Abstract
Background and Purpose
The effects of high-intensity cycling as an adjuvant therapy for early-stage Parkinson's disease (PD) were highlighted recently. However, patients experience difficulties in maintaining these cycling training programs. The present study investigated the efficacy of cycling at a mild-to-moderate intensity in early-stage PD.
Methods
Thirteen PD patients were enrolled for 16 serial cycling sessions over a 2-month period. Motor function was assessed using the Unified Parkinson's Disease Rating Scale part III (UPDRS III) and Timed Up and Go (TUG) test as primary outcomes. The Montreal Cognitive Assessment (MoCA), modified Hoehn and Yahr Stage (mHYS), total UPDRS, Falls Efficacy Scale, New Freezing of Gait Questionnaire, Schwab and England Activities of Daily Living, 39-item Parkinson's Disease Questionnaire, Patient Global Impression of Change, and gait performance were assessed as secondary outcomes.
Results
The age and the age at onset were 59.67±7.24 and 53.23±10.26 years (mean±SD), respectively. The cycling cadence was 53.27±8.92 revolutions per minute. The UPDRS III score improved significantly after 8 training sessions (p=0.011) and 16 training sessions (T2) (p=0.001) in the off-state, and at T2 (p=0.004) in the on-state compared to pretraining (T0). The TUG duration was significantly shorter at T2 than at T0 (p<0.05). The findings of MoCA, total UPDRS, double limb support time, and mHYS (in both the off- and on-states) also improved significantly at T2.
Conclusions
Our pioneer study has demonstrated that a low-intensity progressive cycling exercise can improve motor function in PD, especially akinesia. The beneficial effects were similar to those of high-intensity rehabilitation programs.
Keywords: Parkinson's disease, exercise, cycling, gait
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disorder that is primarily caused by the loss of dopamine neurons, which leads to reductions in the level of the dopamine neurotransmitter. PD presents with impairment of motor function and automaticity. Motor symptoms that may be associated with deficits of automaticity are akinesia, slowness of simple repetitive movements, reduced arm swing and stride length, gait freezing, micrographia, and facial movement deficits.1,2 Dopaminergic medications and surgical interventions—particularly deep-brain stimulation in the advanced stages—are effective treatments for PD patients. However, the current therapeutic interventions only partially relieve motor symptoms, and they do not slow or modify the disease progression. Moreover, dopaminergic drugs may ultimately induce dyskinesia, motor fluctuations, and other psychological behaviors. These complications have a tremendous impact on the quality of life (QOL) of PD patients. Noninvasive rehabilitation modalities are an important adjunct therapy for early-stage PD to prolong the beneficial effects of motor symptomatic control and delay the motor complications.
Physical exercise was proposed as one of the best nonpharmacological strategies with positive influences on PD.3 Many types of physical exercises—including treadmill training, resistance training, biking, tai chi, tango, and boxing—improve motor function and the nonmotor cognitive abilities in PD patients.4 The neurobiological mechanisms underlying the effects of these exercises are not fully understood. Monteiro-Junior et al.5 proposed two hypothetic mechanisms for the effects of physical exercise: 1) stimulating the synthesis of neurotransmitters (e.g., dopamine)6,7 and neurotrophic factors (e.g., brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, fibroblast growth factor 2, and insulin-like growth factor 1),8,9 and 2) reducing chronic oxidative stresses via simulation of mitochondrial biogenesis and the up-regulation of autophagy.10,11 These mechanisms might slow neurodegeneration and possibly resume structural and functional neuroplasticity; together these effects could ultimate result in physical exercise attenuating the motor symptoms of PD.5
Previous studies have demonstrated that long-term, short-term,12,13,14,15 or even single-session16 forced cycling at a higher cadence improved the motor function and gait speed17,18 in PD patients. Long-term aerobic cycling exercise also improved cognitive and procedural functioning in early PD.18,19 However, PD patients experience difficulty with motor skills due to akinesia, and they may perceive strenuous exercise as strained, sloppy, and less efficient.20 Lauhoff et al.21 emphasized that approximately one-third of PD patients were unable to perform moderate-intensity aerobic cycling training. Several studies have demonstrated side effects of strenuous exercise, such as a temporary depression of various aspects of immune function.22,23,24,25 Recent studies found that the responses of blood leukocyte toll-like receptors are impaired in PD.25,26 We therefore hypothesized that cycling training at a mild-to-moderate intensity would be more suitable for PD patients.
The efficacy of long-term, low-intensity progressive cycling has not been investigated previously in PD patients. We designed a pioneer study to investigate the possible improvement of motor function in patients with early-stage PD undergoing 16-session low-intensity cycling for 8 weeks. The primary outcomes for motor function were alterations of the off- and on-states on the Unified Parkinson's Disease Rating Scale part III (UPDRS III)27 and in the Timed Up and Go (TUG) test.28 Secondary outcomes were alterations of the findings for the Montreal Cognitive Assessment (MoCA), modified Hoehn and Yahr Stage (mHYS), on-state total UPDRS, Falls Efficacy Scale (FES), New Freezing of Gait Questionnaire (NFOGQ), Schwab and England Activities of Daily Living (SE-ADL), 39-item Parkinson's Disease Questionnaire (PDQ-39), Patient Global Impression of Change (PGI-C), and gait performance. Possible adverse events were recorded during the study.
METHODS
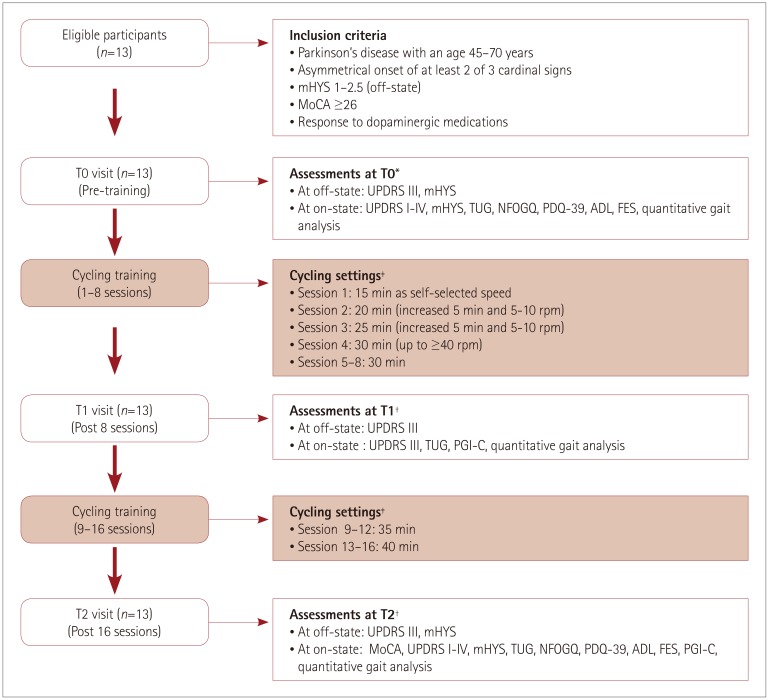
The Institutional Review Board of Chang Gung Medical Foundation approved this study (IRB No. 104-8171A3). Participants signed an informed-consent form prior to participation. Fig. 1 shows the study diagram and outcome measures.
Fig. 1. Study flow diagram and outcome measures. T0: baseline test, T1: midterm test, T2: posttraining test. *Off-state assessment, 12 hours overnight withdrawal of all antiparkinsonian agents (24 hours withdrawal for extended-released dopaminergic agents), †Off-state assessment, 2–3 days later off cycling and 12 hours overnight withdrawal of all antiparkinsonian agents (24 hours withdrawal for extended-released dopaminergic agents), ‡Each session contained a 5-min warm up, main cycling setting, and a 5-min cool down. ADL: Activities of Daily Living, FES: Falls Efficacy Scale, mHYS: modified Hoehn and Yahr Stage, MoCA: Montreal Cognitive Assessment, NFOGQ: New Freezing of Gait Questionnaire, PDQ-39: 39-item Parkinson's Disease Questionnaire, PGI-C: Patient Global Impression of Change, TUG: Timed Up and Go, UPDRS: Unified Parkinson's Disease Rating Scale.
Participants
A neurologist specializing in movement disorders according to the diagnostic criteria proposed by Gelb et al.29 diagnosed patients with idiopathic PD. Patients were eligible if they fulfilled the following criteria: age between 45 and 70 years, asymmetrical onset of at least two of three cardinal signs, early-stage PD (mHYS30 from 1 to 2.5 during the off-state), and MoCA31 score of 26 or higher. Patients were ineligible if they exhibited any of the following characteristics: 1) history of neurological disease other than PD, 2) previous neurosurgery for PD, 3) moderate-to-severe dyskinesia, 4) unstable medical or psychiatric comorbidities, 5) orthopedic conditions restricting exercise, or 6) performing aerobic exercise for longer than 20 min at least three times weekly prior to the study. Patients were maintained on their usual medical treatment throughout the study.
Study procedures
Each patient initially performed a baseline test (T0), which was followed by a midterm test (T1) after 8 training sessions and a posttraining test (T2) after completing 16 training sessions. All clinical assessments were performed after 12 hours of overnight withdrawal of anti-Parkinsonian medications (the withdrawal period was 24 hours for those taking prolonged-release dopaminergic agonists). UPDRS III and mHYS scores were assessed in the off-state on the subsequent morning. Each patient self-administered their medications. The scores for total UPDRS,32 mHYS, TUG, NFOGQ,33 PDQ-39,34 SE-ADL,35 FES,36 and quantitative gait analysis were assessed 40–60 min later in the best on-state. UPDRS subscores were analyzed as follows: tremor (item 20: tremor at rest), rigidity (item 21: rigidity), akinesia (items 23–26: finger taps, hand movements, rapid alternating movements of the hands, and leg agility), and postural instability gait disorder (PIGD) (items 29 and 30: gait and postural stability). PGI-C37 was self-assessed at T1 and T2.
Intervention
Patients were scheduled to perform cycling training twice weekly for 8 weeks, for a total of 16 sessions during their on-states. The intervention program included an initial phase of dose titration for 2 weeks and the late phase of dose maintenance for 6 weeks. Each session included a 5-min warm up, main cycling phase, and a 5-min cool down. A standard stationary bicycle was used. The cycling session began with 15 min of cycling at a self-selected cadence in the titration phase, and it increased in steps of 5 min and 5 to 10 revolutions per minute (rpm). The intensity of cycling reached at least 40 rpm and was maintained at that rate for 30 min in the fourth session. The cycling time was extended by 5 min every 4 sessions in the maintenance phase, in addition to maintaining the 40-rpm cadence, such as 30 min for 5–8 sessions, 35 min for 9–12 sessions, and 40 min for 13–16 sessions. Blood pressure, heart rate (HR), rating of perceived exertion (on the Borg scale),38 and saturation of peripheral oxygen (SpO2) were monitored before, during (in the middle phase), and after each training session. If the HR exceeded 50–55% of the individual's maximum HR (defined as 220 minus the age in years in accordance with the Karnoven formula),39 then the trainer asked the patient to slow the cycling cadence so that the HR reduced to below the maximum HR.
Quantitative gait analysis
Gait performance was analyzed using GAITRite (CIR Systems, Inc., Franklin, NJ, USA) on a 3.66-m-long and 0.9-m-wide instrumented walkway.40 Patients were instructed to walk the length of the walkway twice at their preferred walking speed. The outcomes of the gait assessments were the gait speed, step length, step width, step time, and double limb support time (DLST).
Statistics
All statistical analyses were performed using SPSS (version 22.0, IBM Corp., Armonk, NY, USA) software. Descriptive statistics are reported as mean±SD values. The Friedman test was used to compare the levodopa equivalent daily dosage (LEDD), UPDRS III, TUG, and gait parameters between T0, T1, and T2. The Wilcoxon signed-rank test was applied when a significant difference was detected. This test was used to compare measures of MoCA, mHYS, UPDRS I, UPDRS II, UPDRS IV, total UPDRS, FES, NFOGQ, SE-ADL, and PDQ-39 between T0 and T2. The significance level was set at p<0.05.
RESULTS
Baseline characteristics
Table 1 presents the baseline demographic data of the patients. Thirteen eligible patients (31 % females) were enrolled and completed the study. They were aged 59.67±7.24 years (range: 47–68 years), and their age at onset was 53.23±10.26 years (range: 35–65 years). The disease duration was 6.44±4.04 years (range: 2–14 years), LEDD was 594.62±245.55 mg/daily (range: 350–1010 mg/daily), and the MoCA score was 27.62±1.61 (range: 26–30 in Table 2). All patients remained on the same dosage of anti-Parkinsonism drugs throughout the study.
Table 1. Baseline characteristics.
| Variables | Mean±SD | Range |
|---|---|---|
| AAE (years) | 59.67±7.24 | 47–68 |
| Gender (% of female) | 31% | F/M=4/9 |
| Duration of education (years) | 14.69±2.63 | 12–19 |
| Weight (kg) | 66.04±9.73 | 50–81 |
| Height (cm) | 164.27±4.99 | 155–170 |
| BMI | 24.37±2.61 | 19.29–28.7 |
| AAO (year) | 53.23±10.26 | 35–65 |
| DD (year) | 6.44±4.04 | 2–14 |
| LEDD (mg/daily) | 594.62±245.55 | 350–1,010 |
AAE: age at examination, AAO: age at onset, BMI: body mass index, DD: duration of disease, F: female, LEDD: levodopa equivalent daily dosage, M: male.
Table 2. Primary and secondary outcomes measures assessed at T0, T1, and T2.
| T0 | T1 | T2 | p | p′ | Change | ES | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-training | Post-8 sessions | Post-16 sessions | T0 vs. T1 | T1 vs. T2 | T0 vs. T2 | T0-T1 | T1-T2 | T0-T2 | Post-16 sessions | ||
| Primary outcomes | |||||||||||
| UPDRS III (off) | 23.38±7.97 | 18.85±6.91 | 15.92±6.06 | 0.000 | 0.011 | 0.007 | 0.001 | −4.54±5.21 | −2.92±3.07 | −7.46±4.45 | −1.66 |
| Tremor subscores | 0.85±1.07 | 0.77±1.36 | 0.77±1.09 | 0.939 | 0.705 | 1.00 | 0.564 | −0.08±0.76 | 0.00±0.58 | −0.08±0.49 | −0.16 |
| Rigidity subscores | 4.69±2.93 | 4.77±2.74 | 4.38±2.81 | 0.303 | 0.558 | 0.248 | 0.304 | 0.08±3.52 | −0.38±1.12 | −0.31±3.33 | −0.09 |
| Akinesia subscores | 9.54±3.76 | 6.54±2.85 | 5.54±2.33 | 0.001 | 0.009 | 0.041 | 0.003 | −3.00±3.32 | −1.00±1.53 | −4.00±2.71 | −1.48 |
| PIGD subscores | 1.69±0.85 | 1.23±0.83 | 0.85±0.69 | 0.003 | 0.058 | 0.096 | 0.005 | −0.46±0.78 | −0.38±0.77 | −0.85±0.69 | −1.23 |
| UPDRS III (on) | 15.46±5.56 | 14.00±6.65 | 11.38±5.68 | 0.009 | 0.254 | 0.037 | 0.004 | −1.46±3.97 | −2.62±4.01 | −4.08±3.55 | −1.15 |
| Tremor subscores | 0.31±0.63 | 0.62±1.19 | 0.31±0.75 | 0.779 | 0.414 | 0.414 | 1.000 | 0.31±1.18 | −0.31±1.18 | 0.00±0.41 | 0.00 |
| Rigidity subscores | 3.77±2.62 | 3.31±2.02 | 2.54±2.5 | 0.145 | 0.337 | 0.151 | 0.106 | −0.46±1.61 | −0.77±1.79 | −1.23±2.52 | −0.49 |
| Akinesia subscores | 5.92±2.69 | 5.15±2.88 | 4.46±2.22 | 0.033 | 0.280 | 0.102 | 0.005 | −0.77±2.20 | −0.69±1.60 | −1.46±1.20 | −1.22 |
| PIGD subscores | 1.38±1.12 | 0.92±0.86 | 0.62±0.77 | 0.012 | 0.058 | 0.157 | 0.015 | −0.46±0.78 | −0.31±0.75 | −0.77±0.83 | −0.93 |
| TUG (on) | 7.36±1.19 | 7.18±1.38 | 6.86±0.89 | 0.006 | 0.422 | 0.133 | 0.003 | −0.18±0.70 | −0.32±0.79 | −0.50±0.57 | −0.88 |
| Secondary outcomes | |||||||||||
| MoCA | 27.62±1.61 | - | 29.23±0.83 | - | - | - | 0.004S | - | - | 1.62±1.45 | 1.12 |
| mHYS (off) | 1.96±0.48 | - | 1.62±0.62 | - | - | - | 0.014S | - | - | −0.35±0.38 | −0.92 |
| mHYS (on) | 1.81±0.66 | - | 1.46±0.63 | - | - | - | 0.024S | - | - | −0.35±0.43 | −0.81 |
| UPDRS I (on) | 1.92±1.04 | - | 1.38±0.65 | - | - | - | 0.100S | - | - | −0.54±1.13 | −0.48 |
| UPDRS II (on) | 7.38±3.10 | - | 5.69±3.09 | - | - | - | 0.056S | - | - | −1.69±2.78 | −0.61 |
| UPDRS IV (on) | 0.00±0.00 | - | 0.00±0.00 | - | - | - | 1.000S | - | - | 0.00±0.00 | - |
| Total UPDRS (on) | 24.77±7.78 | - | 18.46±7.92 | - | - | - | 0.005S | - | - | −6.31±5.17 | −1.22 |
| FES | 31.15±12.21 | - | 29.15±10.86 | - | - | - | 0.824S | - | - | −2.00±10.46 | −0.19 |
| NFOGQ | Non-freezer | Non-freezer | Non-freezer | - | - | - | - | - | - | - | - |
| SE-ADL (%) | 91.54±3.76 | - | 91.54±3.76 | 1.000S | - | - | 0.00±4.08 | - | |||
| PDQ-39 | 23.15±15.76 | - | 18.62±11.84 | - | - | - | 0.421S | - | - | −4.54±14.27 | −0.32 |
| PGI-C (%) | |||||||||||
| Very much improved | - | 1, 7.69 | 1, 7.69 | - | - | - | - | - | - | - | - |
| Much improved | - | 4, 30.77 | 5, 38.46 | - | - | - | - | - | - | - | - |
| Minimally improved | - | 7, 53.85 | 6, 46.15 | - | - | - | - | - | - | - | - |
| No change | - | 1, 7.69 | 1, 7.69 | - | - | - | - | - | - | - | - |
Change in negative value indicates improvement: mHYS, UPDRS, TUG, PDQ-39, FES.
Mean±SD, p: Friedman test, α=0.05; p′: Wilcoxon signed rank test, α=0.017; S: Wilcoxon signed rank test, α=0.05; ES (Chohn's d)=mean of change/SD; 0.2 small effect; 0.5 medium effect; 0.8 large effect; T0: Pre-training; T1: Post 8-session training, T2: Post 16-session training; Tremor subscores: item 20 (tremor at rest); Rigidity subscores: item 22 (rigidity); Akinesia subscores; items 23–26 (finger taps, hand movements, rapid alternating movements of the hands, and leg agility); PIGD subscores; items 29–30 (gait, postural stability).
ES: effect size, FES: Falls Efficacy Scale, mHYS: Modified Hoehn and Yahr, MoCA: Montreal Cognitive Assessment, NFOGQ: New Freezing of Gait Questionnaire, PDQ-39: 39-item Parkinson's Disease Questionnaire, PGI-C: Patient Global Impression of Change, SD-ADL: Schwab and England Activities of Daily Living, TUG: Timed Up and Go, UPDRS: Unified Parkinson's Disease Rating Scale.
Cycling exercise training intensity
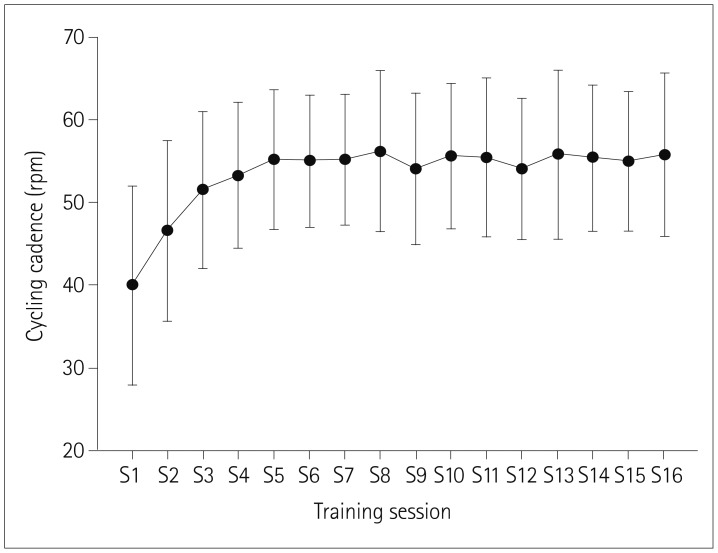
Fig. 2 shows the cycling cadence for the 16 training sessions. The cycling cadence was 40.00±12.03 rpm (range: 22.5–60.0 rpm) at the first training session, which corresponded to the self-selected speed for each participant. Patients reached their individual optimal exercise intensity at the fourth session and maintained this intensity until the final session. The overall cycling cadence was 53.27±8.92 rpm (range: 42–70 rpm), and the estimated power output was 76.14±33.15 watts (range: 48.45–131.71 watts).
Fig. 2. Changes in cycling cadence during 16 training sessions of training (S1–S16).
Primary outcomes
Table 2 lists the results for the primary outcomes. UPDRS III scores, akinesia, and PIGD subscores improved progressively and significantly from T0 to T2 in both the off- and on-states. However, tremor and rigidity subscores did not improve in either state. Notably, UPDRS III scores (p=0.015) and akinesia subscores (p=0.002) improved predominantly in the off-state. This significant difference in the reduction between the on- and off-states indicated that cycling was more beneficial to off-state motor function in PD.
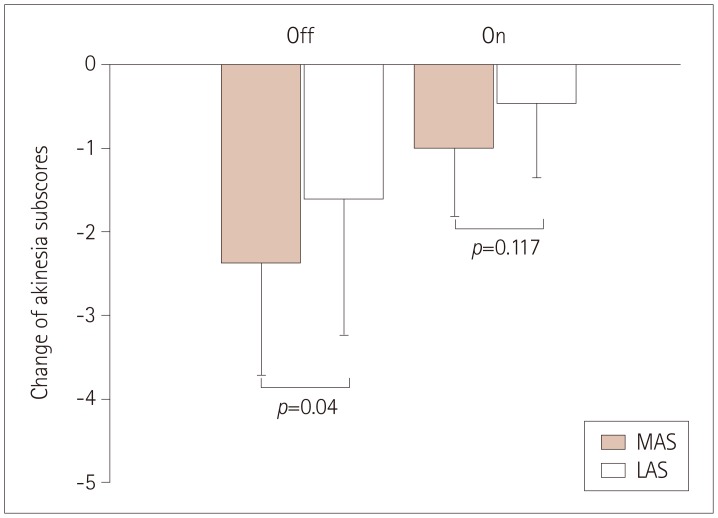
Further analysis revealed that the reductions in the akinesia subscores on the most-affected side (MAS) and less-affected side (LAS) at T2 were 2.38±1.33 and 1.62±1.61, respectively (p=0.04), in the off-state, and 1.00±0.82 and 0.46±0.88 (p=0.117) in the on-state, which indicates that cycling training was more beneficial to off-state motor function on the MAS (Fig. 3). The TUG duration also improved significantly from T0 to T2 in the on-state (p=0.003). The primary outcomes demonstrated that the observed effect sizes were large after 16 sessions of cycling. Two patients complained of mild muscle soreness in the lower extremities during the first cycling session, but this lasted only 2 days and did not recur.
Fig. 3. The improvement of akinesia subscores differed between the MAS and the LAS in both the on- and off-states after 16 sessions of cycling training. LAS: less-affected side, MAS: most-affected side.
Secondary outcomes
Table 2 summarizes the results for the secondary outcomes measures, and Table 3 lists the gait performance outcomes. Outcomes including the MoCA (p=0.004), off-state mHYS (p=0.014), on-state mHYS (p=0.024), and total UPDRS (p=0.005) scores improved significantly from T0 to T2, while the FES, SE-ADL, and PDQ-39 scores did not improve. The DLST became progressively shorter following cycling from T0 to T1 and T2, but other gait parameters did not change significantly. All patients expressed satisfaction with the full course of cycling training. The individual PGI-C scores improved at T1 and T2 in 12 patients (92%).
Table 3. Gait performance assessed at T0, T1, and T2 with preferred speed.
| Gait parameters | T0 | T1 | T2 | p | Change (T0–T1) | Change (T0–T2) |
|---|---|---|---|---|---|---|
| Gait speed (cm/sec) | 109.78±16.45 (103.9) | 117.43±13.27 (111.54) | 115.46±9.67 (113.52) | 0.368 | 7.65±13.82 (6.74) | 5.68±13.11 (4.06) |
| Step length (cm) | 55.57±5.48 (56.06) | 57.83±4.08 (58.29) | 57.77±3.52 (57.41) | 0.125 | 2.25±4.36 (2.45) | 2.19±4.24 (1.89) |
| MAS | 55.05±5.73 (54.05) | 57.56±4.33 (58.01) | 57.29±4.21 (56.38) | 0.232 | 2.51±4.28 (2.74) | 2.23±4.35 (2.46) |
| LAS | 56.09±5.44 (56.39) | 58.09±4.03 (58.8) | 58.25±3.05 (57.85) | 0.794 | 2.00±4.60 (0.93) | 2.15±4.42 (1.90) |
| Step width (cm) | 9.81±2.48 (10.6) | 10.04±2.41 (10.39) | 10.08±2.40 (10.43) | 0.500 | 0.23±0.80 (0.49) | 0.27±0.74 (0.35) |
| MAS | 9.86±2.42 (10.75) | 10.06±2.34 (10.84) | 10.12±2.39 (10.46) | 0.584 | 0.21±0.86 (0.48) | 0.26±0.70 (0.35) |
| LAS | 9.77±2.56 (10.44) | 10.02±2.51 (9.93) | 10.05±2.41 (10.39) | 0.500 | 0.25±0.80 (0.46) | 0.28±0.81 (0.35) |
| Step time (sec) | 0.51±0.04 (0.51) | 0.50±0.04 (0.50) | 0.50±0.03 (0.50) | 0.232 | −0.02±0.03 (−0.02) | −0.01±0.03 (0.00) |
| MAS | 0.52±0.04 (0.51) | 0.50±0.04 (0.50) | 0.51±0.04 (0.50) | 0.199 | −0.02±0.03 (−0.02) | −0.01±0.03 (−0.01) |
| LAS | 0.50±0.04 (0.49) | 0.49±0.04 (0.49) | 0.50±0.03 (0.49) | 0.146 | −0.01±0.02 (−0.01) | 0.00±0.03 (−0.01) |
| Double limb support time (sec) | 0.25±0.04 (0.25) | 0.23±0.04 (0.23)* | 0.23±0.03 (0.23) | 0.023 | −0.02±0.03 (−0.02) | −0.02±0.03 (−0.01) |
| MAS | 0.25±0.04 (0.25) | 0.23±0.04 (0.23)* | 0.23±0.03 (0.23)* | 0.050 | −0.02±0.03 (−0.02) | −0.02±0.03 (−0.01) |
| LAS | 0.25±0.04 (0.25) | 0.23±0.04 (0.23)* | 0.23±0.03 (0.23)* | 0.023 | −0.02±0.03 (−0.02) | −0.02±0.03 (−0.02) |
Mean±SD (medium). p: Friedman test, α=0.05.
*p≤0.05, compared to T0 by using Wilcoxon signed rank test.
LAS: less-affected side, MAS: most-affected side, T0: Pre-training, T1: Post 8-session training, T2: Post 16-session training.
DISCUSSION
To our knowledge, this pioneer study is the first to demonstrate that low-intensity cycling training can improve motor function, especially akinesia, in the off- and on-states. The UPDRS III motor score was reduced 7.46 (31.9% improvement, 7.46/23.38) in the off-state and 4.08 (26.4% improvement, 4.08/15.46) in the on-state. Both of these reductions exceeded the threshold of 2.5 for a minimally clinically important difference (MCID).41 Akinesia subscores on UPDRS III were more than halved (reduced by 54%) in the off-state, which was the main improvement in the remaining subscores. The improvement of the UPDRS III motor score in our patients under low-intensity cycling was similar to that of 35% after an 8-week forced cycling program on a stationary tandem bicycle led by a trainer, and the akinesia score was approximately twofold better than that of 28% reported by Ridgel et al.12 Notably, this score was also approximately twofold higher than the 13.9% improvement of the UPDRS III score in the on-state for three sessions of high-cadence cycling training.15 Akinesia is the most distressing motor disturbance experienced by PD patients, and it may be related to deficits of automaticity42 and affect almost all activities in daily life.43 The improvement in the akinesia subscores predominated over those of the other UPDRS III subscores. Possible explanations were that the motor learning effect was enhanced by these repetitive and alternating lower extremity flexion and extension motions,13 and that motor cortical activation was increased after active training of the upper44 or lower45 extremities, which is thought to be diminished in akinesia.46,47
Mobility and balance also benefited from the present low-intensity progressive cycling training. The coordination of the activities of the quadriceps femoris, popliteal, anterior tibialis, and soleus muscles is crucial to promoting balance and gait performance.48 The TUG duration was significantly shorter (by 0.5 sec) at T2 than at T0 in the present study; this test has been widely used for measuring mobility, balance, and the risk of falling in PD. The present change in the preferred gait speed (5.68 cm/sec) was clinically interesting, which exceeded an MCID of 4 cm/sec for PD patients,49 although no statistical significance was found. The shortened DLST could be related to the improvement of balance control. The faster TUG and gait speed and shorter DLST may have further contributed to the improvement of akinesia and PIGD subscores.
The decreases in UPDRS III scores and akinesia subscores were larger in the off-state than the on-state. Akinesia subscores were reduced more on the MAS than on the LAS. The significant reductions in these scores may indicate that cycling is more beneficial to motor dysfunction in the off-state and on the MAS in PD patients—such a difference in improvements in motor function has not been emphasized previously. Improvement in akinesia was rarely mentioned in previous studies, whereas it might be the most clinically significant improvement in PD patients. mHYS improved significantly after 16 sessions of cycling in both the off- and on-states. These positive results suggest that cycling exercise can slow the progression of PD if such exercise is initiated in very-early-stage or preclinical PD. Long-term regular exercise may also attenuate the severity of early PD.50,51 There are several reports of regular exercise reducing the subsequent PD risk.52,53,54 If exercise can really reduce the risk of PD, then it is probable that the progression of very-early-stage PD could also be slowed.
The mechanisms underlying the improvement of motor function in PD patients after cycling are not known. Several hypothetic explanations can be proposed. Firstly, cycling may enhance both extrinsic and intrinsic feedbacks. For example, complex and variable sensory input increases sensory feedback from the periphery and the subsequent activation of basal ganglia circuits, which may enhance central motor processing.13 Secondly, the pedals of a stationary bicycle inherently offer PD patients the mechanical constraint of a constant movement amplitude.55,56 Thirdly, cycling may correct the coupling between central commands and the biomechanical constraints of the legs.57 These features are consistent with the positive results obtained in the present study that applied progressive cycling at a mild-to-moderate intensity.
Previous clinical and animal studies found that high-intensity exercise was associated with greater positive impacts on PD.16,58,59,60 However, PD patients are already hampered by various motor disabilities (e.g., rigidity and bradykinesia), which may substantially restrict their ability to perform high-intensity exercise, possibly making this impractical even in early-stage PD.
We used an active, lower-cadence, and lower-intensity cycling exercise to overcome these problems and avoid the possible side effects of higher-intensity exercise. The initial cadence in our intervention program was based on the preferences of individual patients, and the cycling duration and intensity were then progressively increased, which contrasts with previous training programs that focused on forced cycling, higher-cadence cycling, and/or aerobic cycling. The mode of training in our study was adjusted so as to optimize the cycling intensity for individual patients, which depended on their physical condition. This methodology allowed our PD patients to maintain a relatively stable and consistent cadence during each session. Ridgel et al.61 also demonstrated that guidance from a trainer acted as a stabilizing influence on patients performing forced cycling. Stabilizing the cadence is an important factor for improving the positive effects of cycling in PD. However, this concept remains to be evaluated in different settings, such as when PD patients are choosing their own cadence.
The findings of this study provide evidence that exercise can reduce the risks of cognitive deficit in PD. The MoCA scores improved significantly (from 27.62 to 29.23) after the 16-session cycling exercise program. This improvement in cognitive function after low-intensity cycling is consistent with previous reports of the effects of various modalities and intensity levels of exercise.4,58,62 The learning effect might be considered a contribution factor. However, the cycling intensity that is the most beneficial to cognition remains to be determined. In contrast to the previous suggestion that a higher intensity of aerobic exercise produces greater cognitive gains, David et al.58 reported that a modified exercise program involving nonaerobic conditioning exercises might improve cognitive outcomes. Our results demonstrate that low-intensity cycling also improved cognitive function in early-stage PD patients. Future studies should investigate the effects of various exercise intensities on cognition and the possible underlying mechanisms.
The QOL and falls self-efficacy did not improve in the present study. We presume that the patients enrolled in this study had a relatively high QOL. Their pretraining PDQ-39 and SE-ADL scores were 23% and 92%, respectively, and both of these metrics have high thresholds for normal scores. The scores indicated that the QOL of the included patients was less impacted by PD disability, and that they were almost completely independent in performing the activities of daily living. Inconsistent effects on mood, QOL, and falls self-efficacy were also found previously in PD for exercise trials.63,64 Some studies found that exercise did not improve the QOL of PD patients, but that combining medications and exercise can transform a potentially debilitating disease into a livable condition.65 Regular exercisers were associated with better QOL, slower progression of disease, lower caregiver burden, and less cognitive decline after 1 year.66 Together these findings indicate that exercise therapy should be emphasized from when the very early symptoms of PD first appear.
The current data indicate that PD patients who perform low-intensity progressive cycling exhibit significant improvements in motor function and cognition. These findings provide important insights that will be helpful for the development of rehabilitation interventions for PD. However, several methodological limitations should be considered when assessing the reported outcomes. Firstly, the extensive measurements for primary and secondary outcomes prevented us from enrolling more patients in a larger-scale study and including matched controls in another arm for comparison. No postintervention follow-up was performed to assess the retention effect. It is ethically difficult to require PD patients to stop physical exercise after a cycling training program if they have benefited from physical activity. Therefore, the possibility of neuroprotection was not determined in the present pioneer study.
Secondly, UPDRS, and particularly UPDRS III, should be applied by investigators who are blinded, rather than the trainer involved in implementing the intervention. We also evaluated the TUG duration and performed quantitative gait analysis in order to avoid subjective bias. The TUG duration was significantly faster than pretraining, and the increase in gait speed after the 16 training sessions exceeded the MCID. Both the subjective and objective data demonstrated a trend of improved mobility.
Thirdly, the off-state motor-function evaluation was completed after anti-Parkinsonian medications had been withdrawn for 12–24 hours. However, such a withdraw might not totally eliminate long-duration responses to medications. All three off-state assessments of the individual patients strictly followed the same procedure and evaluation time point throughout the study period in order to minimize changes in motor function caused by medications.
Fourthly, while the present low-intensity cycling training program provided many clinical benefits to patients with early-stage PD, whether low-intensity cycling training is suitable for advanced-stage PD patients is questionable. Therefore, further large-scale, randomized, and controlled trials are needed to evaluate the efficacy of a low-intensity cycling training regimen in PD patients at different stages. Moreover, the optimal intensity and training duration of low-intensity cycling training also stills needs to be identified and validated in early-stage PD patients.
In conclusion, the present study has demonstrated that low-intensity progressive cycling training is efficient, safe, and feasible for patients with early-stage PD. This mode of cycling exercise improved motor function, especially akinesia, with its beneficial effects being similar to those reported for high-intensity rehabilitation programs. We suggest that low-intensity progressive cycling training could serve as a new therapeutic adjuvant for the treatment of early-stage PD.
Acknowledgements
The authors thank all of the participants for their contributions to this study. This work were supported by the Chang Gung Medical Foundation (grant number CMRPG3F1341, CMRPG3F1342, BMRP127 to CS Lu; CMRPD1G0041 to YJ Chang), and the Ministry of Science and Technology, Taiwan (grant number MOST 106-2218-E-182-003 to YJ Chang).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis. 2015;82:226–234. doi: 10.1016/j.nbd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32:514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- 3.Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J Clin Neurol. 2015;11:212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman JG, Weintraub D. Advances in the treatment of cognitive impairment in Parkinson's disease. Mov Disord. 2015;30:1471–1489. doi: 10.1002/mds.26352. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro-Junior RS, Cevada T, Oliveira BR, Lattari E, Portugal EM, Carvalho A, et al. We need to move more: neurobiological hypotheses of physical exercise as a treatment for Parkinson's disease. Med Hypotheses. 2015;85:537–541. doi: 10.1016/j.mehy.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. doi: 10.1111/j.1600-0404.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- 7.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 8.da Silva PG, Domingues DD, de Carvalho LA, Allodi S, Correa CL. Neurotrophic factors in Parkinson's disease are regulated by exercise: evidence-based practice. J Neurol Sci. 2016;363:5–15. doi: 10.1016/j.jns.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 10.Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 11.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- 12.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23:600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 13.Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson's disease. Exerc Sport Sci Rev. 2011;39:177–186. doi: 10.1097/JES.0b013e31822cc71a. [DOI] [PubMed] [Google Scholar]
- 14.Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson's disease patients. J Physiol Pharmacol. 2014;65:441–448. [PubMed] [Google Scholar]
- 15.Ridgel AL, Phillips RS, Walter BL, Discenzo FM, Loparo KA. Dynamic high-cadence cycling improves motor symptoms in Parkinson's disease. Front Neurol. 2015;6:194. doi: 10.3389/fneur.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridgel AL, Peacock CA, Fickes EJ, Kim CH. Active-assisted cycling improves tremor and bradykinesia in Parkinson's disease. Arch Phys Med Rehabil. 2012;93:2049–2054. doi: 10.1016/j.apmr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 17.McGough EL, Robinson CA, Nelson MD, Houle R, Fraser G, Handley L, et al. A tandem cycling program: feasibility and physical performance outcomes in people with Parkinson disease. J Neurol Phys Ther. 2016;40:223–229. doi: 10.1097/NPT.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 18.Nadeau A, Lungu O, Duchesne C, Robillard MÈ, Bore A, Bobeuf F, et al. A 12-week cycling training regimen improves gait and executive functions concomitantly in people with Parkinson's disease. Front Hum Neurosci. 2017;10:690. doi: 10.3389/fnhum.2016.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchesne C, Lungu O, Nadeau A, Robillard ME, Boré A, Bobeuf F, et al. Enhancing both motor and cognitive functioning in Parkinson's disease: aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70:183–190. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauhoff P, Murphy N, Doherty C, Horgan NF. A controlled clinical trial investigating the effects of cycle ergometry training on exercise tolerance, balance and quality of life in patients with Parkinson's disease. Disabil Rehabil. 2013;35:382–387. doi: 10.3109/09638288.2012.694962. [DOI] [PubMed] [Google Scholar]
- 22.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, et al. Effect of high-versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med. 1994;15:199–206. doi: 10.1055/s-2007-1021047. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen BK, Bruunsgaard H. How physical exercise influences the establishment of infections. Sports Med. 1995;19:393–400. doi: 10.2165/00007256-199519060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyne DB. Regulation of neutrophil function during exercise. Sports Med. 1994;17:245–258. doi: 10.2165/00007256-199417040-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ronsen O, Pedersen BK, Øritsland TR, Bahr R, Kjeldsen-Kragh J. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J Appl Physiol. 2001;91:425–434. doi: 10.1152/jappl.2001.91.1.425. [DOI] [PubMed] [Google Scholar]
- 26.da Silva DJ, Borges AF, Souza PO, de Souza PR, Cardoso CR, Dorta ML, et al. Decreased toll-like receptor 2 and toll-like receptor 7/8-induced cytokines in Parkinson's disease patients. Neuroimmunomodulation. 2016;23:58–66. doi: 10.1159/000443238. [DOI] [PubMed] [Google Scholar]
- 27.Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov Disord. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 28.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 29.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 31.Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, Wadia P, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson's disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson's disease rating scale characteristics and structure. the cooperative multicentric group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 33.Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 34.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(Suppl 1):S10–S14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Martin P, Prieto L, Forjaz MJ. Longitudinal metric properties of disability rating scales for Parkinson's disease. Value Health. 2006;9:386–393. doi: 10.1111/j.1524-4733.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 36.Dewan N, MacDermid JC. Fall efficacy scale-international (FES-I) J Physiother. 2014;60:60. doi: 10.1016/j.jphys.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 38.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 39.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 40.Barker S, Craik R, Freedman W, Herrmann N, Hillstrom H. Accuracy, reliability, and validity of a spatiotemporal gait analysis system. Med Eng Phys. 2006;28:460–467. doi: 10.1016/j.medengphy.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 42.Marsden CD. Slowness of movement in Parkinson's disease. Mov Disord. 1989;4(Suppl 1):S26–S37. doi: 10.1002/mds.870040505. [DOI] [PubMed] [Google Scholar]
- 43.Hallett M. Bradykinesia: why do Parkinson's patients have it and what trouble does it cause? Mov Disord. 2011;26:1579–1581. doi: 10.1002/mds.23730. [DOI] [PubMed] [Google Scholar]
- 44.Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 45.Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- 46.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, et al. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol. 1992;32:749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- 48.Katz-Leurer M, Sender I, Keren O, Dvir Z. The influence of early cycling training on balance in stroke patients at the subacute stage. Results of a preliminary trial. Clin Rehabil. 2006;20:398–405. doi: 10.1191/0269215505cr960oa. [DOI] [PubMed] [Google Scholar]
- 49.Hass CJ, Bishop M, Moscovich M, Stegemöller EL, Skinner J, Malaty IA, et al. Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther. 2014;38:233–238. doi: 10.1097/NPT.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 50.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat Disord. 2012;18(Suppl 1):S147–S150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 53.Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006;21:1419–1424. doi: 10.1002/mds.20998. [DOI] [PubMed] [Google Scholar]
- 56.Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132:2151–2160. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- 57.Lacquaniti F, Grasso R, Zago M. Motor patterns in walking. News Physiol Sci. 1999;14:168–174. doi: 10.1152/physiologyonline.1999.14.4.168. [DOI] [PubMed] [Google Scholar]
- 58.David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, et al. Exercise improves cognition in Parkinson's disease: the PRET-PD randomized, clinical trial. Mov Disord. 2015;30:1657–1663. doi: 10.1002/mds.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridgel AL, Abdar HM, Alberts JL, Discenzo FM, Loparo KA. Variability in cadence during forced cycling predicts motor improvement in individuals with Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2013;21:481–489. doi: 10.1109/TNSRE.2012.2225448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson's disease? J Gerontol A Biol Sci Med Sci. 2011;66:234–240. doi: 10.1093/gerona/glq201. [DOI] [PubMed] [Google Scholar]
- 64.Ebersbach G, Ebersbach A, Edler D, Kaufhold O, Kusch M, Kupsch A, et al. Comparing exercise in Parkinson's disease--the Berlin LSVT®BIG study. Mov Disord. 2010;25:1902–1908. doi: 10.1002/mds.23212. [DOI] [PubMed] [Google Scholar]
- 65.Okun MS. Management of Parkinson disease in 2017: personalized approaches for patient-specific needs. JAMA. 2017;318:791–792. doi: 10.1001/jama.2017.7914. [DOI] [PubMed] [Google Scholar]
- 66.Oguh O, Eisenstein A, Kwasny M, Simuni T. Back to the basics: regular exercise matters in parkinson's disease: results from the National Parkinson Foundation QII registry study. Parkinsonism Relat Disord. 2014;20:1221–1225. doi: 10.1016/j.parkreldis.2014.09.008. [DOI] [PubMed] [Google Scholar]