Abstract
Some porous crystalline solids change their structure upon guest inclusion. Unlocking the potential of these solids for a wide variety of applications requires full characterisation of the response to adsorption and the underlying framework–guest interactions. Here, we introduce an approach to understanding gas uptake in porous metal-organic frameworks (MOFs) by loading liquefied gases at GPa pressures inside the Zn-based framework ZIF-8. An integrated experimental and computational study using high-pressure crystallography, grand canonical Monte Carlo (GCMC) and periodic DFT simulations has revealed six symmetry-independent adsorption sites within the framework and a transition to a high-pressure phase. The cryogenic high-pressure loading method offers a different approach to obtaining atomistic detail on guest molecules. The GCMC simulations provide information on interaction energies of the adsorption sites allowing to classify the sites by energy. DFT calculations reveal the energy barrier of the transition to the high-pressure phase. This combination of techniques provides a holistic approach to understanding both structural and energetic changes upon adsorption in MOFs.
Understanding host–guest interactions and structural changes within porous materials is crucial for enhancing gas storage properties. Here, the authors combine cryogenic loading of gases with high pressure crystallography and computational techniques to obtain atomistic detail of adsorption-induced structural and energetic changes in ZIF-8.
Introduction
Understanding how porous metal organic frameworks (MOFs) interact with guest molecules of commercial interest, and how the framework structure adapts to the presence of these guest molecules, is vital for the further development and commercialisation of MOFs in a wide range of applications1–3. Despite a vast library of synthesised and characterised MOFs, only a small percentage of these frameworks have been investigated extensively to correlate their structure and adsorption properties4. In the MOF subset of the CSD, which contains all of these structures, there are 6, 18, 24, and 23 single crystal structures of MOFs containing modelled Ar, CH4, N2 and O2, respectively—a total of 77 structures. Of these, only 17 were determined at room temperature –3, 9, 3, and 2 for Ar, CH4, N2, and O2, respectively (see Supplementary Fig. 1)4. Whilst reports using techniques such as IR5,6, Raman7 and solid-state NMR spectroscopy8 have inferred structural changes on the binding modes of gases within MOFs, more direct evidence of structural changes can be obtained using in-situ crystallography. Such experiments usually involve a gas capillary cell, where MOFs can be exposed to varying pressures of gas. This equipment is typically housed at dedicated central facilities in order to achieve the highest resolution for the position of guest molecules in the pores9–11. The prototypical MOF chosen for the first ever gas capillary cell experiment was ZIF-8 (Zn6(mIm)12, mIm = 2-methylimidazole)12,13. The structure contains one central nanopore per unit cell, with a volume of ≈2500 Å3 and pore diameter of 11.6 Å14. Connecting these large nanopores are eight six-membered ring (6MR) windows ca. 3.4 Å in diameter, which run through the body diagonals of the unit cell, and six smaller four-membered ring (4MR) windows of ca. 0.8 Å which lie in the faces of the unit cell (Fig. 1)15,16. One reported gas-loading study in ZIF-8 used neutron powder diffraction (NPD) to analyse the adsorption process, and was used to determine the location of six adsorption sites for D217,18. Loading issues for CD4 inhibited data quality preventing the refinement of both the framework and guest molecules. This is a common problem in the study of MOFs and is attributed to a number of factors which include the large void space and weakly scattering guests. These issues make the task of experimentally locating and quantifying adsorption sites with a high degree of confidence challenging19,20.
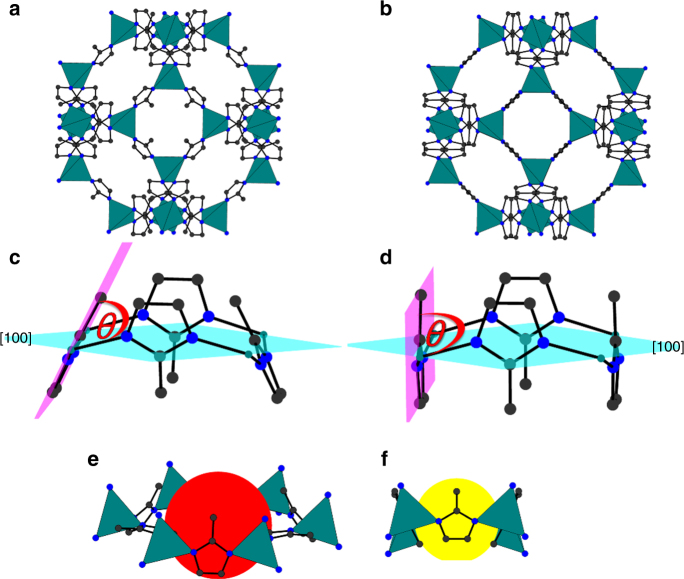
Fig. 1.
Structural differences of ZIF-8-AP and ZIF-8-HP. a ZIF-8-AP with 4MR window in the faces and 6MR windows in the body diagonal. b ZIF-8-HP with mIm linkers rotated by 30° compared to the AP structure c 4MR window showing the opening angle of 68° for ZIF-8-AP, d 4MR window showing the opening angle of 89° for ZIF-8-HP, e 6MR window with pore diameter of 3.0 Å and f 4MR window with pore diameter of 0.8 Å
An alternative to the gas capillary cell is the diamond anvil cell (DAC), a small extreme-pressure device composed of two opposing diamond anvils which can apply GPa pressures to a pressure transmitting medium (PTM) which in turn transmits hydrostatic pressure to a crystalline sample. PTMs are generally liquids or soft solids which, when used to compress porous materials can lead to negative linear compressibility (e.g. in cyanide-bridged frameworks)21,22, pressure-induced amorphisation (as observed in ZIFs)23, and pressured-induced phase transitions, as observed in the high-pressure study of ZIF-813. This last study was the first report to show that ZIF-8 undergoes a displacive phase transition as a result of the PTM penetrating into the pores on increasing pressure (herein, the ambient pressure and high-pressure structures are referred to as ZIF-8-AP and ZIF-8-HP, respectively). The displacive phase transition occurred at 1.47 GPa and is characterised by a re-orientation of the 4MR and 6MR imidazole rings, to form a gate open structure (Fig. 1).
The crystallographic determination of the transition to ZIF-8-HP became instrumental in interpreting the adsorption mechanism in ZIF-8, with the ZIF-8-HP phase being used as a model for in-situ powder diffraction data collected on ZIF-8 with included N2 at more modest pressures (40 kPa)24. This experiment confirmed that the step in the N2 adsorption isotherm at 77 K was induced by the same structural re-arrangement seen in the previous high-pressure study (to ZIF-8-HP). Grand canonical Monte Carlo (GCMC) simulations of an N2 adsorption isotherm in ZIF-8-HP also showed a better agreement in the high-pressure region of the isotherm than with ZIF-8-AP. This demonstrated the power of using high-pressure crystallography in tandem with computational methods to understand adsorption behaviour in MOFs at more modest pressures24.
Using liquefied gases as a PTM in a DAC is common in the fields of high-pressure physics and mineralogy25,26. By using liquefied gases as PTMs, GPa pressures can be reached, facilitating higher occupation of guest molecules inside the pores. This results in atomistic resolution of the position of included gas molecules. In contrast, some gas-loading capillary experiments reported previously for ZIF-817,18 did not report any changes in the framework structure but did report difficulty modelling gas molecules, suggesting only modest levels of gas adsorption. By using GPa pressures, we can also ensure that we can override the particle size dependency associated with the ZIF-8 displacive phase transition27,28. Crucially, the DAC experiments can be carried out routinely using lab sources, making them more accessible than synchrotron gas-cell experiments. Sotelo et al. recently demonstrated the power of liquefied gases as PTMs by loading CH4 and CO2 into the small pore MOF Sc2BDC329. When the pressure of Sc2BDC3-CH4 reached 2.50 GPa, hyperfilling of the framework with supercritical CH4 was possible, allowing the first report of fully occupied CH4 sites in the pores obtained at room temperature. In addition, loading of CO2 to 0.2 GPa revealed a new adsorption site, which had never previously been observed, giving a much better agreement between the calculated uptake from diffraction measurements and experimental isotherm data29.
Here, we present the first combined experimental and computational study of adsorption of small molecules (specifically CH4, Ar, O2 and N2) into ZIF-8. By using the cryogenic loading method we were able to monitor structural changes in the framework upon uptake of these gases, experimentally locating the adsorption sites with high-precision in a MOF with much larger pores29. In addition, we sought to understand the framework responses and energetics of adsorption through plane wave density functional theory (DFT) and GCMC simulations. DFT calculations allowed us to probe the energy landscape of the framework geometry, whilst GCMC simulations of the adsorption process permitted quantification of the adsorption site energies.
Results
Effect of pressure on compressibility, geometry and loading
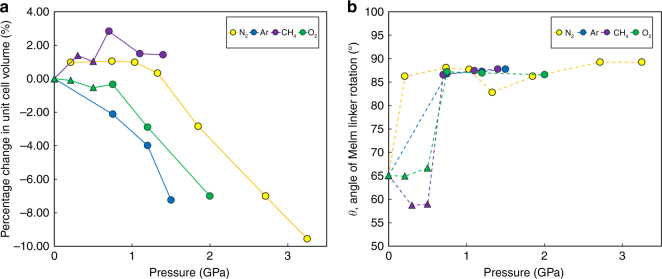
The crystallographic data allowed changes in the framework compressibility, geometry and pore content to be monitored over the pressure range studied. As already highlighted in earlier reports13, the orientation of the mIm linker [defined by the angle θ between the planes of the mIm atoms and the (100) crystallographic plane, see Fig. 1c, d] is particularly sensitive to pressure. Under ambient pressure and temperature conditions, θ is 65.1° and consequently the 4MR and 6MR window diameters measured 0.8 Å and 3.0 Å respectively. Upon sealing the DAC at the lowest possible pressures using CH4 as a PTM (0.30 GPa), the unit cell volume of ZIF-8 increased by 1.39% (Fig. 2). This is indicative of the PTM penetrating into the pores of the framework (Table 1). The increase in electron density was accompanied by a decrease in θ to 58.7°, causing the diameter of the 4MR to decrease from 0.8 to 0.7 Å and the 6MR windows to increase from 3.0 to 3.1 Å. The calculated energy barrier to this small rotation was relatively modest, with an energy penalty of just 0.5 kJ mol-1 per mIm linker (for more information on DFT calculations see Supplementary Fig. 3). On increasing pressure to 0.50 GPa, the unit cell of ZIF-8-AP compressed (by 0.36%) while θ remained essentially unchanged (measuring 58.9°). Such a small change in rotation did not change the 4MR and 6MR window diameters, however the electron density within the pores steadily increased, equating to ca. 33 CH4 molecules/uc (molecules per unit cell). At 0.70 GPa, the unit cell expanded further (by 2.83%), which was accompanied by a displacive phase transition to the ZIF-8-HP phase (Fig. 2), characterised by θ increasing to 86.6°, which caused the diameters of 4MR and 6MR to increase to 2.5 Å and 3.3 Å, respectively13. This was accompanied by a large change in solvent uptake into the pore (equating to ca. 89 CH4 molecules/uc). The energy penalty for the framework to undergo such a large rotation was calculated to be 5.8 kJ mol−1 per mIm linker. The energy of adsorption must, therefore, be greater to overcome this barrier. Increasing the pressure from 0.70 to 1.40 GPa led to compression of the HP phase, decreasing the unit cell volume by 1.40%, while θ continued to increase modestly, measuring 87.8° at 1.40 GPa (which corresponds to a 6.2 kJ mol−1 penalty for rotation per linker). Conversely the 6MR diameter actually decreased in size between 0.70 and 1.40 GPa (by 2.40%) owing to the framework compression. Above 1.40 GPa, crystallinity deteriorated, and no structural data could be extracted.
Fig. 2.
Compression and linker rotation in ZIF-8. a Change in unit cell volume of ZIF-8 with pressure for N2 (yellow), CH4 (red), Argon (blue), O2 (green); b Change in angle of rotation of MeIm linker, θ, in ZIF-8 upon increasing pressure for N2 (yellow), O2 (green), Ar (blue) and CH4 (magenta). Triangles and circles indicate ZIF-8-AP and ZIF-8-HP phases, respectively
Table 1.
Effect of pressure on ZIF-8 for different gases
| Pressure CH4 (GPa) | Unit cell volume (Å3) | % change in uc volume | SAV (Å3) | θ (°) | 4MR diameter (Å) | 6MR diameter (Å) |
|---|---|---|---|---|---|---|
| 0.00 | 4924.5 (2) | 0.00 | 2497 | 65.1 | 0.8 (1) | 3.0 (1) |
| 0.30 | 4993.1 (1) | 1.39 | 2580 | 58.7 | 0.7 (1) | 3.1 (1) |
| 0.50 | 4974.9 (4) | 1.02 | 2551 | 58.9 | 0.7 (1) | 3.1 (1) |
| 0.70a | 5063.8 (4) | 2.83 | 2657 | 86.6 | 2.5 (1) | 3.3 (1) |
| 1.10a | 4998.5 (4) | 1.50 | 2710 | 87.4 | 2.5 (1) | 3.2 (1) |
| 1.40a | 4995.1 (3) | 1.43 | 2586 | 87.8 | 2.5 (1) | 3.2 (1) |
| Pressure O2 (GPa) | Unit cell volume (Å3) | % change in uc volume | SAV (Å3) | θ (°) | 4MR diameter (Å) | 6MR diameter (Å) |
|---|---|---|---|---|---|---|
| 0.00 | 4924.5 (2) | 0.00 | 2514 | 65.1 | 0.8 (1) | 3.0 (1) |
| 0.21 | 4919.8 (5) | −0.10 | 2487 | 64.9 | 0.9 (1) | 2.9 (1) |
| 0.50 | 4898.2 (3) | −0.53 | 2457 | 66.7 | 0.8 (1) | 2.9 (1) |
| 0.75a | 4908.0 (4) | −0.33 | 2522 | 87.2 | 2.2 (1) | 3.6 (1) |
| 1.20a | 4782.0 (9) | −2.89 | 2356 | 86.9 | 2.2 (1) | 3.4 (1) |
| 2.00a | 4579.9 (6) | −7.00 | 2255 | 86.6 | 2.4 (1) | 3.1 (1) |
| Pressure N2 (GPa) | Unit cell volume (Å3) | % change in uc volume | SAV (Å3) | θ (°) | 4MR diameter (Å) | 6MR diameter (Å) |
|---|---|---|---|---|---|---|
| 0.00 | 4924.55 (23) | 0.00 | 2497 | 65.1 | 0.8 (1) | 3.0 (1) |
| 0.21a | 4972.3 (4) | 0.97 | 2548 | 86.2 | 2.5 (1) | 3.4 (1) |
| 0.74a | 4976 (1) | 1.04 | 2525 | 88.0 | 2.3 (1) | 3.5 (1) |
| 1.03a | 4972.8 (9) | 0.98 | 2512 | 87.7 | 2.3 (1) | 3.5 (1) |
| 1.33a | 4940.8 (3) | 0.33 | 2402 | 82.8 | 2.0 (1) | 3.5 (1) |
| 1.85a | 4784.5 (6) | −2.84 | 2364 | 86.2 | 2.0 (1) | 3.5 (1) |
| 2.72a | 4579.3 (5) | −7.01 | 2223 | 89.3 | 2.2 (1) | 3.2 (1) |
| 3.25a | 4454.0 (3) | −9.55 | 2054 | 89.2 | 2.2 (1) | 3.0 (1) |
| Pressure Ar (GPa) | Unit cell volume (Å3) | % change in uc volume | SAV (Å3) | θ (°) | 4MR diameter (Å) | 6MR diameter (Å) |
|---|---|---|---|---|---|---|
| 0.00 | 4924.5 (2) | 0.00 | 2497 | 65.1 | 0.8 (1) | 3.0 (1) |
| 0.75a | 4820.6 (3) | −2.11 | 2458 | 86.7 | 2.5 (1) | 3.3 (1) |
| 1.20a | 4727 (5) | −3.99 | 2405 | 87.3 | 2.4 (1) | 3.3 (1) |
| 1.50a | 4567.9 (4) | −7.24 | 2256 | 87.7 | 2.4 (1) | 3.2 (1) |
Switching the PTM to O2 revealed a similar trend in framework behaviour to that observed with CH4 where there was a compression of the ZIF-8-AP phase marked by the decrease in four parameters: unit cell volume, θ and the diameters of 4MR and 6MR until the onset of the displacive phase transition to the ZIF-8-HP phase (marked by a in Table 1) when all four parameters increased. As the ZIF-8-HP phase was further compressed, the unit cell volume and the diameters of both 4MR and 6MR continued to decrease in size, while θ continued increasing (see Table 1). In the cases of N2 and Ar as PTM, X-ray data is only available after the transition to ZIF-8-HP occurs. These PTM cause the 4MR, 6MR and θ to increase. More in depth information of how each particular gas affected the geometry of ZIF-8 with pressure can be found in Supplementary Note 1.
Adsorption sites and energies in ZIF-8-HP
In addition to monitoring the framework changes that occurred upon gas-loading, information pertaining to the location of adsorbed guest molecules in ZIF-8 could also be obtained, which stands testament to the quality of diffraction data that can be recorded when using liquefied gases as PTMs in DACs. Gas loaded ZIF-8 crystal structures were modelled at 1.40, 0.75, 3.25 and 1.20 GPa in CH4, O2, N2 and Ar PTM, respectively. These pressures were chosen as they met three key criteria: the electron density was at a maximum, the crystallographic data was the most complete and reflections were collected to the highest redundancy (Supplementary Table 1). More details about how the X-ray data were refined can be found in Supplementary Methods. In addition, these crystallographic models and the ZIF-8-AP structure (with all guests removed) were used for GCMC simulations to model the gas uptake. The study of simulated isotherms is an area of active research, which allows theoreticians to explain how structural changes affect the adsorption performance16,30,31. GCMC simulations allowed us to not only determine both the position of gas molecules, but, importantly, the energy of the sites.
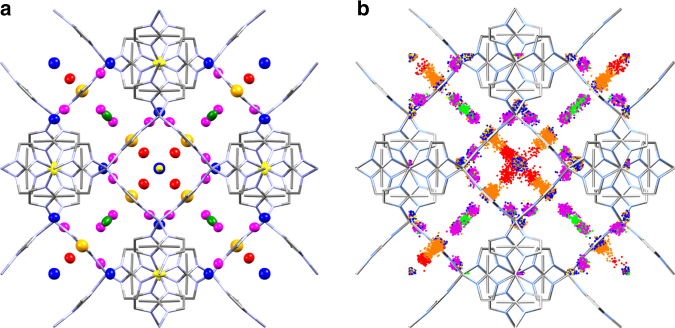
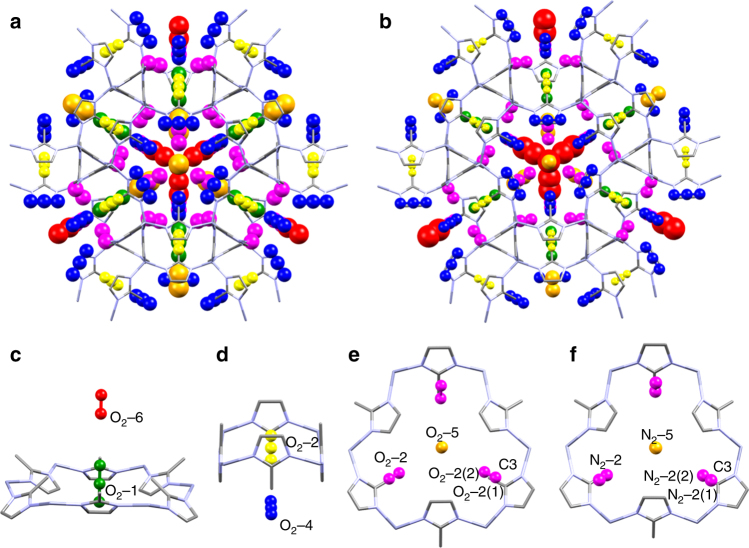
When Ar was used as a PTM at 0.75 GPa, six symmetry-independent adsorption sites could be located within ZIF-8-HP, all of which were fully occupied. The GCMC simulation of Ar with the HP phase gave excellent agreement with those determined from the experimental crystal structure positions (Fig. 3).
Fig. 3.
Comparison of Ar adsorption sites in ZIF-8-HP. a 1.20 GPa (298 K) crystal structure and b GCMC simulations at 100 kPa (at 83 K) of Ar. Colour scheme; Zn (light grey), N (light blue) and C (grey). Hydrogen atoms have been omitted for clarity. In a and b framework atoms are drawn as capped sticks, while Ar atoms are coloured according to the six symmetry-independent sites. In a Ar atoms are drawn with anisotropic displacement parameters (50% probability), while in b Ar atoms are shown as the binned positions with their relative energies from the GCMC simulation
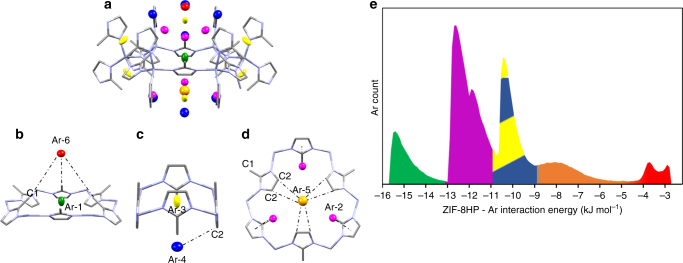
Ar-1 (green, Fig. 4b) resides in the centre of each 6MR window and is the lowest energy (i.e. the most favourable) site, with a framework–argon interaction energy between ca. −16 to −13 kJ mol−1 (Fig. 4e); Ar-2 (magenta, Fig. 4d) is the next most favourable site, and is located between the 4MR and 6MR windows, directly below every other mIm linker in the 6MR window (ca. −13 to −11 kJ mol−1, Fig. 4e). The next two sites have comparable energies of ca. −11 to −9 kJ mol−1: Ar-3 (yellow, Fig. 4c) is found in the centre of the 4MR window whilst Ar-4 (blue, Fig. 4c) sits below this point further into the nanopore. Ar-4 makes a close contact to the 4MR window being at a distance of 3.8118(1) Å from the imidazole C2 carbon (Fig. 4c). Adsorption site Ar-5 (orange, Fig. 4d) sits below the 6MR window equidistant from the mIm ligands, with the shortest contact distance (C2 to Ar-5) measuring 4.8677(1) Å; this long distance is reflected in a broad range of relatively weak energies from −9 to −4 kJ mol−1 (Fig. 4e). Finally Ar-6 (red, Fig. 4b) sits in the centre of the large nanopore above the 6MR windows. The closest non-hydrogen atom contact to Ar-6 measures 5.9078(2) Å, from the methyl (C3) atoms (Fig. 4b). The simulations also confirm that this is a weak binding site, with interaction energies of the order of just ca. 3 kJ mol−1.
Fig. 4.
Crystallographic adsorption sites of Ar in ZIF-8 and their relative computed energies. a Ar adsorption sites in ZIF-8 at 1.20 GPa as shown on a 6MR window for Ar-1 (green), Ar-2 (red), Ar-3 (yellow), Ar-4 (dark blue), Ar-5 (orange), Ar-6 (red). Short contacts for b Ar-6 with the methyl C-atom (C1) and c Ar-4 with the imidazole C-atom (C2) and d Ar-5 (with C2) are drawn as black dotted lines and discussed in the text. e Histogram showing the frequency of guest-framework interaction energies at 100 kPa of guest during GCMC simulation in ZIF-8-Ar using ZIF-8-HP. Sections are coloured according to the adsorption sites Ar-1–Ar-6 (Ar-4 and Ar-5 cover the same energy range). Colour scheme; Zn (light grey), N (light blue) and C (grey). Hydrogen atoms have been omitted for clarity, ellipsoids for Ar-atoms are drawn at 50% probability, whilst the ZIF-8 framework is drawn as capped sticks
Comparison of the energy histograms of framework–guest energies from the GCMC simulations for the ZIF-8-AP and ZIF-8-HP models (see Supplementary Fig. 6) show that the interaction energies are lowered (i.e. are more favourable) in the ZIF-8-HP phase. The difference between the guest-host interaction energies for the AP and HP phases varies between 3 and 7 kJ mol−1 depending on the gas studied (further information on each gas interaction can be found in Supplementary Fig. 6). It is therefore clear that the transition to ZIF-8-HP is driven by the ability to form more favourable guest-host interactions, which, although is not surprising, is gratifying to confirm, but more importantly quantifies the energy gain involved on adsorption.
The position of the six adsorption sites found for Ar are also representative of those found in ZIF-8-HP for CH4 (at 1.40 GPa); a detailed description of the energies of the adsorption sites for this phase can be found in Supplementary Fig. 4, Supplementary Fig. 5 and Supplementary Note 2. Interestingly, this is the first single crystal X-ray structure of ZIF-8 loaded with CH4 in the HP phase. Other studies, at lower pressures of 50 bar, have modelled the electron density of CH4 within PLATON and their results agree with our ZIF-AP-CH4 PLATON results (see Supplementary Table 1)32.
In ZIF-8-HP at 3.25 GPa in N2 and at 1.20 GPa in O2 the adsorption sites are located in the same general positions as Ar, however, some of the sites exhibit positional disorder (Fig. 5). The most energetically favourable (i.e. the site which forms the strongest framework–guest interactions) N2-1 and O2-1 (green, Fig. 5) shows positional disorder perpendicular to the 6MR window, with one atom fully occupied, whilst the other was split across two positions (50% occupied). The shortest non-H contact (from the C2-atom on the mIm ligand) to the central N atom from N2-1 measures 3.44(5) Å. The GCMC simulations agree with the diffraction data showing a range of positions above and below this plane (Fig. 6). N2-2 and O2-2 (magenta, Fig. 5) site shows no crystallographic disorder; it is positioned around a 3-fold axis that goes through the centre of the 6MR window, with each N2- 2 or O2-2 sitting above three of the mIm linkers in the 6MR window. One clear difference between O2-2 and N2-2 is the angle which they make with the mIm linker, defined as the X2-2(1)-X2-2(2)-C3 angle (where X = N or O) (Fig. 5e,f). An angle of 90° would mean the molecule sits parallel to the mIm ring, however for O2-2 the angle is 130° and the respective N2-2 angle is 150°. Consequently it can be seen that N2 will adopt an arrangement closer to an end-on intermolecular interaction (Fig. 5f) than a side-on interaction seen in O2-2 (Fig. 5e).
Fig. 5.
Experimentally-derived unit cell of ZIF-8-HP with N2 and O2 adsorption sites. a Adsorbed N2 molecules at 3.25 GPa, and b adsorbed O2 molecules at 1.20 GPa. In a and b guest shown as ellipsoids (50% probability) and each colour represents symmetry-independent adsorption sites. c 6MR window with sites O2-1 and O2-6. d 4MR window with sites O2-3 and O2-4. e 6MR window with O2-2 and O2-5 showing O2(1)-O2-(2)- C3 angle of 130°. f 6MR window with N2-2 and N2-5, showing N2(1)-N2-(2)- C3 angle of 150°. Note as c and d N2 analogues are similar, their structures are not represented here
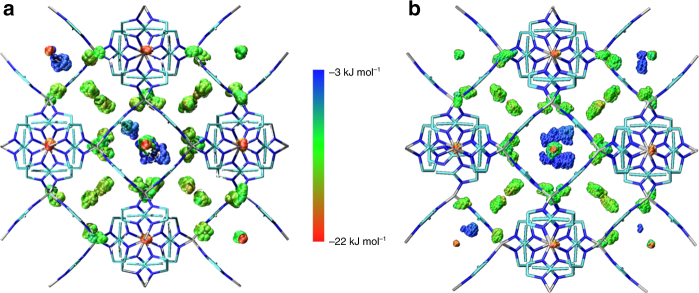
Fig. 6.
GCMC adsorption sites of N2 and O2. a Heat map of N2 in ZIF-8-HP. b Heat map of O2 in ZIF-8-HP. Points are centre of masses of diatomic molecules and the colour represents the framework–fluid interaction energy. Red corresponds to low energy (strong interaction) and blue to high energy (weak interaction)
N2-3 and O2-3 (yellow, Fig. 5) displayed similar disorder to N2-1 and O2-1, but the site was disordered around the plane of the 4MR window, with one fully occupied atom in the plane of the window with a 50% occupied atom above and below the plane. N2-1 to N2-3 and O2-1 to O2-3 are strongly interacting sites, where each diatomic molecule has comparable interaction energies ranging between −22 kJ mol−1 and−12 kJ mol−1 (Fig. 6). This broad range of energies comes from the orientation of the diatomic molecule with respect to the framework. For example in ZIF-8-N2 the interaction energy is reduced if N2-1 does not sit exactly in the plane of the 6MR. In addition, there is an energy penalty if the N2-2 site is found closer to ZIF-8, decreasing from −14 kJ mol−1 to −12 kJ mol−1, as the distance shortens from 4.313 Å to 2.639 Å. From the diffraction data, N2-4 and O2-4 (blue, Fig. 5) were located below the 4MR window, exhibiting similar disorder to N2-1/O2-1 and N2-3/O2-3 and were found to have interaction energies in the region of −12 kJ mol−1 to −9 kJ mol−1. On analysis of the multiple orientations calculated from the GCMC simulations, a wide spread of orientations below the 4-MR ring were observed, where N2-4 and O2-4 are located. This indicates that there is no preferred orientation for either of these pointing toward the methyl group or the C-C backbone of the mIm.
N2-5 and O2-5 (orange Fig. 5) situated below the 6MR window at a distance of 4.10(16) and 4.06(17) from C2, is in close contact with N2-1 or O2-1 with a contact distance of 1.9(3) Å and 1.8(4) Å, for N2-5 and O2-5, respectively. N2-6 and O2-6 (red Fig. 5) sits on the 4-fold axis of rotation going through the 6MR window. The closest guest-framework distance (X…C3, where X = N or O) measures 5.3(2) Å, whilst the closest guest–guest is much shorter measuring 3.8(4) Å and 3.0(2) Å, for N2-6…N2-1 and O2-6…O2-1, respectively. The close contact of these sites with other adsorption sites and the significant distance from the framework may be an indication that these sites are only favourable due to guest–guest interactions rather than interactions with the framework. The GCMC simulations, which inherently have no symmetry imposed on the simulation box unlike the high symmetry (I-3m) imposed in the crystallography, revealed three additional key pieces of information about these sites (N2-5, N2-6, O2-5 and O2-6). Firstly, the positions for these sites were scattered in the pore over the course of the simulation suggesting a flat energy landscape in the centre of the pore. Secondly, the sites were not present at all symmetry equivalent locations throughout the unit cell and lastly, the sites had very weak interaction energies with the framework, around −3 kJ mol−1.
In conclusion, for the first time, high resolution high-pressure single crystal X-ray diffraction studies were combined with GCMC simulations and DFT calculations to understand gas adsorption behaviour. By using a cryogenic method of loading with a DAC, extreme pressures were used as a tool to force liquefied gases into the framework, thus building up occupancy of the gas in the framework. Using high-pressure crystallography, O2, N2, Ar and CH4 were successfully loaded into ZIF-8 and used to determine the adsorption sites inside the framework at room temperature. The wide variety in behaviour confirms the suggestion, in previous crystallographic work by the group and others, that PTMs play a dynamic role in high-pressure studies of MOFs13,33–36. The energies of these crystallographically determined sites were calculated with GCMC simulations. The simulations helped explain a number of unanswered questions from the crystallographic data, including the hierarchy of adsorption sites, the low occupancies observed for some sites and the disorder of the guest molecules. The simulations also gave valuable information to confirm the orientation of the molecules in the pores. This work highlights the necessity of combining high-quality experimental X-ray data with computational methods. With this combined approach, we can monitor the structural changes in a MOF upon uptake of gases, and with theory calculate which interactions are the most favourable. These studies complement conventional adsorption studies by providing a detailed picture of the adsorption mechanism which is essential to understanding the adsorption process of flexible porous materials and their use in practical applications.
Methods
Synthesis of ZIF-8
A solid mixture of zinc(II) nitrate hexahydrate (0.525 g, 1.76 × 10−4 mol) and 2-methylimidazole (mIm; 0.015 g, 1.83 × 10−4 mol) was dissolved in DMF (9 mL) in a 12 mL Teflon-capped vial which was heated at a rate of 200 °C h−1 to 130 °C, held at this temperature for 24 h, and then cooled at a rate of 5 °C h−1 to room temperature. Colourless polyhedral crystals were filtered from the reaction mixture, washed with methanol (3 × 5 mL), and dried in air (30 min).
High-pressure cryogenic loading
Each gas was cryogenically loaded into a DAC using the following basic procedure. A single crystal of ZIF-8 together with a chip of ruby (for pressure calibration) were loaded in a modified Merrill–Bassett DAC with 600 μm culet diamonds and a tungsten gasket37,38. Springs were placed on the pins of the DAC and a calibration performed to determine the open and closed positions of the DAC. The device was then placed inside a cryogenic gas-loading chamber (see Supplementary Fig.2), in the calibrated closed position and the chamber placed in a bath of liquid N2 to equilibrate to 77 K. The gas (N2, O2, Ar or CH4) was purged through the chamber until condensation occurred. The DAC was then opened to a pre-calibrated open position whilst in the bath of liquefied N2 and the sample chamber exposed to the condensed gas for approximately 30 seconds before closing. The DAC was then removed from the bath and allowed to warm to room temperature before the pressure inside the cell was measured using the ruby fluorescence method37.
High-pressure X-ray diffraction
High-pressure diffraction studies were collected on a Bruker APEX II diffractometer with graphite-monochromated Mo Kα radiation (0.71073 Å). Each gas loaded sample was studied over the following pressure regimes: CH4 loaded in ZIF-8 (ZIF-8-CH4) from 0.30 GPa to 1.40 GPa, O2 loaded in ZIF-8 (ZIF-8-O2) from 0.21 GPa to 2.00 GPa, N2 loaded in ZIF-8 (ZIF-8-N2) from 0.21 GPa to 3.25 GPa and Ar loaded in ZIF-8 (ZIF-8-Ar) from 0.75 GPa to 1.50 GPa. Upon increasing pressure further, sample deterioration resulted in loss of resolution. Structure refinements were carried out to the maximum resolution of each sample as determined from the intensity statistics. Data were collected for each pressure point in ω scans in eight settings of 2θ and ϕ, based on the strategy of Dawson et al. with an exposure time per frame of forty seconds and a step size of 0.5°39. The data were integrated using dynamic masks (generated using the programme ECLIPSE), in order to avoid regions of the detector shaded by the DAC, while the absorption corrections for the DAC and sample were carried out using the programme SADABS40,41. The data were then merged in XPREP42.
Crystal structure refinements
Structure refinements were carried out in CRYSTALS starting from the ambient pressure structure of Park et al., (refcode VELVOY)12,43. All framework non-hydrogen atoms were refined anisotropically with thermal similarity and vibrational restraints applied to all non-hydrogen atoms except Zn. The SQUEEZE algorithm was applied (probe radius 1.2 Å, grid spacing 0.2 Å) to calculate the electron density in the pores and give an estimate to the number of guest species in the pore as a function of pressure44. The number of guest molecules was corrected for the residual electron density in the ambient pressure data set, where the crystal had been heated to 80 °C and exposed to vacuum for 12 h. The adsorbed gases were refined with crystallographic models at 0.75 GPa for Ar, 1.40 GPa for CH4, 3.25 GPa for N2 and 0.75 GPa for O2. For the adsorbed gases, Ar and C atoms (for methane) were refined with anisotropic displacement parameters, whereas O2 and N2 were refined isotropically with distance, thermal and vibrational restraints applied. This is due to the increased number of parameters which were required to be refined against the data.
Limiting window diameter analysis of the 4MR and 6MR windows in ZIF-8
Each crystallographic structure was analysed in Mercury using the void analysis tools to determine the limiting pore diameter14. Guest molecules, if present in the pore, were removed before void analysis. The grid spacing was set to 0.2 Å and the probe size was increased until the 4MR windows were no longer accessible to solvent. This probe size diameter corresponds to the largest sphere that can be inserted without overlapping the framework atoms. The process was repeated for the 6MR windows of ZIF-8.
Density functional theory single point energy calculations
All calculations were performed using the CASTEP (version 5.11) simulation package45. The Hamiltonian operator was approximated using the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional augmented with the Tkatchenko-Scheffler dispersion correction46,47. The molecular wavefunction description was provided by ‘on-the-fly’ pseudopotentials and a plane wave basis set operating at 650 eV, which gave convergence to within 4 meV per atom. The electronic structure was sampled at the gamma position only in the Brillouin zone due to the large size of the primitive unit cell (resulting in a k-point sampling grid of no greater than 0.06 Å−1). The ambient pressure crystal structure of ZIF-8 was fully optimised without any symmetry constraints to allow both the relaxation of the atomic positions and the unit cell parameters. The potential energy surface was searched for energy minima by means of the Broyden-Fletcher-Goldfarb-Shanno (BFGS) algorithm48. The structure was considered to be optimised when the energy per atom, maximum force, maximum stress and maximum atomic displacement converged to the values of 0.02 meV/atom, 0.05 eV/Å, 0.1 GPa and 0.002 Å, respectively. Once optimised, the coordinates of the methylimidazole linkers, defined as the angle θ between the planes of the mIm atoms and the (100) crystallographic plane [see Fig. 1c], were rotated through five degree increments up to 30° away from the ambient pressure structure coordinates; at each interval single point energy calculations were carried out at the same level of theory as the geometry optimisation. The data were then fitted to a second order polynomial to interpolate data between the 5° rotations.
Grand canonical Monte Carlo simulations
Gas adsorption was simulated using grand canonical Monte Carlo (GCMC) simulations49, implemented in the multipurpose code MuSiC50. The simulations were carried out using atomistic models of the frameworks ZIF-8-AP12 and ZIF-8-HP13. The framework atoms were fixed at their crystallographic positions. At each pressure, 5 × 107 Monte Carlo steps were performed where each step consists of either a random translation, insertion or deletion, and, for O2 and N2, random rotation. The first 40 % of the steps were used for equilibration and the remaining used to calculate the ensemble averages. Standard Lennard-Jones (LJ) potentials were used to model the dispersive interactions between the framework and gases. The parameters for the force field were obtained from previous work from Fairen-Jimenez, who used a modified version of UFF (known as UFF*)16. Mulliken charges were used as the partial charges on the framework—the advantage of using these charges is that they were derived directly from the periodic DFT calculations, and, thus, capture the periodic nature of the MOF. The TraPPE force field was used to model O2, CH4 and N251, while Ar was modelled using LJ parameters fitted to vapour—liquid data52. Coulombic interactions were included for O2 and N2 and calculated using Wolf Coulombic summations53. Interactions beyond 18 Å were neglected. To calculate the gas-phase fugacity the Peng–Robinson equation of state was used54. To gain better statistics for analysis of the positions of O2 and N2 in ZIF-8-HP, NVT MC simulations were performed with the same parameters and a loading corresponding to 100 kPa of O2 or N2 pressure.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1579394-1579417. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via “http://www.ccdc.cam.ac.uk/data_request/cif” www.ccdc.cam.ac.uk/data_request/cif. All other data underpinning this work is freely available via “http://hdl.handle.net/10283/3047”
Electronic supplementary material
Acknowledgements
C.L.H. thanks the EPSRC and the University of Edinburgh for a studentship. C.A.M. acknowledges the UK Car-Parrinello consortium (EP/P022790/1) for access to the high performance computing resource ARCHER, as managed by the Edinburgh Parallel Computing Centre (EPCC). M.F. acknowledges EPSRC grant number EP/J003999/1, and studentship funding from EPSRC grant number EP/G03673X/1. S.A.M. thanks the EPSRC for EP/K033646/1 and EP/N01331X/1. T.D. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 648283).
Author contributions
S.A.M., T.D. and C.A.M. conceived the project. C.L.H. synthesised the materials, conducted high-pressure crystallographic experiments. C.L.H. and C.W. conducted the cryogenic experiments. M.F. and K.K. designed the cryogenic experiment. C.L.H. and M.J.L. performed the Monte Carlo simulations. C.L.H. performed the DFT calculations. All authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03878-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claire L. Hobday, Email: clh65@bath.ac.uk
Tina Düren, Email: t.duren@bath.ac.uk.
Stephen A. Moggach, Email: s.moggach@ed.ac.uk
References
- 1.Chavan S, et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012;14:1614–1626. doi: 10.1039/C1CP23434J. [DOI] [PubMed] [Google Scholar]
- 2.Liu JW, et al. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014;43:6011–6061. doi: 10.1039/C4CS00094C. [DOI] [PubMed] [Google Scholar]
- 3.Smaldone RA, et al. Metalorganic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010;49:8630–8634. doi: 10.1002/anie.201002343. [DOI] [PubMed] [Google Scholar]
- 4.Moghadam PZ, et al. Development of a Cambridge Structural Database subset: a collection of metal–organic frameworks for past, present, and future. Chem. Mater. 2017;29:2618–2625. doi: 10.1021/acs.chemmater.7b00441. [DOI] [Google Scholar]
- 5.Krap CP, et al. Enhancement of CO2 adsorption and catalytic properties by Fe-Doping of [Ga-2(OH)(2)(L)] (H4L = Biphenyl-3,3 ‘,5,5 ‘-tetracarboxylic Acid), MFM-300(Ga-2) Inorg. Chem. 2016;55:1076–1088. doi: 10.1021/acs.inorgchem.5b02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietzel, P. D. C. et al. Adsorption properties and structure of CO(2) adsorbed on open coordination sites of metal-organic framework Ni(2)(dhtp) from gas adsorption, IR spectroscopy and X-ray diffraction. Chem. Commun., 5125-5127, (2008). [DOI] [PubMed]
- 7.Chen Y, Wang H, Li J, Lockard JV. In situ spectroscopy studies of CO2 adsorption in a dually functionalized microporous metal-organic framework. J. Mater. Chem. A. 2015;3:4945–4953. doi: 10.1039/C4TA05524A. [DOI] [Google Scholar]
- 8.Hoffmann HC, et al. Solid-State NMR spectroscopy of metal-organic framework compounds (MOFs) Materials. 2012;5:2537–2572. doi: 10.3390/ma5122537. [DOI] [Google Scholar]
- 9.Savage M, et al. Selective adsorption of sulfur dioxide in a robust metal-organic framework material. Adv. Mater. 2016;28:8705–8711. doi: 10.1002/adma.201602338. [DOI] [PubMed] [Google Scholar]
- 10.Moreau F, et al. Unravelling exceptional acetylene and carbon dioxide adsorption within a tetra-amide functionalized metal-organic framework. Nat. Commun. 2017;8:14085. doi: 10.1038/ncomms14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salas-Colera E, et al. Design and development of a controlled pressure/temperature set-up for in situ studies of solid-gas processes and reactions in a synchrotron X-ray /powder diffraction station. J. Synchrotron Radiat. 2015;22:42–48. doi: 10.1107/S1600577514021018. [DOI] [PubMed] [Google Scholar]
- 12.Park KS, et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA. 2006;103:10186–10191. doi: 10.1073/pnas.0602439103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moggach SA, Bennett TD, Cheetham AK. The effect of pressure on ZIF-8: increasing pore size with pressure and the formation of a high-pressure phase at 1.47 GPa. Angew. Chem. Int. Ed. 2009;48:7087–7089. doi: 10.1002/anie.200902643. [DOI] [PubMed] [Google Scholar]
- 14.Macrae CF, et al. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008;41:466–470. doi: 10.1107/S0021889807067908. [DOI] [Google Scholar]
- 15.Huang AS, Liu Q, Wang NY, Zhu YQ, Caro J. Bicontinuous zeolitic imidazolate framework ZIF-8@GO membrane with enhanced hydrogen selectivity. J. Am. Chem. Soc. 2014;136:14686–14689. doi: 10.1021/ja5083602. [DOI] [PubMed] [Google Scholar]
- 16.Fairen-Jimenez D, et al. Flexibility and swing effect on the adsorption of energy-related gases on ZIF-8: combined experimental and simulation study. Dalton. Trans. 2012;41:10752–10762. doi: 10.1039/c2dt30774j. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Zhou W, Yildirim T. Hydrogen storage in a prototypical zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2007;129:5314–5315. doi: 10.1021/ja0691932. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Zhou W, Yildirim T. Methane sorption in nanoporous metal-organic frameworks and first-order phase transition of confined methane. J. Phys. Chem. C. 2009;113:3029–3035. doi: 10.1021/jp8103276. [DOI] [Google Scholar]
- 19.Carrington EJ, Vitorica-Yrezabal IJ, Brammer L. Crystallographic studies of gas sorption in metal-organic frameworks. Acta Crystallogr. B. 2014;70:404–422. doi: 10.1107/S2052520614009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JP, Zhu AX, Chen XM. Single-crystal X-ray diffraction and Raman spectroscopy studies of isobaric N-2 adsorption in SOD-type metal-organic zeolites. Chem. Commun. 2012;48:11395–11397. doi: 10.1039/c2cc35544b. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin AL, Keen DA, Tucker MG. Large negative linear compressibility of Ag-3[Co(CN)(6)] Proc. Natl Acad. Sci. USA. 2008;105:18708–18713. doi: 10.1073/pnas.0804789105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairns AB, Thompson AL, Tucker MG, Haines J, Goodwin AL. Rational design of materials with extreme negative compressibility: selective soft-mode frustration in KMn[Ag(CN)(2)](3) J. Am. Chem. Soc. 2012;134:4454–4456. doi: 10.1021/ja204908m. [DOI] [PubMed] [Google Scholar]
- 23.Bennett TD, et al. Structure and properties of an amorphous metal-organic framework. Phys. Rev. Lett. 2010;104:15503. doi: 10.1103/PhysRevLett.104.115503. [DOI] [PubMed] [Google Scholar]
- 24.Fairen-Jimenez D, et al. Opening the gate: framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011;133:8900–8902. doi: 10.1021/ja202154j. [DOI] [PubMed] [Google Scholar]
- 25.Ridley CJ, Kamenev KV. High pressure neutron and X-ray diffraction at low temperatures. Z. Krist. 2014;229:171–199. [Google Scholar]
- 26.Parise JB. High pressure studies. Rev. Mineral. Geochem. 2006;63:205–231. doi: 10.2138/rmg.2006.63.9. [DOI] [Google Scholar]
- 27.Zhang C, Gee JA, Sholl DS, Lively RP. Crystal-size-dependent structural transitions in nanoporous crystals: adsorption-induced transitions in ZIF-8. J. Phys. Chem. C. 2014;118:20727–20733. doi: 10.1021/jp5081466. [DOI] [Google Scholar]
- 28.Tian T, Wharmby MT, Parra JB, Ania CO, Fairen-Jimenez D. Role of crystal size on swing-effect and adsorption induced structure transition of ZIF-8. Dalton. Trans. 2016;45:6893–6900. doi: 10.1039/C6DT00565A. [DOI] [PubMed] [Google Scholar]
- 29.Sotelo J, et al. Locating gases in porous materials: cryogenic loading of fuel-related gases Into a Sc-based metal-organic framework under extreme pressures. Angew. Chem. Int. Ed. 2015;54:13332–13336. doi: 10.1002/anie.201506250. [DOI] [PubMed] [Google Scholar]
- 30.Ania CO, et al. Understanding gas-induced structural deformation of ZIF-8. J. Phys. Chem. Lett. 2012;3:1159–1164. doi: 10.1021/jz300292y. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, et al. Adsorption-induced structural transition of ZIF-8: a combined experimental and simulation study. J. Phys. Chem. C. 2014;118:8445–8454. doi: 10.1021/jp500931g. [DOI] [Google Scholar]
- 32.Shekhah O, et al. The liquid phase epitaxy approach for the successful construction of ultra-thin and defect-free ZIF-8 membranes: pure and mixed gas transport study. Chem. Commun. 2014;50:2089–2092. doi: 10.1039/c3cc47495j. [DOI] [PubMed] [Google Scholar]
- 33.Chapman KW, Halder GJ, Chupas PJ. Guest-dependent high pressure phenomena in a nanoporous metal-organic framework material. J. Am. Chem. Soc. 2008;130:10524–10526. doi: 10.1021/ja804079z. [DOI] [PubMed] [Google Scholar]
- 34.Graham AJ, Allan DR, Muszkiewicz A, Morrison CA, Moggach SA. The effect of high pressure on MOF-5: guest-induced modification of pore size and content at high pressure. Angew. Chem. Int. Ed. 2011;50:11138–11141. doi: 10.1002/anie.201104285. [DOI] [PubMed] [Google Scholar]
- 35.Hobday CL, et al. Tuning the swing effect by chemical functionalization of zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2018;140:382–387. doi: 10.1021/jacs.7b10897. [DOI] [PubMed] [Google Scholar]
- 36.Chapman KW, Halder GJ, Chupas PJ. Pressure-induced amorphization and porosity modification in a metal-organic framework. J. Am. Chem. Soc. 2009;131:17546–17547. doi: 10.1021/ja908415z. [DOI] [PubMed] [Google Scholar]
- 37.Piermarini GJ, Block S, Barnett JD, Forman RA. Calibration of pressure-dependence of R1 ruby fluorescence line to 195 Kbar. J. Appl. Phys. 1975;46:2774–2780. doi: 10.1063/1.321957. [DOI] [Google Scholar]
- 38.Moggach SA, Allan DR, Parsons S, Warren JE. Incorporation of a new design of backing seat and anvil in a Merrill Bassett diamond anvil cell. J. Appl. Cryst. 2008;41:249–251. doi: 10.1107/S0021889808000514. [DOI] [Google Scholar]
- 39.Dawson A, Allan DR, Parsons S, Ruf M. Use of a CCD diffractometer in crystal structure determinations at high pressure. J. Appl. Cryst. 2004;37:410–416. doi: 10.1107/S0021889804007149. [DOI] [Google Scholar]
- 40.ECLIPSE (Madison, Wisconsin, USA, 2006).
- 41.Sheldrick, G. M. (SADABS. University of Göttingen, Germany, 2008).
- 42.XPREP (Madison, Wisconsin, USA, 2004).
- 43.Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ. CRYSTALS version 12: software for guided crystal structure analysis. J. Appl. Cryst. 2003;36:1487–1487. doi: 10.1107/S0021889803021800. [DOI] [Google Scholar]
- 44.Spek AL. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003;36:7–13. doi: 10.1107/S0021889802022112. [DOI] [Google Scholar]
- 45.Clark SJ, et al. First principles methods using CASTEP. Z. Krist. 2005;220:567–570. [Google Scholar]
- 46.Tkatchenko A, Scheffler M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009;102:073005. doi: 10.1103/PhysRevLett.102.073005. [DOI] [PubMed] [Google Scholar]
- 47.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 48.Shanno DF. Conditioning of quasi-Newton methods for function minimization. Math. Comput. 1970;24:647. doi: 10.1090/S0025-5718-1970-0274029-X. [DOI] [Google Scholar]
- 49.Frenkel, D. & Smit, B. Understanding Molecular Simulations. 2nd edn, 1-638 (Academic Press, San Diego, 2002).
- 50.Gupta A, Chempath S, Sanborn MJ, Clark LA, Snurr RQ. Object-oriented programming paradigms for molecular modeling. Mol. Simul. 2003;29:29–46. doi: 10.1080/0892702031000065719. [DOI] [Google Scholar]
- 51.Zhang L, Siepmann JI. Direct calculation of Henry’s law constants from Gibbs ensemble Monte Carlo simulations: nitrogen, oxygen, carbon dioxide and methane in ethanol. Theor. Chem. Acc. 2006;115:391–397. doi: 10.1007/s00214-005-0073-1. [DOI] [Google Scholar]
- 52.McGrath MJ, et al. Vapor-liquid nucleation of argon: exploration of various intermolecular potentials. J. Chem. Phys. 2010;133:084106. doi: 10.1063/1.3474945. [DOI] [PubMed] [Google Scholar]
- 53.Wolf D, Keblinski P, Phillpot SR, Eggebrecht J. Exact method for the simulation of Coulombic systems by spherically truncated, pairwise r(-1) summation. J. Chem. Phys. 1999;110:8254–8282. doi: 10.1063/1.478738. [DOI] [Google Scholar]
- 54.Peng DY, Robinson DB. A New Two-Constant Equation of State. Ind. Eng. Chem. Fund. 1976;15:59–64. doi: 10.1021/i160057a011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1579394-1579417. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via “http://www.ccdc.cam.ac.uk/data_request/cif” www.ccdc.cam.ac.uk/data_request/cif. All other data underpinning this work is freely available via “http://hdl.handle.net/10283/3047”