Abstract
Obesity is considered a global epidemic. Specifically, obesity during pregnancy programs an increased risk of the offspring developing metabolic disorders in addition to the adverse effects on the mother per se. Large numbers of human and animal studies have demonstrated that the gut microbiota plays a pivotal role in obesity and metabolic diseases. Similarly, maternal obesity during pregnancy is associated with alterations in the composition and diversity of the intestine microbial community. Recently, the microbiota in the placenta, amniotic fluid, and meconium in healthy gestations has been investigated, and the results supported the “in utero colonization hypothesis” and challenged the traditional “sterile womb” that has been acknowledged worldwide for more than a century. Thus, the offspring microbiota, which is crucial for the immune and metabolic function and further health in the offspring, might be established prior to birth. As a detrimental intrauterine environment, maternal obesity influences the microbial colonization and increases the risk of metabolic diseases in offspring. This review discusses the role of the microbiota in the impact of maternal obesity during pregnancy on offspring metabolism and further analyzes related probiotic or prebiotic interventions to prevent and treat obesity and metabolic diseases.
Keywords: gut microbiota, maternal obesity, metabolism, offspring, pregnancy
Introduction
Obesity has become an epidemic disease and is associated with tremendous health and economic burden all over the world. The World Health Organization (WHO) newly announced that there were more than 1.9 billion overweight adults worldwide in 2014, and of these, over 600 million were obese. Furthermore, over 300 million women suffer from obesity (WHO Obesity and overweight Fact Sheet No: 311, updated June 2016). A recent study on the global prevalence of obesity showed that 50% of women of childbearing ages and 20–25% of pregnant women in Europe were affected by overweight or obesity [1]. As a major risk factor for metabolic syndrome, diabetes and cardiovascular disease, obesity could lead to various metabolic disorders, including insulin resistance, hyperglycemia, hyperlipidemia, and hypertension. Obesity in women of reproductive age not only gives rise to adverse effects on the mother per se, but also affects the intrauterine environment. Barker first discovered that men with lower birth weights had higher death rates from ischemic heart disease [2] and thus put forward the “the thrifty phenotype hypothesis” [3]. He suggested that maternal under-nutrition during pregnancy and early post-partum would program offspring to adapt to this thrifty environment and develop various dysfunctions. Thereafter, the Developmental Origins of Health and Disease (DOHaD) hypothesis was brought forward in the last decade [4,5], which proposed that an adverse developmental environment in utero and early postnatal life negatively influences long-term health and increases the risk of developing obesity [6], diabetes [7], cardiovascular disease [8], and other chronic diseases. Hence, maternal obesity during pregnancy programs an increased risk of the offspring developing metabolic disturbances. Although obesity-susceptibility genes inherited from the mother may partially explain this phenomenon [9,10], substantial studies have demonstrated that epigenetics might play a role in the effects of maternal health on offspring [11–13]. However, the specific epigenetic regulation mechanism is still unclear as of yet.
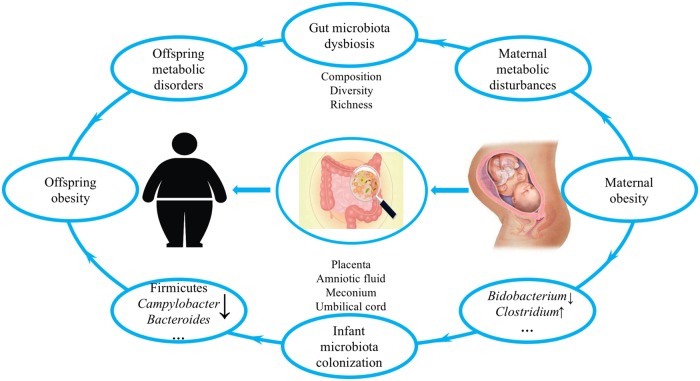
Recently, a growing number of human studies [14,15] and animal experiments [16–18] put forward the hypothesis that the gut microbiota may be identified as a novel factor that plays a significant role in maternal obesity and associated metabolic risks in offspring. During the last few decades, the gut microbiota has become a focus of medical research and has been shown to be intertwined with various diseases, such as obesity [19], diabetes [20], autoimmune diseases [21], cancer [22], and central nervous system diseases [23]. As containing the second genome in the human body, the gut microbiota has important physiological functions such as helping the host absorb fats and fat-soluble vitamins in their diet and digest complex carbohydrates and plant polysaccharides and participating in bile acid-related metabolism. In addition, gut microbiota also plays important roles in maintaining the intestinal epithelial barrier, regulating intestinal permeability, promoting maturation of the enteric nervous system, developing innate immunity, and regulating adaptive immunity [24–27]. In healthy people, the commensal microbiota in the gut is in a balanced symbiosis with the host. However, the equilibrium of this symbiotic relationship is susceptible to the host’s diet, lifestyle, and antibiotics [28]. Substantial human and animal evidence has demonstrated that the composition and diversity of the gut microbiota are altered in obese development [19,29,30]. Some studies have shown a typical increase in the number of Firmicutes and a decrease in the number of Bacteroidetes in obese compared with those in lean individuals and mice [31]. However, some have reported an inversely altered ratio of Firmicutes:Bacteroidetes in obesity [32]. The bacteria that is present or absent and contributes to the development of obesity still has yet to be elucidated. Although the infant gut microbiota used to be widely accepted to be established and colonized postpartum [33,34], in recent years, emerging studies have discovered that the microbiota exists in the placenta, amniotic fluid, umbilical cord blood, and meconium, which strongly indicates that the offspring’s microbiome may be transmitted from the mother prior to birth, and maternal microorganisms are likely to play a vital role in the establishment of the offspring microbiome [35–38]. Hence, maternal obesity during pregnancy is accompanied with gut microbiota dysbiosis with a simultaneous development of metabolic disorders, which could affect microbiota transmission from the mother to offspring and further result in offspring metabolic disturbances (Figure 1). In this review, we discuss the role of the gut microbiota in the impact of maternal obesity during pregnancy on offspring metabolic health and further explore related strategies, including probiotic and prebiotic strategies, to prevent and treat obesity and obesity-related diseases.
Figure 1. Overview of the role of gut microbiota in effects of maternal obesity during pregnancy on offspring metabolism [15,16,93,97].
Impact of obesity during pregnancy on maternal and offspring metabolism
With changes in diet and lifestyle, numerous evidence has demonstrated that the prevalence of obesity in women of childbearing ages is still increasing rapidly [39–41]. Maternal obesity during pregnancy, resulting in a poor intrauterine environment, has adverse outcomes for both the mother and child [42].
Obesity in pregnancy not only affects maternal metabolism, but also leads to detrimental pregnancy outcomes. A population-based prospective cohort study in the Netherlands detected that maternal obesity during pregnancy increased the risks of gestational diabetes, gestational hypertension, preeclampsia, and caesarean section compared to pregnancy with normal weight [43]. Similarly, another systematic review of 22 reviews also showed that gestational diabetes, pre-eclampsia, gestational hypertension, depression, instrumental and caesarean delivery, and surgical site infection were strongly linked with maternal obesity in contrast to women with normal body mass index (BMI). Obesity in pregnancy is also associated with a greater risk of adverse pregnancy consequences, including large-for-gestational age (LGA) babies, preterm birth, fetal defects, congenital anomalies, and perinatal death [44].
Moreover, animal studies also demonstrated that an obesogenic high-fat diet during pregnancy caused maternal hyperinsulinemia and decreased insulin sensitivity [45]. Mina and colleagues also used a high-fat diet prior to and throughout pregnancy and lactation to create obese animal models and found that body fat and plasma corticosterone levels were both elevated in high-fat diet–fed dams [46].
In addition to the impact on maternal health, a body of studies demonstrated that overnutrition during pregnancy increased the susceptibility to metabolic diseases in offspring [42,47]. In the Helsinki Birth Cohort Study, Eriksson et al. [48] indicated that maternal pregnancy BMI was positively associated with offspring health outcomes later in life, particularly cardiovascular disease and type 2 diabetes. Similarly, in another population-based prospective cohort study, Gaillard and his colleagues [49] reported that compared with children from normal-weight mothers, those from obese mothers during pregnancy had elevated risks of childhood obesity and adverse cardiometabolic outcomes, including total body and abdominal fat mass, systolic blood pressure, insulin levels, and high-density lipoprotein cholesterol (HDL-c) levels.
Furthermore, substantial animal studies performed to verify this phenomenon and the concrete mechanisms are emerging. Maternal high-fat diet (HFD) during pregnancy leads to a poor fetal intrauterine developmental environment, which predisposes offspring to develop metabolic disorders. Bringhenti et al. [50] and Wankhade et al. [51] demonstrated that offspring born to HFD-fed dams from weaning to end of lactation, which mimics human continuous high-fat food intake, gained more body weight and adiposity than their control diet-fed littermates. In contrast, Bringhenti et al. showed that offspring from HFD dams had hypercholesterolemia, hypertriacylglycerolemia, and hyperinsulinemia as well as a higher level of leptin with reduced levels of adiponectin. Bringhenti et al. analyzed the mechanisms that led to metabolic abnormalities in the adult offspring through examining islet structure and function and observed increases in the masses of the islet, beta cells, and alpha cells, along with migration of the alpha cells from the edge to the core of the islet and down-regulation of insulin receptor substrate (IRS1), phosphatidylinositide 3-kinase (PI3k), pancreatic and duodenal homeobox 1 (Pdx1), and glucose transporter 2 (Glut2) protein expressions. They suggested that maternal HFD was responsible for impairments in the insulin signaling pathway and altered islet structure in adult offspring. However, Wankhade et al. showed enhanced predisposition to steatohepatitis in offspring born to HFD dams, accompanied by decreased α-diversity in gut microbiota profiles. In addition to the effects of maternal obesity on offspring peripheral metabolic health, a number of studies have shown that central metabolism alterations should also not be ignored. A recent study of a porcine model with the use of positron emission tomography (PET) showed that maternal HFD led to higher brain glucose metabolism and brain insulin receptors (IRβs) at birth in offspring followed by a long-term decrease in brain glucose uptake and glucose transporter 4 (GLUT4) and IRβ expression in minipigs, which may increase the predisposition to metabolic-neurodegenerative diseases in offspring [52].
Hence, obesity during pregnancy serves as a detrimental developmental environment that increases the risk of the development of metabolic diseases in both the mother and offspring. However, the specific mechanisms are not entirely understood. The relationship between the gut microbiota and obesity has been increasingly studied. Meanwhile, the significant role that the gut microbiota in newborns and infants plays in health and development has become increasingly clear. Therefore, the gut microbiota might be a critical factor in the explanation of the phenomenon put forth by the DOHaD Hypothesis.
Maternal obesity during pregnancy and alterations in gut microbiota
In the context of normal pregnancy, bodies undergo substantial changes including immunological adaption and hormonal and metabolic alterations to support the growth and development of the fetus [53]. Similar to the symptoms of metabolic syndrome, increased adiposity, reduced insulin sensitivity, and elevated levels of proinflammatory cytokines in circulation are all common but protective during a healthy pregnancy [54,55]. The commensal microbial community in the human gastrointestinal tract plays crucial roles in the immune response and metabolic homeostasis and, thus, in normal pregnancy [56,57]. In order to explore the role of gut microbiota during pregnancy, Koren et al. [58] analyzed the fecal microbiota of 91 pregnant women from the first to third trimesters with the use of 16S rRNA gene sequences (V1–V2 region) and found that while the microbial diversity was analogous to that of the Human Microbiome Project’s (HMP) normal controls during early pregnancy, significant changes in the microbial community occurred in the third trimester. Specifically, the abundance of Proteobacteria and Actinobacteria was increased but their phylogenetic diversity was reduced. In accordance with the changes in Proteobacteria, which is correlated with inflammation [59], levels of proinflammatory cytokines IFN-γ, IL2, IL6, and TNF-α were significantly increased from the first to third trimester. Furthermore, transference of the microbiota from healthy pregnancies in the third trimester to germ-free mice also resulted in mice with increased adiposity, reduced insulin sensitivity, and a chronic inflammatory condition compared with mice that received microbiota from those in the first trimester [58]. Therefore, the gut microbial community plays a critical role in maternal metabolic adaptations and supports the growth and development of the fetus during normal pregnancy. However, another study done by DiGiulio et al. [60] showed outcomes completely different from Koren’s. They analyzed the microbiota from a case–control study including 49 pregnant women, 15 of whom delivered preterm, with the use of 16S rRNA gene sequences (V3–V5 region). From 40 of these women, they characterized the bacterial taxonomic composition of 3767 specimens collected prospectively and weekly during gestation from the vagina, distal gut, saliva, and teeth/gums rather than a single time point from each of the first and third trimesters as in Koren’s study. Consequently, DiGillio et al. demonstrated that the composition and diversity of the gut microbiota in pregnant women were stable. The distinct differences between Koren’s and DiGiulio’s study outcomes may be partly attributed to heterogeneity in genetics, age, ethnicity, lifestyle, BMI, and gestational age of the participants [61]. Specifically, in Koren’s study, 16 of the women took probiotic supplements during pregnancy, and seven used antibiotics that could greatly influence the composition and diversity of the gut microbiota. Preterm delivery and different 16S rRNA gene sequencing regions might be another two major influencing factors. Furthermore, more studies are needed to clarify the changes in the gut microbiota during normal pregnancy and its effects on fetal and maternal development.
Substantial evidence has demonstrated that the diversity and function of the gut microbiota changes in nonpregnant obesity. To be exact, compared with that observed in lean subjects, the microbial diversity is lower and the ratio of Firmicutes to Bacteroidetes is altered, although controversial, in obese subjects [62–64]. Similarly, changes in the gut microbiota occur in overweight and obese pregnant women when compared with that in normal-weight pregnant women. Collado et al. [65] first compared the composition of the gut microbiota assessed by fluorescent in situ hybridization coupled with flow cytometry (FCM-FISH) and by quantitative real-time polymerase chain reaction (qPCR) in overweight and normal-weight pregnant women from a prospective follow-up study and found significant alterations in the gut microbial community between the two groups, of which Bacteroides and Staphylococcus were the most significantly increased in overweight and obese women. However, another subsequent observational study of 50 pregnant women in Spain analyzed the composition of the gut microbiota by qPCR and showed that Enterobacteriaceae, Escherichia coli, and Staphylococcus numbers were increased but Bifidobacterium and Bacteroides abundances were significantly reduced in overweight and obese pregnant women, which is not consistent with the previous study. The ratio of Bifidobacterium to Clostridium coccoides was significantly lower during pregnancy in overweight and obese women than in normal-weight women. These researchers also analyzed the relationship between gut microbiota composition and biochemical parameters. Increased total bacteria and Staphylococcus numbers were positively related to increased plasma cholesterol levels. Decreased Bacteroides numbers were associated with lower HDL-cholesterol and folic acid levels and higher TAG levels. These associations could be explained by the generation of different short-chain free acids (SCFAs) and regulation of the host gene expression to influence lipid metabolism [66–68]. Lower Bifidobacterium numbers were related to reduced folic acid levels, which may result from the ability of this genus to synthesize and secrete folates in the intestinal environment, providing a complementary endogenous source of this vitamin [69]. Increased Enterobacteriaceae and E. coli numbers were associated with increased ferritin and reduced transferrin [70]. A decrease in transferrin and an increase in ferritin were associated with a decrease in antibacterial activity of serum against Enterobacteria [71]. Thus, distinct changes of the gut microbiota occur in overweight and obese women during pregnancy. These discrepant findings require further confirmation and may be explained by heterogeneity in the gestational age, genetics, dietary intake, and supplementation of the participants and especially by different assessing methods.
In addition, emerging animal studies verified the relationship between maternal obesity and changes in the gut microbiota. In female Sprague–Dawley rats, a high-fat/sucrose diet-induced obesity group had higher blood glucose, plasma insulin, and leptin concentrations and lower peptide-YY (PYY) levels than the lean group. Meanwhile, the composition of the fecal microbiota was altered, with reduced fecal Bidobacterium spp. numbers and increased number of Clostridium clusters XI and I [16]. The phenomenon that gut microbiota shifted physiologically during pregnancy in order to adapt the mother to pregnancy and facilitate optimal fetal growth and development was simultaneously demonstrated in animal study. A recent animal study suggested that there were 26 genera of gut microbiota found to be significantly different in HFD pregnant mice from those in normal diet mice. Of these, 11 genera were also identified to be significantly different between nonpregnant mice fed normal diet and nonpregnant HFD–fed mice, and another 15 genera were independently associated with the pregnant state. Furthermore, these shifts in the gut microbiota could predict the changes in lipid metabolism, glycolysis, and gluconeogenic metabolic pathways [72].
Alterations in the gut microbial community are an attempt to adapt the mother to pregnancy, contribute to the function of the placenta, and promote fetal growth and development in the physiologically pregnant state. During obese pregnancy, as showed above, altered gut microbiota will lead to metabolic disturbances in mother by affecting the production of SCFAs, participating in the metabolism of other substances and regulating gene expression in host, which indirectly could affect the infant’s development and the establishment of its microbiota. Although the altered composition of the gut microbiota is still controversial among different studies, and the concrete mechanisms modulating maternal gut microbial alterations are currently unclear, overweight and obesity during pregnancy result in obvious changes in microbiota composition and concomitant metabolic disorders in the mother.
Establishment of the infant microbial community
The healthy adult human gastrointestinal tract is colonized by more than 1014 bacteria with over 1000 prevalent species, which include 100 times more genes than the human genome [73–75]. The first colonization of gut microbiota in early life is a critical window for development and further health and plays a significant role in programming immune development and metabolic function [35]. For more than a century, the intrauterine environment has been accepted to be sterile, and the microbiota of a neonate has been thought to be colonized during birth and in postnatal life, although there was some early controversy [33,76]. However, the recent “in utero colonization hypothesis” challenges the idea of the traditional “sterile womb” [77]. In the last few years, a multitude of emerging human and animal studies have indicated that the infant microbiome is probably established before birth as specific microbiota and microbial DNA were detected in the placenta, amniotic fluid, umbilical cord, and infant meconium during normal pregnancy with the use of modern sequencing technologies, indicating that the colonization of the infant microbiota was initiated in utero [38,78,79].
In a study of 21 healthy term neonates, a number of bacteria were detected in the meconium first through traditional culture, among which genera Enterococcus fecalis and Staphylococcus were the most predominant [79]. Then, another study collected the meconium of 23 newborns, including 9 neonates from healthy mothers, and 14 born to mothers with diabetes or pre-diabetes. Using 16S rRNA sequencing to profile the meconium microbiota, the study showed that the meconium microbiota of newborns born to healthy mothers was significantly different from adult stool, including a lower proportion of Bacteroidetes and Firmicutes that are the predominant phyla in adult stool samples and a higher proportion of Proteobacteria [80]. This observation suggested that the bacterial composition of the meconium was a proxy for the gut microbiota in utero and supports the theory of microbiota colonization prior to birth. Subsequently, Collado et al. [38] analyzed the microbiota in the maternal placenta, amniotic fluid, and meconium of 15 caesarean-section at-full-term mother–infant pairs and proposed that a unique microbiota exists in the placenta and amniotic fluid, characterized by low abundance, low diversity, and predominant Proteobacteria. The meconium also shared some similarities with the microbial communities in the placenta and amniotic fluid, which could be explained by the fetus swallowing the amniotic fluid. Our latest study first compared the placental microbiota between macrosomic newborns and newborns with normal birth weight and demonstrated that the microbiota in the placenta of normal neonates was indeed significantly different from that in the macrosomic placenta [81].
However, the exact mechanism of maternal–fetal microbial transfer is still unclear and several hypotheses have been put forward. Interestingly, Perez et al. [82] indicated that bacterial translocation of the intestinal mucosa was significantly increased in pregnant mice compared with that in nonpregnant animals in 2007. Then Jiménez et al. [79] orally infused genetically labeled E. fecium strain to pregnant mice and reported that the strain could be detected in the meconium of the infused mice but not in the meconium of the noninfused control group in 2008. Thus, the microbial community in the maternal gastrointestinal tract influences the establishment of the fetal microbiota in the meconium. In another words, the meconium microbial community might originate from the maternal intestine tract. Differently, after analyzing the placental microbiota from 320 subjects, Aagaard et al. [36] suggested that the placental microbiota mainly included microbiota from Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes, and Fusobacteria phyla and were most similar to the human oral microbiota. However, they compared the placental microbiota to the microbiota of other nonpregnant human body sites, including the oral, skin, airway, vaginal, and gut microbiota, and thus, the results were not very convincing. Another more recent study done by Gomez-Arango et al. [83] agreed with Aagaard and demonstrated that the placental microbiota exhibited a higher similarity to the pregnant oral microbiota. What is more, the gut, oral, and placental microbiota they detected by 16S rRNA sequencing were from the same pregnant women [83]. The vaginal microbiota might be another origin of the fetal microbiota by virtue of its proximity to the intrauterine environment. Lactobacilli is the predominant microbiota in the vagina during pregnancy [60,84]. Although Lactobacilli are indeed found in placental membranes [85], the microbiota in utero is diverse, as mentioned previously. Thus, the vagina is at least not the only colonization pathway of the fetal microbiome. The exact origin of the intrauterine microbiota is still unclear and requires more research.
Previous data showed that bacteria could be detected in utero only in cases of intrauterine infection and preterm delivery, and the intrauterine environment was sterile during healthy pregnancy based on microscopy and bacterial culture techniques [33,86,87]. With the development of modern sequencing methods, emerging evidence has demonstrated that microbial communities do exist in the placenta, amniotic fluid, and meconium. However, Perez-Muñoz et al. [77] described the weakness of the evidence supporting the “in utero colonization hypothesis”, including a lack of appropriate controls for contamination, failure of detection approaches to detect low-abundance microbial communities, and no evidence of bacterial viability. Therefore, more studies are needed to reveal the time and mechanism of vertical microbiota transmission and colonization in offspring.
The effects of maternal obesity on the offspring gut microbiota
Both our own studies and other studies have shown that maternal HFD could program a significantly increased predisposition to obesity and metabolic disorders in offspring [46,88–90]. As mentioned above, obesity of pregnant mothers alters the abundance and diversity of the gut microbiota and, thus, as a detrimental intrauterine developmental environment, may influence the establishment of the microbial community in offspring. Large numbers of studies have verified that maternal obesity is associated with gut microbiota dysbiosis in offspring. In a mouse study done by Wankhade et al., a significantly lower α-diversity of the fecal microbial community was identified in offspring of mice with maternal HFD, which was concomitant with a greater weight gain, fatty liver, and increased proinflammatory hepatic transcriptome in the offspring [51]. Furthermore, Bruce-Keller et al. [93] transplanted gut microbiota from mice fed a HFD or control diet for 3 months to mothers. The β-diversity of the gut microbiota in both weaned female and weaned male offspring from the HFD-fed dams was significantly lower than that from the control diet dams, among which the numbers of Firmicutes phylum were reduced. The Firmicutes phylum have the ability to produce butyrate, a main energy source for intestinal epithelium, playing a critical role in maintaining the integrity of the intestinal epithelial barrier [91,92] and associated with neurobehavioral disorder [93]. Studies have shown that abnormal SCFAs produced by gut microbiota dysbiosis could increase host energy extraction from the diet and influence the levels of satiety hormone, which could lead to altered food intake and modify immune cells in the intestinal tract, further influencing metabolic health in the mother and vertically transmitting to the offspring [94–96]. In addition to the effects on intestinal microbiota establishment in early life, in a non-human primate model, Ma et al. [97] demonstrated that maternal HFD compared with a maternal control diet persistently reduced the abundance of Campylobacter until the primates were juveniles.
Moreover, human studies have also consistently indicated that maternal obesity and overnutrition alter the gut microbiota in offspring. In a prospective cohort study done by Chu et al. [15], 163 women in the early third trimester or intrapartum were enrolled and divided into a high-fat group and control group according to a dietary questionnaire covering the past month. The meconium and stool of neonates at delivery and 6 weeks of age were collected and subjected to 16S rRNA gene sequencing. A significant reduction in Bacteroides in the neonatal intestinal microbiota from HFD mothers during gestation was found that persisted to 6 weeks of age [15]. Similarly, another study analyzed the gut microbiota of 77 children born to obese or normal weight mothers and showed that the numbers of Parabacteroides spp. and Oscillibacter spp. were higher in children born to obese mother than in those born to normal weight mothers from a higher socioeconomic status; additionally, the numbers of Blautia spp. and Eubacterium spp. were lower, of which Eubacteriaceae, Oscillibacter, and Blautia have been found to be associated with diet and obesity in previous studies [98,99]. Hence, the increased risk of obesity for children with an obese mother could be explained partially by the transmission of maternal obesogenic intestinal microbes [17]. The changes in the gut microbiota in offspring born to obese mother have still been controversial in recent studies. The differences may be due to heterogeneity in BMI, mode of delivery, feeding methods in early life, and use of antibiotics at delivery. More studies that control for these confounding factors are needed to clarify the specific changes in offspring.
Both animal and human studies have demonstrated that changes in the diversity and abundance of the intestinal microbial community in mothers associated with maternal HFD and obesity were linked with gut microbiota alterations in the offspring in early and later life. In addition, the metabolic health of the offspring was impaired. Thus, the gut microbiota may be a crucial pathogenic mechanism in the maternal obesity programing of an increased susceptibility to obesity and metabolic disorders in offspring. Improvement in the maternal gut microbiota dysbiosis might also promote offspring metabolic health and maintain the balance of the intestinal microbial community.
Intervention with probiotics and prebiotics in the mother
Probiotics
Probiotics have been defined by an international consensus panel as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [100]. Certain specific probiotics can be used to decrease the risk of the development of diseases correlated with increased intestinal permeability, abnormal gut microbiota composition, or impaired immunological or metabolic balance [101]. An increasing number of human and animal studies have shed light on the beneficial effects of probiotic supplements on obesity and metabolic disturbances. A probiotic formulation could ameliorate obesity and obesity-related metabolic disorders by virtue of its action on the composition of the gut microbiota, intestinal barrier integrity, and related metabolites [102–104]. Intervening with probiotics during pregnancy could be the optimum time to reduce the risk of obesity and metabolic disturbances in both the mother and offspring.
A randomized controlled trial (RCT) done in Finland recruited 256 pregnant women without metabolic disorders during their first trimester. Participants were randomized into a control group, dietary counselling intervention group, or dietary counselling with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12) intervention group from the first trimester of pregnancy to 6 months after delivery [105–107]. In the dietary counselling with probiotics group, the fasting blood glucose concentrations were the lowest, and glucose tolerance was better during pregnancy and through the 12-month postpartum period. Furthermore, the frequency of gestational diabetes mellitus (GDM) decreased from 34% in the control group to 13% in the group that received dietary counselling and probiotics. Meanwhile, probiotics-supplemented dietary counselling reduced the risk of central obesity in women at 6 months postpartum. This study suggested that probiotic supplements might be a novel management tool for the prevention and treatment of obesity. In addition, another similar RCT study in Iran with 64 pregnant women with GDM randomized to receive probiotic supplement (Lactobacillus acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31, and Lactobacillus delbrueckii bulgaricus LBY-27) or placebo for 8 weeks also found that the probiotic supplements significantly reduced gestational weight gain and fasting blood glucose and improved insulin sensitivity [108]. Wickens et al. [109] also indicated that probiotic Lactobacillus rhamnosus HN001 supplementation from 14 to 16 weeks’ gestation in women with a history of atopic disease may reduce GDM prevalence from 6.5% (95% CI 3.5, 10.9) in the placebo group to 2.1% (95% CI 0.6, 5.2). However, neither of the above RCT studies examined the effects of maternal probiotic supplements on the metabolic health of the offspring in later life. A follow-up study from birth to 10 years of age with 159 pregnant women in Finland aimed to identify the influences of probiotic (Lactobacillus rhamnosus GG, ATCC 53103) intervention from 4 weeks before delivery to 6 months postnatally on the development of overweight and obesity. Prenatal probiotic supplements restrained excessive weight gain during early life (before 12–24 months) and may have altered the growth pattern in offspring. Gut microbiota modification of probiotics in early life might adjust the energy homeostasis and thus influence the body weight gain and prevent obesity [110].
Rather than examining the effects of probiotics in a normal-weight pregnancy, a double-blind, placebo-controlled, randomized trial in Ireland explored the impact of probiotic intake on obese pregnancy. They recruited 179 pregnant women whose BMI was from 30.0 to 39.9 at early pregnancy and then divided them randomly into groups that received probiotic (Lactobacillus salivarius UCC118) or placebo capsules from 24 to 28 weeks of gestation, and 138 women completed the study. This probiotic strain was chosen by virtue of its existence in the intestinal tract of normal people and its impact on immune reactions [111]. The results showed that the 4-week probiotic capsule intervention during pregnancy had no effect on maternal fasting glucose, the prevalence of impaired glucose tolerance (IGT), infant birth weight or other metabolic profiles or pregnancy outcomes. After adjustments for the use of antibiotics and compliance, the results were still the same [112]. However, there were only 9 cases of GDM and 15 cases of IGT in this study, and thus, the statistical power was limited. Furthermore, the probiotic intervention was only performed for 4 weeks, and the bacteria strain chosen may not have been optimal for obese women during pregnancy as it has merely been studied in the healthy population.
The optimal bacteria strains of probiotics and the concrete mechanisms for their positive effects are still unclear. Current literature is limited to studies with low statistical power and a general lack of consistency in their population characteristics and probiotic strains. We look forward to the results of another RCT study exploring probiotics to prevent gestational diabetes mellitus in overweight and obese women in Australia, which used the same probiotic strains as the first study in Finland. This SPRING trial may demonstrate a role of probiotics in reducing the prevalence of GDM in high-risk pregnant women [113].
Prebiotics
Prebiotics were first defined in 1995 as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon” [114]. After that, the concept of prebiotic was reaffirmed several times, and the latest definition was updated by the International Scientific Association for Probiotics and Prebiotics in December 2016 as “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” This concept indicates that prebiotics include not merely carbohydrates but also substances other than food and that prebiotics could manipulate the microbiota in regions other than in the intestinal tract [115]. Prebiotics are defined to be beneficial to human well-being in terms of both the modulation of the microbiota and the production of metabolites. Prebiotics contribute to health by increasing the growth of Bifidobacteria and Lactobacilli and producing SCFAs, which are considered to be beneficial to human well-being by improving the integrity of the gut barrier, modulating the host immune reaction, and decreasing the abundance of pathogenic bacteria, such as Clostridia [116]. Substantial evidence has demonstrated that prebiotic intake could reduce the risk of developing diarrhea [117], inflammatory bowel disease [118], colon cancer [119], cardiovascular disease [120], obesity [121], metabolic disturbances [122], and other diseases and increase the uptake and bioavailability of calcium and magnesium [123].
Studies on the effects of maternal prebiotic intake during pregnancy on offspring are listed in Table 1. In light of the impact of prebiotic intervention during pregnancy on the metabolic health of mothers and their offspring, several animal studies have been done to shed light on this phenomenon [16,124–126]. First, Maurer et al. [124] explored the influences of a maternal high-prebiotic fiber diet (21.6%) on the expression of genes regulating glucose and lipid metabolism and satiety hormones in offspring. In offspring from dams fed the high-prebiotic fiber diet, the weights of the small intestine and colon were increased significantly at 14 and 21 days; the level of plasma amylin was lower at 7, 14, and 21 days; the expression of intestinal glucose transporters 5 (GLUT5) and Na+-dependent glucose/galactose transporter 1 (SGLT 1) was up-regulated; and the level of glucagon-like peptide-1 (GLP-1) was higher at 21 days than in offspring born to control diet-fed dams. Thus, a maternal high-prebiotic fiber diet might program the development of the intestinal tract and the expression of satiety hormones and genes associated with glucose metabolism in offspring in early life, which could influence the long-term obesity risk. Similarly, Hallam et al. [125] suggested that offspring of dams fed a high-prebiotic fiber diet (21.6%) had a lower plasma lipopolysaccharide level and elevated Bifidobacteria in the gut microbiota until 22 weeks with the use of an animal model and experimental design analogous to Maurer’s. These results indicated that the maternal high-prebiotic fiber diet contributed to offspring health by modulating the composition of the gut microbiota. Meanwhile, Hallam et al. [126] switched offspring from a control diet to a high-fat/sucrose diet for 8 weeks at 14.5 weeks of age and demonstrated that after a high energy challenge, the weight, percentage of fat mass, liver TAG content, and plasma nonesterified fatty acid (NEFA) levels were lower in female offspring of dams fed the high-prebiotic fiber diet. Therefore, maternal prebiotic intake could reduce the risk of obesity and improve long-term metabolic health in offspring. However, only one of the above studies measured changes in the gut microbiota in offspring after prebiotic intervention. Another recent study assessed the impact of prebiotic intervention during pregnancy and lactation on the composition of the maternal gut microbiota and suggested that an intervention with 10% oligofructose in high-fat/sucrose diet-induced obese rats during pregnancy and lactation could improve the maternally altered gut microbiota with a higher relative abundance of Bifidobacterium spp., Bacteroides/Prevotella spp., and Clostridium cluster I as well as reduce the energy intake and gestational weight gain compared with those in the high-fat/sucrose diet group. In addition, the risk of obesity and the metabolic profiles of their offspring were both ameliorated [16]. These observations suggested that early prebiotic intervention in the mother benefited both the maternal and offspring metabolic health and was of great clinical significance. More studies are needed to verify these findings and further clarify the concrete mechanism of the positive effects of prebiotics.
Table 1. Evidence that maternal prebiotic exposures during pregnancy are associated with programming offspring health.
| Maternal prebiotic exposures | Animal model | Effects on offspring | Reference |
|---|---|---|---|
| Galacto-oligosaccharide/inulin mixture | Balb/c mice | Prevents food allergies | [127] |
| Specific mixture of short-chain galacto- and long-chain fructo-oligosaccharides | Balb/c and C57BL/6 mice | Reduces symptoms of allergic asthma | [128] |
| Fructo-oligosaccharide | NC/Nga mice | Diminishes the severity of atopic dermatitis-like skin lesions | [129] |
| Oligofructose | Sprague–Dawley rat | Reduces obesity risk | [16] |
| Oligofructose and inulin | Wistar rat | Decreases fat mass | [125] |
| Inulin and oligofructose | Wistar rat | Regulates expression of circulating metabolism associated hormones and genes | [124] |
| Oligofructose-enriched inulin | Sprague-Dawley rat | Improves bone microarchitecture | [130] |
| Caprine milk oligosaccharides | C57BL/6 mice | Improves the development | [131] |
| Galacto-oligosaccharides-inulin | Balb/cj mice | Enhances intestinal and muscle mass growth | [132] |
| Short-chain fructo-oligosaccharide | Sow | Strengths gut defenses and immune response | [133] |
Conclusion
In summary, the gut microbiota might be the key programming factor in maternal obesity increasing the risk of the development of metabolic disorders in offspring. However, the mechanisms remain unclear. In addition to direct vertical transmission of microbes, microbial dysbiosis influences bile acid metabolism in the host body and changes the metabolites of the microbiota, such as SCFAs, which play important roles in host metabolism and immunity to affect metabolic health in both the mother and offspring [134]. In addition, some scholars have suggested that the microbiota could impact a set of epigenetic factors, including DNA methylation, histone modification and noncoding RNAs, which will further change metabolism-related gene expression in the host [135]. Thus, gut microbiota dysbiosis in obese mothers leads to multiple downstream effects on immune and metabolic functions and further health in offspring.
There have been a few studies that have suggested that probiotics and prebiotics positively modulate the gut microbiota and their metabolites, restrain weight gain, reduce the frequency of gestational diabetes mellitus, and improve blood glucose, insulin sensitivity and glucose metabolism-associated gene expression. Changing the composition of the gut microbiota specifically through the use of probiotics and prebiotics during pregnancy could become a new strategy for reducing the risk of metabolic disorders in both the mother and offspring. Consequently, there is an urgent need for large, well-designed randomized controlled clinical trials to further dissect the use of probiotics and prebiotics as a prevention strategy, in addition to mechanistic studies clarifying potential mechanisms of action in humans.
Abbreviations
- BMI
body mass index
- HDL-c
high-density lipoprotein cholesterol
- HFD
high-fat diet
- IFN-γ
interferon-γ
- IGT
impaired glucose tolerance
- IL2
interleukin 2
- IL6
interleukin 6
- IRβ
insulin receptor
- LGA
large-for-gestational age
- SCFA
short-chain free acid
- TNF-α
tumor necrosis factor-α
Funding
This work was supported by the National Natural Science Foundation of China [81170736 and 81570715].
Author Contribution
All authors contributed to the manuscript and approved the final version.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M. et al. (2012) National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 10, 22 10.1186/1478-7954-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker D.J., Winter P.D., Osmond C., Margetts B. and Simmonds S.J. (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580 10.1016/S0140-6736(89)90710-1 [DOI] [PubMed] [Google Scholar]
- 3.Hales C.N. and Barker D.J. (1992) Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 35, 595–601 10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- 4.Charles M.A., Delpierre C. and Breant B. (2016) Developmental origin of health and adult diseases (dohad): Evolution of a concept over three decades. Med. Sci. (Paris) 32, 15–20 10.1051/medsci/20163201004 [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld C.S. (2017) Homage to the ‘h’ in developmental origins of health and disease. J. Dev. Orig. Health Dis. 8, 8–29 10.1017/S2040174416000465 [DOI] [PubMed] [Google Scholar]
- 6.Mourtakos S.P., Tambalis K.D., Panagiotakos D.B., Antonogeorgos G., Arnaoutis G., Karteroliotis K. et al. (2015) Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: A study of 5,125 children. BMC Pregnancy Childbirth 15, 66 10.1186/s12884-015-0498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson K., Jonsson I., Malmqvist E., Larsson H.E. and Rylander L. (2015) Maternal smoking during pregnancy and offspring type 1 diabetes mellitus risk: Accounting for hla haplotype. Eur. J. Epidemiol. 30, 231–238 10.1007/s10654-014-9985-1 [DOI] [PubMed] [Google Scholar]
- 8.Roberts V.H., Frias A.E. and Grove K.L. (2015) Impact of maternal obesity on fetal programming of cardiovascular disease. Physiology (Bethesda) 30, 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U. et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindorff L.A., Sethupathy P., Junkins H.A., Ramos E.M., Mehta J.P., Collins F.S. et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. U.S.A. 106, 9362–9367 10.1073/pnas.0903103106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunford A.R. and Sangster J.M. (2017) Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab. Syndr. 11, S655–S662 10.1016/j.dsx.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 12.Houshmand-Oeregaard A., Hansen N.S., Hjort L., Kelstrup L., Broholm C., Mathiesen E.R. et al. (2017) Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin. Epigenetics 9, 37 10.1186/s13148-017-0338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang X., Yang Q., Fu X., Rogers C.J., Wang B., Pan H. et al. (2016) Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 594, 4453–4466 10.1113/JP272123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozyrskyj A.L., Kalu R., Koleva P.T. and Bridgman S.L. (2016) Fetal programming of overweight through the microbiome: Boys are disproportionately affected. J. Dev. Orig. Health Dis. 7, 25–34 10.1017/S2040174415001269 [DOI] [PubMed] [Google Scholar]
- 15.Chu D.M., Antony K.M., Ma J., Prince A.L., Showalter L., Moller M. et al. (2016) The early infant gut microbiome varies in association with a maternal high-fat diet. Genome. Med. 8, 77 10.1186/s13073-016-0330-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul H.A., Bomhof M.R., Vogel H.J. and Reimer R.A. (2016) Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 6, 20683 10.1038/srep20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galley J.D., Bailey M., Kamp Dush C., Schoppe-Sullivan S. and Christian L.M. (2014) Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One 9, e113026 10.1371/journal.pone.0113026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J., Xiao X., Zhang Q., Yu M., Xu J., Qi C. et al. (2016) The programming effects of nutrition-induced catch-up growth on gut microbiota and metabolic diseases in adult mice. Microbiologyopen 5, 296–306 10.1002/mbo3.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvit K.B. and Kharchenko N.V. (2017) Gut microbiota changes as a risk factor for obesity. Wiad. Lek. 70, 231–235 [PubMed] [Google Scholar]
- 20.Wang X., Xu X. and Xia Y. (2017) Further analysis reveals new gut microbiome markers of type 2 diabetes mellitus. Antonie Van Leeuwenhoek 110, 445–453 10.1007/s10482-016-0805-3 [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal A., Sarangi A.N., Gaur P., Shukla A. and Aggarwal R. (2017) Gut microbiome in children with enthesitis-related arthritis in a developing country and the effect of probiotic administration. Clin. Exp. Immunol. 187, 480–489 10.1111/cei.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J., Tan Q., Fu Q., Zhou Y., Hu Y., Tang S. et al. (2017) Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential clinical implications. Breast Cancer 24, 220–228 10.1007/s12282-016-0734-z [DOI] [PubMed] [Google Scholar]
- 23.Tremlett H., Bauer K.C., Appel-Cresswell S., Finlay B.B. and Waubant E. (2017) The gut microbiome in human neurological disease: A review. Ann. Neurol. 81, 369–382 10.1002/ana.24901 [DOI] [PubMed] [Google Scholar]
- 24.Natividad J.M. and Verdu E.F. (2013) Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 69, 42–51 10.1016/j.phrs.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Gensollen T., Iyer S.S., Kasper D.L. and Blumberg R.S. (2016) How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J. and Bakker B.M. (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q., Shao W., Zhang C., Xu C., Wang Q., Liu H. et al. (2017) Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 226, 268–276 10.1016/j.envpol.2017.03.068 [DOI] [PubMed] [Google Scholar]
- 28.Thursby E. and Juge N. (2017) Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 10.1042/BCJ20160510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerard P. (2016) Gut microbiota and obesity. Cell. Mol. Life Sci. 73, 147–162 10.1007/s00018-015-2061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiilerich P., Myrmel L.S., Fjaere E., Hao Q., Hugenholtz F., Sonne S.B. et al. (2016) Effect of a long-term high-protein diet on survival, obesity development, and gut microbiota in mice. Am. J. Physiol. Endocrinol. Metab. 310, E886–E899 10.1152/ajpendo.00363.2015 [DOI] [PubMed] [Google Scholar]
- 31.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D. and Gordon J.I. (2005) Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 102, 11070–11075 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwiertz A., Taras D., Schafer K., Beijer S., Bos N.A., Donus C. et al. (2010) Microbiota and scfa in lean and overweight healthy subjects. Obesity (Silver Spring) 18, 190–195 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 33.Escherich T. (1989) The intestinal bacteria of the neonate and breast-fed infant. 1885. Rev. Infect. Dis. 11, 352–356 10.1093/clinids/11.2.352 [DOI] [PubMed] [Google Scholar]
- 34.Harris J.W., Brown J.H., Clark J.G. and Ferguson L.K. (1928) The bacterial content of the uterus at cesarean section. Am. J. Obstet. Gynecol. 16, 7 10.1016/S0002-9378(28)90975-8 [DOI] [Google Scholar]
- 35.Koleva P.T., Kim J.S., Scott J.A. and Kozyrskyj A.L. (2015) Microbial programming of health and disease starts during fetal life. Birth Defects Res. C Embryo Today 105, 265–277 10.1002/bdrc.21117 [DOI] [PubMed] [Google Scholar]
- 36.Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J. and Versalovic J. (2014) The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satokari R., Gronroos T., Laitinen K., Salminen S. and Isolauri E. (2009) Bifidobacterium and lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 48, 8–12 10.1111/j.1472-765X.2008.02475.x [DOI] [PubMed] [Google Scholar]
- 38.Collado M.C., Rautava S., Aakko J., Isolauri E. and Salminen S. (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 6, 23129 10.1038/srep23129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnunen T.I., Skogberg N., Harkanen T., Lundqvist A., Laatikainen T. and Koponen P. (2017) Overweight and abdominal obesity in women of childbearing age of russian, somali and kurdish origin and the general finnish population. J. Public Health (Oxf.), 15, 1–9 10.1093/pubmed/fdx053 [DOI] [PubMed] [Google Scholar]
- 40.Szostak-Wegierek D., Waskiewicz A., Piotrowski W., Stepaniak U., Pajak A., Kwasniewska M. et al. (2017) Metabolic syndrome and its components in polish women of childbearing age: a nationwide study. BMC Public Health 18, 15 10.1186/s12889-017-4564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araya B.M., Padilla O., Garmendia M.L., Atalah E. and Uauy R. (2014) prevalence of obesity among chilean women in childbearing ages. Rev. Med. Chil. 142, 1440–1448 [DOI] [PubMed] [Google Scholar]
- 42.Hussen H.I., Persson M. and Moradi T. (2015) Maternal overweight and obesity are associated with increased risk of type 1 diabetes in offspring of parents without diabetes regardless of ethnicity. Diabetologia 58, 1464–1473 10.1007/s00125-015-3580-1 [DOI] [PubMed] [Google Scholar]
- 43.Gaillard R., Durmus B., Hofman A., Mackenbach J.P., Steegers E.A. and Jaddoe V.W. (2013) Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 21, 1046–1055 10.1002/oby.20088 [DOI] [PubMed] [Google Scholar]
- 44.Marchi J., Berg M., Dencker A., Olander E.K. and Begley C. (2015) Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 16, 621–638 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Twinn D.S., Gascoin G., Musial B., Carr S., Duque-Guimaraes D., Blackmore H.L. et al. (2017) Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 7, 44650 10.1038/srep44650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai M., Jellyman J.K., Han G., Beall M., Lane R.H. and Ross M.G. (2014) Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 211, 237.e231–237.e213 10.1016/j.ajog.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catalano P. and deMouzon S.H. (2015) Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. (Lond.) 39, 642–649 10.1038/ijo.2015.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksson J.G., Sandboge S., Salonen M.K., Kajantie E. and Osmond C. (2014) Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the helsinki birth cohort study. Ann. Med. 46, 434–438 10.3109/07853890.2014.919728 [DOI] [PubMed] [Google Scholar]
- 49.Gaillard R., Steegers E.A., Duijts L., Felix J.F., Hofman A., Franco O.H. et al. (2014) Childhood cardiometabolic outcomes of maternal obesity during pregnancy: The generation R study. Hypertension 63, 683–691 10.1161/HYPERTENSIONAHA.113.02671 [DOI] [PubMed] [Google Scholar]
- 50.Bringhenti I., Ornellas F., Mandarim-de-Lacerda C.A. and Aguila M.B. (2016) The insulin-signaling pathway of the pancreatic islet is impaired in adult mice offspring of mothers fed a high-fat diet. Nutrition 32, 1138–1143 10.1016/j.nut.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 51.Wankhade U.D., Zhong Y., Kang P., Alfaro M., Chintapalli S.V., Thakali K.M. et al. (2017) Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One 12, e0175675 10.1371/journal.pone.0175675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanguinetti E., Liistro T., Mainardi M., Pardini S., Salvadori P.A., Vannucci A. et al. (2016) Maternal high-fat feeding leads to alterations of brain glucose metabolism in the offspring: positron emission tomography study in a porcine model. Diabetologia 59, 813–821 10.1007/s00125-015-3848-5 [DOI] [PubMed] [Google Scholar]
- 53.Newbern D. and Freemark M. (2011) Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18, 409–416 10.1097/MED.0b013e32834c800d [DOI] [PubMed] [Google Scholar]
- 54.Barbour L.A., McCurdy C.E., Hernandez T.L., Kirwan J.P., Catalano P.M. and Friedman J.E. (2007) Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30, S112–S119 10.2337/dc07-s202 [DOI] [PubMed] [Google Scholar]
- 55.Kirwan J.P., Hauguel-De Mouzon S., Lepercq J., Challier J.C., Huston-Presley L., Friedman J.E. et al. (2002) Tnf-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 51, 2207–2213 10.2337/diabetes.51.7.2207 [DOI] [PubMed] [Google Scholar]
- 56.Kelly D., King T. and Aminov R. (2007) Importance of microbial colonization of the gut in early life to the development of immunity. Mutat. Res. 622, 58–69 10.1016/j.mrfmmm.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 57.Smid M.C., Ricks N.M., Panzer A., McCoy A.N., Azcarate-Peril M.A., Keku T.O. et al. (2018) Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol 35, 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Backhed H.K. et al. (2012) Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukhopadhya I., Hansen R., El-Omar E.M. and Hold G.L. (2012) Ibd-what role do proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 9, 219–230 10.1038/nrgastro.2012.14 [DOI] [PubMed] [Google Scholar]
- 60.DiGiulio D.B., Callahan B.J., McMurdie P.J., Costello E.K., Lyell D.J., Robaczewska A. et al. (2015) Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. U.S.A. 112, 11060–11065 10.1073/pnas.1502875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soderborg T.K., Borengasser S.J., Barbour L.A. and Friedman J.E. (2016) Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia 59, 895–906 10.1007/s00125-016-3880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenbaum M., Knight R. and Leibel R.L. (2015) The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 26, 493–501 10.1016/j.tem.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters W.A., Xu Z. and Knight R. (2014) Meta-analyses of human gut microbes associated with obesity and ibd. FEBS Lett. 588, 4223–4233 10.1016/j.febslet.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson E., Ryan P.M., Cryan J.F., Dinan T.G., Ross R.P., Fitzgerald G.F. et al. (2016) Gut microbiota, obesity and diabetes. Postgrad. Med. J. 92, 286–300 10.1136/postgradmedj-2015-133285 [DOI] [PubMed] [Google Scholar]
- 65.Collado M.C., Isolauri E., Laitinen K. and Salminen S. (2008) Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 [DOI] [PubMed] [Google Scholar]
- 66.Ley R.E., Turnbaugh P.J., Klein S. and Gordon J.I. (2006) Microbial ecology: Human gut microbes associated with obesity. Nature 444, 1022–1023 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 67.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R. and Gordon J.I. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 68.Pouteau E., Nguyen P., Ballevre O. and Krempf M. (2003) Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 62, 87–93 10.1079/PNS2003208 [DOI] [PubMed] [Google Scholar]
- 69.Strozzi G.P. and Mogna L. (2008) Quantification of folic acid in human feces after administration of bifidobacterium probiotic strains. J. Clin. Gastroenterol. 42, S179–S184 10.1097/MCG.0b013e31818087d8 [DOI] [PubMed] [Google Scholar]
- 70.Santacruz A., Collado M.C., Garcia-Valdes L., Segura M.T., Martin-Lagos J.A., Anjos T. et al. (2010) Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92 10.1017/S0007114510000176 [DOI] [PubMed] [Google Scholar]
- 71.Jolivet-Gougeon A., Loreal O., Ingels A., Danic B., Ropert M., Bardou-Jacquet E. et al. (2008) Serum transferrin saturation increase is associated with decrease of antibacterial activity of serum in patients with hfe-related genetic hemochromatosis. Am. J. Gastroenterol. 103, 2502–2508 10.1111/j.1572-0241.2008.02036.x [DOI] [PubMed] [Google Scholar]
- 72.Gohir W., Whelan F.J., Surette M.G., Moore C., Schertzer J.D. and Sloboda D.M. (2015) Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 6, 310–320 10.1080/19490976.2015.1086056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C. et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dietert R.R. and Dietert J.M. (2015) The microbiome and sustainable healthcare. Healthcare (Basel) 3, 100–129 10.3390/healthcare3010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marchesi J.R. (2010) Prokaryotic and eukaryotic diversity of the human gut. Adv. Appl. Microbiol. 72, 43–62 10.1016/S0065-2164(10)72002-5 [DOI] [PubMed] [Google Scholar]
- 76.Küstner O. (1877) Beitrag zur lehre von der puerperalen infection der neugeborenen. Archiv. für Gynäkologie 11, 256–263 10.1007/BF01845161 [DOI] [Google Scholar]
- 77.Perez-Munoz M.E., Arrieta M.C., Ramer-Tait A.E. and Walter J. (2017) A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 5, 48 10.1186/s40168-017-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wassenaar T.M. and Panigrahi P. (2014) Is a foetus developing in a sterile environment? Lett. Appl. Microbiol. 59, 572–579 10.1111/lam.12334 [DOI] [PubMed] [Google Scholar]
- 79.Jimenez E., Marin M.L., Martin R., Odriozola J.M., Olivares M., Xaus J. et al. (2008) Is meconium from healthy newborns actually sterile? Res. Microbiol. 159, 187–193 10.1016/j.resmic.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 80.Hu J., Nomura Y., Bashir A., Fernandez-Hernandez H., Itzkowitz S., Pei Z. et al. (2013) Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One 8, e78257 10.1371/journal.pone.0078257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng J., Xiao X.H., Zhang Q., Mao L.L., Yu M., Xu J.P. et al. (2017) Correlation of placental microbiota with fetal macrosomia and clinical characteristics in mothers and newborns. Oncotarget 8, 82314–82325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez P.F., Dore J., Leclerc M., Levenez F., Benyacoub J., Serrant P. et al. (2007) Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 119, e724–e732 10.1542/peds.2006-1649 [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Arango L.F., Barrett H.L., McIntyre H.D., Callaway L.K., Morrison M. and Nitert M.D. (2017) Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci. Rep. 7, 2860 10.1038/s41598-017-03066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Nikita L. et al. (2014) The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2, 4 10.1186/2049-2618-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prince A.L., Ma J., Kannan P.S., Alvarez M., Gisslen T., Harris R.A. et al. (2016) The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 214, 627.e621–627.e616 10.1016/j.ajog.2016.01.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vinturache A.E., Gyamfi-Bannerman C., Hwang J., Mysorekar I.U. and Jacobsson B. (2016) Maternal microbiome - a pathway to preterm birth. Semin. Fetal Neonatal Med. 21, 94–99 10.1016/j.siny.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 87.Keelan J.A. and Payne M.S. (2015) Vaginal microbiota during pregnancy: pathways of risk of preterm delivery in the absence of intrauterine infection? Proc. Natl. Acad. Sci. U.S.A. 112, E6414 10.1073/pnas.1517346112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng J., Xiao X., Zhang Q., Yu M., Xu J. and Wang Z. (2014) Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the ppar pathway in the early life of offspring. Int. J. Mol. Sci. 15, 14967–14983 10.3390/ijms150914967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng J., Xiao X., Zhang Q., Yu M., Xu J., Qi C. et al. (2016) The effects of maternal and post-weaning diet interaction on glucose metabolism and gut microbiota in male mice offspring. Biosci. Rep. 36, e00341, 10.1042/BSR20160103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohta T., Toriniwa Y., Ryumon N., Inaba N., Hirao T., Yamanaka S. et al. (2017) Maternal high-fat diet promotes onset of diabetes in rat offspring. Anim. Sci. J. 88, 149–155 10.1111/asj.12606 [DOI] [PubMed] [Google Scholar]
- 91.Shoaie S., Karlsson F., Mardinoglu A., Nookaew I., Bordel S. and Nielsen J. (2013) Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci. Rep. 3, 2532 10.1038/srep02532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canani R.B., Costanzo M.D., Leone L., Pedata M., Meli R. and Calignano A. (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 17, 1519–1528 10.3748/wjg.v17.i12.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruce-Keller A.J., Fernandez-Kim S.O., Townsend R.L., Kruger C., Carmouche R., Newman S. et al. (2017) Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One 12, e0175577 10.1371/journal.pone.0175577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I. et al. (2011) Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94, 58–65 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang P.V., Hao L., Offermanns S. and Medzhitov R. (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U.S.A. 111, 2247–2252 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J. et al. (2015) Short-chain fatty acids induce both effector and regulatory t cells by suppression of histone deacetylases and regulation of the mtor-s6k pathway. Mucosal. Immunol. 8, 80–93 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma J., Prince A.L., Bader D., Hu M., Ganu R., Baquero K. et al. (2014) High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 5, 3889 10.1038/ncomms4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clarke S.F., Murphy E.F., O’Sullivan O., Ross R.P., O’Toole P.W., Shanahan F. et al. (2013) Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS One 8, e65790 10.1371/journal.pone.0065790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G. et al. (2013) Gut microbiome composition is linked to whole grain-induced immunological improvements. Isme J. 7, 269–280 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B. et al. (2014) Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 101.Isolauri E., Rautava S., Collado M.C. and Salminen S. (2015) Role of probiotics in reducing the risk of gestational diabetes. Diabetes Obes. Metab. 17, 713–719 10.1111/dom.12475 [DOI] [PubMed] [Google Scholar]
- 102.Shin J.H., Nam M.H., Lee H., Lee J.S., Kim H., Chung M.J. et al. (2017) Amelioration of obesity-related characteristics by a probiotic formulation in a high-fat diet-induced obese rat model. Eur. J. Nutr. 60, 1–10 10.1007/s00394-017-1481-4 [DOI] [PubMed] [Google Scholar]
- 103.Bron P.A., Kleerebezem M., Brummer R.J., Cani P.D., Mercenier A., MacDonald T.T. et al. (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 117, 93–107 10.1017/S0007114516004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Muzafar H.M. and Amin K.A. (2017) Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 17, 43 10.1186/s12906-016-1540-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laitinen K., Poussa T. and Isolauri E. (2009) Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br. J. Nutr. 101, 1679–1687 10.1017/S0007114508111461 [DOI] [PubMed] [Google Scholar]
- 106.Ilmonen J., Isolauri E., Poussa T. and Laitinen K. (2011) Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin. Nutr. 30, 156–164 10.1016/j.clnu.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 107.Luoto R., Laitinen K., Nermes M. and Isolauri E. (2010) Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br. J. Nutr. 103, 1792–1799 10.1017/S0007114509993898 [DOI] [PubMed] [Google Scholar]
- 108.Dolatkhah N., Hajifaraji M., Abbasalizadeh F., Aghamohammadzadeh N., Mehrabi Y. and Abbasi M.M. (2015) Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial J. Health Popul. Nutr. 33, 25 10.1186/s41043-015-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wickens K.L., Barthow C.A., Murphy R., Abels P.R., Maude R.M., Stone P.R. et al. (2017) Early pregnancy probiotic supplementation with lactobacillus rhamnosus hn001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br. J. Nutr. 117, 804–813 10.1017/S0007114517000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luoto R., Kalliomaki M., Laitinen K. and Isolauri E. (2010) The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int. J. Obes. (Lond.) 34, 1531–1537 10.1038/ijo.2010.50 [DOI] [PubMed] [Google Scholar]
- 111.Collins J.K., Dunne C., Murphy L., Morrissey D., O’Mahony L. and O’Sullivan E. (2002) A randomised controlled trial of a probiotic lactobacillus strain in healthy adults assessment of its delivery, transit and in uence on microbial flora and enteric immunity. Microb. Ecol. Health Dis. 14, 81–89 10.1080/08910600260081720 [DOI] [Google Scholar]
- 112.Lindsay K.L., Kennelly M., Culliton M., Smith T., Maguire O.C., Shanahan F. et al. (2014) Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (probiotics in pregnancy study). Am. J. Clin. Nutr. 99, 1432–1439 10.3945/ajcn.113.079723 [DOI] [PubMed] [Google Scholar]
- 113.Nitert M.D., Barrett H.L., Foxcroft K., Tremellen A., Wilkinson S., Lingwood B. et al. (2013) Spring: An rct study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Childbirth 13, 50 10.1186/1471-2393-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gibson G.R. and Roberfroid M.B. (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 [DOI] [PubMed] [Google Scholar]
- 115.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J. et al. (2017) Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol Hepatol. 14, 491–502 [DOI] [PubMed] [Google Scholar]
- 116.Slavin J. (2013) Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5, 1417–1435 10.3390/nu5041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Generoso S.V., Lages P.C. and Correia M.I. (2016) Fiber, prebiotics, and diarrhea: What, why, when and how. Curr. Opin. Clin. Nutr. Metab. Care 19, 388–393 10.1097/MCO.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 118.Wasilewski A., Zielinska M., Storr M. and Fichna J. (2015) Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1674–1682 10.1097/MIB.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 119.Qamar T.R., Syed F., Nasir M., Rehman H., Zahid M.N., Liu R.H. et al. (2016) Novel combination of prebiotics galacto-oligosaccharides and inulin-inhibited aberrant crypt foci formation and biomarkers of colon cancer in wistar rats. Nutrients 8, 465, 10.3390/nu8080465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchell C.M., Davy B.M., Halliday T.M., Hulver M.W., Neilson A.P., Ponder M.A. et al. (2015) The effect of prebiotic supplementation with inulin on cardiometabolic health: rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials 45, 328–337 10.1016/j.cct.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carnahan S., Balzer A., Panchal S.K. and Brown L. (2014) Prebiotics in obesity. Panminerva Med. 56, 165–175 [PubMed] [Google Scholar]
- 122.Beserra B.T., Fernandes R., do Rosario V.A., Mocellin M.C., Kuntz M.G. and Trindade E.B. (2015) A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 34, 845–858 10.1016/j.clnu.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 123.Legette L.L., Lee W., Martin B.R., Story J.A., Campbell J.K. and Weaver C.M. (2012) Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J. Food Sci. 77, H88–94 10.1111/j.1750-3841.2011.02612.x [DOI] [PubMed] [Google Scholar]
- 124.Maurer A.D. and Reimer R.A. (2011) Maternal consumption of high-prebiotic fibre or -protein diets during pregnancy and lactation differentially influences satiety hormones and expression of genes involved in glucose and lipid metabolism in offspring in rats. Br. J. Nutr. 105, 329–338 10.1017/S0007114510003533 [DOI] [PubMed] [Google Scholar]
- 125.Hallam M.C., Barile D., Meyrand M., German J.B. and Reimer R.A. (2014) Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity (Silver Spring) 22, 2344–2351 10.1002/oby.20849 [DOI] [PubMed] [Google Scholar]
- 126.Hallam M.C. and Reimer R.A. (2013) A maternal high-protein diet predisposes female offspring to increased fat mass in adulthood whereas a prebiotic fibre diet decreases fat mass in rats. Br. J. Nutr. 110, 1732–1741 10.1017/S0007114513000998 [DOI] [PubMed] [Google Scholar]
- 127.Bouchaud G., Castan L., Chesne J., Braza F., Aubert P., Neunlist M. et al. (2016) Maternal exposure to gos/inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy 71, 68–76 10.1111/all.12777 [DOI] [PubMed] [Google Scholar]
- 128.Hogenkamp A., Thijssen S., van Vlies N. and Garssen J. (2015) Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J. Nutr. 145, 640–646 10.3945/jn.114.197707 [DOI] [PubMed] [Google Scholar]
- 129.Fujiwara R., Takemura N., Watanabe J. and Sonoyama K. (2010) Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of nc/nga mice. Br. J. Nutr. 103, 530–538 10.1017/S000711450999198X [DOI] [PubMed] [Google Scholar]
- 130.Bueno-Vargas P., Manzano M., Diaz-Castro J., Lopez-Aliaga I., Rueda R. and Lopez-Pedrosa J.M. (2016) Maternal dietary supplementation with oligofructose-enriched inulin in gestating/lactating rats preserves maternal bone and improves bone microarchitecture in their offspring. PLoS One 11, e0154120 10.1371/journal.pone.0154120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thum C., McNabb W.C., Young W., Cookson A.L. and Roy N.C. (2016) Prenatal caprine milk oligosaccharide consumption affects the development of mice offspring. Mol. Nutr. Food Res. 60, 2076–2085 10.1002/mnfr.201600118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Desbuards N., Gourbeyre P., Haure-Mirande V., Darmaun D., Champ M. and Bodinier M. (2012) Impact of perinatal prebiotic consumption on gestating mice and their offspring: a preliminary report. Br. J. Nutr. 107, 1245–1248 10.1017/S0007114511004363 [DOI] [PubMed] [Google Scholar]
- 133.Le Bourgot C., Le Normand L., Formal M., Respondek F., Blat S., Apper E. et al. (2017) Maternal short-chain fructo-oligosaccharide supplementation increases intestinal cytokine secretion, goblet cell number, butyrate concentration and lawsonia intracellularis humoral vaccine response in weaned pigs. Br. J. Nutr. 117, 83–92 10.1017/S0007114516004268 [DOI] [PubMed] [Google Scholar]
- 134.Priyadarshini M., Thomas A., Reisetter A.C., Scholtens D.M., Wolever T.M., Josefson J.L. et al. (2014) Maternal short-chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl. Res. 164, 153–157 10.1016/j.trsl.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takahashi K. (2014) Influence of bacteria on epigenetic gene control. Cell. Mol. Life Sci. 71, 1045–1054 10.1007/s00018-013-1487-x [DOI] [PMC free article] [PubMed] [Google Scholar]