Abstract
Tumor infiltrating lymphocytes(TILs) have been shown to be an important prognostic factor in patients with previously untreated head and neck cancer. After organ preservation therapy for laryngeal cancer and subsequent persistence/recurrence, the prognostic value of TILs is unknown. Our goal was to determine if TILs have value as a prognostic biomarker in patients with surgically salvageable persistent/recurrent laryngeal squamous cell carcinoma.
Levels of TILs were quantified on tissue microarrays from 183 patients undergoing salvage total laryngectomy for persistent/recurrent laryngeal cancer after radiation or chemoradiation between 1997–2014. Demographic and clinical data were abstracted. Immunohistology evaluation included CD4, CD8, PDL-1, p16, CD31, Vimentin, EGFR, and p53.
Elevated levels of either CD8 or CD4 positive TILs were associated with improved disease specific survival(CD8: HR 0.46, 95% CI 0.24 – 0.88, CD4: HR 0.43; 95% CI 0.21 – 0.89) and disease free survival(CD8: HR 0.53, 95% CI 0.29 – 0.94, CD4: HR 0.52; 95% CI 0.27 – 0.99). Levels of CD8(HR 0.74; 95% CI 0.47 – 1.17) or CD4(HR 0.66; 95% CI 0.40 – 1.08) TILs were not significantly associated with overall survival. In bivariate analysis, patients with elevated CD4 and/or CD8 TILs had significantly improved disease specific survival(HR 0.42; 95% CI 0.21 – 0.83) and disease free survival(HR 0.45; 95% CI 0.24 – 0.84) compared to patients with low levels of CD4 and CD8. PDL-1, p16, CD31, Vimentin, EGFR, and p53 were not significant prognostic factors. On multivariate analysis, elevated CD8 TILs were associated with improved disease specific survival (HR 0.35; 95% CI 0.14 – 0.88, p=0.02) and disease free survival (HR 0.41; 95% CI 0.17 – 0.96, p=0.04).
CD8, and possibly CD4, positive TILs are associated with favorable disease free and disease specific survival for recurrent/persistent laryngeal cancer.
Keywords: Head and neck cancer, Salvage, Recurrence, Laryngectomy, Tumor infiltrating lymphocytes, CD4, CD8, Survival, Prognosis
Introduction
Recurrent and persistent head and neck squamous cell carcinoma is becoming an increasingly important cohort for head and neck cancer providers. While curative treatment is achieved in many instances, approximately 25–50% of patients with head and neck squamous cell carcinoma will experience recurrence, and the overall median survival for those with recurrent disease who undergo treatment is less than 22 months (1). Treatment for recurrence tends to be difficult, as these tumors are often resistant to standard therapy, complication rates are higher, and prognosis is guarded (2). In patients who are not eligible for salvage therapy with curative intent, median survival is less than 12 months (1).
Laryngeal cancer in particular is increasingly being treated initially with radiation (RT) or chemoradiation (CRT) organ-preservation protocols (3). However, while a significant proportion of these patients achieve cure, a non-trivial proportion experience persistent/recurrent disease. For these patients, salvage surgery is currently the only method for cure, with less than ideal overall survival rates in patients requiring salvage laryngectomy (ranging from 29–66%) (4–7). Additionally, as with other head and neck cancers, salvage surgery results in high rates of complications due to poor healing rates and the increased difficulties of operating in a radiated field (8).
As a result, researchers have attempted to identify biomarkers that will allow for better patient treatment stratification and prognostication. One of the more promising areas of investigation involves the evaluation of biomarkers that reflect the patient’s immune system. It is increasingly appreciated that the immune system plays a key role in head and neck cancer tumorigenesis, progression, and response to therapy. Immunologic signatures have been determined in some subsets of primary head and neck cancers to predict improved survival (9–11). It is unknown, however, whether immunologic biomarkers may demonstrate utility in predicting survival and potentially stratifying salvage treatment options for recurrent/persistent head and neck cancer. Identifying prognostic biomarkers in recurrent head and neck cancer may be useful in providing counseling for patients, considering escalation or de-escalation of care, or adding adjuvant immunotherapeutic agents. Thus, the purpose of this study was to investigate the prognostic role of a panel of immunologic biomarkers in patients with recurrent/persistent laryngeal squamous cell carcinoma after initial RT or CRT.
Methods
Patient population
We performed a single-institution retrospective case series informed by a prospectively maintained database of patients with head and neck cancer. The University of Michigan Hospital and Health Systems IRB approved the protocol (HUM00081554). Inclusion criteria stipulated: 1) biopsy-proven laryngeal squamous cell carcinoma; 2) persistent/recurrent disease at the primary site after radiation (for early stage tumors) or chemoradiation (for advanced stage tumors); 3) total laryngectomy with neck dissections for surgical salvage; and 4) tumor tissue available for creation of tissue microarray. Patients were excluded if they had a second primary tumor necessitating surgery. There were 183 patients who met inclusion criteria, and demographics are shown in Table 1. Patients were staged in accordance to the 7th edition American Joint Committee on Cancer (AJCC) Staging System (10).
Table 1.
Patient Characteristics
| Patient Characteristics (n = 183) | |
|---|---|
|
| |
| Gender | |
| Male | 153 (83.6) |
| Female | 30 (16.4) |
| Ethnicity | |
| White | 161 (88.0) |
| Black/Other/Unknown | 22 (12.0) |
| Initial Cancer | |
| Age at Initial Tumor | 58.63 |
| Initial Site | |
| Glottis | 109 (59.6) |
| Supraglottis | 71 (46.4) |
| Other/Unknown | 3 (1.6) |
| Initial cT stage | |
| cT1 | 46 (25.1) |
| cT2 | 61 (33.3) |
| cT3 | 44 (24.0) |
| cT4 | 18 (9.8) |
| Unk | 14 (7.7) |
| Initial cN status | |
| cN0 | 141 (77.0) |
| cN1 | 15 (8.2) |
| cN2+ | 14 (7.7) |
| Unk | 13 (7.1) |
| Initial Stage | |
| I | 46 (25.1) |
| II | 54 (29.5) |
| III | 44 (24.0) |
| IV | 25 (13.7) |
| Unk | 14 (7.7) |
| Initial Treatment | |
| RT | 112 (61.2) |
| CRT | 71 (38.8) |
| Recurrent Cancer | |
| Age at Recurrence (yrs) | 60.87 |
| Time to Recurrence (mo) | 23.48 |
| Recurrent Site | |
| Glottis | 99 (54.1) |
| Supraglottis | 81 (44.2) |
| Other/Unknown | 3 (1.6) |
| Recurrent cT stage | |
| cT1 | 8 (4.4) |
| cT2 | 80 (43.7) |
| cT3 | 51 (27.9) |
| cT4 | 44 (24.0) |
| Recurrent cN status | |
| cN0 | 159 (86.9) |
| cN1 | 10 (5.5) |
| cN2+ | 14 (7.7) |
| Recurrent cStage | |
| I | 7 (3.8) |
| II | 76 (41.5) |
| III | 49 (26.8) |
| IV | 51 (27.9) |
| Pathologic Data | |
| Recurrent pT stage | |
| pT1 | 6 (3.3) |
| pT2 | 60 (32.8) |
| pT3 | 53 (29.0) |
| pT4 | 64 (35.0) |
| Recurrent pN status | |
| pN0 | 137 (74.9) |
| pN1 | 15 (8.2) |
| pN2+ | 31 (16.9) |
| Recurrent pStage | |
| I | 6 (3.3) |
| II | 53 (29.0) |
| III | 48 (26.2) |
| IV | 76 (41.5) |
Immunohistology
Formalin-fixed paraffin-embedded (FFPE) tissue blocks from salvage surgery and representative hematoxylin-eosin stained slides were reviewed for >70% cellularity. The tissue microarray was constructed with Triplicate 0.7mm diameter cores for each patient (11).
TMA sections were incubated in hot-air oven at 65°C overnight, deparaffinized, rehydrated with xylene, graded alcohols, and buffer immersion steps. Antigen retrieval was carried out by heat-induced epitope retrieval method. The slides were incubated in a preheated pressure cooker with Citrate buffer pH6 or Tris-EDTA buffer pH9 and blocked with horse serum (30 minutes at 25°C). Immunohistochemical staining was completed on a DAKO autostainer using liquid streptavidin biotin horseradish peroxidase and DBA (DAKO labeled avidin-biotin-peroxidase kits) as chromogens. Deparaffinized sections were stained with eight monoclonal antibodies at the following titrations: CD4-1:250 (Abcam Ab846); CD8-1:40 (Nova Castra VP-C320); CD31 – 1:50 (Dako M0823); Vimentin – 1:100 (Dako M0725); PDL1 – 1:200 (Cell signaling E1L3N); p16 – predilute (Ventana 725-4713); EGFR – 1:50 (Invitrogen 280005); and p53 – 1:50 (Cell Marque SP5).
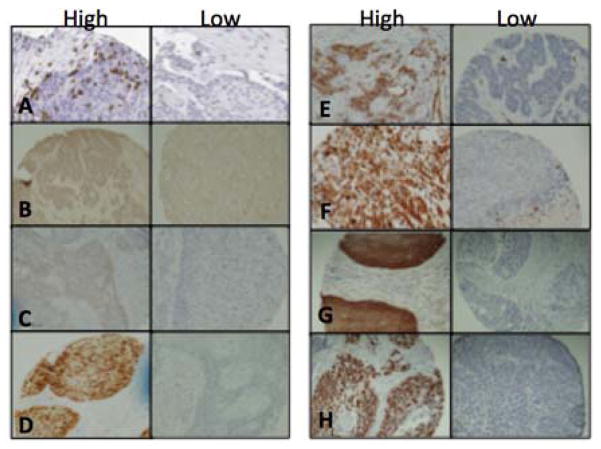
The TMA slides were digitally imaged, scanned, and retrieved with Aperio ImageScope v.12 software. Only cores consisting of >50% tumor parenchyma were counted. The CD4, CD8, PDL1, p16, CD31, Vimentin, EGFR, and p53 positively stained cells in each core were manually counted at 200× magnification (20× objective lens). Examples of positive and negative stains are shown in Figure 1. Only TILs infiltrating in tumor parenchyma were quantified since it has proven to be most reproducible and representative immune response parameter for TILs (12–15). Mean counts per core of triplicate samples for each patient were calculated and used in statistical analysis.
Figure 1.
Representative samples of high and low tumor infiltrating populations. A) CD8; B) CD4; C) PDL1; D) p16; E) CD31; F) Vimentin; G) EGFR; H) p53
Statistical methods
The Kaplan-Meier method and log-rank tests were used for univariate and bivariate outcomes based on overall staining intensity and proportion (p53, EGFR, Vimentin, p16, CD31, PDL1), or high or low levels of each biomarker dichotomized at the median (CD4, CD8), as previously described (9,16,17). SPSS version 24 software (IBM; Armonk, NY) was used to perform statistical analyses. Deaths were confirmed through the electronic medical record and the Social Security Death Index. Primary outcome measures were overall survival (OS; time from salvage laryngectomy to death from any cause), disease-specific survival (DSS; time from salvage laryngectomy to death from any disease recurrence/persistence), and disease-free survival (DFS; time from salvage laryngectomy to any disease recurrence/persistence).
A multivariate analysis was performed to evaluate the correlation of CD4 and CD8 staining with DFS and DSS. Patients with both CD4 and CD8 staining were included in the multivariate analysis. Variables in the model included CD4 and CD8 staining, stage, tumor subsite, initial treatment (RT versus CRT), pre-salvage tracheostomy, grade and margin status, smoking status and ACE 27. Bivariate analysis was performed and variables with a p-value of less than 0.1 in the bivariate analysis were included in the multivariate model. A backward selected binary logistic regression was then performed. An alpha of 0.05 was used for all statistical tests to determine statistical significance.
Results
The estimated 5-year overall, disease specific, and disease free survival for the cohort was 46%, 66%, and 60%, respectively. We then analyzed the effect of demographic and clinical factors (Table 1) on prognosis. We verified that our cohort of patients with advanced T classification and recurrent nodal positivity had worse OS, DSS, and DFS, as we had previously described, and in line with previous investigations (1,5,10,18,19).
Several previously validated and commonly aberrant biomarkers, involved in cell cycle (p16, p53), cell growth (EGFR), vascularity (CD31), and mesenchymal status (vimentin) were evaluated for their association with survival (9,11,20). In our cohort, 5% of tumors stained positive for p16, 99% for EGFR, and 58% for p53 (Table 2). These percentages are consistent with the approximate distribution of each biomarker in primary untreated laryngeal squamous cell carcinoma (20). None of these biomarkers were associated with OS, DSS or DFS in our recurrent/persistent laryngeal squamous cell carcinoma cohort.
Table 2.
Antibody Staining of Patient Cohort
| Stain | Intensity | Patients |
|---|---|---|
| PDL1 | 0 | 161 (99%) |
| ≥1 | 1 (1%) | |
| p16 | 0 | 153 (95%) |
| ≥1 | 8 (5%) | |
| CD31 | 0 | 85 (53%) |
| ≥1.5 | 76 (47%) | |
| Vimentin | 0 | 122 (71%) |
| ≥1 | 50 (29%) | |
| EGFR | 0 | 1 (1%) |
| 1 | 10 (6%) | |
| 2 | 17 (10%) | |
| 3 | 146 (84%) | |
| p53 | 0 | 73 (42%) |
| 1 | 13 (8%) | |
| 2 | 13 (8%) | |
| 3 | 73 (42%) |
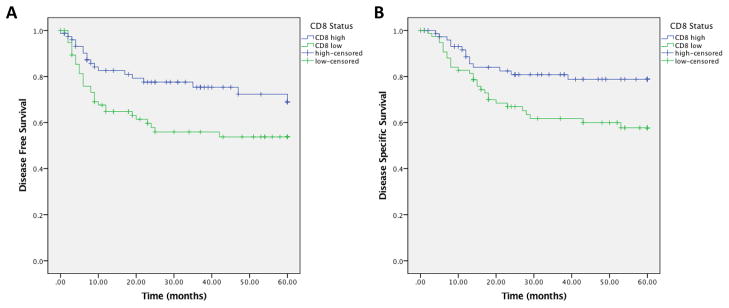
We subsequently sought to characterize the percent of each of CD8 and CD4 TIL populations in each tumor, (dichotomized at the median, as previously described) (9). In total, 155 patients had tumor cores containing greater than 50% of tumor parenchyma that were evaluable for CD8+ cell quantification. There were 68 patients who had low CD8 counts within the tumor parenchyma, and 87 patients who had high CD8 counts (dichotomized at the median). On univariate analysis, elevated levels of CD8 positive TILs were associated with improved DFS (HR 0.53, 95% CI 0.29 – 0.94; p=0.025, Figure 2A) and DSS (HR 0.46, 95% CI 0.24 – 0.88, p=0.015, Figure 2B). We did not see any association with OS.
Figure 2.
CD8 high tumor infiltrating lymphocytes vs. CD8 low tumor infiltrating lymphocytes. 2A) Disease free survival; 2B) Disease specific survival.
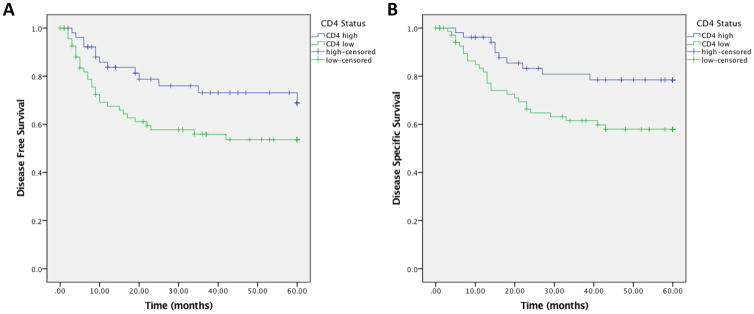
For the tumor cores evaluated for CD4, a total of 138 patients had tumor cores containing greater than 50% tumor parenchyma, with 80 patients who had low levels of CD4 positive tumor infiltrating cells and 58 patients who had high levels of CD4 positive tumor infiltrating cells. On univariate analysis, elevated levels of CD4 positive TILs were also associated with improved DFS (HR 0.52; 95% CI 0.27 – 0.99, p=0.043, Figure 3A) and DSS (HR 0.43; 95% CI 0.21 – 0.89, p=0.019, Figure 3B). Patient characteristics including gender, tumor subsite, initial and recurrent stage, initial treatment modality, smoking status, and alcohol were then correlated to CD4 and CD8 expression. There were no correlations with TIL levels to any of the clinical variables. (Table 3). We subsequently performed bivariate analysis to confirm TIL levels are predictive of survival independent of known clinical prognostic factors. When controlling for smoking, alcohol, recurrent stage, or subsite, elevated levels of CD8 or CD4 positive lymphocytes were still significantly associated with improved DFS and DSS. Overall survival was not found to be significantly different between either CD8 or CD4 high patients or low patients.
Figure 3.
CD4 high tumor infiltrating lymphocytes vs. CD4 low tumor infiltrating lymphocytes. 3A) Disease free survival; 3B) Disease specific survival.
Table 3.
Patient characteristics of CD8 and CD4 cohorts
| CD8 Cohort | CD4 Cohort | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Negative | Positive | Chi-Squared | Negative | Positive | Chi-Squared | ||
|
| |||||||
| Gender | Male | 56 (82%) | 73 (84%) | 0.80 | 64 (83%) | 50 (83%) | 0.97 |
| Female | 12 (18%) | 14 (16%) | 13 (17%) | 10 (17%) | |||
|
| |||||||
| Subsite | Supraglottic | 31 (46%) | 32 (37%) | 0.52 | 36 (47%) | 20 (33%) | 0.32 |
| Glottic | 36 (53%) | 54 (62%) | 39 (51%) | 39 (65%) | |||
| Subglottic | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | |||
| Unknown | 1 (1%) | 1 (1%) | 1 (1%) | 1 (2%) | |||
|
| |||||||
| Initial stage | I–II | 34 (50%) | 49 (56%) | 0.12 | 46 (54%) | 31 (53%) | 0.99 |
| III–IV | 32 (47%) | 27 (31%) | 34 (40%) | 23 (40%) | |||
| Unknown | 2 (3%) | 11 (13%) | 5 (6%) | 4 (7%) | |||
|
| |||||||
| Initial T | T1–2 | 37 (54%) | 53 (61%) | 0.09 | 49 (58%) | 35 (60%) | 0.68 |
| T3–4 | 29 (43%) | 23 (26%) | 31 (36%) | 19 (33%) | |||
| Unknown | 2 (3%) | 11 (13%) | 5 (6%) | 4 (7%) | |||
|
| |||||||
| Initial N | N0 | 51 (75%) | 66 (76%) | 0.19 | 63 (74%) | 46 (79%) | 0.28 |
| N+ | 15 (22%) | 11 (13%) | 18 (21%) | 8 (14%) | |||
| Unknown | 2 (3%) | 10 (11%) | 4 (5%) | 4 (7%) | |||
|
| |||||||
| Recurrent stage | I–II | 22 (32%) | 26 (30%) | 0.74 | 28 (33%) | 20 (34%) | 0.85 |
| III–IV | 46 (68%) | 61 70%) | 57 (67%) | 38 (66%) | |||
|
| |||||||
| Recurrent T | TI–2 | 26 (38%) | 29 (33%) | 0.53 | 30 (35%) | 24 (41%) | 0.46 |
| T3–4 | 42 (62%) | 58 (66%) | 55 (65%) | 34 (59%) | |||
|
| |||||||
| Recurrent N | N0 | 49 (72%) | 63 (72%) | 0.96 | 60 (71%) | 46 (79%) | 0.24 |
| N+ | 19 (28%) | 24 (28%) | 25 (29%) | 12 (21%) | |||
|
| |||||||
| Smoker | Yes | 64 (94%) | 86 (99%) | 0.10 | 74 (96%) | 59 (98%) | 0.44 |
| No | 4 (6%) | 1 (1%) | 3 (4%) | 1 (2%) | |||
|
| |||||||
| Alcohol | Yes | 43 (63%) | 62 (71%) | 0.07 | 52 (68%) | 42 (70%) | 0.70 |
| No | 24 (35%) | 19 (22%) | 20 (26%) | 16 (27%) | |||
| Unknown | 1 (1%) | 6 (7%) | 5 (6%) | 2 (3%) | |||
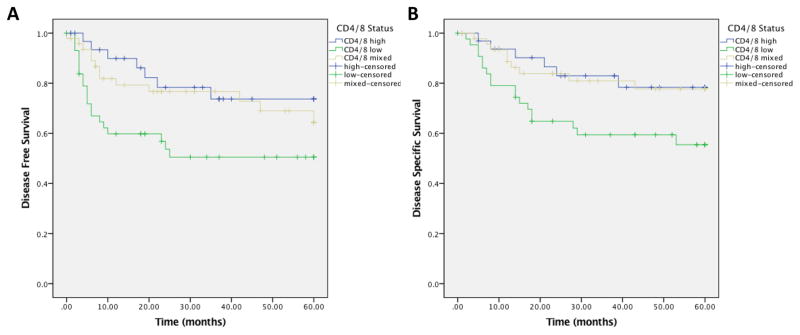
Bivariate analysis was performed to understand the relationship of CD4 and CD8 positive TILs and survival. A total of 126 patients had both CD4 and CD8 stains available in cores with greater than 50% tumor parenchyma, and were included for analysis. Patients with high levels of either CD4 and CD8 TILs had significantly improved DFS (HR 0.45; 95% CI 0.24 – 0.84, p=0.031, Figure 4A) and DSS (HR 0.42; 95% CI 0.21 – 0.83, p=0.036, Figure 4B) compared with the group with both low CD4 and CD8 TIL status. There were no differences in OS between these groups.
Figure 4.
Bivariate analysis of CD8 and CD4 tumor infiltrating lymphocytes. 4A) Disease free survival; 4B) Disease specific survival
A backward selected multivariate binary logistic regression was then performed. In our multivariate models evaluating DFS and DSS, only CD8 staining and overall stage were important variables in the models. Patients with high CD8 TIL status had improved DFS (HR 0.41; 95% CI 0.17 – 0.96, p=0.04) and DSS (HR 0.35; 95% CI 0.14 – 0.88, p=0.02). Not surprisingly, advanced overall stage was associated with worse DFS (p=0.003) and DSS (p=0.005). CD4 TIL status did not reach significance for DFS (p=0.45) or DSS (p=0.71). No other variables showed statistically significant correlation.
The number of vessels in each tumor section were evaluated in order to test the hypothesis that the tumors with a greater number of vessels per area (i.e. increased tumor vascularization) also had a greater number of tumor infiltrating lymphocytes. In 161 evaluable tumors, we found that the number of CD31+ vessels per unit area was not correlated with the number of either CD8 or CD4 tumor infiltrating lymphocytes each tumor. The overall number of vessels per area was also not correlated with any of the survival characteristics, further supporting the conclusion that TIL status may represent an independent biomarker and vascularity does not appear to confound these predictions.
Given the correlation between CD8 or CD4 TILs and survival in our population, as well as the recent FDA approval of anti-PD1 immunotherapeutic agents pembrolizumab and nivolumab in head and neck cancer, we sought to characterize the number of PDL1 positive cells in each specimen. Early data from immunotherapy trials indicates that tumors with >1% of PDL1 positive tumor and immune cells may be more likely to respond to therapy than those without PDL1 positive cells (21,22), In contrast to studies of primary, untreated, mixed head and neck cancer populations that have shown rates of PDL1 positivity as high as 66% (23), our data indicates that only 1 of 162 evaluable tumors was PDL1 positive.
Discussion
The role of the immune system in tumorigenesis, progression, and resistance to treatment in head and neck cancers is becoming increasingly understood as new data regarding the impact of a patient’s immune status on recurrence, response to therapy and survival are reported (19,24). Previously, our group evaluated TILs in mixed head and neck cancer populations, demonstrating that patients with increased levels of CD4 TILs had an improved prognosis (25). Several studies since then have confirmed and expanded on the possible prognostic role of immunological biomarkers in mixed populations of head and neck carcinoma, further showing that CD4 positive TIL (26), CD8 positive TILs (27), S-100 positive dendritic cells (28), CD68 expression (29), or CD3 receptor response (30) correlates with survival in various head and neck carcinomas. However, these studies were all in mixed populations of head and neck carcinoma, and did not address a recurrent cancer population specifically. Moreover, the assessment of immunogenic biomarkers in laryngeal carcinoma in particular has not previously been performed in a robust cohort. Our study is the first to show the independent prognostic value of CD8 positive TILs in patients with recurrent/persistent laryngeal cancer. As such, CD8 (and potentially CD4) status may prove to be a viable and important biomarker for treatment stratification and prognostic guidance for patients with recurrent/persistent laryngeal cancer.
Wansom et al showed that the presence or absence of CD4 positive and CD8 positive TILs correlated with OS and DFS in a cohort including both HPV positive and negative patients (31). More recently, Keck et al performed integrated analysis of a cohort of locoregionally advanced head and neck cancers, and combined with da ta from other large genomic studies including the TCGA, found that patients with higher levels of CD8 positive TILs had improved survival and a specific gene signature, termed the inflamed mesenchymal subtype (11). CD8 positive TIL status demonstrated improved survival, regardless of HPV status. These studies provide evidence the immune system is important in tumor progression and further support the possible critical role that immune markers may have as prognostic biomarkers.
Notably, p16 status did not show significant impact on survival in this population. Laryngeal carcinoma has previously been shown to have a lower overall level of HPV positivity (32). HPV positivity has not been shown definitively to predict or impact survival in laryngeal carcinoma (unlike oropharyngeal carcinoma) (33–34). Although the number of HPV positive patients in our cohort was low, making it difficult to draw definitive conclusions from survival in HPV positive patients with recurrent laryngeal carcinoma, our observations are consistent with previous data.
Biomarkers in patients with recurrent laryngeal squamous cell carcinoma may be increasingly important, particularly as organ preservation becomes more prevalent consideration of treatment algorithms and prognostic guidance is increasingly implemented. Importantly, we have identified subsets of patients based on immunologic biomarker signatures and independent of clinical factors that differ in outcome.
Survival outcomes remain suboptimal among patients with surgically salvageable, recurrent/persistent laryngeal cancer, and many patients may not be surgical candidates. If immune status was shown to be a significant factor in survival of recurrent patients, newly approved immunologic therapies may provide significant benefit for these patients. Subsets of patients who recur/persist or have poor prognostic indicators pre-salvage (i.e. low CD8 TIL status in our cohort) may be prime candidates for immunologic adjuvant therapy. Further characterization of patients who respond to immunotherapy will be integral in the design of such future studies, with particular focus on CD4 and CD8 TIL status as biomarkers for treatment response. Should these CD4 and CD8 TIL biomarkers be further validated, they could prove critically important in helping guide care in regards to counseling on tumor prognosis and providing adjuvant therapy regimens.
Although we find our results encouraging and in line with recent studies demonstrating the importance of TILs and disease prognosis (25–30), there are nevertheless limitations in our study. This was a retrospective cohort study and thus subject to inherit limitations of such study designs. This was a single institution cohort and will benefit from additional validation with external cohorts. Additionally, our study period was over a number of years (from 1997 to 2014), and changes in disease process and treatment may have evolved over time that could lead to some confounding. CD4 status was significant in univariate and bivariate analysis, but did not achieve significance in multivariate analysis. Further studies with larger cohorts may shed light onto the potential of CD4 status as a prognostic biomarker. Nevertheless, we find this data compelling for potential further investigation into this challenging cohort.
Conclusion
CD8 TIL status was associated with a significant improvement in DFS and DSS in patients with recurrent/persistent laryngeal squamous cell carcinoma after RT/CRT. Should this be validated, it could prove critically important to guide therapy, and will be extremely important as the field moves increasingly towards personalized medicine and immunologic adjuvant therapies.
References
- 1.Ho A, Kraus D, Ganly I, Lee N, Shah J, Morris L. Decision making in the management of recurrent head and neck cancer. Head & Neck. 2013;36(1):144–151. doi: 10.1002/hed.23227. [DOI] [PubMed] [Google Scholar]
- 2.Hedberg M, Goh G, Chiosea S, Bauman JE, Freilino ML, Zeng Y, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. Journal of Clinical Investigation. 2015;126(1):169–180. doi: 10.1172/JCI82066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf G, Hong K, Fisher S, Hillman R, Spaulding R, Laramore GE, et al. Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal Cancer. New England Journal of Medicine. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin M, Parsons J, Fein D, Stringer SP, Cassisi NJ, Mendenhall WM, et al. Salvage surgery after radiotherapy failure in T1–T2 squamous cell carcinoma of the glottic larynx. Head & Neck. 1996;18(3):229–235. doi: 10.1002/(SICI)1097-0347(199605/06)18:3<229::AID-HED4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Stoeckli S, Pawlik A, Lipp M, Huber A, Schmid S. Salvage Surgery After Failure of Nonsurgical Therapy for Carcinoma of the Larynx and Hypopharynx. Archives of Otolaryngology–Head & Neck Surgery. 2000;126(12):1473. doi: 10.1001/archotol.126.12.1473. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen K, Godballe C, Hansen O, Bastholt L. Cancer of the Larynx: Treatment Results after Primary Radiotherapy with Salvage Surgery in a Series of 1005 Patients. Acta Oncologica. 2002;41(1):69–76. doi: 10.1080/028418602317314091. [DOI] [PubMed] [Google Scholar]
- 7.Parsons J, Mendenhall W, Stringer S, Cassisi N, Million R. Salvage surgery following radiation failure in squamous cell carcinoma of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 1995;32(3):605–609. doi: 10.1016/0360-3016(95)00527-6. [DOI] [PubMed] [Google Scholar]
- 8.Wolf G, Bradford C, Urba S, Smith A, Eisbruch A, Chepeha DB, et al. Immune Reactivity Does Not Predict Chemotherapy Response, Organ Preservation, or Survival in Advanced Laryngeal Cancer. Laryngoscope. 2002;112(8):1351–1356. doi: 10.1097/00005537-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074–1084. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7. Chicago, IL, USA: Springer Press; 2010. [Google Scholar]
- 11.Keck M, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative Analysis of Head and Neck Cancer Identifies Two Biologically Distinct HPV and Three Non-HPV Subtypes. Clin Cancer Res. 2014;21(4):870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 12.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2013;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–9. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf G, Chepeha D, Bellile E, Nguyen A, Thomas D, McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51(1):90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pretscher D, Distel L, Grabenbauer G, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9(1) doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford CR, Kumar B, Bellile E, Lee J, Taylor J, D’Silva N, et al. Biomarkers in advanced larynx cancer. Laryngoscope. 2014 Jan;124(1):179–87. doi: 10.1002/lary.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008 Jul 1;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin W. Salvage Surgery for Patients With Recurrent Squamous Cell Carcinoma of the Upper Aerodigestive Tract: When Do the Ends Justify the Means? Laryngoscope. 2000;110(S93):1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 19.Birkeland AC, Rosko AJ, Beesley L, Bellile E, Chinn SB, Shuman AG, et al. Preoperative tracheostomy is associated with poor disease-free survival in recurrent laryngeal cancer. Otolaryngol Head Neck Surg. 2017 May;1:194599817709236. doi: 10.1177/0194599817709236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford C, Kumar B, Bellile E, Lee J, Taylor J, D’Silva N, et al. Biomarkers in advanced larynx cancer. Laryngoscope. 2013;124(1):179–187. doi: 10.1002/lary.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow L, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.68.1478. pii: JCO681478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 Blockade Augments Adoptive T-Cell Immunotherapy for Squamous Cell Carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 24.Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016 Sep 19; doi: 10.1200/JCO.2016.68.1478. pii: JCO681478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986:142–152. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 26.Badoual C, Hans S, Rodriguez J, Peyrand S, Klein C, Aqueznay Nel H, et al. Prognostic Value of Tumor-Infiltrating CD4+ T-Cell Subpopulations in Head and Neck Cancers. Clin Cancer Res. 2006;12(2):465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 27.Balermpas P, Rödel F, Rödel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) Intl J Cancer. 2015;138(1):171–181. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 28.Reichert T, Scheuer C, Day R, Wagner W, Whiteside T. The number of intratumoral dendritic cells and ζ-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91(11):2136–2147. [PubMed] [Google Scholar]
- 29.Wolf G, Chepeha D, Bellile E, Nguyen A, Thomas D, McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncology. 2015;51(1):90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuya TY, Nugyen N, McLaren CE, Li KT, Wei WZ, Kim S, et al. Clinical significance of poor CD3 response in head and neck cancer. Clin Cancer Res. 2002 Mar;8(3):745–51. [PubMed] [Google Scholar]
- 31.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, et al. Infiltrating lymphocytes and human papillomavirus-16-associated oropharyngeal cancer. Laryngoscope. 2011;122(1):121–127. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephen J, Divine G, Chen K, Chitale D, Havard S, Worsham M. Significance of p16 in site-specific HPV positive and HPV negative HNSCC. Cancer and Clinical Oncology. 2012;2(1) doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez B, Goodman M, Lynch C, Corzen W, Unger ER, Steinau M, et al. Human Papillomavirus Prevalence in Invasive Laryngeal Cancer in the United States. PloS ONE. 2014;9(12):e115931. doi: 10.1371/journal.pone.0115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephen J, Chen K, Shah V, Havard S, Lu M, Schweitzer VP, et al. Human Papillomavirus Outcomes in an Access-to-Care Laryngeal Cancer Cohort. Otolaryngol Head Neck Surg. 2012;146(5):730–738. doi: 10.1177/0194599811434482. [DOI] [PMC free article] [PubMed] [Google Scholar]