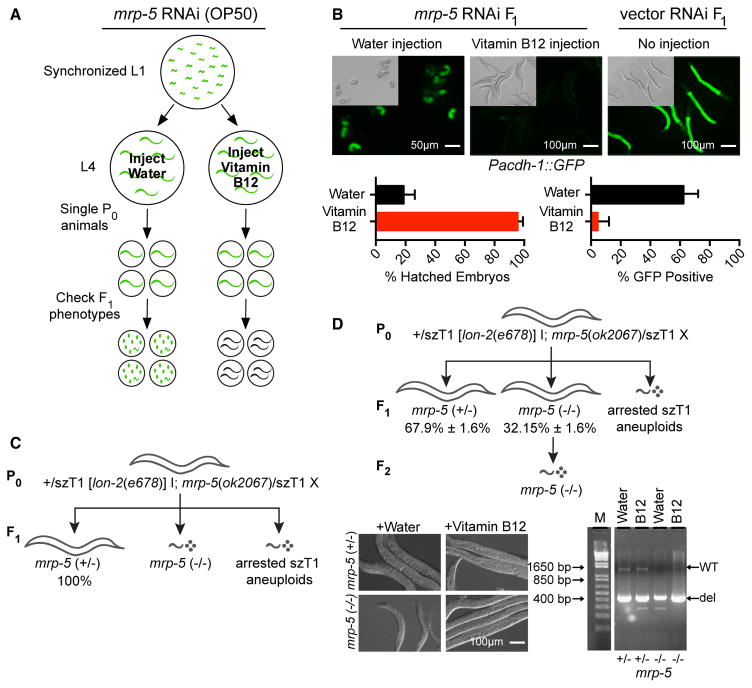
Figure 2. Injection of Vitamin B12 into the Gonad of mrp-5-Deficient Mothers Rescues Lethality in the Offspring.
(A) Experimental design. Briefly, either 3.2 mM vitamin B12 or water (vehicle) as a control was injected into the gonad of Pacdh-1::GFP mothers that were exposed to mrp-5 or vector control RNAi. If the vitamin B12 injection rescues embryonic lethality, live offspring will show low GFP expression, while water-injected animals will show dead embryos with high GFP expression.
(B) Top: DIC and fluorescence microscopy images of embryos from Pacdh-1::GFP mothers subjected to mrp-5 RNAi in E. coli OP50 injected into the gonad with water (left panel) or with 3.2 mM vitamin B12 (middle panel). Animals treated with vector control RNAi without injection are shown as a control (right panel). Scale bars, 50 μm for embryos and 100 μm for adults. Bottom: quantification of lethality and GFP expression in embryos from Pacdh-1::GFP animals described above. Error bars represent SD of the average of three independent experiments.
(C) Diagram of genotypes and phenotypes of the offspring of genetically balanced mrp-5 (+/−) mutant animals. All P0 adult animals are heterozygous for mrp-5. F1 larval arrest or embryonic lethality occurs in mrp-5 (−/−) or szT1 aneuploid animals.
(D) Top: diagram of genotypes and phenotypes of viable offspring from genetically balanced mrp-5 (+/−) mutant animals after vitamin B12 injection. All P0 adult animals are heterozygous for mrp-5. Live F1 animals can be either heterozygous or homozygous for the mrp-5(ok2067) deletion allele. The average percentage and SD of the total number of L1–L2 animals is listed below the genotype. F2 larval arrest or embryonic lethality occurs in all offspring of the F2 generation of mrp-5 (−/−) animals. Bottom left: DIC microscopy images of mrp-5 (+/−) and mrp-5 (−/−) animals with or without vitamin B12 injection. Scale bar, 100 μm. Bottom right: agarose gel image of the genotyping results of heterozygous and homozygous mrp-5 L1–L2 animals with and without injection of vitamin B12.
Injection of vitamin B12 into the gonad of mrp-5 RNAi animals fully and specifically rescued the embryonic viability of their offspring (Figure 2B). In addition, in Pacdh-1::GFP transgenic animals exposed to mrp-5 RNAi, injecting vitamin B12 into the gonad resulted in developed larvae with low levels of GFP, while injection of water resulted in dead embryos with high GFP expression (Figure 2B). We recapitulated these findings with mrp-5(ok2067) deletion mutant animals. Since mrp-5 is an essential gene, the mrp-5 deletion allele is maintained genetically balanced with +/svT1[lon-2(e678)] (Figure 2C). Heterozygous mrp-5 (+/−) mothers injected with vitamin B12 into their gonad produced mrp-5 (−/−) offspring that survived to the adult stage, while animals injected with vehicle control produced mrp-5 (−/−) animals that arrested at the L1–L2 stage (Figure 2D). Importantly, the offspring of the rescued F1 mrp-5 (−/−) animals again exhibited larval arrest, suggesting that, in the absence of mrp-5, vitamin B12 cannot be passed on to the next (F2) generation. Altogether, these findings show that vitamin B12 injected into the gonad of a mrp-5 (+/−) mother can rescue embryonic lethality in her offspring.
Heme Rescues Embryonic Lethality in mrp-5 RNAi Animals
A previous study proposed that mrp-5 encodes a heme transporter (Korolnek et al., 2014). This finding was supported by the observation that embryonic lethality in mrp-5 RNAi animals could be rescued by feeding the animals high doses of heme (Korolnek et al., 2014). How could either feeding of heme or injection of vitamin B12 rescue embryonic lethality of mrp-5 RNAi or mutant animals? ABC transporters are known to be capable of transporting numerous similar types of molecules (Lee and Rosenbaum, 2017; Locher, 2016; Slot et al., 2011). Heme and vitamin B12 share some structural similarities; heme is an iron-containing porphyrin, while vitamin B12 is related to porphyrins and contains cobalt as a metal ion (Figure 3A). Thus, it is possible that MRP-5 transports both molecules. However, if lack of heme and vitamin B12 explains the embryonic lethality of mrp-5 RNAi animals, one would expect that neither cofactor alone would be sufficient for rescue. Our observation that embryonic lethality can be rescued by injection of vitamin B12 into the gonad indicates that lack of vitamin B12 rather than heme may be the cause of embryonic lethality when MRP-5 function is perturbed. To further examine the effects of heme on animal development, we repeated the experiment published in the previous study (Korolnek et al., 2014) and confirmed that feeding 500 μM heme can rescue embryonic lethality in the offspring of mrp-5 RNAi animals (Figure 3B). Interestingly, however, such a high dose of heme was toxic to developing control animals, arresting most animals at the L1–L2 stage, whereas high doses of vitamin B12 were not toxic (Figure S3). Importantly, one can argue that feeding heme does not actually test for transport, because the cofactor would be stuck in the intestine when mrp-5 is perturbed, which would, therefore, not result in the rescue of embryonic development in the offspring.
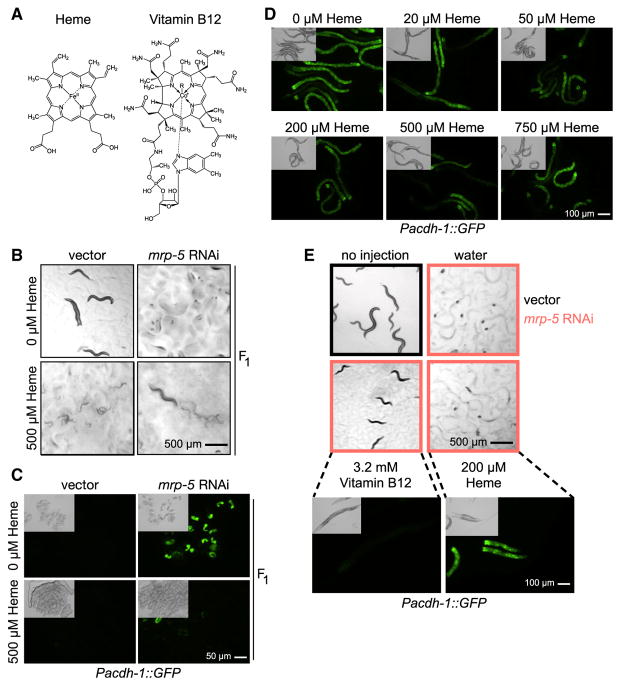
Figure 3. High Concentrations of Heme Rescue Embryonic Lethality in mrp-5 RNAi Animals but Do Not Affect acdh-1 Promoter Activity.
(A) Heme and vitamin B12 are porphyrin or porphyrin-like molecules with structural similarities.
(B) Bright-field images of feeding 500 μM heme to mrp-5 RNAi-treated mothers on E. coli HT115 rescues viability in her offspring and allows them to develop to the L2 stage and later but causes L1 larval arrest and toxicity in vector-treated mothers. 24 singled mothers plus their offspring were analyzed for each condition. Scale bar, 500 μm.
(C) Fluorescence and DIC microscopy images show that feeding 500 μM heme to mrp-5 RNAi-treated mothers on E. coli HT115 reduces Pacdh-1::GFP reporter activity in embryos but not in the embryos of vector control mothers. 50 L1 mothers were seeded onto 6-cm plates, and F1 embryos were collected for imaging. Scale bar, 50 μm.
(D) Fluorescence and DIC microscopy images demonstrate that feeding heme to P0 mothers with E. coli OP50 does not affect Pacdh-1::GFP reporter activity. Scale bar, 100 μm.
(E) Bright-field (top) and fluorescence and DIC microscopy images (bottom) shows that injection of 200 μM heme into mrp-5 RNAi-treated mothers with E. coli HT115 does not rescue embryonic lethality or larval arrest in the offspring, while injection of vitamin B12 leads to viable offspring and repression of the acdh-1 promoter. Scale bars, 500 μm for bright-field microscopy and 100 μm for fluorescence microscopy.
See also Figure S3.
One explanation for the rescue of embryonic development by feeding heme is that this increases vitamin B12 passage through intestinal membranes. Therefore, we next asked whether heme affects the vitamin-B12-responsive Pacdh-1 reporter. We supplemented increasing concentrations of heme and found that the reporter was unaffected by heme in adult animals (Figure 3D). As noted earlier, embryos from Pacdh-1::GFP mothers treated with mrp-5 RNAi without supplemented heme expressed high levels of GFP, suggesting that vitamin B12 cannot enter the embryos and repress the reporter. Importantly, however, GFP levels were greatly reduced in embryos from Pacdh-1::GFP/mrp-5 RNAi animals supplemented with 500 μM heme (Figure 3C). These findings suggest that supplying a high dose of heme facilitates the export of vitamin B12 from the intestine and, therefore, that the cause for embryonic lethality is lack of vitamin B12 and not heme. Indeed, we found that, while injection of vitamin B12 into the gonad rescued embryonic development in Pacdh-1::GFP/mrp-5 RNAi animals, direct injection of 200 μM heme did not rescue. Further, the arrested L1 larvae from Pacdh-1::GFP/mrp-5 RNAi animals injected with 200 μM heme expressed high levels of GFP, indicating low vitamin B12 levels (Figure 3E).
Methionine and SAM Content Is Reduced in Offspring from mrp-5 RNAi Mothers
Although vitamin B12 is a cofactor for two different metabolic enzymes (Figure 1A), our previous studies demonstrated that the developmental delay caused by vitamin B12 deficiency is mainly due to its function as a cofactor for the methionine synthase enzyme METR-1, which generates the methyl donor SAM from methionine (Watson et al., 2014). To determine whether the lack of vitamin B12 or the lack of heme in mrp-5-deficient animals results in defects in the SAM cycle, we measured the levels of methionine and SAM in embryos from mothers treated with mrp-5 or vector control RNAi. We reasoned that differences in methionine and SAM content would be most clear in the presence of supplemented vitamin B12, because the E. coli diet is naturally low in this cofactor. Indeed, we detected a reduced methionine and SAM content in embryos from mothers treated with mrp-5 RNAi relative to control animals in the presence of vitamin B12 (Figures 4A and 4B). Further, SAM levels could be restored to wild-type levels by feeding the animals with 500 μM heme. These results suggest that a defect in the SAM cycle caused by the lack of vitamin B12 in mrp-5-deficient embryos contributes to embryonic lethality.
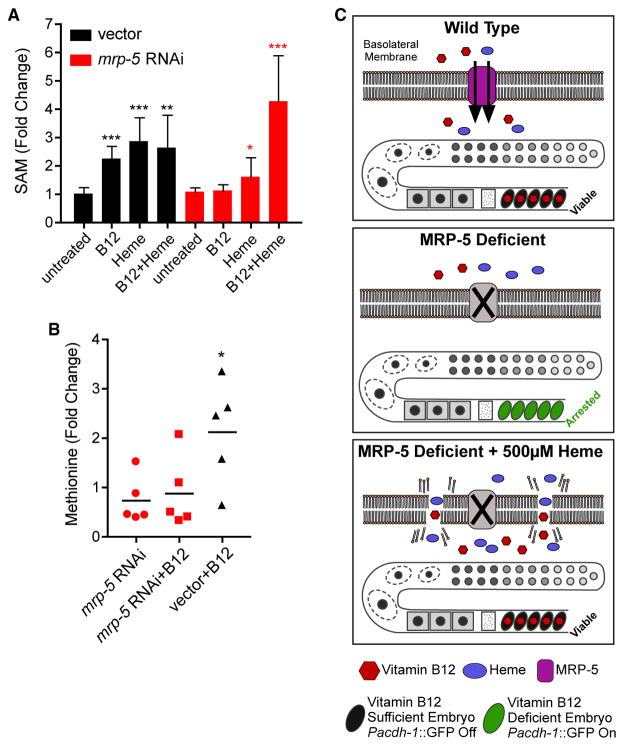
Figure 4. Methionine and S-Adenosylmethionine Content Is Reduced in Offspring from Mothers Treated with mrp-5 RNAi.
(A) SAM content was measured in the embryos of vector or mrp-5 RNAi-treated mothers on E. coli HT115. SAM content was greatly reduced in mrp-5-deficient embryos compared to vector control. Error bars represent SD of the average of three independent experiments. The p value was determined by Student’s t test relative to untreated embryos (*p < 0.05; **p < 0.001; ***p < 0.0001).
(B) Methionine content was measured by GC-MS of vector control or mrp-5 RNAi-treated mothers fed E. coli HT115 with and without 64 nM vitamin B12 supplementation. Each dot represents a biological replicate, and values are relative to vector RNAi without vitamin B12 supplementation. The p value was determined by Student’s t test (*p < 0.05).
(C) Model of MRP-5 function. In wild-type animals, both vitamin B12 and heme are exported from the intestine by MRP-5. Vitamin B12 can enter the embryo and repress the acdh-1 reporter (top). In the absence of MRP-5, neither vitamin B12 nor heme can cross the intestinal membrane, the embryos arrest, and the acdh-1 reporter remains active (middle). The presence of 500 μM heme causes membrane permeability in mrp-5-deficient mothers, leading to vitamin B12 crossing the intestine membrane, entering the embryo to rescue lethality, and repressing the acdh-1 promoter (bottom).
DISCUSSION
We identified MRP-5 as a candidate transporter that exports vitamin B12 from the C. elegans intestine of the mother to support the development of her offspring. MRP-5 is a member of the C. elegans ABC transporter family, and these transporters are known to function as exporters of metabolites and drugs (Locher, 2016; Sheps et al., 2004; Slot et al., 2011). MRP-5 has previously been reported to export heme in C. elegans (Korolnek et al., 2014). C. elegans can synthesize neither vitamin B12 nor heme and depends on dietary supplementation of both cofactors. Loss or reduction of mrp-5 by RNAi knockdown or by genetic mutation results in embryonic or larval lethality. As reported previously, we confirm that this lethality can be rescued by feeding the mother high concentrations of heme. However, it can also be rescued by injecting vitamin B12 directly into the gonad, bypassing the need for import from the intestine and, perhaps, other tissues. Wild-type C. elegans requires only low doses of heme (20 μM) (Rao et al., 2005), and high doses of heme are toxic to many systems (Chiabrando et al., 2014). Indeed, supplementing wild-type animals with a dose of 500 μM heme was toxic to C. elegans as well, whereas high doses of vitamin B12 were not (Figures 3B and S3). One mechanism of heme toxicity is by enhanced membrane permeability (Chiabrando et al., 2014). Therefore, we propose that supplementing mrp-5 RNAi animals with high concentrations of heme leads to increased intestinal membrane permeability, enabling both heme and vitamin B12 to pass through without the need for a dedicated transporter (Figure 4C).
More than 15 human genes involved in vitamin B12 processing have been identified (Nielsen et al., 2012), and four of these encode transporters. One of these is the ABC transporter MRP-1 (or ABCC1), which is localized to intestinal epithelial cells. ABC transporters in human and C. elegans are highly similar in sequence and structure. Therefore, we propose that C. elegans MRP-5 may be a functional ortholog of human MRP1 to export vitamin B12 from the intestine to surrounding tissues.
EXPERIMENTAL PROCEDURES
C. elegans Strains
All C. elegans strains were maintained at standard laboratory conditions as described previously (Brenner, 1974), and strain N2 was used as wild-type. Construction of VL749 (wwIs24 [Pacdh-1::GFP + unc-119(+)]) strain has been previously described (Arda et al., 2010). Strain VL1168 (wwSi1[Pacdh-1::GFP::H2B unc-119(+)] II;avr-14(ad1302) I; unc-119(ed3) III; avr-15(ad1051); glc-1(pk54) V) was generated by mos1-mediated single copy insertion (Frøkjaer-Jensen et al., 2008). Strain wwls47 [mrp5p::MRP-5::GFP + rol-6(+)] was generated by fusion PCR (http://www.wormbook.org). The mrp-5 mutant strain VC1599 (+/szT1 [lon-2(e678)] I; mrp-5(ok2067)/szT1 X) was obtained from the C. elegans Genetics Center (CGC). Bacterial strains E. coli OP50, HT115, and Comamonas aquatica DA1877 were obtained from CGC. The E. coli OP50 RNAi-compatible bacterial strain was generously provided by the Xu lab (Xiao et al., 2015).
RNAi Screen
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to predict C. elegans ABC transporters and solute carrier transporters (Kanehisa et al., 2016). The transporter RNAi mini-library was generated by selecting 215 clones from both the ORFeome and Ahringer RNAi libraries (Kamath et al., 2003; Rual et al., 2004). RNAi screening was carried out on 24-well nematode growth medium (NGM) agar plates containing 2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), 50 μg/mL ampicillin with or without 64 nM Ado-Cbl (vitamin B12). Pacdh-1::GFP animals were grown on regular NGM plates with E. coli OP50 without Ado-Cbl supplementation for several generations before harvesting embryos. Embryos were incubated in M9 buffer overnight to obtain synchronized L1 animals. Approximately 15 L1 animals were added to each well containing individual RNAi clones. Animal phenotypes were observed after 80 hr of incubation, when the F1 generation was at the young adult stage.
Quantitation of Fluorescence
The animal body was outlined, and fluorescence intensity of the animal was determined using ImageJ (NIH). At least five animals per condition were analyzed, and the mean fluorescence per animals was determined.
C. elegans qRT-PCR Experiments
Animals were synchronized by L1 arrest and grown on ampicillin and IPTG plates seeded with either E. coli HT115 (vector RNAi and mrp-5 RNAi) or E. coli OP50 (vector RNAi and mrp-5 RNAi). Approximately 1,000 adult animals were harvested for each condition, in biological duplicate. Animals were washed in M9 buffer, and total RNA was isolated using TRIzol (Invitrogen) followed by DNase I treatment and cleanup using QIAGEN RNeasy columns. cDNA was generated from 1 μg RNA using random primer and M-MuLV reverse transcriptase (New England Biolabs). qPCR was performed in technical duplicates per gene per condition using the Applied Biosystems StepOnePlus Real-Time PCR System and the Fast SYBR Green Master Mix (Thermo Fisher Scientific). Relative transcript abundance was determined using the ΔΔCt method and normalized to averaged ama-1 mRNA expression levels.
Vitamin B12 Injections
Animals were treated with mrp-5 RNAi generated in an E. coli OP50 RNAi compatible strain. Vitamin B12 (3.2 mM) was injected into the gonad of L4 animals using a Narishige microinjection arm attached to the body of a Nikon Eclipse Ti inverted microscope. Sterile water was injected into the control group. After injection, animals were singled and treated with the same RNAi (mrp-5 or vector). Phenotypes were scored after 2 days. mrp-5(ok2067) heterozygotes were singled at the L4 stage. Vitamin B12 was injected as described earlier. After injection, animals were singled, and the phenotype was scored after 5 days.
SAM Measurement
SAM was measured using the Bridge-It S-Adenosyl Methionine Fluorescence Assay Kit purchased from Mediomics following the manufacturer’s recommendations.
Heme Solution Preparation
Hemin is the oxidized form of heme and must be dissolved in acid to make the active form of heme. 10 mM hemin solution was prepared as described previously (Hickman and Winston, 2007). 6.5 mg/mL hemin (Sigma-Aldrich) was dissolved in 0.1 M NaOH and incubated at 37°C for 1 hr. 1 M Tris (pH 7.5) was then added to a final concentration of 0.1 M. The pH was adjusted using HCl, and the hemin solution was stored at 4°C, protected from light, and used within 2 days.
Relative Quantification of Methionine Using Gas Chromatography-Mass Spectrometry
Approximately 150,000 embryos were homogenized with 0.5 mL 200- to 300-μm acid-washed glass beads (Sigma-Aldrich) in 1 mL 80% methanol using a FastPrep-24 bead beater (MP Biomedicals), with intermittent cooling in dry ice/ethanol bath. Samples were then extracted on dry ice for 15 min and centrifuged for 10 min at 20,000 × g, and 250 μL supernatant was dried under vacuum using a SpeedVac concentrator SPD111V (Thermo Fisher Scientific). Derivatization of dried samples was performed first with 20 μL 20 mg/mL methoxyamine hydrochloride (Sigma-Aldrich) in pyridine at 37°C for 90 min, followed by the addition of 50 μL N-methyl-N-(trimethylsilyl)trifluoroacetamide (Sigma-Aldrich) and incubation for 3 hr at 37°C. The derivitazation reaction was completed by incubation for 5 hr at room temperature. Measurements were performed on an Agilent 7890B/5977B single quadrupole GC-MS (gas chromatography-mass spectrometry) equipped with an HP-5ms Ultra Inert capillary column (30 m × 0.25 mm × 0.25 μm). The inlet temperature was set to 230°C, the transfer line was at 280°C, and the MS source and quadrupole were at 230°C and 150°C, respectively. The trimethylsilyl derivative of methionine was quantified as a 176-m/z ion with two qualifier ions of 128 and 293 m/z. Relative quantification of peak areas was done using samples within a linear response range, after mean normalization to total metabolites and blank subtraction.
Supplementary Material
Highlights.
ABC transporter MRP-5 transports vitamin B12 from mother to offspring in C. elegans
mrp-5 mutant embryonic lethality is rescued by vitamin B12 injection into gonad
RNAi of mrp-5 reduces S-adenosylmethionine and methionine content in F1 embryos
Acknowledgments
We thank members of the Walhout lab and Amy Walker for discussion and critical reading of the manuscript. We thank Amy Holdorf for manuscript editing and Shawn Xu for the E. coli OP50 RNAi strain. This work was supported by a grant from the NIH (DK068429) to A.J.M.W. Some bacterial and nematode strains used in this work were provided by the CGC, which is funded by the NIH Office or Research Infrastructure Programs (P40 OD010440).
Footnotes
Supplemental information includes Supplemental Experimental Procedures and three figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.02.100.
DECLARATION OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization, H.N. and A.J.M.W.; Investigation, H.N. and O.P.; Methodology, H.N.; Resources, G.E.G.; Writing – Original Draft, H.N. and A.J.M.W.; Writing – Review & Editing; H.N. and A.J.M.W.; Visualization, H.N. and A.J.M.W.; Funding Acquisition, A.J.M.W.; Supervision, A.J.M.W.
References
- Arda HE, Taubert S, Conine C, Tsuda B, Van Gilst MR, Sequerra R, Doucette-Stam L, Yamamoto KR, Walhout AJM. Functional modularity of nuclear hormone receptors in a C. elegans gene regulatory network. Mol Syst Biol. 2010;6:367. doi: 10.1038/msb.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito T, Matsunaga Y, Yabuta Y, Kawano T, Watanabe F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio. 2013;3:112–117. doi: 10.1016/j.fob.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014;5:61. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008;66:250–255. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- Fall CH, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJ. Micronutrients and fetal growth. J Nutr. 2003;133:1747S–1756S. doi: 10.1093/jn/133.5.1747S. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MJ, Winston F. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol Cell Biol. 2007;27:7414–7424. doi: 10.1128/MCB.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolnek T, Zhang J, Beardsley S, Scheffer GL, Hamza I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014;19:1008–1019. doi: 10.1016/j.cmet.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Rosenbaum DM. Transporters revealed. Cell. 2017;168:951–953. doi: 10.1016/j.cell.2017.02.033. [DOI] [PubMed] [Google Scholar]
- Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJM. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol. 2002;58:275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- Moreno-Garcia MA, Rosenblatt DS, Jerome-Majewska LA. Vitamin B(12) metabolism during pregnancy and in embryonic mouse models. Nutrients. 2013;5:3531–3550. doi: 10.3390/nu5093531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MJ, Rasmussen MR, Andersen CB, Nexø E, Moestrup SK. Vitamin B12 transport from food to the body’s cells–a sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol. 2012;9:345–354. doi: 10.1038/nrgastro.2012.76. [DOI] [PubMed] [Google Scholar]
- Owens S, Fall CH. Consequences of poor maternal micronutrition before and during early pregnancy. Trans R Soc Trop Med Hyg. 2008;102:103–104. doi: 10.1016/j.trstmh.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci USA. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt DS, Cooper BA. Inherited disorders of vitamin B12 utilization. BioEssays. 1990;12:331–334. doi: 10.1002/bies.950120705. [DOI] [PubMed] [Google Scholar]
- Rosenblatt DS, Whitehead VM. Cobalamin and folate deficiency: acquired and hereditary disorders in children. Semin Hematol. 1999;36:19–34. [PubMed] [Google Scholar]
- Rual J-F, Ceron J, Koreth J, Hao T, Nicot A-S, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14(10B):2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelochkov OA, Carrillo-Carrasco N, Venditti C. Propionic acidemia. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews [Internet] University of Washington; 2016. [Google Scholar]
- Sheps JA, Ralph S, Zhao Z, Baillie DL, Ling V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004;5:R15. doi: 10.1186/gb-2004-5-3-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjærven KH, Jakt LM, Dahl JA, Espe M, Aanes H, Hamre K, Fernandes JM. Parental vitamin deficiency affects the embryonic gene expression of immune-, lipid transport- and apolipoprotein genes. Sci Rep. 2016;6:34535. doi: 10.1038/srep34535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs) Essays Biochem. 2011;50:179–207. doi: 10.1042/bse0500179. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJ, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:190–203. doi: 10.1001/jama.2016.19193. [DOI] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Arda HE, Zhu LJ, Walhout AJM. Integration of metabolic and gene regulatory networks modulates the C. elegans dietary response. Cell. 2013;153:253–266. doi: 10.1016/j.cell.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, Walhout AJM. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:759–770. doi: 10.1016/j.cell.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, Olin-Sandoval V, Hoy MJ, Li CH, Louisse T, Yao V, Mori A, Holdorf AD, Troyanskaya OG, Ralser M, Walhout AJ. Metabolic network rewiring of propionate flux compensates vitamin B12 deficiency in C. elegans. eLife. 2016;5:e17670. doi: 10.7554/eLife.17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Chun L, Ronan EA, Friedman DI, Liu J, Xu XZ. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 2015;11:1123–1133. doi: 10.1016/j.celrep.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz LS, Walhout AJM. Worms, bacteria, and micronutrients: an elegant model of our diet. Trends Genet. 2014;30:496–503. doi: 10.1016/j.tig.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.