Abstract
Senescence accelerated mice P1 (SAMP1) is an aging model characterized by shortened lifespan and early signs of senescence. Klotho is an aging-suppressor gene. The purpose of this study is to investigate whether in vivo expression of secreted klotho (Skl) gene attenuates aortic valve fibrosis in SAMP1 mice. SAMP1 mice and age-matched (AKR/J) control mice were used. SAMP1 mice developed obvious fibrosis in aortic valves, namely fibrotic aortic valve disease (FAVD). Serum level of Skl was decreased drastically in SAMP1 mice. Expression of MCP-1, ICAM-1, F4/80, and CD68 was increased in aortic valves of SAMP1 mice, indicating inflammation. An increase in expression of α-SMA (myofibroblast marker), TGFβ-1 and scleraxis (a transcription factor of collagen synthesis) was also found in aortic valves of SAMP1 mice, suggesting that accelerated aging is associated with myofiborblast transition and collagen gene activation. We constructed adeno-associated virus 2 (AAV2) carrying mouse Skl cDNA (AAV2-Skl) for in vivo expression of Skl. Skl gene delivery effectively increased serum Skl of SAMP1 mice to the control level. Skl gene delivery inhibited inflammation and myofibroblastic transition in aortic valves and attenuated FAVD in SAMP1 mice. It is concluded that senescence-related FAVD in SAMP1 mice is associated with a decrease in serum klotho leading to inflammation including macrophage infiltration and TGFβ-1/Scleraxis-driven myofibroblast differentiation in aortic valves. Restoration of serum Skl levels by AAV2-Skl effectively suppresses inflammation and myofibroblastic transition and attenuates aortic valve fibrosis. Skl may be a potential therapeutic target for FAVD.
Keywords: Fibrotic aortic valve disease, Klotho, gene delivery, immunity, inflammation
Introduction
In 2013, more than 30,000 deaths were directly caused by aortic valve disease (AVD) in the USA, making it the second-leading cause of cardiovascular mortality1. Although AVD is an aging related disease, the prevalence of moderate or severe aortic stenosis in the general population >75 years old is almost 3%. While approximately 50% of patients with severe aortic stenosis are referred for aortic valve replacement (AVR), only 40% are actually admitted for AVR. Trans-catheter AVR provides a less-invasive approach than surgical replacement, but it is currently only applied for those patients who were not suitable for traditional AVR surgeries 2. All those put AVD an increasing burden on the health care system as global life expectancy increases3, 4.
Currently, AVD is no longer thought to be a passive process involving fatigue or deterioration of the valve with age but an active, cellular-driven disease that is not an inevitable consequence of aging 5. Although AVD shares several risk factors and mechanisms with vascular diseases (e.g., atherosclerosis), there are fundamental differences between arteries and the aortic valve with respect to disease mechanisms and responses to therapeutic interventions 6. Fibrotic aortic valve disease (FAVD) is an important pathological fibrotic process that eventually leads to aortic valve stiffening and aortic stenosis, but the pathological mechanism of the initiation and development of FAVD remains elusive. This is a critical barrier for the development of effective pharmacological therapy for preventing or curing FAVD.
Klotho(KL) was originally identified as a putative anti-aging gene and is predominately expressed in kidneys7. Both human and mouse SKL gene are alternatively spliced after exon 3, which encodes the KL1 repeat of Klotho, called secreted Klotho (Skl)(≈65kDa) 8. Skl circulates in the blood as a hormone and regulates function in organs and cells that do not express KL 9. The protein level of SKL in the blood declines with the age after 40 years of age10. By age 75, the serum level of Klotho is about a half of what it was at age 40 10. On the other hand, the prevalence of AVD and aortic valve stenosis increases with age 11. Klotho-deficient mice display multiple phenotypes resembling human aging7, 9. A reduction in the level of circulating Klotho is also observed in chronic kidney disease (CKD), hypertension, and diabetes mellitus12–15. However, whether and how Klotho serves as a beneficial and protective factor for FAVD has never been investigated.
The SAMP1/YiFc (SAMP1) murine model was originally derived from brother-sister breeding of AKR/J mice 16, 17. These mice show accelerated senescence and age-dependent pathologies without any experimental manipulation 18, including senile amyloidosis, deficits in learning and memory, degenerative joint disease and decline in immune responsiveness. Here we reported, for the first time, that SAMP1 mice developed severe aortic valve fibrosis which was associated with a decrease in serum Klotho levels. The goal of this study was to determine whether Klotho has beneficial effect on inflammation-related FAVD in SAMP1 mice.
In the present study, we provided evidence that SAMP1 mice carry innate immune defect in the aortic valve. This manifests itself in vivo as an ideal model to study inflammation-induced aortic valve fibrosis. This dysfunctional immune response is specifically present in the aortic valve interstitial cells, including increased monocyte infiltration, macrophage invasion, myofibroblast differentiation and TGFβ1 activation, which eventually leads to collagen deposition on the aortic valve in SAMP1 mice. We also found that serum level of klotho was decreased in SAMP1 mice. AAV delivery of mouse secreted Klotho increased serum Klotho levels, inhibited the inflammatory responses, and attenuated fibrosis on the aortic valve in SAMP1 mice.
Research Design and Methods
The data and analytic methods are available from the corresponding author upon reasonable request.
Animal Study
This study was carried out according to the guidelines of the National Institutes of Health on the Care and Use of Laboratory Animals. This project was approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee (IACUC). All mice were housed in cages at room temperatures (25±1°C) and were provided with Purina laboratory chow (No. 5001) and tap water. After euthanasia, all animals were perfused transcardially with heparinized-PBS under deep anesthesia with ketamine (90 mg/kg) and xylazine (10 mg/kg, IP).
The accelerated senescence–prone strain SAMP1/YitFcs mice and the aged-matched AKR/J mice (controls), from which SAMP1 mice were derived, were purchased from the Jackson Laboratory. At 10 months of age, the animals received a single injection of AAV-SKL or AAV2-GFP via tail veins. The viral particles were delivered IV at 1.2×108 PFU/100 μl per mouse. The animals were divided into 3 groups (5 mice/group): AKR/J mice, SAMP1 mice injected with AAV2-GFP (SAMP1+GFP), and SAMP1 mice injected with AAV2-Skl (SAMP1+Skl).
Construction of Recombinant Adeno-Associated Virus With the Mouse Secreted Klotho (Skl) Gene
The procedure for constructing the recombinant adeno-associated virus (AAV)-2 carrying the mouse secreted Klotho cDNA (AAV2-Skl) was described in our previous studies12, 19. AAV carrying green fluorescent protein (GFP; AAV-GFP) were constructed as control constructs.
Tissue collection
At 24 weeks following AAV delivery, animals were euthanized using an overdose of ketamine (180 mg/kg body weight) and xylazine (20 mg/kg body weight), and blood was collected with heparin as an anticoagulant. Following blood collection, animals were perfused transcardiacally using heparinized saline19, 20. The upper part of the heart along with 3mm of ascending aorta was placed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 24 hours and then embedded in paraffin.
Histological staining
A series of 5-μm cross-sections of the aortic valve were cut and stained using the Masson Trichrome Staining Kit (EMS, Hatfield, PA). Immunohistochemical (IHC) procedures were performed as described in our previous studies13–15, 21–24. Briefly, IHC staining against antibodies of MCP-1 (Cell Signaling, 2029), ICAM-1 (Santa Cruz, sc-8439), F4/80 (Biolegend, 123102), CD-68 (abcam, ab955), TGFβ-1 (Santa Cruz, sc-146), SCXA (abcam, ab58655), α-SMA (abcam, ab7817), TRAP (ProteinTech. 11594-1-AP) and MMP-2 (abcam, ab37150) was performed using the respective ABC staining system (Santa Cruz Biotechnology). Images of aortic valves were collected at the same exposure conditions under a Nikon Eclipse Ti microscope (10x, 20x, and 40x objective). The fractional areas of collagen fibrosis components (blue, trichrome staining; brown, IHC staining) in the aortic valve region were obtained using NIH image J software.
Western Blot Analysis
Western blot analysis was performed as we described in our recent studies.13, 14, 25 Briefly, an equal amount of serum from AKR/J and SAMP1 mice was diluted, treated with 4X Laemmli Sample buffer (Bio-RAD, Hercules, and CA) and loaded on a 4%–20% Express Plus™ PAGE gel (GenScript, NJ). The protein was transferred onto nitrocellulose filters after separation in the gel. Blots were blocked in 1% BSA in TBST for 1 hour at room temperature, and the membranes were incubated with Klotho primary antibody (R&D systems, AF1819) at 4°C overnight. The membranes were incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature. Proteins were visualized, examined and quantified by densitometry using ChemDoc XRS with Quantity One Software (Bio-RAD, Hercules, and CA). Membranes were incubated in Ponceau Stain for internal loading control.
Statistical Analysis
Quantitative data were presented as the Mean ± SE. Data were analyzed using one-way analysis of variance (ANOVA). The Newman–Keuls procedure was used to assess differences between means. Data were expressed as mean ± SEM. p<0.05 was considered significant.
Results
Aortic valve fibrotic formation was associated with decreased serum Klotho levels in SAMP1 mice
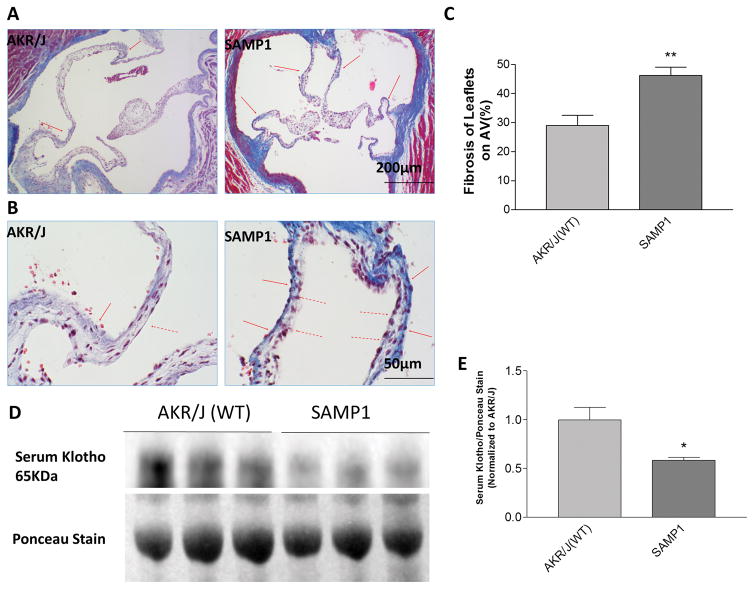
Masson’s trichrome staining of the aortic valves (AoVs) of SAMP1 mice showed significantly increased collagen deposition compared with the AoVs of the age-matched AKR/J mice (Fig. 1A). The collagen deposition preferentially accumulated on the aortic surface (solid arrows, Fig. 1B) compared with the ventricular surface of the leaflets (dashed arrows). This observation indicated a typical development of fibrotic aortic valve disease (FAVD) in the SAMP1 mice. Since SAMP1 is a senescence-accelerated model, we measured serum levels of secreted Klotho (SKL), an anti-aging protein, in SAMP1 and AKR/J mice using immunoblotting. The serum level of SKL in SAMP1 mice is about a half of that of the age-matched AKR/J mice (Fig. 1D, E). Therefore, it would be intriguing to investigate whether the decreased serum level of Klotho contributes to the formation of FAVD in SAMP1 mice.
Figure 1. Aortic valve fibrotic formation was associated with decreased serum Klotho levelsin SAMP1 mice.
(A) Masson’s trichrome staining of aortic valves of AKR/J (WT) and SAMP1 mice. The red arrows indicate collagen deposition on the surface of the leaflets. (B) Higher magnification of aortic valves of AKR/J and SAMP1 mice, which shows asymmetrical sclerosis of aortic valves. The collagen deposition preferentially accumulated on the aortic surface (solid arrows) compared with the ventricular surface of the leaflets (dashed arrows). (C) Quantification of collagen levels in the leaflets of aortic valves (N=4). **p<0.01 vs. AKR/J (t test).(D) Western blot against Klotho in the serum. Ponceau staining was used as a loading control. (E) Quantification of serum Klotho (N=3) indicated that serum Klotho level of SAMP1 mice decreased ~40% compared with age matched AKRJ mice. Data=means±SEM. *p<0.05, *p<0.05vs AKR/J.
In vivo AAV2 delivery of SKL restored the serum Klotho level and attenuated fibrosis in the aortic valve of SAMP1 mice
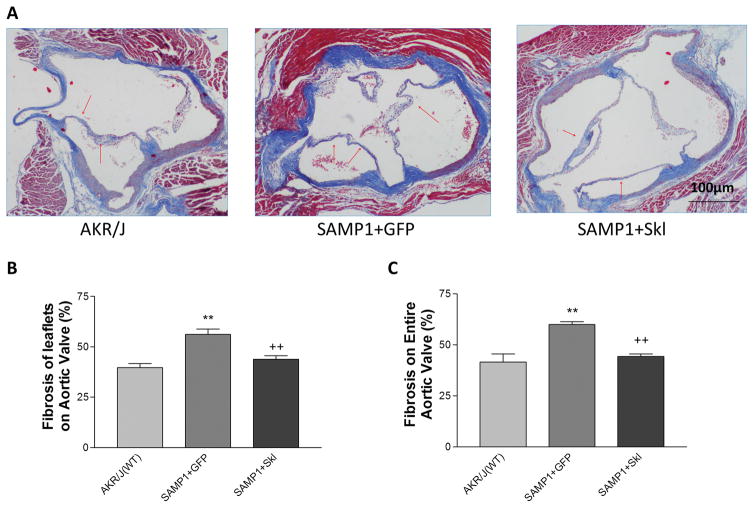
In order to investigate whether an increase in serum secreted Klotho levels rescues SAMP1 mice from FAVD, we overexpressed Skl by in vivo delivery of AAV2-Skl. Indeed, delivery of Skl restored serum level of Klotho of SAMP1 mice to the level of age-matched AKR/J control mice (Fig. S1). On the aortic valves, Masson’s Trichome staining of collagen deposition showed that restoration of serum Klotho levels significantly attenuated fibrosis and collagen accumulation on the leaflets and the entire aortic valves in SAMP1 mice (Fig. 2).
Figure 2. In vivo AAV2 delivery of SKL restored the serum Klotho level and attenuated fibrosis in the aortic valve of SAMP1 mice.
(A) Masson’s trichrome staining (blue) of aortic valves of AKR/J and SAMP1 mice treated with AAV.GFP or AAV.SKL. The red arrows indicate collagen deposition on the surface of the leaflets. (B) Quantification of collagen level in the leaflets (N=4). (C) Quantification of collagen levels of the entire aortic valve region including the aortic root (N=4). Data=means±SEM. **p<0.01 vs. AKR/J; ++p<0.01 vs. SAMP1+GFP.
Restoration of serum Klotho levels inhibited MCP-1 and ICAM-1 expression and decreased macrophage infiltration in SAMP1 mice
We further investigated how increased serum Skl inhibited fibrosis on the aortic valve. Our previous study demonstrated that Klotho deficiency mice fed with high fat diet developed FAVD through AMPKα-mediated activation of Runx226. However, immunohistochemical staining of aortic valves against Runx2, BMP2 and BMP4 antibodies did not show any difference between SAMP1, SAMP1+mskl and AKR/J mouse (Fig. S2), suggesting that the Runx2 pathway may not be involved in the development of FAVD in SAMP1 mice.
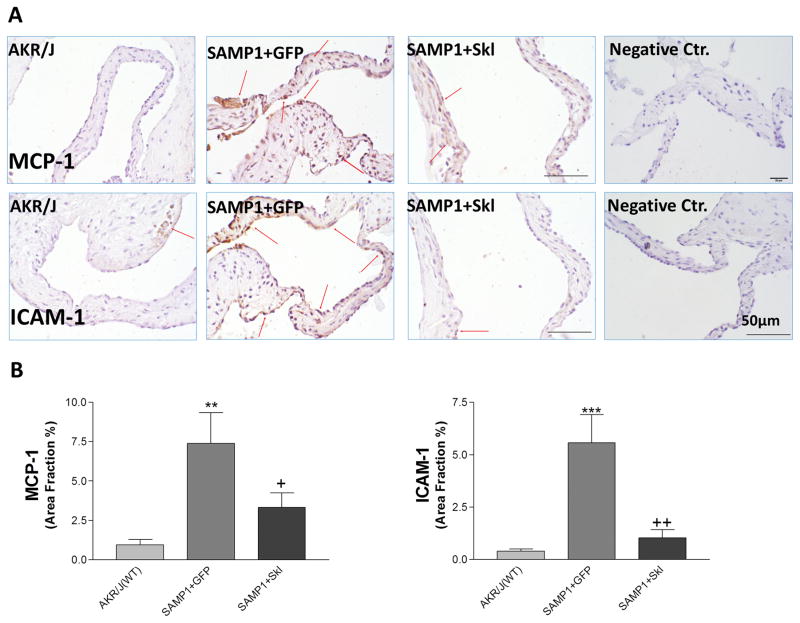
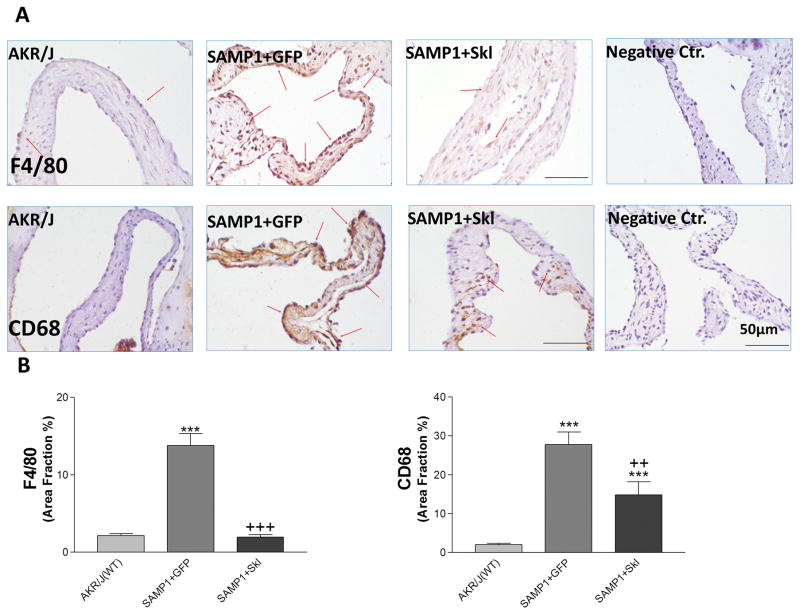
Although SAMP1 is a well-known accelerated aging model, there has been no report about aortic valve diseases in SAMP1 mouse. Monocyte chemoattractant protein 1 (MCP-1) and intercellualr adhesion molecule 1 (ICAM-1) are critical factors that drive monocyte infiltration. Immunohistochemical staining showed that MCP-1 and ICAM-1 levels were increased on the aortic valve of SAMP1 mice, which were decreased significantly by restoration of serum klotho levels (Fig. 3). Consequently, the macrophage marker F4/80 and CD68 was drastically increased in aortic valves of SAMP1 mice (Figure 4). Inflammation would lead to the formation of aortic valve fibrosis in SAMP1 mice. The macrophage infiltration was effectively inhibited by restoration of serum klotho levels (Figure 4). The therapeutic effect of mSKL on FAVD may be attributed in part to its anti-inflammatory action.
Figure 3. Restoration of serum Klotho levels inhibited MCP-1 and ICAM-1 expressionin SAMP1 mice.
(A) IHC staining against MCP-1 and ICAM-1 (brown) on the aortic valves of AKR/J and SAMP1 mice treated with AAV.GFP or AAV.SKL. (B) Quantification of MCP-1 and ICAM-1 expression on the leaflets (N=4). Data=means±SEM. **p<0.01, ***p<0.001 vs. AKR/J; + p<0.05, ++ p<0.01 vs. SAMP1+GFP.
Figure 4. Restoration of serum Klotho levels decreased macrophage infiltration in SAMP1 mice.
(A) IHC staining against F4/80 and CD68 (brown) on the aortic valves of AKR/J and SAMP1 mice treated with AAV.GFP or AAV.SKL. (B) Quantification of F4/80 and CD68 expression on the leaflets (N=4). Data=means±SEM. ***p<0.001 vs. AKR/J; ++p<0.01, +++p<0.001 vs. SAMP1+GFP.
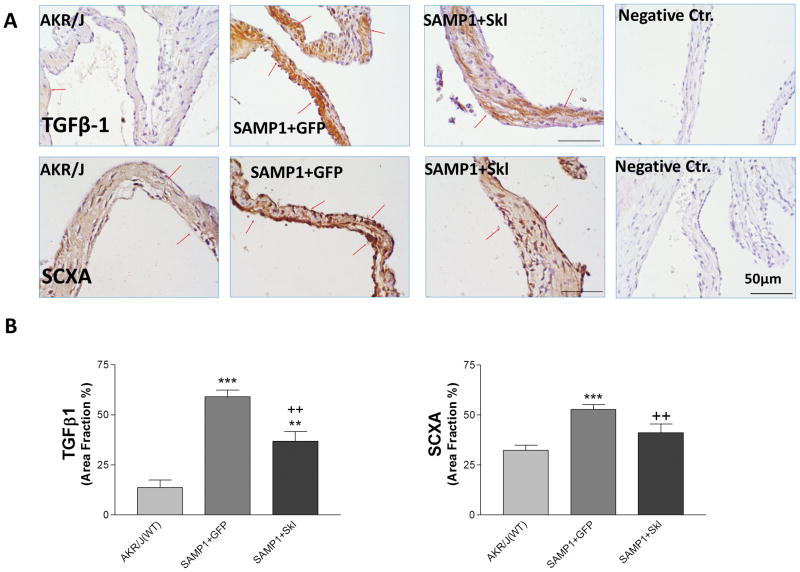
Restoration of serum Klotho levels inhibited upregulation of TGFβ-1 and Scleraxis on the aortic valve
TGFβ-1 plays a central role in several fibrotic diseases in different organs 27, 28 and exhibits anti- or pro-inflammatory roles in immunity. IHC staining of TGFβ-1 on the aortic valve showed that TGFβ-1 was expressed at a very low level in the AKR/J mice but was drastically upregulated in the SAMP1 mice, while restoration of serum Klotho by overexpression of mSKL significantly decreased TGFβ-1 expression on the aortic valves (Fig. 5).
Figure 5. Restoration of serum Klotho levels inhibited upregulation of TGFβ-1 and Scleraxis on the aortic valve.
(A) IHC staining against TGFβ1 and SCXA (brown) on the aortic valves of AKR/J and SAMP1 mice treated with AAV.GFP or AAV.SKL. (B) Quantification of TGFβ1 and SCXA expression on the leaflets (N=4).Data=means±SEM. **p<0.01, ***p<0.001 vs. AKR/J; ++ p<0.01 vs. SAMP1+GFP.
Scleraxis (SCXA)is a member of the basic helix-loop-helix superfamily of transcription factors and has been identified as a potent profibrotic factor regulating collagen genes29–31. It was reported that TGFβ-1 regulated scleraxis expression in cardiac myofibroblasts32. Indeed, upregulation of TGFβ-1 on the aortic valves of SAMP1 mice was associated with increased SCXA expression (Fig. 5), suggesting that the TGFβ-1 and scleraxis pathway may be involved in the collagen over-synthesis. Restoration of serum Klotho attenuated the upregulation of TGFβ-1 and SCXA, suggesting that serum Klotho levels regulate the TGFβ-1-SCXA pathway in collagen synthesis in aortic valves.
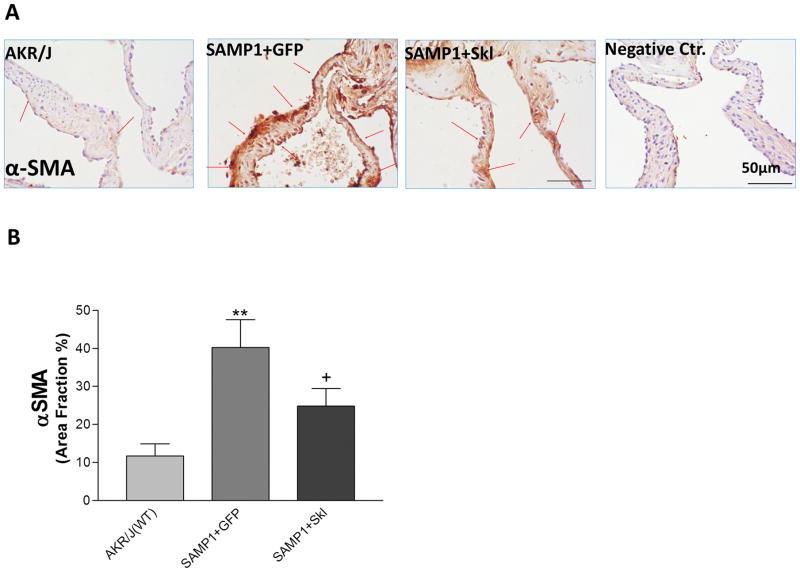
Restoration of serum Klotho inhibited differentiation of aortic valve interstitial cells (AVICs) into myofibroblasts
Myofibroblast differentiation and activation is a critical pathogenetic event leading to human fibrotic diseases 33, 34 and is induced by TGFβ1 35. Smooth muscle actin (α-SMA), a marker of myofibroblasts, was expressed at a very low level on the normal aortic valve but was robustly expressed on the aortic valves of SAMP1 mice (Fig. 6). Thus, activation of myofibroblastsmay playan important role in aortic valve fibrosis formation in this model. Restoration of serum Klotho by in vivo expression of SKL significantly decreased the expression of α-SMA (Fig. 6), suggesting SKL inhibits myofibroblast activation and collagen fibril remodeling in ECM most likely through downregulation of TGFβ1 expression on the aortic valve.
Figure 6. Restoration of serum Klotho inhibited differentiationof aortic valve interstitial cells (AVICs) into myofibroblast.
(A) IHC staining against α-SMA on the aortic valves of AKR/J, SAMP1 mice treated with AAV.GFP or AAV.SKL. (B) Quantification of α-SMA expression on the leaflets (N=4). Data=means±SEM. **p<0.01 vs. AKR/J; +p<0.05 vs SAMP1+GFP.
Restoration of serum Klotho attenuated upregulation of TRAP and MMP-2 expression on the aortic valve
TRAP, an osteoclast marker and MMP-2, a protein involved in ECM remodeling are expressed in a moderate level in the AVICs. Both proteins play important roles in maintaining the structure and mechanic properties of aortic valves. On the aortic valve of SAMP1 mice, IHC against TRAP and MMP-2 antibodies showed that the expression of TRAP and MMP-2 were significantly increased (Fig. S3). The upregulation of TRAP and MMP-2 were attenuated by restoration of serum Klotho (Fig. S3).
Discussion
This study demonstrated for the first time that senescence accelerated mice (SAMP1) developed FAVD which may be a useful model for studying aging-related aortic valve fibrosis. The novel finding is that FAVD may be largely attributed to a decline in serum level of Klotho (Fig. 1&2), an aging-suppressor protein secreted by other organs (e.g., kidneys). Restoration of serum Klotho by in vivo delivery of AAV2.Skl effectively attenuated FAVD in SAMP1 mice. Therefore, Skl may serve as an effective therapeutic target for FAVD. It is known that serum levels of Klotho decrease gradually after age 4010, while the prevalence of aortic stenosis increases with age11. At age 75, serum level of Klotho is only about half of what it was at age 4010. In this study, we found that serum level of Klotho was reduced by ~50% in SAMP1 mice (Fig. 1), which mimics the reduction of Klotho protein levels in the aged population10. Aortic valve disease (AVD) including fibrotic aortic valve disease (FAVD) is a leading cause of adult heart diseases36, 37 and is the most common form of acquired valvular disease in the US11, 38, 39. FAVD eventually leads to aortic valve stiffening and aortic stenosis. Unfortunately, there currently is no effective therapy for FAVD due to unknown etiology. Therefore, pharmacological introduction of secreted Klotho protein may be worthy of further investigations for the treatment of FAVD. Chemical induction of Klotho40 may be tested to treat FAVD.
The SAMP1 mouse is known for its defective immune function and enhanced chronic inflammation in the digestive system41–44. We found a robust inflammatory response in the aortic valves in SAMP1 mice which is characterized by increased levels of MCP-1 and ICAM-1 and infiltration of monocytes/macrophages (Figs. 3&4). MCP-1 and ICAM-1 attracts monocytes to the injury sites and promote a transition of monocytes to macrophages. The inflammatory damage is a critical factor that causes aortic valve fibrotic remodeling. Aortic valve inflammation is likely due to a decrease in serum Klotho levels in SAMP1 mice because restoration of serum Klotho significantly attenuated inflammation (Fig. 3&4). Klotho directly interacts with valvular interstitial cells and regulates their functions and homeostasis26. Klotho deficiency impairs vascular cells45, 46 which elicits immune and inflammatory responses by releasing chemokines or cytokines. This study provides the first evidence that Klotho may serve as an anti-inflammatory factor that inhibits inflammation in aortic valves. The anti-inflammatory action of Klotho may contribute to its anti-fibrotic effect in aortic valves in SAMP1 mice. Although Klotho deficiency may directly cause valvular injury and inflammation, it may also contribute to valvular inflammation indirectly via mechanisms due to Klotho deficiency. For example, Klotho deficiency is known to cause hypertension9 which may contribute to valvular inflammation indirectly. Indeed, we cannot exclud the possibility that the anti-inflammatory effect of Skl in aortic valves may be partially due to a reduction of blood pressure. A further study is needed to test this interesting hypothesis.
A technical challenge for the study of AVD in mice is the limited amount of aortic valve tissue available for molecular assays. We realize the limitation of the immunohistochemical assays, which provides only semi-quantitative analysis. It should be mentioned that in vivo delivery of Skl mainly results in expression of Skl in the kidney12, a major source of Skl in the blood9. Klotho is not expressed in aortic valves or vessels, but the circulating Skl has direct action on valvular and vascular cells9. Thus, serum klotho plays a critical role in the regulation of valvular function. It is interesting that a kidney-derived protein is essential in the maintenance of normal valvular homeostasis.
It should be mentioned that SAMP1 mice developed moderate high blood pressure which was also attenuated by AAV.Skl (Fig. S4). Although Skl has direct action on valvular cells,26 we cannot exclude the possibility that the observed improvement in FAVD may be partially attributed to a reduction of hypertension by AAV.Skl. The limitation of this study is that it cannot determine whether the beneficial effects of AAV.Skl on FAVD is due to the direct actions of Skl on valvular cells or secondary to attenuation of high blood pressure. A further study is warranted to investigate whether there is relationship between hypertension and FAVD.
TGFβ1 and its downstream factor SCXA were upregulated in aortic valves of SAMP1 mice (Fig. 5), which may be attributed to increased valvular inflammation. Activation of myofibroblasts in the AVICs (Fig. 6) which is closely regulated by TGFβ135 also suggests that the inflammatory response may be involved in fibrotic formation in the aortic valves of SAMP1 mice. It is anticipated that activation of myofibroblasts in AVICs caused by inflammation may go through similar molecular mechanisms that govern the myocardial wound healing process 34, 47. Aortic valve is a small but critical piece of apparatus that ensures a unidirectional blood flow in the cardiovascular system. The normal valvular structure and mechanical properties is critical for the maintenance of normal cardiovascular function. Under inflammatory events, activation of myofibroblasts secretes more extracellular matrix (ECM) components including fibrillar collagen proteins47. Increased matrix deposition at this time is required to provide additional structural and tensile strength to the AVICs to prevent aortic valve structural damage. Meanwhile, the matrix-degrading enzymes such as TRAP and MMP-2 also correspondingly increased on the aortic valve of SAMP1 mouse (Fig. S3), suggesting that the AVICs may undergo a compensatory process to maintain the homeostasis of the ECM and normal tissue structure in aortic valves. On the other hand, other MMP enzymes also stimulate matrix deposition.48 Thus, increased MMP-2 activity may be an important mechanism contributing to ECM synthesis. Because fibrosis may promote aortic valve calcification6, this potent self-protective mechanism of AVICs through cell differentiation and ECM remodeling would eventually lead to aortic valve stiffness, stenosis and calcification if this process continues to shift to fibrogenesis and osteogenesis direction.
Scleraxis (SCXA), a member of the basic helix-loop-helix (bHLH) family of transcription factors, is specifically expressed in tendons and ligaments. SCXA is a critical transcription factor for collagen synthesis. This study showed that SCXA is also expressed in aortic valves (Fig. 5). An increase in scleraxis is sufficient to drive expression of the collagen 1α2 gene in primary cardiac fibroblasts.29, 30 Therefore, upregulation of SCXA (Fig. 5) may contribute to the collagen accumulation and ECM remodeling on the aortic valve. Overall, this study sheds light on a new pathway that may mediate the aortic valve fibrotic formation (FAVD) (Fig. S5).
Perspective
The findings suggest that Klotho played an important role in ECM remodeling in aortic valves in SAMP1 mice. This study demonstrated for the first time that restoration of serum Klotho levels attenuated FAVD partly through inhibition of inflammation on the aortic valve. Also, this study may offer novel insights for using anti-aging protein Klotho as a new therapeutic agent to treat FAVD in the aged population.
Supplementary Material
Novelty and Significance.
1. What is new?
It is novel that senescence accelerated mice (SAMP1) developed aortic valve fibrosis, making it an unique animal model for studying fibrotic aortic disease (FAVD).
This study provides the first evidence that secreted Klotho suppresses senescence-associated inflammation and myofibroblastic transition in aortic valves.
The present finding suggests that there may be a potential association between hypertension and FAVD.
2. What is relevant?
It is significant that restoration of serum level of secreted Klotho (SKL)by AAV2-SKL attenuates aortic valve fibrosis in SAMP1 mice. SKL may be a potential therapeutic target for FAVD.
This study reveals that downregulation of serum Klotho may be involved in the pathogenesis of senescence-associated FAVD, an independent risk factor for aortic stenosis and heart failure.
3. Summary
Senescence-related FAVD in SAMP1 mice is associated with a decrease in serum klotho leading to inflammation including macrophage infiltration and TGFβ-1/Scleraxis-driven myofibroblast differentiation in aortic valves. Restoration of serum SKL levels by AAV2-SKL effectively suppresses inflammation and myofibroblastic transition and attenuates aortic valve fibrosis.
Acknowledgments
Source of Funding
This work was supported by NIH R01 HL118558, AG049780, HL122166, HL116863, DK093403, HL105302, and HL102074.
This publication was made possible by NIH Grant Number 1 P30 GM122744-01 from the COBRE Program of the National Institute of General Medical Sciences (NIGMS).
Footnotes
Disclosures: Nothing to disclose
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG investigators Pt. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–91. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res. 2013;113:209–22. doi: 10.1161/CIRCRESAHA.113.300153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 8.Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney international. 2010;77:855–60. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–93. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–7. [PubMed] [Google Scholar]
- 11.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–7. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Sun Z. In Vivo Pancreatic beta-Cell-Specific Expression of Antiaging Gene Klotho: A Novel Approach for Preserving beta-Cells in Type 2 Diabetes. Diabetes. 2015;64:1444–58. doi: 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Sun Z. Antiaging Gene Klotho Attenuates Pancreatic beta-Cell Apoptosis in Type 1 Diabetes. Diabetes. 2015;64:4298–311. doi: 10.2337/db15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Zhou X, Sun Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension. 2015;66:1006–13. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa T, Hosono M, Higuchi K, Aoike A, Kawai K, Takeda T. Immune responses in newly developed short-lived SAM mice. I. Age-associated early decline in immune activities of cultured spleen cells. Immunology. 1987;62:419–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T. A new murine model of accelerated senescence. Mechanisms of ageing and development. 1981;17:183–94. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 18.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. Journal of the American Geriatrics Society. 1991;39:911–9. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 19.Varshney R, Ali Q, Wu C, Sun Z. Monocrotaline-Induced Pulmonary Hypertension Involves Downregulation of Antiaging Protein Klotho and eNOS Activity. Hypertension. 2016;68:1255–1263. doi: 10.1161/HYPERTENSIONAHA.116.08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PG, Sun Z. AAV Delivery of Endothelin-1 shRNA Attenuates Cold-Induced Hypertension. Hum Gene Ther. 2017;28:190–199. doi: 10.1089/hum.2016.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crosswhite P, Chen K, Sun Z. AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension. 2014;64:1141–50. doi: 10.1161/HYPERTENSIONAHA.114.03791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Chen K, Lei H, Sun Z. Klotho Gene Deficiency Causes Salt-Sensitive Hypertension via Monocyte Chemotactic Protein-1/CC Chemokine Receptor 2-Mediated Inflammation. J Am Soc Nephrol. 2015;26:121–32. doi: 10.1681/ASN.2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Chen K, Wang Y, Schuman M, Lei H, Sun Z. Antiaging Gene Klotho Regulates Adrenal CYP11B2 Expression and Aldosterone Synthesis. J Am Soc Nephrol. 2016;27:1765–76. doi: 10.1681/ASN.2015010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan J, Sun Z. The Antiaging Gene Klotho Regulates Proliferation and Differentiation of Adipose-Derived Stem Cells. Stem Cells. 2016;34:1615–25. doi: 10.1002/stem.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Sun Z. Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 beta-cells. Endocrinology. 2012;153:3029–3039. doi: 10.1210/en.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Lin Y, Sun Z. Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKalpha-mediated activation of RUNX2. Aging Cell. 2016;15:853–60. doi: 10.1111/acel.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nature reviews Drug discovery. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochimica et biophysica acta. 2009;1792:746–56. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Bagchi RA, Czubryt MP. Synergistic roles of scleraxis and Smads in the regulation of collagen 1alpha2 gene expression. Biochimica et biophysica acta. 2012;1823:1936–44. doi: 10.1016/j.bbamcr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. Journal of molecular and cellular cardiology. 2009;47:188–95. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. The Journal of biological chemistry. 2007;282:17665–75. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 32.Zeglinski MR, Roche P, Hnatowich M, Jassal DS, Wigle JT, Czubryt MP, Dixon IM. TGFbeta1 regulates Scleraxis expression in primary cardiac myofibroblasts by a Smad-independent mechanism. American journal of physiology Heart and circulatory physiology. 2016;310:H239–49. doi: 10.1152/ajpheart.00584.2015. [DOI] [PubMed] [Google Scholar]
- 33.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–9. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. The New England journal of medicine. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. The Journal of biological chemistry. 2003;278:12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 37.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 38.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner H. Aortic stenosis: medical and surgical management. Heart. 2005;91:1483–8. doi: 10.1136/hrt.2004.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung D, Xu Y, Sun Z. Induction of anti-aging gene klotho with a small chemical compound that demethylates CpG islands. Oncotarget. 2017;8:46745–46755. doi: 10.18632/oncotarget.18608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter BK, Pastorelli L, Brogi M, Garg RR, McBride JA, Rowlett RM, Arrieta MC, Wang XM, Keller EJ, Feldman SH, Mize JR, Cominelli F, Meddings JB, Pizarro TT. Spontaneous, immune-mediated gastric inflammation in SAMP1/YitFc mice, a model of Crohn’s-like gastritis. Gastroenterology. 2011;141:1709–19. doi: 10.1053/j.gastro.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, Chieppa M, Arseneau KO, Ley K, Cominelli F. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–84. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strober W, Nakamura K, Kitani A. The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease. The Journal of clinical investigation. 2001;107:667–70. doi: 10.1172/JCI12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, Imaoka A, Okada Y, Umesaki Y. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y, Chen J, Sun Z. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension. 2016;67:564–73. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: potential therapeutic approaches. Experimental biology and medicine. 2013;238:461–81. doi: 10.1177/1535370213489441. [DOI] [PubMed] [Google Scholar]
- 48.Olszynski K, Zimowska M. Structure and function of matrix metalloproteinases. Postepy Biochem. 2009;55:76–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.